Abstract
Older adults show positive gaze preferences, but to what extent are these preferences malleable? Examining the plasticity of age-related gaze preferences may provide a window into their origins. We therefore designed an attentional training procedure to assess the degree to which we could shift gaze and gaze-related mood in both younger and older adults. Participants completed either a positive or negative dot-probe training. Before and after the attentional training, we obtained measures of fixations to negatively-valenced images along with concurrent mood ratings. We found differential malleability of gaze and mood by age: for young adults, negative training resulted in fewer post-training fixations to the most negative areas of the images, whereas positive training appeared more successful in changing older adults’ fixation patterns. Young adults did not differ in their moods as a function of training, whereas older adults in the train negative group had the worst moods after training. Implications for the etiology of age-related positive gaze preferences are considered.
Keywords: aging, attentional training, emotion, fixation, gaze preferences
Older adults have been observed to show looking preferences toward the positive and away from the negative when gazing at emotional stimuli (Isaacowitz, Wadlinger, Goren, & Wilson, 2006), particularly when in bad moods (Isaacowitz, Toner, Goren, & Wilson, 2008). Gaze preferences are displayed whenever individuals look more at one type of stimuli than another; we refer to this age-related pattern as an overall “positive gaze preference” because fixation is biased toward more positive emotional content. While the downstream effects of these gaze preferences has recently been studied (e.g., Isaacowitz, Toner, & Neupert, 2009), less is known about the origin of the age-related positive preferences. Although a longitudinal study in which individuals are followed over many decades would be one tool for discerning when (and perhaps how) these gaze preferences emerge, another tool for investigating the origins of gaze preferences is to assess their plasticity. As Baltes (1987) has argued, studying the plasticity of a system allows life-span researchers to understand the range of possible development. Thus, investigating the plasticity of gaze preferences can provide a window into their origin by revealing the possible range of developmental outcomes. While this may not specifically identify the causes of the outcome, it can help narrow down the list to which origins are most plausible. In the current study, we tested to what extent we could modify age-related gaze preferences through training. Below, we enumerate three distinct theoretical perspectives that lead to differential predictions about the extent to which such training might actually modify gaze preferences in older and younger individuals.
The Potential Malleability of Gaze Preferences
Why should we believe that gaze preferences are malleable? For one, empirical work in young adult samples has suggested that gaze preferences toward emotional stimuli can change as a function of task as well as training. In one study, young adults modulated their gaze toward emotional images depending on the instructions: those instructed to “manage their emotions” as they viewed the images looked less overall at the images and especially less at negative images (Xing & Isaacowitz, 2006). Similarly, young adults induced into a positive mood state were found to change their gaze pattern to emotional images (Wadlinger & Isaacowitz, 2006). The most direct evidence that young adults’ gaze toward emotional images is malleable comes from a training study: those young adult participants trained to look more at positive words (in positive-neutral word pairs) via a dot-probe training showed changes in their later fixations to emotional images, such that they looked less at the arousing negative areas of interest after the training (Wadlinger & Isaacowitz, 2008).
One reason to believe that older adults’ gaze preferences are malleable comes from the conceptual framework usually invoked to explain findings of age-related positive gaze preferences. Socioemotional selectivity theory (SST: Carstensen, 2006; Carstensen, Isaacowitz, & Charles, 1999) asserts that advancing age brings with it a sense of time being limited, and that when an individual perceives her time as limited, her motivation shifts to prioritize present-oriented hedonic goals related to feeling good and regulating emotions. Findings that older adults may show “positivity effects” in their attention and memory (as termed by Carstensen & Mikels, 2005, for example), favoring positive over negative material, are interpreted as reflecting older adults’ top-down attempts to direct their information processing toward material that will help them achieve their mood regulatory goals. Mather has argued (Mather & Knight, 2005; Knight et al., 2007) that older adults’ use of positivity requires cognitive control, as it involves the top-down modulation of predispositions to focus on the negative. Implicit in the assertion that “positivity effects” such as positive gaze preferences are the product of top-down regulatory mechanisms is the notion that they are malleable and can be flexibly modulated. Indeed, Knight et al. (2007) found that older adults’ positive looking preference could be reversed under certain dual-task conditions, when looking patterns toward emotional stimuli are assessed while the participant also completes another task simultaneously (but see Allard & Isaacowitz, 2008), further supporting the idea that older adults’ positive gaze preferences may be flexible enough to vary by task demands. Therefore, finding plasticity in older adults’ positive gaze preferences would suggest motivation as a plausible causal mechanism underlying such preferences.
Given the above empirical and conceptual reasons to expect plasticity in attention, is there any reason to expect a lack of plasticity? First, older adults in general display less plasticity than their younger counterparts (Baltes, 1987). More directly relevant to the question of fixation preferences, though, is recent evidence that older adults’ preferential processing of positive stimuli may result, at least in part, from age-related changes in neural sensitivity to stimuli of different valence (e.g., Leclerc & Kensinger, 2008). Such changes could, in theory, be due to factors ranging from neural decline to the effects of motivation itself; Cacioppo and colleagues (in press) have recently coined the term “the Aging Brain Model” to connote the idea that certain socioemotional changes with age may be side effects, specifically, of neural decline, which are especially related to age-related reductions in amygdala function. If positive gaze preferences result, not from top-down modulation of emotional responding, but instead, from declines in brain function that overdetermine responses to valenced stimuli, then we might not expect any real malleability in gaze preferences.
A further complication when considering the possible malleability of age-related gaze preferences is that there may be differential malleability by age group. Research on negativity dominance in young adults (e.g., Rozin & Royzman, 2001), and positivity effects in the information processing of older adults (e.g., Carstensen & Mikels, 2005) might suggest that older and young adults are differentially sensitive to positive vs. negative learning, respectively. Socioemotional selectivity theory, for example, suggests that older adults focus more on positive material due to their motivation to optimize their affective state (Carstensen, 2006). Older adults are less sensitive to monetary losses than are young adults (e.g., Samanez-Larkin et al., 2007), and display lower levels of feedback-related negativity in electrophysiological responses than do young adults (Eppinger, Kray, Mock, & Mecklinger, 2008). Such findings suggest that there may, in fact, be differential malleability to valenced learning with age, with older adults appearing to learn more from positive than negative incentives and feedback (at least in tasks involving monetary incentives; see also Denburg et al., 2006; Wood et al., 2005).
The Current Study: Training Gaze and Attention
Therefore, in the current study, we assessed a) to what extent age-related gaze preferences are malleable, and b) whether there might be differential malleability by age. We used a variant of a dot-probe training paradigm first developed by MacLeod and colleagues (2002) in their work on the development of depressogenic attentional biases. In that study, participants were trained using a constrained dot-probe paradigm (“constrained” in the sense that the probe almost always appeared behind the target stimulus type for the training) to look more at negative (training group) or neutral (control group) words in negative-neutral pairs. Training to negative stimuli made young adults more susceptible to the negative mood effects of later stressors. In Wadlinger and Isaacowitz (2008), we slightly modified the original MacLeod paradigm to train young adults to either positive (training group) or neutral (control group) words in positive-neutral pairs.
