Abstract
Activation of mitogen-activated protein kinase (MAPK) in maturing mouse oocytes occurs after synthesis of Mos, a MAPKKK. To investigate whether Mos acts only through MEK1, we microinjected constitutively active forms of MEK1 (MEK1S218D/S222D referred herein as MEK*) and Raf (ΔRaf) into mouse oocytes. In mos–/– oocytes, which do not activate MAPK during meiosis and do not arrest in metaphase II, MEK* and ΔRaf did not rescue MAPK activation and metaphase II arrest, whereas Mos induced a complete rescue. MEK* and ΔRaf induced cleavage arrest of two-cell blastomeres. They induced MAPK activation when protein phosphatases were inhibited by okadaic acid, suggesting that Mos may inhibit protein phosphatases. Finally, in mos–/– oocytes, MEK* induced the phosphorylation of Xp42mapkD324N, a mutant less sensitive to dephosphorylation, showing that a MAPK phosphatase activity is present in mouse oocytes. We demonstrate that active MAPKK or MAPKKK cannot substitute for Mos to activate MAPK in mouse oocytes. We also show that a phosphatase activity inactivates MAPK, and that Mos can overcome this inhibitory activity. Thus Mos activates MAPK through two opposite pathways: activation of MEK1 and inhibition of a phosphatase.
Keywords: meiosis/MEK1/mouse oocyte/phosphatase/Raf-1
Introduction
Mitogen-activated protein kinase (MAPK) belongs to a family of conserved serine/threonine kinases that are activated after the resumption of meiosis in different species of invertebrate and vertebrate oocytes. Several studies performed using Xenopus oocytes have suggested that the activation of the Mos–MAPK pathway is necessary for the resumption of meiosis (Kuang et al., 1991; Kuang and Ashorn, 1993; Carnero et al., 1994; Kosako et al., 1994b; Haccard et al., 1995) and for the cytostatic factor (CSF)-induced arrest in metaphase II (M II) in this species (Haccard et al., 1993; Kosako et al., 1994a). However, it has been shown recently that germinal vesicle breakdown (GVBD) can occur under certain experimental conditions in the absence of detectable MAPK activation (Fisher et al., 1999; Gross et al., 2000). The injection of oligonucleotides against Mos in Xenopus oocytes blocks GVBD (Sagata et al., 1989), suggesting that Mos may have target(s) other than the MAPK kinase MEK. In mouse oocytes, knock-outs for the cmos gene have shown that the Mos–MAPK pathway is not necessary for GVBD but is important for M II arrest (Colledge et al., 1994; Choi et al., 1996; Hashimoto, 1996; Verlhac et al., 1996). Furthermore, the Mos–MAPK pathway is involved in microtubule organization during meiotic maturation of mouse oocytes (Verlhac et al., 1996).
In vertebrates, the MAPK cascade lies downstream of several proto-oncogene products such as Ras (Leevers and Marshall, 1992; Matsuda et al., 1992; Shibuya et al., 1992), Raf-1 (Dent et al., 1992; Howe et al., 1992) and Mos (Nebreda and Hunt, 1993; Posada et al., 1993; Shibuya and Ruderman, 1993). In Xenopus oocytes, several pathways can lead to p42mapk activation. The physiological pathway, which is activated after progesterone stimulation, involves Mos (Nebreda and Hunt, 1993; Posada et al., 1993; Shibuya and Ruderman, 1993). Other pathways involving Ras and Raf-1 would lie on the non-physiological tyrosine receptor pathway. The microinjection of oncogenic mammalian Ras into Xenopus oocytes induces MAPK activation before maturation-promoting factor (MPF) activation, differently from the progesterone pathway where both kinases are activated at the same time, just before GVBD (Nebreda and Hunt, 1993). Thus, the activation of MAPK by oncogenic Ras would occur through the classical Raf-1/MEK1 pathway independently of c-Mos synthesis (Daar et al., 1991; Fabian et al., 1993; Muslin et al., 1993; Fukuda et al., 1994). Whereas exogenous forms of Ras or Raf-1 induce MAPK activation and meiosis resumption, the inhibition of endogenous small G proteins of the Ras family by the lethal toxin from Clostridium sordellii triggers meiotic maturation (Rime et al., 1998). Despite all the studies performed in Xenopus, the relationships between the Mos and the Ras/Raf-1 pathways, as well as the role of small G proteins of the Ras family in triggering MAPK activation and meiosis resumption, still require further investigations.
Whilst many studies have been performed in Xenopus, not much is known about the mechanisms which bring about MAPK activation in mouse oocytes. Protein synthesis (Verlhac et al., 1993; Gavin et al., 1994), and in particular the synthesis of Mos (Verlhac et al., 1996), is necessary for MAPK activation in this species. However, it is still unclear whether Mos acts only by phosphorylating MEK1 to induce MAPK activation in mouse oocytes. Protein phosphatases are also involved, since inhibition of protein phosphatases by okadaic acid (OA) induces a precocious MAPK activation in mouse oocytes (Gavin et al., 1994). Raf-1 is present in mouse oocytes, but it is not activated during meiotic maturation and it does not replace Mos to induce MAPK activation in oocytes from Mos–/– mice (Verlhac et al., 1996).
Here we demonstrate that Mos acts not only through MEK1 phosphorylation but also through the inhibition of MAPK phosphatase activity.
