Abstract
Refuge sharing by otherwise solitary individuals during periods of inactivity is an integral part of social behaviour and has been suggested to be the precursor to more complex social behaviour. We compared social association patterns of active versus inactive sheltering individuals in the social Australian sleepy lizard, Tiliqua rugosa, to empirically test the hypothesis that refuge sharing facilitates social associations while individuals are active. We fitted 18 neighbouring lizards with Global Positioning System (GPS) recorders to continuously monitor social associations among all individuals, based on location records taken every 10 min for 3 months. Based on these spatial data, we constructed three weighted, undirected social networks. Two networks were based on empirical association data (one for active and one for inactive lizards in their refuges), and a third null model network was based on hypothetical random refuge sharing. We found patterns opposite to the predictions of our hypothesis. Most importantly, association strength was higher in active than in inactive sheltering lizards. That is, individual lizards were more likely to associate with other lizards while active than while inactive and in shelters. Thus, refuge sharing did not lead to increased frequencies of social associations while lizards were active, and we did not find any evidence that refuge sharing was a precursor to sleepy lizard social behaviour. Our study of an unusually social reptile provides both quantitative data on the relationship between refuge sharing and social associations during periods of activity and further insights into the evolution of social behaviour in vertebrates.
Keywords: Association, Sleepy lizard, Refuge sharing, Social behaviour, Social networks
Introduction
Refuge sharing or communal roosting during periods of inactivity occurs across many animal taxa (Childress and Herrnkind 1997; Warburg 2000; Visagie et al. 2005), including many bird (Beauchamp 1999), bat (Willis and Brigham 2004), and primate species (Anderson 1998). After associating while inactive, individuals may separate during periods of activity to forage alone (Kerth et al. 2001) or in smaller groups (Kummer 1984), or they remain associated in large groups, as in many communal roosting and flocking bird species (Beauchamp 1999). Individuals that associate while inactive experience reduced predation risk through enhanced vigilance (Lanham and Bull 2004) or dilution of risk (Hamilton 1971). Association during inactivity can also lead to physiological benefits. In heterotherms, close associations or physical contact between individuals reduce cooling rates at night and evaporative water loss in desiccating conditions (du Plessis et al. 1994; Perret 1998; Lancaster et al. 2006; Aubret and Shine 2009). Similarly, Hwang et al. (2007) found that striped skunks (Mephitis mephitis) that overwintered in groups emerged in spring with higher percent body fat than those that overwintered alone. Such benefits should promote mutual tolerance among individuals while they are at sleeping or overwintering sites and may even facilitate cooperation during these periods. Accordingly, sleeping associations are recognised as an integral part of social behaviour (Hamilton 1982; Duffield and Bull 2002; Martin and Martin 2007) and have been suggested to be a precursor to more complex sociality during periods of activity (Warburg 2000; Rasoloharijaona et al. 2003; Shah et al. 2003; Lancaster et al. 2006). However, sleeping associations may also occur independently of any potential benefits if sleeping sites are in short supply. Several previous studies have found that association frequencies increased as the availability of sleeping sites decreased (Childress and Herrnkind 1997; Nieuwoudt et al. 2003; Visagie et al. 2005; Gardner et al. 2007). These results support the environmental constraint hypothesis to explain the evolution of sharing of sleeping sites. Irrespective of the causes leading to sleeping associations, they have potential consequences for social behaviour during periods of activity. In particular, an increased incidence of associating while individuals are inactive might lead to increased social associations while active. Perhaps, the mutual tolerance among individuals in sleeping sites might translate to tolerance while active. This hypothesis about a possible origin for more complex social behaviour remains to be investigated.
Reptiles are frequently ignored in studies of sociality, perhaps because most species appear to be solitary (Brattstrom 1974; Chapple 2003). However, several recent studies have reported highly complex social behaviour among an increasing number of reptile species, particularly lizards (Mouton et al. 1999; Bull 2000; Gardner et al. 2001; Duffield and Bull 2002; Fox et al. 2003; O’Connor and Shine 2003; Stow and Sunnucks 2004; Chapple and Keogh 2006; While et al. 2009a, b). Lizards are important model organisms in the investigation of social behaviour because some selection pressures for social association in other taxa are commonly absent in lizards (Fox et al. 2003). For example, neither obligate biparental care, which selects for social associations in birds (Mock and Fujioka 1990; Kokko 1999) nor infanticide risk, which is important in mammals (van Schaik and Kappeler 1997; van Schaik 2000), is common in lizards. But, infanticide in lizards has been occasionally reported (Lanham and Bull 2000; O’Connor and Shine 2004).
We studied social associations and refuge sharing in the Australian sleepy lizard, Tiliqua rugosa. The sleepy lizard belongs to the Egernia group of scincid lizards that includes many highly social species (Chapple 2003; Gardner et al. 2008). Social organisation within the Egernia group ranges from solitary species to species that form long-term stable family groups consisting of an adult pair and multiple cohorts of their offspring (Duffield and Bull 2002; Chapple 2003). Sleepy lizards form long-term monogamous pair bonds (Bull 1988; Bull et al. 1998; Bull and Burzacott 2006), an intermediate level of social complexity within the Egernia group (While et al. 2009b). Social pair partners are in frequent contact, particularly in a period of up to 8 weeks prior to mating, when males closely follow behind females (within 30 cm) on some days; but pairs are not constantly together (Bull 1988; Bull et al. 1998; Leu et al. 2010a). Social pair partners also share night-time refuges on some nights (Kerr et al. 2003). Although there is extensive home range overlap with neighbouring lizards of both sexes, the core areas of home ranges only overlap among social pair partners (Kerr and Bull 2006a). Individual lizards also associate and interact with non-pair neighbours (Leu et al. 2010a) and can sometimes share refuges with one or more non-pair neighbours at a time (Kerr et al. 2003). However, we have also shown that individual lizards avoid contact with some neighbours while active because an empirically observed social network had fewer connections than expected, if lizards were moving at random in their home ranges (Leu et al. 2010a). In our study site, sleepy lizards use bluebushes, Maireana sedifolia, and burrows dug by mammals as their main overnight refuges (Kerr et al. 2003; Kerr and Bull 2004). Lizards non-randomly choose a set of overnight refuges from among a larger number of available refuges within their home ranges and repeatedly re-use refuges from this set (Kerr et al. 2003).
