Abstract
The capacity to fine-tune cellular bioenergetics with the demands of stem cell maintenance and regeneration is central to normal development and aging and to organismal survival during periods of acute stress. How energy metabolism and stem cell homeostatic processes are coordinated is not well understood. LKB1 acts as an evolutionarily conserved regulator of cellular energy metabolism in eukaryotic cells and functions as the major upstream kinase to phosphorylate AMPK and 12 other AMPK-related kinases 1–3. Whether LKB1 regulates stem cell maintenance remains unknown. Here we show that LKB1 plays an essential role in hematopoietic stem cell (HSC) homeostasis. We demonstrate that ablation of Lkb1 in adult mice results in severe pancytopenia and subsequent lethality. Loss of Lkb1 leads to impaired survival and escape from quiescence of HSCs, resulting in exhaustion of the HSC pool and marked reduction of HSC repopulating potential in vivo. Lkb1 deletion impacted cell proliferation in HSCs, but not more committed compartments, pointing to context specific functions for LKB1 in hematopoiesis. The adverse impact of Lkb1 deletion on hematopoiesis was predominantly cell-autonomous and mTORC1-independent and involves multiple mechanisms converging on mitochondrial apoptosis and possibly down-regulation of PGC-1 coactivators and their transcriptional network which plays critical roles in mitochondrial biogenesis and function. Thus, LKB1 serves as an essential regulator of HSCs and hematopoiesis, and more generally, points to the critical importance of coupling energy metabolism and stem cell homeostasis.
HSCs function to replenish the blood system and maintain hematopoietic homeostasis in response to either physiological or imposed stress demands 4–6. To explore the role of LKB1 in various aspects of HSC biology, we assessed the impact of somatic deletion of Lkb1 in the mouse adult hematopoietic system using Rosa26-CreERT2 deletor mice 7. In this model, the treatment of adult mice with tamoxifen results in complete deletion of Lkb1 in hematopoietic organs (Supplementary Fig. 1a), and associated reductions of phosphorylation of AMPK Thr172 and AMPK substrate Acetyl-CoA carboxylase (ACC) Ser79 (Supplementary Fig. 1b). Strikingly, within 30 days post completing tamoxifen injection (DPI), all tamoxifen-treated Lkb1 L/L, Rosa26-CreERT2 mice (hereafter designated Lkb1 KO) exhibited constitutional signs of weight loss (Supplementary Fig. 2a), lethargy, hunched posture, and ultimately death (Fig. 1a); in contrast, tamoxifen-treated Lkb1 +/+, Rosa26-CreERT2 or Lkb1 L/L mice (collectively hereafter designated Lkb1 WT) remained viable and healthy (Fig. 1a).
