Abstract
This perspective on Boyd et al. (beginning on page XXX in this issue of the journal) discusses recent published research examining the interplay between social stress and breast cancer. Cross-disciplinary studies using genetically defined mouse models and established neonatal and peripubertal paradigms of social stress are illuminating biological programming by diverse early-life experiences for the risk of breast cancer. Understanding the mechanisms underlying this programming can lead to identification of risk factors and sensitive developmental windows, enabling improved prevention and treatment strategies for this devastating disease.
Keywords: breast cancer, stress, mouse models
Recent epidemiologic studies support what common sense suggests: high levels of stress may contribute to disease processes, including breast cancer. Although the results of some epidemiologic studies are mixed, much evidence now points to social stress as a factor in breast cancer progression and a potential component of the higher breast cancer mortality observed in socioeconomically disadvantaged women (1-8). However, the underlying mechanisms remain poorly understood, and controlled laboratory studies are sparse. Among the important questions that remain unanswered: Are early experiences important? If so, how are their effects mediated? Recent studies have taken advantage of genetically defined mouse models to probe the mechanisms underlying stress-related cancer using established experimental paradigms of stress administered during critical periods of maturation of the hypothalamic-pituitary-adrenal (HPA) and -gonadal (HPG) axes, as well as mammary development (Table 1). Two recent such studies, including one from our laboratories, have addressed the interplay between social stress initiated in the peripubertal period (3–6 weeks of age) and mammary tumorigenesis (9, 10). In this issue of the journal, the Kerr laboratory (Boyd et al.) reports a related third study, which extends this line of inquiry to early-life stress paradigms induced by neonatal maternal separation (11). Although these studies differ in the stressor and oncogenic stimulus, together they begin to illuminate the complex mechanisms whereby responses to diverse early-life stress experiences can modulate the developmental and oncogenic processes within the mammary gland. These studies, and the additional issues raised by their findings, have clear implications for long term programming of increased vulnerability to disease in women.
Table 1. Recent studies examining social stressors and mammary tumorigenesis in mouse models.
| Boyd et al. (11) | Williams et al. (9) | Hasen et al. (10) | |
| Stressor | Short- and long-duration separation of neonates from mother | Post-weaning isolated housing | Post-weaning isolated housing |
| Carcinogenic stimulus | DMBA | C3(1)/SV40 large T- antigen transgene | Germline p53 heterozygosity |
| Genetic strain | Balb/c | FVB/N | FVB/N |
| Mammary development | Increased development for both stressors | Not examined | Reduced development |
| Mammary molecular changes | Increased ERα protein with both stressors; no change in ERα mRNA or p53 mRNA/protein at 7.5 weeks of age | Increased mRNA for metabolic regulators, and altered mRNA associated with immunological/inflammatory responses at 15 weeks of age | Increased mRNA for the metabolic regulator, Acyl, and reduced mRNA for the epigenetic regulators, DNMT3b and Mecp2, mRNA at 14 weeks of age |
| Effect of stress on mammary tumors | Long, but not short maternal separation led to increased tumors | Increased tumor burden | Decreased tumor incidence |
Abbreviations: DMBA, 7,12-dimethylbenz[α]anthracene; ERα, estrogen receptor alpha.
