Abstract
Purpose
The midline thalamus is an important component of the circuitry in limbic seizures, but it is unclear how synaptic modulation of the thalamus affects that circuitry. In this study, we wished to understand how synaptic modulation of the thalamus can affect inter-regional signaling and seizure spread in the limbic network.
Methods
We examined the effect of GABA modulation of the mediodorsal (MD) region of the thalamus on responses in the prefrontal cortex (PFC) by stimulation of the subiculum (SB). Muscimol, a GABA-A agonist, was injected into the MD, and the effect on local responses to subiculum stimulation were examined. Evoked potentials were induced in the MD and the PFC by low frequency stimulation of the SB, and seizures were generated in the subiculum by repeated 20 Hz stimulations. The effect of muscimol in the MD on the evoked potentials and seizures was measured.
Key Findings
Thalamic responses to stimulation of the subiculum were reduced in the presence of muscimol. Reduction of the amplitudes of evoked potentials in the MD resulted in an attenuation of the late, thalamic components of the responses in the PFC, as well as of seizure durations.
Significance
Activation of GABA- A receptors in the midline thalamus not only causes changes within the thalamus, but has broader effects on the limbic network. This work provides further evidence that synaptic modulation within the midline thalamus alters system excitability more broadly and reduces seizure activity.
Keywords: GABA, mediodorsal nucleus, subiculum, prefrontal cortex, seizures, limbic system
Introduction
Mesial temporal lobe epilepsy (mTLE) is a common neurological disorder characterized by seizures arising in the limbic network. At present, about 30% of-all epilepsy patients, including 50% of TLE patients (Kwan and Brodie 2000, Stephen et al. 2001), don't respond to medications and require surgery to control their seizures (Kwan and Sperling 2009). For more effective therapies to be developed, a better understanding of the underlying circuitry of seizure activity in the limbic system is needed.
The midline thalamus is a potential candidate for direct clinical intervention in mTLE, because it is connected to most limbic structures involved in mTLE (Ray et al. 1992, Van der Werf et al. 2002, Berendse and Groenewegen 1991, Hoover and Vertes 2007), and work to date has shown that it is a critical component in the initiation and spread of seizure in animal models of mTLE. For example, injection of GABAergic drugs into the thalamus alters seizure activity in animal models (Patel et al. 1988, Miller and Ferendelli 1990, Cassidy and Gale 1998, Bertram et al. 2001, 2008). It has long been assumed that the reason that targeting the thalamus with these drugs is so effective is because it causes a two step reaction: a local change in neuronal activity, which, in turn, leads to broader effects to its afferent connections. However, the basis for why this intervention suppresses seizures has been unclear.
To resolve this question, we sought to identify both the local and widespread effects of GABAergic modulation of the midline thalamus. We hypothesized that modifying synaptic responses in the mediodorsal (MD) region of the midline thalamus would reduce its excitatory effect on components of the seizure circuit.
We pursued this hypothesis by stimulating the subiculum, (the output region of the hippocampus), and recording in both the thalamus and the prefrontal cortex as the GABA-A agonist muscimol was injected into the MD region. The subiculum-prefrontal cortex (SB-PFC) pathway was chosen for these experiments for several reasons. First, both the hippocampus and PFC have been shown to participate in both chronic and kindled models of TLE (Sloan and Bertram 2009). Second, there is a known monosynaptic pathway between the subiculum and the PFC, as well as a polysynaptic path that may also connect the two regions through the midline thalamus. These regions form a potential divergent-convergent circuit, and these connections could form a divergent-convergent excitation amplification circuit that could play a role in limbic seizures.
The goal of this study was to evaluate whether there were measurable circuit effects after modulation of the mediodorsal nucleus by the GABA agonist muscimol.
Materials and Methods
All animals were used in accordance with protocols approved by the Animal Care and Use Committee (ACUC) at the University of Virginia. Naïve adult male Sprague-Dawley rats (Hilltop Laboratories, 250-350 g) were used for all experiments.
