Abstract
Objectives
The purpose of this study was to prospectively examine the relationship between anemia and incident fractures of the hip, spine and all skeletal sites in women from diverse racial and ethnic backgrounds enrolled in the Women's Health Initiative (WHI) Observational Study and Clinical Trials.
Design
Prospective cohort study.
Setting
40 WHI clinical centers across the US.
Participants
Postmenopausal women (n = 160,080), mean age 63.2 (SD: 7.2) years, were recruited and followed for an average of 7.8 years.
Measurements
Anemia was defined as hemoglobin levels at baseline less than 12 g/dL. All fractures were self-reported. Hip fractures were further confirmed by trained physicians using medical records.
Results
Among the participants 8,739 women (5.5%) were anemic. The age-adjusted incidence rate of hip fractures per 10,000 person years were 21.4 in women with anemia and 15.0 in women without anemia; a higher incidence rate for spine or all fractures in anemic women was also observed. After multiple covariates were included in the Cox proportional hazards models, significant increased fracture risk associated with anemia still existed as demonstrated by the hazards ratios (95% confidence interval) of fractures associated with anemia being 1.38 (1.13–1.68), 1.30 (1.09–1.55) and 1.07 (1.01–1.14) for hip, spine and all-types respectively. No significant racial/ethnic difference was found in these relationships.
Conclusion
A significantly increased fracture risk was observed in multi-ethnic postmenopausal women with anemia. Given the high prevalence of anemia in the elderly population, it is important to better understand the relationship and mechanisms linking anemia to fracture risk.
Keywords: fracture risk, anemia, hemoglobin, prospective studies
INTRODUCTION
Anemia is a common health problem in older populations. The World Health Organization (WHO) defines anemia as a hemoglobin level of less than 13g/dL in men, and less than 12g/dL in women.[1, 2] In the Third National Health and Nutrition and Examination Survey (NHANES III) the prevalence of anemia, as defined by WHO criteria, was over 10% for adults age 65 years and older.[3] The prevalence of anemia varies by race/ethnicity and increases with age.[3]
Anemia in older adults has been shown to be associated with disability, declines in physical performance,[4] low muscle strength, reduced muscle density[5] and increased mortality independent from other co-morbidities.[6] In addition, bone density has been found to be significantly lower in older people with anemia as compared to people of the same age. This difference in bone density was persistent even after adjusting for body mass index (BMI).[7] Studies have also demonstrated low muscle mass and strength and higher risk of fall among older people with anemia.[8–11] Elevated fall rates and reduced muscle mass, muscle strength, and bone density are well known risk factors for bone fractures. Therefore, intuitively, anemia may elevate an individual's risk of fractures. To our knowledge no direct evidence are available demonstrating a greater fracture risk in older people with anemia versus those without anemia. It is well known that African Americans have an increased risk for anemia[3] but a lower risk for fractures[12, 13] in comparison to people from other race/ethnic backgrounds. In contrast, both fracture[12, 13] and anemia[3] risks increase with aging. However, whether the association between anemia and fracture varies by age and race/ethnicity is unknown and should be investigated in order to better identify anemic people at increased risk for fractures.
In this prospective cohort study we aimed to investigating the association of anemia with fracture risk in U.S. post-menopausal women from diverse racial and ethnic backgrounds enrolled in the Women's Health Initiative (WHI) Observational Study (OS) and Clinical Trials (CT). The following hypotheses were tested: 1) anemia is associated with an increased fracture risk in post-menopausal women and 2) in spite of the variations in anemia rates by age and/or race/ethnicity, the magnitude of the increased fracture risk associated with anemia is similar in all age and racial/ethnic groups. To evaluate potential mediating factors that link anemia with fractures we have also examined the contribution of falls and self-reported general health (fair/poor health versus good/excellent health) to the relationship between anemia and fracture risk.
METHODS
Participants
This study was conducted using data from the WHI, the largest U.S. health study among postmenopausal women. Between 1993 and 1998, WHI recruited 161,808 participants from 40 WHI clinical centers across the United States. To qualify for the WHI enrollment, women had to be between age of 50–79, post-menopausal and not likely to relocate or die within 3 years of study enrollment. Additional inclusion and exclusion criteria were applied to women in the WHI clinical trials. Details regarding the study design, inclusion and exclusion criteria, recruitment procedures, participants characteristics, intervention regimens, randomization, blinding, and follow-up have been previously published in a number of papers[14–17] for the WHI-OS[18] and WHI-CT[17] arms. The study protocol and consent forms were approved by the institutional review boards for all participating institutions.
Data Collection
Self-administered or interviewer-administered questionnaires were completed by WHI participants for eligibility screening and collection of baseline characteristics (such as demographic, reproductive, and health status information). Physical examinations were conducted and a blood specimen was collected at baseline. The WHI OS women visited the WHI clinical center at 3 year intervals and the WHI CT women visited the clinic every year for follow-up physical measurements and blood collections. The CT participants completed additional questionnaires every 6 months and OS participants every year. The average follow-up time was 7.8 years in this current analysis.
Anemia definition
Hemoglobin levels were measured on all WHI participants at baseline in the context of a complete blood count (CBC) test conducted by designated clinical labs in the surrounding areas of each WHI clinical center. For this analysis, a woman was considered to have anemia if her hemoglobin concentration at baseline was less than 12g/dL, using the WHO criteria. A total of 160,080 study participants had baseline hemoglobin measurements (CT= 68,080, OS=91,999).
Fracture Assessments
During follow-up, 160,934 women (CT = 67,881 and OS = 93,053) had completed at least one medical history update providing information regarding new fracture diagnosis (in the previous 12 months for OS and 6 months for CT participants). Specifically, women were asked, “Since (last reporting date), has a doctor told you that you had a broken, fractured, or crushed bone?” If “Yes” was selected, then they were asked to answer “Which bone did you break, fracture, or crush?” by marking all that apply from the following list: 1) Hip, 2) Upper leg (not hip), 3) Pelvis, 4) Knee (patella), 5) Lower leg or ankle, 6) Foot (not toe), 7) Tailbone (coccyx), 8) Spine or back (vertebra), 9) Lower arm or wrist, 10) Hand (not finger), 11) Elbow, 12) Upper arm or shoulder, 13) Other (specify). The term “all-type” includes any reported fracture from the previous list. Self-reported hip fractures were adjudicated with medical records by trained adjudicators; all non-hip fractures were self-reported without adjudication.
