Abstract
Background
Obesity may cause adverse effects on the respiratory system. The main purpose of this study was to investigate how various measures of obesity are related to arterial blood gases and pulmonary function.
Methods
This is a cross-sectional study of consecutive morbidly obese patients with normal lung function. Blood gas samples were taken from the radial artery after 5 min of rest with subjects sitting upright. Lung function measurements included dynamic spirometry, static lung volumes, and gas diffusing capacity.
Results
The 149 patients (77% women) had a mean (SD) age of 43 years (11 years) and BMI of 45.0 kg/m2 (6.3 kg/m2). The mean expiratory reserve volume (ERV) was less than half (49%) of predicted value, whilst most other lung function values were within predicted range. Forty-two patients had an abnormally low pO2 value (<10.7 kPa [80 mmHg]), while eight patients had a high pCO2 value (>6.0 kPa [45 mmHg]). All anthropometric measures correlated significantly with decreasing pO2 and increasing pCO2 (all P values < 0.05). BMI, neck circumference (NC), and waist circumference (WC) were negatively correlated with ERV (r = −0.25, −0.19, −0.21, respectively, all P values < 0.05). Multiple linear regression showed that BMI, WC, and NC were significantly associated with pO2 after adjustments for age, gender, and pack-years (all P values < 0.001). The models explained 34–36% of the variations in pO2. BMI, NC, and WC were also significantly associated with pCO2 (all P values < 0.05).There was no significant association between waist-to-hip ratio and blood gases (both P values > 0.27).
Conclusions
Both central and overall obesity were associated with unfavorable blood gases and low ERV.
Keywords: Lung function, Anthropometry, Obesity, Blood gases
Introduction
Obesity may cause adverse effects on the respiratory system due to alterations in gas exchange, respiratory mechanics, muscular endurance, and respiratory control [1]. These effects may be linked to a variety of comorbidities such as pulmonary hypertension, diastolic dysfunction, and coronary heart disease. Obesity may also lead to respiratory failure known as obesity hypoventilation syndrome [1]. Pulmonary gas exchange impairment at rest may increase the risk of post-operative pulmonary complications and have negative impact on obesity-related comorbidities.
It has been shown that obesity reduces thoracic wall compliance by restricting diaphragm movement and thoracic cage expansion [2]. Lung compliance is also reduced due to increased blood volume and a combination of alveolar collapse and airway closing in the lung basis [3]. The effects of obesity on total lung capacity (TLC) and vital capacity (VC) are modest, whereas functional residual capacity (FRC) and particularly expiratory reserve volume (ERV) may be severely decreased [1, 4–6]. An observational study of 37 morbidly obese patients showed that massive weight loss following bariatric surgery was associated with a significant improvement of ERV [7].
The effects of various patterns of obesity on pulmonary function have been studied. In obese males, forced vital capacity (FVC), forced expiratory volume (FEV1), and TLC were lower in those with android obesity (i.e., upper body obesity) than in those with gynecoid obesity (i.e., lower body obesity) [8]. It has also been suggested that waist-to-hip ratio (WHR), rather than BMI, explains a large part of the variance in pulmonary gas exchange [9, 10]. Current literature is scarce about the possible relationship between various levels of obesity and pulmonary gas exchange. If increasing levels of extreme obesity have detrimental effects on pulmonary gas exchange, this might have prognostic and therapeutic implications. We aimed to investigate how various measures of overall and abdominal obesity are related to arterial blood gases and pulmonary function in a population of morbidly obese patients with normal lung function.
Material and Methods
Study Design and Population
This is a cross-sectional analysis of baseline data from the non-randomized controlled MOBIL study (Morbid Obesity treatment, Bariatric surgery versus Intense Lifestyle intervention; Clinical Trials.gov number NCT0027314), the primary aim of which was to compare the effects of lifestyle intervention and bariatric surgery on various comorbidities. The study design, methods, and population have been described in detail [11]. Briefly, 224 adult morbidly obese subjects (BMI ≥40 or ≥35 kg/m2 with a weight-related comorbidity), predominantly of European descent, were consecutively screened at our regional tertiary care center. After exclusion of 47 subjects who declined to participate or who had heavy comorbidities, 177 subjects were found potentially eligible for either lifestyle intervention or bariatric surgery. As the aim of this substudy was to explore the specific effects of obesity on blood gases and lung function, 22 subjects with chronic obstructive pulmonary disease (FEV1/FVC <70%) [12] and six subjects with restrictive lung function impairment (FVC <80% of predicted value and TLC <80% of predicted value) [13] were excluded from further analyses. The remaining 149 subjects were defined as having normal lung function and were included in the analysis.
