Abstract
A second degree epidermal scald burn in mice elicits an inflammatory response mediated by natural IgM directed to non-muscle myosin with complement activation that results in ulceration and scarring. We find that such burn injury is associated with early mast cell (MC) degranulation and is absent in WBB6F1-KitW/KitWv mice which lack MCs in a context of other defects due to a mutation of the KIT receptor. To further address a MC role, we used transgenic strains with normal lineage development and a deficiency in a specific secretory granule component. Mouse strains lacking the MC-restricted chymase, mouse MC protease (mMCP)-4, or elastase, mMCP-5, show decreased injury following a second degree scald burn while mice lacking the MC-restricted tryptases, mMCP-6 and mMCP-7, or the MC-specific carboxypeptidase A3 activity are not protected. Histologic sections showed some disruption of the epidermis at the scald site in the protected strains suggesting the possibility of topical reconstitution of full injury. Topical application of recombinant mMCP-5 or human neutrophil elastase to the scalded area increases epidermal injury with subsequent ulceration and scarring, both clinically and morphologically, in mMCP-5-deficent mice. Restoration of injury requires that topical administration of recombinant mMCP-5 occurs within the first h post burn. Importantly, topical application of human MC chymase restores burn injury to scalded mMCP-4-deficient mice but not to mMCP-5-deficient mice revealing non-redundant actions for these two MC proteases in a model of innate inflammatory injury with remodeling.
Introduction
A superficial second degree epidermal burn results in an initial cellular injury that is amplified by an acute inflammatory response that includes edema and an influx of neutrophils (1,2). This inflammatory response is dependent on a natural IgM to uncovered epitopes and complement activation which are also components in the process of ischemic reperfusion (IR) injury of the mouse hind limb skeletal muscle (3-6). In IR injury of mouse hind limb skeletal muscle, we observed that myocyte injury occurred with reperfusion and was accompanied by progressive degranulation of mast cells (MCs) (7). The myocyte injury was known to be diminished in MC-deficient WBB6F1-KitW/KitWv (WWv) mice (8,9) and we observed significant protection in mice with targeted disruption of the elastase, mouse MC protease (mMCP)-5 (7). There was no protection against hind limb IR injury in strains lacking the MC-specific tryptase, mMCP-7, or heparin proteoglycan or other pro-inflammatory functions distributed not only to MCs such as generation of TNFa, expression of transmembrane tryptase (Prss31), and generation of the eicosanoids, prostaglandin D2 or the leukotriene C4 (7).
MCs are highly specialized innate immune effector cells that contain secretory granules in which large amounts of proteases are stored in complexes with serglycin proteoglycans [reviewed in (10,11)]. These proteases represent at least three classes of protease activities in mice (12-14) and in humans (15-17), chymotrypsin-like (chymases), trypsin-like (tryptases) and carboxypeptidase A-like (CPA3) . Rat and mouse skin MCs also contain a unique protease, rMCP-5 and mMCP-5, respectively, which is highly homologous to the chymases, and encoded by a gene in the chymase locus on mouse chromosome 14 (18,19). However, unlike the mouse MC chymases such as mMCP-1 in mucosal MCs and mMCP-4 in skin and connective tissue MCs, rat and mouse MCP-5 have an elastase-like specificity due to the presence of a valine instead of a glycine at position 216 (20-22). Studies with transgenic mice that lack MC-restricted serine proteases have revealed that some of these enzymes have prominent roles in innate immunity and inflammation. For example, mMCP-1-deficient mice have diminished ability to reject adult Trichinella spiralis nematodes from the small intestine (23), and mMCP-6-deficient mice have a reduced capacity to clear peritoneal infection by Klebsiella pneumoniae (24) and to recruit eosinophils to the site of T. spiralis larval encystment (25). These mMCP-6-deficient mice are also protected in models of inflammatory arthritis mediated by immune complexes (26,27). CPA3 mutants with a functionally defective enzyme, are unable to inactivate endothelin-1 or the homologous peptide from snake venom, sarafotoxin 6b (28).
Using a mouse model of an epidermal scald that results in a second degree burn and that depends on natural IgM and serum complement (6), we found that the evolution of the wound from erythema to ulceration to scarring with loss of hair regrowth was prevented in the MC-deficient WWv strain. Significant protection against the progression of burn injury in skin after a scald was observed in mice with targeted disruption of mMCP-4 (chymase) or mMCP-5 (elastase), but not in mice lacking mMCP-6 and -7 (tryptases) or an enzymatically inactive form of CPA3. We then found that application of human MC chymase to the scald site of the mMCP-4-deficient strain and of recombinant mMCP-5 (rmMCP-5) or human neutrophil elastase, but not human chymase, to the scald site of the mMCP-5-deficient strain increased epithelial disruption and restored full burn injury with ulceration and scarring. Furthermore, MC degranulation to release the endogenous proteases occurred in the initial 2 h post scald burn. These findings extend the trauma related functions of natural IgM, complement and MCs to second degree burns with progression to tissue remodeling; a result that may have implications for therapy in thermal injury and for other models of trauma-initiated inflammation-driven tissue pathology.
Materials and Methods
Animals
C57BL/6 mice were obtained from Taconic Laboratories (Germantown, NY) or The Jackson Laboratory (Bar Harbor, ME). MC-sufficient WBB6F1-+/+ and MC-deficient WBB6-KitW/KitWv mice were obtained from The Jackson Laboratory. The mice deficient in mMCP-4 (N7), mMCP-5 (N10) and both mMCP-6 and mMCP-7 (N8) and those with an inactivating mutation of MC-CPA3 (CPA3act−/−, N4) were on a C57BL/6 mouse genetic background, while the mice deficient in mMCP-1 were on a BALB/c background (7,23,24,28-30). These protease deficient strains were maintained in specific pathogen-free colonies at the Dana Farber Institute or in a barrier facility at Taconic Laboratories. Institutional Animal Care and Use Committee approval was obtained for all animal experiments and the studies were conducted in accordance with the National Institutes of Health and Public Health Service guidelines for animal care.
Reagents
Human neutrophil elastase and human MC chymase were obtained from EMD Biosciences (La Jolla, CA) and Enzo Life Sciences International, Inc. (Plymouth Meeting, PA). The chymase substrate S-2586 (MeO-Suc-Arg-Pro-Tyr-pNA) and the tryptase substrate S-2288 (H-D-Ile-Pro-Arg-pNA) were obtained from Chromogenix (Milano, Italy). The elastase substrate Suc-Ala-Ala-Pro-Val-pNA (S-AAPV) and the CPA substrate M-2245 (N-(4-Methoxyphenylazoformyl)-Phe-OH) were obtained from Bachem (Torrance, CA). Mouse Ig, human lung tryptase, and bovine pancreatic CPA were obtained from Sigma-Aldrich (St. Louis, MO). Mouse anti-TNP IgE (C38-2), FITC-conjugated rat anti-mouse IgE (R35-72), and rat anti-mouse CD16/CD32 (2.4G2) were obtained from BD-Biosciences (San Jose, CA). Allophycocyanin-Cy7 conjugated rat anti-mouse Kit (2B8) was obtained from eBioscience (San Diego, CA). Rabbit anti-peptide IgG for each protease was prepared as previously described (31,32).
Recombinant mMCP-5 protein was prepared as described previously (20). Briefly, the cDNAs encoding mMCP-5 were obtained from total RNA extracted from the hearts of male C57B/6 mice (Charles River Labs, Japan) by RT-PCR. After confirmation of the sequences, the cDNA fragments were cloned by transfer vector pFASTbac1 (Invitrogen), and recombinant baculovirus were generated by the Bac-to-Bac™ system (Invitrogen). For expression of the recombinant protein, Tn5 cells were grown in an Erlenmeyer flask (500 ml) to a density of 106 cells/ml in Ex-Cell 405 medium (200 ml/flask, Sigma-Aldrich) supplemented with 50 IU/ml penicillin and 50 mg/ml streptomycin in a rotary shaker (75 rounds/min), and were infected with the recombinant baculovirus at a multiplicity of infection of 1.0. After culture for 3 d at 28 °C, the culture medium was centrifuged and the supernatant was collected as a recombinant protein source. The recombinant pro-mMCP-5 was purified by a column of heparin-cellurofine gel (Seikagaku-kougyo, Japan) and a column of phenyl-sepharose CL-4B (Amersham Biosciences, Japan). The pro-mMCP-5 was activated by treatment with bovine cathepsin C (Sigma-Aldrich) at room temperature for 7 d, purified on a column of heparin-cellurofine gel and dialyzed with excess amount of pyrogen-free PBS for more than 24 hr at 4°C. This preparation was used as the source of the purified rmMCP-5 after determination of protein concentration.
Epidermal scald procedure
The epidermal scald procedure was performed as previously described (6). Interscapular hair was removed from ten-week old male mice and the next day, the mice were anesthetized with an intraperitoneal injection of ketamine (10 mg/kg) and xylazine (20 mg/kg). After full sedation, a burn template, created from a high impact non-heat-conducting plastic container, was used to produce a square-shaped 1 cm2 wound on the interscapular dorsum area (~2.5% of the body surface area). With the template held in position, the shaved skin was scalded in a circulating water bath. After an initial temperature titration all further experimentation was done at 54 °C. The duration of scalding was 25 s when no dressing was applied to the burn, and 35 s when a dressing was applied immediately after the scald to allow for topical application of a protease. Increasing the duration of the scald was necessary to obtain burn injury because the application of the dressing apparently had a cooling or protective effect. All mice were treated for pain twice daily with subcutaneous/intramuscular injections of buprenorphine (0.05 mg/kg) for the first day post burn. The wound area was demarcated by denuded and erythematous skin by d 3.
