Abstract
The integrity of the vascular system is essential for the efficient functioning of the brain. Ageing related structural and functional disturbances in the macro- or microcirculation of the brain make it vulnerable to cognitive dysfunction leading to brain degeneration and dementing illness. Several faltering controls including impairment in autoregulation, neurovascular coupling, blood-brain barrier leakage, decreased cerebrospinal fluid and reduced vascular tone appear responsible for variable degrees of neurodegeneration in old age. There is ample evidence that vascular risk factors are also linked to neurodegenerative processes preceding cognitive decline and dementia. Age is the strongest risk factor for brain degeneration whether it results from vascular or neurodegenerative mechanisms or both. However, several modifiable risks such as cardiovascular disease, hypertension, dyslipidaemia, diabetes and obesity enhance the rate of cognitive decline and increase the risk of Alzheimer’s disease in particular. The ultimate accumulation of brain pathological lesions may be modified by genetic influences such as apoliopoprotein E ε4 allele and the environment. Lifestyle measures that maintain or improve cardiovascular health including consumption of healthy diets, moderate use of alcohol and implementing regular physical exercise are important factors for brain protection.
Keywords: ageing, Alzheimer´s disease, cerebral blood flow, dementia, diabetes, diet, physical activity, stroke, vascular dementia, vascular factors, white matter lesions
INTRODUCTION
The brain comprises only 2% of the body weight yet consumes a critical 20% of the body’s oxygen and other nutrients supplied via the vascular system. The integrity of the vasculature is therefore essential for the optimal functioning of the brain. In addition to the cardiovascular system, brain vascular control mechanisms are vital for the maintenance of the neurovascular milieu, created by nerve terminals, astrocytic endfeet and the microvasculature.1, 2 Numerous molecular signals including vasoactive peptides, gaseous transmitters and growth factors mediate the tight regulation of cerebral blood flow (CBF) in tandem with neural activity. Structural, chemical or functional disturbances in the macro- or microcirculation of the brain would ultimately affect cognitive function and behaviour. Increasing age is the strongest risk factor for brain degeneration and age-related cognitive disorders (Table 1). However, none of the protective mechanisms is immune to the selective or general cumulative effects of ageing and malfunction in one could impact on the other with respect to both time and space (Figure 1).
Table 1.
Risk factors for brain degeneration* during ageing
| Factor | Degree of risk |
|---|---|
| Increasing age | Strongest |
| Family history of dementia or depressive illness | High |
| Down’s syndrome | High |
| Head trauma | Moderate |
| Vascular disease | Moderate |
| Apolipoprotein E-ε4 allele | High |
| Smoking | Moderate |
| Illiteracy | High |
| Sedentary lifestyle | Moderate |
| Female gender | Low |
Collective evidence indicates these are the most widely accepted degrees of risk for AD as the most common cause of dementia. Other less recognized risk factors include lower head circumference, and shorter leg length.4 Majority of evidence suggests more women succumb to AD whereas it appears the opposite for VaD.
Figure 1.
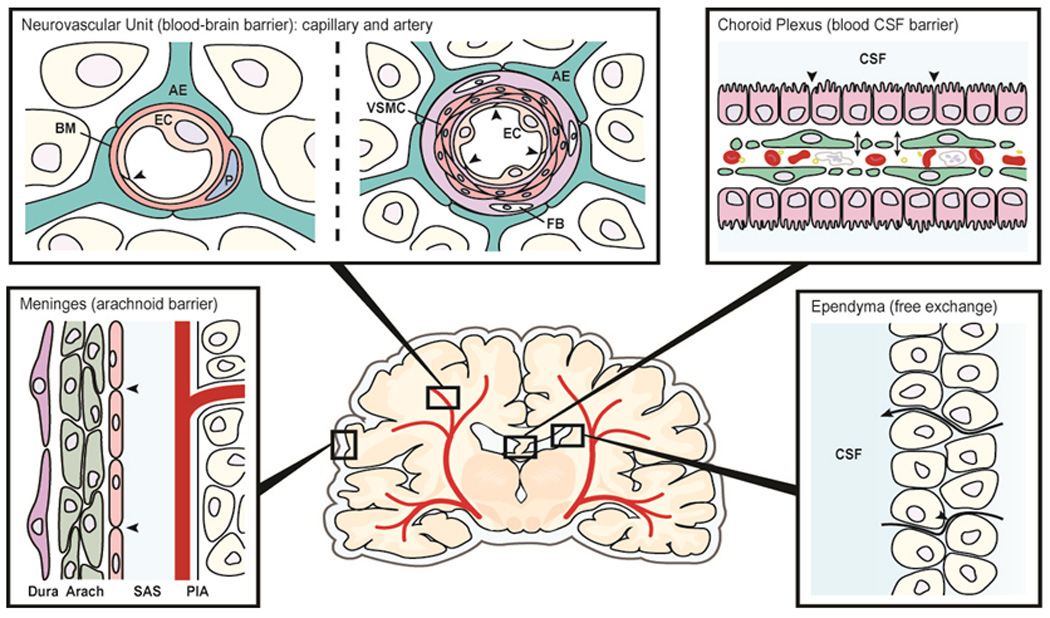
Schematic diagram showing various interfaces/barriers between blood or fluid and brain. Each of the sites can be affected to variable degree by ageing and more selectively as a consequence of neurodegenration. Abbreviations: AE: astrocytic endfoot, BM: basement membrane, EC: endothelial cell, P: pericyte, VSMC: vascular smooth muscle cell, FB: fibroblast, CSF: cerebrospinal fluid, Arach: arachnoid, SAS: subarachnoid space. Modified after Nils-Olof Hagnelius, 2009 (RNK, personal communication).
Various factors may influence the nature and severity of brain degeneration. The degree of cerebral grey matter damage, neuronal death and survival will be dictated by the multiplicity, size and laterality of the tissue injury or the extent of vascular disease. Anatomical features of the circulation including the size of vessels and vascular wall cellular elements e.g. arterioles versus capillaries are important factors in defining the pathology.1 The distribution territories of the anterior, posterior and middle cerebral and the lenticulostriate arteries affect different structures including the angular gyrus, caudate and medial thalamus in the dominant hemisphere, the amygdala and the hippocampus; all structures implicated in forms of cognitive impairment.3 The origin and degree of vascular occlusion or injury and whether this results in ischaemic or haemorrhagic lesions are further factors which define the extent and severity of damage. Alterations in specific genes associated with systemic disease or brain specific proteins and environmental or lifestyle factors may further modify the course of degeneration. This review will consider ageing-related changes at the fluid-tissue surfaces of the brain and various factors which put vascular health at risk to cause brain degeneration and dementia in old age.
Historical aspects
Both cerebrovascular and neurodegenerative diseases increase significantly after 60 years of age in almost all populations world wide.4 Alzheimer’s disease (AD) is widely recognized as the most common cause of dementia predominantly resulting from neurodegenerative changes.5 Cerebrovascular disease causes the second most common form of brain degenerative disorder leading to dementia. Just over 100 years Alois Alzheimer and Emil Kraeplin were among the first neuropsychiatrists who had reasoned that gradual strangulation of the blood supply to the brain was the main cause of organic brain degeneration.6 They surmised that ageing related progressive hardening of the arteries leads to arteriosclerotic dementia. Even until the late sixties5 arteriosclerotic dementia, attributed to cerebral softening with loss of relatively large volume (>50 ml) of tissue was described as a major cause of dementia which was reported to be over-diagnosed clinically. In recent years vascular dementia (VaD) or more precisely vascular cognitive impairment (VCI) has been described that takes into account the consequence of a variety of cerebrovascular lesions or impaired brain perfusion.7 Subcortical ischaemic VaD appears the most significant subtype of VaD 8 which involves small vessel disease and longer survival. The original recognition of subclasses of VaD should probably be credited to Otto Binswanger who described subcortical arteriosclerotic encephalopathy upon pathological verification of cerebral white matter (WM) lesions in patients with hypertensive disease.6 Clinical, neuroimaging and pathological features have facilitated the definition of VaD or VCI subtypes and allowed the appreciation of specific risk factors and the evaluation of treatment or prevention strategies.
Distinguishing between neurodegenerative and vascular processes which result in dementia is pertinent to identifying the causes and treatment options.. However, unselected community-based studies show that brains of demented subjects often exhibit more than one type of pathology. Vascular lesions are frequently found to co-exist with AD-type of lesions in older subjects.9, 10 Even in prospectively assessed AD subjects entirely pure neurodegenerative pathology appears to be an exception, particularly among the oldest old (>85 years).9 Autopsied brains of AD subjects frequently bear cerebrovascular pathology consisting of degenerative microangiopathy, cerebral amyloid angiopathy (CAA), cerebral infarcts and to small extent intracerebral hemorrhages.11 The co-existence of two or more pathologies is not novel to dementia. This was apparent from the description of Alzheimer’s first case of Auguste D. In his original report6 he had written that besides “one or several fibrils in otherwise normal cells, and “numerous small miliary foci …and…storage of peculiar material in the cortex, one sees endothelial proliferation and also occasionally neovascularisation.” While it is unlikely to be known whether the vascular changes represented angiogenesis as a result of ischaemic injury or microvascular degeneration per se the important question is whether the described microvascular pathology was coincident with or causal in the neurodegenerative changes leading to AD. However, it is now apparent that vascular and neurodegenerative pathologies are additive in influencing the clinical presentation of dementia.12 Cerebrovascular disease or strokes appear to not only hasten the pathological processes leading to dementia but also increases the possibility that individuals with Alzheimer lesions in their brains will exhibit dementia.13 Although cerebrovascular disease may be the main cause of degeneration and dementia, it is likely that such an event overcomes the brain's reserves14 in individuals being compromised by neurodegenerative changes related to Alzheimer pathology.
Control and Regulation of cerebral perfusion
The cerebral microvasculature and blood brain barrier (BBB)
Several clinical studies suggest that blood-brain barrier (BBB) dysfunction is involved in the progression and pathogenesis of AD.15, 16 17 The cerebrospinal fluid (CSF)/ serum albumin ratio (commonly used indicators of BBB integrity) is increased in AD patients with particularly co-morbid vascular disease.16, 18 In 85-year-old AD patients,19 higher CSF/serum albumin ratios were reported but the indications of disturbed BBB function were evident even before the onset of disease. The positive correlations between CSF-albumin index and measures of disease progression over 1 year in a subgroup of AD patients also suggest that BBB impairment could modify disease progression.20 The relative dysfunction of the BBB may increase the possibility of substances penetrating the BBB more rapidly to interact with neurons, and initiating a cascade of events involving amyloid accumulation and Alzheimer encephalopathy (Figure 2).