1. SB-Thalamus Pathway
Electrode and Cannula Placement in SB-Thalamus Pathway
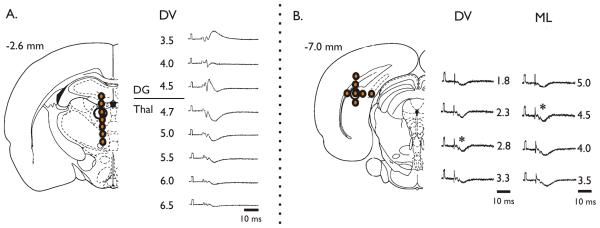
We first sought to show the effect of a GABA agonist on synaptic responses in the thalamus. Animals were put under urethane anesthesia (1.2 g/kg, i.p.) and placed in a multi-arm stereotactic frame. Body temperature was maintained at 37°C by a water blanket controlled with a rectal thermistor. The stimulating and recording electrodes and the cannula for drug perfusion in this study were inserted stereotactically using target coordinates derived from the rat brain atlas of Paxinos and Watson (1998). Initial experiments were performed to define the coordinates for maximal responses. One bipolar, stainless steel stimulating electrode was placed into the ipsilateral subiculum (−6.3 to −7.0 AP, 4.0-5.5 ML, 2.5 to 8.0 mm DV). To find optimal responses, a glass micro-pipette recording electrode (1 MΩ tip resistance) was filled with 0.9 % NaCl and 1% Fast Green dye and placed in the midline thalamus (from Bregma, in mm: −1.8 to −3.2 AP, 0.4 to 0.8 ML, −4.5 to −5.3 DV below dura). For the experiments including drug injections the depths of the stimulating and recording electrodes were adjusted stepwise to achieve a consistent and maximal response (see Figure 1).
Figure 1. Stimulation of Subiculum Induces Field Responses in Mediodorsal Thalamus.
To find optimal responses, pairs of electrode placement depths were compared. (A) The optimal thalamic recording site was found in the mediodorsal nucleus (~5 mm below dura) (B) The optimal stimulation site in the subiculum was found in the dorsal region (~2.8 below dura). The optimal site was found in the dorsal-most region of the subiculum. The asterisks indicate that these were responses from the site used for our analysis.
For local drug injection into the thalamus, a custom holder was used to hold the glass recording electrode and a stainless steel perfusion cannula (25G) close together in the same sagittal plane. Both the electrode and cannula tips were placed 5.0-5.8 mm below the dura so that the tips were less than 1 mm apart. The recording electrode was vertical to the brain surface, while the cannula tip was inserted at an angle (5-12°) toward the recording electrode. The recording electrode was placed first, and the infusion cannula was placed second.
Recording and Drug Delivery in SB-Thalamic Pathway
Evoked potentials in the thalamus were induced by single (0.1-0.2 ms duration monophasic square wave) stimulation of the subiculum. Five responses, from stimulations delivered every 10 seconds, were averaged at each stage of the experiment and used for analysis.
We first obtained a stable maximal amplitude response, and began the infusion of vehicle (ACSF: 127 mM NaCL, 2 mM KCl, 1.5 mM CaCl2, 1.5 mM MgSO4, 25.7 mM NaHCO3, 1.1 mM KH2PO4 and 10 mM Dextrose) for at least 30 min. at 0.1-0.2 μl /min. Muscimol (dissolved into ACSF) was infused through the same cannula by changing syringes using a fluid switch. 0.5 mM muscimol was infused for 30 minutes near the tip of the recording electrode, after which ACSF flow was resumed. Recovery was measured after a minimum of 30 min of wash.
At the end of each experiment, the electrode position was marked for histological confirmation by iontophoresing Fast Green dye from the recording electrode into the surrounding tissue by using a DC current (20-50 μA, 5-15 min). Positive DC current (10V for 5-10 sec) was passed through the negative tip of the stimulating electrode to deposit iron from the electrode into the surrounding tissue. The positive DC current was also passed through the drug infusion cannula to confirm the cannula position by pushing the fast green into the surrounding tissue. Then, the animals were decapitated while still under anesthesia, and the brains were removed and placed into a fixative consisting of 1% potassium ferrocyanide and 4.0% paraformaldehyde. Brains were sectioned and examined qualitatively under a light microscope to confirm electrode placement. For injection studies, placement of the electrodes and cannula were categorized by successful injections (reduction of seizure duration by a minimum of 50% of baseline) to determine whether the effect was site specific.
Measurements of the evoked responses include the peak amplitude of the post-synaptic potential (PSP) from the baseline. Responses were compared using student t-test and paired t-test. Significance was set at the p<.05 level. Data are reported as means ± standard error of the mean (S.E.M.)
