Summary
Intellectual and developmental disabilities (IDD) such as Autistic Spectrum Disorders (ASD) and epilepsies are heterogeneous disorders that have diverse etiologies and pathophysiologies. The high rate of co-occurrence of these disorders, however, suggest potentially shared underlying mechanisms. A number of well-known genetic disorders share epilepsy, intellectual disability and autism as prominent phenotypic features, including tuberous sclerosis, Rett syndrome, and fragile X. In addition, mutations of several genes involved in neurodevelopment, including ARX, DCX, neuroligins and neuropilin2 have been identified in children with epilepsy, IDD, ASD or a combination of thereof. Finally, in animal models, early life seizures can result in cellular and molecular changes that could contribute to learning and behavioral disabilities. Increased understanding of the common genetic, molecular and cellular mechanisms of IDD, ASD and epilepsy may provide insight into their underlying pathophysiology and elucidate new therapeutic approaches for these conditions.
Keywords: Epilepsy, Intellectual Disability, Autism, Synaptic plasticity, hippocampus
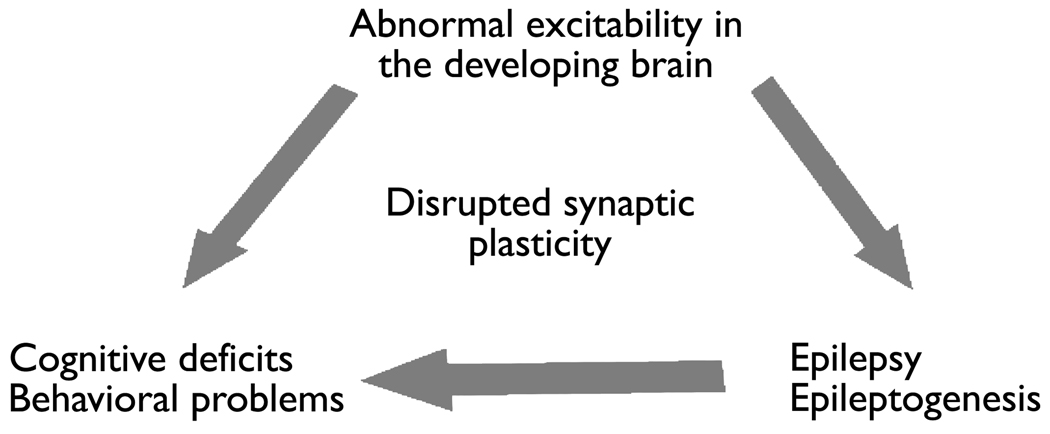
Approximately 30% of children with epilepsy have autism and/or intellectual or developmental disabilities (Tuchman et al., 2009). What might explain the increased association of IDD (intellectual and developmental disabilities), ASD (autistic spectrum disorder), and epilepsy? All three could result from the same pathophysiological mechanisms. For example, it has been proposed that IDD, ASD, and epilepsy can be understood as disorders of synaptic plasticity resulting in a developmental imbalance of excitation and inhibition (Figure 1). This may occur in genetic conditions that result in abnormal excitability and disrupted synaptic plasticity in the developing brain such as fragile X syndrome (FXS), Rett syndrome (RTT), cyclin-dependent kinase-like 5 (CDKL5) mutations, tuberous sclerosis complex (TSC), neuroligin mutations and “interneuronopathies” resulting from aristaless-related homeobox, X-linked (ARX) and Nueropilin 2 (NRP2) gene mutations, all of which include ASD, IDD and epilepsy. In addition, the process of epilepsy development (i.e. epileptogenesis) and/or spontaneous seizures themselves may results in maladaptive synaptic plasticity producing imbalances of excitation and inhibition that contribute to learning and behavioral difficulties. Abnormalities in synaptic plasticity can arrive from alterations in receptors, signaling molecules or neurotropins, many of which are known to be altered after early life seizures and in genetic conditions known to be associated with both ASD and epilepsy (figure 1.)
Figure 1.
There is evidence that epilepsy and disorders of learning and behavior can all arise from abnormal excitability and disrupted synaptic plasticity in the developing brain. This abnormal plasticity can result from genetic conditions. In addition, epilepsy development (epileptogenesis) and/or seizures during early post-natal development may alter synaptic plasticity and contribute to learning and behavioral disorders. Abnormalities in synaptic plasticity can arise from alterations in receptors, signaling molecules or neurotrophins. Multiple of these molecules are known to be altered by early life seizures and genetic conditions associated with IDD, ASD and epilepsy.
Synaptic plasticity describes the process whereby synapses get strengthened by experience or practice. When synapses are activated, depolarization mediated by AMPA receptors allows release of magnesium blockade and calcium entry through NMDA receptors. This triggers calcium dependent activation of kinases and other signaling pathways resulting in enhanced gene transcription and trafficking of receptors that result in faster and stronger synaptic connections. This is known as long-term potentiation and is thought be the cellular basis of learning. Synaptic plasticity depends on a variety of proteins whose genes are disrupted in genetic conditions associated with autism and epilepsy. These include CDKL5 in West syndrome, methyl-CpG binding protein 2 (MeCP2) in Rett syndrome, fragile X mental retardation protein (FMRP) in fragile X mental retardation syndrome, mammalian target of rapamycin (mTOR) in tuberous sclerosis, and reelin in lissencephaly.
Fragile X Syndrome
Fragile X syndrome (FXS) is the most frequent form of inherited mental retardation and often presents with IDD, ASD and epilepsy. The hallmark of FXS pathology is the hyperabundance of dendritic spines with a long, thin, and otherwise immature morphology (Grossman et al., 2006, Irwin et al., 2000). Fragile X results from an expanded triplet repeat in the FMR1 gene. Fmr1 knock-out mice (an animal model for FXS) exhibits a similar excess of long, thin spines (Comery et al., 1997) and display altered learning and behavior, greater susceptibility to seizures, and altered synaptic plasticity (Penagarikano et al., 2007). The FMR1 gene is located on the X-chromosome (Bassell & Warren, 2008), and expanded CGG repeats within the 5’-untranslated region of FMR1 result in FXS (normal length of ~30 triplets, 55–200 triplets in premutations in FXS carriers, 200–800 triplets in affected FXS individuals). The FMR1 gene codes for the fragile X mental retardation protein (FMRP), which is an mRNA-binding protein abundant in the brain that binds to and regulates 4% of brain mRNA, including many RNAs important for synaptic plasticity. FMRP regulates mRNA transport and local protein synthesis in neuronal dendrites and spines that is important for spine development and synaptic plasticity. In the absence of FMRP, excess and dysregulated mRNA translation leads to altered synaptic function and loss of protein synthesis-dependent plasticity. FMRP is also involved in axonal development, synapse formation, and the development and wiring of neuronal circuits (Antar et al., 2004, Bassell & Warren, 2008, Feng et al., 1997, Gibson et al., 2008, Laggerbauer et al., 2001, Li et al., 2001, Lu et al., 2004, Muddashetty et al., 2007, Zalfa et al., 2003).