Figure 2.
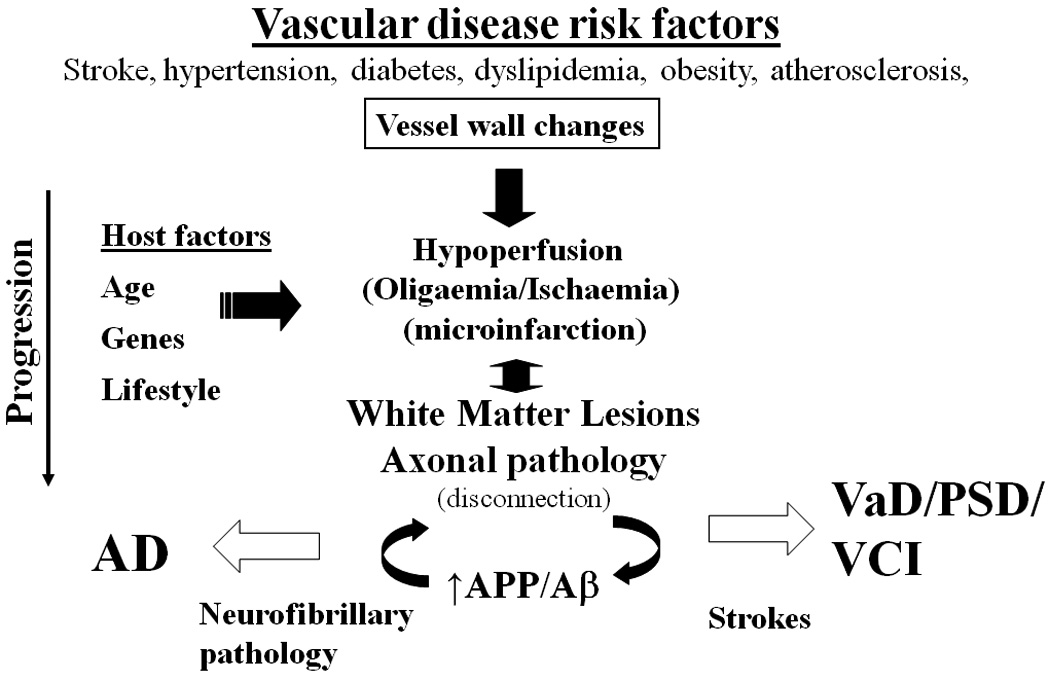
Hypothetical scheme showing the consequences of vascular disease risk clustering factors leading to brain degeneration and dementias. The general pathway may be modified by host influences including genetic, environmental or even perinatal influences. Abbreviations: Aβ, amyloid beta; AD, Alzheimer’s disease, APP, amyloid precursor protein; PSD, post-stroke dementia, VaD, vascular dementia; VCI, vascular cognitive impairment.
Prior biochemical and morphological studies (Table 2) have reported a variety of age-related alterations in the intracranial vessels including the perforating arteries, arterioles and capillaries in animals and man.1, 21 Cerebral arteriosclerosis, focal constrictions and stiffening of the vessel wall resulting from increased collagen fibres and extracellular matrix components result in loss of distensibility or elasticity that would affect brain perfusion. The terminal type arterioles of the deep white matter (WM) are particularly vulnerable targets in the elderly. Thus age-related increases in both the prevalence and severity of tortuosity of vessels in the WM may contribute to a chronic hypoxic state22, 23 which is attributed to the increasing long-term disability in the elderly.24 In addition, cerebral amyloid angiopathy (CAA), microbleeds and atherosclerosis,25, 26 affecting the neocortical perfusing surfaces would not only make the circumscribed neuronal populations behind them vulnerable but also the distal fields of the end-arteries.1 When the vascular changes are compounded by neurodegenerative processes the outcomes are predicted to be worse.
Table 2.
Features of the Cerebral Microvasculature and BBB in Ageing and Neurodegenerative Dementia
| Cellular Feature | Morphological changes | Biochemical markers |
|---|---|---|
| Cerebral endothelium | Loss of cytoplasm and endoplasmic reticulum. Increased pinocytosis. | ↓ glucose transporter-type 1, Na+/K+ ATPase, CD31, CD34 |
| Changes in cytoplasm (oxidative and endoplasmic reticulum stresses | ↑ glucose-6-phosphatase; proteases (endothelin converting enzyme-1) | |
| Endothelial membranes/ microvascular endfeet | ↓ Alkaline Phosphatase, γ-GT, Cholinesterases | |
| Decreased mitochondria | ↓ Carnitine aceytltransferase | |
| Loss of tight junctions | ||
| Vascular basement membranes | Thickening of the extracellular matrix, collagen fibers | ↑ Collagens, perlecans, fibrinogen, matrix metalloproteinases |
| Perivascular cells | Increased astrocytic feet | ↑ GFAP reactivity |
| Increased pericytes | ↑ CD68, macrophage markers | |
| Arteries/arterioles | Loss of vascular smooth muscle cells; increased microthrombi | ↓ α-smooth muscle actin; accumulation of amyloid β |
| Cerebral microvessels | Changes in cerebral endothelium and perivascular cellular elements | ↑ Inflammatory mediators and cytokines |
Results derived from previously published reviews and studies15, 29, 34–36 and unpublished data (Kalaria et al). Arrow indicates decrease or increase.
Abbreviations: AlkP, alkaline phosphatase; CD, clusters of differentiation markers; GFAP, glial fibrillary acid protein; GGT, γ-glutamyl transpeptidase.
Age related changes in cellular elements of the neurovascular unit (Table 2) consisting of focal necrosis of the cerebral endothelium, accumulation of extracellular matrix components within the vascular basement membrane, decreased endothelial mitochondrial density, increased pinocytotic vesicles, loosening of tight junctions, loss of the perivascular nerve plexus, changes in the astrocytic end-feet and selective degeneration of the microvascular tree have also been described.1 These changes are seen to be more intense in brains from AD subjects which also exhibit convolutional abnormalities and "collapsed" or attenuated capillaries in cortical lobes.27 This vascular phenomenon, associated with amyloid β deposits in all cortical lobes, is also evident in Down's syndrome.28 The microvascular changes imply abnormalities in the patency of the brain microvasculature and breach of the BBB. These descriptions support previous conclusions on disturbances in local perfusion and decreased oxygen tension within the neuropil as a consequence such that neurons furthest away from the capillary surface are divested.1 Changes in the dynamics of supporting cells within the neurovascular unit such as astrocytes, microglia and pericytes may bear additional consequences in reactive or compensatory mechanisms. Conversely, evidence from elegant studies by Zlokovic and colleagues29 has indicated that microvascular abnormalities lead to faulty BBB clearance of amyloid β through the deregulated low density lipoprotein-related protein 1 (LRP1) and receptor for advanced glycation endproducts (RAGE)-mediated transport. These impaired clearances of amyloid β and glycation endproducts then lead to aberrant angiogenesis, remodeling of the cerebral microvasculature and eventual arterial dysfunction which in turn initiates neurovascularuncoupling.30
Vascular functional proteins in brain degeneration
Selective changes in key BBB proteins including the glucose and nutrient transporters, neurotransmitter receptors and degrading enzymes (Table 2) have been recorded in the cerebral microvasculature of demented subjects.1, 2, 29 The glucose transporter (type I), an integral protein to the cerebral endothelium, is reduced in the microvasculature of AD subjects compared to ageing controls. Whether amyloid β deposition in brain microvessels in AD directly impairs synthesis or enhances degradation of the protein remains unknown, but profound effects on the permeability and function of the brain endothelium would be expected.15 These in vitro observations are corroborated by positron emission tomography studies showing that the transport of glucose (kinetic parameter k1) into brains of AD subjects is diminished 31. Moreover, uptake of 2-deoxy-D-glucose32 is also decreased further implying impairment of hexokinase activity in the cerebral microvasculature. The decreased activity directly relates to the reduced glucose transporter protein findings and suggests that microvessels deprived of glucose may be using other sources of metabolic fuel. Impairment in glucose transport or metabolism may also induce overactivation of glycogen synthase kinase-3beta and this together with down-regulation of the O-GlcNAcylation process promotes abnormal hyperphosphorylation of tau leading to neurofibrillary pathology.33
Biochemical approaches demonstrate selective changes in markers of the BBB or cerebral microvessels in AD.1, 2, 34 Specific proteins including the cholinesterases and APP cleaving proteases may promote or deregulate amyloid β deposition in the vasculature35 to affect the cerebral microcirculation which is considered a source of neurotoxic and inflammatory mediators.34 It is not unlikely that breakdown of the BBB occurs focally and transiently over a protracted period in association with reactive mechanisms that direct repair and growth.34, 36 For example, vascular endothelial growth factor reactivity is substantially increased in astrocytes and perivascular deposits in brains of subjects with AD implicating angiogenic signals are induced to counter insufficient vascularity or reduced perfusion (oligaemia).37 However, the ensuing pathology probably overwhelms the compensatory mechanisms such that the angiogenic or growth response is stunted.36, 38
The Cerebrospinal Fluid-Blood Barrier
The choroid plexus-cerebrospinal fluid (CSF) system ensures not only CSF secretion and clearance but the constant scrutiny of nutrients and harmful substances to maintain homeostasis.39 Both the quantity (by 50%) and quality of the CSF declines with age and in neurodegenerative disease.40, 41 The choroidal epithelium undergoes atrophy, thickening of the basement membrane, stromal fibrosis and accumulates Biondi rings tangles and lipofuscin with age and more so in AD.42 These changes together with activation of inflammatory mechanisms 43 are responsible for an estimated 50% reduction in turnover and alteration of CSF contents.39 The impaired ability of the choroid plexus to clear molecules from the CSF in older age has potentially serious implications for brain clearance of key proteins including amyloid β in AD subjects.44 This is analogous to the early vascular changes in amyloid precursor protein (APP) in over-expressing transgenic (Tg) mice2 which show that regional specific microvascular anomalies may precede the more profound pathologies which characterise neurodegeneration and AD.
