Abstract
Objectives
To evaluate safety of intravenous tissue plasminogen activator (tPA) delivered without dedicated thrombolytic stroke teams.
Methods
This was a retrospective, observational study of patients treated between 1996 and 2005 at four southeastern Michigan hospital emergency departments (EDs) with a prospectively defined comparison to the National Institute of Neurological Disorders and Stroke (NINDS) tPA stroke study cohort. Main outcome measures were mortality, intracerebral hemorrhage (ICH), systemic hemorrhage, neurologic recovery, and guideline violations.
Results
Two hundred seventy-three consecutive stroke patients were treated by 95 emergency physicians using guidelines and local neurology resources. One-year mortality was 27.8%. Unadjusted Cox model relative risk of mortality compared to the NINDS tPA treatment and placebo groups was 1.20 (95% confidence interval [CI] = 0.87 to 1.64) and 1.04 (95% CI = 0.76 to 1.41), respectively. Rate of significant ICH by computed tomography criteria was 6.6% (OR 1.03, 95% CI = 0.56 to 1.90 compared to NINDS tPA treatment group). The proportion of symptomatic ICH by two other pre-specified sets of clinical criteria was 4.8% and 7.0%. Rate of any ICH within 36 hours of treatment was 9.9% (relative risk [RR] 0.94, 95% CI = 0.58 to 1.51 compared to NINDS tPA group). Occurrence of major systemic hemorrhage (requiring transfusion) was 1.1%. Functional recovery by the modified Rankin Scale score (mRS 0 to 2) at discharge occurred in 38% of patients with a premorbid disability mRS < 2. Guideline deviations occurred in the ED in 26% of patients and in 25% of patients following admission.
Conclusions
In these EDs there was no evidence of increased risk with respect to mortality, ICH, systemic hemorrhage, or worsened functional outcome when tPA was administered without dedicated thrombolytic stroke teams. Additional effort is needed to improve guideline compliance.
Keywords: Stroke, tissue plasminogen activator, safety, emergency department, ICH
INTRODUCTION
Of over 650,000 patients with ischemic stroke in the United States each year,1 15% to 27%2–4 present to hospitals within the 3-hour time window necessary to receive intravenous tissue plasminogen activator (tPA), and 11.5% are estimated fully eligible for treatment.5 Fewer than 2%, however, are actually treated.3,6,7 Reasons for the low practice penetration of thrombolytic therapy include limited data on tPA safety when used outside of acute stroke teams, and the operational constraints on creating acute stroke teams at most community hospitals.
Of 19 published case series4,8–25 on post-approval use of tPA in stroke, nine definitively note any thrombolytic delivery using a “community model” of thrombolytic delivery - where tPA is administered by physicians, including non-neurologists, outside of an acute stroke team.4,8,10,11,18,20,22,23,25 Six studies identified instances of delivery by an emergency or primary care physician (either alone or with telephone consultation with a neurologist).8,18,20,22,23,25 The number of patients treated by non-neurologists in these, however, was small - ranging from six to 53. Two studies reported cautionary findings for “community models” of acute stroke care. One noted an increase in symptomatic intracerebral hemorrhage (ICH) in a series of 70 patients treated by community neurologists,4 and both found increased in-hospital mortality among tPA-treated stroke patients.4,11
Thus, evidence on the safety of thrombolytic delivery in a common U.S. hospital setting an emergency department (ED) without a stroke team that uses local neurological – resources - is quite limited. This potentially contributes to a failure of second-stage knowledge translation in moving randomized controlled trial findings to common clinical practice.
The objective of this study was to evaluate the safety of intravenous tPA use in stroke patients when delivered without a dedicated acute thrombolytic stroke team in a cohort of four hospitals. We prospectively defined historical comparison to the National Institute of Neurological Disorders and Stroke (NINDS) tPA stroke study in order to place the findings in context to results achieved by dedicated stroke researchers. In the comparison, safety was defined as one year mortality and short term disability no worse than the NINDS placebo group, and significant ICH no more frequent than symptomatic ICH in the NINDS treatment group. Survival analysis, as well as mortality at common stroke endpoints (3- and 6-months), is provided for all cohorts.
METHODS
Study Design
This was a retrospective observational cohort study with prospectively defined comparisons to the NINDS tPA stroke study cohort. Each participating hospital’s institutional review board approved the study prior to data collection.
Study Setting and Population
Four secondary and tertiary care hospitals in a contiguous, four-county region of southeastern Michigan participated in the study. In 1996, each hospital implemented a program for emergency-physician-directed stroke treatment using existing resources and neurology consultation as available at each facility, rather than developing an acute stroke response team. The presence of a neurologist or stroke specialist was not required prior to treatment. The hospitals and their physician staffs are independent entities; however, the EDs had affiliations with the University of Michigan over the time course of the study. None of the involved hospitals were designated primary stroke centers during the time period of the study; however, three have subsequently become Joint Commission certified centers. A stroke research team, enrolling subjects at two hospitals (in Ann Arbor only), was available to any of the hospitals for phone consultation regarding tPA use during the study period, but was not responsible for providing standard intravenous tPA treatment. See Table 1 for hospital and ED characteristics, and Appendix A for further detail.
Table 1.
Hospital characteristics.
| Hospital | ||||
|---|---|---|---|---|
| Characteristics | 1 | 2 | 3 | 4 |
| Location - city | Ann Arbor | Ann Arbor | Flint | Jackson |
| Urban/rural/suburban | Suburban | Suburban | Urban | Rural |
| Hospital beds (2004) | 792 | 529 | 463 | 411 |
| ED visits per year (2004)* | 50,918 | 65,259 | 54,707 | 52,500* |
| Neurology residency | Yes | No | No | No |
| Emergency medicine residency | Yes | Yes | Yes | No |
| tPA-treated cases, n (%) | 79 (29) | 127 (47) | 17 (6) | 50 (18) |
For hospitals 1, 2 and 3, this represents adult ED visits only; for hospital 4, this represents total pediatric and adult visits
tPA = tissue plasminogen activator
All patients identified as treated with intravenous tPA for presumed ischemic stroke from a participating hospital ED between January 1, 1996 and January 1, 2005 were included.