Results
MEK1 is present in immature mouse oocytes
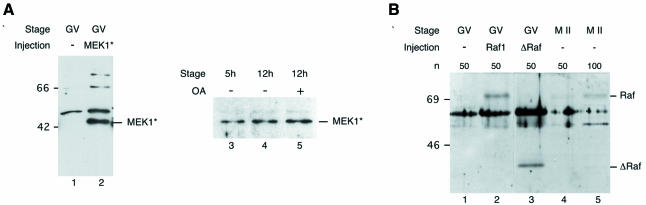
Numerous studies have shown that MAPK activation requires the activation of a MAPK kinase which has to be phosphorylated on two conserved serine residues in order to phosphorylate MAPK on threonine and tyrosine (for a review see Mordret, 1993). In vertebrates, the main pathway leading to MAPK activation involves the MAPK kinase, MEK1 (Mordret, 1993). After immunoblotting of oocytes collected at the germinal vesicle (GV) stage and at the M II stage, the anti-MEK1 antiserum recognized two major proteins of apparent mol. wts 45 and 66 kDa in both GV and M II oocytes (Figure 1, lanes 1 and 2). However, only the lower 45 kDa band corresponds to MEK1 since this signal disappeared when the antiserum was pre-incubated with 15 µM of the immunogenic peptide (Figure 1, lane 3). Thus MEK1 is already present in GV oocytes, and the amount of protein remains constant until the M II arrest in mouse oocytes (Figure 1, lanes 1 and 2).
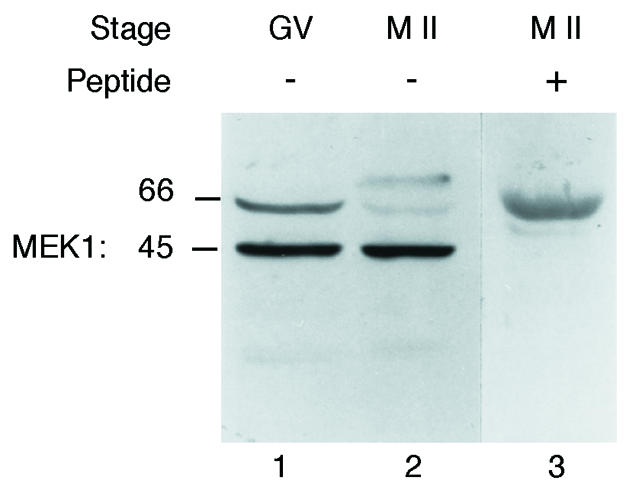
Fig. 1. Identification of MEK1 in mouse oocytes. A total of 250 immature (GV, lane 1) and 250 mature (M II, lane 2) oocytes were immunoblotted using an anti-MEK1 serum. The 250 mature oocytes were immunoblotted using an anti-MEK1 serum pre-incubated with 15 µM of the immunogenic peptide (lane 3).
ΔRaf and MEK* are overexpressed after microinjection into mouse oocytes
Since MEK1 was observed in immature mouse oocytes, it should be possible to activate the MAPK cascade using active forms of MAPK kinase kinase. Thus, we attempted to trigger MAPK activation by microinjecting mRNAs encoding a constitutively active form of Raf-1, ΔRaf. ΔRaf corresponds to a truncated version of the human Raf-1 that encodes only the CR3 catalytic domain (Fabian et al., 1993). We also used a constitutively active form of MEK1, MEK1S218D/S222D, referred to here as MEK*, to activate MAPK directly (Brunet et al., 1994; Pagès et al., 1994).
Microinjected oocytes remained at the GV stage in the presence of dbcAMP for at least 24 h. First we checked that the injection of the mRNAs coding for ΔRaf and for MEK* led to the overexpression of both proteins. Samples of 50 oocytes were collected after overnight expression of microinjected exogenous mRNAs. Using an anti-haemagglutinin (HA) antiserum, recognizing MEK* tagged with HA, we could detect a band of the expected size, ∼45 kDa, in MEK*-injected oocytes that is not present in non-injected oocytes (Figure 2A, compare lanes 1 and 2). This expression is already high 5 h after GVBD and is stable during all the time course of our experiment (Figure 2A, lanes 3 and 4). Using an anti-Raf-1 antibody, we observed that both full-length Raf-1 and truncated ΔRaf were expressed as proteins of the expected mol. wts: 72–74 kDa for Raf-1 and 38 kDa for ΔRaf (Figure 2B, lanes 2 and 3). Thus, although MEK1 and ΔRaf were overexpressed in mouse, they did not induce GVBD in the presence of dbcAMP. The amount of overexpression was estimated by comparing the level of expression of the endogenous and exogenous proteins. ΔRaf was overexpressed ∼13 times (Figure 2B, compare lane 3 with lanes 4 and 5) while MEK* was overexpressed ∼15 times (data not shown).
Fig. 2. (A) Overexpression of constitutively active HA-tagged MEK* after microinjection. Fifty immature oocytes were either not injected (lane 1) or injected with mRNAs encoding HA-tagged MEK* cultured overnight in dbcAMP (lane 2) or cultured for 5 (lane 3) or 12 h (lane 4) after GVBD and then collected. Oocytes microinjected with HA-tagged MEK1S218D/S222D were also cultured for 10 h after GVBD and then incubated for 2 h in OA and collected (lane 5). Samples were then subjected to immunoblotting using an anti-HA antibody. (B) Overexpression of Raf1 and ΔRaf after microinjection. Fifty immature oocytes were either not injected (lane 1) or injected with mRNAs encoding full-length Raf1 (lane 2) or ΔRaf (lane 3), cultured overnight in dbcAMP and then collected. Control M II oocytes were also collected to show the endogenous Raf (50 M II, lane 4; 100 M II, lane 5). Samples were then subjected to immunoblotting using the anti-Raf1 serum.