Cerebral autoregulation
Autoregulation of CBF is the first order mechanism that ensures that the flow and supply of oxygen, glucose and nutrients through the vascular beds remain within the upper and lower limits of the autoregulatory range (50–160 mm Hg) during fluctuations in systemic arterial pressure.3 Cerebral resistance arteries dilate or constrict during changes in arterial pressure. Ageing related changes in the systemic circulation and degenerative changes in the extracerebral resistance arteries1 may shift the lower and upper limits of the autoregulatory plateau to cause hypertensive encephalopathy or cerebral hypoperfusion, which may not necessarily cause ischaemic injury as obvious as in stroke but oligaemia or hypovolemia that leads to disruption of the microcirculation, damage to the cerebral endothelium, breach of the BBB and edema.17 Neurogenic responses via the brainstem autonomic nuclei, which degenerate in age-related brain disorders,45, 46 may also disrupt CBF autoregulation that may increasingly cause orthostatic hypotension and syncope. In addition, pathological changes in vascular wall smooth muscle (myogenic effect) and altered release of metabolic factors could profoundly influence autoregulatory responses, which would have a high impact in the oldest old (>85 years of age). Animal studies reveal that autoregulation fails in Tg mice over-expressing the APP and accumulating Aβ deposits. In the APP Tg mice vascular alterations are observed at 3 months of age much in advance of Aβ deposition and cognitive decline.2
Neurovascular coupling
Functional hyperemia or neural activity coupling with CBF is another vital mechanism to protect the tight dynamic relationship between the cellular elements of the neurovascular unit and homeostasis of the cerebral microenvironment.2 Sensitive neuroimaging methods confirm ageing-related regression in global and regional measures of CBF (~4mL/min/year), cerebral metabolic rate for oxygen, glucose oxidation, cerebral blood volume and not surprisingly CSF secretion and flow.47 In AD, more pronounced regional effects are apparent.2 Under certain pathological and pharmacological conditions, CBF and metabolism are uncoupled and likely result from weaker muscle tone, failure of angiogenesis or breakdown in the molecular communication between neurons and microvessels.38 The latter undergo several changes including loss of pericytes1 that indicate the BBB in the elderly is less likely to compensate for leaks.17 The characteristic deposition of amyloid in cerebral vessels in AD exacerbates vascular dysfunction and promotes hypoperfusion. APP expressing Tg mice also show that the reactivity of the cerebral circulation to endothelium-dependent vasodilators, a major vasoregulatory mechanism, is impaired. This is consistent with the diminished vasodilator responses attributed to altered modulatory function of the endothelium rather than impairments in vascular smooth muscle per se during ageing.48
Vascular Risk Factors in Neurodegeneration and Dementia
Recent advances indicate that dementia risk is modified by perinatal events, education status, nutritional intake, degree of physical activity and cognitive and social engagement.49 Several of these factors impact on adult-onset vascular disorders including strokes, hypertension, atherosclerotic disease, atrial fibrillation, diabetes mellitus, dyslipidemia, hyperinsulinaemia, hyperglycaemia, hyperhomocysteinemia, and obesity (Table 3).50 It is increasingly recognized that factors which increase cardiovascular disease or brain vascular pathology exacerbate the onset or progression of late-onset dementias (Figure 2). While data vary in individual studies that may relate to the clinical diagnosis of dementia, carriers of the apolioprotein E ε4 allele in general appear at greater at risk in the presence of vascular disease. However, the evidence for single clinically defined cardiovascular risk factors being associated with incident AD is inconsistent.51 Some genetic influences or environmental factors may modify the progressive changes which define the final phenotype and burden of brain pathology.11 While randomized or controlled trials of risk factor modification (with multiple simultaneous interventions) are lacking it is encouraging that interventions of cognitive and physical activity were shown to improve cognitive performance and slow cognitive decline.52
Table 3.
Vascular risk factors for Neurodegeneration, Cognitive Impairment and Dementia
| Individual features | Methods of diagnosis | Degree of risk* | Genetic influence† | |
|---|---|---|---|---|
| Stroke(s)/vascular origin | Silent infarcts | Clinical, imaging | +++ | − |
| Transient Ischaemic attacks | Clinical, imaging | +++ | − | |
| Lacunar infarcts | Clinical, imaging | ++ | APOE ε4 | |
| Microinfarcts | Pathological | +++ | ||
| Vascular pathology: atherosclerosis | Carotid arteries | Clinical, imaging | ++ | APOE ε4 |
| Aortic arch | Pathological | ++ | APOE ε4 | |
| Circle of Willis | Pathological | +++ | APOE ε4 | |
| Blood Pressure | Hypertension systolic BP; >130 mm Hg; diastolic >95 mm Hg | Clinical, imaging | +++ | APOE ε4 |
| Hypotension | Clinical, imaging | ++ | − | |
| Heart disease | Coronary artery disease | Pathological | +++ | − |
| Atrial Fibrillation | Clinical, imaging | ++ | − | |
| Dyslipidemia | High cholesterol | Clinical | ++ | APOE ε4 |
| High triglycerides | Clinical, imaging | ++ | − | |
| Homocysteine/ Folate metabolism | Hyperhomocysteinemia (>13 mM) | Clinical | ++ | APOE ε4 |
| Diabetes mellitus | Diabetes Type II | Clinical, imaging, pathological | + | APOE ε4 |
| Insulin dysregulation | Clinical | ++ | − | |
| Metabolic syndrome | Several factors | Clinical | +++ | − |
| Obesity | Body mass index | Clinical, imaging | ++ | − |
| Overweight | Skin-fold thickness | Clinical | ++ | − |
Table lists factors that may directly or indirectly affect vascular tone, resilience and reactivity. Results are summarized from several population-based observational and longitudinal cohort studies. 25, 50, 51, 55, 58, 60, 83, 96, 97, 103, 104, 106, 109, 110, 115, 117
Degree of risk (+++ means high, > 3 fold RR or HR; + means low, < two-fold RR or HR) were derived from published relative risk (RR) and hazard ratios (HR).
The only consistent allelic influence identified.
Abbreviations: APOE, apolipoprotein E.
Strokes, silent infarction and mixed pathologies
In the elderly, stroke episode or history of transient ischaemic attack increases the risk of AD up to three-fold.53–55 Cerebral infarcts appear to accelerate AD progression and account for a large proportion of mixed pathologies cases in older age groups.54, 56–58 Silent brain infarction (without overt neurological sequelae) occurs with high prevalence in the healthy elderly, estimated at ~5% in those 60 yrs of age in both genders.59, 60 Almost 90% of these occur subcortically (and appear as lacunae in basal ganglia) with the rest in the neocortex. Silent infarcts double the risk of dementia, cause steeper cognitive decline (memory performance) and impact on executive dysfunction.61, 62
Patients with cerebrovascular disease who survive long may progress to develop dementia characterized by Alzheimer type of changes.63, 64 Conversely, Henon et al 65 have shown that severe cognitive impairment or low cognitive ability, which could represent AD, is also a risk for cerebrovascular disease or strokes.66 Stroke incidence was highest in those with severe impairment and the association between cognitive impairment and incident stroke appears not to be mediated by systemic vascular disease. The elevated risk of subsequent strokes in older persons with cognitive impairment suggests that cerebrovascular disease may play a direct role in cognitive impairment. Therapeutic monitoring or treatment of the vascular component should ameliorate or prevent worsening of dementia symptoms in individuals with a diagnosis of AD or other insidious neurodegenerative disease.7
Neuropatholgical studies provide further evidence for considerable overlap between AD and cerebrovascular disease.53 High proportions of individuals fulfilling the neuropathological diagnosis of AD have significant cerebrovascular lesions11–13, 67–69 while VaD patients frequently show extensive AD–type changes.70, 71 Thus in one prospective study70 in clinically diagnosed VaD patients at autopsy nearly 60% were found to have AD pathology alone and 40% had AD pathology in combination with cerebrovascular disease. Older patients diagnosed with VaD may accumulate some Alzheimer pathological features e.g. cerebral amyloid β42 levels comparable to those seen in AD.72 The concomitant vascular pathology in terms of lacunae, microinfarcts and arteriosclerosis appear to coexist by more than chance alone.73 For example, studies have emphasised that a significant association exists between cortical microinfarcts and AD (32% cases v 3% controls).57, 74 The microinfarcts were restricted to watershed cortical zones indicating that disturbed haemodynamic factors were involved in the genesis of these vascular lesions which may precede Alzheimer’s symptoms.75 When both neurodegenerative e.g. amyloid β deposits and neurofibrillary tangles and vascular pathologies are present the burden of dementia tends to be associated with small or micro ischemic lesions76, 77 rather than large infarctions. These findings corroborate the importance of microvascular disease rather than macroscopic infarction as the critical substrate in dementia.78
Further clinical importance of the combined neurodegenerative and vascular pathologies is emphasised by the finding that dementia was worse and compounded by the co-existence of both neuropathological features of AD and VaD in elderly nuns.13 In this prospectively assessed cohort with low vascular disease risk and where there was no association between cerebral infarcts and AD type of pathology, it was estimated that an eight-fold greater burden of neocortical NFT would be necessary to develop dementia in the absence of strategic cerebral infarction.13, 79 Similarly, the Religious Orders Study found that although there was no interaction between infarctions and AD pathology13 the presence of one or more infarctions independently increased the odds of dementia by nearly three-fold.80 The Honolulu Aging in Asians study (HAAS) provides additional support for the independence of AD and vascular neuropathology.81 These reports do not show whether ischaemic cerebrovascular disease contributes directly to the development of Alzheimer’s pathology but they indicate that vascular changes have an additive effect and reduces the threshold for dementia diagnosis in patients with an otherwise asymptomatic low-grade Alzheimer’s pathology.82
White Matter Hyperintensities (WMH)
Progressive age-related changes in intracerebral vessels or presence of infarcts may result in WM abnormalities in as many as 96% of those over 65 years of age.83 WM abnormalities are seen as WM74, 84 hypersignals in T2-weighted or fluid attenuated inversion recovery (FLAIR) sequences on magnetic resonance imaging (MRI). Significant risk factors for more severe WMH include older age, history of hypertension, prior stroke, presence of diabetes, smoking habit and clinically silent strokes.85 The pathological correlates of WMH comprise regions of deep WM pallor or myelin loss, axonal disruption, arteriosclerosis within small to medium sized arteries with perivascular spaces. These changes are worsened by oedema and diffuse damage of the BBB with chronic leakage of fluid and macromolecules. Multi-disciplinary correlative studies show that both deep WM and periventricular hyperintensities represent primarily oligaemic tissue damage86 which may be accompanied by a Wallerian type of axonal degeneration secondary to neurodegenerative processes and lead to dementia (Figure 2).
