Abstract
The cecum contains a high concentration of microbes which are a combination of gram negative and gram positive flora. These bacteria range from anaerobic to facultative aerobic to aerobic organisms. In the procedure described in this unit, the ligation of the cecum produces a source of ischemic tissue as well as polymicrobial infection. This combination of ischemic/necrotic tissue and microbial infection distinguishes this multifactorial model from a number of other bacterial sepsis models, including but not limited to: bacteremia secondary to intravenous or intraperitoneal administration; fecal administration or intraperitoneal administration of fecal or bacterial plugs; colonic stents; and bacterial abscess formation.
Introduction
This module will outline the basic protocol(s) for conducting murine cecal ligation and puncture (CLP), expected outcomes, and troubleshooting recommendations. For these experiments, additional mice will be required to undergo sham operation as a comparison with the CLP procedure. In the sham animals, the cecum is exteriorized but neither ligated nor punctured (see below). It is important to note that inherent differences in CLP survival exist among mouse strains [1]. Although CLP has been described in both murine and porcine models, this module will focus on the former [2-4]. The host response to CLP is user dependent and investigators will notice as their technique improves (e.g. improved sterility and decreased tissue trauma) mouse survival often improves. The advantage of this model is that it is very reproducible and relatively quick to perform in experienced hands, and can be modified to study different survival intervals and varying levels of inflammation.
Materials
Material for surgery
Mice
2-0 silk suture
Electric razor
Sterile hollow bore needles, gauge ranging from 22-27
Two 5 inch stainless steel forceps - noncrushing
5 inch stainless steel scissors
Needle driver
BD Autoclip Wound Closing System™
Auto-clips
70% ethanol
Non-sterile gloves
Materials for anesthesia and recovery
0.9% sterile saline
-
Buprenorphine for injection at a dose of 0.05-0.2 mg/kg per mouse
NOTE:
-
1
Buprenorphine can be mixed with the saline to deliver buprenorphine at 0.05-0.2 mg/kg per mouse in 1 mL of saline.
-
2
Buprenorphine is a controlled substance and will require appropriate storage and documentation.
Platform for animal surgery
One liter Induction plexiglass chamber (VetEquip # 941443)
Fisher™ paper tape
Inhalant anesthesia system for veterinary surgery
Desiccant anesthetic scavenger
-
Isoflurane
NOTE: Isoflurane is a controlled substance and will require appropriate storage and documentation.
Tank O2
Thermal Blanket - preferably a water heated blanket, rather than an electric blanket, which can cause skin burns if not carefully controlled.
Procedure
-
After the veterinary anesthesia system has been established, the animals may be placed into the induction chamber. Isoflurane concentration will be between 3.5 to 4.5 % with O2 flow at 2L/min.
NOTE: Observe the mice for the proper anesthetic depth. The mice should be able to lie on their backs while not responding to firm foot squeezing. Over-anesthesia is often manifested by agonal respirations.
NOTE: For larger experiments, we typically place 5 mice into the induction chamber at one time.
-
Scruff the mouse and shave the lower half of their abdomen being careful not to cut its skin. Shaving is done to facilitate the surgery and closure rather than preserve sterility. In addition, 70% ethanol may be used to wipe down the mouse’s abdomen to remove excess hair. Each institutional animal care and use committee may have different requirements regarding sterility and treatment of the incision site. The investigator is responsible to adhere to local regulations regarding preoperative and operative procedures.
NOTE: Do not drench the animal in ethanol as this will predispose the animal to hyperthermia.
-
Using either straight-edge or iris scissors, make a 1 cm midline cut into the skin only, approximately 0.5-1 cm away from xiphoid process (Figure 1). Entry into the peritoneum should be avoided. After the initial cut has been made, the abdominal wall identified and another 1 cm midline cut through the abdominal musculature into peritoneum is performed. In most instances, the cecum will be located directly under the incision and will be easy to exteriorize.
NOTE: The small bowel can be easily transected during entry into the peritoneum. Therefore, care needs to be taken not to inadvertently damage the bowel with the scissors as you enter the abdominal cavity.