For topical application of the proteases, they were diluted in HBSS and 100 μl of the resulting solution was injected into a dressing that was created over the scalded area. The dressing consisted of a 1 cm2 piece of gauze, wrapped inside a Duoderm frame and covered by a Tegaderm dressing (Fisher Scientific). The wet dressing was applied immediately post scald and stayed on the burned area for one hour unless otherwise indicated.
Histology and quantitative assessment of injury
Skin was harvested from the prepared dorsal sites before the burn and at 2 h, 3 d and 12 or 13 d post burn. The tissue was fixed in 4% paraformaldehyde in PBS for 18 h, changed to PBS, and kept at 4 °C. Sections were stained by Masson’s trichrome, or Jones’ stain (methenamine silver-periodic acid-Schiff stain) or for chloroacetate esterase reactivity (CAE) which involves a hematoxlin counterstain as previously described (7).
At 2 h post burn, CAE was employed to highlight MCs and neutrophil infiltration. MCs were examined under high power and quantified in unit areas (1 unit area = 1.2 × 0.9 mm = 1.08 mm2). Nine unit areas of the burn wound on each mouse were evaluated for total MC numbers and used to calculate the mean value (per 9 unit areas) for each individual animal. A MC was determined to be degranulating when 3 or more granules could be found outside of the cell when it was examined under high power (7). Jones’s stain was performed to demonstrate edema which is indicated by decreased intensity of the stain relative to unburned mice. Injured epithelial cells and hair follicles were identified by the cytoplasmic vacuolization observed after CAE
At d 3 post burn, Masson’s trichrome stain was employed to differentiate denatured collagen (red staining) from viable collagen (blue staining) in the dermis of the burn wound. Digital photographs of the burn wound were taken at 10 × magnification (low power field, LPF) and the degree of burn injury was evaluated by Image J software (NIH. Bethesda, MD) based on the staining. The area of the burn was determined by measuring the area of denatured collagen (expressed in μm2). To measure the depth of the burn and degree of edema, the thickness of the injured dermis (indicated by the denatured collagen), the thickness of the viable collagen in the dermis beneath the wound, and the thickness of the hypodermis were measured at three different locations, at the middle, left edge and right edge of the burn. Values are the average of the three measurements and are expressed in um. Injured hair follicles on the photograph were identified by disruption of the normal morphology including epithelial tight junctions, presence of necrotic cells and loss of connection with the skin surface. Values were expressed as the average number of injured hair follicles per 4 high power fields (HPF, 40x). Neutrophils in the same burn wound were quantitated by CAE reactivity and expressed as the total number per 4 HPF.
At d 12 post-burn, Masson’s trichrome or Jone’s stain was employed to demonstrate granulation tissue formation and a decrement of normal collagen in the burn wound. With Masson’s trichrome stain, the thickened epidermis was purple and the granulation tissue in dermis was white in contrast to the surrounding dark blue stained collagen in normal dermis. With Jone’s stain, the epidermis and granulation tissue were pink and collagen in dermis was black. CAE reactivity was employed to show MC and neutrophils.
Characterization of mMCP-4- and mMCP5-deficient strains for dermal MC numbers and proteases
The baseline levels of MC in the interscapular skin, dermis and hypodermis together, were 134 ± 12.8 in WT mice, 97.7 ± 4.9 in mMCP-4 deficient mice, and 72.2 ±14.3 in mMCP-5 deficient mice per 9 unit areas (mean ± SE, n=11, 6 and 5, respectively). The distributions of MCs were similar in all strains with the dermis containing 58.3% in WT mice, 48.4% in mMCP-4-deficient mice, and 47.4% in mMCP-5-deficient mice and the hypodermis containing 41.7% in WT mice, 51.6% in mMCP-4-deficient mice and 52.6% in mMCP-5- deficient mice.
To evaluate protease levels in these strains, lysates were prepared from purified peritoneal MCs obtained from C57BL/6 mice and from the various protease-deficient mice. Mixed peritoneal cells were obtained by lavage with modified Tyrode’s buffer (0.02% KCl, 0.1% NaHCO3, 0.005% NaH2PO4, 0.8% NaCl, 0.1% D-glucose, and 0.1% gelatin) (21), and the Kit+/FcεRI+ MCs were isolated by flow cytometry. Briefly, Fcγ receptors were blocked with anti-CD16/CD32 antibodies (10 μg/ml) and mouse Ig (100 μg/ml) for 10 min before staining. The peritoneal lavage cells were incubated with IgE in HBSS/FBS (2% FBS) for 1 hr, washed twice, and stained with FITC-anti-IgE and allophycocyanin-Cy7-anti-Kit by incubation for 1 hr more. Cells were washed twice with HBSS/FBS and isolated by FACS (MoFlo, Dako-cytomation, Fort Collins, CO) at the Dana Farber Cancer Institute flow cytometry facility. Cells were kept on ice throughout the procedure. Lysates of sorted cells were prepared by sonication, and debris were removed by centrifugation. Sonicates were adjusted to represent equal numbers of cells per ml.
Reactions to determine enzymatic activities were carried out in 96-well plates. Samples of cell lysates or the reference proteases were incubated with chromogenic substrates in a final volume of 200 μl of reaction buffer (100 mM Tris-HCl, 150 mM NaCl, 0.05% Tween-20, and 0.1% BSA, pH 8.5) at 37 °C for 24 h. Chymase activity was measured with substrate S-2586 at 0.5 mM, elastase with S-AAPV at 1 mM, tryptase with S-2288 at 0.5 mM, and CPA3 with M-2245 at 1 mM (20,21,33-35). Enzymatic activities were assessed by measuring the optical density at 405 nm after a 24 h incubation and were expressed as the amount of protease based on standard curves prepared with purified human neutrophil elastase, human MC chymase, human MC tryptase, or bovine CPA, each in the presence of 50 μg/ml of heparin.
The dermal MCs in C57BL/6 (WT) mice express the chymase mMCP-4, the elastase mMCP-5, the MC-specific carboxypeptidase CPA3 and the tryptase mMCP-6 but lack the tryptase mMCP-7 due to a mutation at the exon 2/intron 2 splice site (36,37). The targeted deletion of CPA3 alters the expression other proteases (38) while the CPA3 activity-deficient (CPA3act−/−) strain with non-functional CPA3 is similar to WT mice in the expression of the other proteases (28). As the integrity of the other proteases has not been fully assessed in mMCP-4-, mMCP-5- and mMCP-6/7- targeted strains, we compared the level of chymase (mMCP-4), elastase (mMCP-5), tryptase (mMCP-6), and CPA3 enzymatic activities in purified peritoneal MCs from these strains to those in the MCs from WT mice. The peritoneal MCs isolated from mMCP-4-deficient mice had no detectable chymase activity, normal levels of elastase and CPA3 activities, and enhanced tryptase activity (387% of WT level) (Supplemental Fig. 1 A-D). Peritoneal MCs isolated from mMCP-5-deficient mice had no elastase activity, markedly reduced chymase (10% of WT level) and CPA3 activities (<3% of WT level), but enhanced tryptase activity (421% of WT level). Peritoneal MCs isolated from mMCP-6/7-deficient mice had markedly reduced tryptase activity (14% of WT level) and normal levels of elastase, chymase, and CPA3. The residual tryptic activity is presumably due to the presence of transmembrane tryptase/tryptase γ/Prss31 in these cells. The reduced expression of mMCP-4 in mMCP-5-deficient mice was confirmed by immunoblot analysis using peritoneal MC lysates (Supplemental Fig. 1E)
Statistical analysis
Student’s t test for unpaired samples was used for direct comparisons of means of observations made in various animals. A p value <0.05 was considered significant.
RESULTS
Epidermal scald injury is MC-dependent at 54 °C
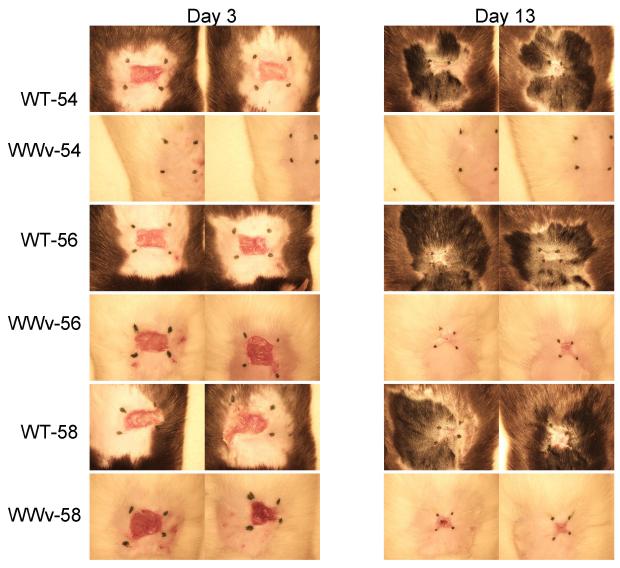
In an initial clinical screen to evaluate whether we could identify an intensity of scald burn injury that required mature cutaneous MCs for development of an ulcer and subsequent remodeling, we compared the injury generated in MC–sufficient (WBB6)F1/J mice versus MC-deficient WWv littermates by exposing a 1cm2 area of dorsal epidermis to water heated to 54 °C, 56 °C, or 58 °C for 25 s. The WWv strain of mice have <1% of the normal number of skin MCs (39). All of the MC-sufficient mice exhibited burn injury by d 3 post scald with erythema and ulceration at each temperature (Fig. 1). Over the next 10 d, the ulcerated skin healed with scarring and loss of hair regrowth. In contrast, after a 54 °C scald the MC-deficient WWv mice showed little or no erythema and no ulceration on d 3 with full reappearance of hair on d 13. However, after a scald burn at 56 °C or 58 °C the ulceration at d 3 and scarring without hair regrowth at d l3 in the MC-deficient mice was similar to that observed in the MC-sufficient controls indicating a MC-independent injury process at these higher temperatures.