Assessments of Covariates
Information on age, race/ethnicity, education, cohabitation, smoking, use of hormone therapy, history of fracture, number of falls in last 12 months, medication or supplement use (corticosteroids, thiazide, estrogen, calcium regulators, calcium, vitamin D, and iron) chronic diseases (depression, diabetes, cardiovascular disease, hypertension, arthritis, osteoporosis, cancer, asthma, and emphysema), number of hospitalization, parental fracture after age 40, parity, self-reported health status (poor/fair health versus good/excellent health), physical activity (total METS/wk) and nutrient intakes (calcium, vitamin D, iron, and total energy) were assessed from baseline questionnaires or follow-up questionnaires. Physical function and depression at baseline were measured using the 10-item Medical Outcomes Study Scale[19] and the shortened Center for Epidemiologic Studies Depression Scale (CES-D),[20] respectively. Weight was measured to the nearest 0.1kg on a balance beam scale with the participant dressed in indoor clothing without shoes. Height was measured to the nearest 0.1 centimeter using a wall-mounted stadiometer. Body mass index (BMI) was calculated as: weight (kg)/height (m)2.[21, 22]
Statistical Analysis
Descriptive analyses were done on baseline characteristics of the study participants and the results were presented as mean (95% confidence interval (CI), median (interquartile range) or frequencies (%) by anemia group at baseline. T-tests, Wilcoxon rank sum or chi-square tests were conducted on the baseline characteristics of the participants. Age-adjusted incidence rates of fractures (all-types and site specific), including 95% CI, were presented by anemia status for total participants and by race/ethnicity. Absolute incidence rate difference (anemia – no anemia) by 10 year age groups was calculated and presented in a bar chart. The likelihood ratio test was used to test the null hypothesis of equal incidence rate difference across age groups.
The Cox-proportional hazards model was used to test differences in the relative risk of fractures by anemia status. Potential confounding effects of covariates, including co-morbidity, falls, physical activity, nutrient intakes, self reported general health status, physical function and demographic characteristics were examined and controlled for when applicable. Potential confounding factors were identified in the marginal analysis if the p-value was less than 0.2, and then examined one by one and as a group in the Cox proportional hazards regression analysis to assess their independent and collective impacts on the relationship between anemia and fracture risk. If a variable significantly affected the hazards ratio (change a hazards ratio more than 10%), then it was considered as a confounding factor and included in the final model. Three sets of models were developed. The first set was adjusted for study components (OS participants versus CT controls and interventions, each separately). The second was adjusted for study assignment/intervention plus race/ethnicity, the only statistically significant confounding factor for all models. The third model set included all the above plus the following: age, height, weight, general health status (fair/poor health versus good/excellent health), baseline number of falls, total calcium intake, total vitamin D intake, total iron intake, physical function, physical activity, smoking, hormone use, fractured after the age of 55, depression, diabetes ever, osteoporosis ever, and cancer ever. The large number of covariates in model 3 was selected from previously reported risk factors for osteoporotic fractures and WHI interventions.[21, 22] The third model set was developed to get a more conservative assessment on the fracture risk after adjusting for the reported factors that are related to fracture risk. Interaction terms of race/ethnicity and anemia, and age group and anemia were tested for possible racial/ethnic and age group variations in the association between anemia and fracture.
Kaplan Meier cumulative incidence probability curves were generated to show longitudinal differences in fracture risk by anemia status.
Stratified analyses were also conducted by race/ethnicity in the Cox proportional hazards regression. All analyses were performed with Stata statistical software (version 10.0, Statacorp, College Station, TX).
RESULTS
A total of 160,080 women who had a baseline hemoglobin measurement were included in this study. Among them, 8,739 women had hemoglobin levels below 12 g/dL. Descriptive analyses by baseline anemia status (Table 1) showed that women with anemia were significantly different from women without anemia in regards to most of the baseline characteristics examined. A markedly higher percent of African American women were anemic. In general, women with anemia were older, had a lower body weight and reported poorer health, lower physical function, lower level of physical activity, higher risk for depression, and a slightly higher frequency of falls. Women with anemia also reported lower intakes of calcium, vitamin D and iron. No significant difference in history of bone fractures at age 55 years or older was observed by baseline anemia status. However, women with anemia versus women without were more likely to report a previous diagnosis of osteoporosis.
Table 1.