FMRP regulates metabotropic glutamate receptor (mGluR)-induced long-term depression (LTD), and in the absence of FMRP, there is excessive AMPA receptor (AMPAR) internalization and exaggerated LTD leading to impaired synaptic excitatory function (“The mGluR theory of fragile X”;(Bear et al., 2004)). Stimulation of group1 mGluRs activates protein phosphatase 2-A (PP2A) and dephosphorylates FMRP, allowing rapid translation of FMRP-associated mRNAs that lead to excessive AMPA receptor internalization, disrupted synaptic function and exaggerated LTD. This is known as the “mGluR theory of fragile X”, and although it adds to our understanding of intellectual impairment in fragile X syndrome, it does not seem to readily explain the CNS hyperexcitability and resultant epilepsy associated with this disorder. Recent studies on group I mGluR-mediated epileptogenesis, however, have begun to reveal a possible connection (Hagerman & Stafstrom, 2009) Bianchi et al. provided compelling evidence that a voltage-gated inward current, I mGluR(V), is the cellular basis for the epileptogenic behavior induced by activation of the mGluR5 receptor (Bianchi et al., 2009, Chuang et al., 2001, Chuang et al., 2000, Chuang et al., 2005). Evidence for a connection between the absence of Fmr1 and epileptogenesis was further supported by a study of neocortical circuits in FMRP knockout mice that found an increased intrinsic excitability in excitatory neurons from the knockout (Gibson et al., 2008). In addition, dysregulation of glutamergic neurons in fragile X syndrome can disrupt the normal actions of inhibitory GABAergic neurons, and result in downregulation of GABA receptor subunits (D’Hulst et al., 2006, El Idrissi et al., 2005, Gantois et al., 2006) and altered expression of a number of enzymes involved in the metabolism of GABA (D’Hulst et al., 2009) that could also contribute to hyperexcitability and epilepsy in the fragile X syndrome.
Tuberous Sclerosis
Tuberous sclerosis complex (TSC) is a neurocutaneous syndrome characterized by benign tumors, early-onset epilepsy, intellectual disability, and autism. TSC results from mutations of hamartin or tuberin (encoded by TSC1 and TSC2 genes), which together inhibit the phosphatidyl inositol 3-kinase (PI3) signaling pathway, involving the mammalian target of rapamycin (mTOR) and a cascade of other downstream kinases and translational factors that stimulate protein translation, cell growth and proliferation. Mutations of hamartin or tuberin in TSC leads to hyperactivation of the mTOR and downstream signaling pathways result in increased cell growth, proliferation and abnormal gene expression. Exact mechanisms of epilepsy, IDD and ASD in TSC are still unknown, but alterations in trafficking of AMPARs, and in expression of specific glutamate and GABA-A receptor subunits and decreases in the glutamate transporter GLT-1 may all contribute to imbalances of excitation and inhibition (White et al., 2001, Wong et al., 2003).
Rett Syndrome
Rett syndrome (RTT) is a postnatal progressive neurodevelopmental disorder that manifests in girls during early childhood. Symptoms appear over stages beginning at 6–18 months and include loss of acquired speech, social skills, purposeful use of the hands and motor skills. Patients typically go on to develop profound cognitive impairment, anxiety, seizures, and a host of autonomic abnormalities (Chahrour et al., 2008). RTT is caused by mutations in the gene encoding methyl-CpG binding protein 2 (MeCP2), a transcriptional regulator involved in chromatin remodeling and the modulation of RNA splicing. In resting neurons, MeCP2 regulates gene expression by binding to methylated CpG dinucleotides and recruiting histone deacetylase (HDAC) co-repressor complexes and chromatin remodeling proteins. This most commonly leads to chromatin compaction, making the promoter inaccessible to the transcriptional machinery and transcriptional repression (Chen et al., 2003), although recent evidence suggests that MeCP2 can also act as a transcriptional activator for some genes (Chahrour et al., 2008). Neuronal activity induces MeCP2 phosphorylation, dissociation of MeCP2 and the corepressor complex from promoters, and target gene expression. In RTT, the absence of MeCP2 causes a loss of activity dependent changes in gene expression that may disrupt synaptic plasticity (Chahrour & Zoghbi, 2007). MeCP2 loss has been shown to induce changes in expression of thousands of genes (Chahrour et al., 2008), although the precise mechanism by which loss of MeCP2 results in either epilepsy, IDD or ASD remains uncertain. Recent evidence suggests that alterations in cortical glutamatergic synaptic responses and excitatory connectivity resulting in a relative excess of inhibition compared to excitation may play an important role (Chao et al., 2007, Dani et al., 2005, Dani et al., 2009).
Neuroligin/Neurexin Mutations
Neuroligins and neurexins are proteins crucial for aligning and activating both excitatory and inhibitory synapses during development. Mutations in a number of these genes, along with the associated Shank3 scaffolding protein, have been implicated in autism. An altered balance between excitatory synapses and inhibitory synapses could affect cognition and social behavior as well as contribute to epilepsy. Mutations in neuroligin (NL)-1, 3 and -4 have been identified in human patients with ASDs (Durand et al., 2007, Jamain et al., 2003). The Arg451→Cys451 substitution in NL-3 found in ASD patients results in enhanced inhibitory transmission and impaired social behavior in knock-in mice (Tabuchi et al., 2007). Overexpression of the mutant NL-1 found in ASD patients depressed the number of excitatory synapses & excitatory synaptic strength and resulted in abnormal social behavior in mice (Chubykin et al., 2007).
Interneuronopathies
ARX
Developmental abnormalities resulting in reduced numbers of cortical and hippocampal interneuron subtypes have been reported to cause both severe early life epilepsies, IDD and autism. In humans, mutations of the aristaless-related homeobox, X-linked (ARX) gene result in several clinical syndromes all of which are associated with intellectual disability, ASD and early-life seizures, most often infantile spasms. In animal models, ARX knockouts have reduced interneuron cell types and a variety of seizure types (absence, myoclonic, generalized tonic-clonic) beginning in early life (Marsh, et al. 2009).
Neuropilin 2 mutations
Neuropilin 2 (NRP2) is a receptor for the axon guidance mediator Semaphorin 3F. Polymorphisms of NRP2 gene have been associated with autism (Wu et al., 2007). NRP2 deficient mice have shorter seizure latency after chemoconvulsants, develop spontaneous seizures, have reductions in specific subsets of hippocampal interneurons (parvalbumin and neuropeptide Y) and reduced dendritic length and complexity on CA1 pyramidal neurons (Gant et al., 2009).