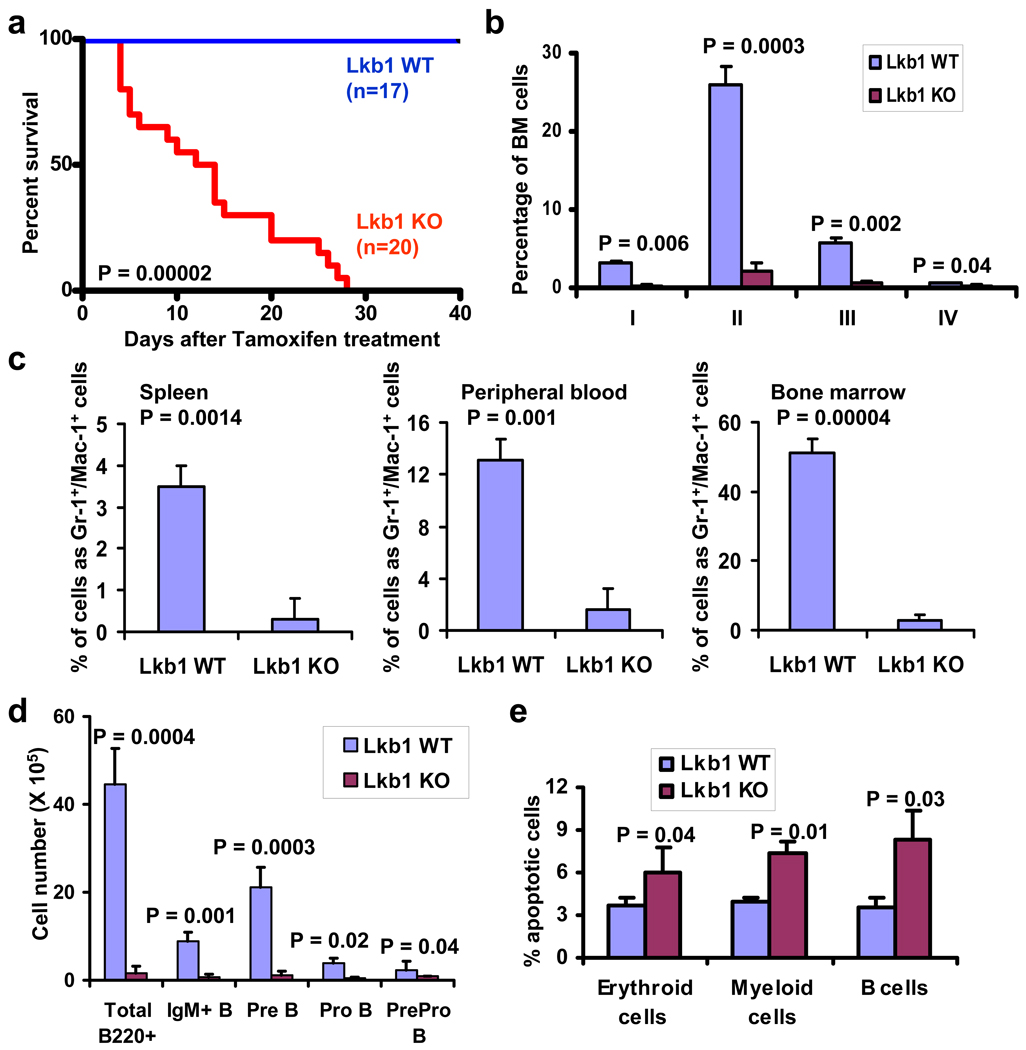
Figure 1. Lkb1 deletion leads to severe pancytopenia phenotype.
a, Kaplan-Meier survival analysis of Lkb1 WT and KO mice. b, c, The percentages of erythroid lineage from bone marrow (b) and Mac-1+/Gr-1+ cells (c) in Lkb1 WT and KO mice at 5 DPI. d, The numbers of bone marrow B cell lineage at 7 DPI from Lkb1 WT and KO mice. e, The percentages of apoptotic cells in erythroid, myeloid and B cells of Lkb1 WT and KO bone marrow cells at 3 DPI. n > 3 (b–e).
Somatic deletion of Lkb1 led to pancytopenia within 1 week after tamoxifen treatment (7 DPI) – evidenced by reduced weight of spleen and thymus, and reduction of the absolute cell number of bone marrow, spleen and thymus (Supplementary Figs. 2b–f). Lkb1 KO mice also developed acute anemia as evidenced by marked decline in red blood cell, hemoglobin and hematocrit counts (Supplementary Figs. 2g–i). While anemic, Lkb1 KO mice had increased non-fasting blood glucose levels (Supplementary Fig. 3), making unlikely that the Lkb1 KO anemia phenotype derives from a profound systemic deficiencies in glucose availability. Further analysis revealed severe reductions in Lkb1 KO Ter119+ cells and erythroid progenitors at all developmental stages (Fig. 1b, Supplementary Figs. 4a–b). Consistent with the pancytopenia phenotype described above, we observed a decline of all hematopoietic lineages examined in the Lkb1 KO mice, including platelets (Supplementary Fig. 4c), Gr-1+/Mac-1+ cells (Fig. 1c and Supplementary Fig. 4d), B cells (Fig. 1d and Supplementary Fig. 4e), and T cells (Supplementary Fig. 4f). To study the underlying mechanism of the multi-lineage defects associated with Lkb1 deletion, we examined the cell survival status of various lineages in Lkb1 WT and KO mice given the important role of LKB1 in the maintenance of cell survival under energy stress in other cellular contexts 8,9. Indeed we observed an increase of cleaved Caspase-3 in Lkb1 KO bone marrow, spleen and thymus samples (Supplementary Fig. 5). Further Annexin V/7AAD analysis revealed increased apoptosis in myeloid, erythroid and B cell populations in Lkb1 KO bone marrow cells (Fig. 1e). In summary, Lkb1 deficiency leads to severe pancytopenia phenotype and impairs the cell survival of multiple lineages.