Study Protocol
Four methods (billing data, pharmacy logs, hospital stroke registries, and Paul Coverdell National Acute Stroke Registry data)26 were used for case identification at each site and are described in Appendix A. Patients initially evaluated or treated in the ED at a non-participating hospital and subsequently transferred to a study hospital for treatment or management were included in the destination hospital at analysis.
Study coordinators, with stroke experience and trained in both NIH Stroke Scale and modified Rankin Scale (mRS) administration (using internationally accepted clinical trial certification materials and tests), confirmed stroke treatment with intravenous tPA prior to data abstraction. They subsequently reviewed each confirmed case using all available hospital and ED paper and/or electronic medical records.
Medical records were then abstracted using a paper data collection form with 148 separate elements (Appendix B) and transferred to an electronic database using double-data entry. Data definitions and decision rules were adapted with permission from the Michigan prototype of the Paul Coverdell stroke registry.
All radiology reports of neuroimaging (computed tomography [CT] or magnetic resonance imaging [MRI]) performed within 10 days of thrombolytic treatment were reviewed. All available CT and MRI images were collected for patients in whom any report described the presence of an intracranial finding other than those directly resulting from ischemic stroke, including, but not restricted to, any suggestion of an ICH.
Twenty percent of charts from each site were systematically selected for dual data abstraction by independent reviewers to assess inter-rater agreement. Ten percent came from the oldest charts (by stroke treatment date) at each site on the basis that these would be most likely to be difficult to read or have missing data elements. An additional 10% were selected from the remaining charts at random. Disagreements between reviewers were adjudicated by physician investigators (PAS, RS) using the process described in Data Supplement 1 (available online).
Neuroimaging
Assessment To identify and classify ICH, two neuroradiologists (SAA, DPL), blinded to treatment course and other interpretations, completed a central analysis of all neuroimaging studies. All hemorrhages were classified using European Cooperative Acute Stroke Study (ECASS) II27 definitions. For cases where images could not be obtained, the original radiology report was used to assess ICH. A third neuroradiologist (EGH) adjudicated disagreements between reviewers on presence or classification of ICH.
Assessment of Stroke Severity and Functional Independence
The National Institutes of Health Stroke Scale (NIHSS) was used to assess pre-treatment stroke severity and was obtained using two methods. First, the medical record was reviewed and the pre-treatment score documented closest in time to the tPA bolus was recorded, if available. No assessment of training or certification of the original hospital personnel documenting the NIHSS score was attempted. Second, study coordinators certified in NIHSS assessment determined an estimated pre-treatment score using previously published guidelines with formal decision rules on source priority and score assignment.28
Functional independence was assessed with the mRS, which uses an ordinal scale from “0” (normal) to “6” (death). Study coordinators determined the scores using all available medical records and defined data decision rules. As this method has not previously been described, we evaluated inter-rater agreement on the sample of charts undergoing dual-abstraction compared to previously reported methods of mRS assessment.
Outcome Measures
The primary outcome was all-cause mortality within one year of treatment, as determined by National Death Index (NDI) query for patients treated from 1996 to 2003, and multi-state vital statistics data for 2004 (NDI data incomplete for 2004). Dates of death allowed analysis of mortality over time.
Secondary outcomes included occurrence of ICH. Three a priori definitions of ICH within 36 hours of treatment were assessed. First, “significant ICH” was defined as a parenchymal hematoma exceeding 30% of the infarct territory with substantial space-occupying effect, or any hemorrhagic lesion identified outside the infarct area (parenchymal hemorrhage type-2 [PH-2] by ECASS II criteria). This is the only type of post-tPA treatment hemorrhage independently associated with clinical deterioration and impaired prognosis.27 Second, “symptomatic ICH” was defined using two separate clinical criteria: any ICH in patients not improved at hospital discharge as compared to condition at arrival, or any ICH accompanied by any documented transient or permanent clinical decline. Third, “any ICH” included any intracranial hemorrhage (symptomatic and asymptomatic) identified within 36 hours of treatment. The neuroimaging time was used as the time of hemorrhage in all cases.
Other secondary safety outcomes included all-cause mortality over time, major systemic hemorrhage, and deviation from strictly interpreted institutional treatment guidelines based on the NINDS tPA trial enrollment criteria29 and the 1996 American Stroke Association30 recommendations. Determination of treatment guideline deviation used explicit rules (i.e. a stroke onset-to-treatment time of 181 minutes would be considered a deviation). Specific criteria are listed in Data Supplement 2. Measures of short-term neurological disability, an additional functional safety outcome, included estimated mRS score at hospital discharge, and qualitative assessment of discharge condition compared to admission.
Data Analysis
Primary and secondary outcome measures, baseline patient characteristics, and acute treatment elements were summarized using descriptive statistics. Any missing data were excluded from analysis. Any patient identified as undergoing subsequent intra-arterial thrombolysis was excluded from the safety analysis. Kappa coefficients were used to evaluate inter-rater reliability.
Comparisons were made using raw patient-level data from the placebo and treatment groups in the original NINDS tPA stroke study data.31 The NINDS tPA stroke study was a randomized, placebo-controlled clinical trial that established the efficacy of tPA as a treatment for acute ischemic stroke when delivered by stroke specialists.29
Regression analyses and stepwise elimination were used to explore the relationships between baseline variables and the primary and secondary outcomes. Baseline characteristics initially included were sex, age (categorized into tertiles), onset to treatment time, stroke severity, pretreatment blood pressure, pretreatment glucose, treating hospital, and available aspects of past medical history. Only the variables age, diabetes, prior stroke, and stroke severity were significant in at least one model. They were considered important on general grounds, and included in all final models for adjustment purposes. Interactions between these four variables and the group indicators (NINDS tPA-treatment, NINDS placebo, and the community-treatment cohorts) were tested and found to be non-significant, and only main effects were included in the final models. The main effect model was also tested by incorporating all first-order interactions between the adjustment covariates, and no lack of fit was found except in the comparison of the community treatment with the NINDS treatment group for the mRS outcome. This is discussed further below. The results reported are also robust to the choice of the link function (e.g. probit and complementary log-log).