ΔRaf did not induce MAPK activation in mouse oocytes treated with puromycin
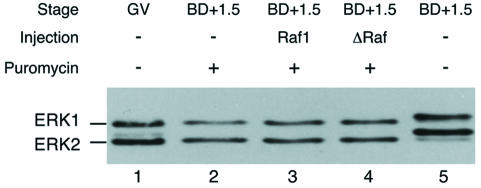
We have shown previously that MAPK activation requires protein synthesis (Verlhac et al., 1993). Since MEK1 is already present in immature mouse oocytes and does not need to be synthesized during meiosis resumption (this study, see above), we attempted to trigger MAPK activation using ΔRaf. We microinjected mRNAs coding for ΔRaf into the cytoplasm of GV stage oocytes that were maintained in prophase by culture in dbcAMP overnight to allow the overexpression of the protein. Then oocytes were transferred to M2 medium without dbcAMP containing 10 µg/ml puromycin to allow GVBD in the absence of protein synthesis (notably without Mos synthesis). Samples were collected 1.5 h after GVBD in these experimental conditions and analysed by immunoblotting using the anti-ERK serum.
The electrophoretic mobility of ERK1 and ERK2 in full-length Raf-1- (which serves as a control for mRNA injection) and ΔRaf-injected oocytes remains identical to their mobility in immature oocytes (Figure 3). This implies that ERK1 and ERK2 are not phosphorylated and thus remain inactive both in non-injected and injected oocytes. Thus, ΔRaf cannot induce MAPK activation in the absence of protein synthesis.
Fig. 3. Overexpression of ΔRaf does not induce MAPK activation in puromycin-treated oocytes. Oocytes were injected with either full-length Raf1 (as an injection control) or ΔRaf, cultured for 12 h in dbcAMP, then washed from dbcAMP and incubated in 10 µg/ml puromycin. Batches of 25 oocytes were immunoblotted with the anti-ERK serum. Lanes 1 and 5, respectively, control GV oocytes and oocytes matured for 1.5 h post-GVBD in M2 medium; lanes 2, 3 and 4, oocytes matured in puromycin-containing medium and collected 1.5 h post-GVBD, either not injected (lane 2) or injected with full-length Raf1 (lane 3) or ΔRaf (lane 4).
MEK* and ΔRaf did not restore MAPK activation and the M II arrest in mos–/– oocytes, while Mos did
ΔRaf was not able to trigger MAPK activation in puromycin-treated mouse oocytes. However, we could not exclude that the synthesis of an unknown protein was required to trigger MAPK activation. Thus, we decided to use a cleaner system to test the ability of ΔRaf or MEK* to trigger MAPK activation. Oocytes from c-mos knockout mice do not activate MAPK and do not arrest in M II (Colledge et al., 1994; Hashimoto et al., 1994; Verlhac et al., 1996). We therefore studied whether ΔRaf or MEK* were able to rescue MAPK activation and the M II arrest in mos–/– oocytes.
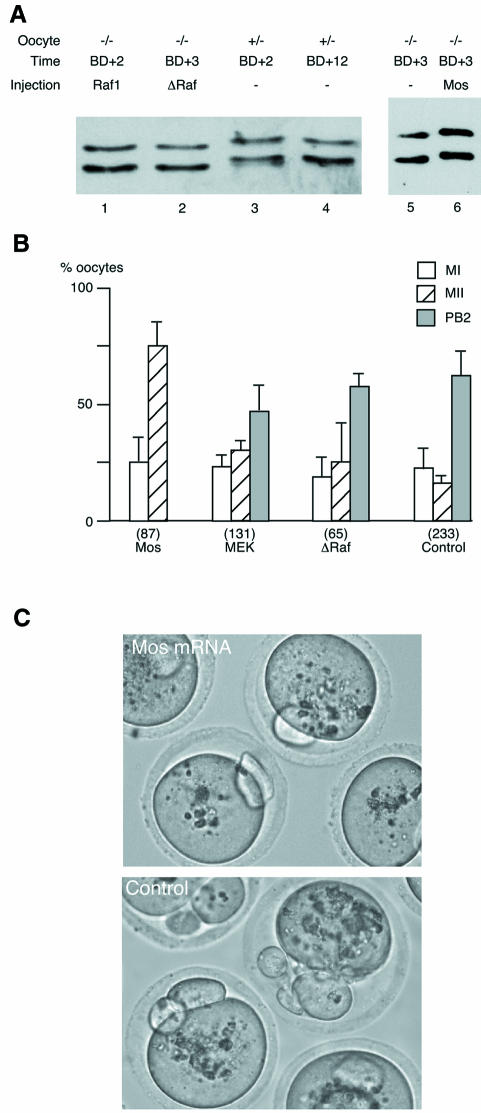
We microinjected mos–/– oocytes at the GV stage in dbcAMP-containing medium. After 6 h, the dbcAMP was removed and the oocytes were allowed to mature overnight. They were then scored for second polar body extrusion and assayed for MAPK activation. Surprisingly, ΔRaf and MEK* were not able to trigger MAPK activation in mos–/– oocytes (Figure 4A, compare lanes 2 and 3 and Figure 6B, lane 3). Moreover, the M II arrest was not restored since MEK*- or ΔRaf-injected mos–/– oocytes spontaneously extruded the second polar body with a frequency comparable to that of control non-injected oocytes (Figure 4B).
