Abstract
This unit describes methods for the digestion of human prostate clinical specimens, dye cycle violet (DCV) staining procedure for the identification, isolation, and quantitation of radiolabeled dihydrotestosterone (DHT) retention of side population cells. The principle of the side population assay is based on differential efflux of DCV, a cell membrane permeable fluorescent dye, by cells with high ATP binding cassette (ABC) transporter activity. Cells with high ABC transporter activity efflux DCV and fall in the lower left quadrant of a flow cytograph are designated as “side population” cells. This unit emphasizes tissue digestion, DCV staining, flow settings for sorting side population cells and quantitation of radiolabeled DHT retention.
Keywords: Prostate, Side population, Dye cycle violet, ABC transporters
INTRODUCTION
Stem cells are hypothesized to be protected from toxins, hypoxia, and radiation (Ailles and Weissman, 2007; Keith and Simon, 2007; Rich and Bao, 2007; Pajonk et al., 2010), one possible mechanism is through expression of ATP binding cassette (ABC) transporters. ABC transporters are a highly conserved superfamily of proteins that efflux toxins, metabolic products and sterols. The biologic function of ABC transporters is to protect the cell from these compounds (Dean, 2009). Additionally, members of the ABC transporter superfamily ABCB1, ABCC1, and ABCG2, that contribute to the side population phenotype, are over expressed in multidrug resistant tumors and are one of the causes for chemotherapy resistance (Dean, 2009). The side population is classically isolated based on efflux of Hoechst 33342 dye by ABC transporter activity and is defined as a small dye negative population in the lower left panel of a flow cytograph (Kim et al., 2002; Scharenberg et al., 2002; Goodell et al., 1996). The side population assay enables isolation of cells by fluorescence activated cell sorting (FACS) with high ABC transporter function for further characterization.
The stem cell enrichment capability of the side population and stem cell characteristics of multipotency, self-renewal, and unlimited proliferative potential was originally defined in the bone marrow (Goodell et al., 1996). Enrichment for stem cells based upon the side population phenotype has been applied to many adult tissues, solid tumors and cell lines. The side population isolated from the bone marrow of mice contains Lin−CD34−c-kit+Sca-1+ hematopoietic stem cells that can repopulate the bone marrow of sublethally irradiated mice (Zhou et al., 2002; Zhou et al., 2001).
This unit describes the methods used for: (i) Digestion of human prostate clinical specimen; (ii) Isolation of side population cells by FACS based on differential efflux of DCV dye from the single cells digested from the clinical prostate specimen; (iii) Quantitation of radiolabeled dihydrotestosterone (DHT) retention in isolated side population and non-side population cells.
BASIC PROTOCOL 1
DIGESTION OF HUMAN PROSTATE CLINICAL SPECIMEN
Note: Approval from the institutional Internal Research Board (IRB) for use of de-identified excess human tissue in accordance with guidelines established at the National Institute of Health is required in these protocols. Approval from the institutional biosafety and radiation committees is also required.
This protocol describes the procedure used for digestion of the human prostate clinical specimen as described previously (Mathew et al., 2009) with some minor modifications. The digestion is performed in two steps: (i) Primary digestion; and (ii) Secondary digestion. Secondary digestion is followed by a gradient centrifugation step in order to enrich for the epithelial cell population.
PRIMARY DIGESTION
Reagents and Materials required
Primary digestion solution (see recipe)
Sterile 1X Phosphate Buffered Saline (PBS) (ice-cold) (see recipe) (Invitrogen)
10% Bleach solution (for disinfection)
Sterilized Pasteur pipettes
Non-tissue culture plate(s) (VWR)
Sterile forceps
Sterile scalpels
Sterilized scissors and tweezers
Sterilized conical flask with a stirrer (50-125 ml flasks are used based upon the amount of specimen received)
10 ml wide-tip pipette(s) (VWR)
Disposable gown
Protective glasses
Face mask
Cold room
Stir plate
Water bath set at 37°C
Laminar air flow bench
Aspiration-filtering tubes connected to plastic vacuum flasks and secondary receptacles.
Procedure
Note: It is best if a dedicated hood can be used for primary digestion and culture because of the high risk of contamination of the digested specimen with cell lines or vice versa. Aspiration wherever required is performed using sterile Pasteur pipettes into a plastic vacuum flask with filtered tubing to avoid contamination of vacuum line.
All materials used in this protocol including scissors, tweezers, conical flask with magnetic stirrer, Pasteur pipettes, and forceps must be sterilized. Scissors, tweezers, and forceps must be soaked in a 10% bleach solution or other appropriate disinfectant for at least 30-60 min and then washed and wrapped in self-seal sterilization pouch (Cardinal Health), autoclave sterilized prior to re-use.
Place the tissue in a non-tissue culture plate and cut into fine pieces, approximately 2 mm3 in size, using sterilized scissors and forceps.
Cut the tissue into smaller pieces, approximately 1 or <2 mm3, with sterile disposable scalpels. Tissue is not of a uniform size at this point.
-
Transfer the cut pieces of tissue, with sterile scalpels into a sterile conical flask with a magnetic stirrer and add sufficient sterile, ice cold 1X PBS to cover the tissue (about 12 ml/gm of tissue) and leave the digestion on a magnetic plate for 5-10 min at 4°C (cold room) at a medium speed setting.
Note: Too high of a speed will cause the conical flask to move away from the magnetic field.
Remove the 1X PBS by carefully aspirating the supernatant once the tissue has settled.
-
Add sufficient volume of primary digestion solution in order to cover the tissue (about 12 ml/gm of tissue) and digest the specimen for 12-16 hrs at 4°C.
Note: Do not exceed 16 hrs of digestion to ensure good yield of epithelial cells.
Note: Clean the surface of the laminar hood with 10% bleach solution or other appropriate disinfectant after each procedure. Additionally throughout entire procedure all spills must be cleaned with an appropriate disinfectant.