2. SB-PFC Pathway
Evoked Potentials
In order to determine whether synaptic modulation of the MD caused a change in a limbic pathway, we evoked field responses in the PFC by SB stimulation. Animal preparation for these experiments was as described above. The stimulating electrode was placed in the dorsal SB (From bregma, in mm: −6.3 to −7.0 AP, 4.0 to −4.5 ML, −2.7 to −3.5 DV), corresponding to the site used in the SB-Thalamus pathway experiments. The glass recording electrode was placed in the ipsilateral medial PFC (2.8 to 3.2 AP, 0.5 ML, −1.5 to −3.5 DV). This recording site was chosen based on previous work that demonstrated strong afferent connections from both the SB and midline thalamus to the PFC, so that there was a likely combination of mono- and polysynaptic components in the response to SB stimulation.
Electrode positions were adjusted to find maximal response amplitudes. Field potentials were obtained through an Axon Smart Probe and a Cyberamp 380. Signals were digitized through a Digidata 1440A and analyzed using Axoscope 8.0 software (Molecular Devices, CA, USA).
Field potentials were elicited by a 120 ms inter-stim interval (i.s.i.) (~8.3 Hz) train of stimulations. This i.s.i. was chosen in order to maximize the likelihood of involvement of the thalamus in the field potential. Direct stimulation of the thalamus at this frequency elicits a thalamus-specific “recruiting response” in which field potential peaks become increasingly augmented (Sloan and Bertram 2009, Dempsey and Morison 1942, Verzeano et al. 1953, Bazhenov et al. 1998). To ensure that all peaks were fully formed, and because response amplitudes increased over the repeated stimulations of the train until plateauing, the 4th stimulation of the train was used for all analyses (five stimulations were given per train). Five trains were averaged for each acquisition, with an inter-train interval of 10 sec. Acquistions were taken every 10 minutes. Stimulations were monophasic square waves of 0.17ms duration with a maximal amplitude of 70V (maximal current 2.3-4.7 mA, at a typical stimulating electrode resistance of 15-30 kΩ). The stimulus intensity was increased until a maximum response was gained, or until 70V was reached.
Amplitudes for each peak were measured from the baseline value occurring immediately prior to the stimulation.
Waveforms generated by this stimulation protocol consisted of multiple peaks: two positive (P1 and P2) and two negative (N1 and N2) peaks. The composition of these evoked field responses is complex, and likely originates from multiple circuits. Because determining a potential thalamic component of these EP's in necessary, we wished to identify a minimal latency in which a polysynaptic response throughout the thalamus would appear in the PFC. We added field response latencies derived from stimulus-recording experiments in the SB-MD pathway from this paper (10.10 +/− 2.5 ms SE), and the MD-PFC pathway from previous work (27.11 +/− 3.1 ms) (Sloan and Bertram 2009). These latencies suggest that a thalamic influence would appear in a PFC field response to subiculum stimulation after approximately 35 ms. Therefore, for the purposes of this study, peaks occurring prior to 35 ms were considered to contain the monosynaptic signal, and those occurring after 35 ms were considered to contain a thalamic influence, among other possible polysynaptic influences (Fig 3C).
Figure 3. Effect of Muscimol injection into the MD on Seizures Generated in the Subiculum.
Injection of muscimol into the MD caused a reduction or ablation of seizures that were generated in the subiculum and recorded in the PFC. (A) Map of injection sites, including successful (seizure durations affected) and unsuccessful (no effect on seizures) injections. Note that successful injections were clustered in and around the MD. (B) Sample seizure showing reduction in seizure duration with muscimol application, and subsequent recovery. (C) Average seizure changes before, during and after a successful injection of muscimol in MD (n=7), showing significant reduction in duration. P<0.05 by paired t-test. (D) Sample unsuccessful injection, in which seizure duration did not change. (E) Average seizure durations before and after unsuccessful injections, showing no significant change (n=5).
Seizures
In the course of this experiment, there was a standard protocol for the induction of the evoked potentials and the seizures. For each evoked potential train that was recorded, seizure afterdischarges were elicited immediately afterward by a 20 Hz train of monophasic pulses of 0.17 ms duration for five seconds at the same intensity that was used for the evoked potentials. Seizures were identified as large, repetitive spike bursts beginning immediately following the end of the stimulus train. Seizure termination was determined by the end of the last spike burst. For each experiment, a minimum of ten stimulus trains were required to induce seizures of a consistent duration. Once a stable seizure duration was achieved, defined as a minimum of three consecutive seizures with durations longer than 10 seconds with less than 30% variation between them, drug was injected.