We asked whether refuge sharing during inactive periods was related to sleepy lizard social associations while active. Such a relationship may indicate that social associations during active periods are facilitated by tolerance for conspecifics while inactive and that refuge sharing is thus an important component of social organisation. We developed three predictions to explore this hypothesis: (1) refuge sharing would occur more frequently than expected by chance; (2) refuge sharing frequencies while inactive and social association frequencies while active would be positively correlated; and (3) social association strengths of active lizards would be lower than social association strengths of inactive refuge sharing lizards. The second prediction was to test whether individuals that often share refuges are more likely to associate while active and out of refuges. However, such a positive correlation might result either from behavioural differences in tolerance, or from the spatial organisation of the population. In particular, high levels of tolerance for conspecifics while inactive may result in higher social association frequencies while active. But, more extensive home range overlap between some neighbour lizards may result in higher frequencies of chance encounters and hence, association frequencies, both while inactive in refuges and while active, may also be higher. The third prediction should differentiate between those two explanations. If the correlation arises from spatial arrangements, we would expect both association frequencies to be influenced to a similar extent. Most importantly, this third prediction allows testing of the directionality of the relationship between refuge sharing and social association of active lizards. If sharing refuges facilitates social associations while individuals are active, and if it is the only deterministic factor, refuge sharing should occur more frequently than social associations while active.
We used light-weight GPS devices to continuously monitor social associations among neighbouring individuals over a period of 3 months. Based on these spatial data, we applied network analysis techniques to derive quantitative measurements of social association strengths among all members of the study population (Krause et al. 2009) to test our predictions. The relationship between refuge sharing and social association of active animals has broader implications for the understanding of the evolution of vertebrate social behaviour. We used our data to examine the hypothesis that refuge sharing has been an evolutionary precursor to more complex sociality in this lizard species and to reflect on the broader relevance of that hypothesis.
Methods
Study animals and site
The sleepy lizard is a large (snout-vent length ≥ 28 cm), long-lived (up to 50 years) scincid lizard endemic to Australia (Bull 1995). It is most active during spring and early summer (September–December; Bull 1987; Firth and Belan 1998), the time when we conducted our study. We observed 18 adult sleepy lizards, the entire resident population in our 700 m × 1000 m study site (33°54′16″S, 139°20′43″E) near Bundey Bore Station, Southern Australia. The study animals were part of a larger population that extended beyond the study site. The area is characterised by chenopod shrubland, dominated by bluebush, M. sedifolia.
GPS tagging of study animals
In August 2007, we caught 18 resident adult lizards (nine males and nine females). They occupied overlapping home ranges, and each had some social interaction with at least one other lizard of the study population (Leu et al. 2010a). We attached a 37-g data logger to the tail of each lizard using surgical adhesive tape. The data loggers included a GPS device and a radio transmitter and weighed 4.9% of an average-sized lizard (750 g). Unique signals from the radio transmitter allowed us to recognise and locate each lizard. Between 15 September and 15 December 2007, the data loggers recorded the GPS location of each lizard every 10 min if it had moved in that period. We synchronised the data recording process among all data loggers, so that all locations were recorded at the same time. We recaptured each lizard fortnightly to download GPS location data and to replace the logger battery. Handling time, less than 60 min per fortnight, was excluded from the data set. In mid-December, we removed the data loggers and released all lizards. We detected no skin damage or irritation where the units were attached, and lizards naturally shed their skin in the following months.
Social network construction
We constructed three weighted, undirected social networks, two based on empirical data and a third null model network based on hypothetical random refuge use. Each individual of the study group was represented by a node in each of the networks. An edge between two nodes indicated that those two individuals had associated during the study period. The edge weight represented the strength of the dyadic association as the relative frequency of association.
One empirical network was based on associations between dyads of lizards while they were inactive in their overnight refuges. The second network was based on associations between dyads of lizards when they were active. The active social network has been previously reported as an unweighted nonzero network (Leu et al. 2010a); but for this study, we derived weighted edges between nodes. In both networks, associations were deduced from spatial proximity with the same two assumptions. First, we considered a social association occurred if lizards were within 2 m of each other. Second, because each GPS device had a median precision of 6 m (Leu et al. 2010a), we considered that two lizards could be within 2 m of each other if the recorded locations were up to 14 m apart. Although this effectively included individuals that could actually have been up to 26 m apart, this distance is within the visual perceptual range of the sleepy lizard (Auburn et al. 2009) and could quickly be covered by lizards that can move more than 2,000 m a day (Kerr and Bull 2006b). Nonetheless, the distance threshold we used may have overestimated the number of social associations. Then, we calculated simple ratio association indices (SRI), which represented the edge weights in the network, by taking the number of occasions when a dyad of lizards was associated and dividing that number by the dyadic sample size, which was the number of times that a distance could be calculated for each dyad of lizards. Thus, all simple association indices were scaled to one, and this took into account that dyadic sample sizes differed among pairs of lizards.
To construct these two networks, we calculated inter-lizard distances on all occasions when both lizards were inactive in overnight refuges (network I) or on all occasions when both lizards were active (network II). Note that dyadic sample sizes for the inactive network were smaller because lizards had only one overnight refuge per day, while, for the active network, there were multiple observations per day when both lizards were active. This disparity in dyadic samples sizes between the networks and the different temporal dependence of sample points was accounted for by calculating SRIs.
Network I described social associations of inactive lizards based on the frequency with which dyads of lizards shared overnight refuges. We deduced the overnight refuge location of each lizard as the last GPS location record on each day (Leu et al. 2010b). The last record marked the end of daily activity because the GPS devices did not record locations when lizards were inactive. Also, no GPS signal was received while lizards were in burrows or under thick shelters, so that the last location record was likely to be close to the refuge rather than in the refuge. Bushes used as refuges have an average canopy area of 4 m2 (Kerr et al. 2003), and mammal burrows are generally more than 2 m long (Leu pers. obs.); so, we considered two lizards located within 2 m of each other to be sharing the same refuge. For each lizard on each night, we calculated distances between its recorded refuge location and the refuge location of all other lizards on that night. We considered that location records 14 m or less apart represented two lizards sharing the same refuge. If a lizard remained inactive in the same refuge over consecutive days, the location was only used once in network calculations. We calculated the refuge sharing SRI, and thus edge weight, as the number of recorded sharing events during the season divided by the number of inter-refuge distances available for each dyad of lizards.
Network II described social associations of active lizards. Using the same distance criterion of 14 m, we deduced the number of social associations among all possible dyads of active lizards during the season. Then, we divided the number of associations by the number of inter-lizard distances available for each active lizard dyad to calculate the active social association SRI. Lizards were considered active if they had moved during the previous 10 min.
Network III was a hypothetical network, based on the assumption that each lizard randomly chose one refuge out of its set of used refuges each night. Based on the location coordinates of used refuges, we calculated for each lizard dyad the refuge overlap, the number of refuges that were used by both lizards over the study period. Two refuge locations were considered to represent the same refuge if they were within 14 m of each other. If lizard A and lizard B use the same n AB refuges in the overlap area of their home ranges, the probability of them sharing one of these refuges on any night will depend on the total number of refuges of each lizard (n A and n B). To assign a network edge weight, we calculated the random sharing index (RSI) for a dyad on a given observation as:
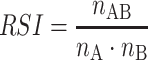 |
The RSI, similar to the SRI calculated above, was also scaled to one. Hence, values were directly comparable among dyads of lizards with different numbers of observations.