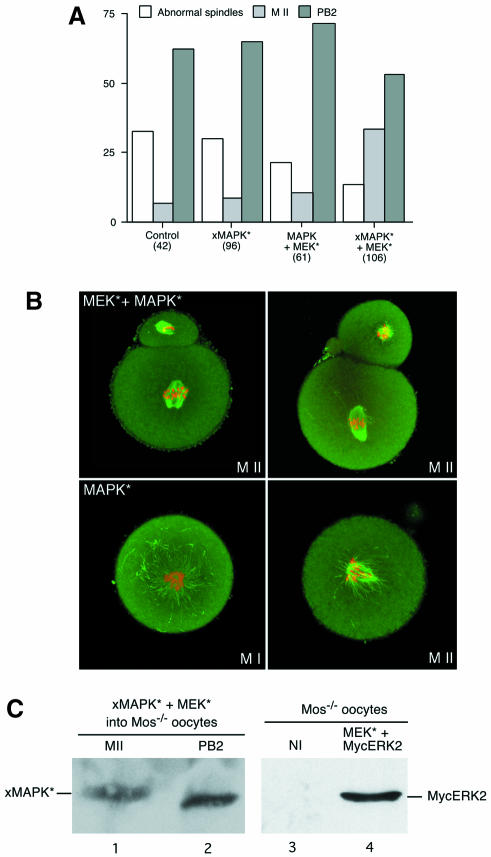
Fig. 4. (A) Overexpression of ΔRaf does not induce MAPK activation in mos–/– oocytes while Mos overexpression does. Mos–/– oocytes were injected with mRNAs encoding either full-length Raf1 (as an injection control), ΔRaf or Mos, cultured for 5 h in dbcAMP then removed from dbcAMP and collected at various times after GVBD. Groups of 25 oocytes were immunoblotted with the anti-ERK serum. Lanes 1 and 2, respectively, Raf1- and ΔRaf-injected mos–/– oocytes collected 3 h after GVBD; lanes 3 and 4, control mos+/– oocytes matured for 2 (lane 3) or 12 h (lane 4) post-GVBD; lanes 5 and 6, respectively, non-injected and Mos-injected mos–/– oocytes collected 3 h after GVBD. (B) Mos, but not MEK* or ΔRaf, restores the M II arrest in mos–/– oocytes. Mos–/– oocytes were either not injected (Control) or were injected with RNAs encoding full-length Mos, constitutively active MEK* or ΔRaf in dbcAMP-containing medium. The oocytes were kept for 5 h in this medium and released in M2 medium for overnight culture. We then scored the oocytes with no polar body (MI), with only one polar body (M II) or with two polar bodies (spontaneously activated oocytes, PB2). The numbers in parentheses represent the total number of oocytes injected. These results correspond to at least three independent experiments. (C) Mos–/– oocytes injected with Mos and arrested in M II (top) and spontaneously activated control non-injected oocytes with two polar bodies (bottom).
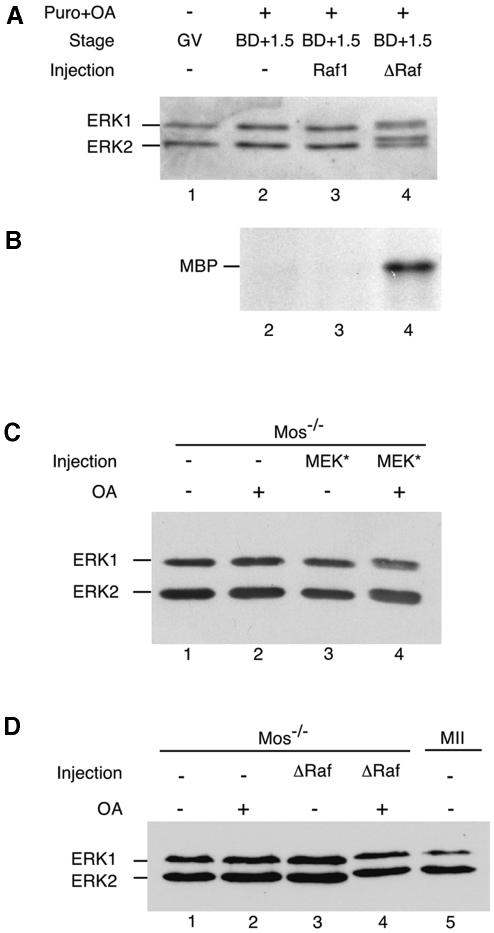
Fig. 6. MEK* and ΔRaf trigger MAPK activation when phosphatases are inhibited by okadaic acid (OA). (A) ERK immunoblot. Oocytes were injected with either full-length Raf1 (as an injection control) or ΔRaf, cultured for 12 h in dbcAMP, then washed from dbcAMP and treated with both puromycin and OA, where they resumed meiosis. Groups of 25 oocytes were immunoblotted with the anti-ERK serum. Lane 1, control GV oocytes; lanes 2, 3 and 4, oocytes matured in puromycin- and OA-containing medium and collected 1.5 h after GVBD, either not injected (lane 2), or injected with full-length Raf1 (lane 3) or ΔRaf (lane 4). (B) MBP kinase assay. Groups of 10 oocytes were subjected to MBP kinase assay. Oocytes, matured in puromycin- and OA-containing medium and collected 1.5 h after GVBD, were either not injected (lane 2), or injected with full-length Raf1 (lane 3) or ΔRaf (lane 4). (C) MEK* triggers MAPK activation in mos–/– oocytes that were cultured in OA. Mos –/– oocytes were either not injected (lanes 1 and 2), or injected with MEK* (lanes 3 and 4), cultured for 5 h in dbcAMP, released in M2 medium for overnight culture and then cultured for 1.5 h with (+) or without (–) OA. Groups of 25 oocytes were immunoblotted with the anti-ERK serum. (D) ΔRaf triggers MAPK activation in mos–/– oocytes that were cultured in OA. Mos –/– oocytes were either not injected (lanes 1 and 2) or injected with ΔRaf (lanes 3 and 4), cultured for 5 h in dbcAMP, released in M2 medium for overnight culture and then cultured for 1.5 h with (+) or without (–) OA. Lane 5: control M II-arrested oocytes. Groups of 25 oocytes were immunoblotted with the anti-ERK serum.
Only the injection of Mos mRNA induced MAPK activation (Figure 4A, compare lanes 5 and 6) and rescued the M II arrest (Figure 4B and C). None of 87 mos–/– oocytes that were injected with Mos extruded a second polar body. Thus we concluded that ΔRaf and MEK* are not able to replace Mos to activate MAPK during meiosis in mouse oocytes.