Baseline characteristics by anemia (N= 160,080)
| No Anemia N= 151,341 | Anemia N= 8,739 | |||
|---|---|---|---|---|
| N | % | N | % | |
| Age at Baseline | *** | |||
| 50–59 | 50,204 | 33.2 | 2,729 | 31.2 |
| 60–69 | 67,998 | 44.9 | 3,853 | 44.1 |
| 70–79 | 33,139 | 21.9 | 2,157 | 24.7 |
| Ethnicity | *** | |||
| American Indian or Alaskan Native | 652 | 0.4 | 52 | 0.6 |
| Asian or Pacific Islander | 3,970 | 2.6 | 170 | 1.9 |
| African-American | 12,101 | 8.0 | 2,316 | 26.6 |
| Hispanic/Latino | 6,118 | 4.1 | 318 | 3.7 |
| White (not of Hispanic origin) | 126,450 | 83.8 | 5,726 | 65.8 |
| Other | 1,673 | 1.1 | 121 | 1.4 |
| Study arm | *** | |||
| OS | 86,459 | 57.1 | 5,540 | 63.4 |
| CT | 64,881 | 42.9 | 3,199 | 36.6 |
| Smoking Status | *** | |||
| never smoked | 75,952 | 50.8 | 4,600 | 53.5 |
| past smoker | 62,760 | 42.0 | 3,639 | 42.4 |
| current smoker | 10,685 | 7.2 | 352 | 4.1 |
| Hormone therapy use | *** | |||
| Never | 66,402 | 43.9 | 3,790 | 43.4 |
| past user | 24,412 | 16.1 | 1,248 | 14.3 |
| current user | 60,402 | 39.9 | 3,694 | 42.3 |
| fracture at age 55+ | ||||
| No | 96,403 | 83.6 | 5,618 | 82.9 |
| yes | 18,850 | 16.4 | 1,156 | 17.1 |
| Depression | *** | |||
| No | 131,118 | 89.0 | 7,354 | 87.5 |
| Yes | 16,119 | 11.0 | 1,052 | 12.5 |
| Diabetes ever | *** | |||
| No | 142,525 | 94.2 | 7,942 | 91.0 |
| Yes | 8,721 | 5.8 | 785 | 9.0 |
| Osteoporosis ever | *** | |||
| No | 137,937 | 92.4 | 7,762 | 90.5 |
| yes | 11,276 | 7.6 | 820 | 9.5 |
| Cancer ever | *** | |||
| no | 136,402 | 90.9 | 7,691 | 88.8 |
| yes | 13,623 | 9.1 | 974 | 11.2 |
| General self-reported health status | *** | |||
| excellent | 25,890 | 17.2 | 1,172 | 13.5 |
| very good | 61,867 | 41.1 | 3,067 | 35.4 |
| good | 49,417 | 32.9 | 3,126 | 36.1 |
| fair | 12,122 | 8.1 | 1,173 | 13.5 |
| poor | 1,087 | 0.7 | 128 | 1.5 |
| Physical Functioning Score † | *** | |||
| <80 | 44,885 | 30.2 | 3,410 | 40.1 |
| 80–90 | 48,307 | 32.5 | 2,440 | 28.7 |
| 91–100 | 55,317 | 37.3 | 2,655 | 31.2 |
| Number of falls in past year | *** | |||
| none | 97,948 | 67.6 | 5,662 | 66.5 |
| 1 time | 29,087 | 20.1 | 1,678 | 19.7 |
| 2 times | 11,977 | 8.3 | 748 | 8.8 |
| 3 or more times | 5,993 | 4.0 | 423 | 5.0 |
|
|
||||
| Mean | SD | Mean | SD | |
|---|---|---|---|---|
| Physical Activity (mets) | 12.4 | 13.7 | 11.7 | 13.6*** |
| Height (cm) | 161.8 | 6.6 | 161.2 | 6.7*** |
| Weight (kg) | 73.6 | 16.8 | 73.0 | 18.3*** |
| BMI (kg/m2) | 28.0 | 5.9 | 27.9 | 6.5 |
| Median | IQR | Median | IQR | |
|---|---|---|---|---|
| Total intake of Iron (mg) | 16.2 | 10.4–27.9 | 15.4 | 9.6–27.7*** |
| Total intake of Calcium (mg) | 1035.0 | 654.6–1,542.9 | 925.4 | 554.5–1,444.2*** |
| Total intake of Vitamin D (IU) | 306.2 | 135.3–552.6 | 257.5 | 113.6–525.6*** |
p≤0.001; calculated using chi-square T-tests, or Wilcoxon rank sum tests IRQ: interquartile range
better physical function with higher score
Absolute Risk for Fractures---Incidence Rates
The age-adjusted incidence rates for all-types, hip, and spinal fractures by baseline anemia status are presented in Table 2. Women with anemia, among the entire cohort and the non-Hispanic white ethnic group, compared to women without anemia had a higher fracture incidence rate of the hip, spine and all-types. Since the numbers of fractures in the anemia group were small among minorities no clear patterns of associations between anemia and higher fracture incidence rates could be identified for these groups.
Table 2.
Incidence rates* of fractures per 10,000 person-years (95% CI) by race/ethnicity
| Hip | Spine | All-types | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Anemia | No Anemia | Anemia | No Anemia | Anemia | No Anemia | |||||||
| #Fx | IR (95% CI) | #Fx | IR (95% CI) | #Fx | IR (95% CI) | #Fx | IR (95% CI) | #Fx | IR (95% CI) | #Fx | IR (95% CI) | |
| Total WHI | 150 | 21.4 (17.9, 24.8) | 1,756 | 15.0 (14.3, 15.7) | 174 | 25.5 (21.7, 29.3) | 2,692 | 23.0 (22.1, 23.9) | 1,449 | 234.3 (222.2, 246.4) | 25,245 | 232.5 (229.6, 235.4) |
| White | 128 | 26.6 (21.9, 31.2) | 1,657 | 16.8 (16.0, 17.7) | 159 | 34.4 (29.0, 39.8) | 2,502 | 25.4 (24.4, 26.4) | 1,129 | 275.9' (259.7, 292.1) | 22,549 | 248.1 (244.9, 251.4) |
| Hispanic | 1 | 4.8 (0, 14.1) | 23 | 5.2 (3.1, 7.3) | 3 | 13.0 (0, 27.9) | 47 | 10.6 (7.5, 13.6) | 33 | 161.0 (105.6, 216.5) | 639 | 150.9 (139.2, 162.6) |
| African American | 14 | 7.4 (3.5, 11.4) | 37 | 4.1 (2.8, 5.4) | 4 | 2.3 (0.04, 4.6) | 51 | 5.6 (4.1, 7.1) | 235 | 142.0 (123.7, 160.2) | 1,215 | 139.4 (131.6, 147.2) |
| Asian | 3 | 16.6 (0, 35.3) | 15 | 5.1 (2.5, 7.6) | 4 | 25.4 (0, 50.9) | 45 | 15.2 (10.8, 19.7) | 21 | 158.5 (86.0, 230.9) | 428 | 151.3 (137.0, 165.6) |
| American Indian | 0 | NA | 8 | 16.6 (5.1, 28.1) | 0 | NA | 10 | 20.6 (7.8, 33.4) | 6 | 153.8 (28.2, 279.3) | 120 | 272.3 (223.6, 321.1) |
age-adjusted; #Fx: number of fractures; IR: incidence rate; CI: confidence interval
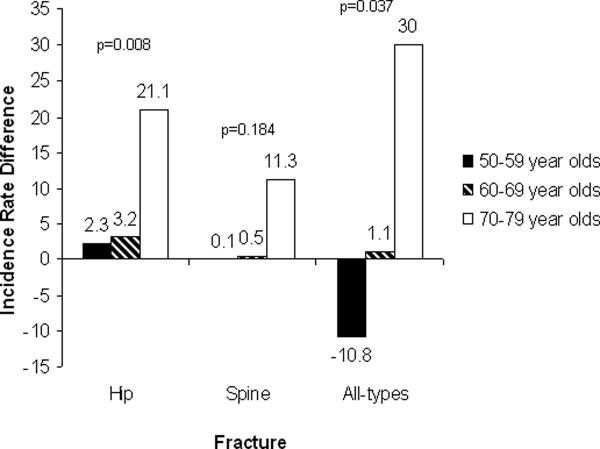
In Figure 1, differences in fracture incidence rates between anemic and non-anemic women are presented by 10 year age groups. The magnitude of increased fracture incidence rate by anemia appears larger in the 70 or older age group in comparison to the younger age groups, especially for the hip and all-types fracture groups.
