Abstract
Background
Children and adolescents, family history positive (FH+) for alcoholism, exhibit differences in brain structure and functional activation when compared to family history negative (FH-) counterparts. Given that frontal brain regions, and associated reciprocal connections with limbic structures, undergo the most dramatic maturational changes during adolescence, the objective of this study was to compare functional brain activation during a frontally-mediated test of response inhibition in 32 adolescents separated into low-risk (FH-) and high-risk (FH+) groups.
Methods
Functional magnetic resonance (fMRI) blood oxygen level dependent (BOLD) data were acquired at 1.5 Tesla during performance of Stroop Color Naming, Word Reading and Interference. Preprocessing and statistical analyses, covaried for age, were conducted in SPM99 using a search territory that included superior, middle, and inferior frontal gyri (trigone region), anterior cingulate gyrus, and left and right amygdala.
Results
Significantly greater activation in the fronto-limbic search territory was observed in FH+ relative to FH- subjects during Stroop Interference. In addition, a significant regression between brain activation and family history density was observed, with a greater density being associated with increased activation in regions including middle frontal gyrus (BA9) and cingulate gyrus (BA24).
Conclusions
These data demonstrate a significant influence of FH status on brain activation during the performance of a response inhibition task, perhaps reflecting a neurobiological vulnerability associated with FH status that may include reduced neuronal efficiency and/or recruitment of additional neuronal resources. These findings are important given that the adolescent developmental period is already associated with reduced inhibitory capacity, even prior to the onset of alcohol use.
Keywords: frontal lobe, fMRI, FH, alcohol abuse, adolescence
Introduction
Adolescence is a time notable for brain re-organization, with white and gray matter tissue volumes each undergoing distinctly different patterns of maturation (Giedd et al., 1996; Jernigan et al., 1991; Pfefferbaum et al., 1994; Reiss et al., 1996). Age-related alterations in white matter typically reflect increased myelination, whereas alterations in gray matter reflect neuronal pruning. These progressive and regressive processes are associated with improved cognitive functioning (Casey et al., 2005; Casey et al., 2000; Paus, 2005), perhaps due in part to increased neuronal efficiency (de Graaf-Peters and Hadders-Algra, 2006; Hua and Smith, 2004). While such changes occur in a rapid fashion across many brain regions, areas that include the prefrontal cortex (PFC) demonstrate prolonged structural and functional refinement that continues into the early twenties (Giedd et al., 1999; Gogtay et al., 2004; Luna et al., 2001; Sowell et al., 2001; Sowell et al., 2004; Sowell et al., 2002; Yurgelun-Todd, 2007). Behavioral manifestations associated with frontal lobe development include improvements in executive functioning, such as strategic planning, impulse control, organized search, abstract reasoning, mental flexibility and self-monitoring. These abilities helps to maintain an appropriate mental set that is necessary for adaptive goal-directed behavior (Luria, 1966; Shallice, 1982; Spreen et al., 1995) that contributes to a successful transition from immaturity to independence.
Higher-order cognitive abilities, such as the regulation of inhibitory control, are subserved by a widely distributed and functionally integrated neurocircuitry (Goldman-Rakic, 1988). Accordingly, functional magnetic resonance imaging (fMRI) studies provide evidence for developmental changes in frontal lobe activation during tasks that require response inhibition, such as the Go No-Go task (Adleman et al., 2002; Casey et al., 1997; Luna and Sweeney, 2004; Marsh et al., 2006; Tamm et al., 2002). The Stroop Color-Word Task (Golden, 1976), also used to investigate response inhibition, has demonstrated robust activation of a network that includes anterior cingulate cortex (ACC), inferior frontal gyrus (IFG), middle frontal gyrus (MFG), medial wall frontal regions, middle temporal gyrus, inferior parietal cortex, insula and basal ganglia (Bench et al., 1993; Gruber et al., 2002; Leung et al., 2000; Pardo et al., 1990; Peterson et al., 1999; Taylor et al., 1997). Accordingly, age-related increases in activation of the left lateral prefrontal cortex (PFC) and ACC (Adleman et al., 2002, 7-22 years), and of the right frontostriatal system (Marsh et al., 2006, 7-57 years), have been reported during Stroop Interference. Thus, the immature yet rapidly developing frontal lobe serves as an inherent neurobiological vulnerability during adolescence, particularly when cognitive demands are high, that could affect navigation of decision-making challenges and the capacity to avoid risky or inappropriate behavior, suboptimal response selection or performance, or harmful consequences.
The onset of alcohol and illicit substance use typically occurs during this period of critical adolescent brain development (Bates and Labouvie, 1997; Johnston et al., 2000). It is known from studies in adult populations that heavy alcohol consumption is associated with deficits across several domains of cognition (Parsons and Nixon, 1998), with executive functioning and memory being the most vulnerable to disruptions by alcohol (Fillmore et al., 2005; Goudriaan et al., 2007; Hartley et al., 2004; Marczinski et al., 2007; Sher et al., 1997; Townshend and Duka, 2005; Weissenborn and Duka, 2003). MRI and fMRI studies have revealed alcohol-related alterations in brain structure (Jang et al., 2007; Paulus et al., 2006; Pfefferbaum et al., 1997; Sullivan and Pfefferbaum, 2005) and brain activation during performance of cognitive tasks (Tapert et al., 2004a; Tapert et al., 2001). While previous studies have likewise documented consequences of adolescent alcohol use on brain structure and cognitive function (Brown et al., 2000; De Bellis et al., 2005; Nagel et al., 2005; Tapert et al., 2004b), it is unclear whether these structural and functional abnormalities are antecedent to the initiation of alcohol use or are the consequence of alcohol use during adolescent brain development.
In order to address this question, previous studies have compared adolescents who are family history positive (FH+) for alcoholism, and who have no or minimal alcohol exposure, with age-matched family history negative (FH-) non-using counterparts. This is an important population to examine, as a positive family history of alcoholism is associated with an earlier initiation and greater magnitude of use (Biederman et al., 2000; Chassin and Barerra, 1993; Clark et al., 2005; Hill et al., 2000; McGue et al., 2001), and a greater prevalence of alcohol use disorders in adolescents and young adults (Chassin et al., 2004; Lieb et al., 2002; Milberger et al., 1999). Although intellectual functioning falls within the average range (Alterman et al., 1989; Johnson and Rolf, 1988; Schuckit et al., 1987), children of alcoholics demonstrate deficits in abstract reasoning and planning, lower IQ scores, and poorer spelling and math performance compared to children of non-alcoholics (Poon et al., 2000). Poorer academic performance has also been reported in at risk adolescents (McGrath et al., 1999; Murphy et al., 1991; Reich et al., 1993; Silveri et al., 2004; Silveri et al., 2008; Vitaro et al., 1996). MRI studies have shown that while whole brain gray and white matter tissue volumes do not differ between FH+ and FH- youth (Silveri et al., 2008), FH+ youth demonstrate reduced amygdalar volumes (Hill et al., 2001), larger cerebellar volumes (Hill et al., 2007b), and reduced right/left orbitofrontal volumes (Hill et al., 2009), relative to FH- youth. Taken together, these studies conducted in FH+ youth suggest evidence for cognitive and neurobiological vulnerabilities associated with increased risk for developing an alcohol abuse problem later in life.
Data from fMRI studies of FH+ children and adolescents are more limited. While no performance differences were observed between FH+ and FH- groups during a spatial working memory (SWM) task, FH+ youth exhibit greater BOLD activation in superior frontal lobe regions during rest and less BOLD activation in the cingulate gyrus (CG) during a simple vigilance condition (Spadoni et al., 2008). Functional activation differences reported for the Go No-Go task demonstrate that FH+ adolescents exhibit less activation in the left MFG relative to FH- counterparts (Schweinsburg et al., 2004). To date, no fMRI studies have compared brain activation in FH+ and FH- youth during performance of the Stroop Color-Word task, thought to be more demanding and effortful than the Go No-Go task due in part to the involvement of conflict monitoring and resolution during task performance (Spreen et al., 2006), and perhaps task-specific differences in functional integration of multiple brain regions (Leung et al., 2000; Pardo et al., 1990; Peterson et al., 1999). Thus, the objective of the current fMRI study was to test the hypothesis that FH status has a significant influence on frontal lobe activation during performance of Stroop Interference, by comparing adolescents stratified into high-risk (FH+) and low-risk (FH-) groups.
Materials and Methods
Participants
The study sample consisted of 32 healthy adolescent volunteers, recruited from the local surrounding communities via advertisement and word of mouth. The overall sample was 59% female, 88% Caucasian (6% African American, 6% Hispanic), typically from middle-upper class socioeconomic status (Hollingshead, 1957), and with a mean age of 13.4 ± 2.9 yrs. (mean ± SD; age ranging from 8 to 19 yrs. old) and a mean education of 7.5 ± 2.8 yrs. (education ranging from 3 to 12 years). The accompanying parent, almost exclusively the mother, underwent a Family History – Epidemiologic (FHE) structured interview to obtain information about the parents and children, as well as an unstructured family interview to obtain information about second-degree relatives. Information from parental interviews was used to stratify subjects by family history status (low risk (FH-) or high risk (FH+)). Subjects met the criteria for FH+ status if there was a positive parental report of either parental or grandparent alcohol abuse (28%, 61% of the sample, respectively) or both parental and grandparent alcohol abuse (11% of the sample). Family expression of alcoholism, or family history density (FHD) of alcoholism, as determined using methods established by Zucker and colleagues (Zucker et al., 1994), was also calculated for each subject, where a single parent with a history of alcoholism contributes 0.5 and a single grandparent contributes 0.25 to the total score, for a possible range of 0 (FH-) to 2 (0.25 – 2.0, FH+). Previous work suggests that FHD may be more sensitive for determining the influence of familial alcohol use disorders than categorical approaches (Stoltenberg et al., 1999). According to these criteria, the FH+ group was comprised of 18 adolescents (family density = 0.43 ± 0.28) and the FH- group was comprised of 14 adolescents (family density = 0.0 ± 0.0). As determined by the family history interview, no cases of premature birth or maternal alcohol or drug use were reported among the enrolled study participants. Demographic data from the study subjects, which did not differ significantly between groups, are presented in Table 1.