In the sleepy lizard, the social unit and the reproductive unit are identical because it is both a genetically monogamous and pair-living species (Bull 1988; Bull 2000; Leu et al. 2010a). Accordingly, we expected that social associations within the social units and that are between social pair partners would be strongly influenced by the reproductive strategy. Beyond the pair bonds, sleepy lizards are part of a stable social neighbourhood. Neighbourhoods are described by decreasing familiarity among individuals with decreasing home range overlap (Kappeler and van Schaik 2002). Sleepy lizard social neighbourhoods have been characterised in previous studies showing that individuals establish stable home ranges with little seasonal shift but extensive overlap among non-pair neighbours (Bull and Freake 1999; Kerr and Bull 2006a) and that non-pair neighbours are in regular contact (Leu et al. 2010a). During movement, sleepy lizards appear to leave individually unique chemical trails that are recognised by conspecifics (Bull et al. 1993; Bull and Lindle 2002), implying that they can make an active choice of whether to avoid or share an already occupied refuge. For instance, during hot days, deep cool refuges are sometimes shared by multiple neighbours (Kerr and Bull 2006a). Thus, in our analysis, we distinguished associations between the male and female of a social pair, from associations among lizards of any sex that were not identified as a social pair. We referred to the latter group as non-pair lizards.
Social network analysis
We first excluded all edges between known pair-living partners from each of the networks. Members of a social unit associate more frequently among each other than with members of other social units (Struhsaker 1969), and we identified the social pairs by an active social association SRI ≥ 0.10 (Leu et al. 2010a). Although this threshold is arbitrary, it captures an association strength dichotomy in our data, with SRIs generally either less than or equal to 0.05 or above 0.10 (Leu et al. 2010a). Then, for each individual, we calculated its node strength with non-pair individuals as the sum of all non-pair edge weights connected to a node (Croft et al. 2008). This node strength represents how well a node is connected within the network. High strength values reflect strong associations with non-pair lizards. High values could either result from many edges with moderate edge weights (i.e. the lizard associates with many other lizards infrequently) or from few edges with high edge weights (i.e. the lizard associates with a few lizards quite regularly).
Because we constructed the three networks from the same local population, they each had the same number of nodes. Because node strength values were calculated from dyadically measured and comparable edge weights, we could make straightforward comparisons between pairs of networks (Croft et al. 2008; James et al. 2009). Our data met the assumption of normality, and we used paired t tests to compare node strength values between pairs of networks. We further used Pearson correlation analyses to determine the relationship of node strength values for individual lizards between pairs of networks. Because network-derived measurements, such as node strength, are relational non-independent data (Croft et al. 2008), we used two sample randomisation tests to estimate the probability that the observed test statistic t or r was obtained by chance. During randomisation, within each network, we randomly reassigned the strength values to all nodes without replacement and recalculated the test statistic. We repeated this procedure 1,000 times to achieve a consistent frequency distribution of the randomly generated t values or r values (Bejder et al. 1998). Following Croft et al. (2008), we calculated Monte Carlo P values as the quotient of the number of times the randomly generated values were more extreme than the observed value.
The edge weight between pair-living partners represented their association intensity in all three networks. Similar to the node strength values, edge weights were relational non-independent data, so we analysed them in the same way as the node strength values, using permutation paired t tests and Pearson correlation analyses with 1,000 permutations. Data were ln-transformed to meet the assumption of normality. Where data were not normally distributed, we presented both mean and median values in the results. We used PopTools (Hood 2008) to analyse node strength and edge weight values and NetDraw (Borgatti 2002) to illustrate our networks.
Results
We made 1,248 observations of lizards in overnight refuges, with 48–85 observations per lizard (mean = 69.3, SE = 2.5, N = 18). Each lizard used a mean of 22 different refuges over the study period (range, 12–39, N = 18). In total, the 18 lizards used 229 different refuges with a mean distance of 25.8 m (SE = 1.2) to the nearest next refuge site. We made 24 observations of two lizards, not defined as a social pair, sharing the same refuge concurrently. Each lizard was observed sharing a refuge with a non-pair partner from 0–10 times during the study (mean = 2.7, SE = 0.6, N = 18, median = 2), with a total of 0–8 different lizards other than the pair partner (mean = 2.0, SE = 0.5, N = 18). Table 1 illustrates the number of associating lizards in relation to sex. There were eight social pairs in our study population. We made 36 observations of these social pairs sharing a refuge with 1–13 sharing events per social pair (mean = 4.5, SE = 1.5, N = 8).
Table 1.
Non-pair social behaviour
| Network | Mean node degree (with males/females only) | |
|---|---|---|
| Empirical refuge sharing | Males | 2.78 (2.00/0.78) |
| Females | 1.22 (0.78/0.44) | |
| Random refuge sharing | Males | 8.78 (4.67/4.11) |
| Females | 7.22 (4.11/3.11) | |
| Active social association | Males | 8.44 (4.22/4.22) |
| Females | 7.78 (4.22/3.56) |
Mean number of individuals (separated into males and females) each sex associated with outside the social pair, shown as node degree
Mean node degree values are based on N = 9 males and N = 9 females
We recorded 539 social associations between non-pair lizards while both were active (range, 3–131 per lizard; mean = 59.9, SE = 9.7, N = 18) and 884 associations between social pair partners (range, 32–372 per pair; mean = 110.5, SE = 38.3, N = 8, median = 78). Figure 1 shows the three networks we constructed, with edges between social pairs removed.
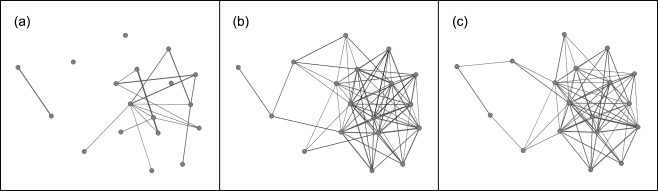
Fig. 1.