Next we examined HSC and hematopoietic progenitor populations in Lkb1 KO mice. Compared with WT controls, serial analysis of Lkb1 KO HSC-enriched LSK cells (Lin−, Sca-1+, c-Kit+) and long-term HSCs (LT-HSCs; CD34− Flt-3− LSKs) in bone marrow showed an acute increase at 1 DPI, but a subsequent decrease in numbers from 4 DPI and thereafter (Figs. 2a–b). BrdU labeling experiment revealed significantly increased percentage of BrdU positive cells in Lkb1 KO LSK cells (Fig. 2c and Supplementary Figs. 6a–b). Notably, Lkb1 deletion increased cell proliferation only in LSK and LSK CD34− cells, but not whole bone marrow and mature lineage cells (Fig. 2b), suggesting an HSC-specific role for LKB1 in the regulation of cell quiescence. The more pronounced function of LKB1 in HSC compartment also aligns well with its more prominent expression levels in HSCs relative to other more committed compartments (Supplementary Figs. 6c–d). Furthermore, 7-AAD/Annexin-V staining of LSK population showed increased apoptosis in Lkb1 KO LSK cells (Supplementary Figs. 6e–f). Together, our results suggest that LKB1 functions to maintain HSC quiescence and survival and that Lkb1 inactivation leads to transient expansion, yet subsequent decline, of bone marrow HSCs.
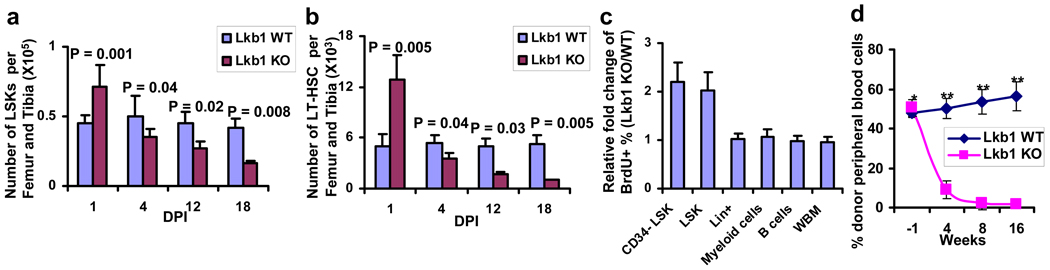
Figure 2. Lkb1 ablation results in reduced HSC reserves and decreased repopulating potential.
a, b, The numbers of LSK cells (a) and LT-HSCs (b) at various DPIs in Lkb1 WT and KO bone marrows. c, The relative fold changes (Lkb1 KO/WT) of the percentages of BrdU+ cells from various hematopoietic cell lineages in Lkb1 WT and KO bone marrows at 1 DPI. n = or > 3 at each time point (a–c). d, The percentages of donor-derived cells in peripheral blood by CD45 staining in 1:1 competitive transplantation. n = 15. *: P > 0.4; **: P < 0.01.
We next performed competitive and noncompetitive transplantation assays to examine the impact of Lkb1 deficiency on HSC repopulating capability in vivo (Supplementary Figs. 7a–b). In the competitive transplantation experiments, we observed that Lkb1 KO transplants showed markedly diminished repopulating capability relative to WT controls (Fig. 2d, and Supplementary Figs. 8a–c). In the noncompetitive experiments, all recipient mice reconstituted with Lkb1L/L, Rosa26-CreERT2 bone marrow cells died within 60 DPI (Supplementary Fig. 8d) with anemia, pancytopenia defects (data not shown), and increased HSC cell cycle entry and subsequent decline (Supplementary Figs. 8e–f). These data collectively indicate that LKB1 exerts a predominant cell-autonomous impact on hematopoietic repopulating potential and homeostasis.