All patients from both the NINDS treatment and placebo groups and community-treatment cohort were included in analyses examining mortality. Survival analysis was conducted using the Kaplan Meier method. Log-rank tests compared survival rates between the study cohort and the NINDS treatment and control groups at 3, 6, and 12 months following the index stroke. Cox regression analysis provided relative risks for mortality adjusted for differences in baseline characteristics between the community-treatment cohort and the NINDS tPA treatment and control cohorts.
In analyses examining ICH, the occurrence of “significant ICH” in the community-treated cohort was specified a priori for comparison to the occurrence of symptomatic ICH within 36 hours of treatment in the NINDS tPA-treated group. Unadjusted comparisons used chi-square tests. Standard logistic regression models were used to make adjusted comparisons.
Standard logistic regression models were also used to make adjusted comparisons in analyses examining functional neurological recovery. The model estimated odds of early functional recovery (defined as a mRS score ≤ 2) versus a poor outcome (mRS > 2). An mRS score of “2” represents a patient with “slight disability; unable to carry out all previous activities but able to look after own affairs without assistance.”32 Functional neurological recovery was defined as mRS score ≤ 2 because early (7-day post-treatment) mRs scores of 0–2 more closely approximate ultimate (90-day post-treatment) mRS outcomes of 0–1.31,33
Comparison was specified a priori between mRS score at hospital discharge in the community-treated cohort, and the 7–10 day mRS score obtained post-treatment in both the NINDS treatment and placebo cohorts. The NINDS tPA treatment cohort, but not the placebo cohort, was restricted in the model to subjects treated between 91–180 minutes to increase homogeneity to the community treated cohort. To include only patients with the potential to meet the study definition of functional recovery (mRS ≤ 2), subjects from both NINDS groups and from the community-treatment cohorts with a pre-stroke mRS of ≥ 2 were excluded from the model. There was some indication of an interaction between age and diabetes in the model for mRS comparing community treatment and NINDS treatment. This interaction did not affect the treatment comparison, however, and because it arose only in this model we took the simpler route of reporting only main effect models in all cases. All analyses were conducted using SAS (SAS Institute, Cary, NC, version 9.1.3).
RESULTS
Emergency Physician Experience
Ninety-five individual emergency physicians treated 273 patients with tPA for acute ischemic stroke during the nine-year study period. Two patients received combination therapy with intravenous tPA followed by intra-arterial tPA at a tertiary center. The maximum number of patients treated by a single physician was 13. The median number of treatments per physician was two (IQR 1–3.5), the mode was one, and the mean was 2.9 patients per physician (SD ± 2.61). Seventy-six percent of treated cases had a documented neurology consultation (40% in person, 36% by phone).
Twenty-eight patients were initially seen at 13 other smaller regional hospitals, and 22 were treated with tPA prior to transfer to one of the four study hospitals. Transferred patients were attributed to their destination hospital for analysis. See Table 1 for treatment data by hospital.
Data Quality
Source data acquisition was excellent, with all identified medical records reviewed. All data concerning a priori specified critical elements were obtained, with nine exceptions (eight post-treatment blood pressure measurements, one premature use of antiplatelet therapy). In 86% of patients, all original CT and MRI images performed within the first ten days following treatment were obtained. Copies of the original neuroradiology interpretations were obtained for all images in 100% of patients.
Dual data abstraction was performed on 29% of charts (over sampled). Raw inter-rater agreement for pre-specified critical and non-critical data elements was 99% and 96%, respectively. With respect to inter-rater agreement for the estimation of the mRS, there were four reviewers involved, and the dual abstractions corresponded primarily to four separate pairs of reviewers. The weighted average of weighted kappa statistics indicated acceptable agreement (0.74, 95% CI = 0.68 to 0.80) and was consistent with previously reported agreement obtained from mRS assessment using a structured interview process.32
Patient Characteristics
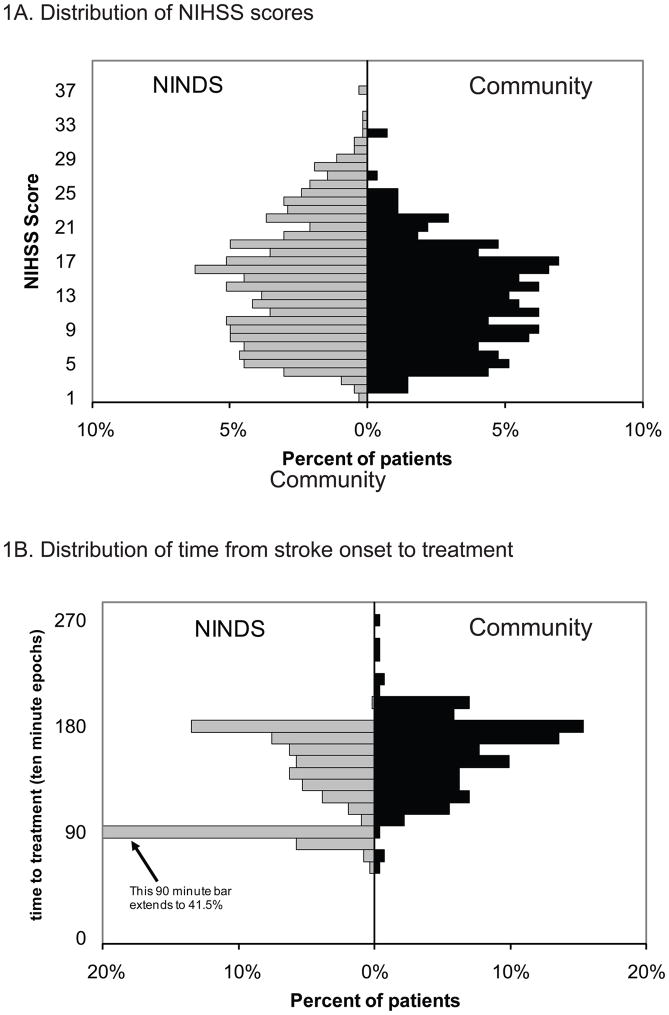
Patient demographics and baseline characteristics are presented in Table 2, and were generally similar to those in the NINDS study. There was a higher proportion of non-Hispanic whites, as well as patients with pre-existing disability, in the community-treated cohort. In the community cohort, 81 of 273 patients (30%) had an mRS score ≥ 2 prior to their presenting stroke, compared to 48 of 624 patients (8%) in the NINDS tPA study. The estimated presenting NIHSS mean score was 13 (SD ± 6). The median was 13 (IQR 8 – 17). The distribution of NIHSS scores is presented in Figure 1A. In the 168 patients with documented presenting NIHSS scores, the mean was 14 (SD ±7) and the median was 14 (IQR 8 – 19).