Using a non-crushing forceps, exteriorize the cecum (Figure 2).
-
At a point approximately 1 cm from the cecal tip, ligate the cecum with a 2-0 silk suture. A hand or instrument tie may be used to perform at least one square knot. Cut the ends of the suture to leave 1 mm ends (Figure 3A).
NOTE: This step is a critical factor in determining the severity of CLP induced sepsis and mouse survival. In a recent report, Ward and colleagues argued that the location of the ligation can be used to vary the severity of the survival response [5]. We have also found that standardization of the volume of feces remaining in the cecum is important as the length of the cecum ligated. However, assuming a constant volume of stool per length of cecum, the longer the length of cecal ligation, the more stool, bacteria and devitalized tissue are present for the intra-abdominal injury. This, in turn, leads to increased mouse mortality.
NOTE: It is very important that the ligation be made below the area where the small bowel (ileum) enters the cecum (ileocecal junction) to prevent obstruction of the mouse’s intestines.
NOTE: For the sham control procedure, a ligation and puncture of the cecum is not done. Rather, the cecum is carefully replaced into the abdomen.
-
Carefully puncture the cecum distal to your ligation with a 27 gauge needle (Figure 3B). The puncture needs to be made in one pass, through and through both sides of the bowel wall.
NOTE: As discussed below, varying the size of the enterotomy can be used to vary the severity of the injury and infection, and thus subsequently alter mortality.
NOTE: At this point, some investigators compress the punctured cecum to express fecal material by gently compressing the ligated cecum with sterile cotton tipped swabs or their fingers, taking care to distribute pressure evenly. The operator must take care not to tear the cecum at the puncture site and increase the size of the holes in the cecum as this will adversely affect your outcome.
-
Replace the ligated and punctured cecum into the abdomen.
NOTE: If stool wipes onto the mouse’s skin during this step, simply remove the stool with a clean disposable cloth (e.g. Kim Wipe™). This will not affect the outcome.
Close the abdominal wall with two 3-0 absorbable polyfilament interrupted sutures (e.g. Vicryl™), using either an instrument or hand tie (Figure 4A). Subsequently, approximate the skin using an auto-stapler (Figure 4B).
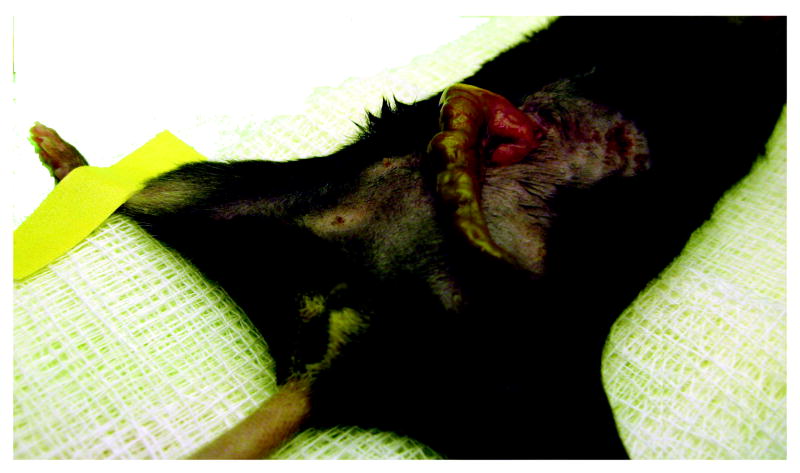
Inject 1 mL of saline/buprenorphine mix into the scruff of the animal’s neck subcutaneously (Figure 5). The use of opioid analgesics is strongly recommended in this model for the first 12-24 hours to ameliorate pain and distress associated with surgical injury.
Place the mouse gently on its back onto the thermal blanket and monitor them.
Return the animals to their cages after they awaken from anesthesia. This is usually indicated by their capacity to independently turn over onto their bellies after being placed on their sides or back.
-
Administer 0.05-0.2 mg/kg buprenorphine subcutaneously every 12 hours × 24 hours or as required. Some institutional animal care and use committees will require analgesic support for up to 48 hours for surgical pain or discomfort.