Networks of the study population. Nodes (circles) represent individuals; edges (lines) are undirected and represent association between individuals; line thickness represents association frequency (scaled to 1) between respective nodes. a empirical refuge sharing network: observed associations while inactive and in refuges, b active social network: observed associations while active, c random refuge sharing network: hypothetical associations while inactive and in refuges
Refuge sharing while inactive overnight
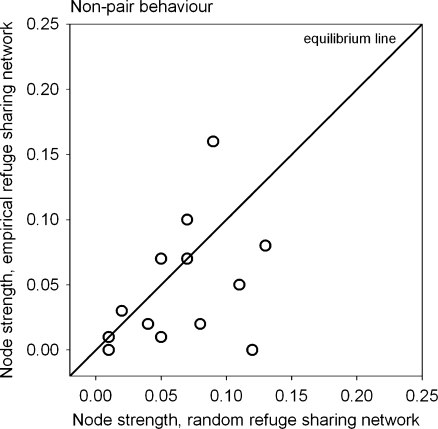
First, we asked whether sleepy lizards shared their refuges more or less frequently than would occur if they used their refuges at random. For non-pair partners, we compared the empirical (Fig. 1a) with the hypothetical (Fig. 1c) refuge sharing network and detected no significant difference in mean node strength, implying non-pair refuge sharing occurred at random (Table 2). However, there was a tendency for marginally higher node strength values in the hypothetical network. Figure 2 shows an equilibrium line that represents random refuge sharing. Data points away from the equilibrium line representing individuals that shared refuges with their non-pair neighbours more (above) or less (below the line) frequently than expected by random refuge use.
Table 2.
Non-pair social behaviour
| Node strength comparison | Observed t 17 | Mean random t 17 (95%CI) | P values |
|---|---|---|---|
| Empirical vs. random refuge sharing | −1.20 | −0.95 (−1.29−0.77) | 0.063 |
| Empirical refuge sharing vs. active social association | −3.55 | −2.54 (−3.26−2.07) | 0.002 |
Randomisation paired t test analysis based on node strength values. All randomisation values are based on 1,000 iterations
CI confidence interval
Fig. 2.
Random versus empirical refuge sharing network. The equilibrium line separates above and below random refuge sharing frequencies between non-pair lizards (measured as node strength). Edges between social pairs had been removed from networks before calculating node strengths. N = 18, some data points depict multiple identical node strength values
In the empirical refuge sharing network, there was no significant difference in mean node strength between sexes (independent t test: observed t 8 = 1.39, mean random t 8 = 0.01, Monte Carlo P = 0.109). Furthermore, neither males nor females shared refuges with non-pair individuals more frequently than expected by chance (Table 3).
Table 3.
Non-pair social behaviour of each sex
| Node strength comparison | Observed t 8 | Mean random t 8 (95%CI) | P values | |
|---|---|---|---|---|
| Empirical vs. random refuge sharing | Males | −0.19 | −0.15 (−0.23−0.11) | 0.101 |
| Females | −1.44 | −1.25 (−2.10−0.92) | 0.196 |
Randomisation paired t test analysis based on node strength values. All randomisation values are based on 1,000 iterations
CI confidence interval
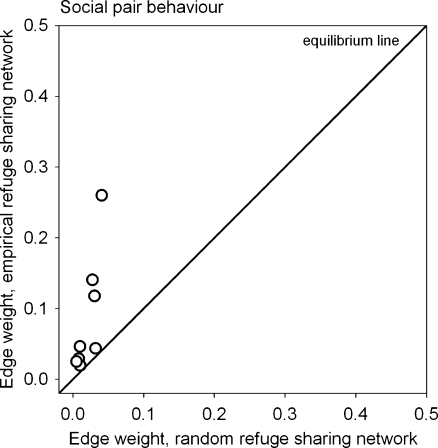
For social pairs, we compared their edge weights in the empirical with the hypothetical refuge sharing network. We found refuge sharing among social pair partners occurred significantly more frequently than if refuges were used at random (Table 4). In Fig. 3, all data points were above the equilibrium line implying that pairs actively sought to share refuges with pair partners. However, pair partners did not share refuges every night, and edge weights were all below 1.
Table 4.
Pair social behaviour
| Edge weight comparison | Observed t 7 | Mean random t 7 (95%CI) | P values |
|---|---|---|---|
| Empirical vs. random refuge sharing | 6.71 | 3.12 (2.23 5.37) | 0.008 |
| Empirical refuge sharing vs. active social association | −6.76 | −2.18 (−3.58−1.61) | <0.001 |
Randomisation paired t test analysis based on ln-transformed edge weight values. All randomisation values are based on 1,000 iterations
CI confidence interval
Fig. 3.
Edge weight values between nodes representing social pairs in the random versus the empirical refuge sharing network. The equilibrium line separates above and below random refuge sharing frequencies (measured as simple ratio association index) among social pair partners
Social association while active
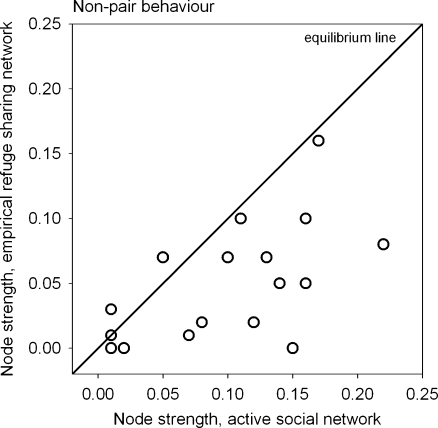
For non-pair lizards, node strength values in the active social network and the empirical refuge sharing network were significantly correlated (Pearson, observed r = 0.56; mean random r = 0.00; Monte Carlo P = 0.005, N = 18). Also, the mean node strength value in the active social network (Fig. 1b) was significantly higher than in the refuge sharing network (Fig. 1a; Table 2). Figure 4 shows that most lizards associated more frequently with non-pair individuals when active than when inactive in refuges.
Fig. 4.
Active social network versus empirical refuge sharing network. Data points below the equilibrium line represent individuals with total association frequencies with non-pair lizards (measured as node strength) that were lower while inactive and in refuges than while active. Edges between social pairs had been removed from networks before calculating node strengths. N = 18, one data point depicts two identical node strength values
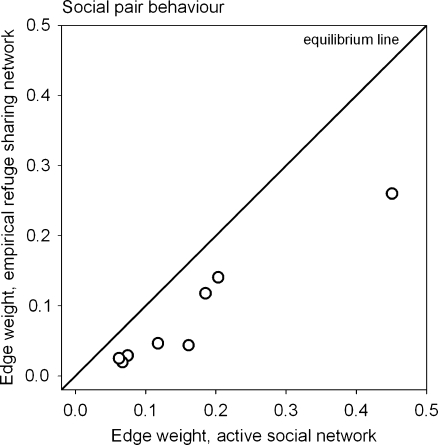
Similarly, we found significant positive correlations for edge weight values between social pair partners while lizards were active and while they were inactive in refuges (Pearson, observed r = 0.95; mean random r = 0.00; Monte Carlo P < 0.001, N = 8). Edge weights between pair partners were significantly higher during active than inactive periods (Table 4; Fig. 5). Lizards associated more frequently with their social pair partners while active than while inactive and in refuges. Thus, pair partners that might have associated during the day often sheltered in different refuges during the night.