Critically, in all cases, the question was whether the constrained dot-probe training had some effect on behavior after the training (e.g., transfer); in the case of the Wadlinger and Isaacowitz (2008) study, the outcome was fixation to unrelated arousing negative stimuli. In this paradigm, malleability of gaze preferences is assessed by determining whether an attentional training shows effects on gaze (these could be termed “directional near-transfer effects” in traditional cognitive training paradigms). Such indirect training methods (as opposed to simply directing people to change their gaze) are common when considering how to change attentional patterns in ways that may promote emotion regulation (see Wadlinger & Isaacowitz, in press) and have proved successful in changing gaze patterns in young adults in previous work (Wadlinger & Isaacowitz, 2008). For example, Shamata meditation’s effects on attention deployment may be assessed, not by examining the individual’s ability to focus on the object of the meditation (e.g., the breath) but rather, on whether someone who has been trained to do so shows subsequent sustained attentional abilities on later tasks. In the current study, we also asked whether constrained dot-probe training could lead to emotion regulation-relevant transfer in the form of changes in viewing patterns to unrelated negative arousing pictures.
There were two key modifications to the training paradigm used in the current study. First, we attempted to use the constrained dot-probe training, not only on young adult participants, but with older participants as well. To our knowledge, this marked the first attempt to extend emotional dot-probe training paradigms to older adults and the first attempt to manipulate older adults’ gaze preferences through training. Second, we varied the make-up of the training: because we had found that older adults naturally showed more fixation toward positive stimuli in positive-neutral pairs and away from negative ones in negative-neutral pairs (e.g., Isaacowitz et al., 2006), we decided to design a training that included both types of pairs (whereas previous studies had used only one or the other). In our training, one group was assigned to train to show a typical “older” gaze pattern: toward the more positive stimulus in each pair (i.e., positive in positive-neutral and neutral in negative-neutral). The other group was assigned to train to show a typical “young” gaze pattern: toward the more negative stimulus in each pair (i.e., neutral in positive-neutral and negative in negative-neutral).
Several hypotheses could be generated from past research. In the full malleability account, both age groups should show transfer effects of the training on gaze patterns, such that the constrained dot-probe training would lead them to show changes in their gaze patterns toward emotional stimuli consistent with the direction of the training: so those of both age groups trained to look at more positive stimuli would develop an attentional avoidance of arousing negative stimuli (and thus a more positive gaze preference), whereas those trained to look more at negative stimuli would become more engaged visually with arousing negative stimuli (thus developing a more negative gaze preference). According to the differential malleability hypothesis, older adults may be most susceptible to positive training, such that they develop more positive gaze preferences after a positive training, whereas young adults may be equally responsive to both types of training. This hypothesis would be most consistent with age-related positivity effects and the motivational account of such effects by socioemotional selectivity theory (Carstensen, 2006). Of course, it was also possible that we would not find any evidence for effects of training on gaze patterns (the no malleability account).
Finally, beyond the question of whether it is possible to shift the gaze patterns that older and young adults display when looking at emotional stimuli, there remains an important functional question: older adults’ display of positivity in attention and memory has been conceptually linked to their attempts to regulate how they feel (e.g., Carstensen & Mikels, 2005), but there is little actual data demonstrating clear links between positivity and mood regulation. Recently, we have shown that positive gaze preferences can help some older adults stave off mood declines, but it depends on the good functioning of the attentional system – especially good executive control (Isaacowitz, Toner, & Neupert, 2009). Therefore, it is important to assess whether training directed at shifting gaze patterns has any corresponding impact on mood as well. Thus, in the current study, in addition to examining the malleability of gaze preferences to negative images, we also considered whether any shifts in gaze might also be associated with changes in mood while viewing the images. We expected that any training that reduced gaze toward arousing negative images would lead to a corresponding improvement in mood during viewing (see also Isaacowitz et al., 2009; Urry, 2010).
In sum, this study attempted to clarify to what extent age-related positive gaze preferences could be shifted through training and whether such shifts impacted mood as well. Understanding the malleability of gaze could help provide support for, or put a constraint on, motivational explanations for age-related shifts in attention toward positive material.
Method
Participants
Seventy-five young adults (28 men, 47 women) aged 18–25 years (M=19.48, SD=1.72) and 84 community-dwelling older adults (22 men, 62 women) aged 61–90 years (M=72.34, SD=6.93) participated. Young adults were recruited through an introductory psychology course and flyers posted on campus. Older adults were recruited through a lifelong learning program and advertisements. Participants received either course credit (some young adults) or a monetary stipend (the remaining young adults and all older adults).
Seven young adults and twenty-three older adults were excluded from all analyses due to incomplete data from trackability issues (e.g., problematic pupils, glare from eyeglasses) and/or other technical problems experienced during their sessions. The remaining 129 participants were successfully tracked for at least 65% of each eye-tracking session and completed the main measures of the study. Comparisons of the trackable and nontrackable participants within each age group revealed no substantial differences on demographic, affective, cognitive, or visual measures.1
Attentional Training Task
The attentional training task utilized a constrained dot-probe design (modified from that of MacLeod et al., 2002 and Wadlinger & Isaacowitz, 2008) in which pairs of words (one neutral word paired with either a negatively or positively valenced word) were presented on the screen for 2 seconds and then disappeared to reveal one or two dots located behind one of the words in the pair. Participants were instructed to respond, as quickly and accurately as possible, to whether they saw one dot or two dots by pressing the corresponding key on the keyboard. There were four conditions, two of which were designed to train attention to positive stimuli and the other two, to train negative. The same words were used in all conditions. In the positive training conditions, the dot-probe task was designed so that the dot(s) appeared more often behind the positively-valenced word in a positive-neutral pair. For the negative training conditions, the converse was true. For each condition of the task, there were 20 pre-test trial pairs, 160 training trial pairs, and 20 post-test trial pairs. All conditions of the task used at least 95% training-congruent word trials to train attention towards the positive or the negative. Because differences between the two types of each training type were minimal (i.e., different orders), they were collapsed into two categories for analyses: an overall positive and an overall negative training category. All of the words were selected from the Affective Norms for English Words (ANEW) manual (Bradley & Lang, 1999) and paired so that they differed only on their valence and arousal. Negative words were indeed significantly lower in valence (M = 2.15, SD = .32), t(298) = −45.65, p < .001, and positive words were significantly higher in valence (M = 7.71, SD = .38), t(298) = 39.96, p < .001, than neutral words (M = 5.08, SD = .60). In addition, both negative (M = 5.57, SD = .82, t(298) = 12.67, p < .001) and positive words (M = 5.50, SD = .99, t(298) = 11.10, p < .001) were significantly more arousing than neutral words (M = 4.33, SD = .79), though the positive and negative words were equally arousing.
Negative Visual Stimuli (Visual Stressor Task)
Two eye-tracking sessions were used: the first provided a pre-training baseline measure of gaze patterns, and the second, after the attentional training task, assessed effects of the training. Seventeen distinct negatively-valenced International Affective Picture System images (IAPS: CSEA-NIMH, 1999) were randomly interspersed with 96 trials of synthetic face pairs (computer-generated faces expressing no emotion paired with the same face expressing an emotion: see Isaacowitz et al., 2006) for each eye-tracking session. The negatively valenced images in each eye-tracking session were designed to be moderately unpleasant and stressful (e.g., a needle stuck in excrement), serving as the most critical trials for examining differences in gaze patterns as a function of age differences in emotion regulation. All images were presented for 4 seconds and centered on the screen with a gray background so that individuals could attend to the images or to the background. Each participant viewed these images in different random orders. Valence and arousal ratings of the IAPS images were taken from the IAPS manual (Lang, Bradley, & Cuthbert, 1999). Luminance levels of the images were measured using Adobe Photoshop CS3 Extended version 10.0.1 photo editing software (Adobe Systems, Inc., San Jose, CA). The pre-training and post-training images did not differ in their content, valence, arousal, or luminance levels.