FIGURE 1.
MC-deficient mice are protected against epidermal scald injury at 54 °C. The clinical response to an epidermal scald of 25 seconds duration in MC-sufficient WBB6F1-+/+ (WT) mice and MC-deficient WWv mice was compared at 3 different temperatures at 3 and 13 d after injury. Pictures show the 2 mice in each group. Hair regrowth at the scald site of the 54 °C treated WWv mice is not seen due to its lack of pigment. The black marks were replaced each day to define the residual injury and do not represent the area of the original scald site. Protection against a visible burn in MC-deficient mice exposed to a 54 °C scald for 25 s was confirmed in 2 further experiments with sufficient and deficient strains with 5 mice per group.
Histological characterization of the scald burn
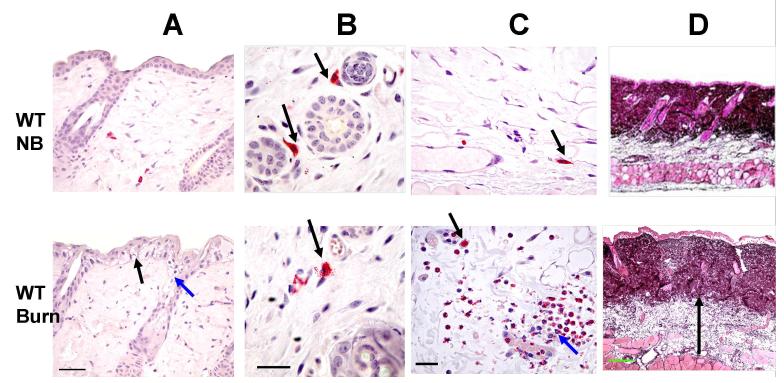
We characterized the histologic changes of a 54 °C scald burn as compared to unburned sites in C57BL/6 (WT) mice 2 h, 3 d, and 12 d post scald. At 2 h post burn, the epidermis showed cytoplasmic vacuolization, and disruption of the tight junctions between the basal cells of the epithelium (black arrow, Fig. 2A). The involved hair follicles showed mild hydropic swelling and disruption of the epithelium of the follicles (red arrow, Fig. 2A). Many of the MCs in the dermis (arrows, Fig. 2B) and hypodermis (arrows, Fig. 2C) were degranulating (48.6 ± 14.3 % and 61.1 ± 14.9 %, respectively, mean ±SE, n=5 from 3 separate experiments). In the hypodermis, there was vasodilatation, vascular stasis, vascular thrombosis, marginated neutrophils within the blood vessels (red arrow) and an influx of neutrophils into the tissue (Fig. 2C). In both the dermis and hypodermis, there was edema. This is apparent in the dermis by the separation of collagen fibers with lighter coloration of the dark stained collagen and in the hypodermis by expansion of the tissue (arrow, Fig 2D).
FIGURE 2.
The histological changes occurring 2 h after a scald burn in the skin of WT C57BL/6 mice. The histologic changes in the shaved skin in WT mice without (top panels, NB, no burn) and with a scald burn of 25 s duration at 54 °C (bottom panels) are compared at 2 h post burn. Sections in A-C are stained for CAE reactivity. A, Epidermis at the burn site shows disruption of the tight junctions between the basal cells of the epithelium (black arrow) and cytoplasmic vacuolization in epithelial cells of the epidermis and the hair follicles (red arrow). B, MCs in the dermis at the shaved site are intact (arrows) whereas MCs are degranulating with extracellular granules 2 h after the scald burn (arrow). C, Similarly, MCs in the hypodermis at the shaved site are intact (arrow) while 2 h after the scald burn they are degranulating (black arrow). There is also neutrophil margination in a blood vessel and influx into the hypodermis (red arrow). D, Jones’ stain shows lighter staining of the collagen matrix of the dermis and expansion of the hypodermis at the scald site due to edema (arrow in bottom panel denotes depth of hypodermis). Scale bars: A=50 μm, B, C=25um, D=200um).
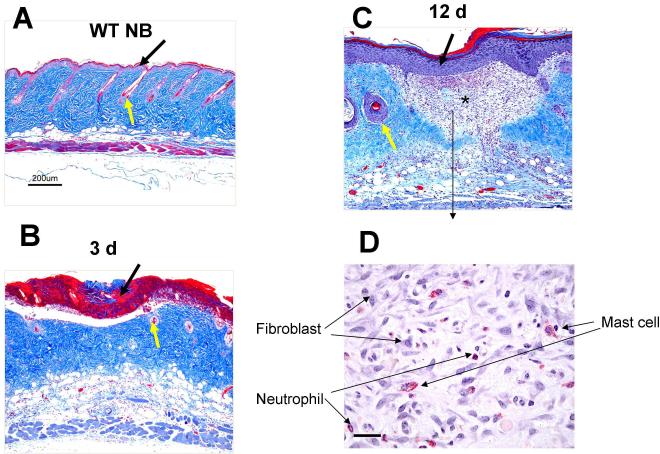
At 3 d post burn, coagulative necrosis at the burn site affected the entire thickness of the epidermis and about half the depth of the dermis (Fig. 3B). The epidermis was denuded, leaving an ulceration demarcated by denatured collagen (stained red, black arrow, Fig.3B). Hair follicles in the dermis were destroyed (yellow arrow, Fig.3B,). The red staining denatured collagen in the dermis was surrounded by a rim of capillary stasis and neutrophil infiltration. The inflammation in the hypodermis was sustained with vasodilatation and scattered neutrophil infiltration.
FIGURE 3.
The histologic changes occurring at 3 d and 12 d after a scald burn in WT mice. A-C are stained with Masson’s trichrome and D is stained for CAE reactivity. A, WT mice with no burn (NB) show intact epithelium (black arrow) and hair follicles (yellow arrow). B, The epidermis 3 d post burn is denuded leaving an ulceration demarcated by denatured collagen (stained red, black arrow), The denatured collagen extends through about one half the dermis reflecting the breadth and depth of the burn and loss of hair follicles (yellow arrow). C, By 12 d post burn, the morphological changes include a thickened epithelium covering the wound (black arrow), granulation tissue without any hair follicles in the dermis (star) and damaged hair follicles at the edge of the scar (yellow arrow). D. High power photograph of the granulation tissue shows accumulation of fibroblasts, MCs and neutrophils. Scale bar: A-C = 200 μm, D=25um).
By 12 d post burn, the morphological changes included a thickened epithelium covering the wound (black arrow, Fig. 3C), no hair follicle regrowth in the dermis with damaged hair follicles at the edge of the scar (yellow arrow, Fig. 3C) and areas of granulation tissue (Fig. 3C, star and 3D). The granulation tissue contained numerous fibroblasts, MCs and neutrophils (Fig. 3D). We evaluated changes in the area of the exposed skin over this time period in other studies by marking the initial burn site corners with a permanent tattoo and noted no contracture of the wound area (data not shown).