For analysis, three evoked potential/ seizure pairs were used for each rat. The first was the recording made just prior to injection of drug (baseline value). The second was the recording with the maximal reduction in seizure duration occurring at least 10 minutes after injection, (to allow for adequate drug diffusion). The third was the recording taken a minimum of 20 min after the seizure had returned to a baseline duration.
Muscimol Suppression of Seizures
Muscimol was injected into the midline thalamus using a Hamilton 25 μl microsyringe with a flat tip. The tip was placed into the ipsilateral mediodorsal nucleus (−1.8 to −2.8 AP, 0.5 ML, −5.0 to −6.0 DV). All drug injections were performed after a baseline of evoked potentials and kindled afterdischarges were achieved. 5.0 mM muscimol, with a standard injection volume of 0.75 μl over five minutes. The concentration used here was higher than that used in the local SB-Thal experiments to ensure that more of the thalamus was exposed to an effective concentration of the drug. Injections were made slowly over the course of three minutes. A successful injection was one in which seizure durations were suppressed by more than 50%.
At the end of the experiment, electrode placements were determined as described above and compared to the results of the injection. Injections outside of the specified region of the MD were ineffective, and were grouped separately from effective injections within the region.
Results
Effect of Local Muscimol in Midline Thalamus on SB-Thal Responses
Evoked field responses to SB stimulation in the midline thalamus were recorded. The most consistent maximal responses were acquired when the stimulating electrode was in the dorsal region of the SB (between −2.5 to −3.5 mm below dura), and when the recording electrode was in the central to dorsal MD nucleus (Figure 1). Standard evoked potentials with this pairing consisted of very brief positive and negative spikes, followed by a much larger and longer negative post-synaptic potential of an average latency of 10.10 +/2.5 ms.
We injected muscimol and recorded the change in the evoked potentials. The muscimol injections caused a marked reduction of the post-synaptic potential which recovered as the drug washed out (Figure 2).
Figure 2. Responses in the Midline Thalamus to Subiculum Stimulation Reduced With Local Injection of Muscimol.
(A) Injection of muscimol into the MD caused a reduction in the local response to subiculum stimulation, which recovered after wash. (B) Average amplitude reduction and recovery with muscimol injection (n=7). P<0.01 by paired t-test.
Effect of GABAergic Modulation of Thalamus on SB-PFC Seizures
In order to ensure that the drug delivery to the MD nucleus was both on target and sufficient to stop seizures, we induced seizures in the SB (the dorsal region used in the previous experiment) and recorded them in the PFC, at an average electrode depth of 2.5 mm (Figure 3A). Seizures were induced by applying regular 20 Hz stimulations at 10 minute intervals. These stimulations caused brief afterdischarges, but with repeated stimulations the duration plateaued with an average duration of 23.48 +/− 2.12 sec. It has been shown in multiple studies that the effect of drug injections on seizures is highly site-specific (Bertram et al. 2008, Patel et al. 1988, Miller and Ferendelli 1990, Cassidy and Gale 1998). Therefore, we could judge the accuracy and effectiveness of an injection by the effect that it had on seizure duration. We confirmed the accuracy of these injections histologically.
Successful injections of muscimol caused a reduction of seizure durations (Figure 2) which lasted for 30-50 minutes before recovering to baseline duration. Successful modulation of seizure duration was considered an indication of an injection within the midline thalamus. Injections outside the region did not induce changes in seizure duration or evoked potentials, but were used as controls for site specificity (Figure 2A). Histological confirmation showed that the sucessful injections (defined as a minimum of a 50% reduction in duration) were within the MD region and that unsuccessful ones were outside this midline region.
Effect of GABAergic Modulation of Thalamus on SB-PFC Evoked Potentials
To determine the network effects of increased inhibition of the MD nucleus, we examined the effect of muscimol injection into the MD on evoked potentials in the SB-PFC pathway (Figure 3). It has been shown previously that stimulation of the subiculum in trains between 7-9 Hz at maximal stimulation intensity yields a complex waveform that is reproducible. This frequency was chosen to maximize the likelihood of thalamic involvement in the response, as stimulation of the thalamus at that frequency is known to induce a progressive response amplification, or “recruiting response” (Sloan and Bertram 2009, Dempsey and Morison 1942, Verzeano et al. 1953, Bazhenov et al. 1998).