Past studies have attempted to assess correlations between the degree of WMH from MRI and cognitive function or disability in the elderly. Although distinctions between periventicular and deep WM may be arbitary24, 87 it appears that the total WMH volume is a critical measure. Most studies with cohorts of variable size reveal declines in frontal lobe functions which include executive tasks, attention, motor and processing speed rather than memory per se and appear to be associated with increased WMH volume.88, 89 In the LADIS cohort executive dysfunction was attributed to WM lesion severity in the non-disabled elderly.24 On the other hand, volume of periventricular WMH rather than deep WMH at baseline and progression was longitudinally associated with decline in processing speed.90 Severe WMH has likewise been reported to increase the risk of disability24 and death91 in community-dwelling elderly without stroke or other neurological disease. Increased WMH and the presence of lacunar infarcts, indicative of subcortical vascular disease, are associated with greater global atrophy.92, 93 An association between WMH severity and medial temporal atrophy (MTA) was reported in one study of siblings affected and at risk for AD94 consistent with demyelination secondary to AD neuronal damage.
Hypertension
Alterations in arterial pressure during ageing have been strongly implicated in brain degeneration and dementia.95 Many studies have confirmed that a history of hypertension during midlife may increase risk of dementia but particularly AD. In the first longitudinal study, Skoog et al96 showed that increased systolic (>160 mmHg) and diastolic (>95 mmHg) blood pressure 10–15 years before were factors in the onset of AD. The risk of developing dementia between the ages of 80 and 85 increased with increasing blood pressure at the age of 70 years even within the lower ranges of blood pressure values obtained. Long-standing increase in blood pressure may increase risk for dementia and AD by inducing small-vessel disease, WM changes and cerebral hypoperfusion by disrupting vasoregulatory functions. However, atherosclerotic disease leading to cerebral hypoperfusion has also been implicated.97 In later life and after onset of dementia blood pressure actually decreases suggesting that cerebral atrophy or intrinsic neuropathological changes are responsible for lowering pressure.
Pathological studies have revealed that hypertensives with elevated systolic and diastolic pressures in midlife exhibit an increased burden of AD type of neurodegenerative lesions including neuritic plaques and neurofibrillary tangles98 and which are also linked to low brain weight.99 A few trials also show that the use of antihypertensives offers some protection from the risk of dementia.100 However, there is no convincing evidence from the currently published trials that blood pressure lowering in late-life prevents the development of dementia or cognitive impairment in hypertensive patients with no apparent prior cerebrovascular disease.101
Atherosclerosis and dyslipidemia
In comparison to the role of hypertension,81 the risk of developing dementia after hypercholesterolemia is less robust. This may relate to how studies are conducted and the age at which cholesterol concentrations are determined.81, 102 However, using doppler scanning, the Rotterdam investigators103 concluded that atherosclerosis exhibited as carotid artery thickening or accumulation of wall plaques in the elderly is a strong risk factor for late onset AD and VaD. On the other hand cholesterol levels measured in midlife were associated with higher risk of AD but not VaD.104 Total cholesterol and low density liopoprotein (LDL) concentrations and a history of diabetes have also been shown to be associated with more rapid cognitive decline in individuals with AD.105 Consistent with the increased risk of AD, subjects with coronary heart disease also exhibit an increased amount of brain amyloidosis.106, 107 It is clear that high cholesterol and other lipids play a role in increasing risk but the course of the converging pathways and how this occurs are debated.108
Diabetes and Metabolic syndrome
Several epidemiological show that a history of adult onset diabetes mellitus increases the risk of cognitive impairment and dementia in the elderly.109, 110 Risk for AD and particularly VaD was reported to be 2–2.5 fold greater among type II diabetics, irrespective of age at which diabetes occurs. Consistent with this a meta-analysis111 showed that the risk for AD was significantly higher (RR 1.4) but almost two fold greater for VaD (RR 2.4). Neuroimaging evidence suggests an association between diabetes and cerebral atrophy and lacunar infarcts but the association with WM lesions is less robust.112 These studies emphasise the link between glucose dysregulation and brain degeneration even in the very old but do not explain the link between diabetes and the AD type of neurodegenerative changes. Several scenarios including impaired insulin signalling induced neurodegenerative changes, advanced glycation of neuronal components, oxidative stress and inflammatory mechanisms have been proposed.
Features of the insulin resistance syndrome have also been associated with low cognitive function113, 114 and with AD.115 The risk is even higher in individuals expressing components of the metabolic syndrome including high blood pressure, increased triglycerides, high blood glucose, low LDL cholesterol and obesity.116, 117 Moreover, obesity and overweight in midlife, measured by body mass index and skin-fold thickness, are strongly associated with an increased risk of both AD and VaD, independent of the development of diabetes or other cardiovascular-related morbidities. Conversely, higher baseline body mass index and slower declining body mass in late life appear to reduce risk of dementia.118 This suggests that a faster decline in body mass index in late life is a preclinical indicator of an underlying dementing illness, especially for those who were initially overweight.118
Vascular health and neuroprotection by prevention
Nutritional factors
Outcomes of large multicentre randomized trials are awaited but the bulk of currently reported observational studies in elderly cohorts119, 120 suggest that development or progression of cognitive impairment and dementia can be slowed by use of healthy foods and vitamin supplements121 and by the avoidance of nutritional deficiencies and balanced calorie restriction. Independent evidence suggests regular consumption of fish is related to lower risk of AD122, 123 and slower rate of cognitive decline.124, 125 Fish may offer protection by countering inflammation and enhancing vascular tone and countering atherosclerosis. Diets that emphasize polyphenols including reservatrol, omega-3 fatty acids, docosahexaenoic and eicosapentaenoic acids, flavonoids and the B vitamins, especially folate, B6 and B12 are implicated as likely to sustain or improve cognitive function in older age.126 Higher adherence to the Mediterranean diet127 consisting of whole grains, fish and olive oil and moderate consumption of alcohol is also associated with lower risk for AD128 and significant improvement in incidence neurodegenerative disorders.129
Findings from clinical and experimental studies show that chronic accumulation of reactive oxygen species in older brains may exhaust antioxidant capacity and trigger neurodegenerative processes as characterized in AD. Thus dietary supplementation with fruit or vegetable extracts high in antioxidants help to decrease the enhanced vulnerability to oxidative stress and improve neuronal communication via increases in neuronal signaling and animal behavior.130 Onset of AD was significantly delayed by use of antioxidant vitamins and polyphenols derived from fruits and vegetables.129 131 Results from the Kame Project suggest that drinking fruit juices which are high in polyphenolic compounds, was associated with lower risk of incident AD.129 Congruent with the notion that vascular health is key to maintaining cognitive function polyphenols from wine, cocoa, coffee, grape seed, blueberries, strawberries, tea, curcumin, pomegranate and green leafy vegetables also have beneficial effects on endothelial function and cardiovascular performance.129 The beneficial actions of resveratrol have been implicated in anti-oxidant defence, regulation of the cell cycle, mitochondrial energy production, vascular reactivity, oncogene suppression and activation of sirtuins (silent information regulator-related enzymes), as anti-ageing inhibitors.132 In contrast to prior assertions that it may be protective there is strong consensus that smoking is a vascular risk factor for dementia because of its association with several degenerative features. These include cerebral infarcts, white matter hyperintensities,102 subcortical atrophy and neuronal death by increasing amyloid burden.133, 134 Although medicinal nicotine may be beneficial133 these findings reinforce the notion that any measure that decreases atherosclerosis and increase brain blood flow are valuable for cognitive function in old age (Figure 2).
Physical activity
Accumulating evidence suggests changes in lifestyle factors such as increasing physical activity will decrease the risk of developing dementia in later life.120, 135 Most studies136, 137 studies show reduced rate of age-related cognitive decline, decreased risk of incident dementia or AD in individuals who exercise regularly. In a prospective cohort study138 higher physical activity was identified to reduce risk of dementia in community-dwelling elderly people. In the largest clinical trial of its kind139 physically active volunteers with memory problems exhibited significant improvement in cognition after a 24-week intervention.
The effects of physical exercise on vascular health may be obvious but neuroimaging studies 140 suggest that the more aerobically fit older adults lose less grey matter. Likewise, whole brain volume was found to be positively associated with aerobic fitness levels among patients with early stage AD.141 Higher aerobic fitness levels also have been shown to be associated with larger hippocampus, increased blood flow, oxygen delivery and better spatial memory150.142 In related studies on prevention, increased physical activity in mid-life136 have been found to be associated with less neocortical atrophy in the elderly. The latter association was independent of atherosclerosis and brain infarcts. The likely mechanisms through which exercise induces brain growth and improves cognition include upregulation of brain-derived neurotrophic factor and preservation of synapses.143
Conclusion
Several lines of evidence suggest a strong role for vascular factors in the aetiology and pathogenesis of brain degeneration. Ageing associated cerebral microangiopathy inflicted at several control levels may explain deficits in autoregulation and neurovascular coupling and can be predicted to occur in subjects developing AD, the most common cause of dementia. Prevailing evidence from population and community based studies suggests that pre-existing features of cardiovascular disease are strongly linked to late-onset dementia. These may lead to cerebral hypoperfusion involving key pathological substrates mainly white matter changes, axonal damage and microinfarction. Unlike age and genetic background, vascular risk factors are modifiable through management and lifestyle changes which then help to protect the brain.
Acknowledgements
I thank Yumi Yamamoto for helping to create Figure 1. Our work has been supported by grants from the Medical Research Council (UK) for G0500247 and G0700718 awards, US National Institutes of Health (NINDS) and the Alzheimer’s Research Trust (UK).
