Abstract
Fibrin (Fn) deposition defines several type 1 immune responses, including delayed-type hypersensitivity and autoimmunity in which PMNs are involved. Fn monomer and fibrinogen (Fg) are multivalent ligands for a variety of cell receptors during cell adhesion. These cell receptors provide critical linkage between thrombosis, inflammation and cancer metastasis under venous flow conditions. However, the mechanisms of Fn-mediated interactions among immune cells and circulating tumor cells remain elusive. By using a cone-plate viscometer shear assay and dual-color flow cytometry, we demonstrated that soluble Fg and Fn had different abilities to enhance heterotypic aggregation between polymorphonuclear leukocytes (PMNs) and Lu1205 melanoma cells in a shear flow, regulated by thrombin levels. In addition, the involvement of integrin αvβ3, Intercellular Adhesion Molecule-1 (ICAM-1) and CD11b/CD18 (Mac-1) in fibrin(ogen)-mediated melanoma-PMN aggregations was explored. Kinetic studies provided evidences that ICAM-1 mediated initial capture of melanoma cells by PMNs, while αvβ3 played a role in sustained adhesion of the two cell types at a shear rate of 62.5 s-1. Quantitative analysis of the melanoma-PMN interactions conducted by a parallel-plate flow chamber assay further revealed that at a shear rate of 20 s-1, αvβ3 had enough contact time to form bonds with Mac-1 via Fn, which could not otherwise occur at a shear rate higher than 62.5 s-1. Our studies have captured a novel finding that leukocytes could be recruited to tumor cells via thrombin-mediated Fn formation within a tumor microenvironment, and αvβ3 and ICAM-1 may participate in multi-step fibrin(ogen)-mediated melanoma cell adhesion within the circulation.
Keywords: Plasma proteins, immuno-microenvironment, leukocyte activation, shear flow, heterotypic cell aggregations, cancer cells
Introduction
Melanoma cancer metastasis is a highly regulated process. Circulation-mediated metastasis requires lodging of cells to distinct sites within vasculature where melanoma cells extensively interact with extracellular environment including platelets, leukocytes and plasma proteins. In face of fluid shear forces, melanoma cells need to express shear-resistant receptors in order to adhere to endothelial cells of the vascular wall. Unlike leukocyte-endothelial cell binding, adhesion between melanoma cells and endothelial cells does not occur via direct receptor-ligand binding, since most of melanoma cells do not express β2 integrins or Sialyl Lewis X (sLex)3 at the levels capable of facilitating the binding to the endothelium (1). Previous studies have reported that platelets and PMNs could facilitate hematogeneous dissemination of melanoma cells by seeding them to the endothelial wall (1-3). However, it is still not well understood how plasma proteins, especially Fg or Fn expressed within a tumor microenvironment, may regulate tumor cell adhesion.
A clear link between hemostasis and tumor metastasis has been discovered by both in vivo and in vitro studies. For example, cancer patients were often shown to have abnormalities of blood coagulations, with elevated levels of Fg and fibrinopeptide A, which is a byproduct of Fn formation (2). Melanoma cells secrete tissue factors, which are the precursors of coagulation cascade leading to Fn production that promotes metastasis by mediating prolonged adhesion of melanoma cells to endothelial cells (4). Effects of Fn monomers on binding of platelets to melanoma cells under flow conditions were examined by several investigators (2, 3, 5). In these studies, Fn was shown to enhance platelet and melanoma cell aggregation by bridging integrin αIIbβ3 on platelets to either integrin αvβ3 (3) or ICAM-1 (2, 5) on melanoma cells. A similar mechanism was seen in Fg-enhanced leukocyte-endothelial cell adhesion through binding of Fg to ICAM-1 on endothelial cell (6). Importantly shown in an animal model of experimental metastasis compared with wild type controls, depletion of Fg reduced sustained adhesion of tumor cells, while treating tumor cells with soluble Fn enhanced lung seeding within the microcirculation (2, 7). Recently, platelet-carcinoma heterotypic aggregation in a suspension under shear conditions was also shown to be interfered by soluble Fn which bound to CD44 on carcinoma cells and diminished CD44-P-selectin interactions (8). Therefore, the enhancement of tumor metastasis by Fg and Fn may be viewed from heterotypic cell-cell adhesion influenced by both mechanical shear and kinetic binding mechanisms.
The fluid shear in circulation that facilitates cell-cell collisions can be translated to a tensile shear force in breaking cellular aggregates (9). Cell-cell adhesion is additionally affected by the intrinsic binding properties between receptors and ligands on respective cell types. Therefore, without a proper affinity between receptors and ligands, cell-cell adhesion cannot occur in an ordered fashion. Most melanoma cells have been shown to lack necessary selectins and integrins that are responsible for the tethering/retaining of tumor cells to the endothelial wall within the circulation. Instead, melanoma cells do express high levels of ICAM-1 that can bind to β2 integrins such as Lymphocyte Function-associated Antigen-1 (LFA-1; CD11a/CD18)) and Mac-1. Recent studies have shown that ICAM-1-expressing melanoma cell could effectively adhere to PMNs in a very cooperative and sequential process, consisting of LFA-1-mediated initial capture and Mac-1-dependent firm adhesion (10).
In the presence of Fg or Fn, adhesion of melanoma cells to PMNs via fibrin(ogen) might follow a similar process mediated by bridging ICAM-1 (on melanoma) to fibrin(ogen) to β2 integrins (on PMN), which is the main focus of this paper. Besides ICAM-1, melanoma cells express αvβ3, which can bind to Fg and Fn both under static and flow conditions (11-13). In melanoma cells, expression of αvβ3 is associated with metastatic phenotypes. Fg contains three potential αvβ3 binding sites at Aα95-98(RGDF), Aα572-575(RGDS) and γC400-411 (12, 13). Many cellular interactions with Fg and Fn occur via binding to one or two of these recognition sequences. Upon thrombin cleavage of fibrinopeptides A, Fg may exposure more cryptic RGD sites that mediate stronger αvβ3 binding. The major ICAM-1 recognition site is located in γ 117-133 of Fg (14). The leukocyte integrin Mac-1 is a high affinity receptor for Fg on stimulated monocytes and PMNs, which has been implicated in many inflammatory responses. Mac-1 interaction site is localized within Fg D domain at a site corresponding to the γ chain190–202 and 377-395 (15).
The binding kinetics of melanoma cells and PMNs under hydrodynamic conditions has been recently investigated in the absence of fibrin(ogen) by using a cone-plate viscometer assay (16). The cell collision frequency, duration of cell-cell contact and shear stresses acting on the cell could also be theoretically determined (9, 17). Since PMNs were shown to be involved in tumor cell extravasation and Fn levels were also shown an increase in cancer patients affected by plasma-contained thrombin (7, 18), we hypothesized in the present study that thrombin-regulated soluble Fn formation might mediate specific processes of melanoma-PMN binding by bridging the two cell types. In this paper, we have examined the binding of melanoma cells to Fn or Fg and its subsequent effects on melanoma-PMN adhesion. In addition, we have provided a quantitative comparison of relative roles in ICAM-1 and αvβ3-mediated melanoma-PMN interactions under various hydrodynamic shear conditions. We have found for the first time, to our best knowledge, that Fg and Fn-enhanced melanoma-PMN binding lies in a potential mechanism of ICAM-1 mediated-initial capture followed by αvβ3-enhanced firm retention of melanoma adhesion to PMNs via fibrin(ogen). This enhancement of PMN-melanoma binding resulted in more melanoma being brought into close proximity of EC, thereby representing a prelude of melanoma adhesion-led invasion and metastasis.
Materials and Methods
Antibodies and reagents
Mouse IgG anti-human monoclonal antibodies (mAbs) against αvβ3 (anti-CD51/61, clone 23C6) and ICAM-1 (clone BBIG-I1) were purchased from R&D systems (Minneapolis, MN). Mouse anti-human mAbs against LFA-1 (anti-CD11a) and Mac-1 (anti-CD11b) were purchased from Invitrogen (Carlsbad, CA). N-formyl-methionyl-leucyl-phenylalanine (fMLF), Fg (Fraction I, type I: from human plasma), GPRP (Gly-Pro-Arg-Pro amide), TRITC (Tetramethylrhodamine isothiocyanate Isomer R), chondroitinase AC II (Arthrobacter aurescens), and BSA (bovine serum albumin) were purchased from Sigma (St Louis, MO). Fibronectin was obtained from VWR. Human serum albumin (HSA) was purchased from Calbiochem (La Jolla, CA). Thrombin bovine (269,300 U/g) was purchased from MP Biomedicals (Solon, Ohio). LDS-751 was purchased from Invitrogen.
Cell culture and preparation
A375m, Lu1205 and WM35 (provided by Dr. Meenhard Herlyn, Wistar Institute, Philadelphia, PA) melanoma cells were grown in DMEM/F12 (Dulbecco’s Modified Eagle Medium Nutrient Mixture F12) and RPMI1640 (GIBCO; Carlsbad, CA), respectively, supplemented with 10% FBS (BioSource; Carlsbad, CA). Prior to each experiment, Lu1205 cells were detached with 0.05% trypsin/EDTA (Invitrogen) and washed twice with fresh medium. The cells were then suspended in fresh media and allowed to recover for 1h while being rocked at a rate of 8 rpm at 37 °C (In this way, ICAM-1 expression was not affected, while integrin αvβ3 could be regenerated after 1 hr’s recovering(Fig. 1 A and B)). In some receptor blocking experiments, Lu1205 cells were pre-treated with respective functional blocking antibody, e.g., anti-αvβ3 (5 μg/ml); anti-hICAM-1 (5 μg/ml) for 30 min at 37 °C, before onset of assays. In selective experiments, to prevent CD44 binding to Fn, specific glycosaminoglycans from CD44 were removed by pre-treating melanoma cells with 0.1 U/ml chondoitinase AC II (which catalyzed the cleavage of N-acetylhexosaminide linkage in chondroitin sulfate and has been shown to significantly suppress binding of CD44s expressing cells to Fn (19)) for 1 hr at 37 °C.
FIGURE 1.
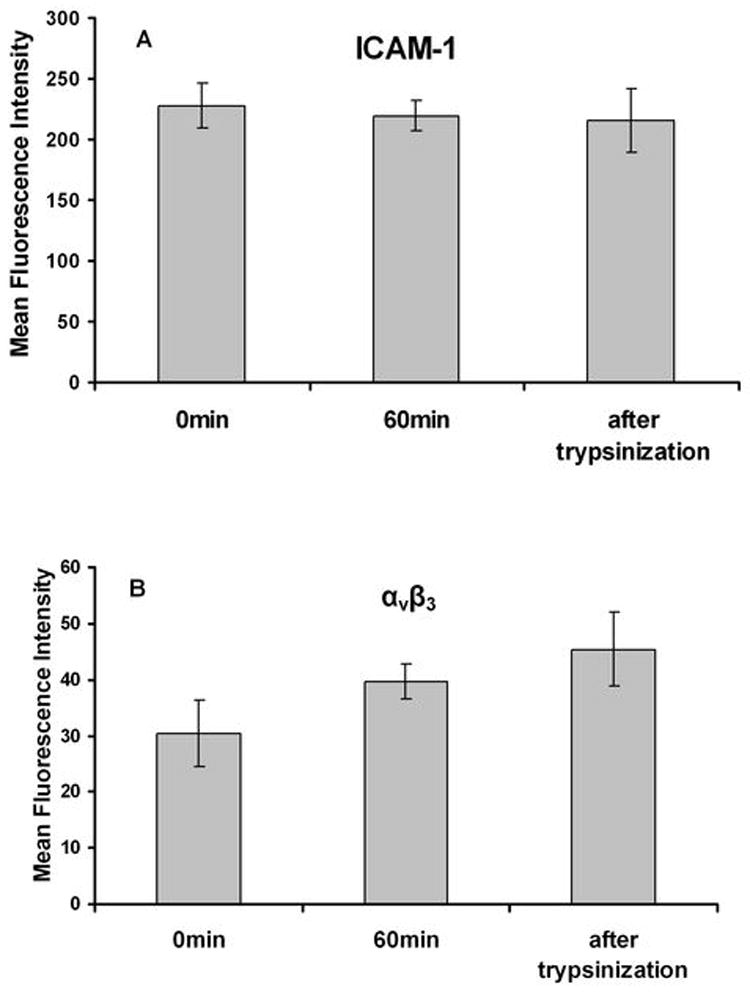
The effect of trypsinization on receptor expression. Lu1205 cells were detached from culture dishes by 0.05% trypsin-EDTA. Cells were incubated for 60 min in medium before being treated with trypsin for 5min. ICAM-1 (A) and αvβ3 (B) expressions on cells at 0 min and 60 min after being detached as well as 5 min after trypsinization were detected by flow cytometry. There were no significant statistical differences between groups.