-
NOTE: The goal of monitoring procedures is to determine when and if the animals enter a prodromal period where death is likely to occur in the next few hours. There are strong ethical and scientific reasons not to allow animals to die spontaneously and euthanize them if and when they become moribund and/or death is imminent. This reduces pain and discomfort to the animals and assures that tissue and blood samples are not lost because of post-mortem changes. Monitor animals every 12 hours for:
Changes in posture - mice will appear hunched early after procedure but this should resolve with time. If it does not, the mice will fail to thrive and you may consider moist chow, more resuscitation with normal saline or euthanasia depending on your protocol.
Failure to right themselves - if mice are placed on their backs, they should right themselves. If they do not, you will need to euthanize them.
Signs of distress - (i.e. pain on touch, agonal breathing). In these cases, you may want to consider more resuscitation or euthanasia of the mouse depending on your protocol.
-
Figure 1.
Midline incision through the skin.
Figure 2.
Exteriorization of the cecum.
Figure 3.
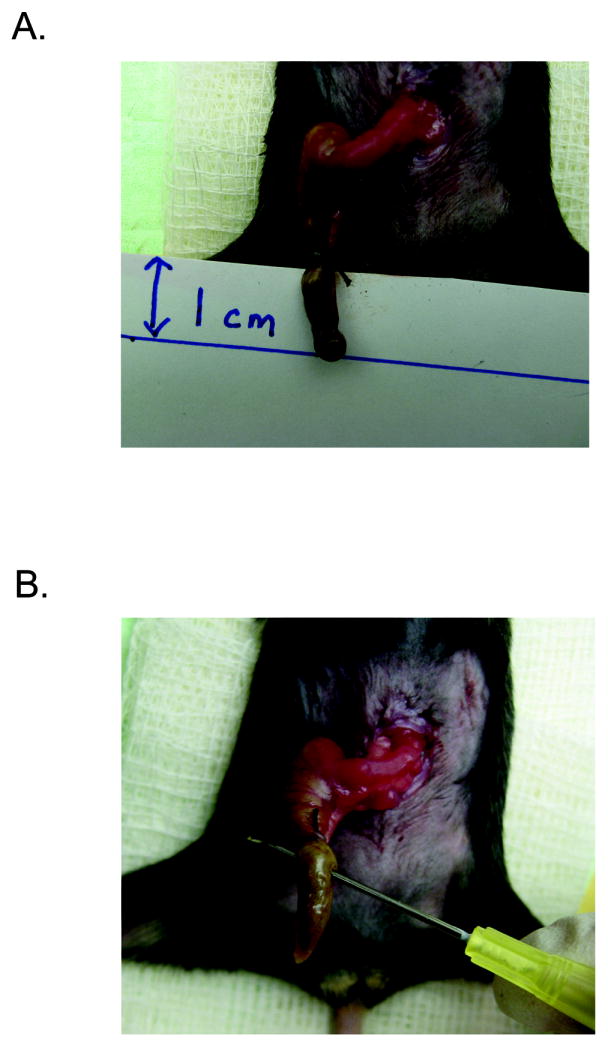
Ligation and needle puncture of the cecum.
A. One centimeter of cecum has been suture ligated. B. The ligated cecum has been punctured through and through with hollow bore needle.
Figure 4.

Closure of the abdomen.
A. Closure of abdominal musculature. B. Closure of skin with auto-stapler.
Figure 5.
Injection of saline/buprenorphine into the scruff of the mouse.
Commentary
Background Information
There are greater than 750,000 cases of sepsis each year due to various etiologies. Mortality due to sepsis remains 20-30% even with significant advances in critical care over the past two decades [6]. Sepsis induces a systemic response that has been characterized by our lab and others as both a pro-inflammatory and anti-inflammatory response [7, 8]. The complex expression of cytokines/chemokines as well as the phenotypic variation of critical effector cells during sepsis is the subject of ongoing work and have been described in the literature [3, 9].