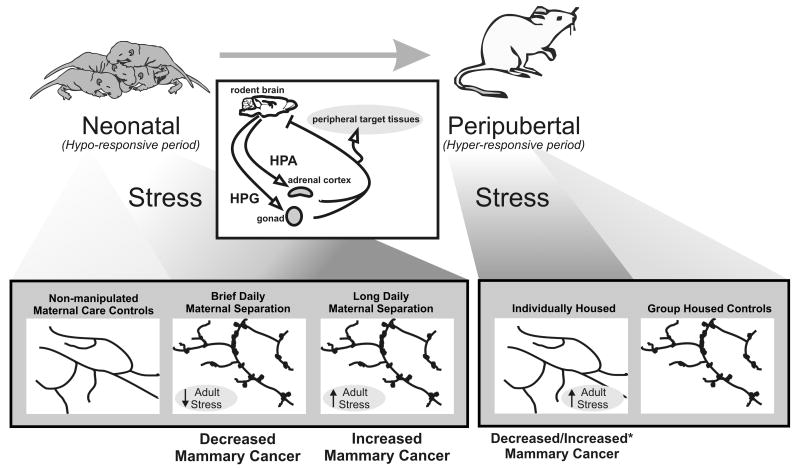
It is well known that stress during the perinatal period, as well as into the peripubertal period, can program lasting differences in neuroendocrine function of the HPA–HPG axes (12). The interactions of the HPA and HPG axes, which are generally antagonistic, provide an important relationship by which stress and the environment can regulate reproductive function and endocrine systems. Since stress can inhibit reproductive function, it provides a possible gateway by which social or other environmental stressors, via modulation of sex steroid hormones, can alter mammary development. In particular, the developmental programming of neuroendocrine function is directly influenced by the neonatal steroid environment and, in some cases, by the early social or maternal environment. There is a critical, or “sensitive,” rather, perinatal period during which the brain is exquisitely sensitive to environmental or endogenous factors, such as steroid hormones, that can differentiate the hypothalamus and thereby alter endocrine systems that last throughout an individual's lifespan. Some components of the neuroendocrine system remain sensitive to steroid hormones into the peripubertal period. As variations in steroid hormones have been linked to breast cancer (13), it is possible that programming of neuroendocrine function by early experiences may alter later breast cancer risk. The varying findings in epidemiologic studies of the potential psychosocial links to breast cancer risk/progression might be related to the timing of stressful experiences during developmental windows for steroid-sensitive brain development. One consequence of different timing is illustrated by the aforementioned studies in mouse models. The effects of chronic stress initiated in the neonatal period on mammary development are opposite to those of chronic stress initiated during the peripubertal period: neonatal maternal separation increases maturation of the ductal tree (11), whereas post-weaning social isolation reduces it (10). Recent studies have shown that variations during mouse development in the amount of maternal care received program estrogen-receptor (14, 15) and glucocorticoid-receptor (16) expression in brain tissue. Since both the HPA and HPG axes appear to be modulated by maternal interactions, it is not surprising that neonatal maternal separation can elicit lasting changes in mammary ductal development. What is intriguing in the comparison among the studies reviewed in this perspective is that differences in the timing and nature of early-life stress experiences have quite distinct consequences for both mammary ductal development and mammary-cancer risk (Fig. 1).
Fig. 1.
Conceptual model of diverse early-life stress experiences and their complex effects on risk of breast cancer. During the neonatal period, both brief (15 minutes) and long (4 hours) daily maternal separations resulted in increased mammary ductal complexity in adulthood compared with mice receiving normal maternal care, presumably as a result of heightened activity/responsiveness of the HPG axis (see adult ductal-morphology diagrams at the bottom of the figure; dark shading indicates stressed females). Neonatal rodents are relatively hyporesponsive to stress. Brief daily maternal separation and consequent attenuation of the HPA axis led to an interesting decrease in mammary cancer. The severe neonatal stressor of long daily maternal separation, however, overrides this hyporesponsiveness and leads to hypersensitivity of the HPA axis and increased mammary-cancer development in adulthood (11). The impact of stress during the peripubertal period, when rodents are hyper-responsive to stress, on breast-cancer incidence or progression is more complex. One study found that chronic stress induced by social isolation during the peripubertal period led to decreased mammary ductal complexity reflecting reduced activity/responsiveness of the HPG axis, which correlated with decreased carcinomas in an estrogen-sensitive model of mammary cancer (10). In a more-aggressive, less-estrogen–sensitive cancer model (marked with an asterisk), similar social isolation during the peripubertal period increased mammary tumor burden (9). As discussed in the text, the differential impact of peripubertal stress on HPA versus HPG programming may also underlie some of these discrepancies. Mammary glands in both of these peripubertal studies displayed similar changes in mRNA for regulators of key metabolic pathways, demonstrating significant effects of chronic stress initiated at this phase of development. The different impact on mammary cancer, however, suggests that the inhibitory effect of heightened stress on the HPG axis can override the deleterious effect (tumor progression) for some cancer types (discussed in the text). Note that mammary morphology in the normally treated control females differed somewhat between the studies reported in refs. 9 and 10. Although the basis for this difference is not clear (cross-fostering of pups, strain background, different ages and/or husbandry of mice, or a combination thereof), both studies show that the social environment during the neonatal or peripubertal period can strikingly alter mammary development that may alter breast-cancer risk. The inset diagram (middle) illustrates the well-documented pathway by which social or environmental cues can impact gonadal (HPG axis) or adrenal (HPA axis) steroid hormone secretions that provide signals to the brain and peripheral targets including the mammary gland. These studies indicate the importance of the timing of the stressor for mammary morphogenesis, and the consequences for tumorigenesis. Further research is needed to determine the effects of stressors and their timing on different breast-cancer subtypes, the consequences of individual variations in temperament in regard to stressor and stressor-timing effects, and the underlying mediators of psychosocial-stress effects in the mammary gland.
