Abstract
A prospective, open label, 3 month study was conducted to evaluate the feasibility and short term clinical effect of subcutaneous erythropoietin injections in patients with anemia and heart failure preserved ejection fraction (HFPEF; EF=55±2). Using a dose adjusted algorithm to affect a rate of rise in hemoglobin not to exceed 0.4 g/dL per week, hemoglobin (10.8±0.3 to 12.2±0.3 gm/dl) and red blood cell volume (1187±55 to 1333±38 ml) increased with an average weekly dose 3926 units. Functional measures increased from baseline [6 minute walk test (289±24 to 331±22 meters), exercise time (432±62 to 571±51 seconds) and peak VO2 (8.2±0.7 to 9.4±0.9 ml/kg/min)], all p<0.05. EDV declined significantly (8% volumetric decrease, 108±3 to 100±3 ml, p = 0.03) but there were no significant changes in LV mass or estimated LVEDP. Pressure volume analysis demonstrated a reduction in V30 (e.g. ventricular capacitance) without significant changes in contractile state.
Keywords: Heart Failure, Anemia, Elderly, Ejection Fraction
Introduction
Anemia is a significant co-morbidity among patients with heart failure both in the setting of a reduced and a preserved ejection fraction (HFPEF)(1–3). In subjects with heart failure there is a relationship between anemia, clinical symptoms, left ventricular (LV) structure, hemodynamics, morbidity, and renal function (4–12). Previous studies in patients with systolic heart failure have demonstrated a significant effect of increasing hemoglobin with either erythropoietin (13–18) or iron (17;19;20) on functional capacity, ventricular structure and quality of life, though a recent randomized trial failed to show clinical benefit in such patients(21). Such studies have not specifically focused on the population with HFPEF who account for more than half of the subjects with chronic heart failure.
Chronic anemia is known to result in compensatory left ventricular hypertrophy, higher myocardial chamber volumes, and a high cardiac output state. These structural and hemodynamic changes could be detrimental in the population with HFPEF, as the clinical syndrome of heart failure may be exacerbated. Thus, correction of anemia may provide significant benefits in terms of preload reduction, less effort intolerance, less dyspnea, leading to improved quality of life. Accordingly, the primary hypothesis of this prospective open label study is that the administration of subcutaneous erythropoietin to a cohort of anemic subjects with HFPEF would be associated with significant changes in left ventricular structure (reduced end diastolic volume, regressed left ventricular mass) and function (LV chamber contractility, ventricular-vascular coupling, stroke volume and cardiac output) as well as improvements in exercise capacity and quality of life.
Methods
Study design and subjects
This was a prospective, open label, twelve week cohort study among community dwelling, independently living, older adult patients with anemia and heart failure with a preserved ejection fraction. Subjects were recruited from internal medicine clinics, as well as specialty cardiology and renal clinics at an urban medical center setting (New York Presbyterian Hospital, New York City.) The diagnosis of heart failure was based on the NHANES CHF Criteria with a score ≥ 3 (22) and were considered to have a preserved ejection fraction if three-dimensional echocardiographically determined ejection fraction was >45%. Anemia was defined as hemoglobin < 12 g/dL.(23) Informed consent was obtained in all subjects. Columbia University Medical Center IRB approved the study.
Patients were excluded from the study if they had uncontrolled hypertension (SBP >160 mmHg and/or DBP > 90 mmHg, resting heart rate >120 bpm, baseline 6 minute walk > 450 meters, valvular heart disease greater than mild by transthoracic echocardiography, infiltrative cardiac disease such as hemochromatosis and amyloidosis, hypertrophic cardiomyopathy, chronic pulmonary disease (FEV1 < 60% predicted), renal failure (GFR < 15 mL/min), hemoglobin < 9 g/dL, exercise limited by angina, claudication or neurological diseases, severe liver dysfunction, cardiac surgery less than 3 months prior, known iron deficiency anemia from chronic GI blood loss, uterine bleeding, or other chronic bleeding, significant alcohol or illicit drug use, known hypercoagulable state or an active hematologic disease. Patients were also excluded if they had a history of deep venous thrombosis or pulmonary embolus within 12 months before study entry, had a history of CVA or TIA within 6 months, or an acute coronary syndrome within 6 months of study entry, had an allergy or sensitivity to human serum albumin, or had a known hypersensitivity to mammalian cell-derived products.
Study drug administration and dosing
Epogen (Epoietin alpha), (Ortho Biotec, Inc) was administered weekly by subcutaneous injection using a pre-specified dosing algorithm (See Supplementary Appendix). The dosing algorithm was designed to make adjustments based on the rate of rise (ROR) of the hemoglobin over a one week period, as well as the absolute hemoglobin value. Subjects initially received active treatment with 10,000 units of erythropoietin given weekly by subcutaneous injection. Subjects were carefully monitored (e.g. every week) when beginning therapy to avoid rapid increases in hemoglobin/hematocrit and/or increasing blood pressure control. No dose adjustments were made for the first three doses of erythropoietin (10,000 units/week) unless the hemoglobin rose too rapidly (greater than 0.3 g/dL) in any given weekly interval.
Blood volume analysis
Blood volume was determined after intravenous administration of iodine131 –labeled albumin (Volumex, Daxor Corp., New York City, New York) as previously described(24;25). Plasma volume was determined as the zero-time volume of distribution of the radiolabeled albumin obtained by semilogarithmic extrapolation of values measured from at least 3 samples drawn twelve minutes after injection at 6-minute intervals. Spun hematocrit was determined from each sample and plasma radioactivity of each sample was measured in a semiautomated counter (BVA-100 Blood Volume Analyzer, Daxor Corp). Blood volume and red blood cell volumes were calculated from the plasma volume measurement and then compared with normal values for age, gender, height, and weight based on the subject’s ideal weight(26).