In conclusion, a number of different genetic mutations can result in IDD, ASD and epilepsy. Many of these mutations cause abnormalities of synaptic plasticity that result in imbalances in excitation and inhibition in the developing brain. In addition to genetic abnormalities that disrupt synaptic plasticity, seizures and epilepsy development (epileptogenesis) in early life may impact synaptic plasticity and potentially contribute to ASD and intellectual disability. What are the changes resulting from epileptogenesis and/or seizures in the developing brain that may alter synaptic plasticity and contribute to IDD and ASD?
Effects of seizures and epileptogenesis on the developing brain
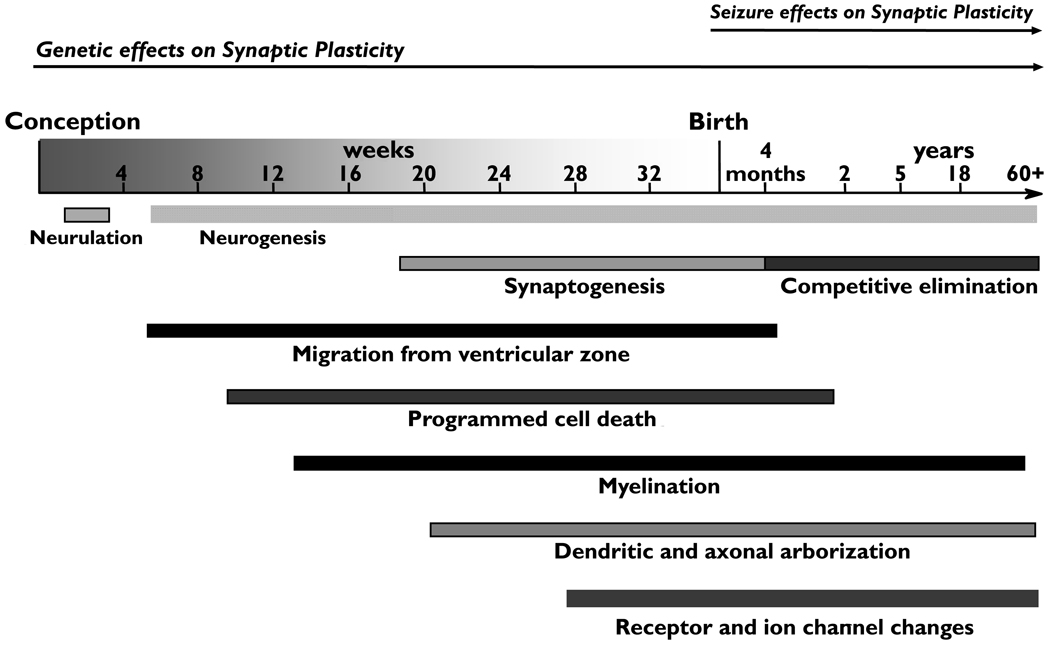
Epileptogenesis is a process that proceeds over months to years in humans, and over days to weeks in rodent models. After an initial precipitating event such as a prolonged febrile seizure or head trauma, there are processes that occur very rapidly including ion channel activation, post-translational changes, and immediate early genes. Next, over a period of days to weeks, there are transcriptional events, neuronal death and inflammation. Over the ensuing weeks, months to years, sprouting, network reorganization, neurogenesis and gliosis occur. These processes may lead to the development of the first spontaneous seizures, and then be recapitulated with each seizure, resulting in perpetuation or progression of epilepsy. Changes associated with epileptogenesis and seizures occur simultaneously with and may disrupt normal activity dependent developmental processes in the brain including synaptic pruning, dendritic and axonal refinement and receptor and ion channel maturations (Figure 2). These effects may occur independently of and in addition to genetic disruptions of synaptic plasticity discussed earlier (Figure 2).
Figure 2.
Changes associated with epileptogenesis and seizures occur simultaneously with and may disrupt normal activity dependent developmental processes in the brain including synaptic pruning, dendritic and axonal refinement and receptor and ion channel maturations. These effects may occur independently of or in addition to genetic disruptions of synaptic plasticity that can have effects beginning very early in development and continue through later life.
There is substantial evidence from animal models that early life-seizures induced by a variety of methods including chemoconvulsants, tetanus toxin, corticotrophin releasing hormone and hyperthermia/fever can all result in later cognitive dysfunction in a subset of animals (Brunson et al., 2001a, Cornejo et al., 2007, de Rogalski Landrot et al., 2001, Dube et al., 2009, Kubova et al., 2004, Lee et al., 2001, Liu et al., 1995, Lynch et al., 2000, Sayin et al., 2004). Until recently, no specific biomarkers were available that could predict the cognitive outcome after early-life seizures. In a recent study, animals that experienced Prolonged Febrile Seizures (PFS) in early life underwent MRI imaging after seizures and then testing of spatial learning and memory in adulthood (Dube et al., 2009). Half of animals exposed to PFS were found to have T2 signal abnormalities in hippocampus, and the spatial learning and memory performances in rats that experienced PFS that also had T2 MRI changes were impaired compared to the rats that had prolonged febrile seizures with no T2 changes, identifying MRI imaging as a possible biomarker for future hippocampal learning and memory dysfunction after early life seizures.
There are many potential effects of seizures and epileptogenesis in the developing brain on synaptic plasticity that could explain the observed effects on cognition and behavior. These include a number of cellular and molecular changes that have been shown to occur during developmental epileptogenesis including cellular changes such as altered neurogenesis and reduced place cell function, molecular changes resulting in altered inhibitory and excitatory neurotransmission, and changes in regulatory and neuromodulatory pathways. Dentate granule cell neurogenesis is critical for learning, and both enhanced and reduced neurogenesis have been reported after early-life seizures, depending on age and model (McCabe et al., 2001, Porter et al., 2004). The location of an animal in his environment is coded by the activity of a category of pyramidal neurons called place cells, a subset of CA1 cells that fire preferentially at particular locations (firing fields) and this encoding is critical for the formation of long-term spatial memory. Recent work examining place cell recordings in CA1 of rats demonstrated that those rats exposed to EPFS that had significant hippocampal MRI changes, had impaired place cell function, including reduced coherence and stability, that correlated with spatial memory deficits on behavioral testing (Dube et al., 2009).