PMN preparation was previously described (1). Following The Pennsylvania State University Institutional `Review Board (IRB)-approved protocols (no. 19311), we collected fresh human blood from healthy adults by venipuncture. PMNs were isolated using a Ficoll-Hypaque (Histopaque, Sigma) density gradient as described by the manufacturer and kept at 4 °C in Dulbecco’s phosphate-buffered saline (DPBS) containing 0.1% HSA for up to 4 hr before an experiment. In selected experiments, PMNs were functionally blocked with anti-LFA-1 (5 μg/ml) and anti-Mac-1 (5 μg/ml).
Fibroblast L-cells that had been transfected to express human E-selectin and ICAM-1 (EI cells; kindly provided by Dr. Scott Simon, UC Davis, CA) were maintained in culture as described elsewhere (1,20). EI cells express ICAM-1 at a level comparable with IL-1β stimulated human umbilical vein endothelial cells (HUVECs) (21) and used as a stable ICAM-1-expressing endothelial monolayer for some of the cell adhesion studies.
Preparation of soluble fibrin solution
Soluble Fn was made freshly in ion-free DPBS prior to each experiment. To produce soluble Fn monomers and prevent coagulation, thrombin cleavage of Fg was initiated in the presence of 4 mM GPRP-NH2 following a published protocol (19, 22). In order to probe the kinetics of Fn production, thrombin concentrations were varied, while keeping a chosen dose from soluble Fg and GPRP. For example, to make 1 ml of Fn solution, 120 μl Fg (25 mg/ml) and 168 μl of GPRP (24 mM) were mixed with either 0 μl, 2.7 μl, 5.5 μl or 207 μl thrombin (10 U/ml, 269,300 U/g) immediately before incubation at 37 °C for 10 min. Then, a two-fold concentrated Fn stock solution was subsequently mixed with cell suspension (containing tumor cells and/or PMNs) 1 sec before experiments at 1:1 ratio to reach a desired Fg, thrombin, GPRP and cell concentrations.
SDS-PAGE for fibrinogen digested products
To characterize thrombin catalyzed digestion of Fg (3mg/ml), the reactions were run at room temperature and terminated at selected intervals by adding 2 × sodium dodecyl sulfate (SDS) running buffer (0.2% bromophenol blue, 4% SDS, 100mM Tris [pH 6.8], 20% glycerol) and 2-mercaptoethanol. The samples were analyzed by SDS-PAGE on 12% gels. Control was prepared by adding SDS and 2-mercaptoethanol to Fg before thrombin and GPRP. Gels were stained with Coomassie Blue and imaged accordingly.
Small interfering RNA (siRNA) targeting ICAM-1 and Integrin αvβ3
100 pmol of duplexed Stealth siRNA (Invitrogen, Carlsbad,CA) was introduced into 1.0×106 1205Lu via nucleofection using an Amaxa Nucleofector using Solution R/program K-17. Transfection efficiency was listed in Table 2 with 80%-90% cell viability. Following siRNA introduction, cells were replated in culture dishes. siRNA sequences (5’ to 3’) were: Scrambled: AAUUCUCCGAACGUGUCACGUGAGA; ICAM-1: UUAUAGAGGUACGUGCUGAGGCCUG; Integrin αv:UUGAUGAGCUCAUAGACAUGGUGGA; Integrin β3: AUAAGCAUCAACAAUGASGCUGGAGG.
Table 2.
| Geometric Mean Fluorescence | ||
|---|---|---|
| Treatment | αvβ3 | ICAM-1 |
| Buffer | 13 | 30.51 |
| Scramble | 12.14 | 40.32 |
| siRNA | 3.11 | 15 |
| KO efficiency* | 77% | 62% |
Knockout efficiency is defined as the ratio of siRNA fluorescence intensity to Scramble fluorescence intensity.
Binding of tumor cells or PMNs to immobilized fibrin or fibrinogen
Immobilized Fg surfaces were generated by absorbing Fg (vWf-, plasminogen-, and fibronectin-free) solution at 2.5 mg/ml in DPBS containing 1.5 mM Ca2+ onto the substrate of a 35-mm polystyrene petri dish (BD, Franklin Lakes, NJ) over night at 4 °C. Similarly, immobilized Fn surfaces were generated by incubating above-mentioned Fg solution with thrombin (2 U/ml) for 2 hr at 37 °C (8). The non-specific binding sites were further blocked by incubating Fg or Fn-coated surfaces with DPBS-1% BSA for 1 hr at room temperature.
To assess circulating cells binding to immobilized fibrin(ogen), a parallel-plate flow assay was adapted following previously published protocols (1), where suspension of either tumor cells or PMNs (5×105 /ml) in DMEM with 0.1% BSA was perfused over the Fn (or Fg)-coated surface for 5 min. Real-time flow digital videos were recorded under a 10× objective (0.48 mm2) to a PC memory by Streampix III (Streampix III, Norpix Inc., Montreal, Canada). The ability of cell binding to immobilized fibrin(ogen) surfaces was quantified by the total number of firmly adhering cells (e.g., adhesion >30 sec) during 5 min-period perfusion under each condition.
Parallel-plate flow assay
Use of a parallel-plate flow assay allowed for direct observation of interactions between PMNs and melanoma cells and determination of kinetic parameters regulating tumor-PMN binding under flow conditions (23). In the present study, PMN binding to immobilized tumor cells was first assayed in order to characterize whether β2 integrins on PMNs bind to αvβ3 or ICAM-1 expressing melanoma cells in the presence or absence of soluble fibrin(ogen). The dishes seeded with melanoma cell monolayer served as a substrate in a parallel-plate flow chamber. The field of view was 800 μm long and 600 μm wide. DMEM alone was first perfused into the chamber to allow the melanoma-cell monolayer to reach equilibrium. fMLF-stimulated PMNs were then allowed to presettle on the monolayer under a low shear rate of 10 s-1 for 2 min before an onset of prescribed experimental shear rates (62.5 s-1 or 20 s-1).
To simulate physiological conditions and assess melanoma adhesion to endothelial cell assisted by PMN, suspension of fMLF-stimulated PMNs and melanoma cells at a ratio of 1:1 in the presence of Fn was perfused over EI monolayer. To evaluate the capacity of PMNs to tether melanoma cells to EC, melanoma adhesion efficiency was employed to quantify melanoma adhesion to EI monolayer as a result of the collision with pre-tethered PMNs. If more than one melanoma cell adhered to a PMN, we counted it as multiple aggregates (e.g. 2 aggregates if 2 melanoma cells adhered to 1 PMN). “Melanoma adhesion efficiency” was expressed by the following ratio:
The adhesion efficiency was then classified based on the durations of melanoma arrests (>1 sec, >3 sec and >5 sec).
Cone-plate viscometer assay
To study binding of melanoma cells to PMNs both in suspension under shear conditions, the flow assay was adapted using a cone-plate viscometer (RotoVisco 1; Haake, Hewington, NH) as previously described (16, 17, 24). This viscometer consists of a stationary plate and a 1°-free rotating cone, capable of generating linear velocity fields with a constant gradient (i.e., shear rate). So when cell suspension was subjected to a shear field within the instrument, the fast moving cells near the rotating cone collide with slowly moving cells near the plate (17). Cell-cell collision generated one bond initially. Once one bond formed, more bonds might form or existing bonds might dissociate when under shear for longer time. The tensile force determined the dissociation rate of a doublet. To later quantify heterotypic cell aggregation, isolated PMNs and melanoma cells were stained with LDS-751 (Red) and TRITC (Orange) respectively for 10 min at 37 °C. Thereafter, PMNs were mixed with melanoma cells in DPBS containing Ca2+/Mg2+ in the presence of 1 μM fMLF to reach a concentration of 5×105 cells/ml for each cell type (1:1). The cell suspension was allowed to equilibrate at 37 °C for 2 min. Fibin(ogen) was added, respectively, into the cell suspension 1 sec before initiation of shear. Shear rates were varied from 62.5 s-1 to 200 s-1 for preset duration of time, ranging from 30 sec to 300 sec. After shear applications, samples were fixed immediately with 1% formaldehyde.
Quantification of heterotypic cell aggregation
An ability of TRITC labeled melanoma cells to form aggregates with LDS-751 labeled PMNs was determined by using the dual-color flow cytometry based on the cellular compositions, which were characterized by cells’ forward scatters, side scatters and fluorescence profiles (Fig. 2) (16). Since the two cell types were labeled with different dyes, their aggregates would appear in the coordinates which are integral multiples of singlet fluorescence values. 5000 events were collected for each case. By applying this method, heterotypic aggregates comprised of single tumor cell (T) with either one PMN (TP1), two PMNs (TP2), or more than three PMNs (TP3+) could be characterized. The extent of heterotypic aggregation was defined as the percentage of bound tumor cells to PMNs in total amount of cells:
FIGURE 2.
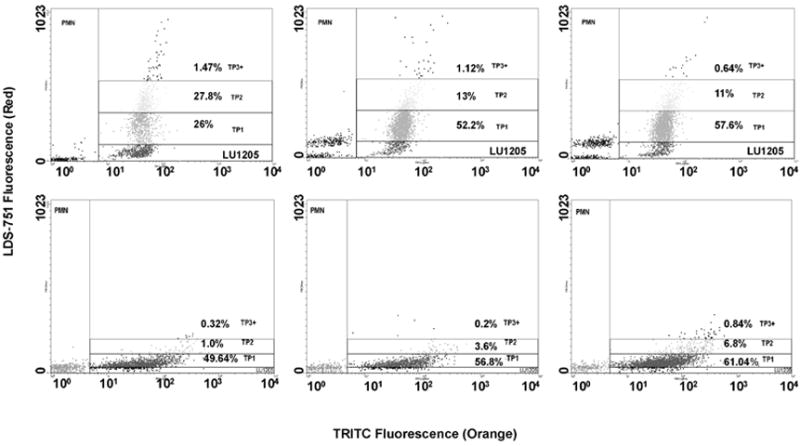
Flow cytometric detection of time-dependent size growth of PMN-melanoma aggregates in the presence of Fn solution made from 0.053 U/ml thrombin, 1.5 mg/ml Fg and 4 mM GPRP. Top three graphs: The TRITC-labeled Lu1205 population was resolved into singlet and aggregates composed of a single Lu1205 cell bound to one PMN (TP1), two PMNs (TP2) or more than three PMNs (TP3+) after 30 sec (left), 180 sec (middle) and 300 sec (right) of shearing at shear rate of 62.5 s-1. Bottom three graphs: Lu1205 cells were incubated with 1 μg/ml anti-ICAM-1 mAb for 1 hr before being labeled with TRITC-linked anti-mouse IgG. Similar to the top panel, these Lu1205 cells were subjected to shear with LDS751-labeled PMNs in the presence of Fn solution for 30 sec (left), 180 sec (middle) and 300 sec (right) at a shear rate of 62.5 s-1. Lu1205 singlet populations (top panel) over the time course were not those Lu1205 cells with low ICAM-1 expression; otherwise the singlet would have clustered to the left (bottom panel). They had the potential to bind to PMNs.
Statistical analysis
Data were obtained from at least three independent experiments and expressed by the means. Statistical significance of difference between means was determined by using Student’s t-test or ANOVA. Turkey’s test was used for post hoc analysis for ANOVA. Probability values of P<0.05 were chosen as statistical significance.