ΔRaf and MEK* induce a cleavage arrest in two-cell mouse embryos
The presence of active MAPK or the activation of the MAPK pathway later during development induces a cleavage arrest (Haccard et al., 1993). To confirm that both MEK* and ΔRaf were able to activate MAPK by a biological assay, we microinjected the mRNAs into blastomeres of late two-cell embryos. Both MEK* and ΔRaf induced a cleavage arrest after injection into a late [50–51 h post-human chorionic gonadotrophin (hCG)] two-cell blastomere (Table I and Figure 5). MEK* induced a cleavage arrest in 56% of the injected blastomeres [∼4 times more than in controls injected with tubulin– green fluorescent protein (GFP) mRNAs]. This cleavage arrest was also observed after a 2-fold dilution of the MEK* mRNA (data not shown). ΔRaf was as potent as Mos in inducing a cleavage arrest: 87% for ΔRaf-injected blastomeres and 88% for the Mos-injected ones. In Xenopus embryos, the cleavage arrest induced by ectopic activation of the MAPK pathway occurs in M-phase. Mouse blastomeres injected with Mos, MEK* or ΔRaf arrest in interphase with decondensed chromatin and interphase microtubules in the two daughter cells of the injected blastomere (Figure 5B). The cleavage arrest induced by MEK*, and probably also by ΔRaf or Mos, is indeed due to the activation of MAPK: using an anti-phospho-ERK antibody, we can detect both ERK1 and ERK2 in their phosphorylated forms after MEK* injection but not in uninjected embryos (Figure 5C, compare lanes 1 and 2 with the positive control in lane 3).
Table I. Percentage of cleavage arrest after injection of mRNAs encoding MEK*, ΔRaf, Mos and tubulin–GFP (as an injection control) into one blastomere of a late two-cell embryo.
| mRNA injected | % cleavage arrest | No. of embryos |
|---|---|---|
| MEK1S218D/S222D | 56 | 100 |
| ΔRaf | 87 | 46 |
| Mos | 88 | 43 |
| Tubulin–GFP | 15 | 91 |
| Non-injected control | 8 | 215 |
Two-cell embryos were injected late, i.e. between 50 and 51 h after hCG injection (which corresponds to 2–3 h before the two- to four-cell division), to avoid degradation of the injected RNAs.
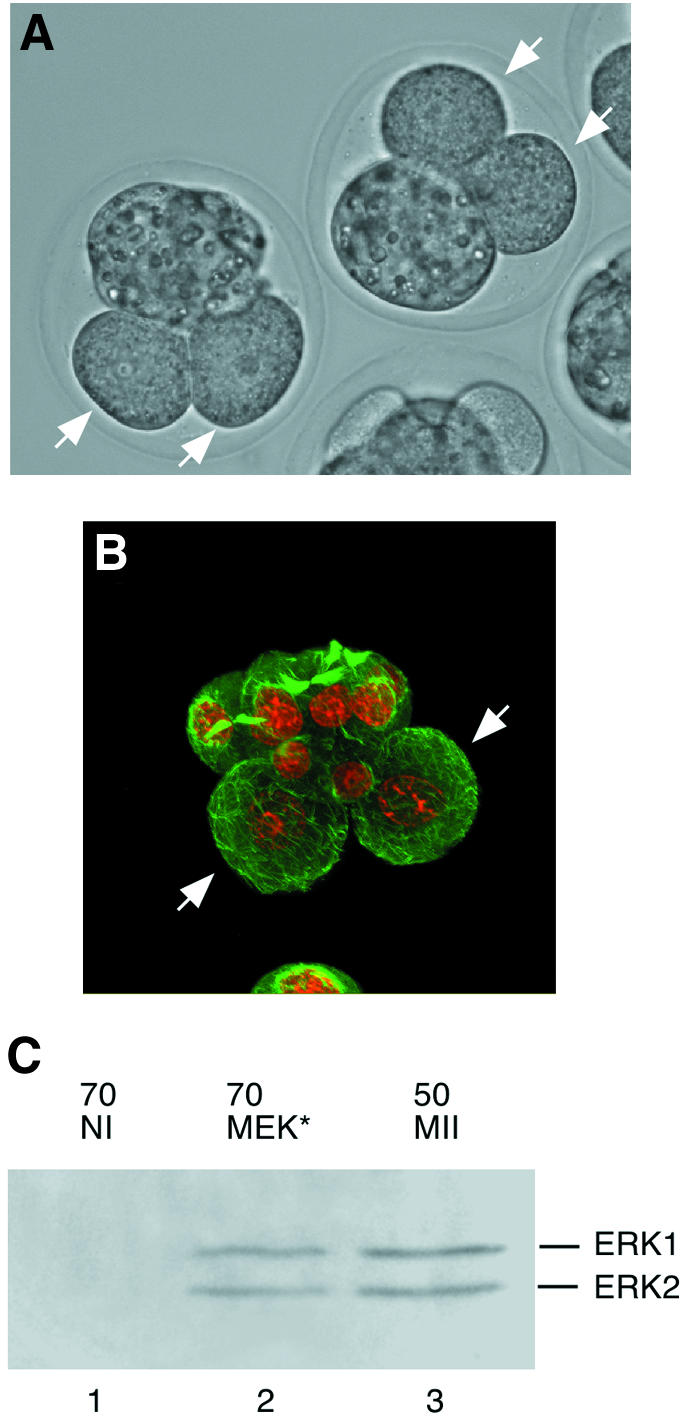
Fig. 5. (A and B) Cleavage arrest induced by microinjection of the MEK* (A) or Mos (B) mRNA into one blastomere of a two-cell embryo, 24 h after injection (white arrows). (A) Phase contrast. (B) Immunofluorescence: microtubules appear in green and chromosomes in red. (C) MEK* induces MAPK phosphorylation after overexpression in one blastomere of two-cell embryos, 24 h after injection. Immuno blotting using anti-phospho-ERK antibody of 70 control uninjected embryos (lane 1), of 70 embryos injected with MEK* into one blastomere of a two-cell stage embryo and collected 24 h after injection (lane 2), and of 50 control M II oocytes (lane 3).