Emerging evidence suggests that early-life seizures can alter the function of neurotransmitter systems and intrinsic neuronal properties that are critical for learning and memory. GABA is the main inhibitory neurotransmitter in the brain and GABA-A receptors mediate most fast synaptic inhibition. Changes in inhibitory neurotransmission are known to affect learning. Enhancement of GABA-A receptor function with benzodiazepines disrupts long-term potentiation (LTP) and memory formation (del Cerro et al., 1992, Seabrook et al., 1997) and GABA-A receptor alpha-subunits have been shown to be key regulators of “critical periods” for cortical plasticity (Fagiolini et al., 2004) and hippocampal dependent spatial learning (Rudolph & Mohler, 2004). Evidence exists for enhanced inhibition after early life seizures that could impair these cognitive processes. On a circuit level, increased paired pulse inhibition in hippocampus has been seen after both hyperthermic and kainate-induced seizures in the postnatal period (Sutula et al., 1992). At the cellular/molecular level, early-life lithium-pilocarpine induced seizures produce an increase in GABA-A receptor expression and a selective increase in the alpha1 subunit in the hippocampal dentate gyrus both immediately and when the animals reach adulthood (Raol et al., 2006, Zhang et al., 2004). These alterations are associated with functional changes including enhanced type I benzodiazepine augmentation of the receptor (Zhang et al., 2004). This is in contrast to the alpha1 subunit expression decrease seen in adult rats following pilocarpine-induced seizures (Brooks-Kayal et al., 1998). These findings suggest that the effects of seizures on expression of GABA-A receptor subunits are age-dependent and that increased GABA-A receptor expression and resulting enhanced inhibition could contribute to cognitive deficits following early-life seizures. While GABAA Receptor activation in mature neurons results in membrane hyperpolarization and inhibition of cell firing, due to developmental changes in the chloride gradient, in immature neurons GABAA receptor activation results in membrane depolarization & is thus excitatory (Cherubini et al., 1990). Depolarizing GABA currents are critical for Ca++ dependent developmental processes including neuronal proliferation, migration, targeting and synaptogenesis (Ben-Air et al., 1997, Leinekugel et al., 1997, LoTurco et al., 1995, Owens et al., 1996). Early-life seizures have recently been shown to accelerate the switch of GABA currents from depolarizing to hyperpolarizing in hippocampal CA1 neurons and has been associated with spatial learning deficits (Galanopoulou, 2008).
Changes in excitatory neurotransmission may also contribute to learning and behavioral differences after early-life seizures. Glutamate is the primary excitatory neurotransmitter in brain and its activity is mediated by a variety of receptor subtypes including NMDA and non-NMDA (AMPA and kainate) ionotropic receptors and metabotropic receptors. Excitatory signaling through both the AMPA and NMDA receptors are critical for different types of LTP and hippocampal learning (Mongillo et al., 2003, Riedel et al., 2003, Schmitt et al., 2005, Yasuda et al., 2003), and mutant mice lacking subtypes of AMPA or NMDA receptors have impaired learning (Mead & Stephens, 2003, Nakazawa et al., 2002). Deficits in excitatory synaptic density and in excitatory signaling through both AMPA and NMDA receptors have been found after early-life seizures. Early-life hypoxia induced seizures have been shown to result in rapid post-transcriptional effects on AMPA receptor subunit (GluR) phosphorylation, including increased phosphorylation of sites in both GluR1 and GluR2 that result in enhanced excitatory synaptic currents due to increased conductance and membrane insertion of Ca-permeable GluR1 containing receptors associated with GluR1 phosphorylation and reduced membrance insertion of Ca-impermeable GluR2 containing receptors (Rakhade et al., 2008). Tetanus-toxin induced seizures in the postnatal period produce long-term effects including a 30% decrease in dendritic spine density on hippocampal CA3 neurons (Jiang et al., 1998) and a 30–40% decrease in NMDA receptor NR1, NR2A, and NR2B subunit proteins in hippocampus (Lee et al., 2001, Swann, 2004). Acute and chronic decreases in AMPA receptor GluR2 subunit expression has been shown after postnatal hypoxia-induced seizures (Sanchez et al., 2001) and lithium-pilocarpine-induced seizures (Zhang et al., 2004). Even a single seizure induced with kainic acid at P7 can result in impairments in spatial memory and impaired CA1 LTP and enhanced LTD (Cornejo et al., 2007). This altered synaptic plasticity is associated with decreases in NMDA receptor NR2A expression and AMPA receptor subunit GluR1 surface expression and increases in the excitatory synaptic anchoring protein PSD-95 (Cornejo et al., 2007).
In addition to effects of early seizures on neurotransmitter receptor systems, they also affect a number of molecules that are essential for intrinsic neuronal function. Prolonged postnatal febrile (hyperthermic) seizures produce a profound, long-lasting enhancement of intrinsic hyperpolarization activated membrane current, Ih, (Chen et al., 2001) due to a decrease in hyperpolarization activated, cyclic nucleotide-gated channel-1 (HCN1) mRNA and simultaneous enhancement of HCN2 mRNA expression in hippocampal CA1 neurons (Brewster et al., 2002, Brewster et al., 2005). These changes are associated with persistent limbic hyperexcitability and a 35% incidence of spontaneous seizures in adulthood (Chen et al., 2001, Dube et al., 2000, Dube et al., 2006). This suggests that a variety of receptors and important cell signaling proteins may be permanently altered following early-life seizures.
Changes in neuromodulatory pathways may also contribute to learning and behavioral differences after early-life seizures. cAMP response element binding protein (CREB) is a key mediator of stimulus-induced changes in gene expression that underlie plasticity of the nervous system and phosphorylation of CREB is required for LTP, learning and memory (Lonze & Ginty, 2002). CREB phosphorylation with learning has been shown to be diminished after repetitive febrile seizures in animals (Chang, et al., 2003). Corticotropin releasing hormone (CRH) is a neuromodulatory peptide released from hippocampal interneurons in response to stress. Early life seizures have been shown to enhance hippocampal CRH mRNA expression in adulthood (Brunson et al., 2001b) and excessive CRH (Chen et al., 2004) and early-life stress (Brunson et al., 2005) have been shown to lead to reductions in dendritic length and arborization as well as progressive cognitive deficits.
Conclusions
Early-life seizures may produce a variety of cellular and molecular changes in hippocampus that may contribute to the enhanced risk of IDD and ASD in patients with early-life seizures and epilepsy. Abnormalities of synaptic plasticity resulting in imbalances of excitatory and inhibitory neurotransmission resulting either from genetic mutations or effects of early life seizures may provide a common mechanism of epilepsy, IDD and ASD and provide a basis for understanding of the frequent co-occurrence of these disorders. Although this concept of a shared pathophysiology can provide a broad framework in which to begin to understand the frequent association of epilepsy, intellectual disability and autism, many questions remain unanswered. Why are epilepsy, ASD and IDD sometimes comorbid, but not always? In what specific brain regions, pathways and cell types and at what point in development do impairments of synaptic plasticity occur, and does the spatial and temporal profile in part determine the specific phenotype? Do identified changes in synaptic plasticity and the cellular and molecular mechanisms that produce them represent new therapeutic targets for treatment of these conditions, and if so what is the developmental window in which these treatments might be effective? Continued research is essential to begin to address these and other gaps in our understanding of the neurobiological basis of the complex association between epilepsy and co-morbid learning and behavioral disorders.
Acknowledgements
This work was supported by NIH funding (RO1 NS 38595).
Footnotes
Disclosure: We confirm that we have read the Journal’s position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
The author does not have any conflicts of interested related to this article to disclose.