In order to test our hypotheses concerning age differences in fixation to unpleasant stimuli before and after the training (i.e., to what extent the training transferred to the task of viewing unpleasant images), it was important to assess fixation patterns, not only to the entire unpleasant image, but to the most negative areas within each image as well. Each IAPS image contains both actively negative parts as well as less negative or even neutral parts; fixations to these different parts would likely have differential impact on mood. Therefore, we created areas of interest (AOIs) to include the most negative areas within each IAPS image (see also Wadlinger & Isaacowitz, 2008). First, a member of the research team evaluated each image and applied a preliminary AOI to the most negative part of it. A pilot sample of 15 raters who were unaware of the study’s hypotheses then rated these AOIs and the remaining parts of each image on their valence and arousal levels using a 9-pt scale on which higher scores indicate positive valence and higher arousal. Paired t-tests comparing the valence of these AOIs to the rest of the images revealed that the AOIs were significantly more negative (M = 3.29, SD = .56) than the remaining areas (M = 4.58, SD = .60), t(33) = −10.05, p < .001, Cohen’s d = −3.50. Furthermore, paired t-tests comparing the arousal ratings of these AOIs to the rest of the images revealed that the AOIs were significantly more arousing (M = 5.32, SD = .65) than the remaining areas (M = 4.06, SD = .60), t(33) = 8.45, p < .001. Comparisons of these AOIs used in the pre-training presentation to those used in the post-training presentation showed that the valence and arousal ratings did not differ between presentations, all p’s > .62. Thus, mean percent fixation to the most negative areas of the IAPS images were computed using these AOIs.
Equipment
An Applied Science Laboratories Model D6 Eye Tracker (Bedford, MA) with facial recognition software was used to record eye movements at a rate of 60Hz. A fixation is defined as an interval in which gaze is focused within 1° visual angle for 100ms or more (Manor & Gordon, 2003). In addition to recording fixations to the most negative areas within, and to the entire, IAPS image, fixations to the gray background bordering the images were coded as “off” fixations. GazeTracker software (EyeTellect, LLC., Charlottesville, VA) was used to present and randomize stimuli on a 17″ display. A potentiometer slider2 and Realterm recording software (Empirisoft Corporation, New York, NY) were used to record participants’ continuous moods (from 0 to 100, best) at a rate of every 1.0 second while participants were viewing the picture stimuli. MATLAB software (The MathWorks, Inc., Natick, Massachusetts) was used to present the dot-probe task.
Mood Measures
Participants were asked to report their moods continuously on the potentiometer slider, which allowed us to extract participants’ specific mood ratings in real-time as they viewed each individual IAPS image. Participants generally tended to look at the images first, and then used the slider to indicate their moods. Therefore, we extracted the mood ratings made during the last second of each image’s presentation to ensure that the participants had enough time to view the image (shown for 4 seconds) and indicate the change in their mood as a function of the image using the slider. These individual mood ratings to each IAPS image were averaged (over the entire second of recording for each slide) to create pre-training IAPS mood ratings and post-training IAPS mood ratings yoked to when the slides were viewed.
Procedure
After providing informed consent, participants reported their age, years of education and rated their current health. Next, participants completed several standard affective measures that were included as covariates in models testing training effects. The Center for Epidemiologic Studies Depression Scale (CES-D: Radloff, 1977) was used to obtain self-reports of depressive symptoms (α = .85). The Life Orientation Test (LOT: Scheier & Carver, 1985) assessed dispositional optimism. The Neuroticism Questionnaire (N-Questionnaire: Bolger & Schilling, 1991) assessed the personality trait of neuroticism. The State-Trait Anxiety Inventory (STAI: Spielberger, Gorsuch, Lushene, Vagg, & Jacobs, 1983) measured state (α = .91) and trait levels of anxiety (α = .90). The Positive and Negative Affect Scale (PANAS: Watson, Clark, & Tellegen, 1988) assessed levels of positive affect (α = .85) and negative affect (α = .85).
Next, participants completed several standard cognitive measures to account for any normative age-related declines in cognitive ability. The Forward Digit-Span and Backward Digit-Span subtests of the Wechsler Adult Intelligence Scale–Revised (Wechsler, 1981) were administered to assess short-term memory. The Digit Symbol Substitution subtest of the Wechsler Adult Intelligence Scale–Revised (Wechsler, 1981) was used to obtain a measure of processing speed. The Mini-Mental State Examination (MMSE: Folstein, Folstein, & McHugh, 1975) was used as a screening tool for symptoms indicative of dementia. Finally, the Shipley Vocabulary Test (Zachary, 1986) was included as a measure of crystallized intelligence.
Next, participants were administered the Snellen vision test for distance acuity, Rosenbaum for near-vision (Rosenbaum, 1984), and Pelli-Robson Contrast Sensitivity Chart (Pelli, Robson & Wilkins, 1988) to assess adequate visual acuity for eye tracking.
After the demographic, affective, cognitive and visual acuity measures, participants were seated in front of the eye tracker and a 17-point calibration was administered to ensure accurate gaze measurement. Participants then rated their current mood from 0 to 100 (best) using the potentiometer slider. After the initial baseline mood measure, participants were asked to use the slider to rate their moods continuously while watching the following eye tracking presentation “naturally, as if they were watching TV at home.” This first eye tracking session (the baseline visual task) was designed to assess their pre-training gaze patterns and emotional responses to negatively-valenced visual stimuli.
After the first eye tracking session, participants were randomly assigned to either a positive-training or negative-training condition and began their attentional training task. Next, after ensuring accuracy of gaze measurement with another 17-pt calibration, participants began their second eye-tracking session, again with the same instructions to watch naturally and to rate their moods continuously using the slider while watching the presentation. Upon completion of the post-training eye tracking, participants indicated their current moods for the last time and completed the Attention Network Test (ANT: Fan, McCandliss, Sommer, Raz, & Posner, 2002) designed to assess three attentional processes: alerting, orienting, and executive control.
Analytic Plan
To discriminate between the three possible accounts of malleability described above, the first step of the analytic plan was to determine whether the training had any impact on gaze patterns, and whether that varied by age group. This was examined with a 2 (age group, young vs. older adults) × 2 (training time, pre vs. post) × 2 (training type, positive vs. negative)mixed-design analysis of variance (ANOVA) on fixation to the most arousing areas of the negative images, with follow-up analyses as appropriate to assess the magnitude and direction of training effects on gaze patterns. To determine whether gaze training impacted mood, we conducted similar analyses focused on change in mood (as rated on the potentiometer slider) from before the training to after the training.
Results
Demographic, Affective and Cognitive Functioning Measures
All participants were native English speakers or had equivalent fluency, with normal or corrected-to-normal vision. In general, both young and older adult participants reported “good” or better present health states. Older adults had higher levels of education, higher scores on the Shipley vocabulary test, higher levels of dispositional optimism, higher positive affect, lower negative affect, lower depressive symptoms, lower levels of neuroticism, lower state and trait anxiety levels, slightly lower scores on the Mini Mental State Exam, slower reaction times to the Attention Network Test, and slightly worse visual acuity and worse contrast-sensitivity than young adults. The specific means and response scales of these measures are noted in Table 1.
Table 1.