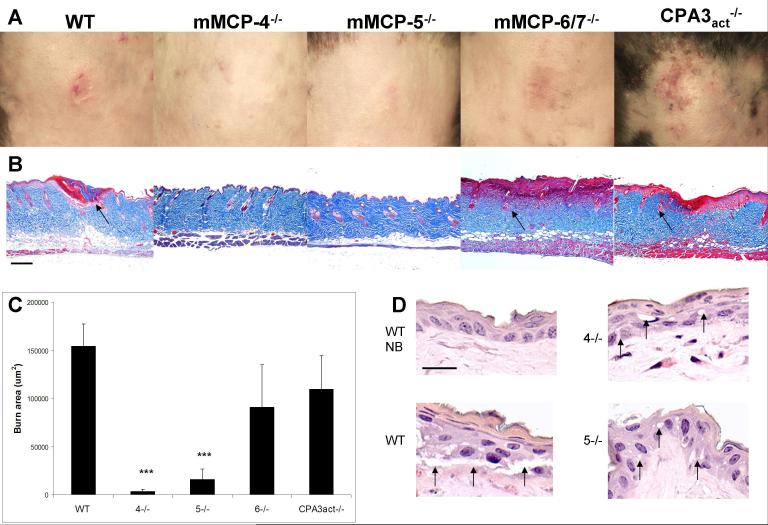
Mice deficient in mMCP-4 and mMCP-5 are protected from epidermal scald injury
In contrast to the clinical presentation of a scald burn with erythema and ulceration in WT C57BL/6 mice, mice lacking the chymase mMCP-4 or the elastase mMCP-5, showed little erythema and no ulceration at 3 d post scald burn (Fig. 4A). Mice lacking the tryptases, mMCP-6/7, or the carboxypeptidase CPA3 activity, responded to the scald burn like the WT with erythema and ulceration. By histology, the scald burn site in mMCP-4−/− or mMCP-5−/− mice in this experiment showed little of the disruption of the epithelium, denaturation of collagen, loss of hair follicles, and edema of the dermis seen in WT, as well as mMCP-6/7-, or CPA3act-deficient mice (Fig. 4B). The mean histologically determined burn area in 20 WT mice pooled from three separate experiments was used to establish the normal range of injury (154.2 ± 23.3 × 103 um2) and this was compared to the injury observed in the various protease deficient strains from one experiment each. The results revealed significant protection in mice lacking mMCP-4 or mMCP-5 (mean area ± SE: 3.1 ± 2.2 × 103 and 15.6 ± 11.1 × 103 um2 with p<0.001 for both, n=4 for both strains), while there was injury similar to WT mice in the mice lacking mMCP-6/7 or CPA3act (90.9 ± 44.0 × 103 and 109.9 ± 34.9 × 103 um2, n= 6 and 5, respectively) (Fig 4C). The aggregate studies at d 3 did show some clinical injury, erythema with ulceration, in 3 of 9 mMCP-4 deficient and 5 of 17 mMCP-5 deficient mice from 3 independent experiments while the injury was similar to WT mice in 9 of 12 mMCP-6/7−/− mice (2 experiments) and 4 of 5 mice with the inactivating mutation in CPA3 (1 experiment). Furthermore, 2 of 2 mice lacking the mucosal MC chymase mMCP-1, which is not expressed in skin MCs, also exhibited full burn injury with ulceration and scarring (data not shown).
FIGURE 4.
The absence of MC-specific secretory granule proteases, mMCP-4 or mMCP-5, protects against epidermal scald injury. A, The progression of clinical injury to ulceration at d 3 occurs in the WT, mMCP-6/7- and CPA3act-deficient mice, but not in mMCP-4- or mMCP-5-deficient mice. B, The corresponding histological sections after Masson’s trichrome staining to assess the breadth and depth of injury by the appearance of denatured collagen (red), injured hair follicles (arrows), and edema of the dermis revealed by thickness and lighter staining. Each picture is from a single mouse representative of the injury characteristic of each strain. C, Burn injury area (red area of denatured collagen in histological sections) in the same groups as in A. Values for the burn area are the mean (± SE) um2. The value for the WT mice is derived from 3 experiments with 20 mice while the other values are from 1 experiment with 4, 4, 6, and 5 mice, respectively. Statistical values are from a t-test relative to the WT value. D, A high magnification of the epidermis in unburned WT (NB) and in burned WT , mMCP-4−/− (4−/−), and mMCP-5−/− (5−/−) mice at 2 h post burn shows disruption of epithelial tight junctions and epithelial vacuolization (arrows) in the burned WT mice but only minimal changes in these parameters mMCP-4−/− and mMCP-5−/− strains. Additional histologic data showing protection in the mMCP-4- and mMCP-5-deficient strains are shown in Fig. 5 and quantitated in Fig. 6. Scale bar: B=200 um, D= 20 um.
The protection against a scald burn in the mMCP-4- and mMCP-5-deficient strains prompted a further histological assessment for epidermal injury at 2 h under a higher power. Whereas there were rare to no vacuoles in the shaved unburned epidermal skin, there was extensive vacuolization of the epidermal cells and disruption of the tight junctions at the scald site 2 h post burn in WT mice. In the scald site of the mMCP-4−/− and mMCP-5−/− strains the vacuoles were few and the disruption of the tight junctions was much less than in the WT mice (Fig 4D). In the mMCP-4−/− mice, the percentage of MCs undergoing degranulation at 2 h post-burn was 24.8 ± 8.0 and 34.2 ± 6.4 % in the dermis and hypodermis, respectively, (mean ± SE, n=4 mice) which is about half of that observed in WT mice, although the differences are not significant given the limited sample size of 4-5 mice. Degranulation also was present in the scalded mMCP-5-deficient mice, but the weak staining of granules due to the low level of the associated proteases prevented reliable quantitation of intact cells and released granules. Thus, these studies implicated the connective tissue type MC and its secretory granule chymase, mMCP-4, and elastase, mMCP-5, but not its tryptases or MC-specific CPA3 in the early events leading to the ulceration and further suggested that these two proteases could play a role in the early epidermal changes.
Topical application of rmMCP-5 or human elastase to the burn site of mMCP-5−/− mice and of human MC chymase to the burn site of mMCP-4−/− mice reconstitutes scald burn injury
To support the findings that two different secretory granule proteases of mouse skin MCs were essential to the ulceration and scarring in our scald burn model, we sought to reconstitute injury by topical application of human chymase to mMCP-4−/− mice and of rmMCP-5 or human neutrophil elastase (elastase) to mMCP-5−/− mice. Each protease was applied to the scald burn site immediately following the burn and the dressing was left in place for a period of one hour. Control mice received a dressing with an equal volume of HBSS. This route of administration seemed reasonable not only because of the known loss of the skin permeability barrier function caused by a scald burn (6) but also by our histologic finding of minimal but distinct disruption of the epidermis in the protected protease deficient mice (Fig. 4D). The mMCP-4−/− mice received a total dose of 10 μg of human chymase and the mMCP-5−/− mice received either a total dose of 1μg of rmMCP-5 or 10μg of human elastase. At d 3 post burn, the scald site in the mMCP-4- and mMCP-5-deficient strains treated with HBSS was without ulceration or significant edema, while the chymase treated site in the mMCP-4−/− mice and the rmMCP-5 or elastase treated sites in the mMCP-5−/− mice showed significant injury on histologic analysis with coagulation necrosis of the epidermis, loss of hair follicles and denaturation of collagen and edema in the dermis (Fig 5).
FIGURE 5.
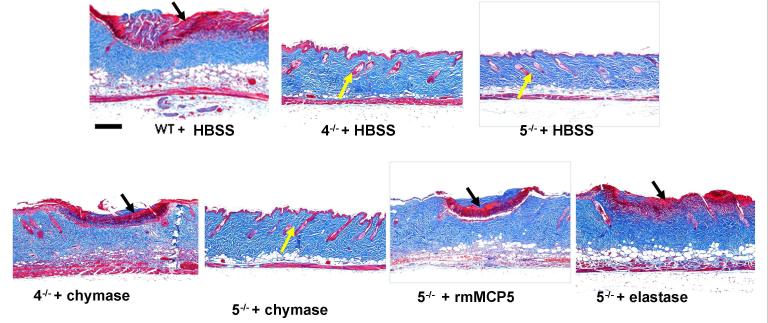
Topical application of human chymase and of recombinant mMCP-5 or human elastase reconstitutes the histologic changes of scald burn injury in mMCP-4−/− and mMCP-5−/− strains, respectively. WT, mMCP-4−/− (4−/−), and mMCP-5−/− (5−/−) mice were burned for 35 s at 54 °C and treated topically with a dressing providing HBSS, human chymase (chymase), human elastase (elastase) or rmMCP-5 for 1 h post burn. Masson’s trichrome staining of scald site is depicted at d 3. The injury is indicated by the red staining denatured collagen (black arrows) while protection is indicated by absence of denatured collagen and the presence of intact hair follicles (yellow arrows). Sections are representative of the aggregate findings for each group presented in Fig 6. Scale bar: 200 um.
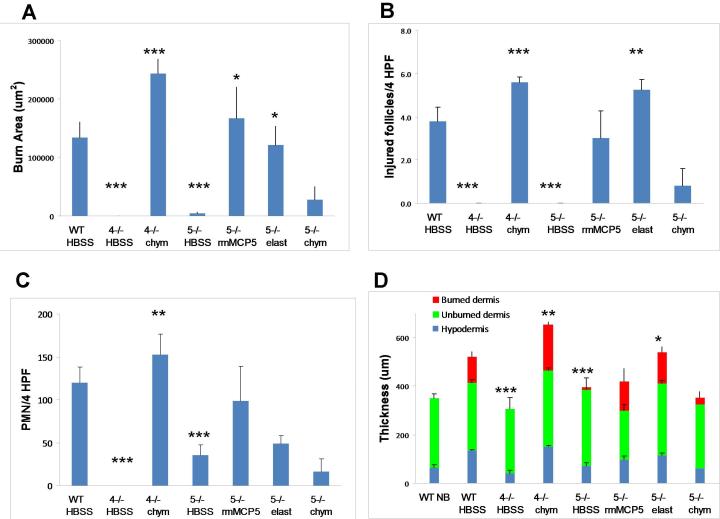
To quantify the changes at d 3 elicited by application of the missing protease activity to the burn site, we compared the mean burn area, neutrophil influx in the hypodermis, number of injured hair follicles and thickness of the skin in these various groups. To establish the range of injury in the HBSS-treated WT mice for comparison to the various protease deficient strains treated with various reagents, we evaluated these values in 16 WT mice from 3 independent experiments with the reconstitution protocol. The mean burn area of HBSS-treated WT mice was 133.8 ± 26.4 × 103 um2 (Fig 6 A), similar to that observed in the non treated WT mice (Fig. 4C). In contrast, the mean burn area of the HBSS-treated mMCP-4- and mMCP-5-deficient strains was significantly less (0 ± 0 and 4.7 ± 1.4 × 103 um2 respectively, mean ± SE, n = 4 from 1 experiment and 7 from 2 experiments, p<0.001 for both, Fig 6A). The mean area of the burn in the mMCP-4−/− mice given topical human chymase (243.1 ± 25.1 × 103 um2) and in the mMCP-5−/− mice given topical rmMCP-5 (167.3 ± 53.1 × 103 um2) or human elastase (121.6 ± 32.0 × 103 um2) was significantly greater than that of their respective protease-deficient, HBSS-treated controls (p<0.001, <0.05 and <0.05, n= 5, 5, 4, respectively, 1 experiment each) and similar to that of the WT mice (Fig 6A). The mMCP-5−/− mice treated with chymase showed no significant increment in burn area (27.2 ± 22.5 × 103 um2, n=5, 1 experiment) relative to the HBSS-treated mMCP-5-deficient mice.