Two and Three Dimensional Echocardiography
Standard two-dimensional transthoracic echocardiography (2-DE) was performed on each subject. End-diastolic measurements of left ventricular internal dimension (LVID), septal thickness (IVS), and posterior wall thickness (LVPWT) were acquired according to the standards of the American Society of Echocardiography(27). Doppler indices of the mitral inflow pattern including peak E wave velocity, peak A wave velocity and isovolumetric relaxation time (IVRT) as well as lateral mitral annual velocities (e′) were recorded for three beats and averaged. Left ventricular filling pressures were estimated by the formula EDP=11.96 +0.596 · E/e′ (28).
The equipment and procedures of freehand three-dimensional transthoracic echocardiography (3-DE) have been previously described in detail (29;30). 3-DE was performed using a conventional real-time echocardiograph, three-dimensional acoustic spatial locater, personal computer, and custom software. The data derived includes left ventricular chamber end-diastolic volume (EDV), myocardial volume (MV), stroke volume (SV), and the ejection fraction (EF = SV/EDV). Myocardial volume was multiplied by 1.05 gm/dl to determine ventricular mass. Echocardiograms were performed by study personnel blinded to clinical information.
Functional parameters
Both a six minute walk test (31;32) and cardiopulmonary exercise test were performed at baseline and after 3 months of study drug treatment by study personnel blinded to clinical information. The total distance walked after six minutes to the nearest meter was recorded. Patients performed an upright bicycle exercise test where the workload was increased every three minutes by 25 Watts according to a standard protocol. After 3 minutes of data at rest, exercise began at a workload of 0 W and increased every 3 minutes by 25 W until symptom-limited peak exercise was reached. Expired gas analysis was performed continuously thoughout the test with the Innocor system (Innovision A/S, Odense, Denmark)(33). Expired gas analysis was performed continuously throughout the test with the Innocor system. Peak VO2 was defined as the highest value of VO2 achieved in the final 30 seconds of exercise.
Quality of life
The Kansas City Cardiomyopathy Questionnaire(34) is a valid, reliable, self-administered, 23-item questionnaire that quantifies physical limitations, symptoms, self-efficacy, social interference and quality of life was employed. KCCQ summary score and sub-scores were calculated (range, 0 to 100; higher scores indicate better health status) at baseline at after 3 months of follow-up.
Echocardiographic Estimates of Ventricular Chamber Properties
The ESPVR and EDPVR were estimated using validated single beat techniques. The slope, defining end-systolic elastance (Ees), and a volume axis intercept (Vo) of the end-systolic pressure-volume relationship were estimated non-invasively by the single-beat method (Ees(sb)) described by Chen et al (35). To account for covariancein Ees and Vo both of which determine the position of the ESPVR, the values of these parameters derived from each subject were used to predict the V120 (the volume to achieve an ESP of 120 mmHg) which is calculated from the Ees and Vo of each patient: V120 = Vo + 120/Ees. Effective arterial elastance (Ea), a lumped index of vascular hemodynamic load primarily related to total peripheral resistance and heart rate, was estimated by Ea ≈SV/Pes,(36) where Pes is the LV end-systolic pressure estimated by 0.9 × SBP(37).
To characterize the end-diastolic pressure-volume relationship (EDPVR, where EDP= α EDVβ; α is a scaling constant and β is a diastolic stiffness constant), a recently developed and validated single-beat approach was used(38). This approach relies on the empiric observation that volume-normalize dEDPVRs share a common shape, thereby allowing estimation of and s to define the entire EDPVR from a single measured pressure–volume point. Measured EDP and EDV were used to derive α and β in each subject. To account for covariance in α and β(39), both of which impact on the shape and position of the EDPVR, the values of these parameters derived from each subject were used to predict the EDV at a common EDP of 30 mmHg to yield a pressure-independent index of heart size or ventricular capacitance (EDV30).
The area between the EDPVR and the ESPVR measured as a function of EDP was used to index overall pump function(40;41). This specific area is called the isovolumic pressure-volume area (PVAiso), is independent of after load and can be calculated analytically as a function of LV following curve fitting of the EDPVR and the ESPVR: PVAiso(V)=∫[Pes(V)−Ped(V)]dV = 0.5Ees(V−Vo)2 − Vm(β/α) eα*(V/V )m, where Pes(V) and Ped(V) are the end-systolic and end-diastolic pressures, respectively, as a function of volume.
Statistical analyses
Results are expressed as mean ± standard error. Changes in principle measures were compared from baseline to 3 month values by a student’s t-test for paired analyses. Associations between changes in hemoglobin and red cell volume measures and outcome variables (LV volumes, functional parameters and quality of life measures) were determined by use of Pearsons’ correlation coefficient. The primary endpoint of the study was changes in left ventricular end diastolic volume (LVEDV) after three months of study. Preliminary data indicates that the mean left ventricular end diastolic volume (LVEDV) in patients with HFPEF is 130+/34 ml as assessed by freehand three dimensional echocardiography. With a total of 10 subjects, we had a 90% power to detect a 13 mL (or 10%) difference after 3 months of therapy at an alpha of 0.05. SAS for Windows (Version 8.0, SAS Institute Inc., Cary, North Carolina) was used for all analyses.