Mean Scores on the Demographic, Affective, and Cognitive Functioning Measures and Tests of Differences Between Age Groups
| Measure | Young Adults | Older Adults | Age Difference |
|---|---|---|---|
| Self-rating of health | 3.99 (.87) | 3.61 (.94) | t(127) = 5.66* |
| Years of education | 13.59 (1.07) | 16.10 (2.14) | t(127) = −8.55*** |
| Snellen visual acuity | 22.68 (6.92) | 34.18 (13.39) | t(127) = −6.22*** |
| Rosenbaum near vision | 20.59 (2.03) | 28.44 (10.23) | t(127) = −6.20*** |
| Pelli-Robson contrast sensitivity | 1.64 (.11) | 1.55 (.16) | t(127) = 4.04*** |
| ANT alerting effect (RT in ms) | 40.52 (26.79) | 35.25 (33.85) | t(124) =.97 |
| ANT orienting effect (RT in ms) | 33.21 (17.97) | 40.46 (29.27) | t(124) = −1.70 |
| ANT conflict effect (RT in ms) | 150.58 (132.89) | 168.69 (106.18) | t(124) = −.84 |
| ANT mean RT (ms) | 539.13 (59.41) | 791.24 (78.56) | t(124) = −20.46*** |
| ANT mean accuracy (percentage correct) | 97.61 (1.75) | 97.68 (2.22) | t(124) =−.19 |
| WAIS Forward Digit Span | 7.60 (1.14) | 7.20 (1.09) | t(127) = 2.07* |
| WAIS Backward Digit Span | 5.51 (1.30) | 5.41 (1.33) | t(127) = .45 |
| WAIS Digit Symbol Substitution | .48 (.08) | .50 (.14) | t(125) = −1.18 |
| MMSE (number correct, out of 30) | 29.50 (1.53) | 28.61 (1.42) | t(127) = 3.43*** |
| Shipley Vocabulary Test (number correct, out of 21) | 13.94 (2.16) | 16.08 (2.11) | t(126) = −5.67*** |
| CES-D | 14.14 (9.72) | 9.40 (5.97) | t(119) = 3.18** |
| N-questionnaire | 15.07 (2.85) | 13.41 (2.20) | t(127) = 3.68*** |
| LOT dispositional optimism | 20.66 (5.32) | 22.93 (4.29) | t(126) = −2.65** |
| STAI state anxiety | 35.87 (9.67) | 27.70 (6.24) | t(127) = 5.62*** |
| STAI trait anxiety | 38.47 (10.29) | 32.25 (7.40) | t(127) = 3.91*** |
| PANAS positive affect | 30.75 (7.92) | 33.51 (6.39) | t(127) = −2.16* |
| PANAS negative affect | 15.76 (5.58) | 11.97 (2.94) | t(127) = 4.76*** |
Note. The sample consisted of 68 young adults (25 men, 43 women; mean age = 19.37, SD = 1.63, range: 18–25) and 61 older adults (13 men, 48 women; mean age = 72.44, SD = 7.23, range: 61–90). Means are given for trackable participants only (see Method). The tests used were as follows: Self-reported current health, ranging from 1 (poor) to 5 (excellent). Snellen chart for visual acuity. Rosenbaum Pocket Vision Screener for near vision (Rosenbaum, 1984). Pelli-Robson Contrast Sensitivity Chart (Pelli, Robson & Wilkins, 1988). Attention Network Test (ANT: Fan, McCandliss, Sommer, Raz, & Posner, 2002). Wechsler Adult Intelligence Scale–Revised (WAIS; Wechsler, 1981). Mini-Mental State Examination (MMSE: Folstein, Folstein, & McHugh, 1975). Shipley Vocabulary Test (Zachary, 1986), means reflect number of correct responses of 21. Center for Epidemiologic Studies Depression Scale (CES-D: Radloff, 1977). Neuroticism Questionnaire (N-Questionnaire: Bolger & Schilling, 1991). Life Orientation Test (LOT: Scheier & Carver, 1985). State-Trait Anxiety Inventory (STAI: Spielberger, 1983). Positive and Negative Affect Schedule (PANAS: Watson, Clark, & Tellegen, 1988). Missing responses are due to technical failures and/or participants not complying with task instructions for proper assessment. All measures except the ANT were completed before eye tracking. RT = reaction time.
p < .001.
p < .01.
p <.05.
Eye Tracking: Did Training Change Gaze Patterns?
To assess to what extent gaze preferences to negative IAPS images varied as a function of age and training, we conducted a 2 × 2 × 2 mixed-design ANOVA, using percent fixations to the most negative areas of the IAPS images as the dependent variable, with 3 factors: age group (between-participants: young, older), time (within-participant: pre-training, post-training), and attentional training type (between-participants: negative, positive). A significant main effect of time emerged, indicating that the average percent fixations to the most negative areas of the IAPS images generally decreased from pre-training (M = 52.01, SD = 11.28) to post-training (M = 43.05, SD = 9.40), F(1,120) = 113.97, p < .001, ηp2 = .487. A significant main effect of age group revealed that young adults generally looked more at the most negative areas of the IAPS images (M = 49.82, SD = 9.78) than did older adults (M = 44.84, SD = 10.40), F(1,120) = 9.63, p < .01, ηp2 = .074. No main effect of training was found, F(1,120) = 1.80, p > .05. No significant age × time interaction was found, F(1,120) = .01, p > .05, and no significant training × time interaction was found, F(1,120) = .40, p > .05. However, the 3-way age × training × time interaction was significant, F(1, 120) = 5.65, p < .05, ηp2 = .045, revealing an interesting pattern of fixation as a function of age and attentional training. As shown in Figure 1, young adults generally fixated more on the most negative areas of the IAPS images than did older adults. Consistent with our hypotheses, the greatest drop in fixation among older adults is seen in the group that received positive training. However, unexpectedly, the greatest drop in fixation among young adults is seen in the group that received negative training.
Figure 1.

Fixations to the most negative areas of the IAPS images from pre-training to post-training, for each training condition and age group.
To understand the nature of this interaction, we examined the means across training groups and within age groups. We found no significant difference at pre-training between the negative and positive training conditions for young adults, F(1, 120) = .28, p > .05, and older adults, F(1, 120) = 2.58, p > .05. Nonetheless, because the older adult groups seemed more different from each other at pre-training than did the young adult groups, we created change in fixation scores from pre-training to post-training. We reasoned that change scores could be especially informative in assessing which groups showed the most change in their gaze patterns, and in what direction. Univariate analysis of variance conducted on the change in fixation to the most negative areas of the IAPS images found a significant age × training interaction, F(1, 120) = 5.65, p < .05, ηp2 = .045. Within the young adult group, there was a significant difference in change scores between those in the train-negative and train-positive conditions, F(1, 65) = 4.60, p < .05, ηp2 = .07, though the difference between training conditions was not significant in the older adults, F(1, 55) = 1.54, p > .05. These results are shown in Figure 2. Of those who received negative attentional training, young adults looked more away from the most negative areas (M = −11.41, SD = 9.48) than their older counterparts (M = −7.60, SD = 9.81). Of those who received positive attentional training, older adults looked more away (M = −10.54, SD = 8.60) from the most negative areas than their younger counterparts (M = −6.35, SD = 9.28). Thus, there was evidence that young adults were more responsive to a negative training; the evidence was not as strong for older adults, but suggested they were more responsive to a positive training. These results are broadly consistent with the differential malleability hypothesis, as the most effective type of training varied by age.
Figure 2.