References
- 1.Kalaria RN. Cerebral vessels in ageing and Alzheimer's disease. Pharmacol Ther. 1996;72:193–214. doi: 10.1016/s0163-7258(96)00116-7. [DOI] [PubMed] [Google Scholar]
- 2.Iadecola C. Neurovascular regulation in the normal brain and in Alzheimer's disease. Nat Rev Neurosci. 2004;5:347–360. doi: 10.1038/nrn1387. [DOI] [PubMed] [Google Scholar]
- 3.Ferrer I, Kaste M, Kalimo H. Vascular diseases. In: Love S, Louis D, Ellison D, editors. Greenfield's Neuropathology. 8th ed. Oxford: Oxford University Press; 2008. pp. 121–240. [Google Scholar]
- 4.Kalaria RN, Maestre GE, Arizaga R, et al. Alzheimer's disease and vascular dementia in developing countries: prevalence, management, and risk factors. Lancet Neurol. 2008;7:812–826. doi: 10.1016/S1474-4422(08)70169-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tomlinson BE. Brain-stem lesions after head injury. J Clin Pathol Suppl (R Coll Pathol) 1970;4:154–165. doi: 10.1136/jcp.s3-4.1.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Berrios GE, Freeman HL. Alzheimer and the dementiaa. In: Berrios GE, editor. Eponymists in Medicine Series. London: Royal Society of Medicine Services; 1991. pp. 69–76. [Google Scholar]
- 7.O'Brien JT, Erkinjuntti T, Reisberg B, et al. Vascular cognitive impairment. Lancet Neurol. 2003;2:89–98. doi: 10.1016/s1474-4422(03)00305-3. [DOI] [PubMed] [Google Scholar]
- 8.Roman GC, Erkinjuntti T, Wallin A, Pantoni L, Chui HC. Subcortical ischaemic vascular dementia. Lancet Neurol. 2002;1:426–436. doi: 10.1016/s1474-4422(02)00190-4. [DOI] [PubMed] [Google Scholar]
- 9.MRC-CFAS. Neuropathology Group of the Medical Research Council Cognitive Function and Ageing Study. Pathological correlates of late-onset dementia in a multicentre, community-based population in England and Wales. Lancet. 2001;357:169–175. doi: 10.1016/s0140-6736(00)03589-3. [DOI] [PubMed] [Google Scholar]
- 10.White L, Petrovitch H, Hardman J, et al. Cerebrovascular pathology and dementia in autopsied Honolulu-Asia Aging Study participants. Ann N Y Acad Sci. 2002;977:9–23. doi: 10.1111/j.1749-6632.2002.tb04794.x. [DOI] [PubMed] [Google Scholar]
- 11.Premkumar DR, Cohen DL, Hedera P, Friedland RP, Kalaria RN. Apolipoprotein E-epsilon4 alleles in cerebral amyloid angiopathy and cerebrovascular pathology associated with Alzheimer's disease. Am J Pathol. 1996;148:2083–2095. [PMC free article] [PubMed] [Google Scholar]
- 12.Heyman A, Fillenbaum GG, Welsh-Bohmer KA, et al. Cerebral infarcts in patients with autopsy-proven Alzheimer's disease: CERAD, part XVIII. Consortium to Establish a Registry for Alzheimer's Disease. Neurology. 1998;51:159–162. doi: 10.1212/wnl.51.1.159. [DOI] [PubMed] [Google Scholar]
- 13.Snowdon DA, Greiner LH, Mortimer JA, Riley KP, Greiner PA, Markesbery WR. Brain infarction and the clinical expression of Alzheimer disease. The Nun Study. JAMA. 1997;277:813–817. [PubMed] [Google Scholar]
- 14.Fratiglioni L, Wang HX. Brain reserve hypothesis in dementia. J Alzheimers Dis. 2007;12:11–22. doi: 10.3233/jad-2007-12103. [DOI] [PubMed] [Google Scholar]
- 15.Kalaria RN. The blood-brain barrier and cerebral microcirculation in Alzheimer disease. Cerebrovasc Brain Metab Rev. 1992;4:226–260. [PubMed] [Google Scholar]
- 16.Blennow K, Wallin A, Fredman P, Karlsson I, Gottfries CG, Svennerholm L. Blood-brain barrier disturbance in patients with Alzheimer's disease is related to vascular factors. Acta Neurol Scand. 1990;81:323–326. doi: 10.1111/j.1600-0404.1990.tb01563.x. [DOI] [PubMed] [Google Scholar]
- 17.Farrall AJ, Wardlaw JM. Blood-brain barrier: Ageing and microvascular disease - systematic review and meta-analysis. Neurobiol Aging. 2007 doi: 10.1016/j.neurobiolaging.2007.07.015. [DOI] [PubMed] [Google Scholar]
- 18.Hampel H, Muller-Spahn F, Berger C, Haberl A, Ackenheil M, Hock C. Evidence of blood-cerebrospinal fluid-barrier impairment in a subgroup of patients with dementia of the Alzheimer type and major depression: a possible indicator for immunoactivation. Dementia. 1995;6:348–354. doi: 10.1159/000106969. [DOI] [PubMed] [Google Scholar]
- 19.Skoog I, Wallin A, Fredman P, et al. A population study on blood-brain barrier function in 85-year-olds: relation to Alzheimer's disease and vascular dementia. Neurology. 1998;50:966–971. doi: 10.1212/wnl.50.4.966. [DOI] [PubMed] [Google Scholar]
- 20.Bowman GL, Kaye JA, Moore M, Waichunas D, Carlson NE, Quinn JF. Blood-brain barrier impairment in Alzheimer disease: stability and functional significance. Neurology. 2007;68:1809–1814. doi: 10.1212/01.wnl.0000262031.18018.1a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kawai M, Kalaria RN, Cras P, et al. Degeneration of vascular muscle cells in cerebral amyloid angiopathy of Alzheimer disease. Brain Res. 1993;623:142–146. doi: 10.1016/0006-8993(93)90021-e. [DOI] [PubMed] [Google Scholar]
- 22.Thore CR, Anstrom JA, Moody DM, Challa VR, Marion MC, Brown WR. Morphometric analysis of arteriolar tortuosity in human cerebral white matter of preterm, young, and aged subjects. J Neuropathol Exp Neurol. 2007;66:337–345. doi: 10.1097/nen.0b013e3180537147. [DOI] [PubMed] [Google Scholar]
- 23.Fernando MS, Simpson JE, Matthews F, et al. White matter lesions in an unselected cohort of the elderly: molecular pathology suggests origin from chronic hypoperfusion injury. Stroke. 2006;37:1391–1398. doi: 10.1161/01.STR.0000221308.94473.14. [DOI] [PubMed] [Google Scholar]
- 24.Pantoni L, Poggesi A, Basile AM, et al. Leukoaraiosis predicts hidden global functioning impairment in nondisabled older people: the LADIS (Leukoaraiosis and Disability in the Elderly) Study. J Am Geriatr Soc. 2006;54:1095–1101. doi: 10.1111/j.1532-5415.2006.00798.x. [DOI] [PubMed] [Google Scholar]
- 25.Beach TG, Wilson JR, Sue LI, et al. Circle of Willis atherosclerosis: association with Alzheimer's disease, neuritic plaques and neurofibrillary tangles. Acta Neuropathol. 2007;113:13–21. doi: 10.1007/s00401-006-0136-y. [DOI] [PubMed] [Google Scholar]
- 26.Roher AE, Esh C, Kokjohn T, Sue L, Beach T. Atherosclerosis and AD: analysis of data from the US National Alzheimer's Coordinating Center. Neurology. 2005;65:974. doi: 10.1212/wnl.65.6.974. author reply 974. [DOI] [PubMed] [Google Scholar]
- 27.Moody DM, Thore CR, Anstrom JA, Challa VR, Langefeld CD, Brown WR. Quantification of afferent vessels shows reduced brain vascular density in subjects with leukoaraiosis. Radiology. 2004;233:883–890. doi: 10.1148/radiol.2333020981. [DOI] [PubMed] [Google Scholar]
- 28.Kalaria RN, Hedera P. Differential degeneration of the cerebral microvasculature in Alzheimer's disease. Neuroreport. 1995;6:477–480. doi: 10.1097/00001756-199502000-00018. [DOI] [PubMed] [Google Scholar]
- 29.Zlokovic BV. The blood-brain barrier in health and chronic neurodegenerative disorders. Neuron. 2008;57:178–201. doi: 10.1016/j.neuron.2008.01.003. [DOI] [PubMed] [Google Scholar]
- 30.Deane R, Zlokovic BV. Role of the blood-brain barrier in the pathogenesis of Alzheimer's disease. Curr Alzheimer Res. 2007;4:191–197. doi: 10.2174/156720507780362245. [DOI] [PubMed] [Google Scholar]
- 31.Jagust WJ, Seab JP, Huesman RH, et al. Diminished glucose transport in Alzheimer's disease: dynamic PET studies. J Cereb Blood Flow Metab. 1991;11:323–330. doi: 10.1038/jcbfm.1991.65. [DOI] [PubMed] [Google Scholar]
- 32.Marcus DL, Freedman ML. Decreased brain glucose metabolism in microvessels from patients with Alzheimer's disease. Ann N Y Acad Sci. 1997;826:248–253. doi: 10.1111/j.1749-6632.1997.tb48476.x. [DOI] [PubMed] [Google Scholar]
- 33.Deng Y, Li B, Liu Y, Iqbal K, Grundke-Iqbal I, Gong CX. Dysregulation of insulin signaling, glucose transporters, O-GlcNAcylation, and phosphorylation of tau and neurofilaments in the brain: Implication for Alzheimer's disease. Am J Pathol. 2009;175:2089–2098. doi: 10.2353/ajpath.2009.090157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Grammas P, Samany PG, Thirumangalakudi L. Thrombin and inflammatory proteins are elevated in Alzheimer's disease microvessels: implications for disease pathogenesis. J Alzheimers Dis. 2006;9:51–58. doi: 10.3233/jad-2006-9105. [DOI] [PubMed] [Google Scholar]
- 35.Kalaria R, Thomas A, Oakley AE, et al. Cerebrovascular amyloidosis and dementia. Curr Med Chem Immun Endoc & Metab Agents. 2003;4:317–327. [Google Scholar]
- 36.Thirumangalakudi L, Samany PG, Owoso A, Wiskar B, Grammas P. Angiogenic proteins are expressed by brain blood vessels in Alzheimer's disease. J Alzheimers Dis. 2006;10:111–118. doi: 10.3233/jad-2006-10114. [DOI] [PubMed] [Google Scholar]