SECONDARY DIGESTION
Reagents and Materials required
Materials used in primary digestion plus:
Secondary digestion solution (thaw at 4°C and then place in the 37°C water bath before use) (see recipe)
Hanks buffered salt solution (HBSS) (Invitrogen)
Fetal Bovine Serum (FBS) (Invitrogen)
10% Bleach solution (for disinfection)
Sterilized sink strainers
50 ml centrifuge tubes (Krackeler)
10 ml and 25 ml pipettes (VWR)
100, 70, and 40 μm BD Falcon strainer(s) (BD Biosciences)
Procedure
Retrieve the conical flask with the primary digest from cold room.
Place a sterile sink strainer over the opening of a 50 ml centrifuge tube labeled “primary digest.”
Using a 10 ml wide-tip pipette, pipette the contents of the flask up and down about 10-20X to break up any undigested tissue.
Transfer the contents of the flask into the labeled 50 ml centrifuge tube.
Use 2-3X the volume of Hanks+5% FBS to rinse out the flask and sink strainer. Collect all liquid in the 50 ml centrifuge tube.
Place the 50 ml centrifuge tube with the primary digestion solution and Hanks on ice.
Using a sterile scalpel place strainer contents on a non-tissue culture plate.
-
Re-cut the contents of the sink strainer on the plate using sterile scalpels.
Note: Tissue re-clumps after primary digestion, so re-cutting is necessary.
-
Return the pieces to the flask containing a stirrer using one of the scalpels.
Note: The pieces of tissue can be transferred into the same conical flask with a stirrer that was used for primary digestion.
Add same volume of secondary digestion solution as the primary digestion solution (about 12 ml/gm of starting tissue).
Place the flask on a stirrer at 37°C (warm room or incubator) at a medium speed setting for 1 hr.
-
Pass the strained liquid from the primary digestion to a new 50 ml centrifuge tube through 100, 70, and then 40 μm pore cell strainers (BD Biosciences) consecutively.
Note: While the secondary digestion is taking place, strain the primary digestion through 100, 70, and then 40 μm pore cell strainers (BD Biosciences) to collect single cells.
If the cell strainer gets clogged, pipette gently up and down, making sure not to puncture the mesh. The consecutive straining steps improve yield of epithelial cells.
Remove filter and place the final tube with the digested cells from primary digestion on ice.
Retrieve the secondary digestion flask after 1 hr incubation at 37°C.
Follow the straining steps (repeat steps 2-5 and 12) done for primary digest solution.
Centrifuge both the 1st digestion and 2nd digestion tubes at 800g for 10 minutes to obtain cell pellets. Perform centrifugation at 4°C for the next centrifugation steps. Add Hanks to balance the tubes.
CELL SUSPENSION
Reagents and Materials required
Epithelial cell culture medium (Invitrogen)
Hanks buffered salt solution (HBSS) (Invitrogen)
Fetal Bovine Serum (FBS) (Invitrogen)
Histopaque-1077 (Sigma)
Autoclaved Pasteur pipettes (VWR)
P1000 and tips (VWR)
15 ml centrifuge tubes (VWR)
5 ml pipettes (VWR)
Procedure
-
Retrieve tubes with the primary and secondary digestion solutions from the centrifuge and carefully aspirate supernatant.
Note: Be careful about rate of aspiration and disturbing the cell pellet.
Resuspend cell pellet in approximately 2 ml of Hanks+5% FBS. Transfer contents of both tubes into a labeled 15 ml centrifuge tube. Let sit for 3 minutes.
-
Establish a Histopaque gradient; add an equal volume (2 ml) of Histopaque to each tube below the cell suspension.
Note: The gradient is established by putting pipette tip with Histopaque all the way to the bottom of the tube and gently pipetting in Histopaque, avoid air bubbles. Lift pipette gently as you add the Histopaque. Gently remove pipette once you are done. Histopaque can be purchased sterile or sterilized by filtering through a 0.22 μm pore filter membrane.
-
Centrifuge the tube at 1500g for 20 minutes at 4°C.
Note: After centrifugation, the epithelial cells are at the Hanks and Histopaque interface.
Aspirate supernatant. Leave some liquid above the interface and be careful to not aspirate the epithelial cells.
Transfer the rest of the liquid including a small fraction of the Histopaque and the epithelial cells at the interface to a labeled 15 ml centrifuge tube.
Add 5 ml ice cold Hanks+5% FBS to the tube. Centrifuge the tube at 800g for 5 minutes to pellet the epithelial cells at 4°C.
Retrieve the centrifuge tube and aspirate the supernatant.
Add appropriate volume (about 1 ml) of epithelial cell culture medium and determine the cell number.
BASIC PROTOCOL 2
DYE CYCLE VIOLET (DCV) STAINING
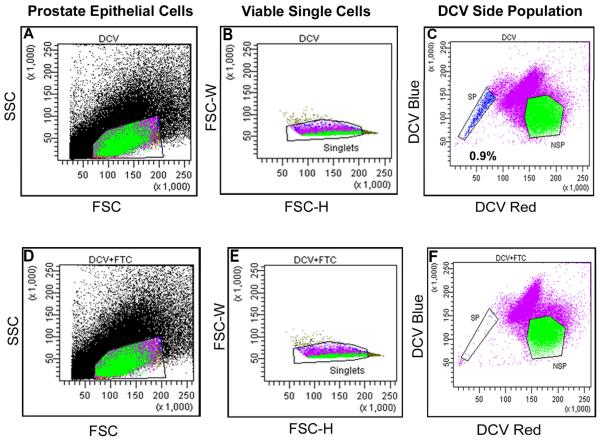
This protocol describes the procedure for DCV staining as previously described (Mathew et al., 2009) with some minor modifications. DCV is a fluorescent dye that is cell membrane permeable. DCV is a substrate for ABCG2 efflux transporter that is excited by a violet laser, whereas Hoechst 33342 requires excitation by an UV laser. DCV is an alternative to Hoechst for identification of the side population. Fumitremorgin C (FTC) is used in all experiments to inhibit ABCG2 function and to determine where DCV effluxing cells are located. Side population cells retain DCV when ABCG2 is inhibited with FTC (Fig. 1F).