Fig. 5.
Edge weight values between nodes representing social pairs in the active social network versus the empirical refuge sharing network. Data points below the equilibrium line represent lower association frequencies (measured as simple ratio association index) among social pair partners while inactive and in refuges than while active
Discussion
Refuge sharing is an integral part of social behaviour across many animal taxa (Hamilton 1982; Warburg 2000; Schradin and Pillay 2005; Visagie et al. 2005; Martin and Martin 2007) and may be an initial phase in the evolution of more complex sociality (Warburg 2000; Rasoloharijaona et al. 2003; Shah et al. 2003; Lancaster et al. 2006). We used a network approach to test whether refuge sharing was related to social associations during active periods in a pair-forming lizard that lives in a stable social neighbourhood. During our study, sleepy lizards shared overnight refuges while inactive and associated while active, both with their social pair partners and with non-pair individuals from the social neighbourhood. Although the relationship between sex and refuge sharing could have a profound effect on sociality, we did not detect any differential refuge sharing behaviour between sexes, at least among non-pair lizards.
Refuge sharing while inactive overnight
Non-pair lizards shared refuges as frequently as expected by chance. Refuge sharing frequencies were low (mean = 3.9% of observed nights per lizard, N = 18), and there was no evidence for an underlying refuge use structure. Thus, sleepy lizards did not exploit the potential physiological or predation-avoiding benefits of refuge sharing with non-pair lizards. However, social pairs shared refuges more often than expected, indicating that any benefits derived from sharing were exploited predominantly among pair partners. Sharing refuges with social pair partners has the same potential benefits as with non-pair lizards, plus access to the reproductive partner, either for male mate guarding (Murray and Bull 2004) or for female mate coercion (Bull and Pamula 1998). If reproductive success is related to association strength with the social partner, the behavioural strategy of sharing refuges would increase individual fitness. These additional benefits may have favoured refuge sharing with pair partners more than with non-pair lizards. However, the frequency of refuge sharing of social pairs was still low (mean = 8.5% of observation nights per pair, N = 8), and pair partners did not shelter together every night. This suggests that any benefits from sharing overnight refuges in the sleepy lizard are relatively low on most nights, for both non-pairs and social pairs.
Social association while active
Empirically derived association strengths of active and of inactive sheltering lizards were correlated both for non-pairs and for social pairs. Lizards that more frequently shared refuges with either non-pair lizards or with their social pair partner also associated with those lizards more often when they were active. These relationships did not simply result from differential home range overlap because association strengths between active and inactive lizards were not equivalent, even though correlated. If association frequencies resulted just from home range overlap, we expected association strength while active and while inactive to be similarly affected by changes in home range overlap.
Social association strength of active lizards was higher than association strength of inactive lizards in overnight refuges both for non-pairs and for social pairs. This result is contrary to the prediction that associations while active result from sharing refuges while inactive. There was no evidence from our study that tolerance developed while sharing refuges led directly to greater association while active. Even highly bonded social pairs were more likely to associate while active than when in overnight refuges.
Why was association strength higher while lizards were active? One explanation for non-social pairs is the typically patchy distribution of food plants in semi-arid environments such as our study site (Webster and Maestre 2004). This may have resulted in direct contacts at feeding sites. If feeding sites are scarcer than refuge sites, then active associations would be more common. For social pairs, the male follows closely behind the female during some of the daily period of lizard activity (Bull 1988). This behaviour has been suggested to enhance mating success of the pair (Bull 2000), although these pair associations are not continuously maintained and partners often separate temporarily (Bull et al. 1998). Temporary separations may reduce the effectiveness of mate guarding, for example. But, separations that occur towards the end of the daily activity period and lead to pair partners sheltering in different refuges may be less costly in terms of lost matings than separating at other times of the day, in particular, if the end of daily activity varies among neighbouring lizards or differs between the sexes. The data suggest that refuge sharing among social partners may be the consequence of a strategy to maintain access to the reproductive partner. This indicates that sexual selection and not natural selection may have ultimately selected for sleepy lizard social organisation.
Refuge sharing and the evolution of sociality
The sleepy lizard belongs to the Egernia group of scincid lizards, which includes many highly social species that form stable family groups (Gardner et al. 2008). In some species, these groups consist of an adult pair and sometimes multiple cohorts of their offspring (Duffield and Bull 2002; Chapple 2003). Many of the studied Egernia species are saxicolous and live in saturated habitats with limited availability of rock crevices used as refuges. Limited refuges coupled with benefits for delayed juvenile dispersal have been suggested to result in refuge sharing behaviour, increased tolerance of conspecifics and the development of social associations in these species (Duffield and Bull 2002; O’Connor and Shine 2003; Stow and Sunnucks 2004; Chapple and Keogh 2006; While et al. 2009b). However, these studies have focused on data from inactive lizards while in refuges, and there are few data on association patterns outside of refuges and while lizards are active.
When we compared social associations between active and sheltering individuals of sleepy lizards, we did not find any evidence that refuge sharing was predictive of social behaviour. There was no evidence that refuge sharing facilitated social association while lizards in our population were active. Instead, we found patterns that were opposite to our predictions. Refuge sharing frequencies were not different from random (for non-pair lizard dyads), and association strength was higher for active individuals than for inactive individuals in refuges, although both strength values were correlated.
The sleepy lizard has a monogamous mating system (Bull et al. 1998; Bull 2000), a social characteristic that it shares with many Egernia species (Gardner et al. 2002; Chapple 2003; While et al. 2009a). This indicates similar selection pressures on reproductive strategies among species in this taxonomic group. Moreover, although their social organisations differ (pair-living in sleepy lizards versus family groups in many Egernia), this seems only due to whether juveniles delay dispersal (in Egernia species) or not (in the sleepy lizard). The phylogeny of the Egernia group suggests that the Tiliqua genus is nested within the Egernia group (Gardner et al. 2008). Hence, it is a more parsimonious explanation that living in stable family groups is the ancestral state and delayed juvenile dispersal and refuge sharing has been secondarily lost during the evolution of sleepy lizard social behaviour. The alternative, with the sleepy lizard social system the ancestral state, would require that the Egernia family structure evolved several times.