FIGURE 6.
The quantitative assessment of burn injury after the topical application of human chymase to mMCP4−/− mice and of rmMCP-5 or human elastase to mMCP-5−/− mice. Mice were burned for 35 s at 54 °C, ,then treated with topically applied HBSS, chymase (chym), rmMCP-5 or elastase (elast) as indicated for 1 h and the injury evaluated on d 3 post scald burn. A, The mean area (±SE) of burn injury area was assessed by Masson’s trichrome staining. The value for the WT mice is derived from 3 experiments with 16 mice. The value for the HBSS-treated mMCP-5−/− is derived from 2 experiments while all others are from 1 experiment. Numbers of mice are 16, 4, 5, 7, 5, 4, and 5 in each group, respectively. B, The mean number ( ±SE) of injured hair follicles (per 4 HPF) in the same animals as in A. C, The mean number (± SE) of neutrophils (per 4 HPF) identified by CAE reactivity in the hypodermis of the same animals as in A. D, The mean depth (± SE) of the skin from the same mice as in A with the depth of the various individual tissues indicated by burned dermis (red), unburned dermis (green) and hypodermis (blue). The mean (± ½ range) values from 2 non burned WT (WT NB) mice are shown for comparison. For statistical analysis, the mMCP-4- and mMCP-5-deficient mice treated with HBSS were compared to the WT controls, while the enzyme reconstituted groups were compared to the respective HBSS-treated strain. *, p<0.05; **, p<0.01; ***, p<0.001.
The number of injured hair follicles in mMCP-4−/− and mMCP-5−/− mice treated with HBSS was negligible and significantly less than the that in WT mice treated with HBSS (p<0.001 for both). The mMCP-4−/− mice treated with chymase or the mMCP-5−/− mice treated with human elastase had a significant increase in injured hair follicles as compared to the same strain treated with HBSS (p<0.001 and p<0.01, respectively) while the response to rmMCP-5 did not reach significance (p=0.08) (Fig. 6B). The mMCP-5−/− mice treated with chymase showed few injured follicles, similar to their HBSS-treated controls. By comparison to burned WT mice, the neutrophil influx into the hypodermis was significantly decreased in mMCP-4−/− and mMCP-5−/− mice (p<0.001 for both). With topical application of chymase the scald burn in mMCP-4−/− mice showed a significant increase in neutrophil influx into the hypodermis while the increase in PMN in the burn of mMCP-5−/− mice treated with either rmMCP-5 or elastase did not reach significance (Fig. 6C). The thickness of the dermis and hypodermis (used as a marker of edema) was significantly less in the mMCP-4−/− and mMCP-5−/− mice given saline (p<0.001 for both) as compared to WT mice and this was significantly increased by topical treatment of the appropriate deficient mice with chymase or elastase, respectively (Fig. 6D). A significant change in thickness was not observed in mMCP-5−/− mice given rmMCP-5 or chymase. Thus the reconstitution of protease-deficient mice with the respective activity of the missing enzyme restored much of the histologic burn injury at 3 d.
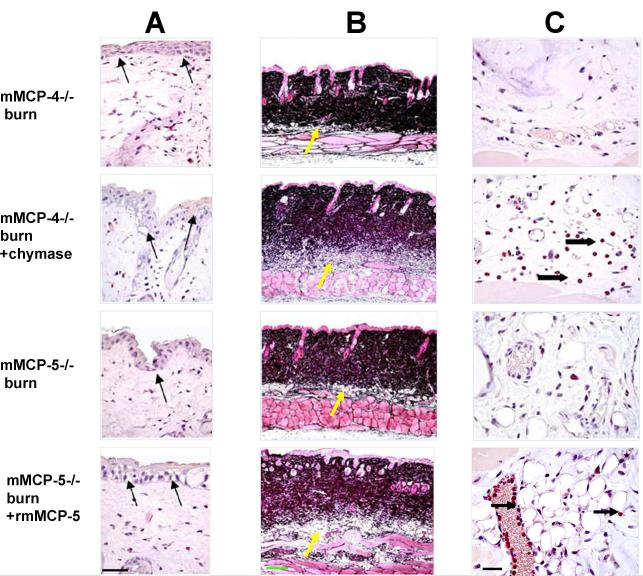
To see if topical protease reconstitution had an effect at 2 h post burn, we compared the histology of the scald site in the deficient strains treated with enzyme or buffer. The minimal but definite disruption of the epidermal basal layer at the burn site in the mMCP-4- and mMCP-5-deficient mice was increased with topical protease treatment (arrows, Fig. 7A), resembling the cytoplasmic vacuolization and disruption of tight junctions seen in a scald injury of a WT strain at the same time point (Fig. 2A, 4C). Similarly, in these protease deficient mice, the topical application of the missing protease function post scald resulted in increased vasodilation and edema in both dermis and hypodermis indicated by lighter staining of dermal collagen and increased hypodermal thickness (Fig. 7B), again resembling the response of the WT strain (Fig. 2C). There was increased neutrophil margination and influx into the hypodermis with chymase treatment of the mMCP-4−/− mice and with rmMCP-5−/− treatment of the mMCP-5−/− mice (Fig. 7C) which is compatible with their presence at this time point in scalded WT mice (Fig. 2D).
FIGURE 7.
The histological changes 2 h after an epidermal scald in the skin of mMCP-4- and mMCP-5-deficient mice without and with topical application of human MC chymase or rmMCP-5, respectively. All mice were treated with topical HBSS without or without a protease for the first h post burn. A, Cytoplasmic vacuolization and disruption of the tight junctions between the basal cells of the epithelium at the scald site is apparent after CAE in the protease-deficient strains and is accentuated with topical application of chymase to the mMCP-4-deficient and of rmMCP-5 to the mMCP-5-deficient strain (arrows). B, Jones’ staining shows increased edema as indicated by the lighter color of the dermis and greater depth of the hypodermis (yellow arrows) in mMCP-4−/− mice treated with chymase, and of mMCP-5−/− mice treated with rmMCP-5 relative to their HBBS-treated protease-deficient controls. C, The CAE reactivity in the hypodermis shows neutrophils (arrows) in the blood vessels and tissue of mMCP-4−/− mice treated with chymase, and in mMCP-5−/− mice treated with rmMCP-5. Scale bar: A=50 um, B=200 um, C= 25 um.
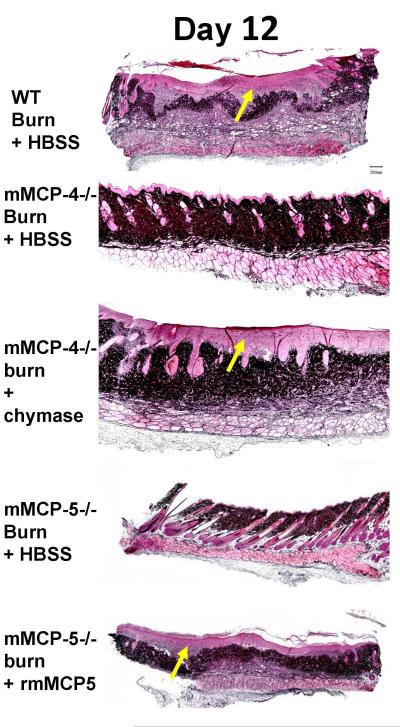
At d 12 post burn, none of the remodeling changes that occur in WT mice were observed in the protected mMCP-4- or mMCP-5-deficient mice, which had a normal epidermal layer and normal dermis with intact hair follicles and sebaceous glands (Fig. 8). In contrast, the scald site in mMCP-4-deficient mice treated with chymase and in the mMCP-5-deficient mice treated with rmMCP-5 showed the thickened epithelium, formation of granulation tissue (light pink staining, yellow arrow) in the dermis and loss of hair follicles comparable to the scalded WT mice (Fig 3B)
FIGURE 8.
The topical application of chymase and rmMCP-5 to the burn site on mMCP-4−/− and mMCP-5−/− mice, respectively, leads to remodeling at d 12. Jones’ stain of the skin reveals thickened epithelium, granulation tissue (light pink, yellow arrows) and loss of hair follicles in WT mice, in mMCP-4−/− mice treated with chymase and in mMCP-5−/− mice treated with rmMCP-5, whereas the protease deficient mice treated with HBSS alone had normal epithelium, hair follicles, and sebaceous glands. Scale bar; 200um.