Table 2.
Demographics and baseline characteristics according to group.
| Characteristic | Community tPA Group (n = 273) | NINDS tPA Group (n = 312) | NINDS Placebo Group (n = 312) |
|---|---|---|---|
| Age (yr) | 68 ± 15 | 68 ± 11 | 66 ± 12 |
| Race or ethnic Group (%) | |||
| White, non-Hispanic | 86 | 66 | 63 |
| Black or African American | 11 | 26 | 29 |
| Hispanic | 1 | 6 | 6 |
| Asian | 1 | 2 | 0 |
| Other | 2 | 0 | 2 |
| Female sex (%) | 45 | 43 | 41 |
| Medical histories (%) | |||
| Stroke | 16 | 14 | 12 |
| TIA | 15 | 17 | 17 |
| Diabetes | 22 | 22 | 20 |
| Hypertension | 72 | 67 | 66 |
| Atrial fibrillation | 23 | 19 | 18 |
| Congestive heart failure | 11 | 15 | 18 |
| Valvular heart disease | 8 | 8 | 6 |
| Smoking in year before stroke | 27 | 34 | 36 |
| Anti-platelet drug use | 43 | 41 | 29 |
| NIHSS (estimated) | |||
| Median | 13 | 14 | 15 |
| Minimum | 2 | 1 | 1 |
| First quartile | 8 | 8 | 9.75 |
| Third quartile | 17 | 19 | 20 |
| Maximum | 32 | 37 | 33 |
| Blood pressure (mm Hg) | |||
| Systolic | 150 ± 22 | 154 ± 22 | 153 ± 21 |
| Diastolic | 77 ± 15 | 85 ± 13 | 86 ± 13 |
| Glucose (mg/dl) | 131 ± 51 | 149 ± 71 | 151 ± 78 |
Plus-minus values are means ± SD. Because of rounding, not all columns total 100%.
NIHSS = National Institutes of Health Stroke Scale
Figure 1. Patient Characteristics.
1A. Distribution of the National Institutes of Health Stroke Scale (NIHSS) scores
1B. Distribution of time from stroke onset to treatment
Average time from onset to treatment was 154 (±32) minutes (median 160; IQR 133 –176). The distribution of time from onset to treatment for patients in this cohort is similar to those in the NINDS 91 to 180 minute treatment group (Figure 1B). Only four patients in the study cohort were treated within 90 minutes of stroke onset.
Occurrence of major systemic hemorrhage (requiring transfusion) was 1.1%. In the NINDS trial, this occurrence was 1.6% (5 of 312 patients).
Mortality
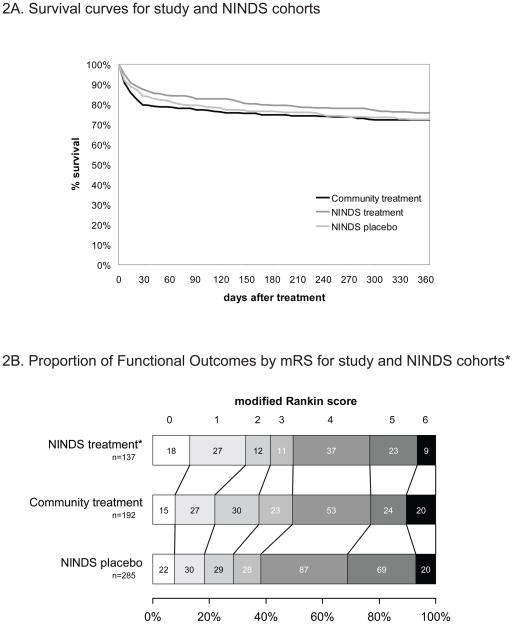
The primary endpoint, mortality at one year was 27.8%, consistent with that in the NINDS tPA-treatment group (24.4%; absolute difference 3.5%, 95% CI = −3.7% to 10.6%), and the NINDS placebo group (27.6%; absolute difference 0.2%, 95% CI = −7.0% to 7.5%). The 3- and 6-month community-treatment mortality rates (22.7% and 25.3% respectively) were also consistent with the NINDS tPA-treatment group (17% and 21%) and NINDS placebo group (21% and 23%). Figure 2A shows survival curves from the Kaplan Meier analyses and group comparisons. There were no significant differences between the curves.
Figure 2. Patient Outcomes.
2A. Survival curves for study and National Institute of Neurological Disorders and Stroke (NINDS) cohorts
2B. Functional outcomes for study and NINDS cohorts*
* Distribution of discharge (mean 8 days) modified Rankin scores in the study cohort compared to those measured at 7–10 days in the NINDS trial. Excludes subjects in all cohorts with pre-stroke mRS > 1. Includes all NINDS subjects treated at 91 – 180 minutes (one tPA and three placebo treated patients excluded from NINDS groups due to missing data).