The waveforms from these trains can be approximately divided into early and late components; the early components primarily contain the monosynaptic signal, while the late components contain any additional polysynaptic influences. We used the 4th response of the stimulus train because the waveforms are completely formed and at their largest amplitudes by that point in the train. The waveforms consist of two positive (P1 and P2) and two negative (N1 and N2) peaks. We grouped these peaks as “early” (P1 and N1, before 35 ms) and “late” (P2 and N2, after 35 ms), and considered the late component to be the more likely to contain a thalamic influence than the early component, as described in the methods (Fig 3C).
Injection of muscimol into the MD nucleus also caused a reduction in late component of the response. The late component, especially the N2 peak, was preferentially affected (Figure 3 D and E). Injections that were outside the midline thalamus did not affect seizure durations and did not alter the evoked potentials (Figure 3F). These results demonstrate that modulation of the MD nucleus has a widespread, network-level effect.
Discussion
This study provides a network-level mechanism for the observation that application of drugs acting on GABA-A receptors in the midline thalamic region modulate limbic seizures. The drugs reduce the responsiveness of the thalamus, which subsequently diminishes the thalamic excitatory drive on other target regions. This additional excitatory drive may be a significant component in the initiation and spread of limbic seizures.
The results of this study, in conjunction with previous studies, suggest that the midline thalamic nuclei may be a key component of a divergent-convergent circuit in which at least two pathways connect one region to another: a direct monosynaptic connection and an indirect, polysynaptic connection that passes through the thalamus (Figure 5). This divergent-convergent circuitry has the capacity to amplify excitation at the target region by prolonging depolarization. Similar circuits have been shown in non-limbic thalamocortical circuits (Reichova and Sherman 2004, Llano and Sherman 2009, Theyel et al. 2010). The convergence of afferent hippocampal and thalamic signals in the PFC has been shown in the PFC (Floresco and Grace 2003). In seizures, this circuitry may facilitate the spread of seizure activity between two regions.
Figure 5. Model of Effect of Increased Inhibition in Midline Thalamus on SB-PFC signaling.
In the normal circuit, there is a direct monosynaptic connection between the hippocampus and the PFC. There is also a disynaptic connection that terminates in the thalamus before reconverging in the PFC. When the thalamic response is reduced, the thalamic output is weakened. This attenuates the functional role of the accessory excitatory thalamic output to the PFC, and also reduces the capacity for seizures to be generated in the SB-PFC pathway.
To our knowledge, this is the first study demonstrating that GABAergic modulation of the midline thalamus has a network-level effect that explains the seizure modulation observed in previous experiments. The thalamus has long been known to participate in generalized seizures (Pellegrini et al. 1978), but explorations into its physiological role in limbic seizures required animal models, as well as a better knowledge of the potential circuit components, that were not available until relatively recently. Injection of muscimol and other GABA-ergic drugs into the thalamus to alter seizure activity in various animal models was described on a number of occasions (Patel et al. 1988, Lee et al. 1989, Miller and Ferrendelli 1990, Garant et al. 1993). Cassidy and Gale showed a site-specific effect of midline thalamic muscimol injection in a model of limbic seizures, in which the seizures were directly initiated in the piriform cortex (Cassidy and Gale 1998). Similar work was also performed recently in a model of awake, kindled rats with seizures generated in the hippocampus (Bertram et al. 2008).
It has remained unclear what role this thalamic region played in limbic seiuzures. Miller and Ferrendelli posited that the thalamus “acts to modify the excitability of other structures more directly involved in the process [of seizure origination and spread]” (1990). The present study provides a mechanism for the success of those experiments. Additionally, this study provides a rationale for findings that structures that send GABAergic projections into the midline thalamus, such as the reticular nucleus (Cavdar 2008, Nanobashvili et al. 2003) and the ventral forebrain (Churchill 1996, Daransart et al. 1999), have the ability to modulate seizures. This study suggests that synaptic modulation in the thalamus may have clinical applications.