The cecum is a pouch of large intestine that connects the terminal ileum (end of the small bowel) to the ascending colon (beginning of the large bowel). The cecum contains a high concentration of microbes which are a combination of gram negative and gram positive flora [10]. These bacteria range from anaerobic to facultative aerobic to aerobic organisms. In the procedure described above, the ligation of the cecum produces a source of ischemic tissue as well as polymicrobial infection [11]. This combination of ischemic/necrotic tissue and microbial infection distinguishes this multifactorial model from a number of other bacterial sepsis models, including but not limited to: bacteremia secondary to intravenous or intraperitoneal administration; fecal administration or intraperitoneal administration of fecal or bacterial plugs; colonic stents; and bacterial abscess formation [5, 11-13]. After replacing the punctured cecum into the peritoneum, fecal contents will begin to spill into the abdomen producing a severe peritonitis. This will cause a local infection followed by a systemic bacteremia. Within 24 hours, bacteria will be detectable in the blood and peritoneum, although this can vary depending on the intensity of the model utilized (i.e. size of the needle for cecal puncture) (Figure 6A) [14].
Figure 6.
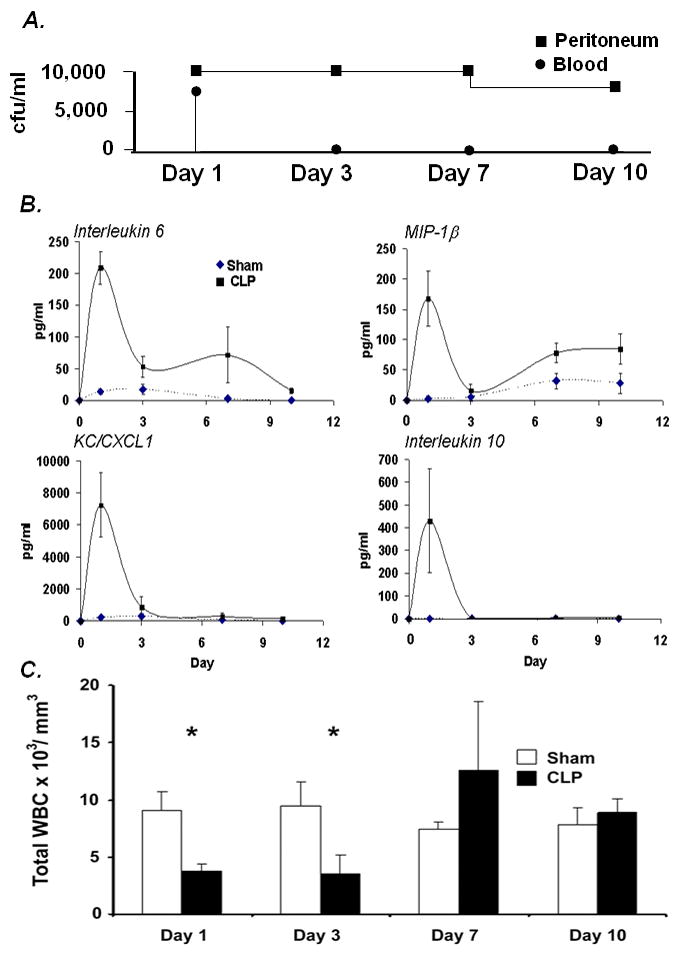
Peritonitis/bacteremia and the immune response after CLP.
A. Blood and Peritoneal Washes cultured on Blood Agar [14] B. Cytokine expression during sepsis [14] C. Peripheral white blood cells (WBCs) in Sham and CLP animals [14].