- 37.Kalaria RN, Cohen DL, Premkumar DR, Nag S, LaManna JC, Lust WD. Vascular endothelial growth factor in Alzheimer's disease and experimental cerebral ischemia. Brain Res Mol Brain Res. 1998;62:101–105. doi: 10.1016/s0169-328x(98)00190-9. [DOI] [PubMed] [Google Scholar]
- 38.Wu Z, Guo H, Chow N, et al. Role of the MEOX2 homeobox gene in neurovascular dysfunction in Alzheimer disease. Nat Med. 2005;11:959–965. doi: 10.1038/nm1287. [DOI] [PubMed] [Google Scholar]
- 39.Emerich DF, Skinner SJ, Borlongan CV, Vasconcellos AV, Thanos CG. The choroid plexus in the rise, fall and repair of the brain. Bioessays. 2005;27:262–274. doi: 10.1002/bies.20193. [DOI] [PubMed] [Google Scholar]
- 40.Serot JM, Bene MC, Faure GC. Choroid plexus, aging of the brain, and Alzheimer's disease. Front Biosci. 2003;8:s515–s521. doi: 10.2741/1085. [DOI] [PubMed] [Google Scholar]
- 41.Redzic ZB, Preston JE, Duncan JA, Chodobski A, Szmydynger-Chodobska J. The choroid plexus-cerebrospinal fluid system: from development to aging. Curr Top Dev Biol. 2005;71:1–52. doi: 10.1016/S0070-2153(05)71001-2. [DOI] [PubMed] [Google Scholar]
- 42.Wen GY, Wisniewski HM, Kascsak RJ. Biondi ring tangles in the choroid plexus of Alzheimer's disease and normal aging brains: a quantitative study. Brain Res. 1999;832:40–46. doi: 10.1016/s0006-8993(99)01466-3. [DOI] [PubMed] [Google Scholar]
- 43.Finch CE. Developmental origins of aging in brain and blood vessels: an overview. Neurobiol Aging. 2005;26:281–291. doi: 10.1016/j.neurobiolaging.2004.03.015. [DOI] [PubMed] [Google Scholar]
- 44.Bateman RJ, Wen G, Morris JC, Holtzman DM. Fluctuations of CSF amyloid-beta levels: implications for a diagnostic and therapeutic biomarker. Neurology. 2007;68:666–669. doi: 10.1212/01.wnl.0000256043.50901.e3. [DOI] [PubMed] [Google Scholar]
- 45.Ballard C, O'Brien J, Barber B, et al. Neurocardiovascular instability, hypotensive episodes, and MRI lesions in neurodegenerative dementia. Ann N Y Acad Sci. 2000;903:442–445. doi: 10.1111/j.1749-6632.2000.tb06396.x. [DOI] [PubMed] [Google Scholar]
- 46.Moretti R, Torre P, Antonello RM, Manganaro D, Vilotti C, Pizzolato G. Risk factors for vascular dementia: hypotension as a key point. Vasc Health Risk Manag. 2008;4:395–402. doi: 10.2147/vhrm.s2434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Stoquart-ElSankari S, Baledent O, Gondry-Jouet C, Makki M, Godefroy O, Meyer ME. Aging effects on cerebral blood and cerebrospinal fluid flows. J Cereb Blood Flow Metab. 2007;27:1563–1572. doi: 10.1038/sj.jcbfm.9600462. [DOI] [PubMed] [Google Scholar]
- 48.Lee TJ, Shirasaki Y, Nickols GA. Altered endothelial modulation of vascular tone in aging and hypertension. Blood Vessels. 1987;24:132–136. doi: 10.1159/000158686. [DOI] [PubMed] [Google Scholar]
- 49.Borenstein AR, Copenhaver CI, Mortimer JA. Early-life risk factors for Alzheimer disease. Alzheimer Dis Assoc Disord. 2006;20:63–72. doi: 10.1097/01.wad.0000201854.62116.d7. [DOI] [PubMed] [Google Scholar]
- 50.Luchsinger JA, Reitz C, Honig LS, Tang MX, Shea S, Mayeux R. Aggregation of vascular risk factors and risk of incident Alzheimer disease. Neurology. 2005;65:545–551. doi: 10.1212/01.wnl.0000172914.08967.dc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Purnell C, Gao S, Callahan CM, Hendrie HC. Cardiovascular risk factors and incident Alzheimer disease: a systematic review of the literature. Alzheimer Dis Assoc Disord. 2009;23:1–10. doi: 10.1097/WAD.0b013e318187541c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Middleton LE, Yaffe K. Promising strategies for the prevention of dementia. Arch Neurol. 2009;66:1210–1215. doi: 10.1001/archneurol.2009.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.de la Torre JC. Alzheimer disease as a vascular disorder: nosological evidence. Stroke. 2002;33:1152–1162. doi: 10.1161/01.str.0000014421.15948.67. [DOI] [PubMed] [Google Scholar]
- 54.Kalaria RN, Ballard C. Stroke and cognition. Curr Atheroscler Rep. 2001;3:334–339. doi: 10.1007/s11883-001-0028-5. [DOI] [PubMed] [Google Scholar]
- 55.Kokmen E, Whisnant JP, O'Fallon WM, Chu CP, Beard CM. Dementia after ischemic stroke: a population-based study in Rochester, Minnesota (1960–1984) Neurology. 1996;46:154–159. doi: 10.1212/wnl.46.1.154. [DOI] [PubMed] [Google Scholar]
- 56.Kalaria RN. The role of cerebral ischemia in Alzheimer's disease. Neurobiol Aging. 2000;21:321–330. doi: 10.1016/s0197-4580(00)00125-1. [DOI] [PubMed] [Google Scholar]
- 57.Suter OC, Sunthorn T, Kraftsik R, et al. Cerebral hypoperfusion generates cortical watershed microinfarcts in Alzheimer disease. Stroke. 2002;33:1986–1992. doi: 10.1161/01.str.0000024523.82311.77. [DOI] [PubMed] [Google Scholar]
- 58.Gold G, Kovari E, Hof PR, Bouras C, Giannakopoulos P. Sorting out the clinical consequences of ischemic lesions in brain aging: a clinicopathological approach. J Neurol Sci. 2007;257:17–22. doi: 10.1016/j.jns.2007.01.020. [DOI] [PubMed] [Google Scholar]
- 59.Vermeer SE, Hollander M, van Dijk EJ, Hofman A, Koudstaal PJ, Breteler MM. Silent brain infarcts and white matter lesions increase stroke risk in the general population: the Rotterdam Scan Study. Stroke. 2003;34:1126–1129. doi: 10.1161/01.STR.0000068408.82115.D2. [DOI] [PubMed] [Google Scholar]
- 60.Vermeer SE, Longstreth WT, Jr, Koudstaal PJ. Silent brain infarcts: a systematic review. Lancet Neurol. 2007;6:611–619. doi: 10.1016/S1474-4422(07)70170-9. [DOI] [PubMed] [Google Scholar]
- 61.Vermeer SE, Prins ND, den Heijer T, Hofman A, Koudstaal PJ, Breteler MM. Silent brain infarcts and the risk of dementia and cognitive decline. N Engl J Med. 2003;348:1215–1222. doi: 10.1056/NEJMoa022066. [DOI] [PubMed] [Google Scholar]
- 62.Prins ND, van Dijk EJ, den Heijer T, et al. Cerebral small-vessel disease and decline in information processing speed, executive function and memory. Brain. 2005;128:2034–2041. doi: 10.1093/brain/awh553. [DOI] [PubMed] [Google Scholar]
- 63.Henon H, Pasquier F, Durieu I, Pruvo JP, Leys D. Medial temporal lobe atrophy in stroke patients: relation to pre-existing dementia. J Neurol Neurosurg Psychiatry. 1998;65:641–647. doi: 10.1136/jnnp.65.5.641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Firbank MJ, Burton EJ, Barber R, et al. Medial temporal atrophy rather than white matter hyperintensities predict cognitive decline in stroke survivors. Neurobiol Aging. 2007;28:1664–1669. doi: 10.1016/j.neurobiolaging.2006.07.009. [DOI] [PubMed] [Google Scholar]
- 65.Henon H, Pasquier F, Durieu I, et al. Preexisting dementia in stroke patients. Baseline frequency, associated factors, and outcome. Stroke. 1997;28:2429–2436. doi: 10.1161/01.str.28.12.2429. [DOI] [PubMed] [Google Scholar]
- 66.Erkinjuntti T. Vascular cognitive deterioration and stroke. Cerebrovasc Dis. 2007;24 Suppl 1:189–194. doi: 10.1159/000107395. [DOI] [PubMed] [Google Scholar]
- 67.Kalaria RN, Ballard C. Overlap between pathology of Alzheimer disease and vascular dementia. Alzheimer Dis Assoc Disord. 1999;13 Suppl 3:S115–S123. doi: 10.1097/00002093-199912003-00017. [DOI] [PubMed] [Google Scholar]
- 68.Barker WW, Luis CA, Kashuba A, et al. Relative frequencies of Alzheimer disease, Lewy body, vascular and frontotemporal dementia, and hippocampal sclerosis in the State of Florida Brain Bank. Alzheimer Dis Assoc Disord. 2002;16:203–212. doi: 10.1097/00002093-200210000-00001. [DOI] [PubMed] [Google Scholar]
- 69.Fu C, Chute DJ, Farag ES, Garakian J, Cummings JL, Vinters HV. Comorbidity in dementia: an autopsy study. Arch Pathol Lab Med. 2004;128:32–38. doi: 10.5858/2004-128-32-CID. [DOI] [PubMed] [Google Scholar]
- 70.Nolan KA, Lino MM, Seligmann AW, Blass JP. Absence of vascular dementia in an autopsy series from a dementia clinic. J Am Geriatr Soc. 1998;46:597–604. doi: 10.1111/j.1532-5415.1998.tb01076.x. [DOI] [PubMed] [Google Scholar]
- 71.Knopman DS, Parisi JE, Boeve BF, et al. Vascular dementia in a population-based autopsy study. Arch Neurol. 2003;60:569–575. doi: 10.1001/archneur.60.4.569. [DOI] [PubMed] [Google Scholar]
- 72.Lewis H, Beher D, Cookson N, et al. Quantification of Alzheimer pathology in ageing and dementia: age-related accumulation of amyloid-beta(42) peptide in vascular dementia. Neuropathol Appl Neurobiol. 2006;32:103–118. doi: 10.1111/j.1365-2990.2006.00696.x. [DOI] [PubMed] [Google Scholar]