Figure 1.
Differences in incidence rates of fracture per 10,000 person-years between anemic and non-anemic women (absolute incidence rate difference= anemia group incidence – no anemia group incidence)
Relative Risk for Fractures---Hazard Ratios
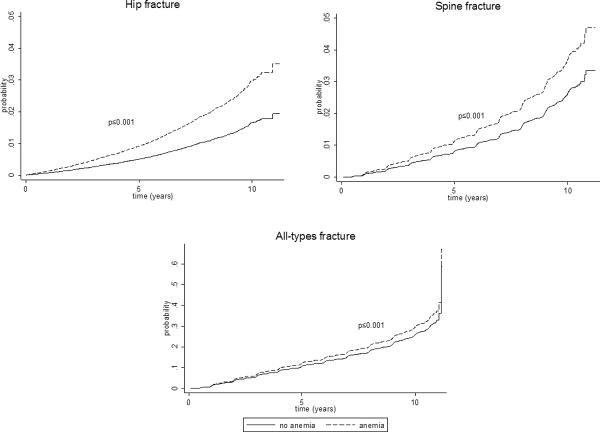
The results from the Cox model indicated that the risk for hip, spinal and all-types of fractures were increased among women with anemia and the largest elevated risk was for hip fractures. After adjusting for study assignment/intervention and the confounding factor, race/ethnicity, the hazards ratios (95%CI) were 1.81 (1.53–2.15), 1.41 (1.20–1.64), and 1.14 (1.08–1.20) for hip, spine and all-types of fractures, respectively (Table 3). The cumulative incidence of fracture based on model 2 is presented in Figure 2, showing greater incidence rate for hip, spine and all-types of fractures even after adjusting for the significant confounder, race/ethnicity.
Table 3.
Risk of fracture by anemia status
| Model 1 (N= 159,218)* | Model 2 (N=158,809)† | Model 3 (N=112,269)‡ | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | HR | 95% CI | HR | 95% CI | |
| Hip | 1.55 | 1.32, 1.84 | 1.81 | 1.53, 2.15 | 1.38 | 1.13, 1.69 |
| Spine | 1.17 | 1.01, 1.37 | 1.41 | 1.20, 1.64 | 1.30 | 1.09, 1.55 |
| All-types | 1.04 | 0.98, 1.09 | 1.14 | 1.08, 1.20 | 1.07 | 1.01, 1.14 |
adjusted for study arm assignment and interventions (observation study participants versus clinical trial controls or interventions)
adjusted for all in model 1 plus race/ethnicity
adjusted for all in model 2 plus age, height, weight, self reported general health (fair/poor health versus good/excellent health), baseline number of falls (none versus one, two, or three or more falls), diabetes ever, osteoporosis ever, cancer ever, total calcium intake, total vitamin D intake, total iron intake, physical function(< 80 score versus 80–90 score or 91–100 score), physical activity (total METS/wk), smoking (never versus past or current smoker), hormone therapy (never versus past or current user), fractured after age 55, and depression
Figure 2.
Cumulative incidence of fracture, adjusted for study arm assignment and race/ethnicity
In model 3 (Table 3), where baseline number of falls, self-reported general health and a large number of other covariates were adjusted for, the hazards ratios (95% CI) were changed slightly to 1.38 (1.13–1.69), 1.30 (1.09–1.55), 1.07 (1.01–1.14) for hip, spine and all-types of fractures, respectively. No significant multiplicative interactive effect of race/ethnicity with anemia on all-types of fracture risk was observed. However, stratified analysis (not shown) showed the direction is the same for these associations among the minorities. The number of fractures was too small for hip and spine regions in minorities so no interactions of race/ethnicity with anemia were examined for these site-specific fractures. The only statistically significant interaction for age group and anemia was found for all types of fractures in the 70–79 year old group.
Sensitivity analysis
We limited the above analyses to non-Hispanic white women (data not shown). Results from the stratified analyses were similar to the results from the entire cohort.