Results
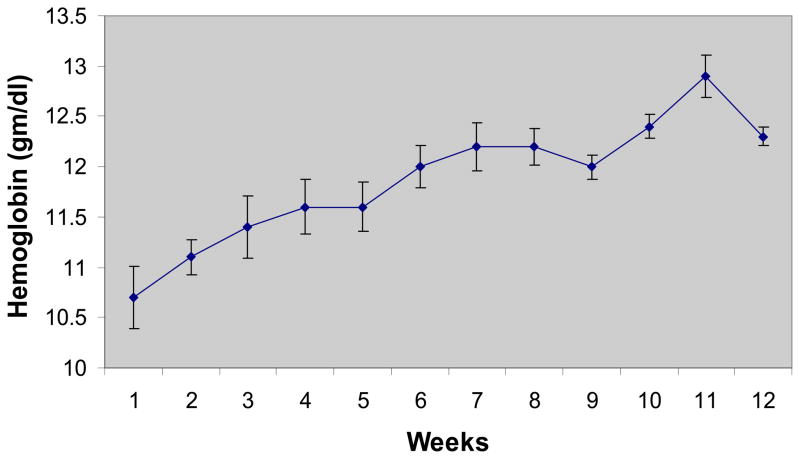
Patients enrolled this study were older adult (average age 68 ± 3 years) predominantly female (92%) subjects with several co-morbidities in addition to HFPEF and anemia (See table 1). All of the subjects had a true anemia (defined by the assay as red cell volume <95% predicted(42)) with an average red cell deficit of 503±57 ml (27±8% deficit). Therapy with erythropoietin was associated with an increase in hemoglobin from 10.8±0.3 to 12.2±0.3 g/dl over the course of the study. The rise in hemoglobin was slow and steady (Figure 1) concordant with the dosing algorithm goals and study target hemoglobin of 12.5 to 13.5 g/dL. The average weekly dose of EPO was 3926 units. Blood volume measurements performed by the I131 tagged albumin method confirmed the increase in red cell volume with erythropoietin treatment (from 1187±55 to 1333±48 mL). While the final hemoglobin was below the target hemoglobin range for the protocol of 12.5 to 13.5 g/dL, 6 of 11 individual patients (55%) reached target hemoglobin
Table 1.
Demographic and Clinical Characteristics
| Parameter | Result |
|---|---|
| Number of Subjects | 12 |
| Age (years) | 68 ± 3 (range 52–86) |
| Gender (% Female) | 92% |
| Race | 8% White |
| 33% Black (nonwhite) | |
| 58% Hispanic (nonwhite) | |
| Co-Morbid Conditions - n (%) | |
| Diabetic | 8 (75%) |
| Coronary Artery Disease | 8 (75%) |
| Obesity | 10(83%) |
| Chronic Renal Insufficiency | 9 (75%) |
| Lab Results | |
| BUN (mg/dl) | 35 ± 7 |
| Creatinine (mg/dl) | 1.7 ± 0.3 |
| eGFR (ml/min) | 55 ± 7 |
| Erythropoietin (mU/ml) | 23 ± 4 |
Figure 1.
Hemoglobin Values over Course of Study. Average weekly hemoglobin values are displayed. Values are mean±SE.
No significant changes were noted in systolic blood pressure and a significant decrease was noted in diastolic blood pressure (from 70 ± 3 mmHg to 64 ± 2 mmHg, p<0.05) during the study period. This was associated with a modest but not significant change in the number of subjects who were prescribed diuretics and calcium channel blockers but no change in the dose of ACE inhibitors and beta blockers. Plasma volume did not differ significantly from baseline to study termination but trended toward a decrease in volume (from 3066 ± 169 mL to 2942 ± 182, p=ns).
In this cohort of patients, LV end diastolic volume (as measured by 3D echocardiography) decreased with erythropoietin treatment, approximately 8 cc (8%) over the three-month period (p=0.03), while LV mass remains unchanged. Pressure Volume relationship of overall cohort pre and post erythropoietin treatment is shown in Figure 2 along with the PVA-iso to EDP relationship. There was a shift in the EDPVR toward smaller volumes, without a significant change in the ESPVR. Overall stroke work, stroke work per grams of ventricular mass and the PVA-Iso area trended toward lower values after 3 months of treatment with EPO.
Figure 2.
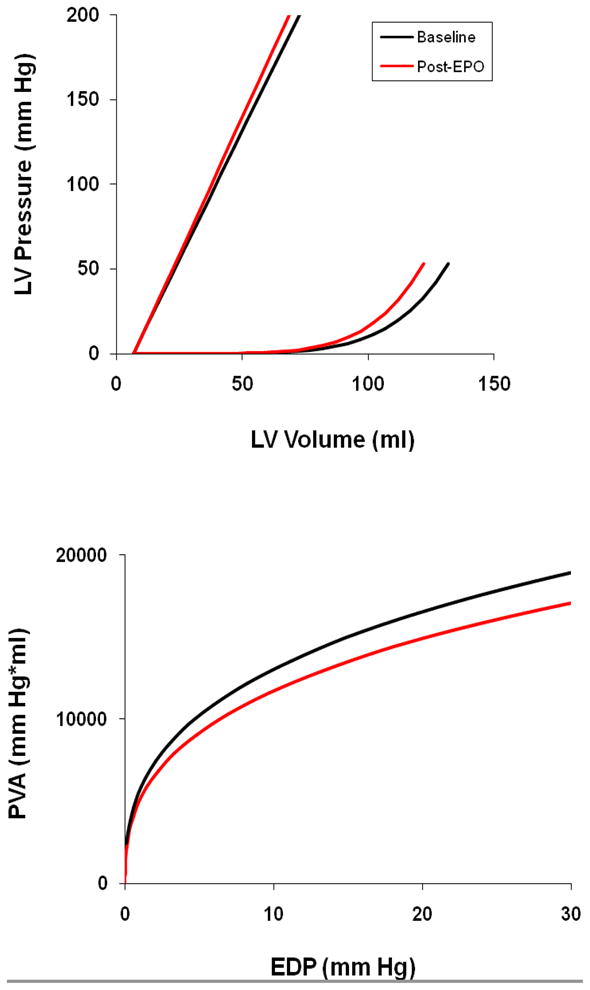
Group average end systolic and end diastolic pressure volume relations for subjects at baseline (black) and after 3 months of treatment with erythropoietin (red) [upper panel]. The isovolumetric pressure volume area versus estimated EDP at baseline (black) and after 3 months of treatment with erythropoietin (red) [lower panel].
All measures of functional capacity improved significantly (p<0.05) with erythropoietin treatment including peak VO2 (15%), exercise time (32%), and six minute walk duration (15%) (Table 2). Finally, eight of nine scales or subscales on the Kansas City Cardiomyopathy Questionnaire (Table 4) improved, with an average increase of 18 percentage points. However, there were no significant associations between changes in hemoglobin or red cell volume and any functional, structural or quality of life measures.