Table 1. Subject Demographics.
| FH+ (n=18) | FH- (n=14) | p | |
|---|---|---|---|
| Age | 13.2 ± 3.2 | 13.8 ± 2.6 | .59 |
| Female | 57% | 61% | - |
| Education | 7.3 ± 3.0 | 7.8 ± 2.6 | .53 |
| Handedness | 17R, 1L | 14R, 0L | - |
| Ethnicity | 93% Caucasian | 83% Caucasian | - |
| Family History Density | 0.43 ± 0.28 | 0.00 ± 0.00 | .0001 |
Data represent mean scores. ± SD.
Procedure
All aspects of the clinical research protocol were reviewed and approved by the Institutional Review Board of McLean Hospital (Belmont, MA, USA). After a complete description of the study, all subjects and their parent(s) or guardian(s) provided written informed assent and consent, respectively, prior to participation. Subjects received monetary compensation for participating in the study.
Clinical Assessment
A trained psychologist conducted diagnostic interviews and clinical assessments. Subjects underwent a structured clinical psychiatric interview, using the Kiddie-Schedule for Affective Disorders and Schizophrenia (K-SADS-E, (Puig-Antich et al., 1980), to rule out Axis I pathologies according to the Diagnostic and Statistical Manual IV (DSM) (e.g., depression, bipolar illness, schizophrenia, conduct disorder, attention deficit disorder). All participating subjects were free of psychiatric diagnoses, neurological illness, severe medical problems, and current or >3 lifetime episodes of alcohol or drug use.
Functional Magnetic Resonance Imaging Protocol
Functional images were acquired for the whole brain on a 1.5 Tesla General Electric Signa LX magnetic resonance scanner (General Electric Medical Systems, Milwaukee, WI), equipped with a birdcage quadrature RF head coil, using echo planar imaging (EPI) blood oxygen level dependent (BOLD) fMRI. Three set of images were generated: a T1-weighted sagittal localizer (spin echo, 256 × 192, 1 NEX, 24 slices, SLT=4mm with a 1-mm gap, TE=19msec, TR=600msec), a dual echo T2-weighted axial series (VEMP, 256 × 192, 0.5 NEX, 54 slices interleaved, SLT=3mm, TE=30/80msec, TR=3000msec) and 3) 3-D fourier transformed spoiled gradient-recalled acquisition images (SPGR, 256 × 192, 124 slices, 1 NEX, SLT=1.5mm, TR=35msec, TE=5msec, flip angle=45°). A neuroradiologist reviewed the clinical images of each subject to rule out neurological structural abnormalities. Sagittal scout images were acquired for alignment and localization using a fast spin echo sequence (FSE) with the following imaging parameters: repetition time (TR) = 3 msec, echo time (TE) = 40 msec, field of view (FOV) = 20cm, matrix size = 64 × 64, slice thickness = 7 mm (1mm gap), and flip angle = 90°. Visual stimuli were projected onto a translucent screen located at the foot of the scanning bed via a magnetically shielded LCD video projector and observed through a mirror mounted on the head coil.
In order to minimize motion associated with vocalization of responses, foam cushions were inserted between the subjects' head and the quadrature head coil for a snug fit. Subjects were fitted with tape across the forehead and chin during landmarking, and head position was rechecked prior to removal from the scanner. While these methods do not reduce fine motion associated with localizing a response, the amount of gross movement is minimized.
Stroop Color-Word fMRI Paradigm
Adolescents completed a version of the Stroop Color-Word Interference Task while undergoing EPI BOLD fMRI. Detailed descriptions of this paradigm have been published previously (Gruber et al., 2002; Killgore et al., 2007). The Stroop test challenges the ability to inhibit inappropriate responses and resist interference using the following conditions: Color Naming (name the color of the block); Word Reading (read words printed in black ink); Interference (name the color of the ink when words are printed in an incongruent color). In a blocked paradigm, each task was completed in a series of three 2.5 min scans. Color Naming consisted of a series of 10 screens each presenting a line of six red, green, and blue colored rectangles (2500 ms stimulus; 500 ms inter-stimulus interval). Word Naming consisted of a series of 10 screens each presenting a line of text comprising six randomly ordered words of “red”, “green”, and “blue” printed in black ink (2500 ms stimulus; 500 ms inter-stimulus interval). Given the longer duration of time required to complete the Color-Word Interference condition, six screens each presented a line of text comprising six printed words of “red”, “green”, and “blue” printed in an incongruent color (4500 ms stimulus; 500 ms inter stimulus interval). This timing sequence was established previously by determining the average reaction time for the completion of six targets in each condition in healthy adult subjects tested off-line (Gruber et al., 2002). Each scanning epoch consisted of five alternating 30s periods (total length, 150s), in which two trials of one Stroop condition were alternated with three rest periods consisting of a simple fixation point (e.g., rest, Color Naming, rest, Color Naming, rest).
Subjects were required to communicate vocal responses via microphone for each series (screen) of each condition (Color Naming, Word Reading and Interference) of the Stroop task. A technician recorded the number of targets incorrectly identified (errors) for each component of the Stroop task, with performance being evaluated by averaging number of errors and percent accuracy over the two trials, within a block of the fMRI paradigm. This yielded a maximum score of 60 (100%) on 10 trials of 6 color blocks (Color Naming), 60 (100%) on 10 trials of 6 words (Word Reading), and 36 (100%) on 6 trials of 6 words written in an incongruent color (Interference).
Image Processing and Data Analysis
Preprocessing and statistical analyses were conducted in SPM99 (Friston et al., 1995) using Matlab (The Mathworks Inc. Sherborn MA, USA). The functional data sets were motion corrected (intra-run realignment) within SPM99 using the first image as the reference. Data that exceeded 2 degrees or 2 millimeters in either the rotational or translational plane was excluded from the analyses. No participants were excluded on this basis. Average motion correction for each subject for each translational and rotational plane was between 0.5 and 1.5mm and 0.5 and 1.5 degrees, respectively, with no differences in average motion being observed between FH+ and FH- groups. After realignment, the image data were normalized to a standard template from Montreal Neurological Institute (MNI) with an isotropic 2×2×2 mm voxel size and smoothed using an isotropic Gaussian kernel (full width half maximum [FWHM] = 10 mm).
The analysis followed a two-step random effects approach in SPM99 in order to permit inference to the population from which the data were collected (Penny et al., 2003). First, a 150-second box-car waveform, convolved with hemodynamic response function, was used as the reference paradigm. Using general linear model and the hemodynamically-corrected reference paradigm, T-score values were calculated for each voxel. Contrasts were set to test for voxel-wise effects of signal differences between conditions, [Color Naming - Fixation], [Word Reading - Fixation], and [Interference-Fixation] - [Word Reading – Fixation], [Interference-Fixation] - [Color Naming-Fixation], and statistical parametric maps (SPM{t}) were calculated for each subject. The following abbreviations are used throughout to represent the aforementioned contrasts, respectively: Color Naming, Word Reading, Interference – Color Naming and Interference – Word Reading.
The whole group (both FH+ and FH- subjects) was first examined for each contrast, and then in the second stage, contrast images were used to compare activation differences between FH+ and FH- adolescents. A region of interest (ROI) approach, as described previously (Killgore et al., 2007), was used for all fMRI analyses. The ROI was restricted to a search territory that included superior, middle, and inferior frontal gyri (trigone region), the ACC, and the left and right amygdala, as defined by a published anatomical atlas (Tzourio-Mazoyer et al., 2002), and as implemented in the Wake Forest University PickAtlas Utility (Maldjian et al., 2003). Regions within this fronto-limbic search territory were selected given their reciprocal connections (Bracht et al., 2009; Stein et al., 2007), and were based on previous fMRI and MRI studies documenting involvement in response inhibition (Leung et al., 2000; Pardo et al., 1990) and error detection (Polli et al., 2008; Polli et al., 2009), as well as structural differences observed in FH+ youth (Hill et al., 2001; Hill et al., 2009). Regions of activation within the ROI were evaluated at an uncorrected threshold of p < .001, with the k (extent) = 20 contiguous voxels. ROI analyses were corrected for multiple comparisons using family-wise error rate implemented within SPM99 using the Pickatlas utility. Significant activations within the ROI at an uncorrected threshold of p < .001 that also survived multiple comparisons corrections at p < .05 are indicated in Tables 3-5. Activation images were superimposed on an average template brain normalized to the standardized coordinate space of the Montreal Neurological Institute (MNI) for visualization. To identify anatomical locations, MNI coordinates were converted to Talairach space using the icbm2tal transform in GingerALE Version 2.0 (http://www.brainmap.org/ale/index.html), and entered into Talairach Daemon Client Version 2.4.2. (Lancaster et al., 2000). Contrast images presented in Figures 1 and 2 were formatted using MRICron (www.cabiatl.com/mricro/mricro/index.html).