With some, but not all, experimental paradigms, effects on mammary development were strongly associated with mammary pathology in adult mice (Table 1). Reduced ductal complexity resulting from post-weaning social isolation was associated with a lower incidence of mammary tumors (10). Conversely, the study of the Kerr laboratory showed that increased ductal complexity induced by prolonged daily neonatal maternal separation was associated with a higher incidence of mammary tumors (11). However, despite similarly increased ductal complexity in females subjected to short daily neonatal maternal separation in the same study, the incidence of invasive mammary carcinomas was reduced (versus mice subjected to prolonged maternal separation or mice receiving normal maternal care; (ref. 11). Therefore, increased ductal development does not necessarily predict increased breast cancer risk. These studies do support the intriguing idea, however, that there are psychosocial links to mammary ductal development and altered risk for mammary carcinomas, albeit complicated ones. Further comparisons among the three studies in Table 1, which include some common elements of experimental design and endpoints, provide tantalizing clues to some mechanisms underlying the early-stress–cancer association with implications for human disease.
These three studies used different models of breast cancer (Table 1), which the literature, albeit incomplete, suggests are differently sensitive to mammogenic hormones. Another study showed that peripubertal ovariectomy has little effect on tumor incidence or latency in C3(1)/SV40 large T-antigen transgenic mice (17). Although mammary morphology was not reported in the Table 1 study employing this model (9), the effect in our study following the same stressor in the same mouse strain (10) would suggest that development was also impaired but did not significantly affect tumorigenesis. In contrast, mammary tumorigenesis in females heterozygotic for p53 may be more dependent on ovarian steroids since oncogenesis in transplanted p53-/- epithelium is very sensitive to ovariectomy (18). Consistently, pubertally isolated p53+/- females with underdeveloped mammary glands developed substantially fewer carcinomas (versus non-isolated mice), even though transcript profiles demonstrated stress-induced shifts in mammary metabolic regulators (10), similar to those reported in socially stressed female C3(1)/SV40 large T-antigen transgenic mice (9). The Boyd et al. study (11) examined tumorigenesis induced by the carcinogen 7,12-dimethylbenz(a)anthracene (DMBA), a model which also is hormonally sensitive (19). Although the activity of the HPG axis was not directly evaluated, the observed enhancement of mammary development in response to neonatal social manipulations likely reflects heightened activity and/or mammary responsiveness. Thus, the experimental design in this report allowed them to observe effects of divergent activity of the HPA axis on hormone-sensitive tumorigenesis in the context of greater mammary development. As predicted, manipulations elevating stress responsiveness increased the incidence of invasive carcinomas. Most intriguing, perinatal paradigms that attenuated adrenal activity partially protected against progression of DMBA-initiated mammary lesions.