Table 2.
Preliminary Clinical Findings
| Baseline (n=11)† | Follow Up (n=11) | |
|---|---|---|
| Adverse Events | N/A | 0 |
| Hemoglobin (g/dL) | 10.8 ± 0.3 | 12.2 ± 0.3* |
| Blood Pressure Parameters (mmHg) | ||
| Systolic Blood Pressure | 140 ± 6 | 138 ± 6 |
| Diastolic Blood Pressure | 70 ± 3 | 64 ± 2* |
| Mean Arterial Pressure | 93 ± 3 | 89 ± 2 |
| Pulse Pressure | 71 ± 5 | 74 ± 7 |
| Medication Use (no. pts (%)) | ||
| Diuretic (any type) | 8 (73) | 10 (91) |
| Loop Diuretic | 5 (56) | 6 (67) |
| Thiazide | 3 (33) | 2 (22) |
| ACE Inhibitor | 7 (78) | 7 (78) |
| Beta Blocker | 6 (67) | 6 (67) |
| Calcium Channel Blocker | 6 (67) | 7 (78) |
| Blood Volume Parameters | ||
| Total Blood Volume (mL) | 4098 ± 214 | 4207 ± 223 |
| Plasma Volume (mL) | 3066 ± 169 | 2942 ± 182 |
| Red Cell Volume (mL) | 1187 ± 55 | 1333 ± 48* |
| Exercise Parameters | ||
| Six Minute Walk Test (m) | 289 ± 24 | 331 ± 22* |
| Bicycle Ergometer Exercise Time (s) | 432 ± 62 | 571 ± 51* |
| VO2 (mL/min) | 628 ± 51 | 773 ± 79* |
| VO2 (mL/kg/min) | 8.2 ± 0.7 | 9.4 ± 0.9* |
| RER | 0.9 ± 0.1 | 0.9 ± 0.1 |
| Peak Exercise HR (bpm) | 96 ± 6 | 107 ± 6 |
Original n=12, one patient discontinued for unrelated medical reasons
p<0.05, Values are Mean±SE
Table 4.
Quality of Life
| Baseline (n=11) | Follow Up (n=11) | |
|---|---|---|
| Clinical Summary | 44 ± 7 | 64 ± 4* |
| Quality of Life | 38 ± 5 | 62 ± 3* |
| Physical Limitations | 44 ± 7 | 65 ± 3* |
| Functional Status | 43 ± 7 | 63 ± 3* |
| Symptom | ||
| Frequency | 43 ± 8 | 60 ± 4* |
| Severity | 45 ± 7 | 61 ± 5* |
| Change | 46 ± 6 | 53 ± 6 |
| Self Efficacy | 46 ± 5 | 61 ± 4* |
| Social Interference | 52 ± 8 | 71 ± 5* |
p<0.01, Values are Mean ± SE
Discussion
This is the first study to describe the effect of EPO treatment in adult elderly patients with HFPEF and anemia. We explored many potential effects of short term therapy including ventricular remodeling, cardiac output, pressure-volume relationships, and ventricular-vascular coupling, as well as other clinical parameters of functional capacity and quality of life. These data demonstrate that correction of mild-moderate anemia with short term EPO is associated with improvements in functional parameters and reductions in left ventricular volumes and ventricular capacitance without significant effects on systolic properties.
Safety and Tolerability
Widespread enthusiasm for the benefits of erythropoietin therapy was dampened by several studies in patients with chronic kidney disease, trauma and cancer who experienced significant adverse effects from erythropoietin therapy including thrombotic events(43) and cardiovascular events in patients targeted to receive a higher hemoglobin(44;45). Meta-analysis has reaffirmed concerns about an increased risk for death and poor blood pressure control(46) in patients targeted for a higher hemoglobin resulting in the issuance of black box warning by the FDA(47). The FDA recommended careful monitoring of hemoglobin values after dose adjustments, careful monitoring and control of blood pressure along with limiting the rate of rise of hemoglobin to less than 1 g/dl in any 2-week period, all of which were taken into account in the design and execution of this pilot/feasibility trial. The dosing algorithm employed called for weekly monitoring of hemoglobin levels, with dose adjustments that were based on the current hemoglobin level as well as rate of rise in the preceding week. This algorithm affected a rise in hemoglobin that was slow and steady and did not result in any significant adverse events (e.g. thrombotic episodes or decompensated heart failure or related hospitalization) or any significant increase in blood pressure. Additionally, the dose of EPO employed was lower than anticipated and differed significantly from current treatment guidelines (48). However, such an approach was associated with significant subject burden and alterations in blood pressure medications in a large percentage of subjects (33%). Whether such an approach will be safe and executable in a larger number of subjects is unknown but is being studied currently(49).
Ventricular Structure and Function
The vast majority of patients with HFPEF have concomitant hypertension and a significant percentage have chronic renal dysfunction and left ventricular hypertrophy, all risk factors for the development of HFPEF in large population based studies(50). Anemia contributes to LVH, which is an important underlying substrate among patients with HFPEF. Accordingly, improving anemia in patients with both chronic renal failure and heart failure in meta-analysis has been shown to reduce left ventricular mass and reduce left ventricular volumes. In these studies, average baseline left ventricular mass was significantly higher (289 grams) than in the current cohort and left ventricular end diastolic volume was larger (148 ml). Meta-analysis demonstrated that EPO resulted in significant decline of 15% in LV mass index and 16% in end diastolic volume(51). The discrepancies between these results and ours could be attributable to several factors, including limited sample size of our study, less severe ventricular remodeling in our subjects, short duration (three months compared with greater than 6 months in most trials) of therapy and limited increase in hemoglobin achieved with therapy in the current study. The use of non-invasive pressure volume analysis, demonstrates despite the absence of changes in LV mass; however, there was a trend toward a reduction in ventricular capacitance concordant with ventricular remodeling.