- 73.Pasquier F, Leys D, Scheltens P. The influence of coincidental vascular pathology on symptomatology and course of Alzheimer's disease. J Neural Transm Suppl. 1998;54:117–127. doi: 10.1007/978-3-7091-7508-8_11. [DOI] [PubMed] [Google Scholar]
- 74.Kalaria RN, Kenny RA, Ballard CG, Perry R, Ince P, Polvikoski T. Towards defining the neuropathological substrates of vascular dementia. J Neurol Sci. 2004;226:75–80. doi: 10.1016/j.jns.2004.09.019. [DOI] [PubMed] [Google Scholar]
- 75.Vermeer SE, Den Heijer T, Koudstaal PJ, Oudkerk M, Hofman A, Breteler MM. Incidence and risk factors of silent brain infarcts in the population-based Rotterdam Scan Study. Stroke. 2003;34:392–396. doi: 10.1161/01.str.0000052631.98405.15. [DOI] [PubMed] [Google Scholar]
- 76.Ballard C, McKeith I, O'Brien J, et al. Neuropathological substrates of dementia and depression in vascular dementia, with a particular focus on cases with small infarct volumes. Dement Geriatr Cogn Disord. 2000;11:59–65. doi: 10.1159/000017215. [DOI] [PubMed] [Google Scholar]
- 77.Esiri MM, Wilcock GK, Morris JH. Neuropathological assessment of the lesions of significance in vascular dementia. J Neurol Neurosurg Psychiatry. 1997;63:749–753. doi: 10.1136/jnnp.63.6.749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Vinters HV, Ellis WG, Zarow C, et al. Neuropathologic substrates of ischemic vascular dementia. J Neuropathol Exp Neurol. 2000;59:931–945. doi: 10.1093/jnen/59.11.931. [DOI] [PubMed] [Google Scholar]
- 79.Esiri MM, Nagy Z, Smith MZ, Barnetson L, Smith AD. Cerebrovascular disease and threshold for dementia in the early stages of Alzheimer's disease. Lancet. 1999;354:919–920. doi: 10.1016/S0140-6736(99)02355-7. [DOI] [PubMed] [Google Scholar]
- 80.Schneider JA, Wilson RS, Bienias JL, Evans DA, Bennett DA. Cerebral infarctions and the likelihood of dementia from Alzheimer disease pathology. Neurology. 2004;62:1148–1155. doi: 10.1212/01.wnl.0000118211.78503.f5. [DOI] [PubMed] [Google Scholar]
- 81.Launer LJ, Petrovitch H, Ross GW, Markesbery W, White LR. AD brain pathology: vascular origins? Results from the HAAS autopsy study. Neurobiol Aging. 2008;29:1587–1590. doi: 10.1016/j.neurobiolaging.2007.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Roman GC, Sachdev P, Royall DR, et al. Vascular cognitive disorder: a new diagnostic category updating vascular cognitive impairment and vascular dementia. J Neurol Sci. 2004;226:81–87. doi: 10.1016/j.jns.2004.09.016. [DOI] [PubMed] [Google Scholar]
- 83.Longstreth WT, Jr, Arnold AM, Beauchamp NJ, Jr, et al. Incidence, manifestations, and predictors of worsening white matter on serial cranial magnetic resonance imaging in the elderly: the Cardiovascular Health Study. Stroke. 2005;36:56–61. doi: 10.1161/01.STR.0000149625.99732.69. [DOI] [PubMed] [Google Scholar]
- 84.Hachinski V, Iadecola C, Petersen RC, et al. National Institute of Neurological Disorders and Stroke-Canadian Stroke Network vascular cognitive impairment harmonization standards. Stroke. 2006;37:2220–2241. doi: 10.1161/01.STR.0000237236.88823.47. [DOI] [PubMed] [Google Scholar]
- 85.Fazekas F, Kleinert R, Offenbacher H, et al. Pathologic correlates of incidental MRI white matter signal hyperintensities. Neurology. 1993;43:1683–1689. doi: 10.1212/wnl.43.9.1683. [DOI] [PubMed] [Google Scholar]
- 86.Young VG, Halliday GM, Kril JJ. Neuropathologic correlates of white matter hyperintensities. Neurology. 2008;71:804–811. doi: 10.1212/01.wnl.0000319691.50117.54. [DOI] [PubMed] [Google Scholar]
- 87.Das RR, Seshadri S, Beiser AS, et al. Prevalence and correlates of silent cerebral infarcts in the Framingham offspring study. Stroke. 2008;39:2929–2935. doi: 10.1161/STROKEAHA.108.516575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Burton EJ, Kenny RA, O'Brien J, et al. White matter hyperintensities are associated with impairment of memory, attention, and global cognitive performance in older stroke patients. Stroke. 2004;35:1270–1275. doi: 10.1161/01.STR.0000126041.99024.86. [DOI] [PubMed] [Google Scholar]
- 89.Au R, Massaro JM, Wolf PA, et al. Association of white matter hyperintensity volume with decreased cognitive functioning: the Framingham Heart Study. Arch Neurol. 2006;63:246–250. doi: 10.1001/archneur.63.2.246. [DOI] [PubMed] [Google Scholar]
- 90.van den Heuvel DM, ten Dam VH, de Craen AJ, et al. Increase in periventricular white matter hyperintensities parallels decline in mental processing speed in a non-demented elderly population. J Neurol Neurosurg Psychiatry. 2006;77:149–153. doi: 10.1136/jnnp.2005.070193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kerber KA, Whitman GT, Brown DL, Baloh RW. Increased risk of death in community-dwelling older people with white matter hyperintensities on MRI. J Neurol Sci. 2006;250:33–38. doi: 10.1016/j.jns.2006.06.022. [DOI] [PubMed] [Google Scholar]
- 92.Brickman AM, Schupf N, Manly JJ, et al. Brain morphology in older African Americans, Caribbean Hispanics, and whites from northern Manhattan. Arch Neurol. 2008;65:1053–1061. doi: 10.1001/archneur.65.8.1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Du AT, Schuff N, Chao LL, et al. White matter lesions are associated with cortical atrophy more than entorhinal and hippocampal atrophy. Neurobiol Aging. 2005;26:553–559. doi: 10.1016/j.neurobiolaging.2004.05.002. [DOI] [PubMed] [Google Scholar]
- 94.Lunetta KL, Erlich PM, Cuenco KT, et al. Heritability of magnetic resonance imaging (MRI) traits in Alzheimer disease cases and their siblings in the MIRAGE study. Alzheimer Dis Assoc Disord. 2007;21:85–91. doi: 10.1097/WAD.0b013e3180653bf7. [DOI] [PubMed] [Google Scholar]
- 95.Cherubini A, Lowenthal DT, Paran E, Mecocci P, Williams LS, Senin U. Hypertension and cognitive function in the elderly. Am J Ther. 2007;14:533–554. doi: 10.1097/MJT.0b013e3180ed6b8f. [DOI] [PubMed] [Google Scholar]
- 96.Skoog I, Lernfelt B, Landahl S, et al. 15-year longitudinal study of blood pressure and dementia. Lancet. 1996;347:1141–1145. doi: 10.1016/s0140-6736(96)90608-x. [DOI] [PubMed] [Google Scholar]
- 97.Qiu C, Winblad B, Fratiglioni L. The age-dependent relation of blood pressure to cognitive function and dementia. Lancet Neurol. 2005;4:487–499. doi: 10.1016/S1474-4422(05)70141-1. [DOI] [PubMed] [Google Scholar]
- 98.Sparks DL, Scheff SW, Liu H, Landers TM, Coyne CM, Hunsaker JC., 3rd Increased incidence of neurofibrillary tangles (NFT) in non-demented individuals with hypertension. J Neurol Sci. 1995;131:162–169. doi: 10.1016/0022-510x(95)00105-b. [DOI] [PubMed] [Google Scholar]
- 99.Petrovitch H, White LR, Izmirilian G, et al. Midlife blood pressure and neuritic plaques, neurofibrillary tangles, and brain weight at death: the HAAS. Honolulu-Asia aging Study. Neurobiol Aging. 2000;21:57–62. doi: 10.1016/s0197-4580(00)00106-8. [DOI] [PubMed] [Google Scholar]
- 100.Freitag MH, Peila R, Masaki K, et al. Midlife pulse pressure and incidence of dementia: the Honolulu-Asia Aging Study. Stroke. 2006;37:33–37. doi: 10.1161/01.STR.0000196941.58869.2d. [DOI] [PubMed] [Google Scholar]
- 101.McGuinness B, Todd S, Passmore P, Bullock R. Blood pressure lowering in patients without prior cerebrovascular disease for prevention of cognitive impairment and dementia. Cochrane Database Syst Rev. 2009 doi: 10.1002/14651858.CD004034.pub3. CD004034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Hughes TF, Ganguli M. Modifiable Midlife Risk Factors for Late-Life Cognitive Impairment and Dementia. Curr Psychiatry Rev. 2009;5:73–92. doi: 10.2174/157340009788167347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Hofman A, Ott A, Breteler MM, et al. Atherosclerosis, apolipoprotein E, and prevalence of dementia and Alzheimer's disease in the Rotterdam Study. Lancet. 1997;349:151–154. doi: 10.1016/S0140-6736(96)09328-2. [DOI] [PubMed] [Google Scholar]
- 104.Craft S. The role of metabolic disorders in Alzheimer disease and vascular dementia: two roads converged. Arch Neurol. 2009;66:300–305. doi: 10.1001/archneurol.2009.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Helzner EP, Luchsinger JA, Scarmeas N, et al. Contribution of vascular risk factors to the progression in Alzheimer disease. Arch Neurol. 2009;66:343–348. doi: 10.1001/archneur.66.3.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Sparks DL, Hunsaker JC, 3rd, Scheff SW, Kryscio RJ, Henson JL, Markesbery WR. Cortical senile plaques in coronary artery disease, aging and Alzheimer's disease. Neurobiol Aging. 1990;11:601–607. doi: 10.1016/0197-4580(90)90024-t. [DOI] [PubMed] [Google Scholar]