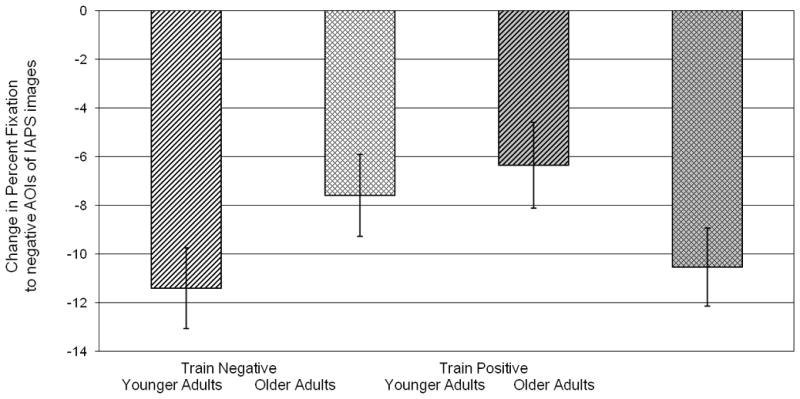
Change in percent fixation to the negative areas of the IAPS images from pre-training to post-training, for each training condition and age group, such that negative values indicate decreased fixation in post-training compared to pre-training. Error bars depict standard errors of the means.
We also considered change patterns in viewing toward the entire negative image, in order to assess whether the training influenced gaze patterns beyond fixations to the most negative areas of the images.3 Looking less at the entire images after training would be indicative of increased looking at the background of the slides. Univariate analysis of variance conducted on change scores to the entire image revealed a significant age × training interaction, F(1, 120) = 7.35, p < .01, ηp2 = .058. As shown in Figure 3, for the negative training groups, older adults had almost no change in gaze (M = −.95, SD = 10.18) compared to young adults, who looked less at the images after training than they did before training (M = −2.95, SD = 7.68). In contrast, within positive training, older adults had negative change scores (M = −3.85, SD = 8.08) whereas young adults actually had positive change scores (M = 2.64, SD = 8.79).
Figure 3.

Change in percent fixation to the entire IAPS images from pre-training to post-training, for each training condition and age group, such that negative values indicate decreased fixation in post-training compared to pre-training. Similarly, positive values indicate increased fixation in post-training compared to pre-training. Error bars depict standard errors of the means.
The original 2 (age group) × 2 (time) × 2 (training type) ANOVA on fixation to the most negative areas of the images were re-run including variables in Table 1 as covariates. Including demographic, vision measures, and attentional functioning as covariates in the model did not yield any significant covariates. Including affective and cognitive measures as covariates in the model revealed some significant covariates; however, patterns of interest remained the same after including these covariates in the models. For example, state anxiety, F(1, 106) = 4.13, p < .05, ηp2 = .037, positive affect, F(1, 106) = 6.10, p < .05, ηp2 = .054, and neuroticism, F(1, 106) = 4.16, p < .05, ηp2 = .038, emerged as significant covariates, but the main effect of age group, F(1, 106) = 5.74, p < .05, ηp2 = .051, and the 3-way interaction of time × age × training, F(1, 106) = 4.68, p < .05, ηp2 = .042, remained significant, whereas the main effect of training, F(1, 106) = .05, p > .05, and the age × training interaction remained nonsignificant, F(1, 106) = .55, p > .05. Furthermore, scores on the Shipley vocabulary test emerged as a significant covariate, F(1, 111) = 8.50, p < .01, ηp2 = .071, but the 3-way interaction of time × age × training remained significant, F(1, 111) = 4.99, p < .05, ηp2 = .043, and the main effect of training, F(1, 111) = 1.20, p > .05, and the age × training interaction remained nonsignificant, F(1, 111) = .44, p > .05.
Finally, due to the seemingly robust nature of the age differences in gaze patterns, we assessed whether the magnitude of these age differences changed over time following the training. We divided the post-training IAPS images into those that appeared in the first half of the slide show and those in the second half (each half was approximately 4 minutes long). There were significant differences between age groups in fixation to the most negative areas in the second half (Mdiff = 6.82, SE = 2.58, t(120) = 2.65, p < .01), and to the entire negative images in both halves (first half: Mdiff =4.98, SE = 1.88, t(83.79)= 2.65, p = .01, second half: Mdiff = 5.84, SE = 2.24, t(83.14) = 2.60, p < .05) 4. This suggests that, if anything, age differences were consistent or rebounded as time passed after training.
Mood Measures: Did Training Change Mood While Viewing Negative Images?
In order to assess the effects of gaze on mood patterns, we conducted independent samples t-tests comparing the moods reported while watching the IAPS images in each presentation for each training condition, separately by age group. For young adults, no significant differences between the pre-training and post-training IAPS yoked moods were found for the different training conditions, all p’s > .54. For older adults, no significant differences in pre-training IAPS yoked moods were found between the positive and negative training groups, t(56) = 1.80, p > .05; however, a significant difference did emerge for the IAPS yoked moods during the second presentation post-training, t(54) = 2.16, p < .05, Cohen’s d = .58. Older adults in the train positive group had higher mood ratings (M = 52.05, SD = 15.07) than their peers in the train negative group (M = 43.69, SD = 13.66). In fact, older adults in the train negative group had the lowest moods post-training when compared to the three other groups (older adult positive, younger adult positive, and younger adult negative) in a contrast analysis (M = 52.06, SD = 17.22), t(116) = 2.29, p < .05, Cohen’s d = .43.
When these analyses were re-run including variables from Table 1 as covariates, no demographic, cognitive (including ANT), or affective variable emerged as a significant covariate, all p’s > .10.
To parallel the temporal analyses described above for gaze patterns, we also considered change in mood from the first to second half of the time period after training. While moods on average dropped from the beginning of the post-training period (M = 51.31, SD = 15.59) to the end (M = 48.30, SD = 17.13), t(118) = −3.76, p < .001, Cohen’s d = .18, there were age differences in the extent to which this was true. Whereas the moods of young adults in both training groups dropped significantly from the start of the post-training period to the end of it (t’s > −2.17, p’s < .04), and older adults in the negative condition seemed to as well (Mdiff = −2.83, t(24) = −1.85, p < .08), older adults in the positive training condition did not show mood declines during this period (p > .48).
Discussion
The current study extends past work on attentional training in young adults to consider whether age-related positive gaze preferences could be shifted with training and whether such shifts in fixation would also produce changes in mood. Our findings most clearly supported a differential malleability account in terms of the impact of the training on downstream gaze patterns relevant to emotion regulation. Young adults who received negative dot-probe training actually showed lessened looking to the highly arousing negative images after training, as compared to their age peers. In contrast, the effects of the training on gaze patterns were not as clear-cut for the older adults, although it appeared that the positive training led to larger changes in viewing patterns toward arousing, negative images than did the negative training for them.
Changes in mood from before to after training were found as well, primarily for the older adult participants. Older adults who received the negative training felt worst when viewing the negative images after training: their mood continued to decline from the start to the end of the period after training. In contrast, older adults in the positive training condition showed stable mood during the post-training trials. Therefore, the overall pattern of findings suggest that the training seemed to have relatively more effect on the gaze patterns of young adults, but on the moods of older adults.
Considering the three differing theoretical perspectives presented above, the no malleability account can be ruled out, because some (though not all) older adults showed shifts in gaze as a function of training. The fact that the older adult negative training group did not show change in looking at entire IAPS images from pre to post-training, and that the young adult positive training group looked more at entire negative images after training, also speaks against the possibility that the training had no impact and any change was due to other factors such as fatigue or stimulus differences in the two picture sets. Similarly, the full malleability account also does not provide a close match to the results, as the pattern of training effects on gaze differed by age and showed age-specific constraints.