References
- Antar LN, Afroz R, Dictenberg JB, Carroll RC, Bassell GJ. Metabotropic glutamate receptor activation regulates fragile x mental retardation protein and FMR1 mRNA localization differentially in dendrites and at synapses. J Neurosci. 2004;24:2648–2655. doi: 10.1523/JNEUROSCI.0099-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassell GJ, Warren ST. Fragile X syndrome: loss of local mRNA regulation alters synaptic development and function. Neuron. 2008;60:201–214. doi: 10.1016/j.neuron.2008.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bear MF, Huber KM, Warren ST. The mGluR theory of fragile X mental retardation. Trends Neurosci. 2004;27:370–377. doi: 10.1016/j.tins.2004.04.009. [DOI] [PubMed] [Google Scholar]
- Ben-Ari Y, Khazipov R, Leinekugel X, Caillard O, Gaiarsa JL. GABAA, NMDA and AMPA receptors: a developmentally regulated ’manage a trois’. Trends Neurosci. 1997;20:523–529. doi: 10.1016/s0166-2236(97)01147-8. [DOI] [PubMed] [Google Scholar]
- Bianchi R, Chuang SC, Zhao W, Young SR, Wong RK. Cellular plasticity for group I mGluR-mediated epileptogensis. J Neurosci. 2009;29:3497–3507. doi: 10.1523/JNEUROSCI.5447-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brewster A, Bender RA, Chen Y, Dube C, Eghbal-Ahmadi M, Baram TZ. Developmental febrile seizures modulate hippocampal gene expression of hyperpolarization-activated channels in an isoform- and cell-specific manner. J Neurosci. 2002;22:4591–45599. doi: 10.1523/JNEUROSCI.22-11-04591.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brewster AL, Bernard JA, Gall CM, Baram TZ. Formation of heteromeric hyperpolarization-activated cyclic nucleotide-gated (HCN) channels in the hippocampus is regulated by developmental seizures. Neurobiol Dis. 2005;19:200–207. doi: 10.1016/j.nbd.2004.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks-Kayal AR, Jin H, Price M, Dichter MA. Developmental expression of GABA(A) receptor subunit mRNAs in individual hippocampal neurons in vitro and in vivo. J Neurochem. 1998;70:1017–1028. doi: 10.1046/j.1471-4159.1998.70031017.x. [DOI] [PubMed] [Google Scholar]
- Brunson K, Eghbal-Ahmadi M, Bender R, Chen Y, Baram T. Long-term, progressive hippocampal cell loss and dysfunction induced by early-life administration of corticotropin-releasing hormone reproduce the effecs of early-life stress. Proc Natl Acad Sci U S A. 2001a;98:8856–8861. doi: 10.1073/pnas.151224898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunson KL, Baram TZ, Bender RA. Hippocampal neurogenesis is not enhanced by lifelong reduction of glucocorticoid levels. Hippocampus. 2005;15:491–501. doi: 10.1002/hipo.20074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunson KL, Eghbal-Ahmadi M, Baram TZ. How do the many etiologies of West syndrome lead to excitability and seizures? The corticotropin releasing hormone excess hypothesis. Brain Dev. 2001b;23:533–538. doi: 10.1016/s0387-7604(01)00312-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chahrour M, Jung SY, Shaw C, Zhou X, Wong ST, Qin J, Zoghbi HY. MeCP2, a key contributor to neurological disease, activates and represses transcription. Science. 2008;320:1224–1229. doi: 10.1126/science.1153252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chahrour M, Zoghbi HY. The story of Rett syndrome: from clinic to neurobiology. Neuron. 2007;56:422–437. doi: 10.1016/j.neuron.2007.10.001. [DOI] [PubMed] [Google Scholar]
- Chang Y, Huang A, Kuo Y, Wang S, Chang Y, Huang C. Febrile seizures impair memory and cAMP response-element binding protein activation. Ann Neurol. 2003;54:706–718. doi: 10.1002/ana.10789. [DOI] [PubMed] [Google Scholar]
- Chao Y, Siller L, Krishnamurthy S, Coxon PR, Bangert U, Gass M, Kjeldgaard L, Patole SN, Lie LH, O'Farrell N, Alsop TA, Houlton A, Horrocks BR. Evaporation and deposition of alkyl-capped silicon nanocrystals in ultrahigh vacuum. Nat Nanotechnol. 2007;2:486–489. doi: 10.1038/nnano.2007.224. [DOI] [PubMed] [Google Scholar]
- Chen WG, Chang Q, Lin Y, Meissner A, West AE, Griffith EC, Jaenisch R, Greenberg ME. Derepression of BDNF transcription involves calcium-dependent phosphorylation of MeCP2. Science. 2003;302:885–889. doi: 10.1126/science.1086446. [DOI] [PubMed] [Google Scholar]
- Chen Y, Bender RA, Brunson KL, Pomper JK, Grigoriadis DE, Wurst W, Baram TZ. Modulation of dendritic differentiation by corticotropin-releasing factor in the developing hippocampus. Proc Natl Acad Sci U S A. 2004;101:15782–15787. doi: 10.1073/pnas.0403975101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Chi Chow J, Lee I. Comparison the cognitive effect of anti-epileptic drugs in seizure-free children with epilepsy before and after drug withdrawal. Epilepsy Res. 2001;44:65–70. doi: 10.1016/s0920-1211(00)00204-7. [DOI] [PubMed] [Google Scholar]
- Cherubini E, Rovira C, Gaiarsa J, Corradetti R, Ben-Ari Y. GABA mediated excitation in immature rat CA3 hippocampal neurons. Int J Dev Neurosci. 1990;8:481–490. doi: 10.1016/0736-5748(90)90080-l. [DOI] [PubMed] [Google Scholar]
- Chuang SC, Bianchi R, Kim D, Shin HS, Wong RK. Group I metabotropic glutamate receptors elicit epileptiform discharges in the hippocampus through PLCbeta1 signaling. J Neurosci. 2001;21:6387–6394. doi: 10.1523/JNEUROSCI.21-16-06387.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuang SC, Bianchi R, Wong RK. Group I mGluR activation turns on a voltage-gated inward current in hippocampal pyramidal cells. J Neurophysiol. 2000;83:2844–2853. doi: 10.1152/jn.2000.83.5.2844. [DOI] [PubMed] [Google Scholar]