Table 3. Foci of Maximally Activated Brain Regions.
| Talairach Coordinates | ||||||
|---|---|---|---|---|---|---|
| Region | BA | x | y | z | Cluster | SPM {t} |
| Color Naming | ||||||
| L. Precentral Gyrus | 4 | -50 | -5 | 41 | 905 | 8.44* |
| R. Insula | 34 | 15 | 4 | 549 | 6.56* | |
| R. Precentral Gyrus | 6 | 41 | -5 | 40 | 788 | 6.19* |
| L. Medial Frontal Gyrus | 6 | -4 | 2 | 58 | 197 | 5.27* |
| R. Cingulate Gyrus | 32 | 10 | 24 | 32 | 301 | 5.06* |
| L. Insula | -31 | 20 | 11 | 128 | 3.99 | |
| Word Reading | ||||||
| R. Insula | 36 | 21 | 3 | 707 | 8.05* | |
| L. Precentral Gyrus | 6 | -48 | -4 | 27 | 208 | 7.64* |
| R. Cingulate Gyrus | 32 | 15 | 18 | 31 | 121 | 5.87* |
| L. Medial Frontal Gyrus | 9 | -20 | 24 | 28 | 47 | 5.33 |
| R. Precentral Gyrus | 6 | 45 | -6 | 28 | 166 | 5.03 |
| L. Insula | -36 | 14 | -2 | 164 | 4.27 | |
| L. Medial Frontal Gyrus | 6 | -4 | -3 | 60 | 21 | 4.05 |
| Interference – Color Naming | ||||||
| L. Middle Frontal Gyrus | 6 | -33 | 1 | 38 | 1408 | 5.77* |
| L. Medial Frontal Gyrus | 9 | -20 | 24 | 26 | 56 | 4.08 |
| R. Precentral Gyrus | 6 | 36 | -1 | 30 | 26 | 3.89 |
| R. Anterior Cingulate Gyrus | 32 | 14 | 29 | 20 | 124 | 3.59 |
| Interference – Word Reading | ||||||
| L. Middle Frontal Gyrus | 46 | -40 | 27 | 17 | 101 | 3.58 |
| L. Precentral Gyrus | 6 | -31 | -1 | 38 | 34 | 3.26 |
L, left hemisphere; R, right hemisphere. BA, Brodmann Area. MNI coordinates transformed into Talairach Space, x=center/left of midline, y=anterior/posterior to anterior commissure, z=superior/inferior to horizontal plane through AC-PC line. SPM {t} scores significant beyond p<.001 (uncorrected) for ROI analyses are reported.
Indicates significance at small volume corrected threshold, p<.05.
Table 5. Family History Effects: Foci of Maximally Activated Brain Regions.
| Talairach Coordinates | ||||||
|---|---|---|---|---|---|---|
| Region | BA | x | y | z | Cluster | SPM {t} |
| FH+: Interference – Color Naming | ||||||
| L. Middle Frontal Gyrus | 6 | -35 | 1 | 38 | 342 | 5.29* |
| L. Insula | -40 | 12 | 17 | 440 | 4.50* | |
| R. Anterior Cingulate Gyrus | 32 | 14 | 29 | 20 | 74 | 4.37 |
| L. Medial Frontal Gyrus | 6 | -18 | 9 | 48 | 35 | 3.90 |
| L. Medial Frontal Gyrus | 9 | -20 | 28 | 25 | 38 | 3.90 |
| R. Middle Frontal Gyrus | 8 | 28 | 15 | 40 | 45 | 3.59 |
| L. Insula | -36 | 16 | -2 | 21 | 3.57 | |
| FH-: Interference – Color Naming | ||||||
| L. Precentral Gyrus | 6 | -38 | -1 | 32 | 43 | 3.32 |
L, left hemisphere; R, right hemisphere. BA, Brodmann Area. MNI coordinates transformed into Talairach Space, x=center/left of midline, y=anterior/posterior to anterior commissure, z=superior/inferior to horizontal plane through AC-PC line. SPM {t} scores significant beyond p<.001 (uncorrected) for ROI analyses are reported.
Indicates significance at small volume corrected threshold, p<.05.
Figure 1. FHD Regression with Brain Activation for Interference – Word Reading.
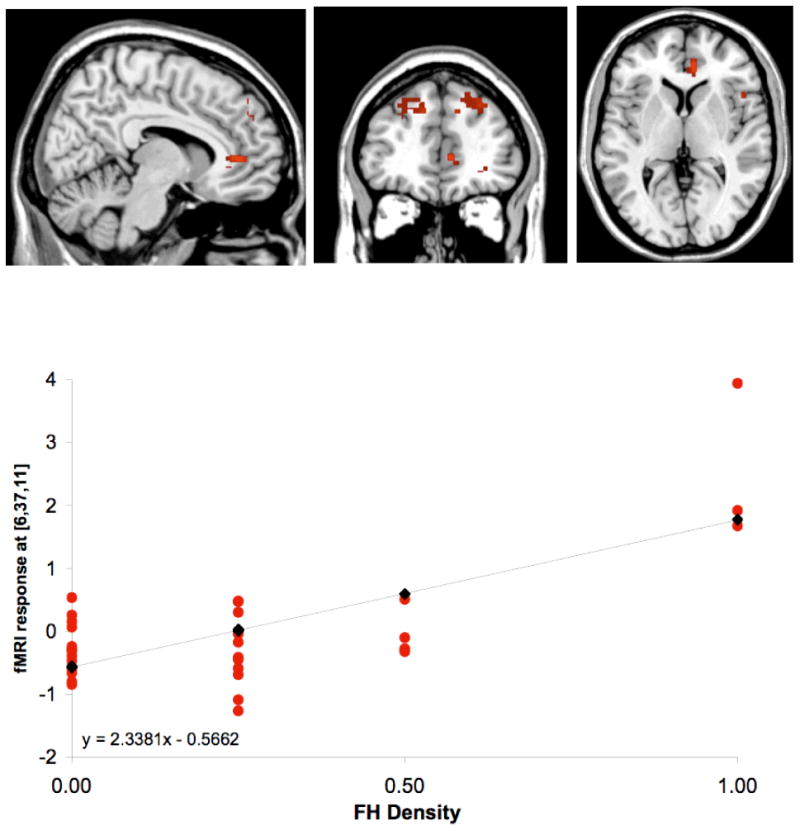
Illustration of SPM maps demonstrating the significant positive regression (p<.05) between greater FHD and greater activation during the Interference condition of the Stroop task [Interference – Word Reading]. Local maxima coordinates were x=6, y=37, z=11, with 75 activated voxels, SPM {t} = 5.49, significant at small volume corrected threshold, p<.05, and containing right ACC (BA32). L, left hemisphere; R, right hemisphere. Red circles represent individuals subjects and black diamonds represent average activation for each FHD group.
Figure 2. FHD Regression with Brain Activation for Interference – Color Naming.
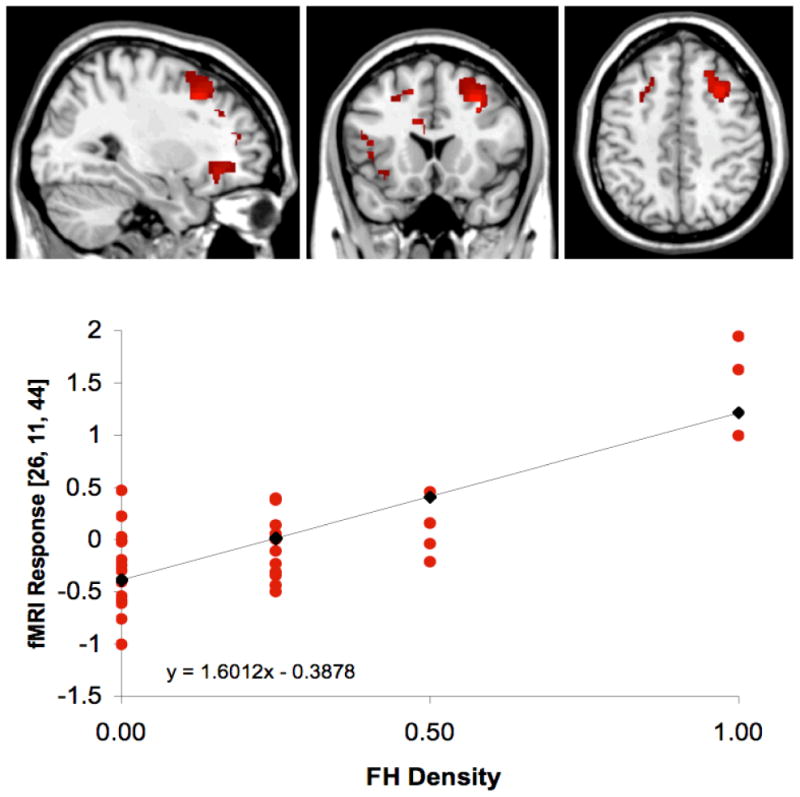
Illustration of SPM maps demonstrating the significant positive regression (p<.05) between greater FHD and greater activation during the Interference condition of the Stroop task [Interference – Color Naming]. Local maxima coordinates were x=26, y=11, z=44, with 1108 activated voxels, SPM {t} = 6.64, significant at small volume corrected threshold, p<.05, and containing right MFG (BA8). L, left hemisphere; R, right hemisphere. Red circles represent individuals subjects and black diamonds represent average activation for each FHD group.