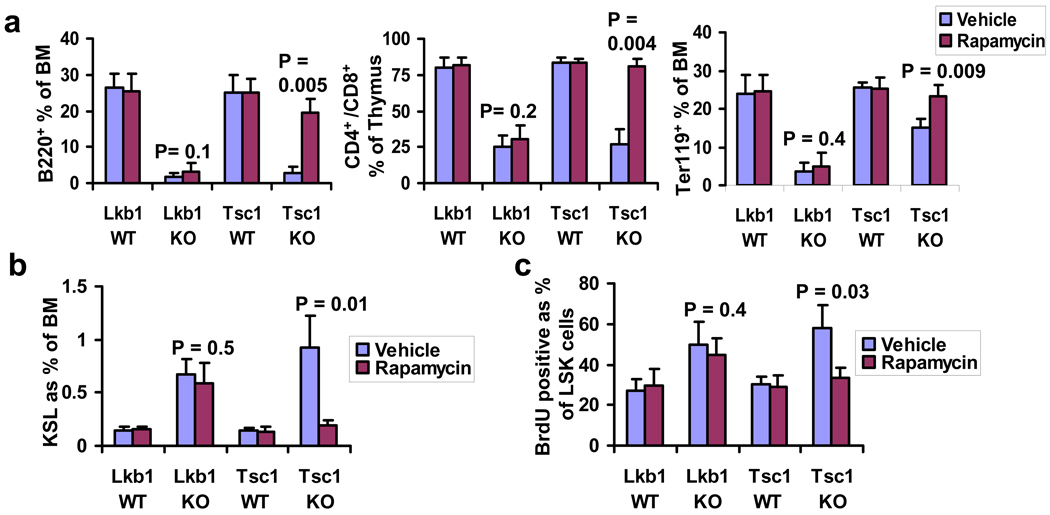
To understand the mechanisms underlying LKB1-directed regulation of HSC homeostasis, we assessed the impact of pharmacological inhibition of mTORC1 signaling on the Lkb1 KO phenotype, given that mTORC1 serves as a key downstream surrogate of LKB1-AMPK signaling 3 and also plays critical roles in the regulation of HSC homeostasis 10–14. Daily rapamycin or vehicle protocols were initiated to Lkb1 or Tsc1 WT and KO mice at the time of tamoxifen treatment 12. Rapamycin treatment significantly rescued multi-lineage defects, HSC cycling increase and expansion phenotypes in Tsc1 KO mice, but had minimal effect on these phenotypes in Lkb1 KO mice (Figs. 3a–c) despite confirmation of abolished S6 phosphorylation in sorted bone marrow B220+ cells, Mac1+ cells and CD34− LSK cells from Lkb1 KO mice (Supplementary Fig. 9). Thus, unlike TSC1, LKB1 regulates hematopoiesis via an mTORC1-independent pathway. Finally, administration of metformin, a known AMPK activator, did not rescue bone marrow/thymus cellularity decline, LSK transient expansion/subsequent depletion phenotype and lineage defects in Lkb1 KO mice (Supplementary Fig. 10). These data, together with AMPK activator A-769662 treatment data from Gurumurthy et al and AMPK KO mice analyses from Nakada et al, suggest that either multiple AMPK-related kinases (including AMPK) cooperatively mediate LKB1 function in HSC homeostasis or non-AMPK dependent processes are operative.
Figure 3. LKB1 regulation of hematopoiesis is TSC-mTORC1-independent.
a, b, c, The percentages of B220+ and Ter119+ populations in bone marrow and thymic CD4+/CD8+ cells (a), bone marrow LSK cells (b), and BrdU+ bone marrow LSK cells (c) from the mice indicated. n = 3 (a–c).