Together, these studies (and important additional studies) suggest a model whereby the early social environment can impact breast cancer. The manipulations resulting in long-term potentiation of the HPA axis reported by the Kerr (11) and Conzen (9) laboratories demonstrate that stress can augment progression of mammary lesions. However, the opposite result reported by our laboratory with a different oncogenic stimulus (10) indicates additional complexity. This contrast suggests that lack of mammary development, reflecting a stress-induced decrease in activity of the HPG axis and/or sensitivity of the mammary gland to these signals, can also influence the outcome of pathologic processes in some circumstances. Sex steroid hormones modulate mammary stem cells, which may contain tumor precursors, and augment proliferation and survival of some types of breast-tumor epithelia (13, 20, 21). A possible explanation for the disparity in these studies is that the outcome of peripubertal stress may differ with the subtype of breast cancer. This hypothesis can be tested by investigating the effects of early stressors in additional defined models of breast-cancer subtypes (22), taking into account any effects on mammary development, particularly in models of hormonally responsive tumorigenesis. An important consideration is that the net impact of peripubertal stress on breast cancer is likely to reflect quantitative effects on the HPA and HPG axes. Specifically, hyperactivation of the HPA axis in the face of modest effects on the HPG axis can increase tumor progression (23). Therefore, the seemingly conflicting studies of peripubertal stress may suggest that, although peripubertal stress may decrease some estrogen-associated breast-cancer risk, it may exacerbate glucocorticoid-associated breast-cancer progression. Additional experimental studies are needed to elucidate the role of different kinds of stress (for example, psycho-social versus physical stress such as in young athletes) experienced by periadolescent females in determination of future breast-cancer risk. Furthermore, the importance of the peripubertal period needs to be examined. What is the effect of initiating social stress on tumorigenesis of different breast-cancer subtypes after mammary ductal morphogenesis is complete? It may be that the peripubertal window is critical for programming either neuroendocrine function or the responsiveness of the mammary gland itself to stress signals, as it is for susceptibility to mutagenic events (24, 25). Future experimental studies using sexually mature female rodents will assist in integrating our understanding of the impact of chronic psychological stress, such as that experienced by adult women caregivers, with biological mechanisms, such as accelerated telomere shortening (26), which has been associated with cancer development or progression (27). Understanding the importance of different developmental windows and net effects on the HPA and HPG axes is essential to understand the implications of these findings for human medicine.
Both the studies of Williams et al. (9) and our group (10) identified mammary transcripts that were altered in response to social stress initiated at puberty. The former group reported altered levels of mRNA for key metabolic regulators (discussed further by Trainor and coauthors; ref. 28). A representative stress-induced transcript was also altered in response to a similar stress in our study, despite a lack of associated pathology in our study (10), suggesting that stress can induce these metabolic changes independently of mammary lesions. We also observed that mammary transcripts for epigenetic regulators (DMNT3b, Mecp2) were reduced by this same stressor. Together, these observations raise several important questions. Are epigenetic regulators in the mammary gland altered by peripubertal stress in other breast cancer models, including C3(1)/SV40 large T-antigen transgenic mice? Which cell type(s) is affected? Are these metabolic and epigenetic regulators markers or mediators of reduced mammary development or chronic stress that may influence lesion progression? Are the changes in regulators only elicited by social stresses initiated at the peripubertal window? What is the effect on the same transcripts in the perinatal stress paradigms employed by Boyd et al (11) or after completion of pubertal endocrine maturation and mammary ductal elongation? How does altered function of the HPA–HPG axes alter these regulators? Some evidence implicates altered levels of glucocorticoids and adrenergic peptides, as well as immunological responses, in stress-related tumor progression (discussed in refs. 9, 28, 29). Are these changes (or changes in as yet unidentified factors) reversible? In light of the potential importance of metabolic changes (discussed further in (refs. 9, 28), and epigenetic changes associated with progenitor populations and cancer (30), these are important areas for future study.
As noted above, the three papers summarized in Table 1 point to intriguing disparities between the timing of social stress and its impact on mammary development and subsequent mammary cancer. The study by Boyd et al. (11) induced stress by maternal separation during the early-neonatal period of hypothalamic differentiation. Our study (10) and that of Williams et al. (9) induced stress by social isolation during the post-weaning period, when social stimuli are less maternally directed and more peer/sibling-dependent. Although newborn rodents are hypo-responsive to stress during the first two weeks of postnatal life, maternal separation remains an effective stressor for neonatal pups during this time period (12). Numerous studies have shown that maternal separation longer than three hours per day can increase HPA activity and can program lasting differences in corticotrophin-releasing–hormone binding sites within the pituitary gland, as well as within the amygdala, hippocampus, and hypothalamus. In contrast to the neonatal period, juvenile rats appear to be hyper-responsive to stress. That is, repeated exposure to a stressor during this period can potentiate the release of glucocorticoids. In adulthood, repeated exposure to the same stressor habituates the mouse to it and thus decreases the glucocorticoid response. Therefore, stressful experiences during these different developmental time periods may have different consequences on the HPA axis and therefore divergent impacts on breast cancer risk. The effects of these varying stress responses on mammary development suggest possible developmental windows for stress effects on programming of hypothalamic endocrine systems (HPA and/or HPG) and subsequent risk of breast cancer. The studies discussed here indicate sensitive windows for altered breast cancer risk, similar to the sensitive windows for neuroendocrine brain programming during early neonatal and, in some cases, peripubertal periods. That is, steroid hormone exposure during the first few weeks of neonatal life can program the HPA–HPG axes; whereas, steroid hormone exposure outside this window has reduced consequences. As mentioned above, maternal or social variation can also program lasting difference in hypothalamic neuroendocrine tissue. Thus, diverse early-life experiences have the potential to permanently alter sensitivity to steroids, and possibly to carcinogens, in a variety of tissues through the lasting consequences they cause in HPA–HPG function.