DISCUSSION
Anemia is common among older people and the prevalence increases with advancing age.[3] Both clinical and epidemiologic research has suggested that anemia is a significant risk factor for morbidity and mortality particularly among older persons. Anemia may be a clinical indicator of disease pathology or may present as a co-morbid condition which increases the severity of existing health conditions.[5, 6, 8, 10, 11, 23–27]
The WHI, with its large sample size and diverse race/ethnic sample, presents a great opportunity to prospectively assess the relationship between anemia and fracture risk in post-menopausal women from different racial and ethnic backgrounds. Our analysis provides direct evidence of a significant increase in risk for hip fracture among U.S. postmenopausal women who present with anemia. The results also suggest elevated spinal and all fracture risks associated with anemia. The increased fracture risk could not be completely explained by co-morbidity, falls, or other life-style factors. The increased fracture risk associated with anemia in our study ranged from 7% to 38% across fracture sites, after including multiple covariates. Our study cannot determine whether the association between anemia and fracture is a causal relationship, but these results highlight the importance of further understanding the underlying mechanisms of this association.
No prior study has prospectively evaluated the relationship between anemia and fracture risk. However, our study results are consistent with the results from the InCHIANTI study in Italy, which showed that anemia was associated with lower bone density, an association that was most significant in older women as compared to older men. Interestingly, anemia-related bone loss was mostly associated with cortical bone loss rather than trabecular bone loss in the InCHIANTI study.[7] In contrast, our study found increased fracture risk at both high trabecular and high cortical composition bone sites, such as spine and hip. In this same Italian cohort, hemoglobin levels were also found to be related to skeletal muscle properties: lower hemoglobin concentrations were correlated with lower skeletal muscle mass and strength.[5] Reduced skeletal muscle mass and strength may have direct impacts on bone density since bone is responsive to mechanical stimulation. Low skeletal muscle mass may also increase the risk of falling. Indeed, findings have unequivocally pointed to a significant association between anemia and falls in older adults. [8, 28, 29] However, in our study, falls did not contribute significantly to the observed association between anemia and fracture risk.
The prevalence of anemia increases with age. In NHANES III one-fifth of women 85 or older presented with anemia. Specifically, the rates of anemia in older women doubled from 10% to 20% when comparing prevalence among 75 to 84 year olds to those over 85 years of age.[3] Our findings are in agreement with previous studies showing an increased prevalence rate of anemia with aging. In addition, we have found that the anemia-associated prevalence rate for hip fracture and all fractures was significantly higher in the 70–79 year old group in comparison to the younger age groups. A similar trend was observed for spinal fractures but the result did not reach the level of statistical significant at the p-value of 0.05. These results suggest that the absolute fracture risk and the difference of absolute risk were greatest within the oldest group of women in our study. The reason for this differential risk relationship between fractures and anemia by age group cannot be determined in this study.
We demonstrated significant race/ethnic differences in the prevalence of anemia in this population supporting the findings from other reports. In NHANES III, the lowest overall prevalence in persons over age 65 was seen in Non-Hispanic whites (9.0%). Mexican Americans (10.4%) had a slightly higher rate, but African Americans (27.8%) had a rate 3 times higher than non-Hispanic whites when the same WHO criteria for anemia was applied.[3] In spite of the strikingly high prevalence of anemia in African Americans, one study has suggested that older African Americans, classified as anemic by WHO criteria, were not at risk for higher mortality and disability; however, an increased risk of death and disability was found in whites with anemia from the same cohort study.[30] Their results suggest racial differences in the association between WHO defined anemia and adverse health events, thus lending support for different anemia cutoff points by race/ethnicity. We did not find race/ethnic differences in the association between anemia and risk for all fractures. However, smaller numbers of fractures in some minority groups have limited our ability to make a conclusive statement.
The underlying mechanisms of the relationship between anemia and fractures are most likely to be complicated and heterogeneous. The association we observed here may be the reflection of direct, indirect or a combination of direct and indirect effects of anemia on fracture risk. Many known risk factors of anemia in older adults, such as androgen insufficiency, chronic inflammation, age-associated renal insufficiency, stem cell aging,[31] and nutrient deficiency including iron, cobalamin (B12) and folate deficiencies, [32, 33] are also known risk factors for osteoporosis and fractures. Thalassemia patients are at higher risk for bone diseases, including severe osteoporosis and fractures,[34] but we do not have information on thalassemia in this current study. Sickle cell disease predisposes an individual to low bone density[35] and hence increases the person's risk for future fractures. It is unlikely that the increased risk of fracture in our study was mainly due to sickle cell disease since only two participants reported having the disease in the baseline questionnaire. In addition to these possible indirect links for the association between anemia and fracture risk, low hemoglobin levels may directly affect precursors of bone cells.[36–38] In future investigations, bone density, geometric structures and bone metabolic markers may be used to help understand the underlying mechanisms related to this increased fracture risk among older anemic women.
Anemia has been considered a modifiable risk factor for adverse health consequence.[39, 40] Although anemia is associated with an increased risk of fractures in our study, whether improving hemoglobin concentration in anemic women can reduce risk for fractures remains to be studied. One prior study[41] showed that after hip fractures women who had hemoglobin levels less than 12 g/dL stayed longer in the hospital and more likely died from the fracture than those with normal hemoglobin levels at the time of hospital admission. Our analysis indicates anemia is associated with higher risk for fractures, but does not provide evidence as to whether a pre-existing anemic condition contributes to longer-term health outcomes of fractures.
In this study only hip fractures were adjudicated. Non-hip fractures were self-reported so they are subject to reporting error. Although the agreement between self-report of fractures and medical record confirmed diagnosis of fractures is estimated to be 70% in this cohort, there was a range of variation in the accuracy of the self-reported fractures by fracture anatomical site,[42] which may have prevented us from accurately assessing the associations between non-hip fractures and anemia. In the current study, spine fractures referred to clinical spinal fractures only, so under-reporting for undiagnosed spinal fractures might have also occurred. It is unknown if these information biases differ by anemia status; hence, their impact on the study findings is difficult to assess. It has been suggested that only anemia linked with renal disease or chronic inflammation are associated with a higher mortality rate.[43] A case-control study in the WHI has suggested that poor renal function, as measured by cystatin, is associated with increased hip fracture risk.[44] Lack of renal function measurements is a limitation of this current study and needs to be addressed in future investigations. Anemia was defined using a single baseline measurement of hemoglobin and may not reflect persistent anemia. No information on inflammation status was collected during the study. Previous studies have shown that one third of the anemia cases in older populations are due to nutrient deficiency; one third to renal insufficiency and chronic inflammation; and one third related to factors that are less understood and difficult to detect in clinical settings.[33] The contributing causes for anemia could not be determined in our study. Whether different subtypes of anemia are associated with fracture risk differently is impossible to be addressed in this study, but remains an interesting research topic for future investigation.