Although much work is yet to be done to prove that the midline thalamus is a useful clinical target in human TLE patients, evidence from both animal models and clinical studies has been encouraging. The corroborating evidence that the midline thalamus plays a role in mTLE seizures in humans is building (Blumenfeld 2004, Bonilha 2005, Guye et al 2006, Mueller 2009). Investigations involving deep brain stimulation of the thalamus have already begun in human patients (Fisher et al. 2010). This work adds evidence that direct pharmacological interventions may also be feasible. However, we stress that this study, along with many previous studies, demonstrates that any injections or interventions must be in precisely the correct location in the thalamus, and that interventions that are outside of the critical midline thalamic region- even if they are close- may be ineffective (Cassidy and Gale 1998, Bertram et al. 2008, Sloan et al 2010-submitted). Site-specificity is a critical consideration for any future clinical therapies involving the midline thalamus.
There are potential limitations in this study. Field potentials, by definition, include responses from many neurons, making it difficult to distinguish between excitatory and inhibitory activity. The exact effects of thalamic modulation on individual cells or sub-populations in the PFC were not resolved. The diversity of cells that receive both hippocampal and thalamic inputs has been shown (Floresco and Grace 2003). For the purposes of this study, an examination of SB-PFC connectivity was sufficient to demonstrate the influence and importance of the midline thalamus on its signaling. In addition, there are always the issues of translating studies of induced seizures that were performed under anesthesia into the clinical realm. The effects of the anesthesia are widespread and the drug may be suppressing activity in other regions. It may be best to view these results as showing how several regions interact to drive the system into a seizure rather than indicating that the thalamus is the only path through which divergent-convergent excitatory amplification can happen. Although many of the previous studies have indicated that manipulation of this thalamic region blunts the generalization of seizures, the data from this report cannot really address whether the MD region is critical for this process. At the moment we can only speak of how the regions may act in concert to induce seizure activity. Whether this same process is involved in the spread of seizures to other regions can only be hypothesized at the present time.
In conclusion, modulation of thalamic inhibition has effects outside of the thalamic region; it has the potential to indirectly reduce normal signaling between cortical regions of the limbic network. This physiological effect should be taken into consideration in studies of basic seizure circuitry.
Figure 4. PFC Responses to Subiculum Stimulation Reduced By Injection of Muscimol into MD.
(A) Stimulation and recording sites for SB-PFC experiments. (B) Stimulation in a train yielded complex field responses in the medial PFC. (C) Average field latencies from polysynaptic circuit suggest a minimal thalamic influence on PFC responses to be approx. 35 ms, suggesting that the “late” component of the response most likely contains influence from the thalamus (see Methods) (D) Injection of muscimol into the MD altered the late component of SB-PFC field potentials. (E) Graph of the differences between P1-N1(early) and P2-N2 (late). The early component did not change significantly, while the late peak was significantly reduced. P<0.05 by paired t-test. (F) Sample responses from an injection outside of the MD, which did not affect seizures (see Figure 3). Note that there was no change in response amplitudes in either component.
Acknowledgements
This work was supported by NIH grants NS025605 and NS064438. We thank John Williamson for his technical support.
Footnotes
None of the authors have any conflicts of interest to disclose.