The epidemiological literature, studies of the Kerr (11) and Conzen (9) laboratories in mouse models discussed above, and studies in other experimental models of cancer (reviewed in ref. 29) support links between chronic stress and tumor progression. Unfortunately, any relationship between stress and lesion development or incidence remains unclear and is difficult to address in existing experimental models. Because of the implications for strategies to prevent not only the initial tumor but also the progression of micrometastases after removal of a primary tumor, the issue of the potential link between chronic stress and tumor risk merits considerable study. The importance of individual variations in temperament and their consequences for cancer development also deserve further investigation. Individual rats with a vigilant temperament are at a greater risk for developing mammary tumors (31). Conversely, individual variations, even within inbred mouse strains, could explain why some juveniles stressed by isolation in our study (10) and some individuals with augmented stress responses in the study of Boyd et al. (11) were resistant to tumorigenesis. The relationships among individual temperaments, neonatal and/or juvenile exposure to stress, and breast-cancer risk is an important area for further exploration, as it could provide models for investigating the effects of individual vulnerability or resilience of women on the development of breast cancer (32).
In summary, in light of recent publications using other defined murine models, the current report from the Kerr laboratory (11) illustrates the importance of early-life psychosocial stress experiences in subsequent mammary oncogenesis. This study, combined with other reports discussed here, underscores the intricate crosstalk between the HPG and HPA axes, which can occur at multiple levels—the hypothalamus, ovaries, and adrenal and mammary glands. Together, these studies offer insight into the many potential mechanisms whereby stress mediators can modulate breast tumorigenesis and point to the need for additional investigation. Models of maternal neglect and social-isolation paradigms both have significant relevance to human disease. Therefore, these animal models can provide reproducible preclinical models to test the intriguing idea that there are sensitive developmental windows for early programming of risk factors for breast cancer. Understanding how breast-cancer risk is altered by responses to the social environment will facilitate development of strategies for prevention not only of cancer but of other diseases that are exacerbated in vulnerable populations and contribute to health disparities in the general population.
Footnotes
Disclosure of Potential Conflicts of Interest: No potential conflicts of interest were disclosed.
References
- 1.Duijts SF, Zeegers MP, Borne BV. The association between stressful life events and breast cancer risk: a meta-analysis. Int J Cancer. 2003;107:1023–9. doi: 10.1002/ijc.11504. [DOI] [PubMed] [Google Scholar]
- 2.Chida Y, Hamer M, Wardle J, Steptoe A. Do stress-related psychosocial factors contribute to cancer incidence and survival? Nat Clin Pract Oncol. 2008;5:466–75. doi: 10.1038/ncponc1134. [DOI] [PubMed] [Google Scholar]
- 3.Antoni MH, Lutgendorf SK, Cole SW, Dhabhar FS, Sephton SE, McDonald PG, Stefanek M, Sood AK. The influence of bio-behavioural factors on tumour biology: pathways and mechanisms. Nat Rev Cancer. 2006;6:240–8. doi: 10.1038/nrc1820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Spiegel D, Giese-Davis J. Depression and cancer: mechanisms and disease progression. Biol Psychiatry. 2003;54:269–82. doi: 10.1016/s0006-3223(03)00566-3. [DOI] [PubMed] [Google Scholar]