WHI is the largest health study ever undertaken among postmenopausal women in the United States. The racially and ethnically diverse cohort makes this study unique in understanding possible differential associations between anemia and fracture risk across racial and ethnic sub-groups. Over the course of the study, 20,006 fractures were diagnosed between January 1999 and September, 2006, making WHI the largest number of reported fractures in post-menopausal women. Hence the study results have provided first hand evidence on the association between anemia and fracture risk in postmenopausal women.
CONCLUSION
In conclusion, in this prospective cohort study we have found that anemia is significantly associated with increased fracture risk in postmenopausal women from the Women's Health Initiative. Given the high prevalence of anemia in older women it is of great public health interest for future research to investigate the mechanisms contributing to this observed association between fracture risk and anemia and to further understand whether age and race/ethnicity modify the relationship between anemia and fracture risk.
ACKNOWLEDGMENTS
SHORT LIST OF WHI INVESTIGATORS Program Office: (National Heart, Lung, and Blood Institute, Bethesda, Maryland) Elizabeth Nabel, Jacques Rossouw, Shari Ludlam, Linda Pottern, Joan McGowan, Leslie Ford, and Nancy Geller.
Clinical Coordinating Center: (Fred Hutchinson Cancer Research Center, Seattle, WA) Ross Prentice, Garnet Anderson, Andrea LaCroix, Charles L. Kooperberg, Ruth E. Patterson, Anne McTiernan; (Wake Forest University School of Medicine, Winston-Salem, NC) Sally Shumaker; (Medical Research Labs, Highland Heights, KY) Evan Stein; (University of California at San Francisco, San Francisco, CA) Steven Cummings.
Clinical Centers: (Albert Einstein College of Medicine, Bronx, NY) Sylvia Wassertheil-Smoller; (Baylor College of Medicine, Houston, TX) Jennifer Hays; (Brigham and Women's Hospital, Harvard Medical School, Boston, MA) JoAnn Manson; (Brown University, Providence, RI) Annlouise R. Assaf; (Emory University, Atlanta, GA) Lawrence Phillips; (Fred Hutchinson Cancer Research Center, Seattle, WA) Shirley Beresford; (George Washington University Medical Center, Washington, DC) Judith Hsia; (Los Angeles Biomedical Research Institute at Harbor- UCLA Medical Center, Torrance, CA) Rowan Chlebowski; (Kaiser Permanente Center for Health Research, Portland, OR) Evelyn Whitlock; (Kaiser Permanente Division of Research, Oakland, CA) Bette Caan; (Medical College of Wisconsin, Milwaukee, WI) Jane Morley Kotchen; (MedStar Research Institute/Howard University, Washington, DC) Barbara V. Howard; (Northwestern University, Chicago/Evanston, IL) Linda Van Horn; (Rush Medical Center, Chicago, IL) Henry Black; (Stanford Prevention Research Center, Stanford, CA) Marcia L. Stefanick; (State University of New York at Stony Brook, Stony Brook, NY) Dorothy Lane; (The Ohio State University, Columbus, OH) Rebecca Jackson; (University of Alabama at Birmingham, Birmingham, AL) Cora E. Lewis; (University of Arizona, Tucson/Phoenix, AZ) Tamsen Bassford; (University at Buffalo, Buffalo, NY) Jean Wactawski-Wende; (University of California at Davis, Sacramento, CA) John Robbins; (University of California at Irvine, CA) F. Allan Hubbell; (University of California at Los Angeles, Los Angeles, CA) Howard Judd; (University of California at San Diego, LaJolla/Chula Vista, CA) Robert D. Langer; (University of Cincinnati, Cincinnati, OH) Margery Gass; (University of Florida, Gainesville/Jacksonville, FL) Marian Limacher; (University of Hawaii, Honolulu, HI) David Curb; (University of Iowa, Iowa City/Davenport, IA) Robert Wallace; (University of Massachusetts/Fallon Clinic, Worcester, MA) Judith Ockene; (University of Medicine and Dentistry of New Jersey, Newark, NJ) Norman Lasser; (University of Miami, Miami, FL) Mary Jo O'Sullivan; (University of Minnesota, Minneapolis, MN) Karen Margolis; (University of Nevada, Reno, NV) Robert Brunner; (University of North Carolina, Chapel Hill, NC) Gerardo Heiss; (University of Pittsburgh, Pittsburgh, PA) Lewis Kuller; (University of Tennessee, Memphis, TN) Karen C. Johnson; (University of Texas Health Science Center, San Antonio, TX) Robert Brzyski; (University of Wisconsin, Madison, WI) Gloria E. Sarto; (Wake Forest University School of Medicine, Winston-Salem, NC) Denise Bonds; (Wayne State University School of Medicine/Hutzel Hospital, Detroit, MI) Susan Hendrix.
Sponsor's Role: This study was supported by National Institute of Aging grant 1R01AG029133-01. The WHI program is funded by the National Heart, Lung and Blood Institute, U.S. Department of Health and Human Services. Sponsor had no role in design, methods, data collection, analysis, or preparation of manuscript.
Appendix
Footnotes
Conflict of Interest: The editor in chief has reviewed the conflict of interest checklist provided by the authors and has determined that the authors have no financial or any other kind of personal conflicts with this paper.