Results
Arrest of melanoma cells and PMNs on immobilized fibrin(ogen) under dynamic flow conditions
Melanoma cells express ICAM-1 and αvβ3, the receptors for fibrin(ogen), whose levels correlate with their respective metastatic potentials (Table 1) (11, 25). To analyze how melanoma cells or PMNs bind to fibrin(ogen) ligands under different flow conditions, a parallel-plate flow system was used where immobilized ligands were present on the substrate of the bottom plate. Perfusion of cell suspension over an immobilized Fn or Fg-coated surface avoided some confounding factors and analysis for cell-cell and cell-soluble protein interactions in a 3-dimensional flow field. When suspended in a binding buffer (DMEM + 0.1%BSA + HEPES), Lu1205 melanoma cells could bind to both immobilized Fn and Fg avidly at both low (62.5 s-1) and high (200 s-1) shear rates (Fig. 3A), although significantly more strongly to Fn than to Fg under low shear condition. Under both shear conditions tested, Lu1205 melanoma cells directly arrested on immobilized Fn or Fg surface without an apparent rolling step. To show the specificity of interactions, Lu1205 cells were also perfused over BSA-coated surface (as a control in Fig. 3A), which resulted in minimal surface-bound arrests, suggesting melanoma cell binding to fibrin(ogen) shown in Fig. 3A was protein specific.
Table 1.
Flow cytometry analysis of ICAM-1 and αvβ3 expressions on WM35, A375m and Lu1205 cellsa
| Receptor | Geometric Mean Fluorescence |
||
|---|---|---|---|
| WM35 (Low metastatic) | A375m (median metastatic ) | Lu1205 (highly metastatic) | |
| Control IgG | 3.75 ± 0.03 | 3.75 ± 0.03 | 3.25 ± 0.49 |
| ICAM-1 | 33.76± 0.25 | 106.76± 10.2 | 165.5 ± 12.8 |
| αvβ3 | 26.22 ± 0.45 | 16.58± 0.36 | 45.5 ± 1.16 |
values are geometric mean fluorescence intensities ±SEM (n > 3).
FIGURE 3.
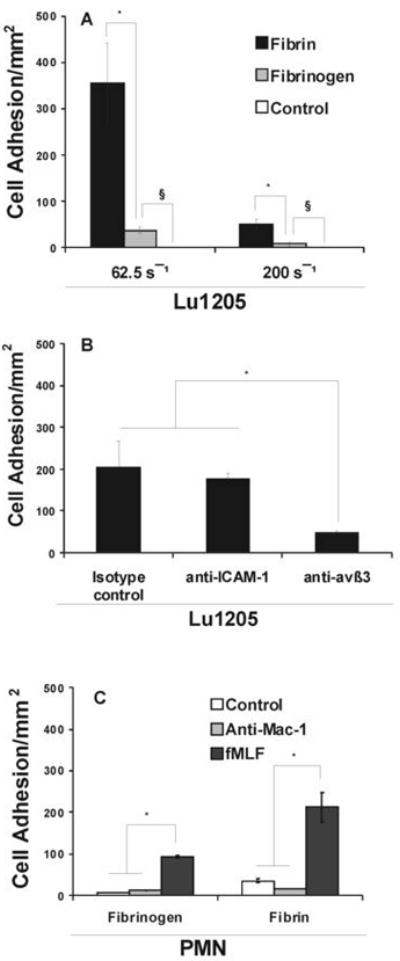
Characterization of Lu1205 and PMN surface receptors for binding to fibrin(ogen) under hydrodynamic shear conditions. (A) Flow differentially affects the arrest of Lu1205 to immobilized Fg and Fn. Lu1205 cells were perfused over immobilized Fn or Fg in a parallel-plate flow chamber. The numbers of firm adhering cells (>30 sec) were quantified. Data represent means ±SEM (n>3). * P<0.05 compared with respective Fg cases under different shear rates. § P<0.05 compared with control cases. Control cases quantified the numbers of Lu1205 adhesion to coated BSA (There were 0 cells adhering to BSA per mm2). (B) Lu1205 cell adhesion to Fn requires αvβ3 and ICAM-1. Data represent means ±SEM (n>3). * P<0.05 compared with the isotype control case. (C) Mac-1 mediates binding of PMNs to fibrin(ogen) under flow. Data represent means ±SEM (n>3). * P<0.05.
To further illustrate cell surface receptors are responsible for binding, Lu1205 were perfused over immobilized Fn at shear rate of 62.5 s-1 in the presence of functional blocking mAbs, respectively against ICAM-1, αvβ3 or IgG isotype control (5 μg/1×106 cells). As shown in Fig. 3B, the ability of Lu1205 cells to arrest on Fn under flow was only marginally affected by ICAM-1 blocking antibody, but much more effectively inhibited by anti-αvβ3 mAb, which demonstrates that αvβ3 could be a more potent receptor than ICAM-1 on melanoma cells to support tumor cell adhesion to Fn and to make shear-resistant bonds.
PMNs were indicated to be capable of binding to either Fg or Fn-coated surface under flow conditions (26, 27). To examine the effect of hydrodynamic shear force and chemoattractant stimulation on PMN binding to fibrin(ogen), non-stimulated or fMLF-stimulated (1 μM; 2 min) PMNs were perfused over Fg or Fn coated surface in a parallel-plate flow chamber at a shear rate of 62.5 s-1. As shown in Fig. 3C, non-stimulated PMNs had a higher affinity for Fn than for Fg during the 5 min perfusion time, while they failed to bind to the BSA-coated surface (as a control; data not shown). In comparison, fMLF, a potent activator for the high-affinity state of β2-integrin, significantly enhanced PMN adhesion to both Fg and Fn (Fig. 3C). Like non-stimulated PMNs, activated PMNs bound to Fn with a higher affinity (220 PMN/mm2) than to Fg (90 PMN/mm2). Functional blocking Mac-1 antibody (5 μg/1×106 cells; for 30 min after being stimulated by fMLF) inhibited PMN binding to fibrin(ogen), which was in an agreement with previous studies (26). Taken together, results from Fig. 3C suggest that PMN adhesion to fibrin(ogen) is Mac-1- dependent.
Tumor cells binding to PMNs is affected by thrombin-mediated Fn formation
Using two-color flow cytometric assays, the heterotypic aggregates between TRITC-labeled tumor cells and LDS-751-labeled PMNs were quantified by their respectively-gated characteristic fluorescence intensities. Some representative fluorescence profiles are shown in Fig. 2. It should be noticed that the melanoma singlet during the shear period represents those with normal expression of ICAM-1. They continue to be recruited to and dissociate from PMNs.
Binding rate of soluble proteins in solution to cell receptors follows an exponential-decay function of protein concentration (28). To approximately find the concentration-dependent Fg binding to melanoma cell and/or PMN receptors, the levels of melanoma-PMN aggregations mediated by soluble Fg with three arbitrary concentrations of 0.5, 1.5 and 3 mg/ml were compared (Fig. 4A). 1.5 mg/ml Fg facilitated significantly higher percentage of Lu1205-PMN heterotypic aggregation than 0.5 mg/ml Fg at 60 sec. However, 3 mg/ml Fg did not further increase the extent of Lu1205-PMN aggregation compared with 1.5 mg/ml Fg concentration, suggesting a possible Fg saturation in mediating receptor-protein binding. Therefore, 1.5 mg/ml concentration was chosen for probing Fg-mediated binding kinetics for the current study.
FIGURE 4.
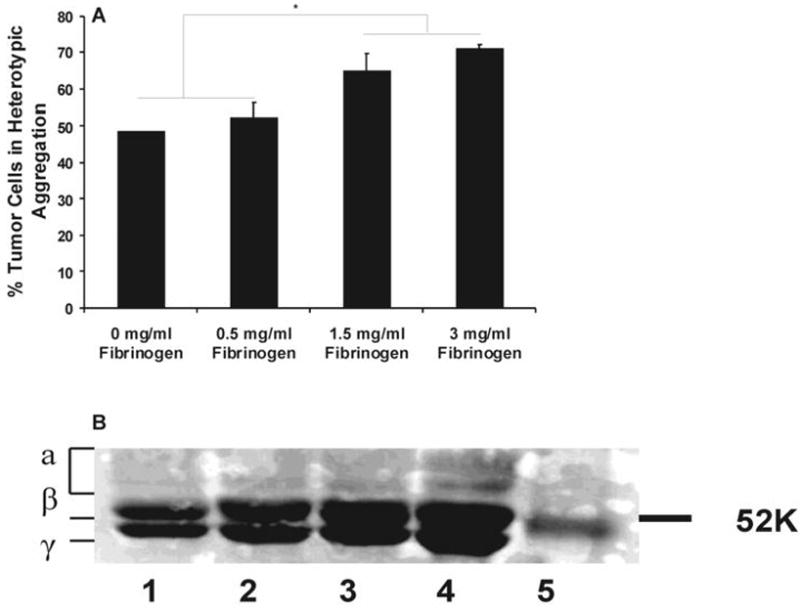
(A) Effect of Fg concentration on PMN-Lu1205 binding. Lu1205 cells were subject to shear with PMNs in the presence of different concentrations of Fg in a cone-plate viscometer at a shear rate of 62.5 s-1. Cell suspensions were fixed with formaldehyde at 60 sec after onset of the experiments. Data represent means ±SEM (n>3). * P<0.05. (B) SDS-PAGE for fibrinogen digested products. Fg was cleaved by thrombin at different levels (0.05 U/ml (Lane 3) and 2 U/ml (Lane 4)) for 10 min. The reaction products were subjected to 12% polyacrylamide gel separation. Separated proteins were stained by Coomassie blue. Lane 1 is fibrinogen, Lane 2 is control and Lane 5 is marker.
To understand potential mechanisms of Fn-mediated melanoma-PMN aggregation, Fn monomers were produced by reacting Fg, thrombin and 4 mM GPRP. Conversion of Fg into Fn is initiated by thrombin-mediated cleavage in two NH2-terminal fibrinopeptides A and B (29). However, the concentrations of thrombin and the reaction time would affect the kinetics of fibrinopeptide and Fn generation and the amounts of generated Fn would have an impact on cell-cell interactions. To examine Fg digested products, the components of Fg-thrombin reaction products were separated by SDS-PAGE to analyze the purity of Fn and mechanisms of thrombin catalysis. 3 mg/ml Fn was cleaved by either 2 U/ml or 0.05 U/ml thrombin in the presence of GPRP for 10min. The reactions were stopped by adding SDS and 2-mercaptoendothenol. Proteins were resolved in 12% SDS-PAGE and stained with Coomassie Blue (Fig. 4B). Fg α, β and γ chains were present on the gel as separate bands and their molecular weights matched well with proposed data (29). After thrombin cleavage, smaller molecular weight α and β chains were generated. In addition, the longer the time and the more thrombin, the more digested products were produced. Thrombin with a concentration of 2 U/ml could cleave all α and β chains within 10 min.
To understand the kinetics of melanoma-PMN aggregation, the percentage of melanoma cells recruited by PMNs was plotted as a function of time. In the absence of soluble Fg (0 mg/ml), the number of aggregates increased with time and maximized at 60 sec, and then rapidly decreased (Fig. 5A). This behavior was similar to WM9 melanoma-PMN binding found previously (16), which might be partially due to a rapid decrease in the cell concentration with time leading to a decrease in cell collision frequency, or due to time-dependent reduction of cell receptor affinity (30).
FIGURE 5.