Figure 1. Side population profile in human clinical prostate specimen (NT1) based upon DCV efflux.
- FSC – Forward Scatter
- SSC – Side Scatter
- FSC-H – Forward Scatter Height
- FSC-W – Forward Scatter Width
- SP – Side Population
- NSP – Non-side Population
Reagents and Materials required
Dye Cycle Violet (DCV) dye (5 mM stock solution) (Invitrogen)
Fumitremorgin C (FTC) (10 mM stock solution) (Alexis Biochemicals)
7-Aminoactinomycin D (7AAD) (10 μl/1 ml cell solution) (Beckman Coulter)
15 ml centrifuge tubes (VWR)
5 ml Polystyrene tubes with cell strainers lids (BD Biosciences)
Procedure
Resuspend the pelleted cells in 1 ml of epithelial culture medium and determine the concentration of cells (number of cells/1 ml).
- Each digested tissue is divided into four tubes including all controls:
- Untreated
- DCV+FTC+7AAD
- DCV+7AAD (Sample to sort)
- 7AAD
-
Each tube should ideally consist of 1×106 cells/0.5 ml.
Note: In the event the amount of the clinical specimen does not produce 4×106 cells for an ideal experiment, divide the cells obtained after digestion so that the sample to sort (iii) DCV+7AAD has enough cells. (See Table 1 for approximate number of viable cells obtained from each tissue sample).
-
In the dark, add 0.5 μl/0.5 ml of 10 mM stock solution of fumitremorgin C (FTC) to obtain a 10 μM final concentration to tube ii (DCV+FTC+7AAD) containing 1×106 cells/0.5 ml.
Note: FTC is light sensitive. So all work must take place with minimal light and samples should be protected from the light at all times.
Vortex the tubes and then place at 37°C for 20 minutes. Cover the tubes to protect them from light.
Vortex cells again at the end of 20 minutes.
-
In the dark, add 1 μl of DCV to tube ii (DCV+FTC+7AAD) and tube iii (DCV+7AAD) with 0.5 ml cell solution to obtain a final concentration of 10 μM.
Note: DCV is light sensitive. 1 μl of DCV added to 500 μl of medium with cells gives a final concentration of 10 μM. If performing for the first time or with a different cell number, determine optimal concentrations of DCV depending on the cell type/density for the assay.
-
Vortex the tubes and place at 37°C for 90 minutes. Cover the tubes to protect them from light. Vortex tubes every 15-20 minutes for the duration of the incubation.
Note: Ensure that all samples are immersed in water in the bath and are maintained at 37°C during the entire incubation period. Water bath should not be used to thaw frozen bottles as this changes temperature drastically.
Centrifuge all samples at 800g for 5 min at 4°C and wash cells with cold 1X PBS before resuspending cells in Hanks+5% FBS buffer.
- Finally, in the dark, add 5 μl of 7AAD to tubes:
-
iiDCV+FTC+7AAD
-
iiiDCV+7AAD
-
iv7AAD
-
ii
Vortex the tubes and keep at 4°C. Cover the tubes to protect them from light.
-
Add 0.5 ml of Hanks+5% FBS to each collection tube used for collecting the sorted cells.
Note: The cells should be placed on ice before being analyzed using flow cytometer, otherwise the loss of DCV dye could lead to detection of false positive side population.
Table 1.
The percentage of side population cells relative to total viable cells from human prostate non-tumor and tumor specimens
| NT/T | Specimen weight (gram) |
% viability |
Total viable cells analyzed |
% SP/viable cells |
Total SP cells sorted |
% NonSP gated |
% Efficiency of SP sorted cells |
|---|---|---|---|---|---|---|---|
| NT1* | 5.820 | 42.1 | 2.50×105 | 0.9 | 5013 | 11.9 | 98 |
| NT2 | 11.423 | 12.4 | 1.60×104 | 0.2 | 341 | 3.2 | 98 |
| NT3 | 3.651 | 86.6 | 1.58×106 | 0.2 | 2514 | 16.6 | 94 |
| NT4 | 1.678 | 26.4 | 1.59×105 | 0.4 | 10856 | 5.1 | 97 |
| NT5 | 4.246 | 12.2 | 9.40×104 | 1.2 | 10173 | 2.0 | 92 |
| T5 | 3.729 | 5.2 | 2.25 ×106 | 0.2 | 11224 | 0.7 | 92 |
| NT6 | 1.988 | 34.5 | 3.53×105 | 1.0 | 12616 | 6.0 | 100 |
| T6 | 1.786 | 36.8 | 4.25×105 | 1.2 | 15292 | 6.6 | 66 |
NT – Histological Non-tumor portion of radical prostatectomy
T – Tumor
SP – Side population
NonSP – Non-side population
NT1 – DCV side population profile is as shown in Fig 1.
% Efficiency – Cells within parameters of gate settings.
BASIC PROTOCOL 3
SORTING SIDE POPULATION CELLS USING FLUORESCENT ACTIVATED CELL SORTING (FACS)
This protocol describes the procedure used for sorting side population cells using FACS as described previously (Mathew et al., 2009) with some minor modifications. The technique utilizes the property of ABC transporter efflux of DCV to identify a small population of viable cells on the lower left panel that are located on a flow cytograph. This minor population of cells is referred to as the side population.
Equipment used
BD FACS Aria II cell sorter
Materials required
5 ml Borosilicate disposable glass culture tubes (Kimble Chase)
Analysis and gating strategy for detecting DCV-labeled cells
The characteristic emission profile of DCV demonstrates strong maxima in both the blue and red regions of the spectra following excitation by a violet laser. The analysis and gating strategy for detecting the DCV stained cells on BD Aria II is as follows:
Note: Procedure may vary due to software and instrumentation.