- 5.McClintock MK, Conzen SD, Gehlert S, Masi C, Olopade F. Mammary cancer and social interactions: identifying multiple environments that regulate gene expression throughout the life span. J Gerontol B Psychol Sci Soc Sci. 2005;60(Spec No 1):32–41. doi: 10.1093/geronb/60.special_issue_1.32. [DOI] [PubMed] [Google Scholar]
- 6.Garssen B. Psychological factors and cancer development: evidence after 30 years of research. Clin Psychol Rev. 2004;24:315–38. doi: 10.1016/j.cpr.2004.01.002. [DOI] [PubMed] [Google Scholar]
- 7.Dalton SO, Boesen EH, Ross L, Schapiro IR, Johansen C. Mind and cancer. do psychological factors cause cancer? Eur J Cancer. 2002;38:1313–23. doi: 10.1016/s0959-8049(02)00099-0. [DOI] [PubMed] [Google Scholar]
- 8.Palesh O, Butler LD, Koopman C, Giese-Davis J, Carlson R, Spiegel D. Stress history and breast cancer recurrence. J Psychosom Res. 2007;63:233–9. doi: 10.1016/j.jpsychores.2007.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Williams JB, Pang D, Delgado B, Kocherginsky M, Tretiakova M, Krausz T, Pan D, He J, McClintock MK, Conzen SD. A model of gene-environment interaction reveals altered mammary gland gene expression and increased tumor growth following social isolation. Cancer Prev Res. 2009;2:850–61. doi: 10.1158/1940-6207.CAPR-08-0238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hasen NS, O'Leary KA, Auger AP, Schuler LA. Social isolation reduces mammary development, tumor incidence and expression of epigenetic regulators in wild type and p53-heterozygotic mice. Cancer Prev Res. 2010;3:620–9. doi: 10.1158/1940-6207.CAPR-09-0225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Boyd AL, Salleh A, Humber B, Yee J, Tomes L, Kerr LR. Neonatal experiences differentially influence mammary gland morphology, estrogen receptor alpha protein levels, and carcinogenesis in Balb/c mice. Cancer Prev Res. 2010;3 doi: 10.1158/1940-6207.CAPR-10-0111. XXX. [DOI] [PubMed] [Google Scholar]
- 12.Lupien SJ, McEwen BS, Gunnar MR, Heim C. Effects of stress throughout the lifespan on the brain, behaviour and cognition. Nat Rev Neurosci. 2009;10:434–45. doi: 10.1038/nrn2639. [DOI] [PubMed] [Google Scholar]
- 13.Hankinson SE, Eliassen AH. Endogenous estrogen, testosterone and progesterone levels in relation to breast cancer risk. J Steroid Biochem Mol Biol. 2007;106:24–30. doi: 10.1016/j.jsbmb.2007.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Champagne FA, Weaver IC, Diorio J, Dymov S, Szyf M, Meaney MJ. Maternal care associated with methylation of the estrogen receptor-alpha1b promoter and estrogen receptor-alpha expression in the medial preoptic area of female offspring. Endocrinology. 2006;147:2909–15. doi: 10.1210/en.2005-1119. [DOI] [PubMed] [Google Scholar]
- 15.Kurian JR, Olesen KM, Auger AP. Sex differences in epigenetic regulation of the estrogen receptor-alpha promoter within the developing preoptic area. Endocrinology. 2010;151:2297–305. doi: 10.1210/en.2009-0649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Weaver IC, Cervoni N, Champagne FA, D'Alessio AC, Sharma S, Seckl JR, Dymov S, Szyf M, Meaney MJ. Epigenetic programming by maternal behavior. Nat Neurosci. 2004;7:847–54. doi: 10.1038/nn1276. [DOI] [PubMed] [Google Scholar]
- 17.Yoshidome K, Shibata MA, Couldrey C, Korach KS, Green JE. Estrogen promotes mammary tumor development in C3(1)/SV40 large T- antigen transgenic mice: paradoxical loss of estrogen receptoralpha expression during tumor progression. Cancer Res. 2000;60:6901–10. [PubMed] [Google Scholar]
- 18.Medina D, Kittrell FS, Shepard A, Contreras A, Rosen JM, Lydon J. Hormone dependence in premalignant mammary progression. Cancer Res. 2003;63:1067–72. [PubMed] [Google Scholar]