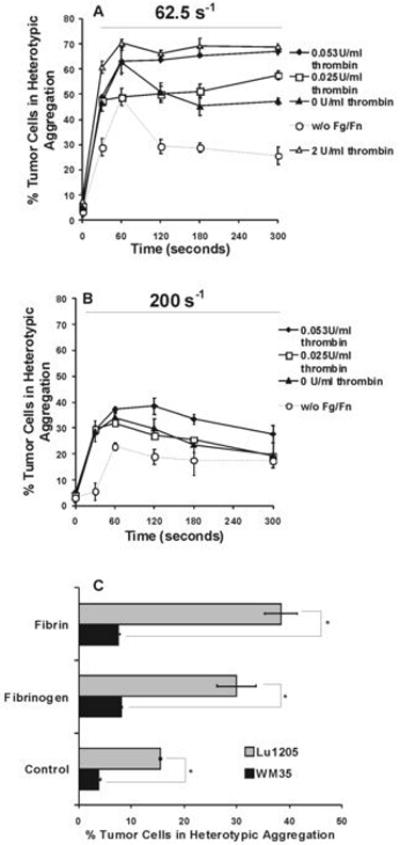
Shear induced adhesion of PMNs to highly-metastatic (Lu1205) and low-metastatic (WM35) melanoma cell lines in the presence of Fg or Fn. Fn solution was made from 1.5 mg/ml Fg and 4 mM GPRP with either 2 U/ml, 0.053 U/ml, 0.025 U/ml, or 0 U/ml thrombin. Control is PMN and melanoma cell binding in the absence of Fg or Fn. Percentage of Lu1205 cells bound to PMNs at shear rates of 62.5 s-1 (A), and 200 s-1 (B) are plotted as a function of time. (C) Comparison of percentages of Lu1205 and WM35 bound to PMNs after 120 sec of shearing at a shear rate of 200 s-1. * P<0.05. Values are means ± SEM (n>3).
Addition of Fg significantly elevated the percentage of heterotypic aggregation at shear rate of 62.5 s-1 (Fig. 5A). The percentage of Lu1205 in aggregation still peaked at 60 sec and descended thereafter, similar to the case in the absence of Fg. Initial capture and subsequent stable adhesion between the two-cell types increased proportionally in the presence of Fg (Fig. 5A) compared with cases without Fg. This implies that soluble Fg largely increased effective forward rate for receptor-ligand binding, while being capable of maintaining the tumor cell-PMN aggregation.
Adding Fn solution (made from 0.025 U/ml thrombin and 1.5 mg/ml Fg) altered the biphasic trend of pure Fg-mediated tumor cell-PMN heterotypic aggregation at 62.5 s-1 (Fig. 5A). The presence of Fn allowed the binding to reach a steady state after the 60 sec, increasing the percentage of aggregates from 48% to 57% which sustained more than 5 min. In comparison, Fn solution made by 0.053 U/ml thrombin boosted the initial melanoma-PMN aggregation to the same level (63%) of the Fg case over a period of 60 sec, followed by a plateau without an apparent dissociation over at least 5 min. However, catalyzing Fg-Fn transformation using 0.025 U/ml or 0.053 U/ml thrombin might result in only a partial conversion, which could make an analysis of Fn-mediated binding mechanism more difficult. Therefore, we also used 2 U/ml thrombin to make apparently more completed Fn solution (8). Indeed, results showed that Fn solution made from 2 U/ml thrombin changed initial capture rate of Lu1205 cells, compared with that made from 0.053 U/ml thrombin. 70% Lu1205 cells were recruited to aggregates within 60 sec. However, the level of prolonged aggregation was not changed. Collectively, these results suggest that thrombin concentration in a tumor microenvironment could be very important and conversion of Fg to Fn exposed some cryptic recognition sites that may stabilize the formed aggregates or other forms of coagulation.
Percentage of aggregation decreased with an increase in shear rates (Fig. 5B). For example, during the first 60 sec, 1000 cell-cell collisions resulted in 38 captures at a low shear rate of 62.5 s-1, while 1000 collisions only resulting in 8 captures at a higher shear rate of 200 s-1. Fg was able to upregulate the extent of adhesion of PMNs to Lu1205 melanoma cells under high shear of 200 s-1 to be comparable to that when Fg was absent under low shear (62.5 s-1) (Fig. 5A). According to the two-body collision theory, increasing shear rate may reduce capture efficiency due to an increase in fluid drag and tensile force in quickly rupturing transient bonds formation (9). Alternatively, increasing shear rate may also decrease the cell-cell encounter duration that limits 20 the bond formation. Despite of this transient feature of cell-cell aggregates at the shear rate of 200 s-1, Fn was still able to slightly prolong the lifetime of aggregates. Fn solution made by 0.053 U/ml thrombin concentration shifted the peak binding from 60 sec to 120 sec at a higher shear rate of 200 s-1. In general, Fn stabilized the transient process in tumor cell-PMN aggregation, given that it profoundly changed the time-dependent profile of fibrin(ogen)-mediated cell aggregation.
Compared with Lu1205 cells, WM35 cells, a low-metastatic melanoma cell line expressing lower amounts of receptors for fibrin(ogen) (Table 1), failed to adhere efficiently to PMNs even in the presence of Fn at 200 s-1 (Fig. 5C), which correlated melanoma-PMN binding abilities with tumor metastatic potentials. Taken together, results from Fig. 5A-C suggested that the recruitment of PMNs to melanoma cells was dependent on thrombin-mediated Fn production in a tumor microenvironment with respect to tumor metastatic phenotypes and circulatory shear rates.
Relative roles of αvβ3, ICAM-1 and Mac-1 in fibrin(ogen)-mediated binding
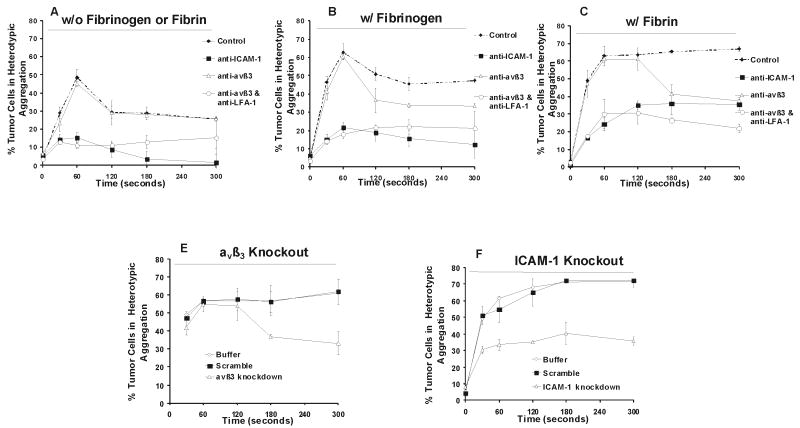
Lu1205 cells were incubated with a panel of αvβ3 functional blocking antibodies before being subjected to shear experiments in the cone-plate viscometer to determine the roles of αvβ3 in heterotypic tumor cell-PMN aggregation. αvβ3 blocking did not affect the rate of Lu1205 binding to PMNs in the absence of fibrin(ogen) (Fig. 6A) , since αvβ3 could not bind to β2 integrins on PMNs directly. Blocking αvβ3 did not affect short term binding between PMNs and Lu1205 melanoma cells in the presence of Fg (Fig. 6B). 50% of Lu1205 cells were still able to bind to PMNs during the first 60 sec, which could be due to the tethering via Fg between some un-identified receptors on the tumor cell and β2 integrins on PMNs, while the transient tethering quickly dissociated after 60 sec, implying that Fg-dependent tethering did not sustain for longer time period during melanoma cell-PMN adhesion (Fig. 6B). Similarly, blocking αvβ3 on melanoma cells had a marginal effect on initial Fn-mediated capture of Lu1205 cells to PMNs, whereas the percentage of aggregates was significantly reduced compared with non-blocking cases after 120 sec (Fig. 6C). Therefore, interaction of αvβ3 with Fn-bound Mac-1 sustained Lu1205-PMN binding. Interestingly, Fn apparently prolonged the duration of αvβ3-independent transient tethering from 60 sec compared with the Fg cases up to 120 sec (Fig. 6A, C).
FIGURE 6.
Relative contribution of αvβ3 and ICAM-1 to time-dependent adhesion of Lu1205 cells to PMNs in the absence of fibrin(ogen) (A), presence of fibrinogen (B), or fibrin (C-E) under 62.5 s-1 shear rate. Lu1205 cells were pre-treated with anti-ICAM-1, αvβ3 and/or CD11a mAb (A-C) or siRNA targeting ICAM-1 or αvβ3 (D-E) before being sheared with PMNs. Fibrin was made from 0.053 U/ml thrombin, 1.5 mg/ml Fg and 4 mM GPRP; Fibrinogen stands for 1.5 mg/ml fibrinogen solution. Values are means ± SEM from three independent experiments.
If αvβ3 mediates the sustained adhesion, a question remains as what receptors on melanoma cells would play roles in tethering tumor cells to PMNs in the presence of soluble fibrin(ogen) under hydrodynamic shear. Fn γ chain contains the ICAM-1 binding sites with a lower affinity than that of RGD sites for integrins (14). Therefore, we rationalized that ICAM-1 and Fn interactions might have a high on-rate and off-rate in bridging melanoma cells to PMNs, which would reduce the traveling speed of melanoma cells and allow a longer contact duration between the two cell types. To investigate the role of ICAM-1 on Fn-mediated melanoma cell and PMN binding, ICAM-1 was blocked by treating Lu1205 cells with functional blocking antibodies anti-ICAM-1 before the shear experiments. Fig. 6A shows that blocking ICAM-1 eliminated native tumor cell-PMN aggregation, given that ICAM-1 was the predominant receptor on Lu1205 melanoma cells that facilitated PMN-melanoma binding (16). The 10% aggregates at 60 sec might represent non-specific binding. Addition of Fg increased the extent of ICAM-1-blocked Lu1205 in heterotypic binding to PMNs by 15% and these aggregates lasted more than 5 min under 62.5 s-1 shear condition, suggesting a strong ability of αvβ3 to bind to Fg (Fig. 6B). Conversion of Fg to Fn further modified the αvβ3-dependent binding kinetics (Fig. 6C). Binding of αvβ3 to Fn-bound Mac-1 slowly increased with time and reached a plateau at 120 sec when 30% tumor cells were recruited to aggregates. These aggregates could sustain 5 min under shear without apparent disaggregation. However, blocking ICAM-1 reduced the rate of initial tethering of PMNs to Lu1205 cells in the presence of Fn by 60% (Fig. 6C, control vs. anti-ICAM-1 case). This implies that the initial tethering (first 60 sec binding after onset of shear) of Lu1205 cells to PMNs was due to ICAM-1 in terms of the high on-rate of ICAM-1-Fn binding.
LFA-1 has been shown to support the initial formation of melanoma-PMN aggregates (1). To distinguish LFA-1/ICAM-1-mediated initial tethering from potential Mac-1/Fn/ICAM-1-mediated events, we subsequently blocked LFA-1 on PMNs in addition to αvβ3 blocking on Lu1205 cells before the shear experiments. It can be seen from Fig. 6A that blocking LFA-1 flattens the biphasic trend of Lu1205-PMN binding behavior. Although Fg slightly enhanced Mac-1-dependent binding (Fig. 6B) in a later time, Fn dramatically enhanced the initial binding (Fig. 6C), indicating that Fn added extra binding sites in tethering PMNs to Lu1205 cells. Taken together, LFA-1 cooperated with Fn-bound Mac-1 to enhance initial capture of tumor cells to PMNs in the presence of Fn.
Since there might be a potential concern about the Fc effect for antibody blocking, cells were subsequently transfected by siRNA to silence fibrin(ogen)-related receptors on Lu1205 cells, αvβ3 and ICAM-1 (knockout efficiencies were shown in Table 2 ), before being subjected to shear experiments. As shown in Fig. 6D, in the presence of Fn, αvβ3-knockout Lu1205 cells remained being associated with PMNs for 120 sec, and ICAM-1 supported Lu1205 aggregation with PMNs up to a peak level. However, after 120 sec, disaggregation proceeded more rapidly than scramble and buffer cases. In contrast, rate of ICAM-1-knockout Lu1205 capture to PMNs was significantly smaller than that of scramble and buffer cases, and αvβ3- mediated cell aggregation preceded very slowly and reached only 50% of the scramble and buffer cases at 5 min (Fig. 6E). These receptor knockdown results verified antibody blocking results, showing the relative roles of ICAM-1 and αvβ3 in recruitment of Lu1205 cells to PMNs.