The immune profile induced by CLP can be divided into both pro- and anti-inflammatory responses. This includes the expression of inflammatory and anti-inflammatory cytokines and chemokines, which are believed to mimic human sepsis (Figure 6B) [14]. The pro-inflammatory phase seen in early sepsis is marked by dramatic increases in the plasma level of several cytokines and chemokines, such as IL-6, MIP-1α, and KC (Figure 6B) [7, 8]. Expression of these inflammatory mediators increases monocyte/ neutrophil bone marrow emigration, activation, and adhesion molecules up regulation, all of which promote leukocyte transmigration to sites of inflammation [7, 8]. These inflammatory mediators also cause a decrease in systemic vascular resistance that can lead to hypotensive shock. Persistence of the hyper-inflammatory response and shock can induce multi-organ system dysfunction and subsequently, death. It has been generally assumed that the early death in this model (within 48 hours) is primarily due to an exaggerated inflammatory response, hypovolemic shock and inadequate tissue perfusion.
Occurring along with the hyper-inflammatory response is an anti-inflammatory and Th2 response. The production of the anti-inflammatory cytokine, IL-10 (see Figure 6B), and the Th2 cytokine, IL-4, by innate immune effector cells, such as macrophages, are typically associated with this phenomenon. In addition, a gradual shift from a pro-inflammatory to an anti-inflammatory state is thought to predispose the septic patient to develop nosocomial infections days to weeks after the initial injury [4]. This “second infectious hit” can lead to further organ compromise, organ failure and death [7, 15, 16]. Also within the first 36-48 hours, considerable apoptosis of secondary lymphoid tissue (i.e. spleen and lymph node) lymphocytes and dendritic cells occurs which many researchers provide as evidence to support the notion of sepsis induced immune suppression [17, 18]. After the first 1-2 days, myelopoiesis/granulopoiesis will increase to replenish peripheral pools of neutrophils that have been depleted via transmigration to the sites of infection and inflammation (Figure 6C). During this time, attempts are made by the host to clear the bacteremia and contain the punctured cecum through fibrin deposition and subsequent abscess formation[19]. The failure to localize the septic focus and/or clear bacteremia will lead to death in most cases. In animals dying late from this model, death is often associated with the failure to adequately sequester the necrotic cecum and develop an abscess.
The observations documented in the CLP model have been verified in some human studies [5, 16, 20]. However, there are several limitations to the translation of the CLP model to clinical sepsis. Although there are many similarities in the pathophysiology induced by CLP in mice and sepsis in humans, the two populations are not the same. For example, most septic patients have one if not several co-morbidities including cancer, atherosclerotic disease, and pulmonary disease [12]. This is in contrast to the mice used for CLP which are for the most part young and healthy [12]. Additionally, most septic patients are on ventilator support whereas mechanical ventilation is not typically integrated into the CLP model [12]. Other significant differences between the two populations also include onset of sepsis and tolerance to hemorrhage [12].
In addition, technique variability and differences in cecal ligation length strongly influence mouse mortality and physiologic outcomes (i.e. cytokine level production and survival) [13, 21-23]. The use of antibiotics, analgesics, and other resuscitation practices can also introduce variability in the model [11, 21-23]. Also, differences in the gauge needle used for puncture can either reduce (smaller gauge) or increase (larger gauge) immediate mortality as a result of CLP [22]. In experienced hands, these differences can also be useful, especially when testing interventions meant to either improve or worsen outcome. For example, a 10% mortality model in the CLP mouse is not of much value when testing therapeutic approaches to reduce mortality, but is extremely helpful when determining which tissue responses are required for survival. Conversely, an LD90 or LD100 model may be more appropriate for evaluating therapies to improve outcome. Thus, the ability to modulate severity of response either by varying the size of the enterotomy or the length of the ligation represents a major strength of the protocol (see Figure 7) [5, 22]. As described, adjustment of the needle puncture and the length of cecum ligation can allow for control of outcome/mortality (see Figure 7). Also, it is important to note that strain specific differences in response to infection exist. For example, the C57BL/6 mouse has been demonstrated to produce Th1 skewed responses where the Balb/C has been demonstrated to produce Th2 responses [1]. Although a review of the literature demonstrates the wide variability of investigator based practices for this particular model, CLP is still thought to be the “gold standard” in the study of adult sepsis in rodent models.
Figure 7.

CLP survival curve using different gauge needles for puncture (Unpublished data).