- Chuang SC, Zhao W, Bauchwitz R, Yan Q, Bianchi R, Wong RK. Prolonged epileptiform discharges induced by altered group I metabotropic glutamate receptor-mediated synaptic responses in hippocampal slices of a fragile X mouse model. J Neurosci. 2005;25:8048–8055. doi: 10.1523/JNEUROSCI.1777-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chubykin AA, Atasoy D, Etherton MR, Brose N, Kavalali ET, Gibson JR, Sudhof TC. Activity-dependent validation of excitatory versus inhibitory synapses by neuroligin-1 versus neuroligin-2. Neuron. 2007;54:919–931. doi: 10.1016/j.neuron.2007.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comery TA, Harris JB, Willems PJ, Oostra BA, Irwin SA, Weiler IJ, Greenough WT. Abnormal dendritic spines in fragile X knockout mice: maturation and pruning deficits. Proc Natl Acad Sci U S A. 1997;94:5401–5404. doi: 10.1073/pnas.94.10.5401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornejo BJ, Mesches MH, Coultrap S, Browning MD, Bentke TA. A single episode of neonatal seizures permanently alters glutamatergic synapses. Ann Neurol. 2007;61:411–426. doi: 10.1002/ana.21071. [DOI] [PubMed] [Google Scholar]
- D'Hulst C, De Geest N, Reeve SP, Van Dam D, De Deyn PP, Hassan BA, Kooy RF. Decreased expression of the GABAA receptor in fragile X syndrome. Brain Res. 2006;1121:238–245. doi: 10.1016/j.brainres.2006.08.115. [DOI] [PubMed] [Google Scholar]
- D'Hulst C, Heulens I, Brouwer JR, Willemsen R, De Geest N, Reeve SP, De Deyn PP, Hassan BA, Kooy RF. Expression of the GABAergic system in animal models for fragile X syndrome and fragile X associated tremor/ataxia syndrome (FXTAS) Brain Res. 2009;1253:176–183. doi: 10.1016/j.brainres.2008.11.075. [DOI] [PubMed] [Google Scholar]
- Dani VS, Chang Q, Maffei A, Turrigiano GG, Jaenisch R, Nelson SB. Reduced cortical activity due to a shift in the balance between excitation and inhibition in a mouse model of Rett syndrome. Proc Natl Acad Sci U S A. 2005;102:12560–12565. doi: 10.1073/pnas.0506071102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dani VS, Nelson SB. Intact long-term potentiation but reduced connectivity between neocortical layer 5 pyramidal neurons in a mouse model of Rett syndrome. J Neurosci. 2009;29:11263–11270. doi: 10.1523/JNEUROSCI.1019-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]; del Cerro S, Jung M, Lynch G. Benzodiazepines block long-term potentiation in slices of hippocampus and piriform cortex. Neuroscience. 1992;49:1–6. doi: 10.1016/0306-4522(92)90071-9. [DOI] [PubMed] [Google Scholar]
- de Rogalski Landrot I, Minokoshi M, Silveira DC, Cha BH, Holmes GL. Recurrent neonatal seizures: relationship of pathology to the electroencephalogram and cognition. Brain Res Dev Brain Res. 2001;129:27–38. doi: 10.1016/s0165-3806(01)00177-8. [DOI] [PubMed] [Google Scholar]
- del Cerro S, Jung M, Lynch G. Benzodiazepines block long-term potentiation in slices of hippocampus and piriform cortex. Neuroscience. 1992;49:1–6. doi: 10.1016/0306-4522(92)90071-9. [DOI] [PubMed] [Google Scholar]
- Dube C, Chen K, Eghbal-Ahmadi M, Brunson K, Soltesz I, Baram TZ. Prolonged febrile seizures in the immature rat model enhance hippocampal excitability long term. Ann Neurol. 2000;47:336–344. [PMC free article] [PubMed] [Google Scholar]
- Dube C, Richichi C, Bender RA, Chung G, Litt B, Baram TZ. Temporal lobe epilepsy after experimental prolonged febrile seizures: prospective analysis. Brain. 2006;129:911–922. doi: 10.1093/brain/awl018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dube CM, Zhou JL, Hamamura M, Zhao Q, Ring A, Abrahams J, McIntyre K, Nalcioglu O, Shatskih T, Baram TZ, Holmes GL. Cognitive dysfunction after experimental febrile seizures. Exp Neurol. 2009;215:167–177. doi: 10.1016/j.expneurol.2008.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durand CM, Betancur C, Boeckers TM, Bockmann J, Chaste P, Fauchereau F, Nygren G, Rastam M, Gillberg IC, Anckarsater H, Sponheim E, Goubran-Botros H, Delorme R, Chabane N, Mouren-Simeoni MC, de Mas P, Bieth E, Roge B, Heron D, Burglen L, Gillberg C, Leboyer M, Bourgeron T. Mutations in the gene encoding the synaptic scaffolding protein SHANK3 are associated with autism spectrum disorders. Nat Genet. 2007;39:25–27. doi: 10.1038/ng1933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Idrissi A, Ding XH, Scalia J, Trenkner E, Brown WT, Dobkin C. Decreased GABA(A) receptor expression in the seizure-prone fragile X mouse. Neurosci Lett. 2005;377:141–146. doi: 10.1016/j.neulet.2004.11.087. [DOI] [PubMed] [Google Scholar]
- Fagiolini M, Fritschy JM, Low K, Mohler H, Rudolph U, Hensch TK. Specific GABAA circuits for visual cortical plasticity. Science. 2004;303:1681–1683. doi: 10.1126/science.1091032. [DOI] [PubMed] [Google Scholar]
- Feng Y, Gutekunst CA, Eberhart DE, Yi H, Warren ST, Hersch SM. Fragile X mental retardation protein: nucleocytoplasmic shuttling and association with somatodendritic ribosomes. J Neurosci. 1997;17:1539–1547. doi: 10.1523/JNEUROSCI.17-05-01539.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galanopoulou AS. Dissociated gender-specific effects of recurrent seizures on GABA sigaling in CA1 pyramidal neurons: role of GABA(A) receptors. J Neurosci. 2008;28:1557–1567. doi: 10.1523/JNEUROSCI.5180-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gant JC, Thibault O, Blalock EM, Yang J, Bachstetter A, Kotick J, Schauwecker PE, Hauser KF, Smith GM, Mervis R, Li Y, Barnes GN. Decreased number of interneurons and increased seizures in neuropilin 2 deficient mice: implications for autism and epilepsy. Epilepsia. 2009;50:629–645. doi: 10.1111/j.1528-1167.2008.01725.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gantois I, Vandesompele J, Speleman F, Reyniers E, D'Hooge R, Severijnen LA, Willemsen R, Tassone F, Kooy RF. Expression profiling suggests underexpression of the GABA(A) receptor subunit delta in the fragile X knockout mouse model. Neurobiol Dis. 2006;21:346–357. doi: 10.1016/j.nbd.2005.07.017. [DOI] [PubMed] [Google Scholar]