Differential Malleability by Age
Why would older adults not respond well to a negative training, while young adults respond to the negative training by actually looking less at negative images? Such differential malleability can be explained by considering the different emotion-relevant processing preferences that have been identified in the two age groups. The notion that different aspects of emotional processing may be differentially salient to young and older adults was part of the original formulation of socioemotional selectivity theory (e.g., Carstensen & Turk-Charles, 1994; Isaacowitz, Charles, & Carstensen, 2000). Older adults’ negative response to negative, and perhaps better response to positive, training is consistent with more recent work in the socioemotional selectivity theory tradition finding that older adults may display “positivity effects” in their information processing, favoring positively- over negatively-valenced stimuli (e.g., Carstensen & Mikels, 2005). Evidence for such positivity effects has consistently been found in eye tracking studies: older adults demonstrate preferences in their sustained attention for positive material, and they tend to look away from negative material (e.g., Isaacowitz et al., 2006a, 2006b). A training paradigm in which the probe appeared behind the more positive word in positive-neutral and negative-neutral word pairs is a good match to the natural looking patterns of older adults and seems to have grabbed their attention most. Exposure to a training that reinforced their existing patterns seems to further develop their looking preference away from the negative, whereas exposure to a training that varied from their natural patterns seems to have worsened their mood. These findings also mesh well with recent work using different types of learning paradigms, primarily involving monetary incentives, in which older adults appear to learn more from positive than negative feedback, in contrast to the younger adult pattern (Denburg et al., 2006; Eppinger et al., 2008; Wood et al., 2005).
On the surface, our finding regarding young adults and negative training seems surprising: a training directing their attention to more negative stimuli actually made them look less at arousing negative pictorial stimuli later. However, the fact that young adults were more susceptible to a negative training in the first place is consistent with a large research tradition, almost exclusively conducted in young adult samples, demonstrating “negativity dominance” (e.g., Baumeister, Bratslavsky, Finkenauer, & Vohs, 2001; Rozin & Royzman, 2001). In that research, (young adult) participants tend to show greater responses of all sorts to negative as compared to neutral and positive stimuli. Thus, our finding that young adults were most responsive to negative training is consistent with a negativity dominance effect in young adults.
However, this does not explain why training to direct attention toward negative words led young adults to actually look less at arousing negative images after training. Rather than making them more susceptible to later visual stressors, negative training seems to have habituated the young adults to negative content, making them less interested in engaging with negative content in the visual stress task shortly after the training. Our findings are somewhat at odds with those of Wadlinger and Isaacowitz (2008), in which a positive attentional training led young adults to look less at arousing negative images post-training. There are several possible reasons for these different findings. First, in the Wadlinger and Isaacowitz study, participants were trained only to positive-neutral word pairs, and no negative words were included in the training. The presence of negative words seems to dramatically change the character of the training effects, perhaps due to negativity dominance effects that prioritize young adults’ processing of negative material. Another difference was that the current study used significantly fewer training trials than did the Wadlinger and Isaacowitz (2008) study. We may have encountered habituation effects in the current paradigm (e.g, Dijksterhuis & Smith, 2002); some previous work has found that affective recovery can follow affective habituation (Britton, Shin, Barrett, Rauch, & Wright, 2008), so the specific number of training trials may distinguish habituated from activated responses to the trained stimuli. While these effects require further investigation, it is notable that including negative stimuli in the training seems to change the effects of the training for young adults. Future work will need to consider the “fit” of training to person, as well as to what extent training effects are due to habituated or activated responses to targets depending on features of the training itself.
Robustness of the Overall Age Difference
It is important to note that, despite changes in looking patterns due to training that varied by age group, the end result was in one key sense similar to the pre-test: in both cases, older adults looked less at the negative areas of negative images than did young adults. The training was apparently not enough to eliminate the overall main effect of age on fixation. This finding suggests an important caveat to the potential malleability of age-related gaze patterns, such that the overall age difference seems resistant to this particular indirect method of training. What might this mean about the origins and functions of such age-related preferences? One possibility is that age-related declines in neural functioning predispose older adults to look less at negative images, which would be consistent with the Aging Brain Model (Cacioppo et al., in press); in this account, no behavioral training can override this neurally-based tendency to be less reactive to high-arousal negative stimuli. However, the robust age effect does not necessarily imply that there is no plausible role for motivation. It is also possible that the goal of avoiding negative information that is predicted by socioemotional selectivity theory has become so chronically activated, or well-practiced, for older adults that they cannot easily shift away from this pattern. Nonetheless, a recent study by Urry et al. (2009) found that older adults’ neural activation to negative stimuli changes as a function of instructions (such as to increase negative response), suggesting malleability of neural function – including the amygdala – as well.
According to temporal analyses, these age differences are, if anything, stronger in the time period later after the training has ended than they are immediately after the end of the training; this suggests a possible rebounding effect from any training and reinforces the resilience of the age difference in gaze patterns, at least in the current paradigm.
Nonetheless, it should be noted that our findings do not imply that older adults’ gaze patterns can never be changed; in fact, we do find evidence for some malleability in gaze for both age groups in our paradigm, which is based on the premise that dot-probe attention training can transfer to other aspects of regulation-relevant emotional processing (Wadlinger & Isaacowitz, 2008; see also MacLeod et al., 2002). The direction of these changes varies as a function of age, however. Furthermore, the robustness of the age difference even after this indirect training is suggestive that some aspects of older adults’ positive gaze preference may be resistant to training, thus providing a potential constraint on the range of possible development, but that conclusion should be considered tentative until other types of training paradigms (perhaps including more explicit ones that actually direct participants to move their gaze toward negative stimuli) are attempted as well.
Mood Effects
We found some evidence that training affected mood states experienced by individuals as they viewed the unpleasant negative images. In particular, the older adults in the negative training condition showed a rather negative mood response to viewing unpleasant images after the training. One possibility is that older adults find engaging with some types of negative material to be particularly aversive (e.g., Blanchard-Fields, 2007); the negative training kept them focused on negative material, and this continued through the visual stressor task, and made them feel bad. While evidence suggests older adults can regulate how they feel in tense situations (e.g., Birditt, Fingerman, & Almeida, 2005), it is likely that the tools they use involve minimizing their exposure to the negative. Our training may have overridden these usual tools, leading older adults to engage with more negative material than they are used to, and thus affecting their mood. Only recently have studies of aging and emotion regulation started to use actually reported affect as a dependent variable, so future research will need to clarify under what conditions older adults do actually develop negative mood states despite overall age differences in regulation (e.g., Stanley & Isaacowitz, 2009).
Limitations and Conclusions
Several limitations of the current study warrant mention. First, the attentional training procedure was certainly short-term in nature, and only potential short-term effects of it were assessed. A longer-term training paradigm might lead to more enduring effects on both gaze and mood. In terms of the visual stressor task, our use of singly-presented images, while consistent with previous work (e.g., Wadlinger & Isaacowitz, 2008), may have constrained variability in fixation patterns. Using paired stimuli might have produced greater variability in gaze to the unpleasant images, and may have resulted in larger training effects. We chose a 4-second time interval for stimulus presentation based on previous work in our lab (e.g., Isaacowitz et al., 2008); using different time intervals, or further decomposing the 4-seconds into smaller time increments (e.g., Isaacowitz, Allard, Murphy, & Schlangel, 2009), might provide a more nuanced view of the time course of trained gaze effects. The fact that all older participants were paid, whereas only some younger participants received monetary payment, may have introduced differential task framing and/or motivation. Finally, we only considered gaze toward negative stimuli; future research should consider training effects for positive stimuli as well.
These findings have implications for the design of interventions to foster more positive looking patterns in individuals who, for some reason, do not show them naturally. This is especially important because distraction-based emotion regulation techniques seem useful, as well as less effortful, than some other ways of regulating emotions, such as reappraisal (see also Wadlinger & Isaacowitz, in press). For young adults, exposure to the negative may actually facilitate long-term positive gaze preferences, such that later arousing, negative stimuli are engaged with less visually. This suggests that exposure-based interventions could lead to long-term use of distraction for emotion regulation by young adults. However, such exposure could actually be deleterious for older adults. For them, engaging with the negative seems to lead to worsened mood over time. Overall, training paradigms appear to require a match to, and perhaps an extension of, naturalistic viewing patterns.