- 19.Jerry DJ, Kittrell FS, Kuperwasser C, Laucirica R, Dickinson ES, Bonilla PJ, Butel JS, Medina D. A mammary-specific model demonstrates the role of the p53 tumor suppressor gene in tumor development. Oncogene. 2000;19:1052–8. doi: 10.1038/sj.onc.1203270. [DOI] [PubMed] [Google Scholar]
- 20.Joshi PA, Jackson HW, Beristain AG, Di Grappa MA, Mote P, Clarke C, Stingl J, Waterhouse PD, Khokha R. Progesterone induces adult mammary stem cell expansion. Nature. 2010;465:803–7. doi: 10.1038/nature09091. [DOI] [PubMed] [Google Scholar]
- 21.Asselin-Labat ML, Vaillant F, Sheridan JM, Pal B, Wu D, Simpson ER, Yasuda H, Smyth GK, Martin TJ, Lindeman GJ, Visvader JE. Control of mammary stem cell function by steroid hormone signalling. Nature. 2010;465:798–802. doi: 10.1038/nature09027. [DOI] [PubMed] [Google Scholar]
- 22.Herschkowitz JI, Simin K, Weigman VJ, Mikaelian I, Usary J, Hu Z, Rasmussen KE, Jones LP, Assefnia S, Chandrasekharan S, Backlund MG, Yin Y, Khramtsov AI, Bastein R, Quackenbush J, Glazer RI, Brown PH, Green JE, Kopelovich L, Furth PA, Palazzo JP, Olopade OI, Bernard PS, Churchill GA, van Dyke T, Perou CM. Identification of conserved gene expression features between murine mammary carcinoma models and human breast tumors. Genome Biol. 2007;8:R76. doi: 10.1186/gb-2007-8-5-r76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hermes GL, Delgado B, Tretiakova M, et al. Social isolation dysregulates endocrine and behavioral stress while increasing malignant burden of spontaneous mammary tumors. Proc Natl Acad Sci U S A. 2009;106:22393–98. doi: 10.1073/pnas.0910753106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Russo J, Yang X, Hu YF, Bove BA, Huang Y, Silva ID, Tahin Q, Wu Y, Higgy N, Zekri A, Russo IH. Biological and molecular basis of human breast cancer. Front Biosci. 1998;3:D944–D960. doi: 10.2741/a335. [DOI] [PubMed] [Google Scholar]
- 25.Land CE, Tokunaga M, Koyama K, Soda M, Preston DL, Nishimori I, Tokuoka S. Incidence of female breast cancer among atomic bomb survivors, Hiroshima and Nagasaki, 1950-1990. Radiat Res. 2003;160:707–17. doi: 10.1667/rr3082. [DOI] [PubMed] [Google Scholar]
- 26.Epel ES, Blackburn EH, Lin J, et al. Accelerated telomere shortening in response to life stress. Proc Natl Acad Sci U S A. 2004;101:17312–15. doi: 10.1073/pnas.0407162101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Blackburn EH, Tlsty TD, Lippman SM. Unprecedented opportunities and promise for cancer prevention research. Cancer Prev Res. 2010;3:394–402. doi: 10.1158/1940-6207.CAPR-10-0051. [DOI] [PubMed] [Google Scholar]
- 28.Trainor BC, Sweeney C, Cardiff R. Isolating the effects of social interactions on cancer biology. Cancer Prev Res. 2009;2:843–6. doi: 10.1158/1940-6207.CAPR-09-0167. [DOI] [PubMed] [Google Scholar]
- 29.Armaiz-Pena GN, Lutgendorf SK, Cole SW, Sood AK. Neuroendocrine modulation of cancer progression. Brain Behav Immun. 2009;23:10–15. doi: 10.1016/j.bbi.2008.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bloushtain-Qimron N, Yao J, Shipitsin M, Maruyama R, Polyak K. Epigenetic patterns of embryonic and adult stem cells. Cell Cycle. 2009;8:809–17. doi: 10.4161/cc.8.6.7938. [DOI] [PubMed] [Google Scholar]
- 31.Cavigelli SA, Yee JR, McClintock MK. Infant temperament predicts life span in female rats that develop spontaneous tumors. Horm Behav. 2006;50:454–62. doi: 10.1016/j.yhbeh.2006.06.001. [DOI] [PubMed] [Google Scholar]
- 32.Feder A, Nestler EJ, Charney DS. Psychobiology and molecular genetics of resilience. Nat Rev Neurosci. 2009;10:446–57. doi: 10.1038/nrn2649. [DOI] [PMC free article] [PubMed] [Google Scholar]