Performance data (number of errors and percent accuracy) for the Color Naming, Word Reading and Interference components of the Stroop task were examined between FH+ and FH- adolescent using a one-way analysis of variance (ANOVA) using SPSS 11.0 (SPSS, Chicago, IL), with α set at .05. No significant group differences or correlations with FHD were observed for performance data on any of the three components of the Stroop task (Table 2).
Table 2. Stroop Performance.
| FH+ (n=18) | FH- (n=14) | ||||
|---|---|---|---|---|---|
| Errors | Percent Accuracy | Errors | Percent Accuracy | p | |
| Color Naming | 12.5 ± 9.9 | 79.2 ± 16.6 | 11.2 ± 9.3 | 81.3 ± 15.4 | .72 |
| Word Reading | 5.7 ± 8.0 | 90.5 ± 13.4 | 3.7 ± 5.3 | 93.8 ± 8.9 | .43 |
| Interference | 6.3 ± 5.6 | 82.7 ± 15.5 | 6.8 ± 7.3 | 81.1 ± 20.4 | .80 |
Data represent mean ± SD. No significant differences (p < .05) were observed.
Results
Whole Sample ROI Analysis
ROI analysis of the whole study sample (n=32), with age included as a covariate, revealed activation patterns for Color Naming and Word Reading contrasts that included bilateral precentral gyrus (PG) and insula, left MeFG, and right CG (Table 3), with Word Reading also including additional activation in the BA9 region of the left MeFG (Table 3).
The Interference – Color Naming contrast for the whole study sample demonstrated additional activation in the left MFG, left MeFG, right PG and the right ACC, whereas the Interference – Word Reading contrast demonstrated increased activation in the left PG and left MFG as compared to the Color Naming and Word Reading tasks alone (Table 3).
Categorical Family History Effects
No significant FH activation differences were observed during Color Naming, however during Word Reading, FH+ subjects activated significantly more regions of the search territory, including bilateral ACC, bilateral MFG and left IFG than FH- subjects (Table 4). Although no FH differences were observed when the Interference – Word Reading contrast was examined, significant differences were evident for the Interference – Color Naming contrast. As reported in Table 5, the FH+ group demonstrated significantly greater activation in bilateral MFG, left insula, right ACC, and left MeFG relative to FH- counterparts, whereas FH- subjects demonstrated only increased activation in the PG as compared to the FH+ group.
Table 4. Family History Effects: Foci of Maximally Activated Brain Regions.
| Talairach Coordinates | ||||||
|---|---|---|---|---|---|---|
| Region | BA | x | y | z | Cluster | SPM {t} |
| FH+: Word Reading | ||||||
| L. Anterior Cingulate | BA24 | -9 | 21 | 22 | 21 | 4.10 |
| L. Anterior Cingulate | BA32 | -7 | 41 | 12 | 91 | 3.79 |
| R. Middle Frontal Gyrus | BA9 | 27 | 36 | 24 | 57 | 3.77 |
| L. Middle Frontal Gyrus | BA46 | -40 | 23 | 18 | 33 | 3.61 |
| L. Inferior Frontal Gyrus | BA45 | -44 | 18 | 11 | 35 | 3.50 |
| R. Anterior Cingulate | BA32 | 14 | 37 | 13 | 22 | 3.33 |
L, left hemisphere; R, right hemisphere. BA, Brodmann Area. MNI coordinates transformed into Talairach Space, x=center/left of midline, y=anterior/posterior to anterior commissure, z=superior/inferior to horizontal plane through AC-PC line. SPM {t} scores significant beyond p<.001 (uncorrected) for ROI analyses are reported.
Indicates significance at small volume corrected threshold, p<.05.
FHD Regression Analyses
Regression analyses conducted for the calculated FHD measure and brain activation for the Interference – Word Reading and Interference – Color Naming contrasts revealed significant relationships. For the Interference – Word Reading contrast, greater family density was associated with enhanced activation in the right ACC, right MFG, left superior frontal gyrus (SFG) and right PG (Table 6). An illustration of the positive regression of FHD as a function of brain activation for Interference – Word Reading at the local maxima in the right ACC (x=6, y=37, z=11) is presented in Figure 1.
Table 6. Family History Density: Regression with Brain Activation.
| Talairach Coordinates | |||||||
|---|---|---|---|---|---|---|---|
| Region | BA | x | y | z | Cluster | SPM {t} | |
| Positive Regression: FHD and Interference – Word Reading | |||||||
| R. Anterior Cingulate Gyrus | 32 | 6 | 37 | 11 | 75 | 5.49* | |
| R. Middle Frontal Gyrus | 6 | 28 | 5 | 47 | 603 | 4.84 | |
| L. Superior Frontal Gyrus | 8 | -3 | 22 | 51 | 57 | 4.78 | |
| R. Middle Frontal Gyrus | 9 | 45 | 12 | 37 | 68 | 4.48 | |
| R. Precentral Gyrus | 44 | 43 | 14 | 10 | 21 | 3.59 | |
| Negative Regression: FHD and Interference – Word Reading | |||||||
| R. Superior Frontal Gyrus | 6 | -6 | -6 | 65 | 74 | 4.04 | |
L, left hemisphere; R, right hemisphere. BA, Brodmann Area. MNI coordinates transformed into Talairach Space, x=center/left of midline, y=anterior/posterior to anterior commissure, z=superior/inferior to horizontal plane through AC-PC line. SPM {t} scores significant beyond p<.001 (uncorrected) for ROI analyses are reported.
Indicates significance at small volume corrected threshold, p<.05.
For the Interference – Color Naming contrast, greater family density was associated with enhanced activation in the left and right MFG, left SFG, left CG, left insula and right PG (Table 7). An illustration of the positive regression of FHD as a function of brain activation for Interference – Color Naming at the local maxima in the right MFG (x=26, y=11, z=44) is presented in Figure 2.
Table 7. Family History Density: Regression with Brain Activation.
| Talairach Coordinates | ||||||
|---|---|---|---|---|---|---|
| Region | BA | x | y | z | Cluster | SPM {t} |
| Positive Regression: FHD and Interference – Color Naming | ||||||
| R. Middle Frontal Gyrus | 8 | 26 | 11 | 44 | 1108 | 6.64* |
| L. Superior Frontal Gyrus | 6 | -13 | 15 | 49 | 198 | 4.83 |
| L. Cingulate Gyrus | 24 | -12 | 11 | 30 | 59 | 4.60 |
| R. Precentral Gyrus | 6 | 39 | -7 | 35 | 98 | 4.55 |
| L. Insula | -38 | 16 | 14 | 255 | 4.48* | |
| L. Middle Frontal Gyrus | 9 | -29 | 32 | 27 | 456 | 4.14 |
| Negative Regression: FHD and Interference – Color Naming | ||||||
| L. Superior Frontal Gyrus | 6 | -6 | -6 | 67 | 223 | 5.55 |
| L. Precentral Gyrus | 6 | -24 | -13 | 64 | 29 | 4.75 |
| R. Medial Frontal Gyrus | 6 | 6 | -27 | 65 | 47 | 3.54 |
L, left hemisphere; R, right hemisphere. BA, Brodmann Area. MNI coordinates transformed into Talairach Space, x=center/left of midline, y=anterior/posterior to anterior commissure, z=superior/inferior to horizontal plane through AC-PC line. SPM {t} scores significant beyond p<.001 (uncorrected) for ROI analyses are reported.
Indicates significance at small volume corrected threshold, p<.05.
Significant negative correlations were also observed, with a greater FHD being associated with less activation in the right SFG for Interference – Word Reading (Table 6) and left SFG, left PG, and right MeFG for Interference – Color Naming (Table 7).
Error Rate Regression Analyses
Regression analyses conducted for Interference error rate and brain activation for the Interference – Color Naming contrast revealed a significant positive relationship, with increased error rate being associated with greater activation in the left MFG (BA46, 28 voxels, local maxima x=-46, y=30, z=23, SPM {t} = 3.98). A significant negative regression was not observed. When the regression of error rate and brain activation was conducted separately for each FH group, significant positive regressions were observed for left SFG (BA10, 29 voxels; x=-27, y=49, z=26; SPM {t} =4.09) and left MFG (BA46, 84 voxels; x=-46, y=30, z=24; SPM {t} =4.06) in the FH- group, however, no significant areas of activation that correlated with error rate were observed for the FH+ group.
Discussion
The current findings are consistent with previous Stroop fMRI studies demonstrating activation of a network of frontal lobe regions during performance of the Interference condition (Gruber et al., 2002; Leung et al., 2000; Pardo et al., 1990; Peterson et al., 1999). With age included as a covariate, the contrast of Interference – Color Naming revealed a network of brain activation that was unique from that observed during Color Naming, Word Reading, and Interference – Word Reading. Both Interference contrasts demonstrated increased recruitment of the PG, but also additional engagement of the PFC (BA9, involved in executive functions and cognitive control) and dorsal ACC (BA32, involved in decision-making) for Interference – Color Naming and increased activation of the DLPFC (BA 46, involved in executive functions) for Interference – Word Reading. These results confirm that in the current sample of adolescent subjects, performance of a cognitive task requiring response inhibition, Stroop Interference, is subserved by enhanced recruitment of frontal lobe neurocircuitry that is unique from the Color Naming and Word Reading subtests alone.