The current study demonstrates that gaze patterns to unpleasant, arousing images can be shifted to some degree using a training paradigm involving focusing attention on emotional words. Thus, affective training that changes gaze is possible, even in older adults. It is notable that such effects emerged even after a relatively brief training. However, the nature of these effects varied by age: to the extent that looking less at these negative images can be considered adaptive (e.g., Wadlinger & Isaacowitz, 2008), the negative training was more adaptive for the young adults but seemed maladaptive for the older adults. This finding was qualified, though, by an overall robust age effect, such that older adults looked less at the unpleasant images before and after training. Thus, there is malleability, but the nature of it varies by age, and it is constrained by overarching age differences in level of gaze to unpleasant images. The malleability findings are consistent with motivational effects on gaze, as motivational effects would require gaze flexibility so that goals can actually shift gaze patterns during real-time viewing. The robust nature of the overall age effect could be taken as support for the Aging Brain Model, such that aging may be associated with decreased neural reactivity to negative stimuli, and the lessened gaze patterns to negative stimuli simply reflect these underlying neural changes. However, such patterns could also result from chronic top-down deployment of attention in mood-congruent ways (e.g., Kryla-Lighthall & Mather, 2009). It will remain for future research to distinguish between these two potential explanations; the current findings set the stage for making such distinctions and further clarifying how aging acts on attentional systems involved in affect and its regulation.
Acknowledgments
This work was supported by National Institute on Aging Grant R01 AG026323 to DMI. The authors thank Kaitlin Toner and members of the Emotion Lab for their assistance with the running of this project, as well as Gregory Samanez-Larkin for his valuable feedback.
Footnotes
The seven nontrackable young adults reported slightly higher mean years of education than their trackable peers, Mdifference = 1.27, t(73) = 2.74, p < .01, as these nontrackable YAs were upperclassmen whereas the majority of the trackable YAs were underclassmen. No other demographic, affective, cognitive and visual measures were significantly different between trackable and nontrackable young adults. Nontrackable older adults had lower mean conflict effect scores on the ANT task (M = 114.16, SD = 88.55) than their trackable counterparts (M = 168.69, SD = 106.18), F(1,77) = 4.09, p < .05, and reported fewer depressive symptoms as measured by the CESD (M = 6.37, SD = 4.88) than their trackable peers (M = 9.40, SD = 5.97), F(1,75) = 4.00, p < .05.
A potentiometer slider has been used in previous studies (e.g., Isaacowitz et al., 2009) to record and measure participants’ self-reports of mood continuously in real-time. The potentiometer slider is comprised of a rectangular black box and a raised button marked with a notch that slides from one side to the other. Participants were instructed to slide this button horizontally along a continuum, such that moving the notch to the left most side would indicate that they are feeling “extremely unpleasant” (mood recorded as “0”) and moving the notch to the right-most side would indicate that they are feeling “extremely pleasant” (mood recorded as “100”), with a midpoint for feeling “neutral” (mood recorded as “50”). Participants were encouraged to use the full possible range of responses between the endpoints.
We also examined patterns in viewing the entire negative image using the raw fixation scores. The results showed a similar pattern, but did not reach statistical significance.
The degrees of freedom are adjusted due to unequal variances.
Publisher's Disclaimer: The following manuscript is the final accepted manuscript. It has not been subjected to the final copyediting, fact-checking, and proofreading required for formal publication. It is not the definitive, publisher-authenticated version. The American Psychological Association and its Council of Editors disclaim any responsibility or liabilities for errors or omissions of this manuscript version, any version derived from this manuscript by NIH, or other third parties. The published version is available at www.apa.org/pubs/journals/EMO
References
- Allard ES, Isaacowitz DM. Are preferences in emotional processing affected by distraction? Examining the age-related positivity effect in visual fixation within a dual-task paradigm. Aging, Neuropsychology, and Cognition. 2008;15:725–743. doi: 10.1080/13825580802348562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baltes P. Theoretical propositions of life-span developmental psychology: On the dynamics between growth and decline. Developmental Psychology. 1987;23:611–626. [Google Scholar]
- Baumeister RF, Bratslavsky E, Finkenauer C, Vohs KD. Bad is stronger than good. Review of General Psychology. 2001;5:323–370. [Google Scholar]
- Birditt KS, Fingerman KL, Almeida DM. Age differences in exposure and reactions to interpersonal tensions: A daily diary study. Psychology and Aging. 2005;20:330–340. doi: 10.1037/0882-7974.20.2.330. [DOI] [PubMed] [Google Scholar]
- Blanchard-Fields F. Everyday problem solving and emotion: An adult developmental perspective. Current Directions in Psychological Science. 2007;16:26–31. [Google Scholar]
- Bolger N, Schilling E. Personality and the problems of everyday life: The role of neuroticism in exposure and reactivity to daily stressors. Journal of Personality. 1991;59:355–386. doi: 10.1111/j.1467-6494.1991.tb00253.x. [DOI] [PubMed] [Google Scholar]
- Bradley MM, Lang PJ. Affective norms for English words (ANEW): Instruction manual and affective ratings (Tech. Rep. No. C-1) Gainesville: Center for Research in Psychophysiology, University of Florida; 1999. [Google Scholar]
- Britton JC, Shin LM, Barrett LF, Rauch SL, Wright CI. Amygdala and fusiform gyrus temporal dynamics: Responses to negative facial expressions. BMC Neuroscience. 2008;9:44–49. doi: 10.1186/1471-2202-9-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cacioppo JT, Berntson GG, Bechara A, Tranel D, Hawkley LC. Could an aging brain contribute to subjective well being?: The value added by a social neuroscience perspective. In: Tadorov A, Fiske ST, Prentice D, editors. Social Neuroscience: Toward understanding the underpinnings of the social mind. New York: Oxford University Press; in press. [Google Scholar]
- Carstensen LL. The influence of a sense of time on human development. Science. 2006;312:1913–1915. doi: 10.1126/science.1127488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carstensen LL, Isaacowitz DM, Charles S. Taking time seriously: a theory of socioemotional selectivity. American Psychologist. 1999;54:165–181. doi: 10.1037//0003-066x.54.3.165. [DOI] [PubMed] [Google Scholar]
- Carstensen LL, Mikels J. At the intersection of emotion and cognition: Aging and the positivity effect. Current Directions in Psychological Science. 2005;14:117–121. [Google Scholar]
- Carstensen LL, Turk-Charles S. The salience of emotion across the adult life span. Psychology and Aging. 1994;9:259–264. [PubMed] [Google Scholar]
- Center for the Study of Emotion and Attention [CSEA-NIMH] International affective picture system: Digitized photographs. Gainsville, FL: The Center for Research in Psychophysiology, University of Florida; 1999. [Google Scholar]