In contrast to many Egernia species that experience limited refuge availability, the sleepy lizard occupies habitats that include many potential refuges (Kerr et al. 2003). Refuge sharing may have become non-adaptive because the cost of locating a conspecific in one of many possible refuges may outweigh the benefit arising from sharing the refuge. Furthermore, sharing refuges with many other individuals at the same time allows transmission of some pathogens and parasites (Corner et al. 2003). For example, in sleepy lizards, infestation levels of some parasites are higher among lizards that use other lizard's refuges (Leu et al. 2010b). Similarly, the abundance of directly transmitted parasites depends on direct contact frequencies among host individuals (Altizer et al. 2003). High parasite infestation levels are costly for host individuals (Møller 1993; Main and Bull 2000), which among other factors may have selected for low refuge sharing frequencies and early juvenile dispersal. Accordingly, sleepy lizard parents and offspring separate soon after birth and are not in direct contact, although their home ranges overlap in the first spring (Bull and Baghurst 1998). Hence, while retaining the monogamous mating system, the sleepy lizard evolved into a pair-living species that associates more frequently when active than when sharing refuges.
Comparing social association patterns of active and inactive individuals allowed us to empirically test predictions directly derived from the hypothesis that refuge sharing has played an important role during the evolution of lizard sociality. Our results, based on the current population structure, provide evidence that refuge sharing has not been the precursor to sleepy lizard social behaviour and provide a deeper insight into the evolution of sociality in the highly social Egernia lizard clade. More broadly, the study suggests the utility of applying social network theory to testing hypotheses concerning the evolution of sociality.
Acknowledgements
The study was approved by the Flinders University Animal Welfare Committee (approval no. 478 E232) in compliance with the Australian Code of Practice for the use of animals for scientific purposes and conducted under the Department of Environment and Heritage Permit to Undertake Scientific Research (permit no. A23436 15). All procedures carried out in this study conformed to the current laws of Australia. This research was supported by funds from the Australian Research Council and the Holsworth Wildlife Research Endowment. S.T.L. was funded by the German Academic Exchange Service. We thank Anne Goldizen and two anonymous referees who provided valuable feedback on the manuscript. We thank Ron and Leona Clark for allowing access to their land, Geoff Cottrell for maintaining the data loggers, and Dale Burzacott for logistical support at the field site.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
References
- Altizer S, Nunn CL, Thrall PH, Gittleman JL, Antonovics J, Cunningham AA, Dobson AP, Ezenwa V, Jones KE, Pedersen AB, Poss M, Pulliam JRC. Social organization and parasite risk in mammals: Integrating theory and empirical studies. Annu Rev Ecol Evol Syst. 2003;34:517–547. doi: 10.1146/annurev.ecolsys.34.030102.151725. [DOI] [Google Scholar]
- Anderson JR. Sleep, sleeping sites, and sleep-related activities: awakening to their significance. Am J Primatol. 1998;46:63–75. doi: 10.1002/(SICI)1098-2345(1998)46:1<63::AID-AJP5>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- Aubret F, Shine R. Causes and consequences of aggregation by neonatal tiger snakes (Notechis scutatus, Elapidae) Austral Ecol. 2009;34:210–217. doi: 10.1111/j.1442-9993.2008.01923.x. [DOI] [Google Scholar]
- Auburn ZM, Bull CM, Kerr GD. The visual perceptual range of a lizard, Tiliqua rugosa. J Ethol. 2009;27:75–81. doi: 10.1007/s10164-008-0086-z. [DOI] [Google Scholar]
- Beauchamp G. The evolution of communal roosting in birds: origin and secondary losses. Behav Ecol. 1999;10:675–687. doi: 10.1093/beheco/10.6.675. [DOI] [Google Scholar]
- Bejder L, Fletcher D, Bräger S. A method for testing association patterns of social animals. Anim Behav. 1998;56:719–725. doi: 10.1006/anbe.1998.0802. [DOI] [PubMed] [Google Scholar]
- Borgatti SP (2002) Netdraw network visualization. Analytic Technologies, Harvard, MA
- Brattstrom BH. The evolution of reptilian social behavior. Am Zool. 1974;14:35–49. [Google Scholar]
- Bull CM. A population study of the viviparous Australian lizard, Trachydosaurus rugosus (Scincidae) Copeia. 1987;3:749–757. doi: 10.2307/1445669. [DOI] [Google Scholar]
- Bull CM. Mate fidelity in an Australian lizard Trachydosaurus rugosus. Behav Ecol Sociobiol. 1988;23:45–49. doi: 10.1007/BF00303057. [DOI] [Google Scholar]
- Bull CM. Population ecology of the sleepy lizard, Tiliqua rugosa, at Mt Mary, South Australia. Aust J Ecol. 1995;20:393–402. doi: 10.1111/j.1442-9993.1995.tb00555.x. [DOI] [Google Scholar]
- Bull CM. Monogamy in lizards. Behav Processes. 2000;51:7–20. doi: 10.1016/S0376-6357(00)00115-7. [DOI] [PubMed] [Google Scholar]
- Bull CM, Baghurst BC. Home range overlap of mothers and their offspring in the sleepy lizard, Tiliqua rugosa. Behav Ecol Sociobiol. 1998;42:357–362. doi: 10.1007/s002650050448. [DOI] [Google Scholar]
- Bull CM, Burzacott D. The influence of parasites on the retention of long-term partnerships in the Australian sleepy lizard, Tiliqua rugosa. Oecologia. 2006;146:675–680. doi: 10.1007/s00442-005-0224-z. [DOI] [PubMed] [Google Scholar]
- Bull CM, Freake MJ. Home-range fidelity in the Australian sleepy lizard, Tiliqua rugosa. Aust J Zool. 1999;47:125–132. doi: 10.1071/ZO99021. [DOI] [Google Scholar]
- Bull CM, Lindle C. Following trails of partners in the monogamous lizard, Tiliqua rugosa. Acta ethologica. 2002;5:25–28. doi: 10.1007/s10211-002-0063-4. [DOI] [Google Scholar]