Mac-1 is involved in fibrin binding, while LFA-1 and CD44 are not
CD44 on carcinoma cells had an ability to bind to soluble Fn under flow conditions (8). Lu1205 melanoma cells express standard form of CD44 (CD44s; data not shown). Biochemical studies revealed that CD44s-fibrin(ogen) interaction was dependent on N-linked glycans which had a high affinity for Fn-β chain, which was distinct from Mac-1, ICAM-1 and αvβ3 recognition sites on Fn (19, 31). To test whether other receptors, including CD44s, contribute to Fn-mediated PMN-Lu1205 heterotypic aggregation, ICAM-1 and αvβ3 on Lu1205 cells were simultaneously blocked before shear experiments. Fig. 7A shows during 5min’s shear, αvβ3 supported 44% adhesion, ICAM-1 mediated 38% adhesion, while receptors other than these two supported 17% adhesion, indicating CD44s might not play as much roles as αvβ3 and ICAM-1 in Fn-mediated PMN-Lu1205 melanoma cell heterotypic aggregation.
FIGURE 7.
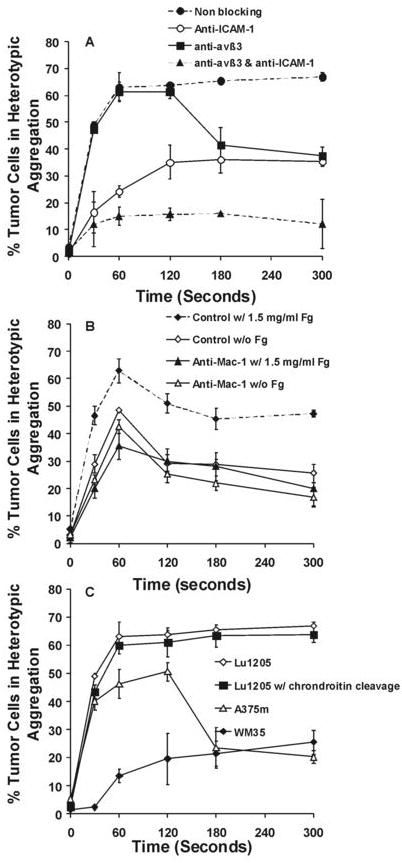
Receptors on Lu1205 cells other than ICAM-1 and αvβ3 played marginal roles in Lu1205 and PMN heterotypic aggregation. (A) The effect of ICAM-1 and CD51/61 blocking on Lu1205 and PMN heterotypic aggregation in the presence of Fn. Fibrin solution was made from 0.053 U/ml thrombin, 1.5 mg/ml Fg and 4 mM GPRP. Values are means ± SEM from three independent experiments. (B) The effect of Mac-1 blocking on Lu1205 and PMN heterotypic aggregation in the presence or absence of Fg. Values are means ± SEM (n>3). (C) The abilities of melanoma cells with different metastatic potentials and different receptor expressions to bind to PMNs in the presence of Fn. Lu1205 expresses high levels of ICAM-1 and αvβ3, A375m expresses median levels of ICAM-1 and low levels of αvβ3, and WM35 expresses median levels of αvβ3 and low levels of ICAM-1. Cells were pretreated with 1 U/ml chondroitinase AC II for 60 min at 37 °C to remove terminal chondroitin sulfate and dermatan sulfate residues (on CD44). Values are means ± SEM (n>3).
Pre-treatment of PMNs with monoclonal anti-CD11b antibody inhibited the rate of their adhesion to Lu1205 cells via Fg by 50%, while the same antibody did not significantly affect the rate of their adhesion without Fg and slightly reduced longer term binding by 35% (Fig. 7B). This implied that Mac-1 participated in Fg and Fn-mediated initial tether of PMNs to Lu1205, but only involved in sustained phase of native binding between PMN and Lu1205. Interestingly, at 60 sec, when Mac-1 was blocked, Fg seemed to inhibit LFA-1 binding (anti-Mac-1 w/ 1.5mg/ml Fg vs. anti-Mac-1 w/o Fg case). This may result from the occupancy of free binding sites on ICAM-1 by Fg.
To further demonstrate the roles of ICAM-1 and αvβ3 in Fn binding, while rule out the role of CD44, adhesive phenotypes among several melanoma cells with different metastatic potential were compared. This included Lu1205 cells expressing ICAM-1 and αvβ3, A375 cells expressing high ICAM-1 (low αvβ3) and WM35 cells expressing high αvβ3 (low ICAM-1) (Table 1). Before shear experiments, these cells were pre-treated with chondoitinase AC II which has been suggested to cleave binding motifs, like chondoitin sulfate and dermatan sulfate, on CD44 responsible for binding to Fn (19). Consistent with antibody blocking and siRNA knockout assays, the kinetics of WM35 binding mainly reflected that of αvβ3, whereas A375m binding was primarily contributed by ICAM-1 and reiterated the kinetics of ICAM-1 binding (Fig. 7C). Their adhesive phenotypes did not seem to be affected by enzymatic treatment targeting CD44.
Fibrin(ogen) alters the mechanics of receptor-mediated melanoma cell adhesion to PMN and PMN-facilitated melanoma adhesion to EC
To further elucidate the relative roles of αvβ3, ICAM-1 and Mac-1 in fibrin(ogen)-mediated binding, direct video-microscopy observation of PMN-Lu1205 adhesion was obtained using a parallel-plate flow chamber assay where a confluent Lu1205 cell monolayer served as a substrate of the flow chamber and PMNs were perfused over Lu1205 cells under two shear rates of 62.5 s-1 and 20 s-1, respectively. The kinetics of PMN binding to immobilized Lu1205 cells assayed by a parallel-plate flow chamber was not identical to the binding kinetics characterized in cone-plate viscometer shear assays where both PMNs and tumor cells were in suspension. This is because cone-plate assay data reflected a statistical snapshot of the aggregate formation at a chosen time point while a parallel-plate assay provided a direct quantification in a real-time individual PMN-Lu1205 aggregation formation. For example, Fig. 8A clearly showed that Fg only enhanced the longer-period binding (i.e. sustained adhesion) between PMNs and Lu1205 melanoma cells (statistically significant for length of stop >3 sec), while Fn significantly enhanced both shorter (i.e., initial tethered adhesion) and longer-period binding, which corroborated what we found in Fig. 8A when both cell types were in suspension under shear. In addition, in the presence of Fn, functional blocking of αvβ3 on tumor cells significantly reduced firm adhesion (longer-period >3 sec) of PMNs to Lu1205 cells, while blocking ICAM-1 on tumor cells only reduced initial capture ability (significant for time <3 sec) of Lu1205 cells under 62.5 s-1 (Fig. 8B). This is again consistent with results from the cone-plate shear assay (Fig. 6C). Blocking ICAM-1 on Lu1205 cells did not significantly affect longer-period tumor cell binding to PMNs, suggesting it was the αvβ3 that supported the firm adhesion in the presence of Fn. αvβ3 on tumor cells was also shown to be sufficient in mediating optimal PMN-Lu1205 binding under very-low shear (20 s-1) which allows a longer contact time between PMN-Lu1205 (Fig. 8C). In contrast to Lu1205, non-metastatic WM35 melanoma cells failed to support appreciable PMN adhesion even in the presence of Fn (Fig. 8D), demonstrating the metastatic tumor specificity.
FIGURE 8.
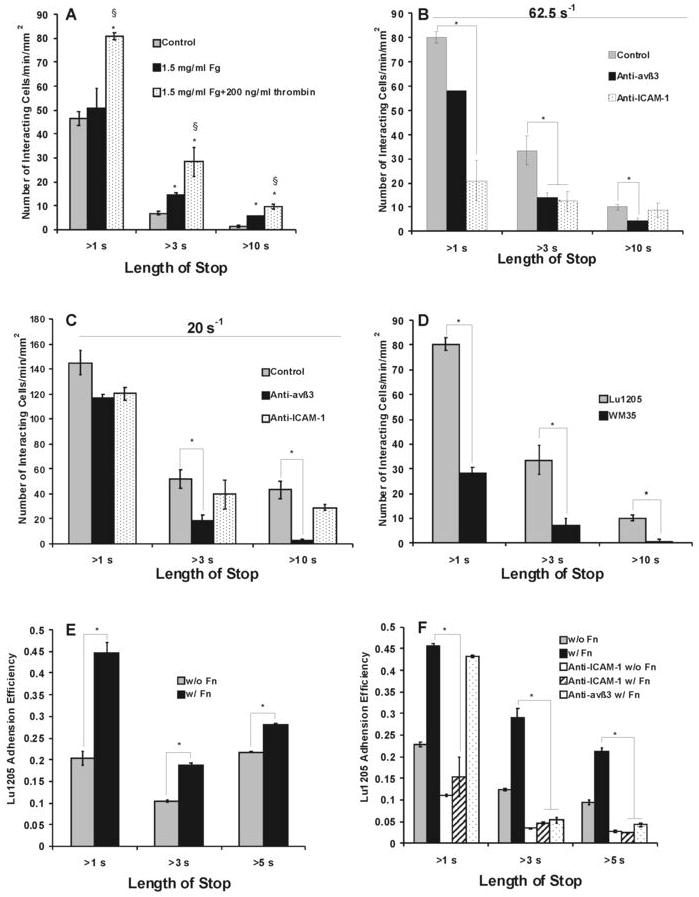
Relative contributions of αvβ3 and ICAM-1 to fibrin(ogen)-mediated PMN-melanoma-EC interaction under flow in parallel plate. (A-D) The number of PMNs contacting with Lu1205 or WM35 cells for more than 1 sec (initial tethering), 3 sec (transient adhesion) and 10 sec (long-term adhesion): (A) Fibrin(ogen) enhanced PMN and melanoma cell interaction. Data are means ± SEM (n>3). *P<0.05 compared with control. § P<0.05 compared with fibrinogen cases; (B-C) Roles of αvβ3 and ICAM-1 in different phases of adhesion of PMNs to Lu1205 cells under shear rate of 62.5 s-1 (B), or 20 s-1 (C). Values are means ± SEM (n>3). *P<0.05 compared with fibrin only cases; (D) PMNs bind weakly to non-metastatic melanoma cell line WM35. Values are means ± SEM (n>3). *P<0.05 compared with Lu1205 cases. (E-F) The adhesion efficiency of Lu1205 cells binding to EI cells for more than 1 sec (initial tethering), 3 sec (transient adhesion) and 5 sec (long-term adhesion) as a result of Lu1205 and PMN collisions: (E) Role of soluble fibrin(ogen) on melanoma adhesion efficiency to EI cells via PMNs at shear rate of 62.5 s-1; (F) Roles of αvβ3 and ICAM-1 in Lu1205 adhesion at the shear rate of 62.5 s-1. *P < 0.05 compared with the “Lu1205+Fn” case. Values are mean ± SEM (n>3).
Fibrin(ogen) mediates melanoma-PMN aggregation, which may increase melanoma tethering to the endothelial wall and subsequently facilitate melanoma cell extravasation from the circulation (32). The mechanisms of bringing melanoma into close proximity to the endothelial cells by pre-tethered PMNs were studied in parallel plate flow assays with EI cells as a monolayer. In these experiments, it was observed that most of the melanoma cell binding to the EI under flow was enhanced by tethered PMNs. To normalize the resulting melanoma adhesion with respect to frequency of PMN-melanoma collision, a parameter, adhesion efficiency, was adopted (Materials and Methods, Fig. 8E). The time lengths of melanoma arrest on EI cells varied and were categorized to >1 sec, >3 sec and >5 sec. Fn affected melanoma adhesion to EI cells via tethered PMN more than Fg did (Fig. 8E). The 1 sec short-term melanoma-PMN tethering on EI cells was mediated by ICAM-1 on the melanoma (less by αvβ3); αvβ3 maintained the bonds between PMN and melanoma, thereby settling melanoma on EI monolayer (Fig. 8F). These results indicate that thrombin-mediated Fg conversion to Fn plays influential roles in melanoma-PMN-EC adhesion, mechanistically via the receptors of ICAM-1 and αvβ3 on melanoma.