Anemia results in reductions in systemic vascular resistance, increases in sympathetic nervous system activation and expansion of plasma volume which can augment preload volume, contributing to the effort intolerance and dyspnea experienced by subjects with HFPEF. Accordingly, correction of anemia would ameliorate these hemodynamic changes resulting in declines in EDV, stroke volume and cardiac output. Accordingly, treatment of anemia through an amelioration of altered loading conditions would be anticipated to reduce ventricular work, as shown in the reduction in the pressure volume area (Figure 1, bottom panel).
Functional Capacity and Quality of Life
Effects of EPO on quality of life have been limited predominantly to the cancer population, with a Cochrane Review suggesting that therapy may improve Quality of Life and functional class(52) in this population. As well in pre-dialysis patients and dialysis patients, EPO corrects anemia and also improves quality of life and sub-maximal exercise performance(53–55). However, for heart failure subjects limited data are available. Early un-blinded studies and phase II results using EPO in patients with systolic heart failure have found overall significant improvements in exercise capacity and quality of life(56;57). However, more recent randomized trials did not demonstrate a significant benefit on exercise duration, New York Heart Association class, or quality of life score compared with placebo(21). Since a majority of heart failure cases involve subjects with a preserved ejection fraction who have a similar decrement in quality of life as subjects with systolic heart failure(58), characterizing the role of EPO in this population is warranted. While the data from this open label trial are encouraging, with almost all scales and subscales on the KCCQ demonstrating statistically significant increases in scores (concordant with improved quality of life), compatible with a moderate to large clinical benefit (59), given the un-blinded and open label nature of this study, causality can not be concluded.
Study limitations
This study is limited by its open label design, its limited sample size, and the limited duration of treatment. While the sample size is limited, our study demonstrated the feasibility of performing clinical trials in a patient population previously unstudied and not typically included in clinical trials. Such preliminary data is difficult to obtain for reasons of patient accessibility to study facilities, study subject burden, and patient comorbidities. Our findings can be considered a prerequisite in order to determine relative safety and feasibility of the approach prior to the undertaking of larger randomized phase II-III clinical trials, which are currently underway(49). Notably, in order to safely raise hemoglobin with weekly injections, the study participants were at their target hemoglobin only at the very end of the study period (generally week 11 and 12), and were still anemic for the first two months of the study, which may have blunted the true effect of EPO (or raised hemoglobin) on ventricular remodeling. While we found that the increase in hemoglobin was accompanied by significant increases in functional capacity, improvements in quality of life and reductions in ventricular volumes, these associations cannot be directly attributed to the intervention given the open label study design and absence of statistical correlation and may have occurred by chance or are attributable to some other confounding factor (e.g. medication adjustment).
Conclusions
In a population previously unstudied (older adults with HFPEF and concomitant anemia), erythropoietin was well tolerated and effected a significant increase in hemoglobin and red cell volume without significant increases in blood pressure or other adverse effects during the three month study period. Additionally, there were improvements in exercise capacity, improvements in quality of life and reduced ventricular capacitance during the course of the trial. These data suggest that ongoing evaluation of erythropoietin therapy in subjects with HFPEF and anemia is warranted.
Supplementary Material
Table 3.
Echocardiographc and Physiologic Variables
| Baseline (n=11) | Follow Up (n=11) | |
|---|---|---|
| 2D Echocardiographic Parameters | ||
| Mitral Valve E Velocity | 98 ± 7 | 77 ± 6* |
| Mitral Valve A Velocity | 100 ± 9 | 93 ± 7 |
| E/A Ratio | 1.1 ± 0.1 | 0.8 ± 0.1* |
| E′ (Tissue Doppler) | 13 ± 0.8 | 11 ± 1 |
| 3D Echocardiographic Parameters | ||
| SV (mL) | 59 ± 2 | 54 ± 2* |
| EDV (mL) | 108 ± 3 | 100 ± 3* |
| Ejection Fraction (%) | 55 ± 2 | 54 ± 1 |
| LV Mass (g) | 167 ± 7 | 164 ± 6 |
| Pressure-Volume Parameters | ||
| Estimated LVEDP (mmHg) | 16 ± 0.4 | 16 ± 0.3 |
| Single Beat Ees (mm Hg/ml) | 3.0 ± 0.2 | 3.3 ± 0.2 |
| Single Beat Vo (mL) | 7 ± 3 | 7 ± 2 |
| Pes/ESV (mmHg/mL) | 2.6 ± 0.2 | 2.7 ± 0.1 |
| V120 (ml) | 47 ± 3 | 45 ± 3 |
| V30 (ml) | 119 ± 4 | 111 ± 4† |
| Stroke Work (mL mmHg) | 5441 ± 310 | 4898 ± 253 |
| Stroke Work/Mass Ratio (mL mmHg/g) | 33 ± 2 | 30 ± 1 |
| PVA-Iso-20 mm Hg (ml * mm Hg) | 16239 ± 1035 | 14876 ± 988 |
p<0.05
p = 0.054. Values are Mean±SE
Acknowledgments
This research was supported by the NIH/NIA (R01AG027518-01A1). Dr. Rose Cohen was supported by the ACCF/Merck Fellowship 2005-2006 and a grant from the New York Academy of Medicine Glorney Raisbeck Fellowship 2006.