We confirm that we have read the Journal's position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
References
- Bazhenov M, Timofeev I, Steriade M, Sejnowski TJ. Cellular and network models of intrathalamic augmenting responses during 10Hz stimulation. J Neurophysiol. 1998;79:2730–2748. doi: 10.1152/jn.1998.79.5.2730. [DOI] [PubMed] [Google Scholar]
- Berendse HW, Groenewegen HJ. Restricted cortical termination fields of the midline and intralaminar thalamic nuclei in the rat. Neuroscience. 1991;42:73–102. doi: 10.1016/0306-4522(91)90151-d. [DOI] [PubMed] [Google Scholar]
- Bertram EH, Mangan PS, Zhang DX, Scott CA, Williamson JM. The midline thalamus: alterations and potential role in limbic epilepsy. Epilepsia. 2001;42:967–978. doi: 10.1046/j.1528-1157.2001.042008967.x. [DOI] [PubMed] [Google Scholar]
- Bertram EH, Zhang D, Williamson JW. Multiple roles of midline dorsal thalamic nuclei in induction and spread of limbic seizures. Epilepsia. 2008;2:256–268. doi: 10.1111/j.1528-1167.2007.01408.x. [DOI] [PubMed] [Google Scholar]
- Blumenfeld H, McNally KA, Vanderhill SD, Paige AL, Chung R, Davis K, Norden AD, Stokking R, Studholme C, Novotny EJ, Jr, Zubal IG, Spencer SS. Positive and negative network correlations in temporal lobe epilepsy. Cereb Cortex. 2004;14:892–902. doi: 10.1093/cercor/bhh048. [DOI] [PubMed] [Google Scholar]
- Bonilha L, Rorden C, Castellano G, Cendes F, Li LM. Voxel-based morphometry of the thalamus in patients with refractory medial temporal lobe epilepsy. Neuroimage. 2005;25:1016–1021. doi: 10.1016/j.neuroimage.2004.11.050. [DOI] [PubMed] [Google Scholar]
- Cassidy RM, Gale K. Mediodorsal thalamus plays a critical role in the development of limbic motor seizures. J Neurosci. 1998;18:9002–9008. doi: 10.1523/JNEUROSCI.18-21-09002.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavdar S, Onat FY, Cakmak YO, Yananli HR, Gulcebi M, Aker R. The pathways connecting the hippocampal formation, the thalamic reuniens nucleus and the thalamic reticular nucleus in the rat. J Anat. 2008;212:249–256. doi: 10.1111/j.1469-7580.2008.00858.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Churchill L, Zahm DS, Kalivas PW. The mediodorsal nucleus of the thalamus in rats- I. Forebrain gabaergic innervation. Neuroscience. 1996;70:93–102. doi: 10.1016/0306-4522(95)00351-i. [DOI] [PubMed] [Google Scholar]
- Deransart C, Riban V, Lê BT, Hechler V, Marescaux C, Depaulis A. Evidence for the involvement of the pallidum in the modulation of seizures in a genetic model of absence epilepsy in the rat. Neurosci Lett. 1999;265:131–134. doi: 10.1016/s0304-3940(99)00113-5. [DOI] [PubMed] [Google Scholar]
- Fisher R, the SANTE Study Group Electrical stimulation of the anterior nucleus of thalamus for treatment of refractory epilepsy. Epilepsia. 2010;51:899–908. doi: 10.1111/j.1528-1167.2010.02536.x. 40 others. [DOI] [PubMed] [Google Scholar]
- Floresco SB, Grace AA. Gating of hippocampal-evoked activity in prefrontal cortical neurons by inputs from the mediodorsal thalamus and ventral tegmental area. J Neurosci. 2003;23:3930–3943. doi: 10.1523/JNEUROSCI.23-09-03930.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garant DS, Xu SG, Sperber EF, Moshe SL. The influence of thalamic GABA transmission on susceptibility of adult rats to flurothyl induced seizures. Epilepsy Res. 1993;15:185–192. doi: 10.1016/0920-1211(93)90055-c. [DOI] [PubMed] [Google Scholar]
- Guye M, Regis J, Tamura M, Wednling F, McGonical A, Chauvel P, Bartolomei F. The role of corticothalamic coupling in human temporal lobe epilepsy. Brain. 2006;129:1917–1928. doi: 10.1093/brain/awl151. [DOI] [PubMed] [Google Scholar]
- Hoover WB, Vertes RP. Anatomical analysis of anatomic projections to the medial prefrontal cortex of the rat. Brain Struct Funct. 2007;212:149–179. doi: 10.1007/s00429-007-0150-4. [DOI] [PubMed] [Google Scholar]
- Hughes JR. Absence seizures: a review of recent reports with new concepts. Ep and Behav. 2009;15:404–412. doi: 10.1016/j.yebeh.2009.06.007. [DOI] [PubMed] [Google Scholar]