DCV is excited by a violet diode laser at 408 nm (Coherent Inc.). Therefore, analyze the cells detected as DCV containing based on high fluorescence emission of DCV in the blue fluorescence through a 450/40 nm bandpass (BP) filter and the red fluorescence through a 650 nm longpass (LP) filter.
For sorting, exclude the non-viable cells based upon uptake of 7AAD. Utilize a 488 nm SapphireTM laser to excite 7AAD; with emission monitored through a 695/40 nm bandpass filter.
The gating strategy for analysis or flow sorting of the side population is the same. Use dual-parametric analysis of Forward Scatter (FSC) versus Side Scatter (SSC) as the primary gate to identify cells in the appropriate size range, and to eliminate debris.
Evaluate the cells falling in the primary gate subsequently by analysis of SSC height versus SSC width, followed by FSC height vs. FSC width, for doublet discrimination.
In addition, evaluate cells within the primary gate for 7AAD uptake to eliminate dead cells.
The live, singlet cells are displayed in a dual-parametric presentation of DCV red fluorescence (x-axis) vs. DCV blue fluorescence (y-axis).
-
Finally, determine the location of the side population based upon loss of the putative side population in a population pre-incubated with FTC (See Figure 1). The side population appears in DCV alone stained sample and disappears when the cells were stained with DCV in the presence of FTC due to inhibition of ABCG2 pump.
Note: The DCV+FTC+7AAD tube is used for gating side population.
Use back gating of the side population to confirm the proper location and size of the gates in all histograms. When back gating display only the side population cells and non-side population cells in each histogram in order to position and/or resize the gates. This technique improves sort efficiency.
-
Collect sorted cells in a borosilicate disposable glass culture tubes containing 0.5 ml Hanks solution.
Note: Do not use polystyrene round-bottomed tubes, because during cell sorting the flow droplets may stick to the side walls of the tube. The use of glass tubes for collecting the samples avoids this problem.
-
Gate for side population and viable cells using the available software.
Note: The entire cell sorting and sample collection is performed at 4°C in a sterile environment. Analysis software is chosen based upon availability. Software used to generate Figure 1 was BD FACSDiva software version 6.1.2.
BASIC PROTOCOL 4
QUANTITATION OF RETENTION OF RADIOLABELED ANDROGENS (DHT)
This protocol describes the procedure used for quantitation of retention of radiolabeled dihydrotestesterone as described previously (Huss et al., 2005) with some minor modifications. Isolated viable side and non-side population cells can be used for a number of assays comparing the two phenotypes. This protocol is an example of the retention assay that can be performed with any effluxed radiolabeled molecule (e.g. steroids, taxols, or chemotherapic agents). Transported compounds can be screened using membrane vesicles prepared from cells that over express the transporter of interest. Membrane vesicles over expressing specific ABC-transporters in baculovirus-infected Sf9 insect cells are commercially available (BD Biosciences) and are a powerful tool for screening compounds that can be effluxed due to high and human specific protein expression.
Note: Optimization of concentrations and incubation times may need to be performed for each experiment.
Reagents and Materials required
Epithelial cell culture media (Invitrogen)
[3H]dihydrotestosterone
Fumitremorgin C (FTC) (Alexis Biochemicals)
Scintillation counter
Scintillation fluid
Assay tubes
15 ml centrifuge tubes (VWR)
2N NaOH
2N HCl
Procedure
-
Divide side population cells into the following tubes:
- Side population cells + FTC + [3H]dihydrotestosterone
- Side population cells + [3H]dihydrotestosterone
Note: Repeat for non-side population cells and the unsorted epithelial cells.
-
Pre-incubate isolated side population cells, non-side population cells, and/or unsorted epithelial cells with 10 μM concentration of FTC for 20 min at 37°C.
Note: Use same number of cells for each treatment group. About 103-104 cells per 0.5 ml sample would be an optimal cell number to perform the assay.
After pre-incubation, incubate the cells with 3 nmol/L of [3H]dihydrotestosterone with/without FTC for 2 hrs at 37°C.
Wash the cells with PBS to resuspend the pellet by centrifugation at 800g for min at 4°C.
Remove PBS and lyse the cells with 2N NaOH.
Neutralize the lysates with equal amount of 2N HCl.
Transfer neutralized lysates to scintillation counter tube and fill with appropriate amount of scintillation fluid.
Measure radioactivity in scintillation counter.
Calculate fold increase of [3H]DHT retained in cells with FTC inhibition compared to without inhibitor for each phenotype tested.
Note: Retained [3H]DHT is quantified by liquid scintillation counting. [3H] cannot be detected with Geiger counter, swipe tests should be performed on regular basis to monitor for contamination.
Radioactivity = cpm/counting efficiency X specific activity
cpm – counts per minute
REAGENTS AND SOLUTIONS
Solutions for human prostate clinical specimen digestion
Prepare dispase (Invitrogen) stock solution (48 units (U)/ml) to be used for preparation of the primary and secondary digestion solutions.
20X (48 U/ml) dispase stock solution
Dissolve 5 g of dispase at a 1.76 U/mg concentration in 183.3 ml volume of dulbecco’s phosphate buffered saline (DPBS) (Invitrogen) to obtain a final concentration of 48 U/ml. Aliquot and store at −20°C until used.
Note: Filter through a 0.22 μm membrane filter.
Primary digestion solution
Prepare dispase solution (2.4 U/ml) from the 20X dispase stock solution. Dilute the 20X stock solution to 1X with DPBS. Filter through 0.22 μm filter membrane, aliquot and store at −20°C. Thaw the solution as needed.
Note: Filter through a 0.22 μm membrane filter.
Secondary digestion solution
The final concentration is:
Collagenase II 0.28% w/v
DNase 0.01% w/v
Dispase 2.4 U/ml
Preparation
To obtain the above-mentioned final concentrations of each ingredient, to a sterile 500 ml bottle add the following:
239.24 ml of DBPS.
0.03571 g of DNase.