REFERENCES
- 1.Ershler WB. Inflammation gone awry: aging or disease. Blood. 2005;105:2247. [Google Scholar]
- 2.World Health Organization . Nutritional anemia: report of a World Health Organization Scientific Group. World Health Organization Geneva; Switzerland: 1968. [Google Scholar]
- 3.Guralnik JM, Eisenstaedt RS, Ferrucci L, et al. Prevalence of anemia in persons 65 years and older in the United States: Evidence for a high rate of unexplained anemia. Blood. 2004;104:2263–2268. doi: 10.1182/blood-2004-05-1812. [DOI] [PubMed] [Google Scholar]
- 4.Penninx BW, Pahor M, Cesari M, et al. Anemia is associated with disability and decreased physical performance and muscle strength in the elderly. J Am Geriatr Soc. 2004;52:719–724. doi: 10.1111/j.1532-5415.2004.52208.x. [DOI] [PubMed] [Google Scholar]
- 5.Cesari M, Penninx BW, Lauretani F, et al. Hemoglobin levels and skeletal muscle: results from the InCHIANTI study. J Gerontol A Biol Sci Med Sci. 2004;59:249–254. doi: 10.1093/gerona/59.3.m249. [DOI] [PubMed] [Google Scholar]
- 6.Chaves PH, Xue QL, Guralnik JM, et al. What constitutes normal hemoglobin concentration in community-dwelling disabled older women? J Am Geriatr Soc. 2004;52:1811–1816. doi: 10.1111/j.1532-5415.2004.52502.x. [DOI] [PubMed] [Google Scholar]
- 7.Cesari M, Pahor M, Lauretani F, et al. Bone density and hemoglobin levels in older persons: results from the InCHIANTI study. Osteoporos Int. 2005;16:691–699. doi: 10.1007/s00198-004-1739-6. [DOI] [PubMed] [Google Scholar]
- 8.Dharmarajan TS, Avula S, Norkus EP. Anemia increases risk for falls in hospitalized older adults: An evaluation of falls in 362 hospitalized, ambulatory, long-term care, and community patients. J Am Med Dir Assoc. 2006;7:287–293. doi: 10.1016/j.jamda.2005.10.010. [DOI] [PubMed] [Google Scholar]
- 9.Dharmarajan TS, Avula S, Norkus EP. Anemia increases risk for falls in hospitalized older adults: an evaluation of falls in 362 hospitalized, ambulatory, long-term care, and community patients. J Am Med Dir Assoc. 2007;8(3 Suppl 2):9–15. doi: 10.1016/j.jamda.2006.12.015. [DOI] [PubMed] [Google Scholar]
- 10.De Breucker S, Nkodo Mekongo YP, Ibebeke B, et al. Falls of older individuals: medical assessment. Rev Med Brux. 2007;28:177–182. [PubMed] [Google Scholar]
- 11.Penninx BW, Pluijm SM, Lips P, et al. Late-life anemia is associated with increased risk of recurrent falls. J Am Geriatr Soc. 2005;53:2106–2111. doi: 10.1111/j.1532-5415.2005.00491.x. [DOI] [PubMed] [Google Scholar]
- 12.Araujo AB, Travison TG, Harris SS, et al. Race/ethnic differences in bone mineral density in men. Osteoporos Int. 2007;18:943–953. doi: 10.1007/s00198-006-0321-9. [DOI] [PubMed] [Google Scholar]
- 13.Baron JA, Barrett J, Malenka D, et al. Racial differences in fracture risk. Epidemiology. 1994;5:42–47. doi: 10.1097/00001648-199401000-00008. [DOI] [PubMed] [Google Scholar]
- 14.Design of the Women's Health Initiative clinical trial and observational study. The Women's Health Initiative Study Group Control Clin Trials. 1998;19:61–109. doi: 10.1016/s0197-2456(97)00078-0. [DOI] [PubMed] [Google Scholar]
- 15.Anderson GL, Manson J, Wallace R, et al. Implementation of the Women's Health Initiative study design. Ann Epidemiol. 2003;13(9 Suppl):S5–17. doi: 10.1016/s1047-2797(03)00043-7. [DOI] [PubMed] [Google Scholar]
- 16.Curb JD, Mc Tiernan A, Heckbert SR, et al. Outcomes ascertainment and adjudication methods in the Women's Health Initiative. Ann Epidemiol. 2003;13(9 Suppl):S122–128. doi: 10.1016/s1047-2797(03)00048-6. [DOI] [PubMed] [Google Scholar]
- 17.Hays J, Hunt JR, Hubbell FA, et al. The Women's Health Initiative recruitment methods and results. Ann Epidemiol. 2003;13(9 Suppl):S18–77. doi: 10.1016/s1047-2797(03)00042-5. [DOI] [PubMed] [Google Scholar]
- 18.Langer RD, White E, Lewis CE, et al. The Women's Health Initiative Observational Study: Baseline characteristics of participants and reliability of baseline measures. Ann Epidemiol. 2003;13(9 Suppl):S107–121. doi: 10.1016/s1047-2797(03)00047-4. [DOI] [PubMed] [Google Scholar]
- 19.Ware JE, Jr., Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care. 1992;30:473–483. [PubMed] [Google Scholar]
- 20.Burnam MA, Wells KB, Leake B, et al. Development of a brief screening instrument for detecting depressive disorders. Med Care. 1988;26:775–789. doi: 10.1097/00005650-198808000-00004. [DOI] [PubMed] [Google Scholar]
- 21.Cauley JA, Wu L, Wampler NS, et al. Clinical risk factors for fractures in multi-ethnic women: The Women's Health Initiative. J Bone Miner Res. 2007;22:1816–1826. doi: 10.1359/jbmr.070713. [DOI] [PubMed] [Google Scholar]
- 22.Robbins J, Aragaki AK, Kooperberg C, et al. Factors associated with 5-year risk of hip fracture in postmenopausal women. JAMA. 2007;298:2389–2398. doi: 10.1001/jama.298.20.2389. [DOI] [PubMed] [Google Scholar]