Critical Parameters and Troubleshooting
Mice
An often understated parameter in this or any technique is the care and/or origin of the animals. We have found that allowing the mice to equilibrate to the diurnal cycle and feed at the home institution for 1-2 weeks before manipulation will decrease variability in this model. This will decrease the stress from shipping as well as allow the animals to become accustomed to the mouse chow. It is also important that these mice be kept under specific pathogen free guidelines.
Surgery
The outcome of this procedure is largely dependent on the investigator’s experience with the technique. Therefore it may be necessary to conduct several trial runs in order to establish the model. Additionally, as the investigator becomes more familiar with the technique, the exteriorization of the cecum will become easier and will reduce the time of the procedure as well as possible damage to surrounding structures. It should be noted that excessive manipulation of bowel will result in bleeding and/or perforation as it is incredibly fragile. Also, manipulation of the bowel without puncture or ligation has been shown to cause release of pro-inflammatory cytokines and inflammation [24].
Anesthesia
Isoflurane is our anesthetic of choice for this procedure. It provides rapid onset, adequate depth, and rapid awakening. Care should be taken to ensure that the depth of the anesthetized mouse is not excessive, as agonal respiration and subsequent death can occur. In the past, we have used pentobarbital injection with success.
Resuscitation, post-operative analgesia, and antibiotics
We routinely inject our animals postoperatively with 1 mL of normal saline once. It is important to resuscitate them as they will be hypovolemic from the laparotomy, anesthesia, and ensuing shock. Also, we mix the saline and buprenorphine so that the animals receive only one injection. Additionally, every 12 hours after surgery, we administer buprenorphine (0.05 mg/kg BW) for postoperative analgesia for the first 48 hours. In our practice, we do not administer buprenorphine longer than 48 hours due to possible effect on the immune response and on assay outcomes. Several groups also use broad spectrum antibiotics in their CLP model either to investigate antibiotic efficacy or as standard post surgical treatment (see Table 1) [25-28]. As mentioned above, this is reasonable considering septic patients are routinely placed on antibiotics. Investigator choice will dictate whether to include this in their model of CLP.
Table 1.
Antibiotics used as standard post surgical treatment
| Reference | Antibiotic (Dosage) and schedule |
|---|---|
| Zanotti-Cavazzoni et al., 2009 [26] | Ceftriaxone (30 mg/kg) and clindamycin (30 mg/kg) intramuscular every 6 hours |
| Enoh et al.,2007 [27] | Imipenem/cilastin (25 mg/kg) intraperitoneal every 8 hours |
| Benjamim et al., 2003 [25] | Imipenem/cilastin (10 mg/kg) intraperitoneal every 8 hours |
| Turnbull et al., 2003 [28] | Metronidazole (12.5 mg/kg) and Ceftriaxone (25 mg/kg) intraperitoneal every 12 hours |
The use of analgesics is a controversial topic and should be discussed with the local institutional animal use and care committee. We routinely use opiates in the immediate post-operative period to reduce surgical pain and discomfort. We believe that opiates administered after this period are of little value, since sepsis appears not to be associated with localized pain or discomfort, but rather malaise and weakness. Opiates tend to suppress feeding behavior and activity, so it is often difficult to judge the moribund nature of the animals when they are receiving opiate treatment. Rather, we monitor the animals closely for behavioral signs of decompensation and use euthanasia as a means to reduce discomfort and pain. Nonsteroidal anti-inflammatory drugs may be contraindicated because of their direct effects on the inflammatory response.
Anticipated Results
Following CLP, serum levels of cytokines and chemokine levels rise rapidly. Neutrophils and other innate immune effector cells are recruited to the peritoneum to contain the septic focus. Mortality will start to occur as rapidly as 18-24 hours after CLP. Of note, all of the sham animals used as experimental controls should survive the procedure. Inflammatory changes (i.e. increases in cytokine and chemokine levels) should be seen in the sham mice, however, they should be significantly different than those measured in CLP mice (Figure 6A). Additionally, CLP mortality and outcome can be adjusted by varying the size of needle used for the cecal puncture, as seen in Figure 7.