The current results also support the hypothesis that FH status has a significant influence on brain activation during Stroop Interference. Although no FH effects were observed for Color Naming, the Interference – Color Naming contrast demonstrated greater activation within the region of interest in FH+ adolescents relative to FH- counterparts. Regardless of whether a categorical approach comparing FH+ versus FH- subjects or a regression analysis including FHD (Zucker et al., 1994) was used, areas within the search territory exhibiting greater activation during Stroop Interference in FH+ youth included BA6, BA8 and BA9, and left insula. Activation of these regions of the premotor cortex (BA6/8) and PFC (BA9) has been reported previously in fMRI studies of Stroop Interference (Adelman et al., 2002; Leung et al., 2000; Peterson et al., 1999). Activation of the insula has also been reported previously during Stroop Interference (Leung et al., 2000). Both approaches revealed enhanced recruitment of the cingulate cortex in FH+ youth, however, the categorical approach revealed greater activation in the right ACC (BA32) and the FHD regression approach revealed greater activation in the left CG (BA24). The ventral cingulate cortex (BA24), which is part of the limbic system that has connections to the amygdala, hippocampus and orbito-frontal cortex, and dorsal ACC (BA32), which is involved in decision-making, have been implicated in Stroop performance. No categorical group differences were observed for the Interference – Word Reading contrast, however, the FHD regression analysis demonstrated that a denser family history was associated with greater activation in the ACC, MFG, SFG, and PG, and less activation in the BA6 region of the SFG.
While the Interference – Color Naming contrast provided evidence for increased neurobiological recruitment associated with response inhibition that is dissociable from color naming ability between FH groups, the Interference – Word Reading contrast provided supporting evidence for increased recruitment specific to response inhibition, albeit not as clearly dissociable from FH effects associated with word reading ability. It is noteworthy that greater activation in the ACC, dorsal (BA32) and ventral (BA24) portions, and bilateral MFG (BA9/46) and IFG was observed in FH+ youth during the simple Word Reading component of the Stroop task. This pattern of altered activation was of a lesser magnitude when compared to the FH-related alterations observed for Interference (relative to Color Naming or Word Reading), but could nonetheless reflect a developmental vulnerability in neuronal resource allotment during a simple information-processing task that is considered to have a high degree of automaticity (Protopapas et al., 2007). These surprising findings should be interpreted cautiously, particularly given a lack of FH differences in activation during the simple Color Naming condition, and in light of similar task performance between groups.
Enhanced recruitment of brain regions in FH+ youth is consistent with reports that compensation of brain activity (increased BOLD signal) occurs in the affected and adjacent regions, when a region of the brain is temporarily fatigued or otherwise compromised, in order to sustain roughly equivalent levels of performance on cognitively demanding tasks (Chang et al., 2008; Drummond et al., 2005; Gruber et al., 2002; Kanayama et al., 2004). Taken together, these findings suggest evidence for increased neuronal recruitment in FH+ youth during performance of a response inhibition task, despite the absence of significant performance differences between groups. There was some modest evidence for a significant regression between error rate and greater activation of the left MFG (BA46) and left SFG (BA10), however this relationship was driven by FH- youth. This is consistent with findings observed by Marsh and colleagues (Marsh et al., 2006), who reported a significant correlation between increased activation in the BA10/46 regions and poorer performance on Stroop Interference.
The findings in this report contrast with the findings from Schweinsburg and colleagues (Schweinsburg et al., 2004), who reported significantly less frontal lobe activation in FH+ adolescents relative to FH- counterparts during Go No-Go performance. These conflicting results are not surprising, as performance of these tasks require unique response components that are task-specific, e.g., Go No-Go requires withholding a motor response, whereas the Stroop Interference task used in this study requires vocalizing a less automatic response (naming ink color) while inhibiting a more automatic tendency (reading words). Differential activation of neural circuits between FH groups may therefore not generalize across response inhibition tasks that require different sensory or response demands (Stevens et al., 2007). These findings highlight the necessity of utilizing different challenge paradigms in order to identify elements of response inhibition that might be mediated by a common neural network, as well as differences in network dynamics that vary as a function of cognitive demand. It is plausible that frontal lobe activation is influenced by FH status, indicating that FH+ youth experience a neurobiological change on a network level, in the absence of performance differences.
There are a number of factors that must be considered when interpreting these study findings. First, while the current investigation includes a moderate sample size for neuroimaging studies, the sample size provided limited power for the investigation of sex effects on functional brain activation. Future studies should address the potential interaction between FH status and sex, particularly in light of the reports of significant sex differences in brain structure and function during adolescence (Gallagher et al., 2000; Halpern, 1992; Silveri et al., 2004; Silveri et al., 2006; Silveri et al., 2008; Yurgelun-Todd et al., 2002). The sample was well characterized, in that all adolescent subjects in this study reported less than three episodes of lifetime alcohol use and no lifetime use of other psychoactive substances, had middle to upper class SES status, regardless of FH status, and did not meet criteria for psychiatric conditions such as attention deficit disorder or conduct disorder. Furthermore, inclusion of age as a covariate in all SPM analyses likely minimized the possibility that FH group differences were influenced by age (given an age range of 8 to 19), which could be associated with the developmental time course of frontal lobe maturation. Although there are inherent limitations associated with the fMRI block design (Amaro and Barker, 2006), Leung and colleagues (Leung et al., 2000) have reported similar, albeit less robust, activation in frontal brain networks during Stroop performance when using a block design in comparison to an event related design.
Risk associated with FH status may have been minimized as a result of the methods used to establish a family history of alcohol abuse. Our categorization was based on a structured interview with a single parent, a method that has been shown to be less sensitive for detecting accurate family history status than interviewing multiple family members (Rice et al., 1995). Furthermore, the majority of adolescents in the FH+ group were from simplex families with alcohol dependence, that is, families where only a single relative, parent or grandparent, was identified as meeting the criteria for a positive family history of alcohol dependence (88%). It has been suggested by Hill and colleagues (Hill et al., 2007a), that a greater family loading of alcoholism (multiplex family history of alcoholism) is associated with a greater genetic susceptibility for developing an alcohol use disorder. Our sample therefore would be expected to have a lesser genetic loading than subjects drawn from multiplex families and, as a result, would be expected to exhibit more subtle activation differences. Importantly, complementary FH results were observed in the present study when both categorical and FHD (Zucker et al., 1994) regression approaches were employed to examine the influence of FH effects on brain activation during Stroop Interference.
Within the framework of characterizing brain reorganization and rapid improvements in cognition during the adolescent period, studies identifying structural, functional and cognitive deficits in adolescents with high- versus low-risk for future alcoholism suggest a potential neurobiological vulnerability that may be present prior to the initiation of alcohol use (Hill et al., 2007b; Schweinsburg et al., 2004; Silveri et al., 2008; Spadoni et al., 2008). While the adolescent developmental period is already associated with reduced inhibitory capacity, having a positive family history may confer risk for future alcoholism by impacting adolescent maturation of frontal networks which is necessary to develop the capability to evaluate and appropriately modulate response inhibition, as well as emotional responses (Luna and Sweeney, 2004; Rubia et al., 2000; Yurgelun-Todd, 2007). Resulting difficulties with cognitive control could therefore place FH+ adolescents at even greater risk when faced with decision-making challenges that include when to begin drinking alcohol, which is well established to influence the escalation of alcohol consumption and risk for developing an alcohol abuse disorder later in life (Brown and Tapert, 2004; Chassin et al., 2004; Grant and Dawson, 1997; Hill et al., 2000).
Acknowledgments
This work was supported by NIAAA K01 grant AA014651 (MMS), NIDA R01 grants DA 020269 and DA 12483 (DYT), and a grant from the Charles H. Hood Foundation (DYT).