All participants gave informed written consent before enrolment. The study was approved by the Regional Ethics Committee for Medical Research (S-05715).
Data Source and Measurements
All subjects were examined by a physician. Demographic data and medical history, including smoking habits (recorded as pack-years), and use of bronchodilators were recorded. Height, weight, neck circumference (NC), waist circumference (WC), and hip circumference (HC) were measured with subjects in an upright position wearing light clothes and no shoes. Indexes were calculated for BMI (weight divided by the square of height, kilograms per square meter (kg/m2)) and WHR (WC divided by HC). NC was measured at the cricoid cartilage and WC at the level midway between the lowest rib margin and the iliac crest.
Blood gas samples were taken from the radial artery after at least 5 min of rest and with the subjects in a sitting position. The sampling was carried out by either a physician (AMG) or two experienced nurses. For analyses we used an ABL 735 Radiometer (Copenhagen, Denmark) calibrated in accordance with the manufacturer's specifications. Arterial blood gas values for pO2 < 10.7 kPa (80 mmHg) and for pCO2 > 6.0 kPa (45 mmHg) were defined as abnormal [14].
Lung function measurements included dynamic spirometry, static lung volumes, and gas diffusion capacity. All tests were carried out by two experienced nurses according to the guidelines recommended by the ATS-ERS task force [15–17] and with subjects sitting in an upright position wearing a nose clip. The Jaeger Master Lab (Eric Jaeger, Wurzburg, Germany) was used in all tests and was calibrated daily using a 1-L syringe. Recorded variables were: FVC, FEV1, FEV1/FVC, diffusion capacity for carbon monoxide (DLCO), DLCO divided by alveolar lung volume (DLCO/VA), TLC, VC, inspiratory capacity (IC), intrathoracic gas volume (ITGV), ERV, and residual volume (RV).
The reference values for FVC, FEV1, DLCO, TLC, VC, IC, ITGV, and RV are those recommended by the European Respiratory Society (ERS) [13]. These reference values have been derived from studies of healthy, non-smoking adults of European descent [13]. However, since the ERS offers no reference values for ERV, the Jaeger Master Lab reference values were used instead. Sixteen lung volume tests were rejected (12 due to technical problems and four due to compliance problems), and one single breath test was rejected (due to non-compliance). All tests were carried out between 9 and 10 a.m. No bronchodilator drug was allowed prior to lung function testing.
Statistical Analysis
Independent samples t test or Mann-Whitney test were used for the comparison of continuous data, whereas Chi-square test was used for categorical data. Spearman's correlations were calculated to assess bivariate relationships between anthropometric measures, blood gases, and lung function tests. Multiple linear regression analyses were used to assess the effect of each anthropometric measure on arterial blood gases after adjustment for confounding factors (age, gender, pack-years). We fitted eight separate multiple linear regression models with either pO2 or pCO2 as the dependent variable, and either BMI, NC, WC, or WHR as the independent variable adjusted for confounders. No violation of the assumptions of normality or multicollinearity was revealed in the models. Two-tailed P values are reported, with P values below 0.05 considered statistically significant. Semi-partial (part) correlation coefficients were squared in order to estimate the percentage of total variance in the dependent variable explained by a given independent variable. The analyses were carried out using SPSS 16.0 (SPSS, Chicago, IL).
Results
Demographic, clinical, and anthropometric characteristics of the 149 morbidly obese patients are shown in Table 1. Forty subjects (27%) had physician-diagnosed asthma and had been prescribed brochodilators; 14 used on-demand short acting bronchodilators, while 26 used the combination of inhaled steroids and long acting beta-2-agonists.
Table 1.