Time Considerations
We typically place 5 mice into the anesthetic induction chamber at one time. This will allow the investigator to start the next surgery while the mouse from the previous procedure is recovering. The CLP procedure should take less than 5 minutes per mouse. Depending on how much anesthetic the mice have received, recovering mice may take up to 10 minutes to fully awaken from anesthesia. Also, as technical proficiency improves, the time per procedure will decrease.
Acknowledgments
Supported in part by grants GM-40586-22, GM-63041-10 and GM-81923-2, awarded by the National Institute of General Medical Sciences. AGC, MJD, and KKS were recipients of a National Institute of General Medical Sciences training grant in burns and trauma (T32 GM-008721-12).
References
- 1.Watanabe H, et al. Innate immune response in Th1- and Th2-dominant mouse strains. Shock. 2004;22(5):460–6. doi: 10.1097/01.shk.0000142249.08135.e9. [DOI] [PubMed] [Google Scholar]
- 2.Witek-Janusek L, Ratmeyer JK. Sepsis in the young rat: maternal milk protects during cecal ligation and puncture sepsis but not during endotoxemia. Circ Shock. 1991;33(4):200–6. [PubMed] [Google Scholar]
- 3.Ertel W, et al. The complex pattern of cytokines in sepsis. Association between prostaglandins, cachectin, and interleukins. Ann Surg. 1991;214(2):141–8. doi: 10.1097/00000658-199108000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Scumpia PO, et al. Increased natural CD4+CD25+ regulatory T cells and their suppressor activity do not contribute to mortality in murine polymicrobial sepsis. J Immunol. 2006;177(11):7943–9. doi: 10.4049/jimmunol.177.11.7943. [DOI] [PubMed] [Google Scholar]
- 5.Rittirsch D, et al. Immunodesign of experimental sepsis by cecal ligation and puncture. Nat Protoc. 2009;4(1):31–6. doi: 10.1038/nprot.2008.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Martin GS, et al. The epidemiology of sepsis in the United States from 1979 through 2000. N Engl J Med. 2003;348(16):1546–54. doi: 10.1056/NEJMoa022139. [DOI] [PubMed] [Google Scholar]
- 7.Mannick JA, Rodrick ML, Lederer JA. The immunologic response to injury. J Am Coll Surg. 2001;193(3):237–44. doi: 10.1016/s1072-7515(01)01011-0. [DOI] [PubMed] [Google Scholar]
- 8.Okazaki Y, Matsukawa A. Pathophysiology of sepsis and recent patents on the diagnosis, treatment and prophylaxis for sepsis. Recent Pat Inflamm Allergy Drug Discov. 2009;3(1):26–32. doi: 10.2174/187221309787158416. [DOI] [PubMed] [Google Scholar]
- 9.Scumpia PO, et al. CD11c+ dendritic cells are required for survival in murine polymicrobial sepsis. J Immunol. 2005;175(5):3282–6. doi: 10.4049/jimmunol.175.5.3282. [DOI] [PubMed] [Google Scholar]
- 10.Townsend Courtney M, Jr MD, DB R, M, Mark Evers B, MD, Mattox Kenneth L., MD . Sabiston Textbook of Surgery: Expert Consult Premium Edition: Enhanced Online Features and Print (Sabiston Textbook of Surgery: The Biological Basis of Modern Practicsurgical Practice) (Hardcover) 18. Saunders; Nov 6, 2007. p. 2100. [Google Scholar]
- 11.Hubbard WJ, et al. Cecal ligation and puncture. Shock. 2005;24(Suppl 1):52–7. doi: 10.1097/01.shk.0000191414.94461.7e. [DOI] [PubMed] [Google Scholar]
- 12.Esmon CT. Why do animal models (sometimes) fail to mimic human sepsis? Crit Care Med. 2004;32(5 Suppl):S219–22. doi: 10.1097/01.ccm.0000127036.27343.48. [DOI] [PubMed] [Google Scholar]