Unadjusted Cox model relative risk (RR) for mortality at one year was 1.20 (95% CI = 0.87 to 1.64) in the study cohort compared to the NINDS tPA group, and 1.04 (95% CI = 0.76 to 1.41) compared to the NINDS placebo group. In the Cox regression analysis of survival, patients who were older, diabetic, or had higher baseline stroke severity (NIHSS score) were at greater risk for death at 12 months. Having adjusted for those covariates, there were still no significant differences in mortality risk among the three groups (community treatment, NINDS treatment, NINDS placebo; see Table 3).
Table 3.
Safety of tPA Use.
| Unadjusted | Adjusted* | |||||||
|---|---|---|---|---|---|---|---|---|
| Community vs. NINDS treatment | Community vs. NINDS placebo | Community vs. NINDS treatment | Community vs. NINDS placebo | |||||
| One-year mortality (Cox model) RR (95% CI) | 1.20 | (0.87–1.64) | 1.04 | (0.76–1.41) | 1.25 | (0.90–1.75) | 1.09 | (0.79–1.52) |
| Intracerebral Hemorrhage (sICH within 36 h) OR (95% CI) | 1.03 | (0.53–1.98) | 1.02 | (0.51–2.07) | ||||
| Functional recovery (mRS ≤ 2) OR (95% CI) | 0.83 | (0.53–1.29) | 1.46 | (0.99–2.15) | 0.67 | (0.39–1.16) | 1.07 | (0.67–1.70) |
Outcomes adjusted for baseline variables: age, diabetes, stroke severity, and prior stroke. sICH = significant/symptomatic intracerebral hemorrhage, RR = relative risk; OR = odds ratio; mRS = modified Rankin Scale; NINDS = National Institute of Neurological Disorders and Stroke
In-hospital mortality among community-treated patients, a potential signal of treatment complications, was consistent with both NINDS treatment and placebo groups with 13.2%, 10.9%, and 12.5%, of patients dying prior to discharge, respectively.
Intracerebral Hemorrhage
In the community-treated cohort, the proportion of significant ICH within 36 hours of treatment by CT criteria (ECASS PH2) was 6.6% (OR 1.03, 95% CI = 0.53 to 1.98 compared to the NINDS tPA-treatment group with symptomatic hemorrhage within 36 hours). The proportions of symptomatic ICH by the other pre-specified clinical criteria were 4.8% (any ICH within 36 hours of treatment in patients not improved at hospital discharge) and 7.0% (any ICH within 36 hours of treatment preceded by any documented transient or permanent clinical decline). The proportion of any ICH (symptomatic and asymptomatic) within 36 hours of treatment was 9.9% (OR 0.94, 95% CI = 0.58 to 1.51, compared to NINDS tPA-treatment group). These unadjusted secondary safety measures were consistent with the experience of the NINDS trial, where the proportions of symptomatic and total ICH within 36 hours of treatment were 6.4% and 10.9%, respectively.
In the binary logistic regression model for estimating the odds of having a significant hemorrhage (PH-2), patients over 80 years of age (highest tertile) were found to have greater risk compared to those ages 63 years or younger (lowest tertile). After adjusting for these variables, there was still no significant difference in the incidence of symptomatic hemorrhage in those treated with tPA in the NINDS trial compared to the incidence of significant hemorrhage (PH2) in the community cohort (Table 3). No interactions between baseline variables and the NINDS tPA treatment group were detected.
tPA-Use Guideline Deviations
Guideline deviations occurred in the ED in 26% (n = 71) of patients (61% treated beyond 3 hours; 11% hypertension; 7% each thrombocytopenia, hypoglycemia, or seizure). Among the 46 patients treated beyond 180 minutes, the median time beyond the treatment window was 15 minutes (IQR 5–20). In nine (20%) of these patients, there was specific acknowledgement or consent documented in the chart regarding treatment beyond 180 minutes. Following admission, guideline deviations occurred in 25% of patients in the first 24 hours (36% blood pressure violations, 39% premature (< 24 hours) use of anti-platelet agents, 25% premature use of anticoagulants).
Neurological Recovery
In evaluating neurological recovery, 192, 138, and 288 patients from the community-treatment, NINDS tPA-treatment, and NINDS placebo cohorts met the specified time and pre-stroke mRS exclusion criteria for inclusion in the regression model (see Data Analysis for further detail).
The mRS scores at discharge are presented by cohort in Figure 2B. Complete or near-complete functional neurological recovery (mRS 0 to 2 at discharge – mean 8 days post-treatment [SD ±7.7]; median 6 days [IQR 4–9]) was achieved in 38% of patients with premorbid disability mRS scores < 2. In both the unadjusted and adjusted models, the chance of early functional neurological recovery not significantly different in the community-treated cohort compared to the NINDS placebo group or the comparison NINDS tPA-treatment group (Table 3). Patients who were older and had more severe strokes were at greater risk for poor outcomes.
Qualitative condition at discharge relative to stroke presentation, as determined by the study coordinators, was: normal (11%), improved (64%), unchanged (6%), worse (7%), and dead (13%). Discharge disposition was home (38%), rehabilitation (30%), assisted living or nursing home (16%), other acute care hospital (3%), and death (13%).
DISCUSSION
This population-based study of stroke treatments at four community hospitals in a four-county region exemplifies the “community model” of acute stroke care, in which a large number of emergency physicians treated patients with tPA using standard neurology consulting practices and protocols. Consequently, the individual experience of most emergency physicians was limited.