We also evaluated the timing needed for topical application of rmMCP-5 to restore clinical burn injury in mMCP-5-deficient mice at d 3. We applied 400 ng of the recombinant enzyme for 1 h immediately after injury or after a delay of 1, 3, 6, or 24 h. Each of 9 mMCP-5-deficient mice receiving rmMCP-5 immediately post burn or with a delay of 1 hr exhibited erythema and ulceration comparable to the WT mice treated in parallel. However when the topical application of rmMCP-5 was delayed to 3 h or more, 13 of 14 mMCP-5−/− mice exhibited protection from clinical injury. We also noted that 10 ug of topically applied elastase failed to reconstitute the clinical burn in the mMCP-4-deficient strain (data not shown).
Discussion
The findings that two secretory granule serine proteases of cutaneous and connective tissue MCs, mMCP-4, a chymase, and mMCP-5, an elastase, are required for a scald injury to progress to a second degree burn in the mice manifested by ulceration and subsequent scaring without hair regrowth extends the role of these proteases previously recognized as critical to IR injury in hind limb muscle (7). Both models also require natural IgM and activation of the complement pathway, implicating an innate autologous pathway to injury (4-6). The progressive histologic changes at the site of the scald burn in WT mice include disruption of the epidermal basal layer, degranulation of MCs and edema of the dermis and hypodermis at 2 h, denuding of the epidermis with wound erosion and destruction of the hair follicles by d 3, and remodeling with failure to regrow hair by 9-13 d.
The scald burn injury to the epidermis is evident as early as 2 h post burn by some vacuolization and disruption of the tight junctions between the epidermal cells at the scald site even in the protease deficient strains which failed to show progression of the injury to ulceration and remodeling. This suggested that there could be an early permeability change in the epidermis that might allow a topical approach to restore the protease dependent component of the full response to burn injury in the protected mMCP-4- and mMCP-5-deficient strains. Indeed, the topical application immediately post scald of rmMCP-5 or human neutrophil elastase to the mMCP-5-deficient mouse or of human MC chymase to the mMCP-4-deficient mouse increased the epidermal disruption and fully restored the downstream aspects of the burn injury. The resultant edema of dermis and hypodermis and influx of neutrophils at 2 h post burn, ulceration with collagen denaturation of the dermis and necrosis of the hair follicles at 3 d and scar formation without hair regrowth at 9-13 d resembled the burn injury of WT mice. The requirement for the specificity of both proteases for scald burn progression is supported by the failure of chymase to restore clinical and histologic progression of injury in the mMCP-5-deficient strain and the failure of elastase to reconstitute the clinical injury in the mMCP-4-deficient strain. The aggregate data suggest that MC activation with non redundant protease-dependent epidermal injury initiates or is an essential component of the processes leading to ulceration and remodeling of the burn site.
The availability of mouse strains deficient in different secretory granule proteases was critical to our study for two major reasons. First, these strains have none of the hematologic and cellular defects due to the mutation of Kit in the WWv strain. For the burn model, this difference could be important because neutrophil infiltration does occur early in the dermis and hypodermis of the burn site in WT mice and the WWv stain is neutropenic and has impaired neutrophil mobilization to tissues (40,41). Second, the range of mice with targeted deletion of MC-specific proteases and normal MC numbers allowed identification of the particular secretory granule proteases participating in the scald burn injury model.
Skin and connective tissue MCs of most mouse strains express the tryptases mMCP-6 and mMCP-7, the chymase mMCP-4, the elastase mMCP-5, and the exopeptidase CPA3, whereas the T cell-dependent intraepithelial MCs characterized in the jejunual mucosa during the height of a helminth infection preferentially express the chymases mMCP-1 and mMCP-2, but no elastase, neither of the tryptases nor CPA3. Only the mice lacking the cutaneous MC chymase mMCP-4 or the elastase mMCP-5 were significantly protected against the scald injury at the clinical and morphologic levels. Because an earlier report demonstrated that the mutation of granzyme B within the chymase locus on chromosome 14 disrupted the expression of other genes in the locus (42), we evaluated the injury in mice with a mutant mMCP-1 gene, which is present in the same locus as the mMCP-4 and mMCP-5 genes (19). The lack of protection against scald burn in the mMCP-1-deficient mice indicates that only the respective mutations of mMCP-4 and mMCP-5 in the locus are each important.
mMCP-4 and mMCP-5 are highly homologous serine proteases that are encoded by different genes on mouse chromosome 14C1 that give them distinct substrate specificities. The mouse and rat chymases, mMCP-4 and rMCP-4, represent homologues of human MC chymase-1 on the basis of sequence homology and similarities in substrate specificity, including their ability to generate the angiotensin II fragment from angiotensin I (33,43-45). The mouse and rat MC proteases, mMCP-5 and rMCP-5, each have a distinct elastase substrate specificity due to a change of glycine to valine at position 216 (20,21,46). Surprisingly, the MC-specific exopeptidase CPA3 is coordinately expressed with mMCP-5, and the expression of each depends on the presence of the other through an as yet undefined post-transcriptional mechanism (28,29,38). However, in the recently developed mutant with enzymatically inactive CPA3 protein, the expression of mMCP-5 is intact(28), and the normal level of burn injury in these mice indicates that the absence of CPA3 in the mMCP-5−/− mice does not account for their protection against the scald burn. We have also observed that mMCP-4 is poorly expressed in mMCP-5-deficient MCs as assessed by both measurement of enzymatic activity and immunoblots of serosal MC, and this secondary deficiency may contribute to the small size of the secretory granule by histochemical detection (7). That topical rmMCP-5 or human elastase but not human chymase restored scald burn injury to the mMCP-5-deficient strain indicates that the level of mMCP-4 in this strain is sufficient to satisfy the non-redundant requirement for mMCP-4. We found that mMCP-4-deficient MCs were selectively deficient in only that protease as originally reported (32) and reconstituted full burn injury with the homologous human MC chymase. Taken together these data indicate that these two proteases act non-redundantly in the pathway leading to the inflammation, ulceration and tissue remodeling of our scald burn model.
Even with histologic evidence of some scald-induced epidermal injury in the protected mMCP-4- and mMCP-5-deficient mice, it is likely that only a small fraction of the protease applied for one hour post burn is absorbed into the tissue. Hence, it seems reasonable to suggest that this activity could be provided endogenously by the activation of MCs at the burn site in the first 2 h. That the absence of either mMCP-4 or mMCP-5 abrogates development of a second degree burn despite some scald-induced epidermal injury argues for their early action in facilitating a second degree burn and this is nicely supported by exogenous protease-specific reconstitution within the first h post scald. The role of these proteases could be in amplifying exposure of the tissue epitope for natural IgM, augmenting the required activation and function of the complement pathway or mediating paracrine activation of the MCs (47,48). Furthermore, these proteases could have direct tissue effects such as activation of matrix metalloproteases by either mMCP-4 (30) or mMCP-5 with concomitant inactivation of TIMP-1 (49).
Since neutrophils were absent in the hind limb IR injury of the mouse that is also dependent on MCs (9) and their mMCP-5 (7) and mMCP-4 proteases (T. Shi & M. Gurish, unpublished data), we believe that the critical elastase function in both models is mediated by mMCP-5 and that neutrophil-derived elastase encoded on mouse chromosome 10 (50) does not provide a redundant function either in time or location. Further that the mutation that created the elastolytic function also is retained in the rat chymase locus, favors a biologic purpose for non-redundant functions of the MC-specific proteases, mMCP-4 and mMCP-5.The recognition that natural IgM, complement and two MC proteases can mediate injury as different as a second degree scald burn of skin and IR necrosis of hind limb muscle suggest function of an innate triad in trauma-induced inflammatory processes. It may be that local permeability and cellular injury allow for the lectin pathway to activate complement with consequent MC degranulation (4,51,52) which targets a profound downstream process leading to termination of the ulcer with scarring but not contracture.
Supplementary Material
SUPPLEMENTAL FIGURE 1. Evaluation of the enzymatic activities and secretory granule proteases in MCs from the MC protease-deficient mouse strains with targeted disruption of mMCP-4, mMCP-5 or mMCP-6. Using small synthetic substrates, the (A) elastase, (B) chymase, (C) tryptase, and (D) carboxypeptidase A3 (CPA3) activities in purified peritoneal MCs from WT , mMCP-4-deficient (4−/−), mMCP-5-deficient (5−/−), and mMCP-6/7-deficient (6/7−/−) mice are shown. A-D, Activities are the means (± SE) from 4 WT, 4 mMCP-4−/−, and 4 mMCP-5−/− mice, and the mean (± ½ range) from 2 mMCP-6/7−/− mice. E, Immunoblot analysis of peritoneal lavage cell lysates showing weak expression of mMCP-4 and no expression of either mMCP-5 or CPA3 in mMCP-5−/− mice while mMCP-4−/− mice only lack expression of mMCP-4. Cell lysates are from C57BL/6 (lane 1), mMCP-4−/− (lane 2) or mMCP-5−/− (lane 3) mice. Equal cell number equivalents were loaded in each lane. Parallel blots were probed with the indicated antibodies. Lane M shows the 30Kd molecular weight marker.