Demographic, anthropometric, and clinical characteristics of 149 morbidly obese patients
| Variables | Values |
|---|---|
| Females | 114 (77) |
| Age [years] | 43 (11) |
| Weight [kg] | 130 (23) |
| BMI [kg/m2] | 45.0 (6.3) |
| Neck circumference [cm] | 42 (4) |
| Waist circumference [cm] | 133 (14) |
| Waist-to-hip ratio | 0.97 (0.09) |
| Bronchodilator medication | 40 (27) |
| Never-smokers | 61 (41) |
| Pack-years | 9 (13) |
Values are given as mean (SD) or n (%)
The spirometry results, lung volumes, and blood gases are shown in Table 2. All lung function values except ERV, ITGV, and IC were within predicted range. The individual pO2 and pCO2 values ranged from 7.7 kPa (58 mmHg) to 15.3 kPa (115 mmHg) and from 3.6 kPa (27 mmHg) to 6.5 kPa (49 mmHg), respectively. Forty-two patients (28%) had abnormally low pO2 values (<10.7 kPa [80 mmHg]) and eight patients (5%) had abnormally high pCO2 values (>6.0 kPa [45 mmHg]).
Table 2.
Lung function and arterial blood gases in 149 morbidly obese patients
| Variables | Values |
|---|---|
| Spirometry | |
| Forced vital capacity | 103 (15) |
| Forced expiratory volume the first second | 97 (15) |
| Gas diffusing capacity | |
| Diffusing capacity for carbon monoxide [DLCO] | 91 (14) |
| DLCO divided by alveolar lung volume | 105 (16) |
| Static lung volumes | |
| Total lung capacity | 98 (12) |
| Vital capacity | 101 (15) |
| Inspiratory capacity | 123 (22) |
| Intrathoracic gas volume | 78 (16) |
| Expiratory reserve volume | 49 (34) |
| Residual volume | 96 (28) |
| Blood gases and pH | |
| pCO2 [kPa/mmHg] | 5.2/39 (0.5/4) |
| pO2 [kPa/mmHg] | 11.5/87 (1.5/11) |
| pH | 7.41 (0.02) |
Except for blood gases, values are referred as “percent predicted” and given as mean (SD)
BMI, NC, and WC correlated significantly and negatively with ERV (r = −0.25, −0.19 and −0.21, all p < 0.05), while NC correlated significantly and negatively with VC (r = −0.23, p = 0.007). WHR did not correlate significantly with any lung volume index, and no significant correlations were observed between anthropometrics measures and IC or RV (data not shown).
Figure 1 shows that all anthropometric measures correlated significantly with decreasing pO2 (all P values < 0.01), and increasing pCO2 (all P values < 0.05).
Fig. 1.
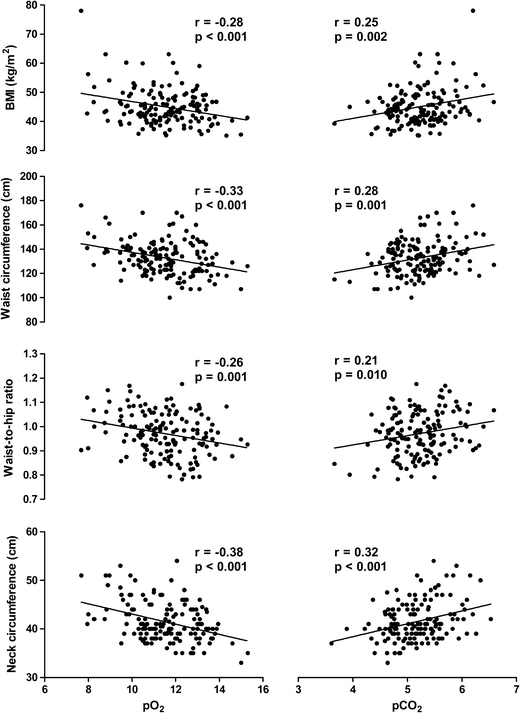
The scatter plots show the relationship between anthropometric measures and blood gases
ERV and RV correlated significantly with pO2 and pCO2 (Fig. 2).
Fig. 2.
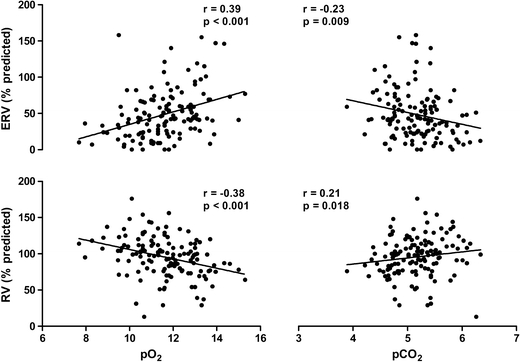
The scatter plots show the relationship between expiratory reserve volume, residual volume, and blood gases
The multiple linear regression analyses showed that increasing levels of BMI, WC, and NC were significantly associated with decreasing pO2 after adjustments for age, gender, and pack-years (Tab 3). The three multivariate models explained 34–36% of the variation in pO2. Based on part correlations BMI explained 9%, and NC and WC explained 6% of the variations in pO2.