These data fail to provide evidence that tPA delivery in this cohort is less safe than delivery by the specialized acute stroke teams in the NINDS tPA stroke study. Safety was confirmed on both primary and secondary outcome measures. Survival at one year, nearly identical in our cohort to that in the NINDS tPA trial, was selected as the primary outcome because it is intuitively important, patient oriented, objective, and reliably obtained. The one-year survival curve for the community treatment cohort was not significantly different from those of the NINDS tPA treatment group or the NINDS placebo group.34
The proportion of tPA-associated ICH is an important secondary outcome, as physician concern over this complication reinforces barriers to acceptance of thrombolytic use in stroke, including physician agreement, motivation, and outcome expectancy. Addressing these barriers is essential to adoption of new treatment guidelines and necessary for effective knowledge translation to broaden community usage.35 Our ICH findings are in clear contrast to data from substantially smaller case series that suggested the occurrence of post-treatment ICH may be unacceptably high in community practice, where experience may be diluted among a larger number of providers.4,11,18
We found a lower proportion of total (symptomatic and asymptomatic) ICH in the study cohort compared to the NINDS treatment group. This may be an artifact resulting from mandatory repeat CT scanning in the NINDS study, but not in clinical practice. In the community-treated cohort, 82% of all patients underwent post-treatment neuroimaging. The likelihood that some entirely asymptomatic ICH was missed probably highlights the lack of clinical relevance of this measure.
Of greater importance is the proportion of significant or symptomatic hemorrhage, which was conservatively defined using one imaging and two clinical criteria in this study. The findings were robust, with all three measures consistent with those in the NINDS tPA stroke study. It is unlikely that substantial numbers of patients with significant or symptomatic ICH were missed due to lack of repeat neuroimaging given the high mortality (61% at 90-days) associated with symptomatic ICH in the NINDS tPA stroke study, and our findings of equivalent 90-day post-treatment mortality. Patients with good neurological outcomes were most likely not to have follow-up neuroimaging performed. Patients who were dead at hospital discharge were least likely to not have follow-up neuroimaging (three patients).
The prevalence of guideline deviations is a process-oriented secondary outcome measure, and was found to be considerable. This represents an opportunity for improvement through systems of quality management and continuing education. The most prevalent guideline deviation in the ED was treatment outside the three-hour window. Although 20% of these violations were documented as deliberate, the vast majority were not. Similarly, while a third of the out-of-window treatments were less than 5 minutes late, defined by the most conservative estimate of time-last-seen-normal, many were substantially greater.
Given the other safety outcomes and recently published data suggesting safety and efficacy of tPA use in stroke between 3 and 4.5 hours from onset,36 the clinical implications of these violations seems limited, but suggests a continuing need for process improvement. Our data offer new insight into the frequency of deviations from accepted post-thrombolytic treatment guidelines in the critical first 24 hours, and emphasize extending efforts on guideline adherence to the inpatient setting.
In addition to meeting the mortality and ICH criteria for safety, the community treatment cohort also demonstrated safety in functional disability, which, measured conservatively, was not worse than the NINDS placebo group. This methodology was explicitly designed to determine safety, and not to look for signals of efficacy. It was therefore conservatively biased against demonstrating improvements as compared to the NINDS placebo group. This bias was manifest in the use of short-term rather than 90-day post-treatment outcomes, and in the adjusted analysis by the assumption that all undocumented portions of the NIHSS were normal. Although limited in this manner, the unadjusted data do suggest - but cannot confirm - better functional recovery in the community delivery of tPA cohort as compared to placebo in the NINDS trial.
LIMITATIONS
This study was designed to evaluate safety rather than efficacy. Ultimately, safety is most important when considered in the context of the potential for clinical recovery. Data indicate, however, that emergency physician concerns regarding use of tPA in stroke focus primarily on safety, particularly risk of ICH.37 The study’s retrospective design allows robust evaluation of multiple safety measures, but only limited confidence in outcome data related to efficacy.
Assessment of efficacy in this cohort is quite limited due to the lack of uniform measures of long-term outcome. Estimations of NIHSS, an important variable in all of the adjusted analyses, were biased toward lower scores as any component not explicitly charted was assumed normal. This may explain differences found between the unadjusted and adjusted analyses. Retrospective estimation of mRS score is common, but has not been previously validated and inter-rater agreement was modest. Agreement for mRS scores between reviewers in our study was comparable to an accepted structured interview process, although the potential for substantial variation remains. Additionally, because estimations of mRS in this study were performed at hospital discharge, they may be higher and more variable than at 90 days post-treatment, a common stroke outcome assessment point.
In the regression models, it was not possible to adjust for treatment clustering by physician given the small number of treatments per physician in the study dataset and the use of historical NINDS data. Although treatment hospital was included in the initial model it was dropped for lack of effect; this may incompletely account for potential clustering of patients within hospitals in the final model.
An additional potential limitation involves the inclusion of 22 patients who were treated with intravenous tPA at emergency departments elsewhere and immediately transferred to one of the four study hospitals. Reanalysis excluding these patients did not change any of the findings. See Data Supplement 1, Table, for further detail on the results.
Another potential limitation is that the cohorts compared are sequential rather than contemporaneous (1991 to 1994 for the NINDS study, and 1996–2005 for this observational study).
Finally, while these findings represent the largest series to date of patients treated without an acute thrombolytic team, care should be taken in generalizing the findings. The absolute number of hospitals in the cohort is small, and the participating hospitals have varying levels of affiliation with an academic medical center with an emergency medicine residency and a strong interest in stroke.
CONCLUSIONS
In this cohort, there was no evidence of increased risk when tPA use in stroke was directed by emergency physicians, using protocols and available neurology consultation methods, in hospitals without acute thrombolytic stroke teams. Additional work is needed to improve guideline compliance both in the emergency department and in-patient settings, and to increase speed of delivery.
Supplementary Material
Acknowledgments
Funding Sources: Awards N006648 from the Michigan Department of Community Health and 5 RO1 NS050372 from the National Institute of Neurological Disorders and Stroke supported the study.
Role of the Sponsor: Neither the Michigan Department of Community Health nor the National Institute of Neurological Disorders and Stroke had a role in the design and conduct of the study, the collection, management, analysis, and interpretation of the data, or in the preparation, review, and approval of the manuscript, other than with respect to the review of the grant application at the initiation of the project.
Additional Contributions: The authors would like to thank Lynn Blythe, BS and Kari Moore, MS for their data management contributions as compensated study personnel. We thank Rodney Smith, MD and Susan Hickenbottom, MD of St. Joseph Mercy Hospital, Ann Arbor, MI and Taruna Aurora, MD of Hurley Medical Center, Flint, MI for their administrative support of the research. No compensation was provided or received from these individuals for their assistance.