- Bull CM, Pamula Y. Enhanced vigilance in monogamous pairs of the lizard, Tiliqua rugosa. Behav Ecol. 1998;9:452–455. doi: 10.1093/beheco/9.5.452. [DOI] [Google Scholar]
- Bull CM, Bedford GS, Schulz BA. How do sleepy lizards find each other? Herpetologica. 1993;49:294–300. [Google Scholar]
- Bull CM, Cooper SJB, Baghurst BC. Social monogamy and extra-pair fertilization in an Australian lizard, Tiliqua rugosa. Behav Ecol Sociobiol. 1998;44:63–72. doi: 10.1007/s002650050515. [DOI] [Google Scholar]
- Chapple DG. Ecology, life-history, and behavior in the Australian scincid genus Egernia, with comments on the evolution of complex sociality in lizards. Herpetol Monogr. 2003;17:145–180. doi: 10.1655/0733-1347(2003)017[0145:ELABIT]2.0.CO;2. [DOI] [Google Scholar]
- Chapple DG, Keogh JS. Group structure and stability in social aggregations of White's Skink, Egernia whitii. Ethology. 2006;112:247–257. doi: 10.1111/j.1439-0310.2006.01153.x. [DOI] [Google Scholar]
- Childress MJ, Herrnkind WF. Den sharing by juvenile Caribbean spiny lobsters (Panulirus argus) in nursery habitat: cooperation or coincidence? Mar Freshw Res. 1997;48:751–758. doi: 10.1071/MF97158. [DOI] [Google Scholar]
- Corner LAL, Pfeiffer DU, Morris RS. Social-network analysis of Mycobacterium bovis transmission among captive brushtail possums (Trichosurus vulpecula) Prev Vet Med. 2003;59:147–167. doi: 10.1016/S0167-5877(03)00075-8. [DOI] [PubMed] [Google Scholar]
- Croft DP, James R, Krause J. Exploring animal social networks. Princeton: Princeton University Press; 2008. [Google Scholar]
- du Plessis MA, Weathers WW, Koenig WD. Energetic benefits of communal roosting by acorn woodpeckers during the nonbreeding season. Condor. 1994;96:631–637. doi: 10.2307/1369466. [DOI] [Google Scholar]
- Duffield G, Bull CM. Stable social aggregations in an Australian lizard, Egernia stokesii. Naturwissenschaften. 2002;89:424–427. doi: 10.1007/s00114-002-0346-7. [DOI] [PubMed] [Google Scholar]
- Firth BT, Belan I. Daily and seasonal rhythms in selected body temperatures in the Australian lizard Tiliqua rugosa (Scincidae): field and laboratory observations. Physiol Zool. 1998;71:303–311. doi: 10.1086/515919. [DOI] [PubMed] [Google Scholar]
- Fox SF, McCoy JK, Baird TA, eds. (2003) Lizard social behavior. The John Hopkins University Press, Baltimore
- Gardner MG, Bull CM, Cooper SJB, Duffield GA. Genetic evidence for a family structure in stable social aggregations of the Australian lizard Egernia stokesii. Mol Ecol. 2001;10:175–183. doi: 10.1046/j.1365-294X.2001.01171.x. [DOI] [PubMed] [Google Scholar]
- Gardner MG, Bull CM, Cooper SJB. High levels of genetic monogamy in the group-living Australian lizard Egernia stokesii. Mol Ecol. 2002;11:1787–1794. doi: 10.1046/j.1365-294X.2002.01552.x. [DOI] [PubMed] [Google Scholar]
- Gardner M, Bull CM, Fenner A, Murray K, Donnellan SC. Consistent social structure within aggregations of the Australian lizard, Egernia stokesii across seven disconnected rocky outcrops. J Ethol. 2007;25:263–270. doi: 10.1007/s10164-006-0022-z. [DOI] [Google Scholar]
- Gardner MG, Hugall AF, Donnellan SC, Hutchinson MN, Foster R. Molecular systematics of social skinks: phylogeny and taxonomy of the Egernia group (Reptilia: Scincidae) Zool J Linn Soc. 2008;154:781–794. doi: 10.1111/j.1096-3642.2008.00422.x. [DOI] [Google Scholar]
- Hamilton WD. Geometry for the selfish herd. J Theor Biol. 1971;31:295–311. doi: 10.1016/0022-5193(71)90189-5. [DOI] [PubMed] [Google Scholar]
- Hamilton WJ., III Baboon sleeping site preferences and relationships to primate grouping patterns. Am J Primatol. 1982;3:41–53. doi: 10.1002/ajp.1350030104. [DOI] [PubMed] [Google Scholar]
- Hood GM (2008) PopTools version 3.0.6. URL http://www.cse.csiro.au/poptools
- Hwang YT, Larivière S, Messier F. Energetic consequences and ecological significance of heterothermy and social thermoregulation in striped skunks (Mephitis mephitis) Physiol Biochem Zool. 2007;80:138–145. doi: 10.1086/509211. [DOI] [PubMed] [Google Scholar]
- James R, Croft DP, Krause J. Potential banana skins in animal social network analysis. Behav Ecol Sociobiol. 2009;63:989–997. doi: 10.1007/s00265-009-0742-5. [DOI] [Google Scholar]
- Kappeler PM, van Schaik CP. Evolution of primate social systems. Int J Primatol. 2002;23:707–740. doi: 10.1023/A:1015520830318. [DOI] [Google Scholar]
- Kerr GD, Bull CM. Field observations of extended locomotor activity at sub-optimal body temperatures in a diurnal heliothermic lizard (Tiliqua rugosa) J Zool. 2004;264:179–188. doi: 10.1017/S0952836904005734. [DOI] [Google Scholar]
- Kerr GD, Bull CM. Exclusive core areas in overlapping ranges of the sleepy lizard, Tiliqua rugosa. Behav Ecol. 2006;17:380–391. doi: 10.1093/beheco/arj041. [DOI] [Google Scholar]
- Kerr GD, Bull CM. Movement patterns in the monogamous sleepy lizard (Tiliqua rugosa): effects of gender, drought, time of year and time of day. J Zool. 2006;269:137–147. doi: 10.1111/j.1469-7998.2006.00091.x. [DOI] [Google Scholar]
- Kerr GD, Bull CM, Burzacott D. Refuge sites used by the scincid lizard Tiliqua rugosa. Austral Ecol. 2003;28:152–160. doi: 10.1046/j.1442-9993.2003.01268.x. [DOI] [Google Scholar]
- Kerth G, Wagner M, König B. Roosting together, foraging apart: information transfer about food is unlikely to explain sociality in female Bechstein's bats (Myotis bechsteinii) Behav Ecol Sociobiol. 2001;50:283–291. doi: 10.1007/s002650100352. [DOI] [Google Scholar]
- Kokko H. Cuckoldry and the stability of biparental care. Ecol Lett. 1999;2:247–255. doi: 10.1046/j.1461-0248.1999.00075.x. [DOI] [Google Scholar]
- Krause J, Lusseau D, James R. Animal social networks: an introduction. Behav Ecol Sociobiol. 2009;63:967–973. doi: 10.1007/s00265-009-0747-0. [DOI] [Google Scholar]
- Kummer H. From laboratory to desert and back: a social system of Hamadryas baboons. Anim Behav. 1984;32:965–971. doi: 10.1016/S0003-3472(84)80208-0. [DOI] [Google Scholar]