The above observations prompted transcriptome analysis of sorted LSK cells from Lkb1 WT and KO bone marrows at 1 DPI to gain further mechanistic insight of LKB1 regulation of HSC homeostasis (Supplementary Fig. 11a). Ingenuity pathway analysis of 570 significantly differentially expressed genes revealed significant enrichment of genes involved in G1/S cell cycle checkpoint regulation (Supplementary Fig. 11b, and Supplementary Table 1) including up-regulation of Cyclin D1, Cyclin D2, Cyclin E1, Cdc25A, E2F1, Cdk4 and Skp2, which would serve to synergistically promote cell cycling of Lkb1 KO LSKs. Most notably, there was prominent representation of LXR/RXR, VDR/RXR and PPAR metabolism pathways (Supplementary Fig. 11b). To identify key networks regulated by LKB1 in a TSC-mTORC1-independent manner, we further conducted comparative analysis of Lkb1 and Tsc1 12 HSC transcriptome datasets (Supplementary Fig. 11a), which revealed that, although G1/S cell cycle checkpoint was enriched in both datasets, the LXR/RXR, VDR/RXR and PPAR metabolism pathways were distinctively enriched in the Lkb1 transcriptome dataset (Supplementary Fig. 11c). In addition, promoter analysis of Lkb1 HSC transcriptome dataset identified E2F, nuclear respiratory factor 1 (NRF1) and PPARγ motifs as the most significantly enriched promoter binding elements in Lkb1 LSK transcriptome dataset (1.4x, 2.1x and 1.4x, respectively). The link to PPARγ and NRF1 is notable given that peroxisome proliferator-activated receptor-coactivators, PGC-1α and PGC-1β, are the principal transcriptional coactivator for PPARγ and NRF1 15, and that the PGC-1s are regulated by LKB1 16.
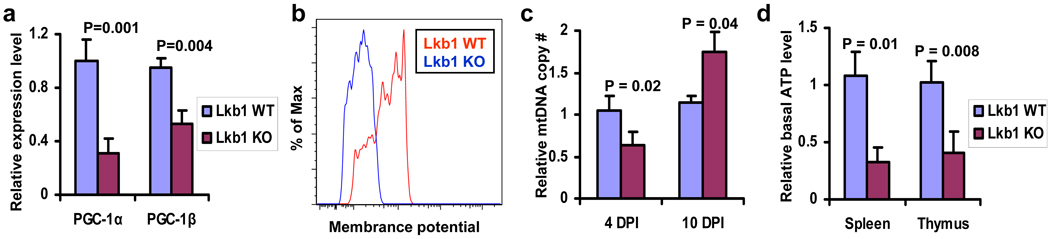
Prompted by this observation, we next investigated whether Lkb1 deletion impacted on PGC-1, a master transcriptional regulator of mitochondrial biogenesis 17, and its associated biological processes in the hematopoietic system. We found the expression levels of both PGC-1α and PGC-1 β were down-regulated in Lkb1 KO LSKs (Fig. 4a), which coincided with decreased mitochondrial membrane potential and DNA content in Lkb1 KO LSKs at 4 DPI (Figs. 4b–c). Interestingly, we observed increased mitochondrial DNA content in Lkb1 KO LSKs at later time point (10 DPI), possibly reflecting compensatory (secondary) effects that occur in the wake of mitochondriopathy (Fig. 4c). Finally, we found that the basal ATP levels in spleen and thymus were profoundly decreased in Lkb1 KO mice (Fig. 4d). Although our data raises the possibility that Lkb1 deficiency and associated dysregulation of PGC-1 impair mitochondrial function, the presence of apoptosis in these Lkb1−/− HSCs does not allow us to exclude the possibility that mitochondrial dysfunction reflects in part an ongoing apoptotic process caused by Lkb1 deletion.