References
- Adleman NE, Menon V, Blasey CM, White CD, Warsofsky IS, Glover GH, Reiss AL. A Developmental fMRI Study of the Stroop Color-Word Task. Neuroimage. 2002;16:61–75. doi: 10.1006/nimg.2001.1046. [DOI] [PubMed] [Google Scholar]
- Alterman AI, Searles JS, Hall JG. Failure to find differences in drinking behavior as a function of familial risk for alcoholism: a replication. J Abnorm Psychol. 1989;98:50–53. doi: 10.1037//0021-843x.98.1.50. [DOI] [PubMed] [Google Scholar]
- Amaro E, Jr, Barker GJ. Study design in fMRI: basic principles. Brain Cogn. 2006;60:220–232. doi: 10.1016/j.bandc.2005.11.009. [DOI] [PubMed] [Google Scholar]
- Bates ME, Labouvie EW. Adolescent risk factors and the prediction of persistent alcohol and drug use into adulthood. Alcohol Clin Exp Res. 1997;21:944–950. [PubMed] [Google Scholar]
- Bench CJ, Frith CD, Grasby PM, Friston KJ, Paulesu E, Frackowiak RS, Dolan RJ. Investigations of the functional anatomy of attention using the Stroop test. Neuropsychologia. 1993;31:907–922. doi: 10.1016/0028-3932(93)90147-r. [DOI] [PubMed] [Google Scholar]
- Biederman J, Faraone SV, Monuteaux MC, Feighner JA. Patterns of alcohol and drug use in adolescents can be predicted by parental substance use disorders. Pediatrics. 2000;106:792–797. doi: 10.1542/peds.106.4.792. [DOI] [PubMed] [Google Scholar]
- Bracht T, Tuscher O, Schnell S, Kreher B, Rusch N, Glauche V, Lieb K, Ebert D, Il'yasov KA, Hennig J, Weiller C, van Elst LT, Saur D. Extraction of prefronto-amygdalar pathways by combining probability maps. Psychiatry Res. 2009;174:217–222. doi: 10.1016/j.pscychresns.2009.05.001. [DOI] [PubMed] [Google Scholar]
- Brown SA, Tapert SF. Adolescence and the trajectory of alcohol use: basic to clinical studies. Ann N Y Acad Sci. 2004;1021:234–244. doi: 10.1196/annals.1308.028. [DOI] [PubMed] [Google Scholar]
- Brown SA, Tapert SF, Granholm E, Delis DC. Neurocognitive functioning of adolescents: effects of protracted alcohol use. Alcohol Clin Exp Res. 2000;24:164–171. [PubMed] [Google Scholar]
- Casey BJ, Galvan A, Hare TA. Changes in cerebral functional organization during cognitive development. Curr Opin Neurobiol. 2005;15:239–244. doi: 10.1016/j.conb.2005.03.012. [DOI] [PubMed] [Google Scholar]
- Casey BJ, Giedd JN, Thomas KM. Structural and functional brain development and its relation to cognitive development. Biol Psychol. 2000;54:241–257. doi: 10.1016/s0301-0511(00)00058-2. [DOI] [PubMed] [Google Scholar]
- Casey BJ, Trainor R, Giedd J, Vauss Y, Vaituzis CK, Hamburger S, Kozuch P, Rapoport JL. The role of the anterior cingulate in automatic and controlled processes: a developmental neuroanatomical study. Dev Psychobiol. 1997;30:61–69. [PubMed] [Google Scholar]
- Chang L, Yakupov R, Nakama H, Stokes B, Ernst T. Antiretroviral treatment is associated with increased attentional load-dependent brain activation in HIV patients. J Neuroimmune Pharmacol. 2008;3:95–104. doi: 10.1007/s11481-007-9092-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chassin L, Barerra M. Substance use escalation and substance use restraint among adolescent children of alcoholics. Psychol Addict Behav. 1993;7:3–20. [Google Scholar]
- Chassin L, Flora DB, King KM. Trajectories of alcohol and drug use and dependence from adolescence to adulthood: The effects of familial alcoholism and personality. J Abnorm Psychol. 2004;113:483–498. doi: 10.1037/0021-843X.113.4.483. [DOI] [PubMed] [Google Scholar]
- Clark DB, Cornelius JR, Kirisci L, Tarter RE. Childhood risk categories for adolescent substance involvement: a general liability typology. Drug Alcohol Depend. 2005;77:13–21. doi: 10.1016/j.drugalcdep.2004.06.008. [DOI] [PubMed] [Google Scholar]
- De Bellis MD, Narasimhan A, Thatcher DL, Keshavan MS, Soloff P, Clark DB. Prefrontal cortex, thalamus, and cerebellar volumes in adolescents and young adults with adolescent-onset alcohol use disorders and comorbid mental disorders. Alcohol Clin Exp Res. 2005;29:1590–1600. doi: 10.1097/01.alc.0000179368.87886.76. [DOI] [PubMed] [Google Scholar]
- de Graaf-Peters VB, Hadders-Algra M. Ontogeny of the human central nervous system: what is happening when? Early Hum Dev. 2006;82:257–266. doi: 10.1016/j.earlhumdev.2005.10.013. [DOI] [PubMed] [Google Scholar]
- Drummond SP, Meloy MJ, Yanagi MA, Orff HJ, Brown GG. Compensatory recruitment after sleep deprivation and the relationship with performance. Psychiatry Res. 2005;140:211–223. doi: 10.1016/j.pscychresns.2005.06.007. [DOI] [PubMed] [Google Scholar]
- Fillmore MT, Marczinski CA, Bowman AM. Acute tolerance to alcohol effects on inhibitory and activational mechanisms of behavioral control. J Stud Alcohol. 2005;66:663–672. doi: 10.15288/jsa.2005.66.663. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Frith CD, Frackowiak RS, Turner R. Characterizing dynamic brain responses with fMRI: a multivariate approach. Neuroimage. 1995;2:166–172. doi: 10.1006/nimg.1995.1019. [DOI] [PubMed] [Google Scholar]
- Gallagher AM, De Lisi R, Holst PC, McGillicuddy-De Lisi AV, Morely M, Cahalan C. Gender differences in advanced mathematical problem solving. J Exp Child Psychol. 2000;75:165–190. doi: 10.1006/jecp.1999.2532. [DOI] [PubMed] [Google Scholar]
- Giedd JN, Blumenthal J, Jeffries NO, Castellanos FX, Liu H, Zijdenbos A, Paus T, Evans AC, Rapoport JL. Brain development during childhood and adolescence: a longitudinal MRI study. Nat Neurosci. 1999;2:861–863. doi: 10.1038/13158. [DOI] [PubMed] [Google Scholar]
- Giedd JN, Snell JW, Lange N, Rajapakse JC, Casey BJ, Kozuch PL, Vaituzis AC, Vauss YC, Hamburger SD, Kaysen D, Rapoport JL. Quantitative magnetic resonance imaging of human brain development: ages 4-18. Cereb Cortex. 1996;6:551–560. doi: 10.1093/cercor/6.4.551. [DOI] [PubMed] [Google Scholar]
- Gogtay N, Giedd JN, Lusk L, Hayashi KM, Greenstein D, Vaituzis AC, Nugent TF, 3rd, Herman DH, Clasen LS, Toga AW, Rapoport JL, Thompson PM. Dynamic mapping of human cortical development during childhood through early adulthood. Proc Natl Acad Sci U S A. 2004;101:8174–8179. doi: 10.1073/pnas.0402680101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golden CJ. Identification of brain disorders by the Stroop Color and Word Test. J Clin Psychol. 1976;32:654–658. doi: 10.1002/1097-4679(197607)32:3<654::aid-jclp2270320336>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Goldman-Rakic PS. Topography of cognition: parallel distributed networks in primate association cortex. Annu Rev Neurosci. 1988;11:137–156. doi: 10.1146/annurev.ne.11.030188.001033. [DOI] [PubMed] [Google Scholar]
- Goudriaan AE, Grekin ER, Sher KJ. Decision making and binge drinking: a longitudinal study. Alcohol Clin Exp Res. 2007;31:928–938. doi: 10.1111/j.1530-0277.2007.00378.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant BF, Dawson DA. Age at onset of alcohol use and its association with DSM-IV alcohol abuse and dependence: results from the National Longitudinal Alcohol Epidemiologic Survey. J Subst Abuse. 1997;9:103–110. doi: 10.1016/s0899-3289(97)90009-2. [DOI] [PubMed] [Google Scholar]
- Gruber SA, Rogowska J, Holcomb P, Soraci S, Yurgelun-Todd DA. Stroop performance in normal control subjects: An fMRI study. NeuroImage. 2002;16:349–360. doi: 10.1006/nimg.2002.1089. [DOI] [PubMed] [Google Scholar]
- Halpern DF. Sex differences in cognitive abilities. 2nd. Erlbaum; Hillsdale: 1992. [Google Scholar]
- Hartley DE, Elsabagh S, File SE. Binge drinking and sex: effects on mood and cognitive function in healthy young volunteers. Pharmacol Biochem Behav. 2004;78:611–619. doi: 10.1016/j.pbb.2004.04.027. [DOI] [PubMed] [Google Scholar]
- Hill SY, De Bellis MD, Keshavan MS, Lowers L, Shen S, Hall J, Pitts T. Right amygdala volume in adolescent and young adult offspring from families at high risk for developing alcoholism. Biol Psychiatry. 2001;49:894–905. doi: 10.1016/s0006-3223(01)01088-5. [DOI] [PubMed] [Google Scholar]
- Hill SY, Hoffman EK, Zezza N, Thalamuthu A, Weeks DE, Matthews AG, Mukhopadhyay I. Dopaminergic mutations: Within-family association and linkage in multiplex alcohol dependence families. Am J Med Genet B Neuropsychiatr Genet. 2007a;147B:517–526. doi: 10.1002/ajmg.b.30630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill SY, Muddasani S, Prasad K, Nutche J, Steinhauer SR, Scanlon J, McDermott M, Keshavan M. Cerebellar volume in offspring from multiplex alcohol dependence families. Biol Psychiatry. 2007b;61:41–47. doi: 10.1016/j.biopsych.2006.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill SY, Shen S, Lowers L, Locke J. Factors predicting the onset of adolescent drinking in families at high risk for developing alcoholism. Biol Psychiatry. 2000;48:265–275. doi: 10.1016/s0006-3223(00)00841-6. [DOI] [PubMed] [Google Scholar]