In summary, our studies showed that fibrin(ogen) could enhance the binding between PMNs and melanoma cells under shear conditions; ICAM-1 and αvβ3 contributed to this process. Under hydrodynamic conditions, ICAM-1/Fn/Mac-1 and ICAM-1/LFA-1 bonds promoted effective cell-cell tethering and prolonged initial cell-cell contact duration, while αvβ3/Fn/Mac-1 bonds enhanced cell-cell sustained adhesion, demonstrating cooperative roles of ICAM-1 and αvβ3 in tumor cell-PMN adhesion in the presence of Fn.
Discussion
In the present study, we have demonstrated that: 1) melanoma cells and PMNs can attach to immobilized Fg and Fn under venous flow conditions. αvβ3 on Lu1205 melanoma cells and Mac-1 on PMNs are the primary receptors for binding to immobilized Fg and Fn; 2) soluble Fg and Fn can enhance the hetero-aggregation between melanoma cells and PMNs as the bridging molecules, although the kinetics of Fn-mediated binding is different from that of Fg; 3) αvβ3 and ICAM-1 cooperate to mediate PMN and melanoma cell binding in the presence of soluble Fg or Fn under flow conditions, where ICAM-1 mediates initial tethering while αvβ3 contributes to firm adhesion (Fig. 9).
FIGURE 9.
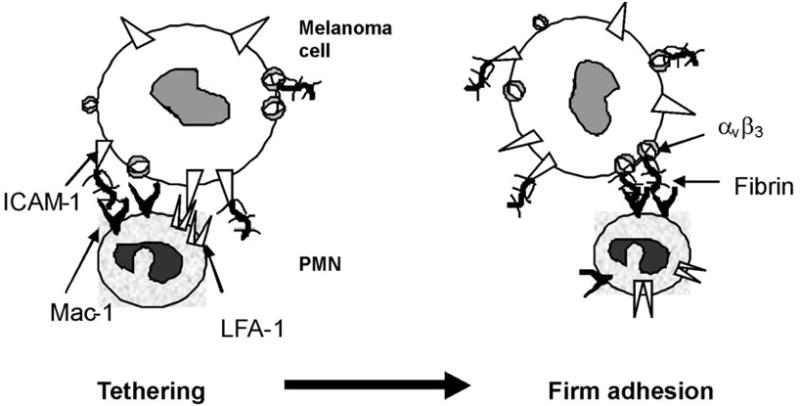
A model concluded from the present studies on PMN-Lu1205 heterotypic aggregation in the presence of Fn under shear conditions. Fn-bound ICAM-1 quickly binds to Mac-1 on PMNs, thereby increasing the cell-cell contact duration and allowing Fn-bound αvβ3 to mediate firm adhesion between PMN-Lu1205.
Soluble fibrin or fibrinogen, as a dimeric molecule, supports both initial capture and stable adhesion of melanoma cells to neutrophils under flow conditions
Tumor cells and PMNs in a cone-plate viscometer are subject to a uniform shear field, resulting in mutual collisions (9, 17). This millisecond scale of shear-dependent encounter has been shown to be able to mediate receptor-ligand bond formation between melanoma cells and PMNs in the absence of fibrin(ogen) (16). Increasing shear force opposes bond formation by decreasing the cell-cell contact duration but by increasing the cell-cell contact area. If the ensemble strength of receptor-ligand bonds outweigh tensile force, the heterotypic tumor cell-PMN aggregates form; otherwise, the aggregates dissociate. We now have shown that Fg mainly enhances the initial tethering of melanoma cells to PMNs, while Fn profoundly changes the kinetics of cell-cell binding, stabilizing melanoma-PMN doublets for several minutes. Results from Fig. 5A show that the extent of aggregation was increased with an increasing amount of Fg. However, this trend would be reversed after the concentration of soluble Fg reaches a certain level, since high amounts of free ligands tend to saturate counter-receptors on mutual cell types instead of bridging two cell receptors.
Tumor cell-mediated activation of coagulation cascade is a key factor for hematogenous metastasis. In this process, Fn plays various roles. Fn has also been shown to support migration of different cell types, including monocytes and fibrinoblasts. Its proteolytic products are chemotactic and angiogenic reagents. In addition, Fn is related to tumor stroma formation and invasion. Since thrombin generation is a determinant for Fn formation, it is interesting to see how thrombin affects tumor cell adhesion. Enzyme-linked immunosorbent assay (ELISA) results have shown that thrombin in plasma was increased when Lu1205 and A2058 melanoma cells were cultured or co-cultured with HUVECs (data not shown). Thrombin enhances tumor cell adhesion to platelets and facilitated tumor cell metastasis in vivo (33). Thrombin may also promote tumor cell metastasis through an Fg-independent mechanism (7). The possible effect of thrombin on Fg can be described using a Michaelis-Menten scheme where Fg binds to thrombin with certain rates and affinities (34). The rate of conversion of Fg to Fn is dependent on the concentration of thrombin. To understand the potential role of thrombin on conversion of Fg to Fn, we separated Fn digested products with SDS-PAGE. Most of α and β chains were cleaved by thrombin to produce lower molecular weight products. This demonstrated the functionality of thrombin and purity of Fn produced by 2 U/ml thrombin. Thrombin concentration of 2 U/ml was assumed to convert all the 1.5 mg/ml Fg to Fn within a 10min incubation time period (8). To probe the sensitivity of cell adhesion to Fn concentration, we have varied the concentrations of thrombin for Fn generation. We tried a range of thrombin concentrations, varying from 0.0265 U/ml to 2 U/ml. Results have shown that Fn solution made from 0.053 U/ml thrombin significantly enhanced αvβ3-mediated firm adhesion between melanoma cells and PMNs, suggesting that cryptic αvβ3 binding sites are exposed by thrombin catalysis. When thrombin level was further increased to 2U/ml from 0.053 U/ml, only the initial tethering phase was affected. This could imply that more ICAM-1 binding sites were available when melanoma cells were subject to higher concentration of thrombin. Therefore, we speculate that αvβ3 binding sites could be first exposed upon conversion of Fg to Fn, while it may require higher dose of thrombin to make ICAM-1 binding sites accessible. This hypothesis needs further biochemical analysis for proof. The fact that Fn has a higher affinity than Fg for receptors on melanoma cells agrees with the results from other cell types where CD44 on carcinoma cells had a higher affinity for Fn than Fg (8, 19, 31). Thrombin may have a potential effect on β2 integrin expression on PMNs. Therefore, the residual thrombin in our Fn solution may itself affect PMN-melanoma cell aggregation. However, it should be noted that all PMNs used in the current studies were pre-stimulated with fMLF which boosted the Mac-1 expression to maximal levels when we conducted cone-plate and parallel plate assays (35). In this way, we can preclude the possibility that the observed Fn-mediated aggregation was the pseudo-image of thrombin-stimulated PMN-melanoma binding.
The concept of Fn-mediated melanoma cell and PMN aggregation under flow conditions is intriguing. Several earlier studies demonstrated that Fn might mediate platelet-tumor cell adhesion (3, 5). These platelet-tumor cell microemboli may tether tumor cells to the endothelial wall, thereby contributing to tumor metastasis. Other similar evidence of Fn-mediated cell-cell interaction came from the studies of leukocyte adhesion to endothelial cell, where Mac-1 on PMNs and ICAM-1 on the endothelial cells may be the respective receptors for Fg (6, 25).
To our best knowledge, the present study has first shown that thrombin-regulated Fn formation can enhance PMN and melanoma cell aggregation under flow conditions. An earlier study reported that soluble Fn would actually reduce the monocyte adherence to A375 melanoma cells under flow conditions (22). However, there are several differences between these studies and the current work. The melanoma cell line A375 used in (22) was reported to express ICAM-1 but not αvβ3, while Lu1205 cells used in the current study express αvβ3 in addition to ICAM-1, which was shown to have a high affinity for fibrin(ogen)-mediated firm adhesion of melanoma cells. In addition, experimental methods used in (22) were different from the current study. In (22), monocytes were perfused over tumor cell monolayer for 1 hr in a parallel plate flow chamber. In contrast, we quantified PMN-Lu1205 heterotypic aggregation within short time periods (several minutes). In a cone-plate assay, both Fn and Fg were able to significantly enhance aggregation between αvβ3-blocked Lu1205 cells and PMNs within 5 min shear (Fig. 6A, B&C). By 60 sec, Fn and Fg increased percentage of aggregation from 45% to 60%; By 5 min, Fn and Fg increased percentage of aggregation from 27% to 37% and 35%, respectively. This result implies that Fn promotes Mac-1/ICAM-1 bond formation by possibly adding extra binding sites between Mac-1 and ICAM-1. However, during the long-term shear, Mac-1/Fn/ICAM-1 bonds dissociate (22). Without αvβ3, Fn-occupied ICAM-1 or Mac-1 could become obstacles to normal Mac-1/ICAM-1 bond formation, thereby reducing aggregation.
αvβ3 integrin contributes to fibrin-mediated prolonged-stable adhesion between melanoma cells and PMNs
Melanoma cells do not express selectins or sLex sugar groups at the levels necessary for cells to attach to the endothelial wall under venous flow conditions. Recent studies have shown that fibrinolytic factors and Fn deposition were both associated with hematogenous metastasis of murine melanoma cells (7). Fn is likely to be deposited on the surface of endothelium or platelets upon inflammation or tissue damage. Melanoma cells attach to Fg via αvβ3 and αvβ1, to von Willebrand Factor via αvβ3, and to fibronectin via αvβ3, αvβ1 and α5β1, respectively, under static conditions. In addition, the present study using a parallel-plate flow chamber to examine Lu1205 melanoma cells binding to immobilized fibrin(ogen) have indicated that αvβ3 was a major contributing receptor for Fg and Fn binding especially under low shear rates, which agrees well with a previous study on M21 melanoma cells adhesion to fibrin(ogen) (11). More importantly, melanoma cells could engage with fibrin(ogen) firmly without apparent “cell rolling”, which is potentially important for the arrest of sLex-negative melanoma cells the endothelial cells under venous flow conditions.
The expression of αvβ3 is associated with malignant phenotype of tumor, which promotes tumor cells, endothelial cells and fibroblast migration and invasion by interacting with fibrin(ogen) and its plasminogen-lytic products (36, 37). When αvβ3 on melanoma cells were blocked, Fn-mediated sustained aggregation between melanoma cells and PMNs was almost obligated. This demonstrated that αvβ3 which have a high affinity for Fn-mediated stable-firm adhesion between PMNs and melanoma cells under hydrodynamic conditions. The high affinity of αvβ3 for fibrin(ogen) is evident from biochemical and structural analysis. There are three putative αvβ3 binding sites on Fg (13), which are RGDS at the COOH terminus of α chain Aα 572-575, RGDF at NH2 terminus of α chain Aα 95-98 and dodecapeptide at the COOH terminus of γ chain γ400-411. These domains bind to immobilized αvβ3 so strongly that they do not dissociate once they bind. In particular, RGDS site at Aα 572-575 has stronger affinity for αvβ3 than dodecapeptide. Thrombin treatment of Fg on its α chain may induce conformational change and expose these RGD sites that are inaccessible to integrins in native structures. In addition, fibrin(ogen) may activate αvβ3 and induce cluster of integrins on cell-cell contact regions, further increasing the stability of cell aggregates (38). In agreement with our heterotypic aggregation studies and model about fibrin(ogen)-mediated PMN-dependent melanoma adhesion, soluble Fg enhanced the melanoma cell arrest to immobilized Fg, serving as cross-linking ligand for αvβ3 between attached and circulating tumor cells (11). More in-depth kinetic analysis of αvβ3- fibrin(ogen) interaction is needed in order to better understand the bond strength.