Acknowledgements
None
This work was supported by NIH grants GM 052585 and HL36110, and by grants from the Swedish Medical Research Council (VR) and the Goran Gustafsson Foundation. RA was supported by The University of Texas M. D. Anderson Cancer Center Physician Scientist Program. YK works for Teijin Pharma Limited
Abbreviations used in this paper
- CPA3
carboxypeptidase A3
- HPF
high power field
- IR
ischemia reperfusion
- LPF
low power field
- MC
mast cell
- mMCP
mouse MC protease
- rmMCP-5
recombinant mMCP-5
- WWv
WBB6F1-KitW/KitWv
Footnotes
Disclosures The authors have no financial conflicts of interest to declare.
References
- 1.Horton JW, Mileski WJ, White DJ, Lipsky P. Monoclonal Antibody to Intercellular Adhesion Molecule-1 Reduces Cardiac Contractile Dysfunction after Burn Injury in Rabbits. J. Surg. Res. 1996;64:49–56. doi: 10.1006/jsre.1996.0305. [DOI] [PubMed] [Google Scholar]
- 2.Nwariaku FEM, Mileski WJM, Lightfoot EJ, Sikes PJB, Lipsky PEM. Alterations in Leukocyte Adhesion Molecule Expression after Burn Injury. J. Trauma. 1995;39:285–288. doi: 10.1097/00005373-199508000-00016. [DOI] [PubMed] [Google Scholar]
- 3.Weiser MR, Williams JP, Moore FD, Jr., Kobzik L, Ma M, Hechtman HB, Carroll MC. Reperfusion injury of ischemic skeletal muscle is mediated by natural antibody and complement. J. Exp. Med. 1996;183:2343–2348. doi: 10.1084/jem.183.5.2343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chan RK, Ibrahim SI, Takahashi K, Kwon E, McCormack M, Ezekowitz A, Carroll MC, Moore FD, Jr., Austen WG., Jr. The differing roles of the classical and mannose-binding lectin complement pathways in the events following skeletal muscle ischemia-reperfusion. J. Immunol. 2006;177:8080–8085. doi: 10.4049/jimmunol.177.11.8080. [DOI] [PubMed] [Google Scholar]
- 5.Chan RK, Verna N, Afnan J, Zhang M, Ibrahim S, Carroll MC, Moore FD., Jr. Attenuation of skeletal muscle reperfusion injury with intravenous 12 amino acid peptides that bind to pathogenic IgM. Surgery. 2006;139:236–243. doi: 10.1016/j.surg.2005.05.028. [DOI] [PubMed] [Google Scholar]
- 6.Suber F, Carroll MC, Moore FD., Jr. Innate response to self-antigen significantly exacerbates burn wound depth. Proc. Natl. Acad. Sci. USA. 2007;104:3973–3977. doi: 10.1073/pnas.0609026104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Abonia JP, Friend DS, Austen WG, Jr., Moore FD, Jr., Carroll MC, Chan R, Afnan J, Humbles A, Gerard C, Knight P, Kanaoka Y, Yasuda S, Morokawa N, Austen KF, Stevens RL, Gurish MF. Mast cell protease 5 mediates ischemia-reperfusion injury of mouse skeletal muscle. J. Immunol. 2005;174:7285–7291. doi: 10.4049/jimmunol.174.11.7285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mukundan C, Gurish MF, Austen KF, Hechtman HB, Friend DS. Mast cell mediation of muscle and pulmonary injury following hindlimb ischemia-reperfusion. J. Histochem. Cytochem. 2001;49:1055–1056. doi: 10.1177/002215540104900813. [DOI] [PubMed] [Google Scholar]
- 9.Bortolotto SK, Morrison WA, Han X, Messina A. Mast cells play a pivotal role in ischaemia reperfusion injury to skeletal muscles. Lab. Investig. 2004;84:1103–1111. doi: 10.1038/labinvest.3700126. [DOI] [PubMed] [Google Scholar]
- 10.Stevens RL, Adachi R. Protease-proteoglycan complexes of mouse and human mast cells and importance of their beta-tryptase-heparin complexes in inflammation and innate immunity. Immunol Rev. 2007;217:155–167. doi: 10.1111/j.1600-065X.2007.00525.x. [DOI] [PubMed] [Google Scholar]
- 11.Pejler G, Abrink M, Ringvall M, Wernersson S. Mast cell proteases. Adv. Immunol. 2007;95:167–255. doi: 10.1016/S0065-2776(07)95006-3. [DOI] [PubMed] [Google Scholar]
- 12.Serafin WE, Dayton ET, Gravallese PM, Austen KF, Stevens RL. Carboxypeptidase A in mouse mast cells. Identification, characterization, and use as a differentiation marker. J. Immunol. 1987;139:3771–3776. [PubMed] [Google Scholar]
- 13.Reynolds DS, Stevens RL, Lane WS, Carr MH, Austen KF, Serafin WE. Different mouse mast cell populations express various combinations of at least six distinct mast cell serine proteases. Proc. Natl. Acad. Sci. USA. 1990;87:3230–3234. doi: 10.1073/pnas.87.8.3230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Reynolds DS, Stevens RL, Gurley DS, Lane WS, Austen KF, Serafin WE. Isolation and molecular cloning of mast cell carboxypeptidase A. A novel member of the carboxypeptidase gene family. J. Biol. Chem. 1989;264:20094–20099. [PubMed] [Google Scholar]
- 15.Weidner N, Austen KF. Heterogeneity of mast cells at multiple body sites. Fluorescent determination of avidin binding and immunofluorescent determination of chymase, tryptase, and carboxypeptidase content. Pathol. Res. Pract. 1993;189:156–162. doi: 10.1016/S0344-0338(11)80086-5. [DOI] [PubMed] [Google Scholar]
- 16.Irani AM, Craig SS, DeBlois G, Elson CO, Schechter NM, Schwartz LB. Deficiency of the tryptase-positive, chymase-negative mast cell type in gastrointestinal mucosa of patients with defective T lymphocyte function. J. Immunol. 1987;138:4381–4386. [PubMed] [Google Scholar]
- 17.Kaartinen M, Penttila A, Kovanen PT. Mast cells of two types differing in neutral protease composition in the human aortic intima. Demonstration of tryptase- and tryptase/chymase-containing mast cells in normal intimas, fatty streaks, and the shoulder region of atheromas. Arterioscler. Thromb. 1994;14:966–972. doi: 10.1161/01.atv.14.6.966. [DOI] [PubMed] [Google Scholar]
- 18.McNeil HP, Austen KF, Somerville LL, Gurish MF, Stevens RL. Molecular cloning of the mouse mast cell protease-5 gene. A novel secretory granule protease expressed early in the differentiation of serosal mast cells. J. Biol. Chem. 1991;266:20316–20322. [PubMed] [Google Scholar]
- 19.Gurish MF, Nadeau JH, Johnson KR, McNeil HP, Grattan KM, Austen KF, Stevens RL. A closely linked complex of mouse mast cell-specific chymase genes on chromosome 14. J. Biol. Chem. 1993;268:11372–11379. [PubMed] [Google Scholar]
- 20.Kunori Y, Koizumi M, Masegi T, Kasai H, Kawabata H, Yamazaki Y, Fukamizu A. Rodent alpha-chymases are elastase-like proteases. Eur. J. Biochem. 2002;269:5921–5930. doi: 10.1046/j.1432-1033.2002.03316.x. [DOI] [PubMed] [Google Scholar]
- 21.Karlson U, Pejler G, Tomasini-Johansson B, Hellman L. Extended substrate specificity of rat mast cell protease 5, a rodent alpha-chymase with elastase-like primary specificity. J. Biol. Chem. 2003;278:39625–39631. doi: 10.1074/jbc.M301512200. [DOI] [PubMed] [Google Scholar]
- 22.Solivan S, Selwood T, Wang ZM, Schechter NM. Evidence for diversity of substrate specificity among members of the chymase family of serine proteases. FEBS Lett. 2002;512:133–138. doi: 10.1016/s0014-5793(02)02242-1. [DOI] [PubMed] [Google Scholar]
- 23.Knight PA, Wright SH, Lawrence CE, Paterson YY, Miller HR. Delayed expulsion of the nematode Trichinella spiralis in mice lacking the mucosal mast cell-specific granule chymase, mouse mast cell protease-1. J. Exp. Med. 2000;192:1849–1856. doi: 10.1084/jem.192.12.1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Thakurdas SM, Melicoff E, Sansores-Garcia L, Moreira DC, Petrova Y, Stevens RL, Adachi R. The mast cell-restricted tryptase mMCP-6 has a critical immunoprotective role in bacterial infections. J Biol. Chem. 2007;282:20809–20815. doi: 10.1074/jbc.M611842200. [DOI] [PubMed] [Google Scholar]
- 25.Shin K, Watts GF, Oettgen HC, Friend DS, Pemberton AD, Gurish MF, Lee DM. Mouse mast cell tryptase mMCP-6 is a critical link between adaptive and innate immunity in the chronic phase of Trichinella spiralis infection. J. Immunol. 2008;180:4885–4891. doi: 10.4049/jimmunol.180.7.4885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shin K, Nigrovic PA, Crish J, Boilard E, McNeil HP, Larabee KS, Adachi R, Gurish MF, Gobezie R, Stevens RL, Lee DM. Mast cells contribute to autoimmune inflammatory arthritis via their tryptase/heparin complexes. J. Immunol. 2009;182:647–656. doi: 10.4049/jimmunol.182.1.647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McNeil HP, Shin K, Campbell IK, Wicks IP, Adachi R, Lee DM, Stevens RL. The mouse mast cell-restricted tetramer-forming tryptases mouse mast cell protease 6 and mouse mast cell protease 7 are critical mediators in inflammatory arthritis. Arthritis Rheum. 2008;58:2338–2346. doi: 10.1002/art.23639. [DOI] [PubMed] [Google Scholar]