These results show that both MEK* and ΔRaf are active after injection of the corresponding mRNAs. Therefore, the lack of rescue of the c-mos knockout phenotype by both MEK* and ΔRaf cannot be explained by a lack of activity of both proteins.
ΔRaf and MEK* induce MAPK activation after okadaic acid treatment
As mentioned in the Introduction, phosphatases sensitive to OA are involved in the control of MAPK activation in mouse oocytes (Gavin et al., 1994). To test whether these phosphatases inhibit MAPK activation by MEK* or ΔRaf during meiosis, we treated MEK*- and ΔRaf-injected oocytes with OA. Wild-type oocytes treated with puromycin and OA do not activate MAPK (Figure 6A, lane 2). However, if they expressed ΔRaf (but not Raf-1), we observed both MAPK phosphorylation (Figure 6A, compare lanes 3 and 4) and activation, as evidenced by MBP kinase activity (Figure 6B, compare lanes 3 and 4).
Similarly, mos–/– oocytes did not activate MAPK after MEK* or ΔRaf injection, or OA treatment alone (Figure 6C and D, lanes 3 and 2, respectively). However, we observed MAPK activation in oocytes microinjected with MEK* or ΔRaf and then incubated for 2 h in OA (Figure 6C and D, lane 4). We can exclude the possibility that somehow OA treatment could stabilize the overexpressed proteins since, after treament with OA, we do not observe significant accumulation of HA-tagged MEK* in microinjected oocytes compared with untreated oocytes (Figure 2A, compare lanes 4 and 5).
These results show that both MEK* and ΔRaf can activate endogenous MAPK when OA-sensitive phosphatases are inhibited.
Mos induces meiosis resumption in dbcAMP
An additional observation suggests that Mos acts through a pathway dependent upon the inhibition of OA-sensitive phosphatases. Mos injection triggers GVBD in mouse oocytes in the presence of dbcAMP, like OA (Gavin et al., 1992). As shown in Figure 7, only Mos, and neither MEK* nor ΔRaf, induced GVBD efficiently (up to 75% after 8 h) in the presence of dbcAMP.
Fig. 7. (A) Effect of Mos, MEK* and ΔRaf mRNAs microinjection on meiosis resumption in medium containing dbcAMP. The numbers in parentheses represent the total number of oocytes injected. The results of at least three independent experiments were summed. (B) Kinetics of GVBD induction after Mos mRNA injection in oocytes maintained in dbcAMP. Scoring of GVBD was perfomed every hour on groups of 30 injected oocytes maintained in dbcAMP.
Co-expression of the sevenmaker mutation, Xp42mapkD324N, and MEK* rescued the Mos phenotype and activated the exogenous MAPK
To demonstrate that a phosphatase able to dephosphorylate MAPK is active after MEK* or ΔRaf injection but not after Mos injection, we co-expressed both MEK* and Xp42mapkD324N (Umbhauer et al., 1995). This sevenmaker mutation in the Xenopus p42 MAPK is known to produce a protein less sensitive to phosphatases (Brunner et al., 1994; Chu et al., 1996). By itself, the Xp42mapkD324N was not able to rescue the M II arrest (Figure 8A). However, when co-expressed with MEK*, it induced an increase in the percentage of M II-arrested oocytes, from 8.1 to 33.2% (Figure 8A). The ratio M II/PB2 was 0.6 in MEK* and Xp42mapkD324N co-injected oocytes compared with 0.1 in Xp42mapkD324N-injected, non-injected mos–/– and MEK* and wild-type MycERK2 co-injected oocytes (Figure 8A). Moreover, co-expression of Xp42mapkD324N and MEK* reduced the percentage of abnormal spindles leading to an M I or M II arrest in mos–/– oocytes (Figure 8B). These M-phase arrests are due to the activation of the spindle assembly checkpoint (SAC) and not to CSF, as described previously (Verlhac et al., 1996), and are amplified by the long incubation time in dbcAMP required for the overexpression of the exogenous proteins. Finally, the electrophoretic mobility of Xp42mapkD324N was retarded in M II-arrested mos–/– oocytes microinjected with Xp42mapkD324N and MEK* (Figure 8C, lane 1) but not in those oocytes that had extruded the second polar body (Figure 8C, lane 2). The high level of overexpression of the Xp42mapkD324N allows its detection without detecting the endogenous MAPK (Figure 8C). We also checked that our MycERK2 was indeed expressed after co-injection with MEK* (Figure 8C, compare lanes 3 and 4). These results show that only the co-injection of Xp42mapkD324N and MEK* allowed the activation of the exogenous Xp42mapkD324N and rescued the CSF arrest in M II. It also rescued many spindle abnormalities that cause an M I or M II arrest in mos–/– oocytes by activating the SAC. Finally, our results demonstrate that a phosphatase, which dephosphorylates MAPK, is active in mos–/– oocytes.