- Gibson JR, Bartley AF, Hays SA, Huber KM. Imbalance of neocortical excitation and inhibition and altered UP states reflect network hyperexcitability in the mouse model of fragile X syndrome. J Neurophysiol. 2008;100:2615–2626. doi: 10.1152/jn.90752.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossman AW, Aldridge GM, Weiler IJ, Greenough WT. Local protein synthesis and spine morphogenesis: Fragile X syndrome and beyond. J Neurosci. 2006;26:7151–7155. doi: 10.1523/JNEUROSCI.1790-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagerman PJ, Stafstrom CE. Origins of epilepsy in fragile x syndrome. Epilepsy Curr. 2009;9:108–112. doi: 10.1111/j.1535-7511.2009.01309.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irwin SA, Galvez R, Greenough WT. Dendritic spine structural anomalies in fragile-X mental retardation syndrome. Cereb Cortex. 2000;10:1038–1044. doi: 10.1093/cercor/10.10.1038. [DOI] [PubMed] [Google Scholar]
- Jamain S, Quach H, Betancur C, Rastam M, Colineaux C, Gillberg IC, Soderstrom H, Giros B, Leboyer M, Gillberg C, Bourgeron T. Mutations of the X-linked genes encoding neuroligins NLGN3 and NLGN4 are associated with autism. Nat Genet. 2003;34:27–29. doi: 10.1038/ng1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang M, Lee CL, Smith KL, Swann JW. Spine loss and other persistent alterations of hippocampal pyramidal cell dendrites in a model of early-onset epilepsy. J Neurosci. 1998;18:8356–8368. doi: 10.1523/JNEUROSCI.18-20-08356.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubova H, Mares P, Suchomelova L, Brozek G, Druga R, Pitkanen A. Status epilepticus in immature rats leads to behavioral and cognitive impairement and epileptogenesis. Eur J Neurosci. 2004;19:3255–3265. doi: 10.1111/j.0953-816X.2004.03410.x. [DOI] [PubMed] [Google Scholar]
- Laggerbauer B, Ostareck D, Keidel EM, Ostareck-Lederer A, Fischer U. Evidence that fragile X mental retardation protein is a negative regulator of translation. Hum Mol Genet. 2001;10:329–338. doi: 10.1093/hmg/10.4.329. [DOI] [PubMed] [Google Scholar]
- Lee CL, Hannay J, Hrachovy R, Rashid S, Antalffy B, Swann JW. Spatial learning deficits without hippocampal neuronal loss in a model of early-onset epilepsy. Neuroscience. 2001;107:71–84. doi: 10.1016/s0306-4522(01)00327-x. [DOI] [PubMed] [Google Scholar]
- Leinekugal X, Medina I, Khalilov I, Ben-Ari Y, Khazipov R. Ca2+ oscillations mediated by the synergistic excitatory actions GABA(A) and NMDA receptors in the neonatal hippocampus. Neuron. 1997;18:243–255. doi: 10.1016/s0896-6273(00)80265-2. [DOI] [PubMed] [Google Scholar]
- Li Z, Zhang Y, Ku L, Wilkinson KD, Warren ST, Feng Y. The fragile X mental retardation protein inhibits translation via interacting with mRNA. Nucleic Acids Res. 2001;29:2276–2283. doi: 10.1093/nar/29.11.2276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu AW, Delgado-Escueta AV, Serratosa JM, Alonso ME, Medina MT, Gee MN, Cordova S, Zhao HZ, Spellman JM, Peek JR, et al. Juvenile myoclonic epilepsy locus in chromosome 6p21.2-p11: linkage convulsions and electroencephalography trait. Am J Hum Genet. 1995;57:368–381. [PMC free article] [PubMed] [Google Scholar]
- Lonze BE, Ginty DD. Function and regulation of CREB family transcription factors in the nervous system. Neuron. 2002;35:605–623. doi: 10.1016/s0896-6273(02)00828-0. [DOI] [PubMed] [Google Scholar]
- LoTurco JJ, Owens DF, Heath MJ, Daivs MB, Kreigstein AR. GABA and dlutamate depolarize cortical progenitor cellsa nd inhibit DNA synthesis. Neuron. 1995;15:1287–1298. doi: 10.1016/0896-6273(95)90008-x. [DOI] [PubMed] [Google Scholar]
- Lu R, Wang H, Liang Z, Ku L, O'Donnell WT, Li W, Warren ST, Feng Y. The fragile X protein controls microtubule-associated protein 1B translation and microtubule stability in brain neuron development. Proc Natl Acad Sci U S A. 2004;101:15201–15206. doi: 10.1073/pnas.0404995101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch M, Sayin U, Bownds J, Janumpalli S, Sutula T. Long-term consequences of early postnatal seizures on hippocampal learning and plasticity. Eur J Neurosci. 2000;12:2252–2264. doi: 10.1046/j.1460-9568.2000.00117.x. [DOI] [PubMed] [Google Scholar]
- Marsh E, Fulp C, Gomez E, Nasrallah I, Minarcik J, Sudi J, Christian SL, Mancini G, Labosky P, Dobyns W, Brooks-Kayal A, Golden JA. Targeted loss of Arx results in a developmental epilepsy mouse model and recapitulates the human phenotype in heterozygous females. Brain. 2009;132:1563–1576. doi: 10.1093/brain/awp107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mead AN, Stephens DN. Involvement of AMPA receptor GluR2 subunits in stimulus-reward learning: evidence from glutamate receptor gria2 knock-out mice. J Neurosci. 2003;23:9500–9507. doi: 10.1523/JNEUROSCI.23-29-09500.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCabe BK, Silveira DC, Cilio MR, Bha BH, Liu X, Sogawa Y, Holmes GL. Reduced neurogenesis after neonatal seizures. J Neurosci. 2001;21(6):2094–2103. doi: 10.1523/JNEUROSCI.21-06-02094.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mongillo G, Amit DJ, Brunel N. Retrospective and prospective persistent activity induced by Hebbian learning in a recurrent cortical network. Eur J Neurosci. 2003;18:2011–2024. doi: 10.1046/j.1460-9568.2003.02908.x. [DOI] [PubMed] [Google Scholar]
- Muddashetty RS, Kelic S, Gross C, Xu M, Bassell GJ. Dysregulated metabotropic glutamate receptor-dependent translation of AMPA receptor and postsynaptic density-95 mRNAs at synapses in a mouse model of fragile X syndrome. J Neurosci. 2007;27:5338–5348. doi: 10.1523/JNEUROSCI.0937-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]; Nakazawa K, Quirk MC, Chitwood RA, Watanabe M, Yeckel MF, Sun LD, Kato A, Carr CA, Johnston D, Wilson MA, Tonegawa S. Requirement for hippocampal CA3 NMDA receptors in associative memory recall. Science. 2002;297:211–218. doi: 10.1126/science.1071795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakazawa K, Quirk MC, Chitwood RA, Watanabe M, Yeckel MF, Sun LD, Kato A, Carr CA, Johnston D, Wilson MA, Tonegawa S. Requiremetn for hippocampal CA3 NMDA receptors in associative memory recall. Science. 2002;297:211–218. doi: 10.1126/science.1071795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owens D, Boyce L, Davis M, Kriegstein A. Excitatory GABA respones in embryonic and neonatal cortical slices demonstrated by gramicidin perforated-patch recordings and calcium imaging. J Neurosci. 1996;16:6414–6423. doi: 10.1523/JNEUROSCI.16-20-06414.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penagarikano O, Mulle JG, Warren ST. The pathophysiology of fragile x syndrome. Annu Rev Genomics Hum Genet. 2007;8:109–129. doi: 10.1146/annurev.genom.8.080706.092249. [DOI] [PubMed] [Google Scholar]