- 107.Soneira CF, Scott TM. Severe cardiovascular disease and Alzheimer's disease: senile plaque formation in cortical areas. Clin Anat. 1996;9:118–127. doi: 10.1002/(SICI)1098-2353(1996)9:2<118::AID-CA4>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 108.Launer LJ, White LR, Petrovitch H, Ross GW, Curb JD. Cholesterol and neuropathologic markers of AD: a population-based autopsy study. Neurology. 2001;57:1447–1452. doi: 10.1212/wnl.57.8.1447. [DOI] [PubMed] [Google Scholar]
- 109.Leibson CL, Rocca WA, Hanson VA, et al. Risk of dementia among persons with diabetes mellitus: a population-based cohort study. Am J Epidemiol. 1997;145:301–308. doi: 10.1093/oxfordjournals.aje.a009106. [DOI] [PubMed] [Google Scholar]
- 110.Ott A, Stolk RP, Hofman A, van Harskamp F, Grobbee DE, Breteler MM. Association of diabetes mellitus and dementia: the Rotterdam Study. Diabetologia. 1996;39:1392–1397. doi: 10.1007/s001250050588. [DOI] [PubMed] [Google Scholar]
- 111.Lu FP, Lin KP, Kuo HK. Diabetes and the risk of multi-system aging phenotypes: a systematic review and meta-analysis. PLoS One. 2009;4:e4144. doi: 10.1371/journal.pone.0004144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.van Harten B, de Leeuw FE, Weinstein HC, Scheltens P, Biessels GJ. Brain imaging in patients with diabetes: a systematic review. Diabetes Care. 2006;29:2539–2548. doi: 10.2337/dc06-1637. [DOI] [PubMed] [Google Scholar]
- 113.Kalmijn S, Feskens EJ, Launer LJ, Stijnen T, Kromhout D. Glucose intolerance, hyperinsulinaemia and cognitive function in a general population of elderly men. Diabetologia. 1995;38:1096–1102. doi: 10.1007/BF00402181. [DOI] [PubMed] [Google Scholar]
- 114.Kalmijn S, Foley D, White L, et al. Metabolic cardiovascular syndrome and risk of dementia in Japanese-American elderly men. The Honolulu-Asia aging study. Arterioscler Thromb Vasc Biol. 2000;20:2255–2260. doi: 10.1161/01.atv.20.10.2255. [DOI] [PubMed] [Google Scholar]
- 115.Kuusisto J, Koivisto K, Mykkanen L, et al. Association between features of the insulin resistance syndrome and Alzheimer's disease independently of apolipoprotein E4 phenotype: cross sectional population based study. BMJ. 1997;315:1045–1049. doi: 10.1136/bmj.315.7115.1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Whitmer RA. The epidemiology of adiposity and dementia. Curr Alzheimer Res. 2007;4:117–122. doi: 10.2174/156720507780362065. [DOI] [PubMed] [Google Scholar]
- 117.Whitmer RA, Gunderson EP, Quesenberry CP, Jr, Zhou J, Yaffe K. Body mass index in midlife and risk of Alzheimer disease and vascular dementia. Curr Alzheimer Res. 2007;4:103–109. doi: 10.2174/156720507780362047. [DOI] [PubMed] [Google Scholar]
- 118.Hughes TF, Borenstein AR, Schofield E, Wu Y, Larson EB. Association between late-life body mass index and dementia: The Kame Project. Neurology. 2009;72:1741–1746. doi: 10.1212/WNL.0b013e3181a60a58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Burgener SC, Buettner L, Coen Buckwalter K, et al. Evidence supporting nutritional interventions for persons in early stage Alzheimer's disease (AD) J Nutr Health Aging. 2008;12:18–21. doi: 10.1007/BF02982159. [DOI] [PubMed] [Google Scholar]
- 120.Solfrizzi V, Capurso C, D'Introno A, et al. Lifestyle-related factors in predementia and dementia syndromes. Expert Rev Neurother. 2008;8:133–158. doi: 10.1586/14737175.8.1.133. [DOI] [PubMed] [Google Scholar]
- 121.Morris MC, Evans DA, Bienias JL, Tangney CC, Wilson RS. Vitamin E and cognitive decline in older persons. Arch Neurol. 2002;59:1125–1132. doi: 10.1001/archneur.59.7.1125. [DOI] [PubMed] [Google Scholar]
- 122.Morris MC, Evans DA, Bienias JL, et al. Consumption of fish and n-3 fatty acids and risk of incident Alzheimer disease. Arch Neurol. 2003;60:940–946. doi: 10.1001/archneur.60.7.940. [DOI] [PubMed] [Google Scholar]
- 123.Huang TL, Zandi PP, Tucker KL, et al. Benefits of fatty fish on dementia risk are stronger for those without APOE epsilon4. Neurology. 2005;65:1409–1414. doi: 10.1212/01.wnl.0000183148.34197.2e. [DOI] [PubMed] [Google Scholar]
- 124.van Gelder BM, Tijhuis M, Kalmijn S, Kromhout D. Fish consumption, n-3 fatty acids, and subsequent 5-y cognitive decline in elderly men: the Zutphen Elderly Study. Am J Clin Nutr. 2007;85:1142–1147. doi: 10.1093/ajcn/85.4.1142. [DOI] [PubMed] [Google Scholar]
- 125.Morris MC, Evans DA, Tangney CC, Bienias JL, Wilson RS. Fish consumption and cognitive decline with age in a large community study. Arch Neurol. 2005;62:1849–1853. doi: 10.1001/archneur.62.12.noc50161. [DOI] [PubMed] [Google Scholar]
- 126.Kidd PM. Alzheimer's disease, amnestic mild cognitive impairment, and age-associated memory impairment: current understanding and progress toward integrative prevention. Altern Med Rev. 2008;13:85–115. [PubMed] [Google Scholar]
- 127.Sofi F, Cesari F, Abbate R, Gensini GF, Casini A. Adherence to Mediterranean diet and health status: meta-analysis. BMJ. 2008;337:a1344. doi: 10.1136/bmj.a1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Scarmeas N, Stern Y, Tang MX, Mayeux R, Luchsinger JA. Mediterranean diet and risk for Alzheimer's disease. Ann Neurol. 2006;59:912–921. doi: 10.1002/ana.20854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Luchsinger JA, Noble JM, Scarmeas N. Diet and Alzheimer's disease. Curr Neurol Neurosci Rep. 2007;7:366–372. doi: 10.1007/s11910-007-0057-8. [DOI] [PubMed] [Google Scholar]
- 130.Joseph JA, Shukitt-Hale B, Willis LM. Grape juice, berries, and walnuts affect brain aging and behavior. J Nutr. 2009;139:1813S–1817S. doi: 10.3945/jn.109.108266. [DOI] [PubMed] [Google Scholar]
- 131.Zandi PP, Anthony JC, Khachaturian AS, et al. Reduced risk of Alzheimer disease in users of antioxidant vitamin supplements: the Cache County Study. Arch Neurol. 2004;61:82–88. doi: 10.1001/archneur.61.1.82. [DOI] [PubMed] [Google Scholar]
- 132.Markus MA, Morris BJ. Resveratrol in prevention and treatment of common clinical conditions of aging. Clin Interv Aging. 2008;3:331–339. [PMC free article] [PubMed] [Google Scholar]
- 133.Swan GE, Lessov-Schlaggar CN. The effects of tobacco smoke and nicotine on cognition and the brain. Neuropsychol Rev. 2007;17:259–273. doi: 10.1007/s11065-007-9035-9. [DOI] [PubMed] [Google Scholar]
- 134.Tyas SL, White LR, Petrovitch H, et al. Mid-life smoking and late-life dementia: the Honolulu-Asia Aging Study. Neurobiol Aging. 2003;24:589–596. doi: 10.1016/s0197-4580(02)00156-2. [DOI] [PubMed] [Google Scholar]
- 135.Flicker L. Life style interventions to reduce the risk of dementia. Maturitas. 2009;63:319–322. doi: 10.1016/j.maturitas.2009.06.008. [DOI] [PubMed] [Google Scholar]
- 136.Rovio S, Spulber G, Nieminen LJ, et al. The effect of midlife physical activity on structural brain changes in the elderly. Neurobiol Aging. 2008 doi: 10.1016/j.neurobiolaging.2008.10.007. [DOI] [PubMed] [Google Scholar]
- 137.Podewils LJ, Guallar E, Kuller LH, et al. Physical activity, APOE genotype, and dementia risk: findings from the Cardiovascular Health Cognition Study. Am J Epidemiol. 2005;161:639–651. doi: 10.1093/aje/kwi092. [DOI] [PubMed] [Google Scholar]
- 138.Scarmeas N, Luchsinger JA, Schupf N, et al. Physical activity, diet, and risk of Alzheimer disease. JAMA. 2009;302:627–637. doi: 10.1001/jama.2009.1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Lautenschlager NT, Cox KL, Flicker L, et al. Effect of physical activity on cognitive function in older adults at risk for Alzheimer disease: a randomized trial. JAMA. 2008;300:1027–1037. doi: 10.1001/jama.300.9.1027. [DOI] [PubMed] [Google Scholar]
- 140.Colcombe SJ, Erickson KI, Raz N, et al. Aerobic fitness reduces brain tissue loss in aging humans. J Gerontol A Biol Sci Med Sci. 2003;58:176–180. doi: 10.1093/gerona/58.2.m176. [DOI] [PubMed] [Google Scholar]
- 141.Burns JM, Cronk BB, Anderson HS, et al. Cardiorespiratory fitness and brain atrophy in early Alzheimer disease. Neurology. 2008;71:210–216. doi: 10.1212/01.wnl.0000317094.86209.cb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Colcombe SJ, Erickson KI, Scalf PE, et al. Aerobic exercise training increases brain volume in aging humans. J Gerontol A Biol Sci Med Sci. 2006;61:1166–1170. doi: 10.1093/gerona/61.11.1166. [DOI] [PubMed] [Google Scholar]
- 143.Nagahara AH, Merrill DA, Coppola G, et al. Neuroprotective effects of brain-derived neurotrophic factor in rodent and primate models of Alzheimer's disease. Nat Med. 2009;15:331–337. doi: 10.1038/nm.1912. [DOI] [PMC free article] [PubMed] [Google Scholar]