- 28.Schneider LA, Schlenner SM, Feyerabend TB, Wunderlin M, Rodewald HR. Molecular mechanism of mast cell mediated innate defense against endothelin and snake venom sarafotoxin. J. Exp. Med. 2007;204:2629–2639. doi: 10.1084/jem.20071262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stevens RL, Qui D, McNeil HP, Friend D, Hunt J, Austen KF, Zhang M. Transgenic mice that possess a disrupted mast cell protease 5 (mMCP-5) gene cannot store carboxypeptidase A in their granules. FASEB J. 1996;10:1307. [Google Scholar]
- 30.Tchougounova E, Lundequist A, Fajardo I, Winberg JO, Abrink M, Pejler G. A key role for mast cell chymase in the activation of pro-matrix metalloprotease-9 and pro-matrix metalloprotease-2. J. Biol. Chem. 2005;280:9291–9296. doi: 10.1074/jbc.M410396200. [DOI] [PubMed] [Google Scholar]
- 31.McNeil HP, Frenkel DP, Austen KF, Friend DS, Stevens RL. Translation and granule localization of mouse mast cell protease-5. Immunodetection with specific antipeptide Ig. J. Immunol. 1992;149:2466–2472. [PubMed] [Google Scholar]
- 32.Tchougounova E, Pejler G, Abrink M. The chymase, mouse mast cell protease 4, constitutes the major chymotrypsin-like activity in peritoneum and ear tissue. A role for mouse mast cell protease 4 in thrombin regulation and fibronectin turnover. J. Exp. Med. 2003;198:423–431. doi: 10.1084/jem.20030671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Karlson U, Pejler G, Froman G, Hellman L. Rat mast cell protease 4 is a beta-chymase with unusually stringent substrate recognition profile. J. Biol. Chem. 2002;277:18579–18585. doi: 10.1074/jbc.M110356200. [DOI] [PubMed] [Google Scholar]
- 34.Hallgren J, Karlson U, Poorafshar M, Hellman L, Pejler G. Mechanism for activation of mouse mast cell tryptase: dependence on heparin and acidic pH for formation of active tetramers of mouse mast cell protease 6. Biochem. (Mosc) 2000;39:13068–13077. doi: 10.1021/bi000973b. [DOI] [PubMed] [Google Scholar]
- 35.Henningsson F, Wolters P, Chapman HA, Caughey GH, Pejler G. Mast cell cathepsins C and S control levels of carboxypeptidase A and the chymase, mouse mast cell protease 5. Biol. Chem. 2003;384:1527–1531. doi: 10.1515/BC.2003.169. [DOI] [PubMed] [Google Scholar]
- 36.Hunt J, Stevens RL. Mouse mast cell proteases. In: Galli SJ, Kitamura Y, Yamamoto S, Greaves M, editors. Biological and molecular aspects of mast cell and basophil differentiation and function. Raven Press, Limited; New York City: 1995. pp. 149–160. [Google Scholar]
- 37.Hunt JE, Stevens RL, Austen KF, Zhang J, Xia Z, Ghildyal N. Natural disruption of the mouse mast cell protease 7 gene in the C57BL/6 mouse. J. Biol. Chem. 1996;271:2851–2855. doi: 10.1074/jbc.271.5.2851. [DOI] [PubMed] [Google Scholar]
- 38.Feyerabend TB, Hausser H, Tietz A, Blum C, Hellman L, Straus AH, Takahashi HK, Morgan ES, Dvorak AM, Fehling HJ, Rodewald HR. Loss of histochemical identity in mast cells lacking carboxypeptidase A. Mol. Cell Biol. 2005;25:6199–6210. doi: 10.1128/MCB.25.14.6199-6210.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kitamura Y, Go S, Hatanaka K. Decrease of mast cells in W/Wv mice and their increase by bone marrow transplantation. Blood. 1978;52:447–452. [PubMed] [Google Scholar]
- 40.Chervenick PA, Boggs DR. Decreased neutrophils and megakaryocytes in anemic mice of genotype W/W. J. Cell. Physiol. 1969;73:25–30. doi: 10.1002/jcp.1040730104. [DOI] [PubMed] [Google Scholar]
- 41.Zhou JS, Xing W, Friend DS, Austen KF, Katz HR. Mast cell deficiency in Kit(W-sh) mice does not impair antibody-mediated arthritis. J. Exp. Med. 2007;204:2797–2802. doi: 10.1084/jem.20071391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pham CT, MacIvor DM, Hug BA, Heusel JW, Ley TJ. Long-range disruption of gene expression by a selectable marker cassette. Proc. Natl. Acad. Sci. USA. 1996;93:13090–13095. doi: 10.1073/pnas.93.23.13090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kunori Y, Muroga Y, Iidaka M, Mitsuhashi H, Kamimura T, Fukamizu A. Species differences in angiotensin II generation and degradation by mast cell chymases. J Recept. Signal. Transduct. Res. 2005;25:35–44. doi: 10.1081/rrs-200054355. [DOI] [PubMed] [Google Scholar]
- 44.Caughey GH, Raymond WW, Wolters PJ. Angiotensin II generation by mast cell alpha- and beta-chymases. Biochim. Biophys. Acta. 2000;1480:245–257. doi: 10.1016/s0167-4838(00)00076-5. [DOI] [PubMed] [Google Scholar]
- 45.Andersson MK, Karlson U, Hellman L. The extended cleavage specificity of the rodent [beta]-chymases rMCP-1 and mMCP-4 reveal major functional similarities to the human mast cell chymase. Mol. Immunol. 2008;45:766–775. doi: 10.1016/j.molimm.2007.06.360. [DOI] [PubMed] [Google Scholar]
- 46.Huang RY, Blom T, Hellman L. Cloning and structural analysis of MMCP-1, MMCP-4 and MMCP-5, three mouse mast cell-specific serine proteases. Eur. J. Immunol. 1991;21:1611–1621. doi: 10.1002/eji.1830210706. [DOI] [PubMed] [Google Scholar]
- 47.Schwartz LB, Kawahara MS, Hugli TE, Vik D, Fearon DT, Austen KF. Generation of C3a anaphylatoxin from human C3 by human mast cell tryptase. J. Immunol. 1983;130:1891–1895. [PubMed] [Google Scholar]
- 48.Schick B, Austen KF, Schwartz LB. Activation of rat serosal mast cells by chymase, an endogenous secretory granule protease. J. Immunol. 1984;132:2571–2577. [PubMed] [Google Scholar]
- 49.Jackson PL, Xu X, Wilson L, Weathington NM, Clancy JP, Blalock JE, Gaggar A. Human Neutrophil Elastase-Mediated Cleavage Sites of MMP-9 and TIMP-1: Implications to Cystic Fibrosis Proteolytic Dysfunction. Mol. Med. 2010;16:159–166. doi: 10.2119/molmed.2009.00109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Belaaouaj A, Walsh B, Jenkins N, Copeland N, Shapiro S. Characterization of the mouse neutrophil elastase gene and localization to Chromosome 10. Mamm. Genome. 1997;8:5–8. doi: 10.1007/s003359900337. [DOI] [PubMed] [Google Scholar]
- 51.el Lati SG, Dahinden CA, Church MK. Complement peptides C3a- and C5a-induced mediator release from dissociated human skin mast cells. J. Invest. Dermatol. 1994;102:803–806. doi: 10.1111/1523-1747.ep12378589. [DOI] [PubMed] [Google Scholar]
- 52.Kimura T, Andoh A, Fujiyama Y, Saotome T, Bamba T. A blockade of complement activation prevents rapid intestinal ischaemia-reperfusion injury by modulating mucosal mast cell degranulation in rats. Clin. Exp. Immunol. 1998;111:484–490. doi: 10.1046/j.1365-2249.1998.00518.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
SUPPLEMENTAL FIGURE 1. Evaluation of the enzymatic activities and secretory granule proteases in MCs from the MC protease-deficient mouse strains with targeted disruption of mMCP-4, mMCP-5 or mMCP-6. Using small synthetic substrates, the (A) elastase, (B) chymase, (C) tryptase, and (D) carboxypeptidase A3 (CPA3) activities in purified peritoneal MCs from WT , mMCP-4-deficient (4−/−), mMCP-5-deficient (5−/−), and mMCP-6/7-deficient (6/7−/−) mice are shown. A-D, Activities are the means (± SE) from 4 WT, 4 mMCP-4−/−, and 4 mMCP-5−/− mice, and the mean (± ½ range) from 2 mMCP-6/7−/− mice. E, Immunoblot analysis of peritoneal lavage cell lysates showing weak expression of mMCP-4 and no expression of either mMCP-5 or CPA3 in mMCP-5−/− mice while mMCP-4−/− mice only lack expression of mMCP-4. Cell lysates are from C57BL/6 (lane 1), mMCP-4−/− (lane 2) or mMCP-5−/− (lane 3) mice. Equal cell number equivalents were loaded in each lane. Parallel blots were probed with the indicated antibodies. Lane M shows the 30Kd molecular weight marker.