Fig. 8. The co-injection of MEK* and Xp42mapkD324N in mos–/– oocytes rescues the M II arrest. (A) Mos–/– oocytes were either not injected (control), injected with RNAs encoding Xp42mapkD324N alone (xMAPK*), co-injected with wild-type MycERK2 and constitutively active MEK* (MAPK + MEK*), or co-injected with RNAs encoding Xp42mapkD324N and constitutively active MEK* (xMAPK* + MEK*) in dbcAMP-containing medium. After 5 h incubation in dbcAMP to allow overexpression of the exogenous proteins, the oocytes were transferred to M2 medium for overnight culture. The oocytes were then scored for the presence of polar bodies and normal bipolar spindles (see B): 0 or 1 polar body with abnormal spindles (arrested in M I or M II, abnormal spindles); one polar body and a normal spindle (M II); or two polar bodies (spontaneously activated oocytes, PB2). The numbers in parentheses represent the total number of oocytes injected. (B) Immunofluorescence staining of microtubules and chromatin in mos–/– oocytes microinjected with MEK* and Xp42mapkD324N (MEK* + xMAPK*) or Xp42mapkD324N alone (xMAPK*). Oocytes were microinjected in dbcAMP and kept in the drug for 5 h. The oocytes were transferred to M2 medium for overnight culture and fixed 16 h after GVBD. Microtubules appear in green and chromosomes in red. Top: normal spindles in oocytes injected with MEK* and Xp42mapkD324N that extruded one polar body (M II). Bottom: abnormal spindles in oocytes injected with Xp42mapkD324N that did not extrude the first polar body (M I, left) or extruded only one polar body (M II, right). (C) M II-arrested mos–/– oocytes co-injected with MEK* and Xp42mapkD324N show Xp42mapkD324N phosphorylation whereas the activated ones do not. Oocytes treated as in (A) were collected separately, i.e. M II-arrested (M II, lane 1) and activated (PB2, lane 2). Groups of 15 oocytes were immunoblotted with the anti-ERK serum. These results correspond to two independent experiments. The MycERK2 is overexpressed after co-injection with MEK* into mos–/– oocytes. Fifty mos–/– oocytes either not injected (NI, lane 3) or co-injected with MEK* and MycERK2 (MEK* + MycERK2, lane 4) were scored after second polar body extrusion, then collected and subjected to immunoblotting using the anti-Myc antibody.
Discussion
MEK1 and ΔRaf overexpression in mouse oocytes does not trigger MAPK activation
In this study, we analysed the mechanisms leading to MAPK activation after meiosis resumption in mouse oocytes. Using overexpressed constitutively active kinases (MEK* and ΔRaf) in oocytes devoid of MAPK activity (wild-type oocytes treated with puromycin or oocytes from Mos–/– mice), we show the existence of another pathway, which cannot be overcome by MEK1 activation.
It was very surprising to observe that ΔRaf, which is able to activate MAPK in Xenopus oocytes (Fabian et al., 1993), was not able to trigger MAPK activation in mouse oocytes treated with puromycin. We found that overexpression of both ΔRaf (Fabian et al., 1993) and MEK* (Brunet et al., 1994; Pagès et al., 1994), the kinase directly upstream of MAPK, was not able to activate MAPK in mos–/– oocytes. We checked that both proteins were indeed overexpressed in the oocytes. We also checked that ΔRaf and MEK* were active, as shown by their ability to induce a cleavage arrest efficiently in late two-cell embryos through MAPK phosphorylation. However, mouse blastomeres arrested in interphase, in contrast to Xenopus blastomeres that were arrested in M phase. This difference might be due to the fact that the G1 and G2 phases of the cell cycle appear as early as the first embryonic mitotic cycle in the mouse, while they appear later in Xenopus, at the mid-blastula transition (when the embryo is composed of thousands of cells). Indeed, it has been shown in starfish embryos and in Xenopus cycling egg extracts that active MAPK can arrest the mitotic cycle in the G1 or G2 phase of the cell cycle (Abrieu et al., 1997; Tachibana et al., 1997; Bitangcol et al., 1998).
We overexpressed a mutant MEK* that has been shown to have a basal activity 5-fold higher than serum- stimulated wild-type MEK1 (Brunet et al., 1994). Since we overexpressed it ∼15 times compared with the endogenous MEK1, we produced a MAPK kinase activity that was at least 10-fold (actually maximum 75-fold) higher than the endogenous MEK1 activity. Yet this mutant could not induce MAPK activation in mos–/– oocytes. This suggested that the pathway leading to MAPK activation was not limited to a direct activation of the Mos–MEK1–MAPK pathway. Our data also suggested that Mos had other target(s) beside MEK1, since ΔRaf or MEK* could not compensate for its function. Moreover, by rescuing both MAPK activation and M II arrest in mos–/– oocytes with Mos overexpression, we complete the demonstration that Mos is physiologically involved in the CSF arrest, probably through MAPK activation.
MAPK phosphatases are active in Mos–/– oocytes
Overexpression of a constitutively active MAPK kinase (MEK*), which directly phosphorylates MAPK, does not trigger MAPK activation in mos–/– oocytes. It is therefore likely that a MAPK phosphatase able to dephosphorylate MAPK immediately is present and active in mouse oocytes. To test this hypothesis directly, we co-injected into mos–/– oocytes both MEK* and a mutant form of MAPK, Xp42mapkD324N (Umbhauer et al., 1995), which is less sensitive to phosphatases (Brunner et al., 1994; Chu et al., 1996). This co-injection, and not the co-injection of MEK* and wild-type ERK2, was able to rescue the CSF arrest, increasing the proportion of mos–/– oocytes arrested in M II with a bipolar spindle. It also rescued some spindle defects that normally occur in mos–/– oocytes (Verlhac et al., 1996). However, this rescue was not complete. This is probably due to the fact that MEK* phosphorylates both the endogenous MAPK, whose phosphorylation turns over rapidly, and the exogenous MAPK, whose phosphorylation turns over more slowly. Moreover, since we are overexpressing a Xenopus protein in mouse oocytes, Xp42mapkD324N may not be able to recognize all the physiological substrates of the endogenous mouse MAPK. Nevertheless, we observed a phosphorylation of Xp42mapkD324N by MEK* in M II-arrested mos–/– oocytes. By using a MAPK less sensitive to phosphatases, we show that MEK* is active during meiosis and that a phosphatase activity able to counteract the activity of MEK* or ΔRaf is present in mos–/– oocytes.