17.86 ml of 48U/ml dispase (From stock solution).
To a 1 g bottle of collagenase II add a total of 100 ml of DPBS. Dissolve completely.
Dissolve dispase and DNase in 25 ml of DPBS. Add to 100 ml collagenase II. Mix well.
Mix the two solutions in 500 ml sterile bottle and make final volume to 357.1 ml in DPBS.
Aliquot and store at −20°C. Thaw the solution as needed.
Note: Filter through a 0.22 μm membrane filter.
2N NaOH
Dissolve 0.8 g of sodium hydroxide (anhydrous) in 10 ml of sterile distilled water. Vortex briefly to ensure mixing of solution. The solution can be stored at room temperature.
Note: Filter through a 0.22 μm membrane filter.
1X PBS
10X PBS is purchased from Invitrogen and then diluted 1X with distilled water to obtain 1X PBS which is used in all experiments.
Fumitremorgin C
To 250 μg fumitremorgin C add 65.9 μl of dimethly sulfoxide (DMSO) to obtain a stock solution of 10 mM. Aliquot and store at 4 °C. Protect from light.
COMMENTARY
A. Background Information
Detection of cells with ABC transporter efflux capabilities is performed based upon efflux of fluorescent substrates. Fluorescent dye exclusion was first demonstrated in hematopoietic stem cells isolated from bone marrow by FACS based upon rhodamine 123 dye efflux (Udomsakdi et al., 1991). The side population cells, located in the lower left quadrant of the flow cytograph, effectively efflux the cell membrane permeable Hoechst 33342 and segregate from cells that retain Hoechst 33342. Hoechst 33342 emits within the Red and Blue spectrum upon binding DNA and UV excitation (Goodell et al., 1996). Several ABC transporters can contribute to defining the side population, but ABCB1 (formally Mdr1 and p-glycoprotein) and ABCG2 (formally Bcrp) are the main contributors (Zhou et al., 2001).
The side population assay enriches for stem cells in many organs, however this may not be universal for all somatic stem cells or cancer stem cells. All cells isolated based on the side population phenotype need to be appropriately characterized to determine if they contain stem cell properties. The side population assay is a functional assay for viable cells with high transporter activity. The stem cells outside of the side population lack high transporter activity but can still retain true stem cell properties. Cells within the side population are resistant to chemotherapy (Bertolini et al., 2009; Chikazawa et al., 2010; Dean et al., 2005; Hirschmann-Jax et al., 2004; Wulf et al., 2001) and radiation (Woodward et al., 2007). In addition to cell protection mechanisms, the ABC transporters have been implicated in protection from differentiation signals and efflux of many sterols. In Dictystelium cells, an efflux pump (RhT), similar to the ABC transporters, efflux differentiation-inducing factor (DIF-1) (Good and Kuspa, 2000). ABCB1 mediates intracellular cholesterol transport, a mechanism by which progesterone interaction with ABCB1 inhibits the esterification of cholesterol (Debry et al., 1997). Our previous work showed that ABCG2-expressing cells have low retention of dihydrotestosterone (DHT) (Huss et al., 2005). Inhibitors of ABCG2 in the presence of androgen also increased nuclear AR expression. ABCB1-mediated efflux of DHT in prostate cancer cell lines demonstrated modulation of prostate specific antigen (PSA) expression (Fedoruk et al., 2004).
Identification of the prostate side population has been performed in prostate tissue obtained from patients undergoing radical prostatectomy, cystoprostatectomy, and trans-urethral resection of the prostate (TURPs) for benign prostate, BPH and malignant disease (Bhatt et al., 2003; Brown et al., 2007; Mathew et al., 2009). The side population and cells expressing ABCG2 (isolated by magnetic beads conjugated to an antibody against ABCG2) were isolated from the prostate. Affymetrix Array analysis indicates considerable overlap of gene expression between the side population and ABCG2 expressing cells from the prostate (Pascal et al. 2007). In the prostate the ABC transporters, ABCG1, ABCG2, ABCB1, and ABCE1 were expressed at higher levels in the side population compared to ABCG2 expressing cells (Pascal et al. 2007).
B. Critical Parameters
Successful isolation of the side population from solid tissue depends on timely excess and proper digestion of fresh human tissue. The procurement of human prostate specimen used in these protocols is as described by Morrison et al. (2009). Minor modifications may be needed for other types of tissue. While many insights can be gained from experiments performed in cell lines, the side population phenotype in cell lines probably does not represent a stem cell phenotype. Optimum tissue digestion results in single cells to be used in flow cytometry, with little debris and highly enriched for epithelial cells. Dispase is a very mild enzyme that allows for a relatively convenient digestion period, 12-16 hours, as most of the procured tissues at our institution are received in the laboratory in the afternoon. Dispase digestion results in sheets of cells and very few single cells, a more stringent digestion is required for a single cell suspension. Epithelial cell enrichment is required prior to staining, if culturing is not performed, to remove debris.
The original side population phenotype is based on the excitation by a UV laser of Hoechst 33342 bound to DNA (Goodell et al., 1996). UV lasers are not available on all flow cytometry instruments, whereas the more common violet laser is available on most flow cytometers. Unfortunately the emission in the 395- to 410 nm is not optimal for Hoechst 33342 excitation for a clear side population analysis. The cell permeable Dye Cycle Violet (DCV) excitation and emission is with the more common violet laser thus providing an alternative substrate to Hoechst 33342 for side population analysis (Telford et al., 2007). Depending on the laser availability and other considerations, such as overlapping emissions if other analysis are being performed in tandem, there is a choice between DCV and Hoechst 33342 for side population determination (Telford, 2010). If Hoechst 33342 staining procedure is considered for isolation of side population, detailed information about Hoechst 33342 dye concentration, incubation time, and cell number is as described by Goodell (2005) and Lin and Goodell, (2006).