- Lancaster JR, Wilson P, Espinoza RE. Physiological benefits as precursors of sociality: why banded geckos band. Anim Behav. 2006;72:199–207. doi: 10.1016/j.anbehav.2006.01.010. [DOI] [Google Scholar]
- Lanham EJ, Bull CM. Maternal care and infanticide in the Australian skink, Egernia stokesii. Herpetol rev. 2000;31:151–152. [Google Scholar]
- Lanham EJ, Bull CM. Enhanced vigilance in groups in Egernia stokesii, a lizard with stable social aggregations. J Zool. 2004;263:95–99. doi: 10.1017/S0952836904004923. [DOI] [Google Scholar]
- Leu ST, Bashford J, Kappeler PM, Bull CM. Association networks reveal social organization in the sleepy lizard. Anim Behav. 2010;79:217–225. doi: 10.1016/j.anbehav.2009.11.002. [DOI] [Google Scholar]
- Leu ST, Kappeler PM, Bull CM. Refuge sharing network predicts ectoparasite load in a lizard. Behav Ecol Sociobiol. 2010;64:1495–1503. doi: 10.1007/s00265-010-0964-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Main AR, Bull CM. The impact of tick parasites on the behaviour of the lizard Tiliqua rugosa. Oecologia. 2000;122:574–581. doi: 10.1007/s004420050981. [DOI] [PubMed] [Google Scholar]
- Martin JK, Martin AA. Resource distribution influences mating system in the bobuck (Trichosurus cunninghami: Marsupialia) Oecologia. 2007;154:227–236. doi: 10.1007/s00442-007-0823-y. [DOI] [PubMed] [Google Scholar]
- Mock DW, Fujioka M. Monogamy and long-term pair bonding in vertebrates. Trends Ecol Evol. 1990;5:39–43. doi: 10.1016/0169-5347(90)90045-F. [DOI] [PubMed] [Google Scholar]
- Møller AP. Ectoparasites increase the cost of reproduction in their hosts. J Anim Ecol. 1993;62:309–322. doi: 10.2307/5362. [DOI] [Google Scholar]
- Murray K, Bull CM. Aggressiveness during monogamous pairing in the sleepy lizard, Tiliqua rugosa: a test of the mate guarding hypothesis. Acta ethologica. 2004;7:19–27. doi: 10.1007/s10211-004-0092-2. [DOI] [Google Scholar]
- Nieuwoudt CJ, Mouton PLFN, Flemming AF. Aggregation behaviour and movement patterns in the large-scaled girdled lizard, Cordylus macropholis. Amphib-Reptil. 2003;24:345–357. doi: 10.1163/156853803322440808. [DOI] [Google Scholar]
- O’Connor D, Shine R. Lizards in ‘nuclear families’: a novel reptilian social system in Egernia saxatilis (Scincidae) Mol Ecol. 2003;12:743–752. doi: 10.1046/j.1365-294X.2003.01777.x. [DOI] [PubMed] [Google Scholar]
- O’Connor DE, Shine R. Parental care protects against infanticide in the lizard Egernia saxatilis (Scincidae) Anim Behav. 2004;68:1361–1369. doi: 10.1016/j.anbehav.2004.02.014. [DOI] [Google Scholar]
- Perret M. Energetic advantage of nest-sharing in a solitary primate, the lesser mouse lemur (Microcebus murinus) J Mammal. 1998;79:1093–1102. doi: 10.2307/1383001. [DOI] [Google Scholar]
- Mouton PlFN, Flemming AF, Kanga EM. Grouping behaviour, tail-biting behaviour and sexual dimorphism in the armadillo lizard (Cordylus cataphractus) from South Africa. J Zool. 1999;249:1–10. doi: 10.1111/j.1469-7998.1999.tb01055.x. [DOI] [Google Scholar]
- Rasoloharijaona S, Rakotosamimanana B, Randrianambinina B, Zimmermann E. Pair-specific usage of sleeping sites and their implications for social organization in a nocturnal Malagasy primate, the Milne Edwards’ sportive lemur (Lepilemur edwardsi) Am J Phys Anthropol. 2003;122:251–258. doi: 10.1002/ajpa.10281. [DOI] [PubMed] [Google Scholar]
- Schradin C, Pillay N. Intraspecific variation in the spatial and social organization of the African striped mouse. J Mammal. 2005;86:99–107. doi: 10.1644/1545-1542(2005)086<0099:IVITSA>2.0.CO;2. [DOI] [Google Scholar]
- Shah B, Shine R, Hudson S, Kearney M. Sociality in lizards: why do thick-tailed geckos (Nephrurus milii) aggregate? Behaviour. 2003;140:1039–1052. doi: 10.1163/156853903322589632. [DOI] [Google Scholar]
- Stow AJ, Sunnucks P. High mate and site fidelity in Cunningham’s skinks (Egernia cunninghami) in natural and fragmented habitat. Mol Ecol. 2004;13:419–430. doi: 10.1046/j.1365-294X.2003.02061.x. [DOI] [PubMed] [Google Scholar]
- Struhsaker TT. Correlates of ecology and social organization among African cercopithecines. Folia Primatol. 1969;11:80–118. doi: 10.1159/000155259. [DOI] [PubMed] [Google Scholar]
- van Schaik CP. Vulnerability to infanticide by males: patterns among mammals. In: van Schaik CP, Janson CH, editors. Infanticide by males and its implications. Cambridge: Cambridge University Press; 2000. pp. 61–71. [Google Scholar]
- van Schaik CP, Kappeler PM. Infanticide risk and the evolution of male–female association in primates. Proc R Soc Lond B Biol Sci. 1997;264:1687–1694. doi: 10.1098/rspb.1997.0234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visagie L, PlFN M, Bauwens D. Experimental analysis of grouping behaviour in cordylid lizards. Herpetol J. 2005;15:91–96. [Google Scholar]
- Warburg MR. Intra- and interspecific cohabitation of scorpions in the field and the effect of density, food, and shelter on their interactions. J Ethol. 2000;18:59–63. doi: 10.1007/s101640070026. [DOI] [Google Scholar]
- Webster R, Maestre FT. Spatial analysis of semi-arid patchy vegetation by the cumulative distribution of patch boundary spacings and transition probabilities. Environ Ecol Stat. 2004;11:257–281. doi: 10.1023/B:EEST.0000038015.83910.37. [DOI] [Google Scholar]
- While GM, Uller T, Wapstra E. Family conflict and the evolution of sociality in reptiles. Behav Ecol. 2009;20:245–250. doi: 10.1093/beheco/arp015. [DOI] [Google Scholar]
- While GM, Uller T, Wapstra E. Within-population variation in social strategies characterize the social and mating system of an Australian lizard, Egernia whitii. Austral Ecol. 2009;34:938–949. doi: 10.1111/j.1442-9993.2009.02002.x. [DOI] [Google Scholar]
- Willis CKR, Brigham RM. Roost switching, roost sharing and social cohesion: forest-dwelling big brown bats, Eptesicus fuscus, conform to the fission-fusion model. Anim Behav. 2004;68:495–505. doi: 10.1016/j.anbehav.2003.08.028. [DOI] [Google Scholar]