- Denburg NL, Recknor EC, Bechara A, Tranel D. Psychophysiological anticipation of positive outcomes promotes advantageous decision-making in normal older persons. International Journal of Psychophysiology. 2006;61:19–25. doi: 10.1016/j.ijpsycho.2005.10.021. [DOI] [PubMed] [Google Scholar]
- Dijksterhuis A, Smith PK. Affective habituation: Subliminal exposure to extreme stimuli decreases their extremity. Emotion. 2002;2:203–214. doi: 10.1037/1528-3542.2.3.203. [DOI] [PubMed] [Google Scholar]
- Eppinger B, Kray J, Mock B, Mecklinger A. Better or worse than expected? Aging, learning, and the ERN. Neuropsychologia. 2008;46:521–539. doi: 10.1016/j.neuropsychologia.2007.09.001. [DOI] [PubMed] [Google Scholar]
- Fan J, McCandliss B, Sommer T, Raz A, Posner M. Testing the efficiency and independence of attentional networks. Journal of Cognitive Neuroscience. 2002;14:340–347. doi: 10.1162/089892902317361886. [DOI] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”: A practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Isaacowitz DM, Allard ES, Murphy NA, Schlangel M. The time course of age-related preferences towards positive and negative stimuli. Journal of Gerontology: Psychological Sciences. 2009;64:188–192. doi: 10.1093/geronb/gbn036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isaacowitz DM, Charles ST, Carstensen LL. Emotion and cognition. In: Craik FIM, Salthouse TA, editors. The Handbook of Aging and Cognition. 2. Mahwah, N.J: Lawrence Erlbaum Associates; 2000. pp. 593–631. [Google Scholar]
- Isaacowitz DM, Toner K, Goren D, Wilson H. Looking while unhappy: Mood-congruent gaze in young adults, positive gaze in older adults. Psychological Science. 2008;19:848–853. doi: 10.1111/j.1467-9280.2008.02167.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isaacowitz DM, Toner K, Neupert SD. Use of gaze for real-time mood regulation: Effects of age and attentional functioning. Psychology and Aging. 2009;24:989–994. doi: 10.1037/a0017706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isaacowitz DM, Wadlinger HA, Goren D, Wilson HR. Is there an age-related positivity effect in visual attention? A comparison of two methodologies. Emotion. 2006;6:511–516. doi: 10.1037/1528-3542.6.3.511. [DOI] [PubMed] [Google Scholar]
- Isaacowitz DM, Wadlinger HA, Goren D, Wilson HR. Selective preference in visual fixation away from negative images in old age? An eye tracking study. Psychology and Aging. 2006;21:40–48. doi: 10.1037/0882-7974.21.1.40. [DOI] [PubMed] [Google Scholar]
- Knight M, Seymour T, Gaunt J, Baker C, Nesmith K, Mather M. Aging and goal-directed emotional attention: Distraction reverses emotional biases. Emotion. 2007;7:705–714. doi: 10.1037/1528-3542.7.4.705. [DOI] [PubMed] [Google Scholar]
- Kryla-Lighthall N, Mather M. The role of cognitive control in older adults’ emotional well-being. In: Berngtson V, Gans D, Putney N, Silverstein M, editors. Handbook of Theories of Aging. 2. New York: Springer Publishing; 2009. pp. 323–344. [Google Scholar]
- Lang PJ, Bradley MM, Cuthbert B. International Affective Picture System (IAPS): Technical manual and affective ratings. Gainesville, FL: University of Florida, The Center for Research in Psychophysiology; 1999. [Google Scholar]
- Leclerc CM, Kensinger EA. Age-related differences in medial prefrontal activation in response to emotional images. Cognitive, Affective & Behavioral Neuroscience. 2008;8:153–164. doi: 10.3758/cabn.8.2.153. [DOI] [PubMed] [Google Scholar]
- MacLeod C, Rutherford E, Campbell L, Ebsworthy G, Holker L. Selective attention and emotional vulnerability: Assessing the causal bias of their association through the experimental manipulation of the attentional bias. Journal of Abnormal Psychology. 2002;111:107–123. [PubMed] [Google Scholar]
- Manor BR, Gordon E. Defining the temporal threshold for ocular fixation in free-viewing visuocognitive tasks. Journal of Neuroscience Methods. 2003;128:85–94. doi: 10.1016/s0165-0270(03)00151-1. [DOI] [PubMed] [Google Scholar]
- Mather M, Knight M. Goal-directed memory: The role of cognitive control in older adults’ emotional memory. Psychology and Aging. 2005;20:554–570. doi: 10.1037/0882-7974.20.4.554. [DOI] [PubMed] [Google Scholar]
- Pelli DG, Robson JG, Wilkins AJ. The design of a new letter chart for measuring contrast sensitivity. Clinical Vision Science. 1988;2:187–199. [Google Scholar]
- Radloff L. The CES-D scale: A self-report depression scale for research in the general population. Applied Psychological Measurement. 1977;1:385–401. [Google Scholar]
- Rosenbaum JG. The biggest reward for my invention isn’t money. Medical Economics. 1984;61:152–163. [Google Scholar]
- Rozin P, Royzman E. Negativity bias, negativity dominance, and contagion. Personality and Social Psychology Review. 2001;5:296–320. [Google Scholar]
- Samanez-Larkin GR, Gibbs SEB, Khanna K, Nielsen L, Carstensen LL, Knutson B. Anticipation of monetary gain but not loss in healthy older adults. Nature Neuroscience. 2007;10:787–791. doi: 10.1038/nn1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheier MF, Carver CS. Optimism, coping, and health: Assessment and implications of generalized coping expectancies. Health Psychology. 1985;4:219–247. doi: 10.1037//0278-6133.4.3.219. [DOI] [PubMed] [Google Scholar]
- Spielberger CD, Gorsuch RL, Lushene R, Vagg PR, Jacobs GA. Manual for the state-trait anxiety inventory. Palo Alto, CA: Consulting Psychologists Press; 1983. [Google Scholar]
- Stanley JT, Isaacowitz DM. Age-related differences in profiles of mood-change trajectories. Manuscript submitted for publication. 2009 doi: 10.1037/a0021023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urry HL. Seeing, thinking, and feeling: Emotion-regulating effects of gaze-directed cognitive reappraisal. Emotion. 2010;10:125–135. doi: 10.1037/a0017434. [DOI] [PubMed] [Google Scholar]
- Urry HL, van Reekum CM, Johnstone T, Davidson RJ. Individual differences in some (but not all) medial prefrontal regions reflect cognitive demand while regulating unpleasant emotion. NeuroImage. 2009;47:852–863. doi: 10.1016/j.neuroimage.2009.05.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wadlinger HA, Isaacowitz DM. Positive mood broadens visual attention to positive stimuli. Motivation and Emotion. 2006;30:89–101. doi: 10.1007/s11031-006-9021-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wadlinger HA, Isaacowitz DM. Fixing our focus: Training attention to regulate emotion. Personality and Social Psychology Review. doi: 10.1177/1088868310365565. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wadlinger HA, Isaacowitz DM. Looking happy: The experimental manipulation of a positive visual attention bias. Emotion. 2008;8:121–126. doi: 10.1037/1528-3542.8.1.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson D, Clark L, Tellegen A. Development and validation of brief measures of positive and negative affect: the PANAS scales. Journal of Personality and Social Psychology. 1988;54:1063–1070. doi: 10.1037//0022-3514.54.6.1063. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Manual for the Wechsler Adult Intelligence Scale–Revised. New York: Psychological Corp; 1981. [Google Scholar]
- Wood S, Busemeyer J, Koling A, Cox CR, Davis H. Older adults as adaptive decision makers: Evidence from the Iowa Gambling Task. Psychology and Aging. 2005;20:220–225. doi: 10.1037/0882-7974.20.2.220. [DOI] [PubMed] [Google Scholar]
- Xing C, Isaacowitz DM. Aiming at happiness: How motivation affects attention to and memory for emotional images. Motivation and Emotion. 2006;30:249–256. [Google Scholar]
- Zachary R. Shipley Institute of Living Scale, revised manual. Los Angeles: Western Psychological Services; 1986. [Google Scholar]