Mac-1 on PMNs serves as a counter-receptor for ICAM-1 and fibrin(ogen)
Mac-1 on leukocytes, especially PMNs, is a high affinity receptor for fibrin(ogen), mediating PMN adhesion to inflamed endothelial cells (39). Previously, Mac-1 was shown to mediate PMN homotypic aggregation under venous flow conditions (40). It is novel in the present study to show that Mac-1 also played roles in Fn-mediated heterotypic aggregation between melanoma cells and PMNs. Fg contains many recognition sites for Mac-1 within D domain, including two peptide sequences, γ190-202 and γ377-395 (41), which are called P1 and P2. Mac-1 may non-covalently bind to sites other than P1 and P2 (42). However, Mac-1 has stronger avidity for soluble Fn or immobilized Fg. This was suggested by the cryptic theory where pulling out P2 region from the central domain of Fg γ-module would regulate the binding affinity of Fg for Mac-1. This partially explains our melanoma-PMN aggregation results showing that Fn mediates stronger binding than Fg does. Although some studies also showed that PMNs have a higher possibility to form long-lasting bonds with immobilized ICAM-1 than immobilized Fg (27), we cannot rule out the possibility that Fn could enhance PMNs and tumor cell binding by inserting additional bonds between Mac-1 and ICAM-1 instead of replacing the existing bonds.
Collectively, our study demonstrated that PMN could tether melanoma cells to the vascular endothelial wall through the formation of shear-resist bonds. The most intriguing part of the present study is to demonstrate how a tumor microenvironment affects immune cell responses and cancer cell adhesion, especially when being subject to thrombin-regulated fibrin(ogen) immunoediting. This is one of several possible settings where tumor cells can capitalize on hemostatic components for the metastasis. This study has shed light on possible molecular mechanisms in fibrin(ogen)-mediated PMN-melanoma interactions under flow conditions and potential therapeutic importance of Fn receptors in melanoma cell adhesion.
Potential roles of selectin ligands, P-selectin, L-selectin and VCAM-1 on melanoma cell adhesion
Conventionally, the binding of P-selectin to leukocytes requires sialylated and fucosylated carbohydrates on PSGL-1. This binding is supported by sLex-like motifs on O-linked glycans of PSGL-1. Analysis of human metastatic melanoma lesions showed an elevated expression of sLea and sLex (43). However, further exploration of the interaction between melanoma and endothelial cells revealed that melanoma may use other proteoglycan and oligosaccharide to bind to P-selectin and E-selectin, including heparin sulfate under flow (44). Using mAbs failed to detect any apparent expression of sLex on small cell lung cancer and neuroblastoma cells (45). Therefore, it is of interest to compare PMN-mediated, Fn-mediated and selectin-mediated melanoma adhesion to endothelial cells under flow. From our adhesion studies using parallel-plate assays, most melanoma arresting onto the EI monolayer were associated with PMNs. In addition, Fn enhanced PMN-facilitated melanoma adhesion in higher magnitude than did melanoma direct adhesion to endothelial cells (data not shown). In spite of importance of PMN and Fn, selectins and VCAM-1 on endothelial cells should not be ignored. VLA-4 which is expressed by melanoma has ligand VCAM-1 on endothelial cells. Upregulation of VCAM-1 expression was associated with increased experimental metastasis in vivo (46, 47). VCAM-1/VLA-4 interaction mediated melanoma adhesion to endothelial cells under low shear condition (48, 49). Surprisingly, high level of VLA-4 may restrict cells to detach as a prelude to invasion, thereby inhibiting tumor cell metastasis. Selectin roles in melanoma metastasis were also demonstrated in animal models. For example, L-selectin plays a role in nonlymphoma tumor cell localization to lymph node (50). Although P-selectin was traditionally considered as a receptor for platelet-melanoma binding, its role in endothelial cell-melanoma binding was studied in P-selectin-deficient mice whose lung metastasis was significantly attenuated (51).
Tether behavior exhibited by fibrin(ogen)-ICAM-1 bonds may be an indication that in absence of selectin ligands, melanoma cells may explore other mechanisms for their successful transmigration. There have been no biomechanical models or biochemical basis for this interaction so far. Fast association and dissociation rates are prerequisites for PSGL-1-P-selectin-mediated PMN tether to endothelial cells under flow conditions. Such a rapid kinetic property was not witnessed in Mac-1/Fn and αvβ3/Fn binding measured by SPR. Based on the current study, it is may be possible that effective tumor adhesion to PMN and endothelial cells in the presence of multivalent plasma protein does not require a rapid association rate. It is intriguing to think whether it is unique among Fn mediated cell-cell interactions or in a similar in magnitude to reported selectin-PSGL-1 binding.
Potential roles of receptors in melanoma metastasis
During their passage through the endothelial cell wall, tumor cells undergo extensive interactions with host cells including PMNs. PMNs cause a dense Fn accumulation around them under flow conditions with a platelet-independent mechanism in which Mac-1 may play a role (52). This may contribute to binding of melanoma to PMNs and arrest of melanoma to endothelial cells. A dose- and time- dependent increase in ICAM-1 on melanoma by TNF-α treatment resulted in enhancement of in vivo metastasis (53). This TNF-α inducible increase in metastasis could be reversed by knocking down ICAM-1 on cell surface. Apparent contradictory results came from in vivo studies with ICAM-1 knockout mice, which showed that ICAM-1 deletion enhanced pulmonary metastasis (54, 55). However, this genetic deletion was conducted in acceptor mice side but not on melanoma side. Since T cells use receptors for ICAM-1 to bind/migrate into tissue and mediate tumor killing processes, reduced ICAM-1 expression may inhibit T cell migration and T cell-mediated tumor rejection. Most likely, the reduced tumor killing might compensate for the reduced tumor adhesion to endothelial cells in ICAM-1 knockout mice, leading to the enhanced melanoma lesions observed in tissue outside the vasculature. A better understanding of involvement of receptors in melanoma adhesion to endothelial cells via Fn and PMNs in circulation may come from examination of melanoma arrest within vasculature.
Acknowledgments
The authors thank Dr. M. Herlyn (Wistar Institute, Philadelphia, PA) for providing WM35 melanoma cells and Dr. Scott Simon (UC Davis, CA) for providing EI cells. We also acknowledge Dr. Hari Muddana and Dr. Shile Liang for many helpful discussions and technical supports.
Footnotes
This study was funded by NIH CA-127892, ACS RSG-04-053-01-GMC, and The Foreman Foundation for Melanoma Research (G.P.R); as well as NIH CA-125707 and NSF CBET-0729091 (C.D.).
Abbreviations used in this paper: sLex, Sialyl Lewis X; PMN, polymorphonuclear leukocyte; Fg, fibrinogen; Fn, fibrin; GPRP, Gly-Pro-Arg-Pro amide; TRITC, Tetramethylrhodamine isothiocyanate Isomer R; ICAM-1, Inter Cellular Adhesion Molecule-1; ELISA, Enzyme-linked immunosorbent assay.
References
- 1.Liang S, Slattery MJ, Dong C. Shear stress and shear rate differentially affect the multi-step process of leukocyte-facilitated melanoma adhesion. Exp Cell Res. 2005;310:282–292. doi: 10.1016/j.yexcr.2005.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Biggerstaff P, Seth N, Amirkhosravi A, Amaya M, Fogarty S, Meyer TV, Siddiqui F, Francis JL. Soluble fibrin augments platelet/tumor cell adherence in vitro and in vivo, and enhances experimental metastasis. Clin Exp Metastasis. 1999;17:723–730. doi: 10.1023/a:1006763827882. [DOI] [PubMed] [Google Scholar]
- 3.Felding-Habermann B, Habermann R, Saldívar E, Ruggeri ZM. Role of b3 Integrins in Melanoma Cell Adhesion to Activated Platelets under Flow. J Biol Chem. 1996;271:5892–5900. doi: 10.1074/jbc.271.10.5892. [DOI] [PubMed] [Google Scholar]
- 4.Mueller BM, Reisfeld RA, Edgington TS, Ruf W. Expression of tissue factor by melanoma cells promotes efficient hematogenous metastasis. Proc Natl Acad Sci. 1992;89:11832–11836. doi: 10.1073/pnas.89.24.11832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Biggerstaff JP, Seth NB, Meyer TV, Amirkhosravi A, Francis JL. Fibrin monomer increases platelet adherence to tumor cells in a flowing system: a possible role in metastasis? Thromb Res. 1998;92(6 Suppl 2):S53–58. doi: 10.1016/s0049-3848(98)00161-3. [DOI] [PubMed] [Google Scholar]
- 6.Languino LR, Plescia J, Duperray A, Brian AA, Plow EF, Geltosky JE, Altieri DC. Fibrinogen mediates leukocyte adhesion to vascular endothelium through an ICAM-1-dependent pathway. Cell. 1993;73:1423–1434. doi: 10.1016/0092-8674(93)90367-y. [DOI] [PubMed] [Google Scholar]
- 7.Palumbo JS, Kombrinck KW, Drew AF, Grimes TS, Kiser JH, Degen JL, Bugge TH. Fibrinogen is an important determinant of the metastatic potential of circulating tumor cells. Blood. 2000;96:3302–3309. [PubMed] [Google Scholar]
- 8.Alves CS, Burdick MM, Thomas SN, Pawar P, Konstantopoulos K. The dual role of CD44 as a functional P-selectin ligand and fibrin receptor in colon carcinoma cell adhesion. Am J Physiol Cell Physiol. 2008;294:C907–C916. doi: 10.1152/ajpcell.00463.2007. [DOI] [PubMed] [Google Scholar]
- 9.Goldsmith HL, Quinn TA, Drury G, Spanos C, McIntosh FA, Simon SI. Dynamics of Neutrophil Aggregation in Couette Flow Revealed by Videomicroscopy: Effect of Shear Rate on Two-Body Collision Efficiency and Doublet Lifetime. Biophys J. 2001;81:2020–2034. doi: 10.1016/S0006-3495(01)75852-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liang S, Slattery M, Wagner D, Simon SI, Dong C. Hydrodynamic shear rate regulates melanoma-leukocyte aggregations, melanoma adhesion to the endothelium and subsequent extravasation. Ann Biomed Eng. 2008;36:661–671. doi: 10.1007/s10439-008-9445-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pilch J, Habermann R, Felding-Habermann B. Unique Ability of Integrin avb3 to Support Tumor Cell Arrest under Dynamic Flow Conditions. J Biol Chem. 2002;277:21930–21938. doi: 10.1074/jbc.M201630200. [DOI] [PubMed] [Google Scholar]
- 12.Felding-Habermann B, Zaverio M, Ruggeri T, Cheresh DA. Distinct Biological Consequences of Integrin avb3-mediated Melanoma Cell Adhesion to Fibrinogen and Its Plasmic Fragments. J Biol Chem. 1992;267:5070–5077. [PubMed] [Google Scholar]
- 13.Yokoyama K, Erickson HP, Ikeda Y, Takada Y. Identification of Amino Acid Sequences in Fibrinogen g-Chain and Tenascin C C-terminal Domains Critical for Binding to Integrin avb3. J Biol Chem. 2000;275:16891–16898. doi: 10.1074/jbc.M000610200. [DOI] [PubMed] [Google Scholar]