In this unit we describe the method used for the digestion of the human prostate clinical specimen, isolation of cells with the side population phenotype based on DCV efflux, and quantitation of radiolabeled DHT retention based upon ABC transporter efflux. Successful isolation and appropriate yield of side population cells depend on the following: (i) preparation of the clinical sample and (ii) cellular staining. The side population assay is based on differential efflux of DCV dye, therefore attention to tissue digestion to prepare single epithelial cell suspension, cell density, DCV dye concentration, staining time, staining temperature, and flow cytometry gating strategies are all critical steps. Specifically:
The tissue must be washed with 1X PBS before addition of the primary digestion solution to remove any debris that could inhibit digestion.
The smaller pieces the tissue is cut (approximately 2 mm3 in size) ensures proper disaggregation and sieving through the 100, 70 and 40 μm strainers. Straining the tissue initially through a sterile sink strainer facilitates future straining steps.
Primary tissue digestion should not exceed 16 hrs to avoid excessive digestion of the sample, more than 16 hrs increases cell death. Primary digestion must be done at 4°C for the dispase to be active.
Presence of DNase in the secondary digestion solution is important as necrotic and dead cells in the sample release DNA that can cause clumping of the live cells. DNase helps prevent this situation.
Ensure that the secondary digestion is performed at 37°C (Basic protocol 1; step 11, procedure for secondary tissue digestion).
Yield of epithelial cells is directly correlated to straining steps (Basic protocol 1; steps 2-5 and 12, procedure for secondary digestion).
Histopaque gradient is important to ensure removal of red blood cells and lymphocytes. Presence of blood cells may fall in the side population region in the scatter plot and cause difficulty in gating.
Use low volumes of vehicles (Dimethyl sulfoxide (DMSO)) for treatment or dissolving the inhibitor or other drugs to reduce the vehicle effect.
If detecting more than one marker, do not choose antibodies that fluoresce in the same fluorochrome region. This will cause difficulty in compensation and detecting the cells with the specific parameters being analyzed.
Positive control such as hematopoietic cells ensures that the experimental conditions are working (Basic protocols 2 and 3).
Once the staining is complete (Basic protocol 2; Step 8), perform the remaining protocol at 4°C to prevent loss of DCV dye retention in non-side population cells with low ABC transporter function. Loss of DCV dye retention leads to a false positive side population.
Use a cell viability marker to ensure exclusion of dead cells. In the method described in this unit, we used 7AAD as a viability marker since it does not interfere with DCV fluorochrome.
Leave the samples on ice (4°C) for ~1 hr, but not much longer, after Basic protocol 2; step 11, before sorting, (Basic protocol 3), to achieve dye equilibrium.
Additionally, it is important to maintain sterile conditions throughout the cell sorting procedure if the sorted cells are planned for sterile procedures such as cell culture or in vivo experiments.
Basic protocol 4 (steps 5 and 6): Be sure to perform the lysate preparation under ice-cold conditions.
Note: Since the side population is transitory, each investigator must optimize the time required for staining, concentration of dye used based on the type of sample being used. Too high of dye concentration, or too long of incubation time may cause the side population to disappear.
C. Troubleshooting Guide
Potential problems with protocols described in this unit include the following:
| Problem | Possible cause | Possible solution |
|---|---|---|
|
Human prostate digestion Low yield of epithelial cells |
Low quality starting specimen. | Avoid extensive time between tissue procurement and starting of digestion. |
| Over digestion of the tissue specimen. |
Follow appropriate digestion time which should not exceed 16 hrs. |
|
| Exces specimen (for example greater than 3 g). |
Use a sink strainer in the first step if necessary. |
|
| Improper straining and/or gradient centrifugation. |
Care must be taken in removing the pellet from the interface after gradient centrifugation. Follow all straining steps. |
|
|
DCV Staining and FACS No definitive side population |
Loss of DCV after staining procedure. |
Keep the samples on ice before FACS |
| Concentration of inhibitor is too high causing a shift in the population. |
Optimize the concentrations of dye and inhibitor based on the cell type being investigated. Always use a positive control when testing for the presence of side population in a cell type for the first time. |
|
| No difference between inhibitor + DCV and DCV alone samples. |
Check the settings in the FACS to ensure proper detection of the cells. |
|
|
Radiolabeling with DHT Low radioactive detection |
Loss of [3H]DHT after retention. |
Maintain cold conditions once radiolabeled DHT is in cells. |
| No cells retain [3H]DHT. | Use optimal concentration of inhibitor(s) and cell number. |
|
| Cell lysate preparation | Ensure the quality of NaOH solution in order to properly lyse the cells. |
D. Anticipated Results
Detecting cells with high ABCG2 activity based on efflux of DCV dye depends on the quality of the clinical specimen and its preparation. Working with a clinical specimen of 1 g will yield approximately 1×106 epithelial cells upon digestion. A range of 0.2-1.2% viable cells have a side population phenotype isolated by FACS (see Table 1). The number of side population cells collected is not always related to the amount of starting material. NT2 was 11.423 g but yielded only 341 side population cells with a low viability (see Table 1). The percent efficiency of sorted side population cells is defined as the efficiency of the flow cytometer to sort the cells based on the parameters set at the time of gating. A low efficient sort signifies that the tissue specimen has more cells which were outside the gating parameters. The percent efficiency of T6 specimen is 66% indicating a large number of cells were not collected based on the parameters set at gating (see Table 1). Proper staining technique and the use of an appropriate viability marker that does not interfere with DCV dye improves the detection of live cells. Collecting viable cells with a side population phenotype with high efficiency and the non-side population counterpart is essential for studies such as measuring the radiolabeled DHT retention, drug resistance, or stem cell properties.