Footnotes
Prior Presentations: Presented at the ASA International Stroke Conference, San Francisco, California, February 7-9, 2007 and the Fourth Mediterranean Emergency Medicine Congress, Sorrento, Italy, September 15-19, 2007.
Disclaimer: The views expressed in this article are those of the authors and not necessarily those of the State of Michigan or the U.S. government.
References
- 1.Rosamond W, Flegal K, Furie K, et al. Heart disease and stroke statistics--2008 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2008;117(4):e25–146. doi: 10.1161/CIRCULATIONAHA.107.187998. [DOI] [PubMed] [Google Scholar]
- 2.Barber PA, Zhang J, Demchuk AM, Hill MD, Buchan AM. Why are stroke patients excluded from TPA therapy? An analysis of patient eligibility. Neurology. 2001;56(8):1015–20. doi: 10.1212/wnl.56.8.1015. [DOI] [PubMed] [Google Scholar]
- 3.Katzan IL, Hammer MD, Hixson ED, Furlan AJ, Abou-Chebl A, Nadzam DM. Utilization of intravenous tissue plasminogen activator for acute ischemic stroke. Arch Neurol. 2004;61(3):346–50. doi: 10.1001/archneur.61.3.346. [DOI] [PubMed] [Google Scholar]
- 4.Katzan IL, Furlan AJ, Lloyd LE, et al. Use of tissue-type plasminogen activator for acute ischemic stroke: the Cleveland area experience. JAMA. 2000;283(9):1151–8. doi: 10.1001/jama.283.9.1151. [DOI] [PubMed] [Google Scholar]
- 5.Hills NK, Johnston SC. Why are eligible thrombolysis candidates left untreated? Am J Prev Med. 2006;31(6 Suppl 2):S210–6. doi: 10.1016/j.amepre.2006.08.004. [DOI] [PubMed] [Google Scholar]
- 6.Schumacher HC, Bateman BT, Boden-Albala B, et al. Use of thrombolysis in acute ischemic stroke: analysis of the nationwide inpatient sample 1999 to 2004. Ann Emerg Med. 2007;50(2):99–107. doi: 10.1016/j.annemergmed.2007.01.021. [DOI] [PubMed] [Google Scholar]
- 7.Kleindorfer D, Xu Y, Moomaw CJ, Khatri P, Adeoye O, Hornung R. US geographic distribution of rt-PA utilization by hospital for acute ischemic stroke. Stroke. 2009;40(11):3580–4. doi: 10.1161/STROKEAHA.109.554626. [DOI] [PubMed] [Google Scholar]
- 8.Akins PT, Delemos C, Wentworth D, Byer J, Schorer SJ, Atkinson RP. Can emergency department physicians safely and effectively initiate thrombolysis for acute ischemic stroke? Neurology. 2000;55(12):1801–5. doi: 10.1212/wnl.55.12.1801. [DOI] [PubMed] [Google Scholar]
- 9.Albers GW, Bates VE, Clark WM, Bell R, Verro P, Hamilton SA. Intravenous tissue-type plasminogen activator for treatment of acute stroke: the Standard Treatment with Alteplase to Reverse Stroke (STARS) study. JAMA. 2000;283(9):1145–50. doi: 10.1001/jama.283.9.1145. [DOI] [PubMed] [Google Scholar]
- 10.Asimos AW, Norton HJ, Price MF, Cheek WM. Therapeutic yield and outcomes of a community teaching hospital code stroke protocol. Acad Emerg Med. 2004;11:361–70. doi: 10.1197/j.aem.2003.12.016. [DOI] [PubMed] [Google Scholar]
- 11.Bravata DM, Kim N, Concato J, Krumholz HM, Brass LM. Thrombolysis for acute stroke in routine clinical practice. Arch Intern Med. 2002;162(17):1994–2001. doi: 10.1001/archinte.162.17.1994. [DOI] [PubMed] [Google Scholar]
- 12.Buchan AM, Barber PA, Newcommon N, et al. Effectiveness of t-PA in acute ischemic stroke: outcome relates to appropriateness. Neurology. 2000;54(3):679–84. doi: 10.1212/wnl.54.3.679. [DOI] [PubMed] [Google Scholar]
- 13.Chapman KM, Woolfenden AR, Graeb D, et al. Intravenous tissue plasminogen activator for acute ischemic stroke: a Canadian hospital's experience. Stroke. 2000;31(12):2920–4. doi: 10.1161/01.str.31.12.2920. [DOI] [PubMed] [Google Scholar]
- 14.Grotta JC, Burgin WS, El-Mitwalli A, et al. Intravenous tissue-type plasminogen activator therapy for ischemic stroke: Houston experience 1996 to 2000. Arch Neurol. 2001;58(12):2009–13. doi: 10.1001/archneur.58.12.2009. [DOI] [PubMed] [Google Scholar]
- 15.Hill MD, Buchan AM. Thrombolysis for acute ischemic stroke: results of the Canadian alteplase for stroke effectiveness study. CMAJ. 2005;172(10):1307–12. doi: 10.1503/cmaj.1041561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Katzan IL, Hammer MD, Furlan AJ, Hixson ED, Nadzam DM. Quality improvement and tissue-type plasminogen activator for acute ischemic stroke: a Cleveland update. Stroke. 2003;34(3):799–800. doi: 10.1161/01.STR.0000056944.42686.1E. [DOI] [PubMed] [Google Scholar]
- 17.Koennecke HC, Nohr R, Leistner S, Marx P. Intravenous tPA for ischemic stroke team performance over time, safety, and efficacy in a single-center, 2-year experience. Stroke. 2001;32(5):1074–8. doi: 10.1161/01.str.32.5.1074. [DOI] [PubMed] [Google Scholar]
- 18.Lopez-Yunez AM, Bruno A, Williams LS, Yilmaz E, Zurru C, Biller J. Protocol violations in community-based rTPA stroke treatment are associated with symptomatic intracerebral hemorrhage. Stroke. 2001;32(1):12–6. doi: 10.1161/01.str.32.1.12. [DOI] [PubMed] [Google Scholar]