- 23.Balducci L, Ershler WB, Krantz S. Anemia in the elderly-clinical findings and impact on health. Crit Rev Oncol Hematol. 2006;58:156–165. doi: 10.1016/j.critrevonc.2005.09.003. [DOI] [PubMed] [Google Scholar]
- 24.Dharmarajan TS. Falls and fractures linked to anemia, delirium, osteomalacia, medications, and more: The path to success is strewn with obstacles! J Am Med Dir Assoc. 2007;8:549–550. doi: 10.1016/j.jamda.2007.07.006. [DOI] [PubMed] [Google Scholar]
- 25.Izaks GJ, Westendorp RG, Knook DL. The definition of anemia in older persons. JAMA. 1999;281:1714–1717. doi: 10.1001/jama.281.18.1714. [DOI] [PubMed] [Google Scholar]
- 26.Landi F, Russo A, Danese P, et al. Anemia status, hemoglobin concentration, and mortality in nursing home older residents. J Am Med Dir Assoc. 2007;8:322–327. doi: 10.1016/j.jamda.2007.01.028. [DOI] [PubMed] [Google Scholar]
- 27.Penninx BW, Pahor M, Woodman RC, et al. Anemia in old age is associated with increased mortality and hospitalization. J Gerontol A Biol Sci Med Sci. 2006;61:474–479. doi: 10.1093/gerona/61.5.474. [DOI] [PubMed] [Google Scholar]
- 28.Dharmarajan TS, Norkus EP. Mild anemia and the risk of falls in older adults from nursing homes and the community. J Am Med Dir Assoc. 2004;5:395–400. doi: 10.1097/01.JAM.0000144734.84172.89. [DOI] [PubMed] [Google Scholar]
- 29.Duh MS, Mody SH, Lefebvre P, et al. Anaemia and the risk of injurious falls in a community-dwelling elderly population. Drugs Aging. 2008;25:325–334. doi: 10.2165/00002512-200825040-00005. [DOI] [PubMed] [Google Scholar]
- 30.Patel KV, Harris TB, Faulhaber M, et al. Racial variation in the relationship of anemia with mortality and mobility disability among older adults. Blood. 2007;109:4663–4670. doi: 10.1182/blood-2006-10-055384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Makipour S, Kanapuru B, Ershler WB. Unexplained anemia in the elderly. Semin Hematol. 2008;45:250–254. doi: 10.1053/j.seminhematol.2008.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Carmel R. Nutritional anemias and the elderly. Semin Hematol. 2008;45:225–234. doi: 10.1053/j.seminhematol.2008.07.009. [DOI] [PubMed] [Google Scholar]
- 33.Patel KV, Harris TB, Newman AB, et al. Further epidemiologic research on anemia in older adults is needed. Blood. 2008;111:2941–2942. [Google Scholar]
- 34.Vichinsky EP. The morbidity of bone disease in thalassemia. Ann N Y Acad Sci. 1998;850:344–348. doi: 10.1111/j.1749-6632.1998.tb10491.x. [DOI] [PubMed] [Google Scholar]
- 35.Sarrai M, Duroseau H, D'Augustine J, et al. Bone mass density in adults with sickle cell disease. Br J Haematol. 2007;136:666–672. doi: 10.1111/j.1365-2141.2006.06487.x. [DOI] [PubMed] [Google Scholar]
- 36.Steinbrech DS, Mehrara BJ, Saadeh PB, et al. Hypoxia regulates VEGF expression and cellular proliferation by osteoblasts in vitro. Plast Reconstr Surg. 1999;104:738–747. doi: 10.1097/00006534-199909030-00019. [DOI] [PubMed] [Google Scholar]
- 37.Wang Y, Wan C, Gilbert SR, et al. Oxygen sensing and osteogenesis. Ann N Y Acad Sci. 2007;1117:1–11. doi: 10.1196/annals.1402.049. [DOI] [PubMed] [Google Scholar]
- 38.D'Ippolito G, Diabira S, Howard GA, et al. Low oxygen tension inhibits osteogenic differentiation and enhances stemness of human MIAMI cells. Bone. 2006;39:513–522. doi: 10.1016/j.bone.2006.02.061. [DOI] [PubMed] [Google Scholar]
- 39.Woodman R, Ferrucci L, Guralnik J. Anemia in older adults. Curr Opin Hematol. 2005;12:123–128. doi: 10.1097/01.moh.0000154030.13020.85. [DOI] [PubMed] [Google Scholar]
- 40.Agnihotri P, Telfer M, Butt Z, et al. Chronic anemia and fatigue in elderly patients: Results of a randomized, double-blind, placebo-controlled, crossover exploratory study with epoetin alfa. J Am Geriatr Soc. 2007;55:1557–1565. doi: 10.1111/j.1532-5415.2007.01357.x. [DOI] [PubMed] [Google Scholar]
- 41.Gruson KI, Aharonoff GB, Egol KA, et al. The relationship between admission hemoglobin level and outcome after hip fracture. J Orthop Trauma. 2002;16:39–44. doi: 10.1097/00005131-200201000-00009. [DOI] [PubMed] [Google Scholar]
- 42.Chen Z, Kooperberg C, Pettinger MB, et al. Validity of self-report for fractures among a multiethnic cohort of postmenopausal women: Results from the Women's Health Initiative observational study and clinical trials. Menopause. 2004;11:244–245. doi: 10.1097/01.gme.0000094210.15096.fd. [DOI] [PubMed] [Google Scholar]
- 43.Semba RD, Ricks MO, Ferrucci L, et al. Types of anemia and mortality among older disabled women living in the community:The Women's Health and Aging Study I. Aging Clin Exp Res. 2007;19:259–264. doi: 10.1007/bf03324699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.LaCroix AZ, Lee JS, Wu L, et al. Women's Health Initiative Observational. Cystatin-C, renal function, and incidence of hip fracture in postmenopausal women. J Am Geriatr Soc. 2008;56:1434–1441. doi: 10.1111/j.1532-5415.2008.01807.x. [DOI] [PMC free article] [PubMed] [Google Scholar]