Figure 4. Lkb1 deletion diminishes mitochondrial biogenesis and energy production in the hematopoietic system.
a, b, c, The relative expression levels of PGC-1α/β at 2 DPI (a), the mitochondrial membrane potential at 4 DPI (b), and the relative mitochondria DNA copy numbers at 4 and 10 DPI (c) of Lkb1 WT and KO bone marrow LSKs. d, The relative basal ATP levels in spleen and thymus from Lkb1 WT and KO mice at 4 DPI. n = 3 (a–d).
In conclusion, our results reveal an essential role of LKB1 in the maintenance of HSC homeostasis. Somatic deletion of Lkb1 in the hematopoietic system impairs HSC quiescence and survival and leads to metabolic catastrophe, resulting in pancytopenia and rapid animal death. Mechanistically, we propose that LKB1 maintains HSC homeostasis through multiple mechanisms governing mitochondrial function, cell survival, and cell cycle regulation via LKB1 regulation of AMPK and other AMPK-related kinases and their downstream effectors (including PGC-1). Our findings align with those of Nakada et al and Gurumurthy et al. and provide a broad framework to understand the integration of energy signaling and mitochondrial physiology in the maintenance of HSC homeostasis.
Methods Summary
The Lkb1 L/L mice were described previously 18. Rosa26CreERT2 mice were provided by A. Berns 7. Lkb1 L/+, Rosa26CreERT2+ mice were backcrossed 6 generations into C57BL/Ka-CD45.2:Thy-1.1 background, which were then intercrossed to generate mice of the desired genotypes. Recipients in transplantation assays were adult C57BL/Ka-CD45.1:Thy-1.2 mice (Jackson Laboratory). Tamoxifen treatment, phenotypic analyses of mice, rapamycin administration, flow cytometric analysis, and transplantation assays were performed as previously described 12. All animal manipulations were performed with Harvard/DFCI’s Institutional Animal Care and Use Committee (IACUC) approval. For microarray analysis, the RNA from sorted LSK cells (10,000–20,000) was extracted and the cDNA was analyzed on the Affymetrix 430 2.0 platform. Quantitative real-time PCR was performed on the Stratagene Mx3000P utilizing the Quantitative SYBR Green PCR kit. Full methods and the associated references are available in the online version of the paper.
Supplementary Material
Acknowledgements
We are grateful to Anton Berns for providing Rosa26-CreERT2 mouse strain. We are also grateful to Shan (Julia) Zhou for the assistance in the animal facility and Carol Lim for the assistance with genotyping. We thank Suzan Lazo-Kallanian, John Daley and Peter Schow for assistance with flow cytometry. We also thank Nabeel Bardeesy and Sean Morrison for communicating unpublished information, Alexander Stegh for helpful comments on apoptosis, and Daisuke Nakada for Western blotting protocol from sorted HSCs. This research was supported by U01CA141508 (R. A. D. and L. C.), R21CA135057 (R. A. D. and B. G.), and DOD TSCRP Career Transition Award (TS093049) (B. G.). B. G. and J. H. are the Research Fellows of the Leukemia & Lymphoma Society. Y. A. W. is supported by Multiple Myeloma Research Foundation. R. A. D. was supported by an American Cancer Society Research Professorship and the Robert A. and Renee E. Belfer Foundation.
Footnotes
Supplementary Information is linked to the online version of the paper at www.nature.com/nature.
Author Contributions
B. G. and R. A. D. designed the experiments, interpreted the data, and wrote the manuscript. B. G., S. J., J. H., L. Z., E. F. performed experiments. Y. L. and L. C. conducted the microarray and promoter analyses. E. S. and S. C. contributed reagents. L. C. and Y. A. W. contributed to the writing of the manuscript.
Author Information
Reprints and permissions information is available at www.nature.com/reprints. The authors declare no competing financial interests. Completed microarray data are deposited on the GEO website under super series accession number GSE24765.