- 13.Buras JA, Holzmann B, Sitkovsky M. Animal models of sepsis: setting the stage. Nat Rev Drug Discov. 2005;4(10):854–65. doi: 10.1038/nrd1854. [DOI] [PubMed] [Google Scholar]
- 14.Delano MJ, et al. MyD88-dependent expansion of an immature GR-1(+)CD11b(+) population induces T cell suppression and Th2 polarization in sepsis. J Exp Med. 2007;204(6):1463–74. doi: 10.1084/jem.20062602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lederer JA, Rodrick ML, Mannick JA. The effects of injury on the adaptive immune response. Shock. 1999;11(3):153–9. doi: 10.1097/00024382-199903000-00001. [DOI] [PubMed] [Google Scholar]
- 16.Hotchkiss RS, Karl IE. The pathophysiology and treatment of sepsis. N Engl J Med. 2003;348(2):138–50. doi: 10.1056/NEJMra021333. [DOI] [PubMed] [Google Scholar]
- 17.Hotchkiss RS, et al. Apoptosis in lymphoid and parenchymal cells during sepsis: findings in normal and T- and B-cell-deficient mice. Crit Care Med. 1997;25(8):1298–307. doi: 10.1097/00003246-199708000-00015. [DOI] [PubMed] [Google Scholar]
- 18.Efron PA, et al. Increased lymphoid tissue apoptosis in baboons with bacteremic shock. Shock. 2004;21(6):566–71. doi: 10.1097/01.shk.0000126648.58732.8c. [DOI] [PubMed] [Google Scholar]
- 19.Reijnen MM, Bleichrodt RP, van Goor H. Pathophysiology of intra-abdominal adhesion and abscess formation, and the effect of hyaluronan. Br J Surg. 2003;90(5):533–41. doi: 10.1002/bjs.4141. [DOI] [PubMed] [Google Scholar]
- 20.Weber SU, et al. Induction of Bim and Bid gene expression during accelerated apoptosis in severe sepsis. Crit Care. 2008;12(5):R128. doi: 10.1186/cc7088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hollenberg SM. Mouse models of resuscitated shock. Shock. 2005;24(Suppl 1):58–63. doi: 10.1097/01.shk.0000191415.02085.48. [DOI] [PubMed] [Google Scholar]
- 22.Rittirsch D, Hoesel LM, Ward PA. The disconnect between animal models of sepsis and human sepsis. J Leukoc Biol. 2007;81(1):137–43. doi: 10.1189/jlb.0806542. [DOI] [PubMed] [Google Scholar]
- 23.Poli-de-Figueiredo LF, et al. Experimental models of sepsis and their clinical relevance. Shock. 2008;30(Suppl 1):53–9. doi: 10.1097/SHK.0b013e318181a343. [DOI] [PubMed] [Google Scholar]
- 24.Harada T, et al. Ethyl pyruvate ameliorates ileus induced by bowel manipulation in mice. Surgery. 2005;138(3):530–7. doi: 10.1016/j.surg.2005.04.006. [DOI] [PubMed] [Google Scholar]
- 25.Benjamim CF, et al. Septic mice are susceptible to pulmonary aspergillosis. Am J Pathol. 2003;163(6):2605–17. doi: 10.1016/S0002-9440(10)63615-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zanotti-Cavazzoni SL, et al. Fluid resuscitation influences cardiovascular performance and mortality in a murine model of sepsis. Intensive Care Med. 2009;35(4):748–54. doi: 10.1007/s00134-008-1360-9. [DOI] [PubMed] [Google Scholar]
- 27.Enoh VT, et al. Mice depleted of alphabeta but not gammadelta T cells are resistant to mortality caused by cecal ligation and puncture. Shock. 2007;27(5):507–19. doi: 10.1097/SHK.0b013e31802b5d9f. [DOI] [PubMed] [Google Scholar]
- 28.Turnbull IR, et al. Effects of age on mortality and antibiotic efficacy in cecal ligation and puncture. Shock. 2003;19(4):310–3. doi: 10.1097/00024382-200304000-00003. [DOI] [PubMed] [Google Scholar]