E. Time Considerations
The amount of time required to measure the protective effect of ABC transporters on prostate cells is divided into three phases: (i) Tissue digestion; (ii) Cellular staining and cell sorting; and finally (iv) Measuring androgens (DHT) retained in ABC transporters. The first phase of the protocol, tissue digestion, is divided into cutting the tissue specimen, washing with 1X PBS, primary and secondary digestions steps. The total time required to cut a 1 gm tissue specimen is approximately 20-30 min. The primary digestion takes from 12-16 hrs. This is followed by straining the primary digest solution, and incubation for 1 hr with secondary digest solution. Straining the secondary digest solution and gradient centrifugation followed by collecting the cell suspension takes approximately 3 hrs. The second phase consisting of cell staining and sorting (Basic protocols 2 and 3) takes 3 hrs and 2 hrs respectively. With experience a senior investigator can perform the entire straining and cell pellet collecting steps in Basic protocol 1 in approximately 2 hrs; however the first time takes considerably longer. The incubation times are critical and if shortened impairs the quality of the staining. The final phase of procedure for measuring the radiolabeled androgens (DHT) is performed in 2-3 hrs. With experience the individual protocols mentioned in this unit can be performed in a single day. The entire procedure of tissue digestion; staining; cell sorting; and finally measuring radioactivity can take from 2-3 days.
Key References.
Mathew, G., Timm, E.A. Jr., Sotomayor, P., Godoy, A., Montecinos, V.P., Smith, G.J., and Huss, W.J. 2009. ABCG2-mediated DyeCycle Violet efflux defined side population in benign and malignant prostate. Cell Cycle. 8:1053-61.
Initial reference for prostate tissue digestion and prostate DCV side population analysis.
Zhou, S., Schuetz, J.D., Bunting, K.D., Colapietro, A.M., Sampath, J., Morris, J.J., Lagutina, I., Grosveld, G.C., Osawa, M., Nakauchi, H., and Sorrentino, B.P. 2001. The ABC transporter Bcrp1/ABCG2 is expressed in a wide variety of stem cells and is a molecular determinant of the side-population phenotype. Nat. Med. 7:1028-34.
Demonstrates ABCG2 is required for mouse hematopoietic side population.
Telford, W.G., Bradford, J., Godfrey, W., Robey, R.W., and Bates, S.E. 2007. Side population analysis using a violet-excited cell-permeable DNA binding dye. Stem Cells 25:1029-1036.
Compares Hoechst 33342 and DCV side population.
Goodell, M.A. 2005. Stem cell identification and sorting using the Hoechst 33342 side population (SP). Curr. Protoc. Cytom. Chapter9:Unit9:18.
Lin, K.K., and Goodell, M.A. 2006. Purification of hematopoietic stem cells using the side population. Methods Enzymol. 420:255-264.
Telford, W.G. 2010. Stem cell side population analysis and sorting using DyeCycle violet. Curr. Protoc. Cytom. Chapter 9: Unit9 30.
Provide excellent protocols for Hoechst 33342 and DCV based side population analysis.
Acknowledgements
We thank Mame Diop, Department of Pharmacology and Therapeutics, for assistance with tissue digestion; Earl Timm, Jr., Department of Flow Cytometry, for assistance with side population analysis; and the Departments of Urologic Oncology and Pathology, Pathology Resource Network, for providing clinical prostate specimens, at Roswell Park Cancer Institute.
Literature Cited
- Ailles LE, Weissman IL. Cancer stem cells in solid tumors. Curr Opin Biotechnol. 2007;18:460–466. doi: 10.1016/j.copbio.2007.10.007. [DOI] [PubMed] [Google Scholar]
- Bertolini G, Roz L, Perego P, Tortoreto M, Fontanella E, Gatti L, Pratesi G, Fabbri A, Andriani F, Tinelli S, Roz E, Caserini R, Lo Vullo S, Camerini T, Mariani L, Delia D, Calabro E, Pastorino U, Sozzi G. Highly tumorigenic lung cancer CD133+ cells display stem-like features and are spared by cisplatin treatment. Proc Natl Acad Sci U.S.A. 2009;106:16281–16286. doi: 10.1073/pnas.0905653106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhatt RI, Brown MD, Hart CA, Gilmore P, Ramani VA, George NJ, Clarke NW. Novel method for the isolation and characterisation of the putative prostatic stem cell. Cytometry. 2003;54A:89–99. doi: 10.1002/cyto.a.10058. [DOI] [PubMed] [Google Scholar]
- Brown MD, Gilmore PE, Hart CA, Samuel JD, Ramani VA, George NJ, Clarke NW. Characterization of benign and malignant prostate epithelial Hoechst 33342 side populations. Prostate. 2007;67:1384–1396. doi: 10.1002/pros.20620. [DOI] [PubMed] [Google Scholar]
- Chikazawa N, Tanaka H, Tasaka T, Nakamura M, Tanaka M, Onishi H, Katano M. Inhibition of Wnt signaling pathway decreases chemotherapy-resistant side-population colon cancer cells. Anticancer. 2010;30:2041–2048. [PubMed] [Google Scholar]
- Dean M. ABC transporters, drug resistance, and cancer stem cells. J. Mammary Gland Biol. Neoplasia. 2009;14:3–9. doi: 10.1007/s10911-009-9109-9. [DOI] [PubMed] [Google Scholar]
- Dean M, Fojo T, Bates S. Tumour stem cells and drug resistance. Nat. Rev. Cancer. 2005;5:275–84. doi: 10.1038/nrc1590. [DOI] [PubMed] [Google Scholar]
- Debry P, Nash EA, Neklason DW, Metherall JE. Role of Multidrug Resistance P-glycoproteins in Cholesterol Esterification. Journal of Biological Chemistry. 1997;272:1026–1031. doi: 10.1074/jbc.272.2.1026. [DOI] [PubMed] [Google Scholar]