References
- 1.Alessi DR, Sakamoto K, Bayascas JR. LKB1-dependent signaling pathways. Annu Rev Biochem. 2006;75:137–163. doi: 10.1146/annurev.biochem.75.103004.142702. [DOI] [PubMed] [Google Scholar]
- 2.Hardie DG. AMP-activated/SNF1 protein kinases: conserved guardians of cellular energy. Nat Rev Mol Cell Biol. 2007;8:774–785. doi: 10.1038/nrm2249. [DOI] [PubMed] [Google Scholar]
- 3.Shackelford DB, Shaw RJ. The LKB1-AMPK pathway: metabolism and growth control in tumour suppression. Nat Rev Cancer. 2009;9:563–575. doi: 10.1038/nrc2676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Adams GB, Scadden DT. The hematopoietic stem cell in its place. Nat Immunol. 2006;7:333–337. doi: 10.1038/ni1331. [DOI] [PubMed] [Google Scholar]
- 5.Moore KA, Lemischka IR. Stem cells and their niches. Science. 2006;311:1880–1885. doi: 10.1126/science.1110542. [DOI] [PubMed] [Google Scholar]
- 6.Orkin SH, Zon LI. Hematopoiesis: an evolving paradigm for stem cell biology. Cell. 2008;132:631–644. doi: 10.1016/j.cell.2008.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vooijs M, Jonkers J, Berns A. A highly efficient ligand-regulated Cre recombinase mouse line shows that LoxP recombination is position dependent. EMBO Rep. 2001;2:292–297. doi: 10.1093/embo-reports/kve064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shaw RJ, et al. The tumor suppressor LKB1 kinase directly activates AMP-activated kinase and regulates apoptosis in response to energy stress. Proc Natl Acad Sci U S A. 2004;101:3329–3335. doi: 10.1073/pnas.0308061100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Corradetti MN, Inoki K, Bardeesy N, DePinho RA, Guan KL. Regulation of the TSC pathway by LKB1: evidence of a molecular link between tuberous sclerosis complex and Peutz-Jeghers syndrome. Genes Dev. 2004;18:1533–1538. doi: 10.1101/gad.1199104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen C, et al. TSC-mTOR maintains quiescence and function of hematopoietic stem cells by repressing mitochondrial biogenesis and reactive oxygen species. J Exp Med. 2008;205:2397–2408. doi: 10.1084/jem.20081297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gan B, DePinho RA. mTORC1 signaling governs hematopoietic stem cell quiescence. Cell Cycle. 2009;8:1003–1006. doi: 10.4161/cc.8.7.8045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gan B, et al. mTORC1-dependent and -independent regulation of stem cell renewal, differentiation, and mobilization. Proc Natl Acad Sci U S A. 2008;105:19384–19389. doi: 10.1073/pnas.0810584105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ito K, Bernardi R, Pandolfi PP. A novel signaling network as a critical rheostat for the biology and maintenance of the normal stem cell and the cancer-initiating cell. Curr Opin Genet Dev. 2009;19:51–59. doi: 10.1016/j.gde.2009.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yilmaz OH, et al. Pten dependence distinguishes haematopoietic stem cells from leukaemia-initiating cells. Nature. 2006;441:475–482. doi: 10.1038/nature04703. [DOI] [PubMed] [Google Scholar]
- 15.Puigserver P, Spiegelman BM. Peroxisome proliferator-activated receptor-gamma coactivator 1 alpha (PGC-1 alpha): transcriptional coactivator and metabolic regulator. Endocr Rev. 2003;24:78–90. doi: 10.1210/er.2002-0012. [DOI] [PubMed] [Google Scholar]
- 16.Shaw RJ, et al. The kinase LKB1 mediates glucose homeostasis in liver and therapeutic effects of metformin. Science. 2005;310:1642–1646. doi: 10.1126/science.1120781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kelly DP, Scarpulla RC. Transcriptional regulatory circuits controlling mitochondrial biogenesis and function. Genes Dev. 2004;18:357–368. doi: 10.1101/gad.1177604. [DOI] [PubMed] [Google Scholar]
- 18.Bardeesy N, et al. Loss of the Lkb1 tumour suppressor provokes intestinal polyposis but resistance to transformation. Nature. 2002;419:162–167. doi: 10.1038/nature01045. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.