- Kim CH, Koo B-B, Chung CK, Lee J-M, Kim JS, Lee SK. Thalamic changes in temporal lobe epilepsy with and without hippocampal sclerosis: predictors for long-term surgical outcome. Brain. 2010;128:395–404. doi: 10.1093/brain/awh358. [DOI] [PubMed] [Google Scholar]
- Kwan P, Brodie MJ. Early identification of refractory epilepsy. N Engl J Med. 2000;342:314–319. doi: 10.1056/NEJM200002033420503. [DOI] [PubMed] [Google Scholar]
- Kwan P, Sperling MR. Refractory seizures: try additional antiepileptic drugs (after two have failed) or go directly to early surgery evaluation? Epilepsia. 2009;50(Suppl 8):57–62. doi: 10.1111/j.1528-1167.2009.02237.x. [DOI] [PubMed] [Google Scholar]
- Lee S-C, Cruikshank SJ, Connors BW. Electrical and chemical synapses between relay neurons in developing thalamus. J Physiol. 2010;588:2403–2415. doi: 10.1113/jphysiol.2010.187096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller JW, Ferrendelli JA. The central median nucleus: thalamic site of seizure regulation. Brain Res. 1990;508:297–300. doi: 10.1016/0006-8993(90)90411-4. [DOI] [PubMed] [Google Scholar]
- Morison RS, Dempsey EW. Mechanism of thalamocortical augmentation and repetition. Am J Physiol. 1943;138:297–308. [Google Scholar]
- Mueller SG, Laxer KD, Barakos J, Cheong I, Finlay D, Garcia P, Cardenas-Nicolson V, Weiner MW. Involvement of the thalamocortical network in TLE with and without mesiotemporal sclerosis. Epilepsia. 2009 doi: 10.1111/j.1528-1167.2009.02413.x. DOI 10.1111/j.1528-1167.2009.02413.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nanobashvili Z, Chachua T, Nanobashvili A, Bilanishvili I, Lindvall O, Kokaia Z. Suppression of limbic motor seizures by electrical stimulation in thalamic reticular nucleus. Exp. Neurol. 2003;181:224–230. doi: 10.1016/s0014-4886(03)00045-1. [DOI] [PubMed] [Google Scholar]
- Patel S, Millan MH, Meldrum BS. Decrease in excitatory transmission within the lateral habenula and the mediodorsal thalamus protects against limbic seizures in rats. Exp. Neurol. 1988;101:63–74. doi: 10.1016/0014-4886(88)90065-9. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. 4th ed. Academic Press; San Diego, CA, USA: 1998. [Google Scholar]
- Pellegrini A, Musgrave J, Gloor P. Role of afferent input of subcortical origin in the genesis of bilaterally synchronous epileptic discharges of feline generalized penicillin epilepsy. Exp. Neurol. 1979;64:155–173. doi: 10.1016/0014-4886(79)90012-8. [DOI] [PubMed] [Google Scholar]
- Sloan DM, Bertram EH. Changes in midline thalamic recruiting responses in the prefrontal cortex of the rat during the development of chronic limbic seizures. Epilepsia. 2009;3:556–565. doi: 10.1111/j.1528-1167.2008.01790.x. [DOI] [PubMed] [Google Scholar]
- Stephen LJ, Kwan P, Brodie MJ. Does the cause of localization-related epilepsy influence the response to anti-epileptic drug treatment? Epilepsia. 2001;42:357–362. doi: 10.1046/j.1528-1157.2001.29000.x. [DOI] [PubMed] [Google Scholar]
- Ray JP, Russchen FT, Fuller TA, Price JL. Sources of presumptive glutamatergic/aspartic afferents to the mediodorsal nucleus of the thalamus in the rat. J Comp Neuro. 1992;32:435–456. doi: 10.1002/cne.903200403. [DOI] [PubMed] [Google Scholar]
- Reichova I, Sherman SM. Somatosensory corticothalamic projections: distinguishing drivers from modulators. J Neurophysiol. 2004;92:2185–2197. doi: 10.1152/jn.00322.2004. [DOI] [PubMed] [Google Scholar]
- Theyel BB, Llano DA, Sherman SM. The corticothalamocortical circuit drives higher-order cortex in the mouse. Nature Neuro. 2010;13:84–88. doi: 10.1038/nn.2449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Der Werf YD, Witter MP, Groenwegen HJ. The intralaminar and midline nuclei of the thalamus. anatomical functional evidence for participation in processes of arousal and awareness. Brain Res. Rev. 2002;39:107–14. doi: 10.1016/s0165-0173(02)00181-9. [DOI] [PubMed] [Google Scholar]
- Verzeano M, Lindsley DB, Magoun HW. Nature of recruiting response. J Neurophysiol. 1953;16:183–195. doi: 10.1152/jn.1953.16.2.183. [DOI] [PubMed] [Google Scholar]