- Hill SY, Wang S, Kostelnik B, Carter H, Holmes B, McDermott M, Zezza N, Stiffler S, Keshavan MS. Disruption of orbitofrontal cortex laterality in offspring from multiplex alcohol dependence families. Biol Psychiatry. 2009;65:129–136. doi: 10.1016/j.biopsych.2008.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollingshead AB. Two factor index of social position. Author; New Haven: 1957. [Google Scholar]
- Hua JY, Smith SJ. Neural activity and the dynamics of central nervous system development. Nat Neurosci. 2004;7:327–332. doi: 10.1038/nn1218. [DOI] [PubMed] [Google Scholar]
- Jang DP, Namkoong K, Kim JJ, Park S, Kim IY, Kim SI, Kim YB, Cho ZH, Lee E. The relationship between brain morphometry and neuropsychological performance in alcohol dependence. Neurosci Lett. 2007;428:21–26. doi: 10.1016/j.neulet.2007.09.047. [DOI] [PubMed] [Google Scholar]
- Jernigan TL, Trauner DA, Hesselink JR, Tallal PA. Maturation of human cerebrum observed in vivo during adolescence. Brain. 1991;114:2037–2049. doi: 10.1093/brain/114.5.2037. [DOI] [PubMed] [Google Scholar]
- Johnson JL, Rolf JE. Cognitive functioning in children from alcoholic and non-alcoholic families. Br J Addict. 1988;83:849–857. doi: 10.1111/j.1360-0443.1988.tb00520.x. [DOI] [PubMed] [Google Scholar]
- Johnston LD, O'Malley PM, Bachman JG. Volume 1: Secondary school students (NIH publication No 00-4802) Vol. 1. National Institute on Drug Abuse; Rockville: 2000. Monitoring the Future national survey results on drug use, 1975-1999. [Google Scholar]
- Kanayama G, Rogowska J, Pope HG, Gruber SA, Yurgelun-Todd DA. Spatial working memory in heavy cannabis users: a functional magnetic resonance imaging study. Psychopharmacology (Berl) 2004;176:239–247. doi: 10.1007/s00213-004-1885-8. [DOI] [PubMed] [Google Scholar]
- Killgore WD, Gruber SA, Yurgelun-Todd DA. Depressed mood and lateralized prefrontal activity during a Stroop task in adolescent children. Neurosci Lett. 2007;416:43–48. doi: 10.1016/j.neulet.2007.01.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancaster JL, Woldorff MG, Parsons LM, Liotti M, Freitas CS, Rainey L, Kochunov PV, Nickerson D, Mikiten SA, Fox PT. Automated Talairach atlas labels for functional brain mapping. Hum Brain Mapp. 2000;10:120–131. doi: 10.1002/1097-0193(200007)10:3<120::AID-HBM30>3.0.CO;2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung HC, Skudlarski P, Gatenby JC, Peterson BS, Gore JC. An event-related functional MRI study of the stroop color word interference task. Cereb Cortex. 2000;10:552–560. doi: 10.1093/cercor/10.6.552. [DOI] [PubMed] [Google Scholar]
- Lieb R, Merikangas KR, Hofler M, Pfister H, Isensee B, Wittchen HU. Parental alcohol use disorders and alcohol use and disorders in offspring: a community study. Psychol Med. 2002;32:63–78. doi: 10.1017/s0033291701004883. [DOI] [PubMed] [Google Scholar]
- Luna B, Sweeney JA. The emergence of collaborative brain function: FMRI studies of the development of response inhibition. Ann N Y Acad Sci. 2004;1021:296–309. doi: 10.1196/annals.1308.035. [DOI] [PubMed] [Google Scholar]
- Luna B, Thulborn KR, Munoz DP, Merriam EP, Garver KE, Minshew NJ, Keshavan MS, Genovese CR, Eddy WF, Sweeney JA. Maturation of widely distributed brain function subserves cognitive development. Neuroimage. 2001;13:786–793. doi: 10.1006/nimg.2000.0743. [DOI] [PubMed] [Google Scholar]
- Luria AR. Higher cortical fucntions in man. Basic Books; New York: 1966. [Google Scholar]
- Maldjian JA, Laurienti PJ, Kraft RA, Burdette JH. An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. Neuroimage. 2003;19:1233–1239. doi: 10.1016/s1053-8119(03)00169-1. [DOI] [PubMed] [Google Scholar]
- Marczinski CA, Combs SW, Fillmore MT. Increased sensitivity to the disinhibiting effects of alcohol in binge drinkers. Psychol Addict Behav. 2007;21:346–354. doi: 10.1037/0893-164X.21.3.346. [DOI] [PubMed] [Google Scholar]
- Marsh R, Zhu H, Schultz RT, Quackenbush G, Royal J, Skudlarski P, Peterson BS. A developmental fMRI study of self-regulatory control. Hum Brain Mapp. 2006;27:848–863. doi: 10.1002/hbm.20225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGrath CE, Watson AL, Chassin L. Academic achievement in adolescent children of alcoholics. J Stud Alcohol. 1999;60:18–26. doi: 10.15288/jsa.1999.60.18. [DOI] [PubMed] [Google Scholar]
- McGue M, Iacono WG, Legrand LN, Elkins I. Origins and consequences of age at first drink. II. Familial risk and heritability. Alcohol Clin Exp Res. 2001;25:1166–1173. [PubMed] [Google Scholar]
- Milberger S, Faraone SV, Biederman J, Chu MP, Feighner JA. Substance use disorders in high-risk adolescent offspring. Am J Addict. 1999;8:211–219. doi: 10.1080/105504999305820. [DOI] [PubMed] [Google Scholar]
- Murphy RT, O'Farrell TJ, Floyd FJ, Connors GJ. School adjustment of children of alcoholic fathers: comparison to normal controls. Addict Behav. 1991;16:275–287. doi: 10.1016/0306-4603(91)90020-i. [DOI] [PubMed] [Google Scholar]
- Nagel BJ, Schweinsburg AD, Phan V, Tapert SF. Reduced hippocampal volume among adolescents with alcohol use disorders without psychiatric comorbidity. Psychiatry Res. 2005;139:181–190. doi: 10.1016/j.pscychresns.2005.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardo JV, Pardo PJ, Janer KW, Raichle ME. The anterior cingulate cortex mediates processing selection in the Stroop attentional conflict paradigm. Proc Natl Acad Sci U S A. 1990;87:256–259. doi: 10.1073/pnas.87.1.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons OA, Nixon SJ. Cognitive functioning in sober social drinkers: a review of the research since 1986. J Stud Alcohol. 1998;59:180–190. doi: 10.15288/jsa.1998.59.180. [DOI] [PubMed] [Google Scholar]
- Paulus MP, Tapert SF, Pulido C, Schuckit MA. Alcohol attenuates load-related activation during a working memory task: relation to level of response to alcohol. Alcohol Clin Exp Res. 2006;30:1363–1371. doi: 10.1111/j.1530-0277.2006.00164.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paus T. Mapping brain maturation and cognitive development during adolescence. Trends Cogn Sci. 2005;9:60–68. doi: 10.1016/j.tics.2004.12.008. [DOI] [PubMed] [Google Scholar]
- Penny WD, Holmes AP, Friston KJ. Random effects analysis. Academic Press; 2003. [Google Scholar]
- Peterson BS, Skudlarski P, Gatenby JC, Zhang H, Anderson AW, Gore JC. An fMRI study of Stroop word-color interference: evidence for cingulate subregions subserving multiple distributed attentional systems. Biol Psychiatry. 1999;45:1237–1258. doi: 10.1016/s0006-3223(99)00056-6. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Mathalon DH, Sullivan EV, Rawles JM, Zipursky RB, Lim KO. A quantitative magnetic resonance imaging study of changes in brain morphology from infancy to late adulthood. Arch Neurol. 1994;51:874–887. doi: 10.1001/archneur.1994.00540210046012. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Sullivan EV, Mathalon DH, Lim KO. Frontal lobe volume loss observed with magnetic resonance imaging in older chronic alcoholics. Alcohol Clin Exp Res. 1997;21:521–529. doi: 10.1111/j.1530-0277.1997.tb03798.x. [DOI] [PubMed] [Google Scholar]
- Polli FE, Barton JJ, Thakkar KN, Greve DN, Goff DC, Rauch SL, Manoach DS. Reduced error-related activation in two anterior cingulate circuits is related to impaired performance in schizophrenia. Brain. 2008;131:971–986. doi: 10.1093/brain/awm307. [DOI] [PubMed] [Google Scholar]
- Polli FE, Wright CI, Milad MR, Dickerson BC, Vangel M, Barton JJ, Rauch SL, Manoach DS. Hemispheric differences in amygdala contributions to response monitoring. Neuroreport. 2009;20:398–402. doi: 10.1097/WNR.0b013e328324edb8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poon E, Ellis DA, Fitzgerald HE, Zucker RA. Intellectual, cognitive, and academic performance among sons of alcoholics, during the early school years: differences related to subtypes of familial alcoholism. Alcohol Clin Exp Res. 2000;24:1020–1027. [PubMed] [Google Scholar]