- Porter B, M M, Brooks-Kayal A. Fate of newborn dentate granule cells after early life status epilepticus. Epilepsia. 2004;45:13–19. doi: 10.1111/j.0013-9580.2004.23903.x. [DOI] [PubMed] [Google Scholar]
- Rakhade SN, Zhou C, Aujla PK, Fishman R, Sucher NJ, Jensen FE. Early alterations of AMPA receptors mediate synaptic potentiation induced by neonatal seizures. J Neurosci. 2008;28:7979–7990. doi: 10.1523/JNEUROSCI.1734-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raol YH, Zhang G, Lund IV, Porter BE, Maronski MA, Brooks-Kayal AR. Increased GABA(A)-receptor alpha1-subunit expression in hippocampal dentate gyrus after early-life status epilepticus. Epilepsia. 2006;47:1665–1673. doi: 10.1111/j.1528-1167.2006.00640.x. [DOI] [PubMed] [Google Scholar]
- Riedel G, Platt B, Micheau J. Glutamate receptor function in learning and memory. Behav Brain Res. 2003;140:1–47. doi: 10.1016/s0166-4328(02)00272-3. [DOI] [PubMed] [Google Scholar]
- Rudolph U, Mohler H. Analysis of GABAA receptor function and dissection of the pharmacology of benzodiazepines and general anesthetics through mouse genetics. Annu Rev Pharmacol Toxicol. 2004;44:475–498. doi: 10.1146/annurev.pharmtox.44.101802.121429. [DOI] [PubMed] [Google Scholar]
- Sanchez RM, Koh S, Rio C, Wang C, Lamperti ED, Sharma D, Corfas G, Jensen FE. Decreased glutamate receptor 2 expression and enhanced epileptogenesis in immature rat hippocampus after perinatal hypoxia-induced seizures. J Neurosci. 2001;21:8154–8163. doi: 10.1523/JNEUROSCI.21-20-08154.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sayin U, Sutula TP, Stafstrom CE. Seizures in the developing brain cause adverse long-term effects on spatial learning and anxiety. Epilepsia. 2004;45:1539–1548. doi: 10.1111/j.0013-9580.2004.54903.x. [DOI] [PubMed] [Google Scholar]
- Schmitt WB, Sprengel R, Mack V, Draft RW, Seeburg PH, Deacon RM, Rawlins JN, Bannerman DM. Restoration of spatial working memory by genetic rescue of GluR-A-deficient mice. Nat Neurosci. 2005;8:270–272. doi: 10.1038/nn1412. [DOI] [PubMed] [Google Scholar]
- Seabrook GR, Easter A, Dawson GR, Bowery BJ. Modulation of long-term potentiation in CA1 region of mouse hippocampal brain slices by GABAA receptor benzodiazepine site ligands. Neuropharmacology. 1997;36:823–830. doi: 10.1016/s0028-3908(97)00040-3. [DOI] [PubMed] [Google Scholar]
- Sutula T, Cavazos J, Golarai G. Alteration of long-lasting structural and functional effects of kainic acid in the hippocampus by brief treatment with phenobarbital. J Neurosci. 1992;12:4173–4187. doi: 10.1523/JNEUROSCI.12-11-04173.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swann JW. The effects of seizures on the connectivity and circuitry of the developing brain. Ment Retard Dev Disabil Res Rev. 2004;10:96–100. doi: 10.1002/mrdd.20018. [DOI] [PubMed] [Google Scholar]
- Tabuchi K, Blundell J, Etherton MR, Hammer RE, Liu X, Powell CM, Sudhof TC. A neuroligin-3 mutation implicated in autism increases inhibitory synaptic transmission in mice. Science. 2007;318:71–76. doi: 10.1126/science.1146221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuchman R, Moshe SL, Rapin I. Convulsing toward the pathophysiology of autism. Brain Dev. 2009;31:95–103. doi: 10.1016/j.braindev.2008.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White R, Hua Y, Scheithauer B, Lynch DR, Henske EP, Crino PB. Selective alterations in glutamate and GABA receptor subunit mRNA expression in dysplastic neurons and giant cells of cortical tubers. Ann Neurol. 2001;49:67–78. doi: 10.1002/1531-8249(200101)49:1<67::aid-ana10>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]; Wong M, Ess KC, Uhlmann EJ, Jansen LA, Li W, Crino PB, Mennerick S, Yamada KA, Gutmann DH. Impaired glial glutamate transport in a mouse tuberous sclerosis epilepsy model. Ann Neurol. 2003;54:251–256. doi: 10.1002/ana.10648. [DOI] [PubMed] [Google Scholar]
- Wong M, Ess KC, Uhlmann EJ, Jansen LA, Li W, Crino PB, Mennerick S, Yamada KA, Gutmann DH. Impaired glial glutamate transport in a mouse tuberous sclerosis epilepsy model. Ann Neurol. 2003;54:251–256. doi: 10.1002/ana.10648. [DOI] [PubMed] [Google Scholar]
- Wu S, Yue W, Jia M, Ruan Y, Lu T, Gong X, Shuang M, Liu J, Yang X, Zhang D. Association of the neuropilin-2 (NRP2) gene polymorphisms with autism in Chinese Han population. Am J Med Genet B Neuropsychiatr Genet. 2007;144B:492–495. doi: 10.1002/ajmg.b.30495. [DOI] [PubMed] [Google Scholar]
- Yasuda H, Barth AL, Stellwagen D, Malenka RC. A developmental switch in the signaling cascades for LTP induction. Nat Neurosci. 2003;6:15–16. doi: 10.1038/nn985. [DOI] [PubMed] [Google Scholar]
- Zalfa F, Giorgi M, Primerano B, Moro A, Di Penta A, Reis S, Oostra B, Bagni C. The fragile X syndrome protein FMRP associates with BC1 RNA and regulates the translation of specific mRNAs at synapses. Cell. 2003;112:317–327. doi: 10.1016/s0092-8674(03)00079-5. [DOI] [PubMed] [Google Scholar]
- Zhang G, Raol YS, Hsu FC, Brooks-Kayal AR. Long-term alterations in glutamate receptor and transporter expression following early-life seizures are associated with increased seizure susceptibility. J Neurochem. 2004;88:91–101. doi: 10.1046/j.1471-4159.2003.02124.x. [DOI] [PubMed] [Google Scholar]