- Fedoruk MN, Gimenez-Bonafe P, Guns ES, Mayer LD, Nelson CC. P-glycoprotein increases the efflux of the androgen dihydrotestosterone and reduces androgen responsive gene activity in prostate tumor cells. Prostate. 2004;59:77–90. doi: 10.1002/pros.10354. [DOI] [PubMed] [Google Scholar]
- Goodell MA, Brose G, Paradis G, Conner AS, Mulligan RC. Isolation and functional properties of murine hematopoietic stem cells that are replicating in vivo. J. Exp. Med. 1996;183:1797–1806. doi: 10.1084/jem.183.4.1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Good JR, Kuspa A. Evidence that a cell-type-specific efflux pump regulates cell differentiation in Dictyostelium. Dev. Biol. 2000;220:53–61. doi: 10.1006/dbio.2000.9611. [DOI] [PubMed] [Google Scholar]
- Goodell MA. Stem cell identification and sorting using the Hoechst 33342 side population (SP) Curr. Protoc. Cytom. 2005 doi: 10.1002/0471142956.cy0918s34. Chapter9:Unit9:18. [DOI] [PubMed] [Google Scholar]
- Hirschmann-Jax C, Foster AE, Wulf GG, Nuchtern JG, Jax TW, Gobel U, Goodell MA, Brenner MK. A distinct “side population” of cells with high drug efflux capacity in human tumor cells. Proc. Natl. Acad. Sci. U.S.A. 2004;101:14228–14233. doi: 10.1073/pnas.0400067101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huss WJ, Gray DR, Greenberg NM, Mohler JL, Smith GJ. Breast cancer resistance protein-mediated efflux of androgen in putative benign and malignant prostate stem cells. Cancer Res. 2005;65:6640–6650. doi: 10.1158/0008-5472.CAN-04-2548. [DOI] [PubMed] [Google Scholar]
- Keith B, Simon MC. Hypoxia-inducible factors, stem cells, and cancer. Cell. 2007;129:465–472. doi: 10.1016/j.cell.2007.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim M, Turnquist H, Jackson J, Sgagias M, Yan Y, Gong M, Dean M, Sharp JG, Cowan K. The multidrug resistance transporter ABCG2 (breast cancer resistance protein 1) effluxes Hoechst 33342 and is overexpressed in hematopoietic stem cells. Clin Cancer Res. 2002;8:22–28. [PubMed] [Google Scholar]
- Lin KK, Goodell MA. Purification of hematopoietic stem cells using the side population. Methods Enzymol. 2006;420:255–264. doi: 10.1016/S0076-6879(06)20011-9. [DOI] [PubMed] [Google Scholar]
- Mathew G, Timm EA, Jr., Sotomayor P, Godoy A, Montecinos VP, Smith GJ, Huss WJ. ABCG2-mediated DyeCycle Violet efflux defined side population in benign and malignant prostate. Cell Cycle. 2009;8:1053–1061. doi: 10.4161/cc.8.7.8043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison C, Cheney R, Johnson CS, Smith G, Mohler JL. Central quadrant procurement of radical prostatectomy specimens. Prostate. 2009;69:770–773. doi: 10.1002/pros.20925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pajonk F, Vlashi E, McBride WH. Radiation resistance of cancer stem cells: the 4 R’s of radiobiology revisited. StemCells. 2010;28:639–648. doi: 10.1002/stem.318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascal LE, Oudes AJ, Petersen TW, Goo YA, Walashek LS, True LD, Liu AY. Molecular and cellular characterization of ABCG2 in the prostate. BMC Urol. 2007;7:6. doi: 10.1186/1471-2490-7-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rich JN, Bao S. Chemotherapy and cancer stem cells. Cell Stem Cell. 2007;1:353–355. doi: 10.1016/j.stem.2007.09.011. [DOI] [PubMed] [Google Scholar]
- Scharenberg CW, Harkey MA, Torok-Storb B. The ABCG2 transporter is an efficient Hoechst 33342 efflux pump and is preferentially expressed by immature human hematopoietic progenitors. Blood. 2002;99:507–512. doi: 10.1182/blood.v99.2.507. [DOI] [PubMed] [Google Scholar]
- Telford WG. Stem cell side population analysis and sorting using DyeCycle violet. Curr. Protoc. Cytom. 2010 doi: 10.1002/0471142956.cy0930s51. Chapter 9: Unit9 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Telford WG, Bradford J, Godfrey W, Robey RW, Bates SE. Side population analysis using a violet-excited cell-permeable DNA binding dye. Stem Cells. 2007;25:1029–1036. doi: 10.1634/stemcells.2006-0567. [DOI] [PubMed] [Google Scholar]
- Udomsakdi C, Eaves CJ, Sutherland HJ, Lansdorp PM. Separation of functionally distinct subpopulations of primitive human hematopoietic cells using rhodamine-123. Exp. Hematol. 1991;19:338–342. [PubMed] [Google Scholar]
- Woodward WA, Chen MS, Behbod F, Alfaro MP, Buchholz TA, Rosen JM. WNT/beta-catenin mediates radiation resistance of mouse mammary progenitor cells. Proceedings of the National Academy of Sciences. 2007;104:618–623. doi: 10.1073/pnas.0606599104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wulf GG, Wang RY, Kuehnle I, Weidner D, Marini F, Brenner MK, Andreeff M, Goodell MA. A leukemic stem cell with intrinsic drug efflux capacity in acute myeloid leukemia. Blood. 2001;98:1166–1173. doi: 10.1182/blood.v98.4.1166. [DOI] [PubMed] [Google Scholar]
- Zhou S, Morris JJ, Barnes Y, Lan L, Schuetz JD, Sorrentino BP. Bcrp1 gene expression is required for normal numbers of side population stem cells in mice, and confers relative protection to mitoxantrone in hematopoietic cells in vivo. Proc Natl Acad Sci U.S.A. 2002;99:12339–12344. doi: 10.1073/pnas.192276999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou S, Schuetz JD, Bunting KD, Colapietro AM, Sampath J, Morris JJ, Lagutina I, Grosveld GC, Osawa M, Nakauchi H, Sorrentino BP. The ABC transporter Bcrp1/ABCG2 is expressed in a wide variety of stem cells and is a molecular determinant of the side-population phenotype. Nat. Med. 2001;7:1028–1034. doi: 10.1038/nm0901-1028. [DOI] [PubMed] [Google Scholar]