Reference List
- 1.Ezekowitz JA. Anemia in patients with advanced heart failure. J Am Coll Cardiol. 2007 Jun 12;49(23):2301. doi: 10.1016/j.jacc.2007.04.004. [DOI] [PubMed] [Google Scholar]
- 2.Lang CC, Mancini DM. Non-cardiac comorbidities in chronic heart failure. Heart. 2007 Jun;93(6):665–671. doi: 10.1136/hrt.2005.068296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tang YD, Katz SD. Anemia in chronic heart failure: prevalence, etiology, clinical correlates, and treatment options. Circulation. 2006 May 23;113(20):2454–2461. doi: 10.1161/CIRCULATIONAHA.105.583666. [DOI] [PubMed] [Google Scholar]
- 4.Luthi JC, Flanders WD, Burnier M, Burnand B, McClellan WM. Anemia and chronic kidney disease are associated with poor outcomes in heart failure patients. BMC Nephrol. 2006;7:3. doi: 10.1186/1471-2369-7-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Grigorian SL, Varela RA, Garcia-Acuna JM, Mazon RP, Virgos LA, Gonzalez-Juanatey JR. Anaemia is associated with higher mortality among patients with heart failure with preserved systolic function. Heart. 2006 Jun;92(6):780–784. doi: 10.1136/hrt.2005.064394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.O’Meara E, Clayton T, McEntegart MB, et al. Clinical correlates and consequences of anemia in a broad spectrum of patients with heart failure: results of the Candesartan in Heart Failure: Assessment of Reduction in Mortality and Morbidity (CHARM) Program. Circulation. 2006 Feb 21;113(7):986–994. doi: 10.1161/CIRCULATIONAHA.105.582577. [DOI] [PubMed] [Google Scholar]
- 7.Gardner RS, McDonagh TA. The prognostic value of anemia, right-heart catheterization and neurohormones in chronic heart failure. Expert Rev Cardiovasc Ther. 2006 Jan;4(1):51–57. doi: 10.1586/14779072.4.1.51. [DOI] [PubMed] [Google Scholar]
- 8.Ralli S, Horwich TB, Fonarow GC. Relationship between anemia, cardiac troponin I, and B-type natriuretic peptide levels and mortality in patients with advanced heart failure. Am Heart J. 2005 Dec;150(6):1220–1227. doi: 10.1016/j.ahj.2005.01.049. [DOI] [PubMed] [Google Scholar]
- 9.Anand I, McMurray JJ, Whitmore J, et al. Anemia and its relationship to clinical outcome in heart failure. Circulation. 2004 Jul 13;110(2):149–154. doi: 10.1161/01.CIR.0000134279.79571.73. [DOI] [PubMed] [Google Scholar]
- 10.Brucks S, Little WC, Chao T, et al. Relation of anemia to diastolic heart failure and the effect on outcome. AJC. 2004 Apr 15;93(8):1055–1057. doi: 10.1016/j.amjcard.2003.12.062. [DOI] [PubMed] [Google Scholar]
- 11.Silverberg D, Wexler D, Blum M, Schwartz D, Iaina A. The association between congestive heart failure and chronic renal disease. Curr Opin Nephrol Hypertens. 2004 Mar;13(2):163–170. doi: 10.1097/00041552-200403000-00004. [DOI] [PubMed] [Google Scholar]
- 12.Ezekowitz JA, McAlister FA, Armstrong PW. Anemia is common in heart failure and is associated with poor outcomes: insights from a cohort of 12 065 patients with new-onset heart failure. Circulation. 2003 Jan 21;107(2):223–225. doi: 10.1161/01.cir.0000052622.51963.fc. [DOI] [PubMed] [Google Scholar]
- 13.Palazzuoli A, Silverberg DS, Iovine F, et al. Effects of beta-erythropoietin treatment on left ventricular remodeling, systolic function, and B-type natriuretic peptide levels in patients with the cardiorenal anemia syndrome. Am Heart J. 2007 Oct;154(4):645–15. doi: 10.1016/j.ahj.2007.07.022. [DOI] [PubMed] [Google Scholar]
- 14.Palazzuoli A, Silverberg D, Iovine F, et al. Erythropoietin improves anemia exercise tolerance and renal function and reduces B-type natriuretic peptide and hospitalization in patients with heart failure and anemia. Am Heart J. 2006 Dec;152(6):1096–15. doi: 10.1016/j.ahj.2006.08.005. [DOI] [PubMed] [Google Scholar]
- 15.Mancini DM, Katz SD, Lang CC, LaManca J, Hudaihed A, Androne AS. Effect of erythropoietin on exercise capacity in patients with moderate to severe chronic heart failure. Circulation. 2003 Jan 21;107(2):294–299. doi: 10.1161/01.cir.0000044914.42696.6a. [DOI] [PubMed] [Google Scholar]
- 16.Silverberg DS, Wexler D, Blum M, Iaina A. The cardio renal anemia syndrome: correcting anemia in patients with resistant congestive heart failure can improve both cardiac and renal function and reduce hospitalizations. Clin Nephrol. 2003 Jul;60(Suppl 1):S93–102. [PubMed] [Google Scholar]
- 17.Silverberg DS, Wexler D, Blum M, et al. Effect of correction of anemia with erythropoietin and intravenous iron in resistant heart failure in octogenarians. Isr Med Assoc J. 2003 May;5(5):337–339. [PubMed] [Google Scholar]
- 18.Silverberg DS, Wexler D, Sheps D, et al. The effect of correction of mild anemia in severe, resistant congestive heart failure using subcutaneous erythropoietin and intravenous iron: a randomized controlled study. J Am Coll Cardiol. 2001 Jun 1;37(7):1775–1780. doi: 10.1016/s0735-1097(01)01248-7. [DOI] [PubMed] [Google Scholar]