Multiple linear regression (Table 3) showed that increasing BMI, NC, and WC were significantly associated with increasing pCO2. The three models explained 20–23% of the variation in pCO2. Based on part correlations BMI explained 6%, and NC and WC explained 3% of the variations in pCO2. There were no significant associations between WHR and blood gases in the multivariable analyses.
Table 3.
The relationship between various anthropometric measures and arterial blood gases in 149 morbidly obese subjects with normal lung function
| Dependent variables | Independent variables | Standardized beta | P values | R-square |
|---|---|---|---|---|
| pO2 | BMI | −0.292 | <0.001 | 0.36 |
| NC | −0.317 | <0.001 | 0.34 | |
| WC | −0.270 | <0.001 | 0.36 | |
| WHR | −0.096 | 0.268 | 0.29 | |
| pCO2 | BMI | 0.250 | 0.001 | 0.23 |
| NC | 0.225 | 0.023 | 0.20 | |
| WC | 0.205 | 0.014 | 0.20 | |
| WHR | 0.031 | 0.739 | 0.17 |
Each anthropometric measure was adjusted for age, gender, and pack-years in eight separate multiple linear regression analyses
BMI denotes body mass index, NC neck circumference, WC waist circumference, WHR waist-to-hip ratio
Discussion
Key Results
The main and novel findings of this study are that both measures of overall (BMI) and central obesity (WC, NC) were significantly associated with lower pO2 and higher pCO2 among morbidly obese subjects with normal lung function. In addition, BMI, NC, and WC correlated negatively with ERV.
Interpretation
Our results are partly in contrast to those of Zavorsky et al. [9] who found that increasing WHR, but not BMI, was associated with detrimental changes in blood gas values. This discrepancy might have several explanations. Firstly, the previous study was small (n = 25) and included a heterogeneous sample of subjects with either normal (n = 18) or abnormal (n = 7) lung function, but the analysis was not stratified according to lung function. Conversely, we examined 149 subjects, all with normal lung function. Secondly, in contrast to our study, clinically relevant confounding factors were neither addressed nor adjusted for in the previous study. Notably, we too could demonstrate statistically significant associations between WHR and blood gases in the univariate analyses, but these associations did not remain significant after adjustment for gender, age, and pack-years.
Our findings partly confirm the preliminary results from a cross-sectional study of 76 morbidly obese Chinese subjects that showed a significant association between BMI and blood gases [18]. Our results extend those findings to be valid in white morbidly obese patients independent of confounding factors. No measure of central obesity was addressed in the Chinese study.
Another more recent cross-sectional study of 150 morbidly obese Chinese adults with normal lung function demonstrated that only WC—in contrast to BMI and WHR—was associated with reductions in FEV1, FVC, TLC, and VC [19]. However, this study did not address the relationship between anthropometric measures and arterial blood gases.
Possible Mechanisms
Abdominal fat raises intra-abdominal pressure causing cephalad movement of the diaphragm, and thus reducing chest volume. Morbidly obese subjects have an abnormally high alveolar-to-arterial oxygen partial pressure difference and reduced pO2 [9, 10], which reflects a ventilation–perfusion mismatch as the lower parts of the lungs are underventilated and overperfused in obese subjects. Accordingly, profound weight loss after bariatric surgery has been associated with increased pO2 [7, 10]. Both WC and BMI reflect the amount of abdominal fat [20], and consequently increasing BMI and WC may be associated with atelectases and ventilation-perfusion mismatching, which may have an impact on blood gases.
In accordance with a previous study of morbidly obese patients, most lung function values were within predicted range whilst the mean ERV was less than 50% of predicted value [21].