- 14.Altieri DC, Duperray A, Plescia J, Thornton GB, Languino LR. Structural Recognition of a Novel Fibrinogen γChain Sequence (117–133) by Intercellular Adhesion Molecule-1 Mediates Leukocyte-Endothelium Interaction. J Biol Chem. 1995;270:696–699. doi: 10.1074/jbc.270.2.696. [DOI] [PubMed] [Google Scholar]
- 15.Lishko VK, Podolnikova NP, Yakubenko VP, Yakovlev S, Medved L, Yadav SP, Ugarova TP. Multiple binding sites in fibrinogen for integrin alphaMbeta2 (Mac-1) J Biol Chem. 2004;279:44897–44906. doi: 10.1074/jbc.M408012200. [DOI] [PubMed] [Google Scholar]
- 16.Liang S, Fu C, Wagner D, Guo H, Zhan D, Dong C, Long M. Two-dimensional kinetics of β2-integrin and ICAM-1 bindings between neutrophils and melanoma cells in a shear flow. Am J Physiol Cell Physiol. 2008;294:C743–C753. doi: 10.1152/ajpcell.00250.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hentzen ER, Neelamegham S, Kansas GS, Benanti JA, McIntire LV, Smith CW, Simon SI. Sequential binding of CD11a/CD18 and CD11b/CD18 defines neutrophil capture and stable adhesion to intercellular adhesion molecule-1. Blood. 2000;95:911–920. [PubMed] [Google Scholar]
- 18.Liang S, Sharma A, Peng HH, Robertson G, Dong C. Targeting mutant (V600E) B-Raf in melanoma interrupts immunoediting of leukocyte functions and melanoma extravasation. Cancer Res. 2007;67:5814–5820. doi: 10.1158/0008-5472.CAN-06-4233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Alves CS, Yakovlev S, Medved L, Konstantopoulos K. Biomolecular characterization of CD44-fibrin(ogen) binding: distinct molecular requirements mediate binding of standard and variant isoforms of CD44 to immobilized fibrin(ogen) J Biol Chem. 2009;28:1177–1189. doi: 10.1074/jbc.M805144200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Simon SI, Hu Y, Vestweber D, Smith CW. Neutrophil tethering on E-selectin activates h2 integrin binding to ICAM-1 through a mitogenactivated protein kinase signal transduction pathway. J Immunol. 2000;164:4348–4435. doi: 10.4049/jimmunol.164.8.4348. [DOI] [PubMed] [Google Scholar]
- 21.Gopalan PK, Smith CW, Lu H, Berg E, Mcintire LV, Simon SI. PMN CD18-dependent arrest on ICAM-1 in shear flow can be activated through L-selectin. J Immunol. 1997;158:367–375. [PubMed] [Google Scholar]
- 22.Biggerstaff JP, Weidow B, Vidosh J, Dexheimer J, Patel S, Patel P. Soluble fibrin inhibits monocyte adherence and cytotoxicity against tumor cells: implications for cancer metastasis. Thromb J. 2006;4:12. doi: 10.1186/1477-9560-4-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hoskins MH, Dong C. Kinetics analysis of binding between melanoma cells and neutrophils. Mol Cell Biomech. 2006;3:79–87. [PMC free article] [PubMed] [Google Scholar]
- 24.Jadhav S, Bochner BS, Konstantopoulos K. Hydrodynamic shear regulates the kinetics and receptor specificity of polymorphonuclear leukocyte-colon carcinoma cell adhesive interactions. J Immunol. 2001;167:5986–5993. doi: 10.4049/jimmunol.167.10.5986. [DOI] [PubMed] [Google Scholar]
- 25.Duperray A, Languino LR, Plescia J, McDowalli A, Hoggi N, Craig AG, Berendt AR, Altieri DC. Molecular Identification of a Novel Fibrinogen Binding Site on the First Domain of ICAM-1 Regulating Leukocyte-Endothelium Bridging. J Biol Chem. 1997;272:435–441. doi: 10.1074/jbc.272.1.435. [DOI] [PubMed] [Google Scholar]
- 26.Kuijper PH, Gallardo Torres HI, van der Linden JA, Lammers JW, Sixma JJ, Zwaginga JJ, Koenderman L. Neutrophil adhesion to fibrinogen and fibrin under flow conditions is diminished by activation and L-selectin shedding. Blood. 1997;89:2131–2138. [PubMed] [Google Scholar]
- 27.Kadash KE, Lawrence MB, Diamond SL. Neutrophil string formation: hydrodynamic thresholding and cellular deformation during cell collisions. Biophys J. 2004;86:4030–4039. doi: 10.1529/biophysj.103.035782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li P, Selvaraj P, Zhu C. Analysis of Competition Binding between Soluble and Membrane-Bound Ligands for Cell Surface Receptors. Biophys J. 1999;77:3394–3406. doi: 10.1016/S0006-3495(99)77171-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yakovlev S, Gorlatov S, Ingham K, Medved L. Interaction of Fibrin(ogen) with Heparin: Further Characterization and Localization of the Heparin-Binding Site. Biochemistry. 2003;42:7709–7716. doi: 10.1021/bi0344073. [DOI] [PubMed] [Google Scholar]
- 30.Neelamegham S, Taylor AD, Hellums JD, Dembo M, Smith CW, Simon SI. Modeling the reversible kinetics of neutrophil aggregation under hydrodynamic shear. Biophys J. 1997;72:1527–1540. doi: 10.1016/S0006-3495(97)78801-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Konstantopoulos K, Thomas SN. Cancer cells in transit: the vascular interactions of tumor cells. Annu Rev Biomed Eng. 2009;11:177–202. doi: 10.1146/annurev-bioeng-061008-124949. [DOI] [PubMed] [Google Scholar]
- 32.Slattery MJ, Liang S, Dong C. Distinct role of hydrodynamic shear in leukocyte-facilitated tumor cell extravasation. Am J Physiol Cell Physiol. 2005;288:C831–C839. doi: 10.1152/ajpcell.00439.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nierodzik ML, Kajumo F, Karpatkin S. Effect of thrombin treatment of tumor cells on adhesion of tumor cells to platelets in vitro and tumor metastasis in vivo. Cancer Res. 1992;52:3267–3272. [PubMed] [Google Scholar]
- 34.Scheraga HA. The thrombin-fibrinogen interaction. Biophys Chem. 2004;112:117–130. doi: 10.1016/j.bpc.2004.07.011. [DOI] [PubMed] [Google Scholar]
- 35.Neelamegham S, Taylor AD, Burns AR, Smith CW, Simon SI. Hydrodynamic Shear Shows Distinct Roles for LFA-1 and Mac-1 in Neutrophil Adhesion to Intercellular Adhesion Molecule-1. Blood. 1998;92:1626–1638. [PubMed] [Google Scholar]
- 36.Gailit J, Clarke C, Newman D, Tonnesen MG, Mosesson MW, Clark RAF. Human Fibroblasts Bind Directly to Fibrinogen at RGD Sites through Integrin avb3. Exp Cell Res. 1997;232:118–126. doi: 10.1006/excr.1997.3512. [DOI] [PubMed] [Google Scholar]
- 37.Leavesley I, Ferguson GD, Wayner EA, Cheresh DA. Requirement of the Integrin/ 3 Subunit for Carcinoma Cell Spreading or Migration on Vitronectin and Fibrinogen. J Cell Biol. 1992;117:1101–1107. doi: 10.1083/jcb.117.5.1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cluzel C, Saltel F, Lussi J, Paulhe F, Imhof BA, Wehrle-Haller B. The mechanisms and dynamics of (alpha)v(beta)3 integrin clustering in living cells. J Cell Biol. 2005;171:383–392. doi: 10.1083/jcb.200503017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Diamond MS, Springer TA. A Subpopulation of Mac-1 CDllb/CD18 Molecules Mediates Neutrophil Adhesion to ICAM-1 and Fibrinogen. J Cell Biol. 1993;120:545–556. doi: 10.1083/jcb.120.2.545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Simon SI, Chambers JD, Butcher E, Sklar LA. Neutrophil aggregation is beta 2-integrin- and L-selectin-dependent in blood and isolated cells. J Immunol. 1992;149:2765–2771. [PubMed] [Google Scholar]
- 41.Mosesson MW. Fibrinogen and fibrin structure and functions. J Thromb Haemost. 2005;3:1894–1904. doi: 10.1111/j.1538-7836.2005.01365.x. [DOI] [PubMed] [Google Scholar]
- 42.Yakovlev S, Zhang L, Ugarova T, Medved L. Interaction of fibrin(ogen) with leukocyte receptor alpha M beta 2 (Mac-1): further characterization and identification of a novel binding region within the central domain of the fibrinogen gamma-module. Biochemistry. 2005;44:617–626. doi: 10.1021/bi048266w. [DOI] [PubMed] [Google Scholar]
- 43.Kageshita T, Hirai S, Rimara T, Hanai N, Olita S, Ono T. Association between Sialyl Lewis3 Expression and Tumor Progression in Melanoma. Cancer Res. 1995;55:1748–1751. [PubMed] [Google Scholar]
- 44.Ma YQ, Geng JG. Heparan sulfate-like proteoglycans mediate adhesion of human malignant melanoma A375 cells to P-selectin under flow. J Immunol. 2000;165(1):558–65. doi: 10.4049/jimmunol.165.1.558. [DOI] [PubMed] [Google Scholar]
- 45.Stone JP, Wagner DD. P-selectin mediates adhesion of P-selectin to neuroblastoma and small cell lung cancer. J Clin Invest. 1993;92:804. doi: 10.1172/JCI116654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Okahara H, Yagita H, Miyake K, Okumura K. Involvement of Very Late Activation Antigen 4 (VLA-4) and Vascular Cell Adhesion Molecule 1 (VCAM-1) in Tumor Necrosis Factor a Enhancement of Experimental Metastasis. Cancer Res. 1994;54:3233–3236. [PubMed] [Google Scholar]
- 47.Vidal-Vanaclocha F, Fantuzzi G, Mendoza L, Fuentes AM, Anasagasti MJ, Martín J, Carrascal T, Walsh P, Reznikov LL, Kim SH, Novick D, Rubinstein M, Dinarello CA. IL-18 regulates IL-1b-dependent hepatic melanoma metastasis via vascular cell adhesion molecule-1. Proc Natl Acad Sci U S A. 2000;97(2):734–739. doi: 10.1073/pnas.97.2.734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Liang Shile, Dong Cheng. Integrin VLA-4 enhances sialyl-Lewisx/a-negative melanoma adhesion to and extravasation through the endothelium under low flow conditions. Am J Physiol Cell Physiol. 2008;295(3):C701–C707. doi: 10.1152/ajpcell.00245.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mould P, Askari JA, Craig SE, Garratt AN, Clement J, Humphries MJ. Integrin α4β1 –mediated Melanoma Cell Adhesion and Migration on Vascular Cell Adhesion Molecule-1(VCAM-1) and the Alternatively Spliced IIICS Region of Fibronectin. J Biol Chem. 1994;269:27224–27230. [PubMed] [Google Scholar]
- 50.Qian F, Hanahan D, Weissman IL. L-selectin can facilitate metastasis to lymph nodes ina transgenic mouse model of carcinogenesis. Proc Natl Acad Sci U S A. 2001;98:3976–3981. doi: 10.1073/pnas.061633698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ludwig RJ, Boehme B, Podda M, Henschler R, Jager E, Tandi C, Boehncke Wolf-Henning, Zollner TM, Kaufmann R, Gille J. Endothelial P-Selectin as a Target of Heparin Action in Experimental Melanoma Lung Metastasis. Cancer Res. 2004;64:2743–2750. doi: 10.1158/0008-5472.can-03-1054. [DOI] [PubMed] [Google Scholar]
- 52.Goel Mukul S, Scott L. Diamond Neutrophil Enhancement of Fibrin Deposition Under Flow Through Platelet-Dependent and -Independent Mechanisms. Arteriosclerosis Thrombosis and Vascular Biology. 2001;21:2093. doi: 10.1161/hq1201.100255. [DOI] [PubMed] [Google Scholar]
- 53.Miele ME, Bennett CF, Miller BE, Welch DR. Enhanced metastatic ability of TNF-alpha-treated malignant melanoma cells is reduced by intercellular adhesion molecule-1 (ICAM-1, Cd54) antisense oligonucleotides. Exp Cell Res. 1994;214:231–241. doi: 10.1006/excr.1994.1253. [DOI] [PubMed] [Google Scholar]
- 54.Yamada M, Yanaba K, Hasegawa M, Matsushita Y, Horikawa M, Komura K, Matsushita T, Kawasuji A, Fujita T, Takehara K, Steeber DA, Tedder TF, Sato S. Regulation of local and metastatic host-mediated anti-tumour mechanisms by l-selectin and intercellular adhesion molecule-1. Clin Exp Immunol. 2006;143(2):216–227. doi: 10.1111/j.1365-2249.2005.02989.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Marvin MR, Southall JC, Trokhan S, DeRosa C, Chabot J. Liver metastases are enhanced in homozygous deletionally mutant ICAM-1 or LFA-1 mice. J Surg Res. 1998;80(2):143–148. doi: 10.1006/jsre.1998.5322. [DOI] [PubMed] [Google Scholar]