- 19.Toblli JE, Lombrana A, Duarte P, Di GF. Intravenous iron reduces NT-pro-brain natriuretic peptide in anemic patients with chronic heart failure and renal insufficiency. J Am Coll Cardiol. 2007 Oct 23;50(17):1657–1665. doi: 10.1016/j.jacc.2007.07.029. [DOI] [PubMed] [Google Scholar]
- 20.Bolger AP, Bartlett FR, Penston HS, et al. Intravenous iron alone for the treatment of anemia in patients with chronic heart failure. J Am Coll Cardiol. 2006 Sep 19;48(6):1225–1227. doi: 10.1016/j.jacc.2006.07.015. [DOI] [PubMed] [Google Scholar]
- 21.Ghali JK, Anand IS, Abraham WT, et al. Randomized Double-Blind Trial of Darbepoetin Alfa in Patients With Symptomatic Heart Failure and Anemia. Circulation. 2008 Jan 29;117(4):526–535. doi: 10.1161/CIRCULATIONAHA.107.698514. [DOI] [PubMed] [Google Scholar]
- 22.Schocken DD, Arrieta MI, Leaverton PE, Ross EA. Prevalence and mortality rate of congestive heart failure in the United States. J Am Coll Cardiol. 1992 Aug;20(2):301–306. doi: 10.1016/0735-1097(92)90094-4. [DOI] [PubMed] [Google Scholar]
- 23.World Health Organization. Nutritional Anaemias: Report of a WHO Scientific Group. Geneva, Switzerland: 1968. 2007. [PubMed] [Google Scholar]
- 24.Feldschuh J, Enson Y. Prediction of the normal blood volume. Relation of blood volume to body habitus. Circulation. 1977 Oct;56(4 Pt 1):605–612. doi: 10.1161/01.cir.56.4.605. [DOI] [PubMed] [Google Scholar]
- 25.Katz SD. Blood volume assessment in the diagnosis and treatment of chronic heart failure. Am J Med Sci. 2007 Jul;334(1):47–52. doi: 10.1097/MAJ.0b013e3180ca8c41. [DOI] [PubMed] [Google Scholar]
- 26.Feldschuh J, Katz S. The importance of correct norms in blood volume measurement. Am J Med Sci. 2007 Jul;334(1):41–46. doi: 10.1097/MAJ.0b013e318063c707. [DOI] [PubMed] [Google Scholar]
- 27.Sahn DJ, DeMaria A, Kisslo J, Weyman A. Recommendations regarding quantitation in M-mode echocardiography: results of a survey of echocardiographic measurements. Circulation. 1978 Dec;58(6):1072–1083. doi: 10.1161/01.cir.58.6.1072. [DOI] [PubMed] [Google Scholar]
- 28.Ommen SR, Nishimura RA, Appleton CP, et al. Clinical utility of Doppler echocardiography and tissue Doppler imaging in the estimation of left ventricular filling pressures: A comparative simultaneous Doppler-catheterization study. Circulation. 2000 Oct 10;102(15):1788–1794. doi: 10.1161/01.cir.102.15.1788. [DOI] [PubMed] [Google Scholar]
- 29.Gopal AS, King DL, Katz J, Boxt LM, King DL, Jr, Shao MY. Three-dimensional echocardiographic volume computation by polyhedral surface reconstruction: in vitro validation and comparison to magnetic resonance imaging. J Am Soc Echocardiogr. 1992 Mar;5(2):115–124. doi: 10.1016/s0894-7317(14)80541-5. [DOI] [PubMed] [Google Scholar]
- 30.King DL. Three-dimensional Ultrasonic Imaging of Animal Soft Tissue. 4,100,916. United States patent. 1978 Jul 18;
- 31.Guyatt GH, Pugsley SO, Sullivan MJ, et al. Effect of encouragement on walking test performance. Thorax. 1984 Nov;39(11):818–822. doi: 10.1136/thx.39.11.818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cahalin LP, Mathier MA, Semigran MJ, Dec GW, DiSalvo TG. The six-minute walk test predicts peak oxygen uptake and survival in patients with advanced heart failure. Chest. 1996 Aug;110(2):325–332. doi: 10.1378/chest.110.2.325. [DOI] [PubMed] [Google Scholar]
- 33.Lang CC, Karlin P, Haythe J, Tsao L, Mancini DM. Ease of noninvasive measurement of cardiac output coupled with peak VO2 determination at rest and during exercise in patients with heart failure. AJC. 2007 Feb 1;99(3):404–405. doi: 10.1016/j.amjcard.2006.08.047. [DOI] [PubMed] [Google Scholar]
- 34.Green CP, Porter CB, Bresnahan DR, Spertus JA. Development and evaluation of the Kansas City Cardiomyopathy Questionnaire: a new health status measure for heart failure. J Am Coll Cardiol. 2000 Apr;35(5):1245–1255. doi: 10.1016/s0735-1097(00)00531-3. [DOI] [PubMed] [Google Scholar]
- 35.Chen CH, Fetics B, Nevo E, et al. Noninvasive single-beat determination of left ventricular end-systolic elastance in humans. J Am Coll Cardiol. 2001 Dec;38(7):2028–2034. doi: 10.1016/s0735-1097(01)01651-5. [DOI] [PubMed] [Google Scholar]
- 36.Sunagawa K, Maughan WL, Burkhoff D, Sagawa K. Left ventricular interaction with arterial load studied in isolated canine ventricle. Am J Physiol. 1983 Nov;245(5 Pt 1):H773–H780. doi: 10.1152/ajpheart.1983.245.5.H773. [DOI] [PubMed] [Google Scholar]
- 37.Kelly RP, Ting CT, Yang TM, et al. Effective arterial elastance as index of arterial vascular load in humans. Circulation. 1992 Aug;86(2):513–521. doi: 10.1161/01.cir.86.2.513. [DOI] [PubMed] [Google Scholar]
- 38.Klotz S, Hay I, Dickstein ML, et al. Single-beat estimation of end-diastolic pressure-volume relationship: a novel method with potential for noninvasive application. Am J Physiol Heart Circ Physiol. 2006 Jul;291(1):H403–H412. doi: 10.1152/ajpheart.01240.2005. [DOI] [PubMed] [Google Scholar]