Further, ERV was significantly correlated with BMI, NC, and WC. A decrease in ERV may lead to a decrease in FRC which in turn may fall below closing volume. Thus, morbidly obese subjects breathe near their residual volume. The collapse of small airways leads to increasing V/Q mismatch and hypoxia as described above. The relationship between reduced ERV and hypoxia in morbidly obese subjects has previously been shown [7]. In a study aimed to assess the effects of BMI on lung volumes [22], FRC and ERV were found to decrease exponentially with increasing BMI, and the greatest effects were on subjects with BMI >30 kg/m2. Notably, both FRC and ERV were significantly lower in moderately obese women and men (BMI, 30–35 kg/m2) than in their normal weight counterparts (BMI, 20–25 kg/m2) of comparable age. In this way, even moderate obesity lead to decreased ERV which may affect blood gases.
Notably, although we excluded patients with abnormal lung function from the present analysis, one out of four patients (27%) had been prescribed bronchodilator drugs due to asthma as diagnosed by their physician. This finding is in line with a previous study which reported a prevalence of asthma of 24% among morbidly obese subjects [21]. Several biological mechanisms may explain the relationship between asthma and obesity including those referred above [23].
Generalizability and Possible Implications
Our study demonstrates that in morbidly obese subjects, increasing levels of obesity are associated with worsening of blood gases. This could imply that clinicians should use spirometry and blood gas analyses to identify morbidly obese patients at particularly high risk for post-operative complications—for instance after bariatric surgery. Consequently, it might be wise to measure lung function and blood gases routinely in this patient group.
Limitations
Our study has limitations. Firstly, the cross-sectional design is unsuitable for establishing a cause–effect relationship. Secondly, our results may not be valid in non-white populations. Finally, we cannot exclude the possibility that the referral of patients to a tertiary care center might have introduced a sampling bias which may limit the generalizability of our results.
Conclusion
Our study of white morbidly obese subjects with normal lung function, demonstrates that increasing levels of BMI, WC, and NC are significantly associated with decreasing pO2 and increasing pCO2 even after adjustments for age, gender, and pack-years. In addition, both central and overall obesity are associated with low ERV.
Acknowledgements
We acknowledge the help from Dag Hofsø in designing the figures, and Berit Mossing Bjørkås, Heidi Omre Fon, Liv Randi Alu Andersen, and Elda Furulund Borg for their assistance with sampling and logistics. Thanks are due to Matthew McGee for proofreading the manuscript. Finally, we greatly acknowledge the patients who participated in the study.
Conflict of Interest
No potential conflicts of interest relevant to this article were reported.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Abbreviation
- COPD
Chronic obstructive pulmonary disease
- DLCO
Diffusing capacity for carbon monoxide
- ERV
Expiratory reserve volume
- FEV1
Forced expiratory volume the first second
- FRC
Functional residual capacity
- FVC
Forced vital capacity
- HC
Hip circumference
- IC
Inspiratory capacity
- ITGV
Intrathoracic gas volume
- NC
Neck circumference
- TLC
Total lung capacity
- VA
Alveolar lung volume
- VC
Vital capacity
- WC
Waist circumference
- WHR
Waist-to-hip ratio
- RV
Residual volume
Footnotes
Clinical Trials.gov number NCT0027314
References
- 1.Bickelmann AG, Burwell CS, Robin ED, et al. Extreme obesity associated with alveolar hypoventilation; a Pickwickian syndrome. Am J Med. 1956;21:811–18. doi: 10.1016/0002-9343(56)90094-8. [DOI] [PubMed] [Google Scholar]
- 2.Naimark A, Cherniack RM. Compliance of the respiratory system and its components in health and obesity. J Appl Physiol. 1960;15:377–82. doi: 10.1152/jappl.1960.15.3.377. [DOI] [PubMed] [Google Scholar]