- Protopapas A, Archonti A, Skaloumbakas C. Reading ability is negatively related to Stroop interference. Cogn Psychol. 2007;54:251–282. doi: 10.1016/j.cogpsych.2006.07.003. [DOI] [PubMed] [Google Scholar]
- Puig-Antich J, Orvaschel H, Tabrizi M, Chambers W. The Schedule for Affective Disorders and Schizophrenia for School-Aged Children - Epidemiologic Version (Kiddie-SADS-E) New York State Psychiatric Institute and Yale University School of Medicine; New York: 1980. [Google Scholar]
- Reich W, Earls F, Frankel O, Shayka JJ. Psychopathology in children of alcoholics. J Am Acad Child Adolesc Psychiatry. 1993;32:995–1002. doi: 10.1097/00004583-199309000-00017. [DOI] [PubMed] [Google Scholar]
- Reiss AL, Abrams MT, Singer HS, Ross JL, Denckla MB. Brain development, gender and IQ in children. A volumetric imaging study. Brain. 1996;119:1763–1774. doi: 10.1093/brain/119.5.1763. [DOI] [PubMed] [Google Scholar]
- Rice JP, Reich T, Bucholz KK, Neuman RJ, Fishman R, Rochberg N, Hesselbrock VM, Nurnberger JI, Jr, Schuckit MA, Begleiter H. Comparison of direct interview and family history diagnoses of alcohol dependence. Alcohol Clin Exp Res. 1995;19:1018–1023. doi: 10.1111/j.1530-0277.1995.tb00983.x. [DOI] [PubMed] [Google Scholar]
- Rubia K, Overmeyer S, Taylor E, Brammer M, Williams SC, Simmons A, Andrew C, Bullmore ET. Functional frontalisation with age: mapping neurodevelopmental trajectories with fMRI. Neurosci Biobehav Rev. 2000;24:13–19. doi: 10.1016/s0149-7634(99)00055-x. [DOI] [PubMed] [Google Scholar]
- Schuckit MA, Butters N, Lyn L, Irwin M. Neuropsychologic deficits and the risk for alcoholism. Neuropsychopharmacology. 1987;1:45–53. doi: 10.1016/0893-133x(87)90009-1. [DOI] [PubMed] [Google Scholar]
- Schweinsburg AD, Paulus MP, Barlett VC, Killeen LA, Caldwell LC, Pulido C, Brown SA, Tapert SF. An FMRI study of response inhibition in youths with a family history of alcoholism. Ann N Y Acad Sci. 2004;1021:391–394. doi: 10.1196/annals.1308.050. [DOI] [PubMed] [Google Scholar]
- Shallice T. Specific impairments of planning. Philos Trans R Soc Lond B Biol Sci. 1982;298:199–209. doi: 10.1098/rstb.1982.0082. [DOI] [PubMed] [Google Scholar]
- Sher KJ, Martin ED, Wood PK, Rutledge PC. Alcohol use disorders and neuropsychological functioning in first-year undergraduates. Exp Clin Psychopharmacol. 1997;5:304–315. doi: 10.1037//1064-1297.5.3.304. [DOI] [PubMed] [Google Scholar]
- Silveri MM, Tzilos GK, Pimentel PJ, Yurgelun-Todd DA. Trajectories of adolescent emotional and cognitive development: effects of sex and risk for drug use. Ann N Y Acad Sci. 2004;1021:363–370. doi: 10.1196/annals.1308.046. [DOI] [PubMed] [Google Scholar]
- Silveri MM, Rohan ML, Pimentel PJ, Gruber SA, Rosso IM, Yurgelun-Todd DA. Sex differences in the relationship between frontal white matter microstructure and impulsivity in adolescents. Magn Reson Imaging. 2006;24:833–841. doi: 10.1016/j.mri.2006.03.012. [DOI] [PubMed] [Google Scholar]
- Silveri MM, Tzilos GK, Yurgelun-Todd DA. Relationship between white matter volume and cognitive performance during adolescence: effects of age, sex and risk for drug use. Addiction. 2008;103:1509–1520. doi: 10.1111/j.1360-0443.2008.02272.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sowell ER, Thompson PM, Tessner KD, Toga AW. Mapping continued brain growth and gray matter density reduction in dorsal frontal cortex: Inverse relationships during postadolescent brain maturation. J Neurosci. 2001;21:8819–8829. doi: 10.1523/JNEUROSCI.21-22-08819.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sowell ER, Thompson PM, Toga AW. Mapping changes in the human cortex throughout the span of life. Neuroscientist. 2004;10:372–392. doi: 10.1177/1073858404263960. [DOI] [PubMed] [Google Scholar]
- Sowell ER, Trauner DA, Gamst A, Jernigan TL. Development of cortical and subcortical brain structures in childhood and adolescence: a structural MRI study. Dev Med Child Neurol. 2002;44:4–16. doi: 10.1017/s0012162201001591. [DOI] [PubMed] [Google Scholar]
- Spadoni AD, Norman AL, Schweinsburg AD, Tapert SF. Effects of family history of alcohol use disorders on spatial working memory BOLD response in adolescents. Alcohol Clin Exp Res. 2008;32:1135–1145. doi: 10.1111/j.1530-0277.2008.00694.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spreen O, Risser AH, Edgell D. Developmental Neuropsychology. Oxford University Press; Oxford: 1995. Development of functional systems; pp. 37–56. [Google Scholar]
- Spreen O, Strauss E, Sherman EMS. A compendium of neuropsychological tests: Administration, norms, and commentary. Oxford University Press; Oxford: 2006. pp. 477–499. [Google Scholar]
- Stein JL, Wiedholz LM, Bassett DS, Weinberger DR, Zink CF, Mattay VS, Meyer-Lindenberg A. A validated network of effective amygdala connectivity. NeuroImage. 2007;36:736–745. doi: 10.1016/j.neuroimage.2007.03.022. [DOI] [PubMed] [Google Scholar]
- Stevens MC, Kiehl KA, Pearlson GD, Calhoun VD. Functional neural networks underlying response inhibition in adolescents and adults. Behav Brain Res. 2007;181:12–22. doi: 10.1016/j.bbr.2007.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoltenberg SF, Hill EM, Mudd SA, Blow FC, Zucker RA. Birth cohort differences in features of antisocial alcoholism among men and women. Alcohol Clin Exp Res. 1999;23:1884–1891. [PubMed] [Google Scholar]
- Sullivan EV, Pfefferbaum A. Neurocircuitry in alcoholism: a substrate of disruption and repair. Psychopharmacology (Berl) 2005;180:583–594. doi: 10.1007/s00213-005-2267-6. [DOI] [PubMed] [Google Scholar]
- Tamm L, Menon V, Reiss AL. Maturation of brain function associated with response inhibition. J Am Acad Child Adolesc Psychiatry. 2002;41:1231–1238. doi: 10.1097/00004583-200210000-00013. [DOI] [PubMed] [Google Scholar]
- Tapert SF, Brown GG, Baratta MV, Brown SA. fMRI BOLD response to alcohol stimuli in alcohol dependent young women. Addict Behav. 2004a;29:33–50. doi: 10.1016/j.addbeh.2003.07.003. [DOI] [PubMed] [Google Scholar]
- Tapert SF, Brown GG, Kindermann SS, Cheung EH, Frank LR, Brown SA. fMRI measurement of brain dysfunction in alcohol-dependent young women. Alcohol Clin Exp Res. 2001;25:236–245. [PubMed] [Google Scholar]
- Tapert SF, Schweinsburg AD, Barlett VC, Brown SA, Frank LR, Brown GG, Meloy MJ. Blood oxygen level dependent response and spatial working memory in adolescents with alcohol use disorders. Alcohol Clin Exp Res. 2004b;28:1577–1586. doi: 10.1097/01.alc.0000141812.81234.a6. [DOI] [PubMed] [Google Scholar]
- Taylor SF, Kornblum S, Lauber EJ, Minoshima S, Koeppe RA. Isolation of specific interference processing in the Stroop task: PET activation studies. Neuroimage. 1997;6:81–92. doi: 10.1006/nimg.1997.0285. [DOI] [PubMed] [Google Scholar]
- Townshend JM, Duka T. Binge drinking, cognitive performance and mood in a population of young social drinkers. Alcohol Clin Exp Res. 2005;29:317–325. doi: 10.1097/01.alc.0000156453.05028.f5. [DOI] [PubMed] [Google Scholar]
- Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, Mazoyer B, Joliot M. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 2002;15:273–289. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- Vitaro F, Dobkin PL, Carbonneau R, Tremblay RE. Personal and familial characteristics of resilient sons of male alcoholics. Addiction. 1996;91:1161–1177. doi: 10.1046/j.1360-0443.1996.91811618.x. [DOI] [PubMed] [Google Scholar]
- Weissenborn R, Duka T. Acute alcohol effects on cognitive function in social drinkers: their relationship to drinking habits. Psychopharmacology (Berl) 2003;165:306–312. doi: 10.1007/s00213-002-1281-1. [DOI] [PubMed] [Google Scholar]
- Yurgelun-Todd D. Emotional and cognitive changes during adolescence. Curr Opin Neurobiol. 2007;17:251–257. doi: 10.1016/j.conb.2007.03.009. [DOI] [PubMed] [Google Scholar]
- Yurgelun-Todd DA, Killgore WD, Young AD. Sex differences in cerebral tissue volume and cognitive performance during adolescence. Psychol Rep. 2002;91:743–757. doi: 10.2466/pr0.2002.91.3.743. [DOI] [PubMed] [Google Scholar]
- Zucker RA, Ellis DA, Fitzgerald HE. Developmental evidence for at least two alcoholisms. I. Biopsychosocial variation among pathways into symptomatic difficulty. Ann N Y Acad Sci. 1994;708:134–146. doi: 10.1111/j.1749-6632.1994.tb24706.x. [DOI] [PubMed] [Google Scholar]