- 39.Burkhoff D, Mirsky I, Suga H. Assessment of systolic and diastolic ventricular properties via pressure-volume analysis: a guide for clinical, translational, and basic researchers. Am J Physiol Heart Circ Physiol. 2005 Aug;289(2):H501–H512. doi: 10.1152/ajpheart.00138.2005. [DOI] [PubMed] [Google Scholar]
- 40.Suga H, Goto Y, Futaki S, et al. Systolic pressure-volume area (PVA) as the energy of contraction in Starling’s law of the heart. Heart Vessels. 1991;6(2):65–70. doi: 10.1007/BF02058751. [DOI] [PubMed] [Google Scholar]
- 41.Todaka K, Wang J, Yi GH, et al. Impact of exercise training on ventricular properties in a canine model of congestive heart failure. Am J Physiol. 1997 Mar;272(3 Pt 2):H1382–H1390. doi: 10.1152/ajpheart.1997.272.3.H1382. [DOI] [PubMed] [Google Scholar]
- 42.Androne AS, Katz SD, Lund L, et al. Hemodilution is common in patients with advanced heart failure. Circulation. 2003 Jan 21;107(2):226–229. doi: 10.1161/01.cir.0000052623.16194.80. [DOI] [PubMed] [Google Scholar]
- 43.Corwin HL, Gettinger A, Fabian TC, et al. Efficacy and safety of epoetin alfa in critically ill patients. N Engl J Med. 2007 Sep 6;357(10):965–976. doi: 10.1056/NEJMoa071533. [DOI] [PubMed] [Google Scholar]
- 44.Singh AK, Szczech L, Tang KL, et al. Correction of anemia with epoetin alfa in chronic kidney disease. N Engl J Med. 2006 Nov 16;355(20):2085–2098. doi: 10.1056/NEJMoa065485. [DOI] [PubMed] [Google Scholar]
- 45.Drueke TB, Locatelli F, Clyne N, et al. Normalization of hemoglobin level in patients with chronic kidney disease and anemia. N Engl J Med. 2006 Nov 16;355(20):2071–2084. doi: 10.1056/NEJMoa062276. [DOI] [PubMed] [Google Scholar]
- 46.Phrommintikul A, Haas SJ, Elsik M, Krum H. Mortality and target haemoglobin concentrations in anaemic patients with chronic kidney disease treated with erythropoietin: a meta-analysis. Lancet. 2007 Feb 3;369(9559):381–388. doi: 10.1016/S0140-6736(07)60194-9. [DOI] [PubMed] [Google Scholar]
- 47.Information on Erythropoiesis Stimulating Agents (ESA) (marketed as Procrit, Epogen, and Aranesp) 2007. [Google Scholar]
- 48.II. Clinical practice guidelines and clinical practice recommendations for anemia in chronic kidney disease in adults. Am J Kidney Dis. 2006 May;47(5 Suppl 3):S16–S85. doi: 10.1053/j.ajkd.2006.03.011. [DOI] [PubMed] [Google Scholar]
- 49.Anemia in Heart Failure with a Normal Ejection Fraction (HFNEF) NCT00286182. http://clinicaltrials.gov/ct2/show/NCT00286182?term=hfpef&rank=2.
- 50.Gottdiener JS, Arnold AM, Aurigemma GP, et al. Predictors of congestive heart failure in the elderly: the Cardiovascular Health Study. J Am Coll Cardiol. 2000 May;35(6):1628–1637. doi: 10.1016/s0735-1097(00)00582-9. [DOI] [PubMed] [Google Scholar]
- 51.Jones M, Schenkel B, Just J. Epoetin alfa’s effect on left ventricular hypertrophy and subsequent mortality. Int J Cardiol. 2005 Apr 20;100(2):253–265. doi: 10.1016/j.ijcard.2004.08.051. [DOI] [PubMed] [Google Scholar]
- 52.Bohlius J, Wilson J, Seidenfeld J, et al. Erythropoietin or darbepoetin for patients with cancer. Cochrane Database Syst Rev. 2006;3:CD003407. doi: 10.1002/14651858.CD003407.pub4. [DOI] [PubMed] [Google Scholar]
- 53.Cody J, Daly C, Campbell M, et al. Frequency of administration of recombinant human erythropoietin for anaemia of end-stage renal disease in dialysis patients. Cochrane Database Syst Rev. 2005;(3):CD003895. doi: 10.1002/14651858.CD003895.pub2. [DOI] [PubMed] [Google Scholar]
- 54.Cody J, Daly C, Campbell M, et al. Recombinant human erythropoietin for chronic renal failure anaemia in pre-dialysis patients. Cochrane Database Syst Rev. 2005;(3):CD003266. doi: 10.1002/14651858.CD003266.pub2. [DOI] [PubMed] [Google Scholar]
- 55.Jones M, Ibels L, Schenkel B, Zagari M. Impact of epoetin alfa on clinical end points in patients with chronic renal failure: a meta-analysis. Kidney Int. 2004 Mar;65(3):757–767. doi: 10.1111/j.1523-1755.2004.00450.x. [DOI] [PubMed] [Google Scholar]
- 56.Mancini DM, Katz SD, Lang CC, LaManca J, Hudaihed A, Androne AS. Effect of erythropoietin on exercise capacity in patients with moderate to severe chronic heart failure. Circulation. 2003 Jan 21;107(2):294–299. doi: 10.1161/01.cir.0000044914.42696.6a. [DOI] [PubMed] [Google Scholar]
- 57.Ponikowski P, Anker SD, Szachniewicz J, et al. Effect of darbepoetin alfa on exercise tolerance in anemic patients with symptomatic chronic heart failure: a randomized, double-blind, placebo-controlled trial. J Am Coll Cardiol. 2007 Feb 20;49(7):753–762. doi: 10.1016/j.jacc.2006.11.024. [DOI] [PubMed] [Google Scholar]
- 58.Kitzman DW, Little WC, Brubaker PH, et al. Pathophysiological characterization of isolated diastolic heart failure in comparison to systolic heart failure. JAMA. 2002 Nov 6;288(17):2144–2150. doi: 10.1001/jama.288.17.2144. [DOI] [PubMed] [Google Scholar]
- 59.Spertus J, Peterson E, Conard MW, et al. Monitoring clinical changes in patients with heart failure: a comparison of methods. Am Heart J. 2005 Oct;150(4):707–715. doi: 10.1016/j.ahj.2004.12.010. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.