- 19.Reed SD, Cramer SC, Blough DK, Meyer K, Jarvik JG. Treatment with tissue plasminogen activator and inpatient mortality rates for patients with ischemic stroke treated in community hospitals. Stroke. 2001;32(8):1832–40. doi: 10.1161/01.str.32.8.1832. [DOI] [PubMed] [Google Scholar]
- 20.Rymer MM, Thurtchley D, Summers D. Expanded modes of tissue plasminogen activator delivery in a comprehensive stroke center increases regional acute stroke interventions. Stroke. 2003;34(6):e58–60. doi: 10.1161/01.STR.0000071107.66925.93. [DOI] [PubMed] [Google Scholar]
- 21.Schmulling S, Grond M, Rudolf J, Heiss WD. One-year follow-up in acute stroke patients treated with rtPA in clinical routine. Stroke. 2000;31(7):1552–4. doi: 10.1161/01.str.31.7.1552. [DOI] [PubMed] [Google Scholar]
- 22.Smith RW, Scott PA, Grant RJ, Chudnofsky CR, Frederiksen SM. Emergency physician treatment of acute stroke with recombinant tissue plasminogen activator: a retrospective analysis. Acad Emerg Med. 1999;6:618–25. doi: 10.1111/j.1553-2712.1999.tb00416.x. [DOI] [PubMed] [Google Scholar]
- 23.Tanne D, Bates VE, Verro P, et al. Initial clinical experience with IV tissue plasminogen activator for acute ischemic stroke: a multicenter survey. Neurology. 1999;53(2):424–7. doi: 10.1212/wnl.53.2.424. [DOI] [PubMed] [Google Scholar]
- 24.Wahlgren N, Ahmed N, Davalos A, et al. Thrombolysis with alteplase for acute ischaemic stroke in the Safe Implementation of Thrombolysis in Stroke-Monitoring Study (SITS-MOST): an observational study. Lancet. 2007;369(9558):275–82. doi: 10.1016/S0140-6736(07)60149-4. [DOI] [PubMed] [Google Scholar]
- 25.Wang DZ, Rose JA, Honings DS, Garwacki DJ, Milbrandt JC. Treating acute stroke patients with intravenous tPA. The OSF stroke network experience. Stroke. 2000;31(1):77–81. doi: 10.1161/01.str.31.1.77. [DOI] [PubMed] [Google Scholar]
- 26.Centers for Disease Control and Prevention. [Accessed Jul 2, 2010.];Paul Coverdell National Acute Stroke Registry homepage. Available at: http://www.cdc.gov/dhdsp/stroke_registry.htm.
- 27.Berger C, Fiorelli M, Steiner T, et al. Hemorrhagic transformation of ischemic brain tissue: asymptomatic or symptomatic? Stroke. 2001;32(6):1330–5. doi: 10.1161/01.str.32.6.1330. [DOI] [PubMed] [Google Scholar]
- 28.Williams LS, Yilmaz EY, Lopez-Yunez AM. Retrospective assessment of initial stroke severity with the NIH Stroke Scale. Stroke. 2000;31(4):858–62. doi: 10.1161/01.str.31.4.858. [DOI] [PubMed] [Google Scholar]
- 29.The National Institute of Neurological Disorders and Stroke rt-PA Stroke Study Group. Tissue plasminogen activator for acute ischemic stroke. New Engl J Med. 1995;333(24):1581–7. doi: 10.1056/NEJM199512143332401. [DOI] [PubMed] [Google Scholar]
- 30.Adams HP, Brott TG, Furlan AJ, et al. Guidelines for thrombolytic therapy for acute stroke: a supplement to the guidelines for the management of patients with acute ischemic stroke. Circulation. 1996;94(5):1167–74. doi: 10.1161/01.cir.94.5.1167. [DOI] [PubMed] [Google Scholar]
- 31.U.S. Department of Commerce. National Technical Information Service. Available on CD ROM from www.ntis.gov. Product # PB2006–500032.
- 32.Wilson JTL, Hareendran A, Grant M, et al. Improving the assessment of outcomes in stroke: use of a structured interview to assign grades on the modified Rankin Scale. Stroke. 2002;33(9):2243–6. doi: 10.1161/01.str.0000027437.22450.bd. [DOI] [PubMed] [Google Scholar]
- 33.Ovbiagele B, Saver JL. Day-90 acute ischemic stroke outcomes can be derived from early functional activity level. Cerebrovasc Dis. 2010;29(1):50–6. doi: 10.1159/000255974. [DOI] [PubMed] [Google Scholar]
- 34.Kwiatkowski TG, Libman RB, Frankel M, et al. Effects of tissue plasminogen activator for acute ischemic stroke at one year. National Institute of Neurological Disorders and Stroke Recombinant Tissue Plasminogen Activator Stroke Study Group. N Engl J Med. 1999;340(23):1781–7. doi: 10.1056/NEJM199906103402302. [DOI] [PubMed] [Google Scholar]
- 35.Cabana MD, Rand CS, Powe NR, et al. Why don't physicians follow clinical practice guidelines? A framework for improvement. JAMA. 1999;282(15):1458–65. doi: 10.1001/jama.282.15.1458. [DOI] [PubMed] [Google Scholar]
- 36.Hacke W, Kaste M, Bluhmki E, et al. Thrombolysis with alteplase 3 to 4.5 hours after acute ischemic stroke. N Engl J Med. 2008;359(13):1317–29. doi: 10.1056/NEJMoa0804656. [DOI] [PubMed] [Google Scholar]
- 37.Brown DL, Barsan WG, Lisabeth LD, Gallery ME, Morgenstern LB. Survey of emergency physicians about recombinant tissue plasminogen activator for acute ischemic stroke. Ann Emerg Med. 2005;46(1):56–60. doi: 10.1016/j.annemergmed.2004.12.025. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.