- 3.Parameswaran K, Todd DC, Soth M. Altered respiratory physiology in obesity. Can Respir J. 2006;13:203–10. doi: 10.1155/2006/834786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kress JP, Pohlman AS, Alverdy J, et al. The impact of morbid obesity on oxygen cost of breathing (VO(2RESP)) at rest. Am J Respir Crit Care Med. 1999;160:883–6. doi: 10.1164/ajrccm.160.3.9902058. [DOI] [PubMed] [Google Scholar]
- 5.Koenig SM. Pulmonary complications of obesity. Am J Med Sci. 2001;321:249–79. doi: 10.1097/00000441-200104000-00006. [DOI] [PubMed] [Google Scholar]
- 6.Laaban JP, Cassuto D, Orvoen-Frija E, et al. Respiratory complications of massive obesity. Rev Prat. 1992;42:469–76. [PubMed] [Google Scholar]
- 7.Vaughan RW, Cork RC, Hollander D. The effect of massive weight loss on arterial oxygenation and pulmonary function tests. Anesthesiology. 1981;54:325–8. doi: 10.1097/00000542-198104000-00013. [DOI] [PubMed] [Google Scholar]
- 8.Collins LC, Hoberty PD, Walker JF, et al. The effect of body fat distribution on pulmonary function tests. Chest. 1995;107:1298–302. doi: 10.1378/chest.107.5.1298. [DOI] [PubMed] [Google Scholar]
- 9.Zavorsky GS, Murias JM, Kim dJ, et al. Waist-to-hip ratio is associated with pulmonary gas exchange in the morbidly obese. Chest. 2007;131:362–7. doi: 10.1378/chest.06-1513. [DOI] [PubMed] [Google Scholar]
- 10.Zavorsky GS, Hoffman SL. Pulmonary gas exchange in the morbidly obese. Obes Rev. 2008;9:326–39. doi: 10.1111/j.1467-789X.2008.00471.x. [DOI] [PubMed] [Google Scholar]
- 11.Hofso D, Ueland T, Hager H, et al. Inflammatory mediators in morbidly obese subjects: associations with glucose abnormalities and changes after oral glucose. Eur J Endocrinol. 2009;161:451–8. doi: 10.1530/EJE-09-0421. [DOI] [PubMed] [Google Scholar]
- 12.GOLD Guidelines. Executive Summary: Global Strategy for Diagnosis, Management, and Prevention of COPD 2010 (http://www.goldcopd.org/Guidelineitem.asp?l1=2&l2=1&intId=2180)
- 13.Quanjer PH, Tammeling GJ, Cotes JE, et al. Lung volumes and forced ventilatory flows. Report working party standardization of lung function tests, European community for steel and coal. Official statement of the European respiratory society. Eur Respir J Suppl. 1993;16:5–40. [PubMed] [Google Scholar]
- 14.Williams AJ. ABC of oxygen: assessing and interpreting arterial blood gases and acid-base balance. BMJ. 1998;317:1213–16. doi: 10.1136/bmj.317.7167.1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Miller MR, Hankinson J, Brusasco V, et al. Standardisation of spirometry. Eur Respir J. 2005;26:319–38. doi: 10.1183/09031936.05.00034805. [DOI] [PubMed] [Google Scholar]
- 16.Wanger J, Clausen JL, Coates A, et al. Standardisation of the measurement of lung volumes. Eur Respir J. 2005;26:511–22. doi: 10.1183/09031936.05.00035005. [DOI] [PubMed] [Google Scholar]
- 17.MacIntyre N, Crapo RO, Viegi G, et al. Standardisation of the single-breath determination of carbon monoxide uptake in the lung. Eur Respir J. 2005;26:720–35. doi: 10.1183/09031936.05.00034905. [DOI] [PubMed] [Google Scholar]
- 18.Chiang A, Mehrishi S, Ganaraj J et al. Relationship between body mass index and arterial blood gases. Chest. 2003:123S–4S.
- 19.Wei YF, Wu HD, Chang CY, et al. The impact of various anthropometric measurements of obesity on pulmonary function in candidates for surgery. Obes Surg. 2010;20:589–94. doi: 10.1007/s11695-009-9961-0. [DOI] [PubMed] [Google Scholar]
- 20.Janssen I, Heymsfield SB, Allison DB, et al. Body mass index and waist circumference independently contribute to the prediction of nonabdominal, abdominal subcutaneous, and visceral fat. Am J Clin Nutr. 2002;75:683–8. doi: 10.1093/ajcn/75.4.683. [DOI] [PubMed] [Google Scholar]
- 21.Saliman JA, Benditt JO, Flum DR, et al. Pulmonary function in the morbidly obese. Surg Obes Relat Dis. 2008;4:632–9. doi: 10.1016/j.soard.2008.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jones RL, Nzekwu MM. The effects of body mass index on lung volumes. Chest. 2006;130:827–33. doi: 10.1378/chest.130.3.827. [DOI] [PubMed] [Google Scholar]
- 23.Shore SA. Obesity and asthma: possible mechanisms. J Allergy Clin Immunol. 2008;121:1087–93. doi: 10.1016/j.jaci.2008.03.004. [DOI] [PubMed] [Google Scholar]