Inhibition of OA-sensitive phosphatase(s) by Mos is involved in MAPK activation
Mouse oocytes treated with OA activate MAPK more rapidly than non-treated ones (Gavin et al., 1994), suggesting that an OA-sensitive phosphatase is active in maturing mouse oocytes and slows down MAPK activation.
Microinjection of either mos–/– oocytes or puromycin-treated wild-type oocytes with MEK* or ΔRaf did not activate MAPK. However, when these oocytes were incubated with OA, both MEK* and ΔRaf were able to activate MAPK. This demonstrates that there is (are) an (some) OA-sensitive phosphatase(s) that is (are) involved in the dephosphorylation of MAPK in mouse oocytes. Only Mos is able to inhibit this (these) OA-sensitive phosphatase(s). The fact that Mos (like OA) triggers GVBD in oocytes cultured in dbcAMP also favours the hypothesis that Mos acts through the inhibition of OA-sensitive phosphatase(s). It is intriguing that in mouse oocytes, where Mos is not required for GVBD (Colledge et al., 1994; Hashimoto et al., 1994), Mos triggers GVBD in the presence of active protein kinase A (PKA), while, in Xenopus oocytes, where Mos is required for GVBD (Sagata et al., 1988), it does not induce meiosis resumption in IBMX (Faure et al., 1998). These results suggest that PKA (or Mos) may not act on the same target(s) in Xenopus and mouse oocytes.
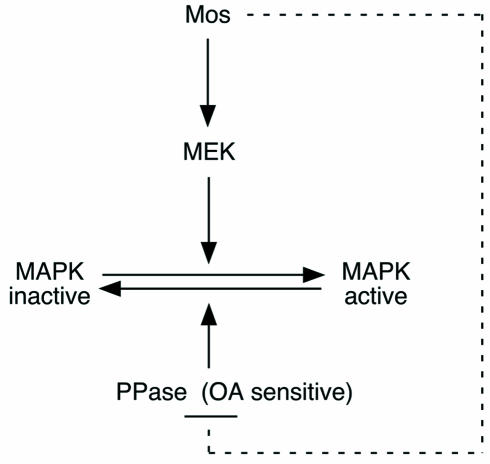
In mouse oocytes, if an (some) OA-sensitive phosphatase(s) is (are) not inhibited by Mos, then MAPK activation does not occur, even if upstream components of the activating pathway are overexpressed. This led us to propose a double pathway for MAPK activation by Mos: MEK1 phosphorylation and OA-sensitive phosphatase(s) inactivation (Figure 9). However, this model might also be true in Xenopus oocytes. It has been shown recently that under certain experimental conditions, MAPK activation is not strictly necessary for GVBD (Fisher et al., 1999; Gross et al., 2000), although injection of oligonucleotides against Mos in Xenopus oocytes consistently blocks GVBD (Sagata et al., 1989), suggesting that Mos could have target(s) other than MEK in Xenopus oocytes. This (these) target(s) could inhibit an OA-sensitive phosphatase pathway involved in GVBD.
Fig. 9. Proposed scheme of the pathway leading to MAPK activation in mouse oocytes.
Proteins of the MAPK family are activated during M-phase of the first embryonic cycle in sea urchin embryos (Chiri et al., 1998; Philipova and Whitaker, 1998). However, their level of activation is much lower in mitosis than in meiosis. As mentioned previously, high levels of MAPK activity seem to be deleterious for cell cycle progression during mitotic cell cycles. The control of activators as well as inhibitors of the MAPK pathway by the same molecule, Mos, may be a very powerful system to ensure that very high levels of MAPK activity will only occur in meiosis, and not when the embryo enters the mitotic cycles.
Several protein phosphatases able to dephosphorylate MAPK in vitro and in vivo when overexpressed have been identified, but very little is known about the physiological MAPK phosphatases involved during meiosis (Alessi et al., 1993; Sun et al., 1993; Minshull et al., 1994). The specificity of the phosphatases varies from tyrosine- or threonine-specific (for a review see Keyse, 1998) to serine/threonine phosphatases (Sturgill et al., 1988; Anderson et al., 1990). By directly measuring the MAPK phosphatase activity, Sohaskey and Ferrell (1999) have shown recently in Xenopus oocytes that there are indeed MAPK phosphatases that are active during meiosis. In this work, they show that at least two distinct phosphatases mediate MAPK inactivation in oocytes. One phosphatase dephosphorylates Thr183 while the other dephosphorylates Tyr185. Both phosphatases are still unknown, one being a tyrosine phosphatase and the other one a PP2A-like threonine phosphatase (sensitive to OA). Interestingly, MAPK molecules phosphorylated only on threonine appear less stable than those phosphorylated only on tyrosine (Sohaskey and Ferrell, 1999). This could suggest that, in mouse oocytes, Mos regulates a PP2A-like OA-sensitive phosphatase(s) and thus inhibits the dephosphorylation of Thr183, resulting in the stabilization of MAPK (Sohaskey and Ferrell, 1999).
Materials and methods
Collection and culture of mouse oocytes
Immature oocytes arrested at prophase I of meiosis were obtained by removing ovaries from 7-week-old KE, OF1 and Mos–/– female mice. KE mice are characterized by a slow rate of oocyte maturation, which facilitated some of our experiments (Polanski, 1986). Oocytes were removed and cultured as previously described (Verlhac et al., 1996). Immunofluorescence was carried out as previously described (Polanski et al., 1998).
Drug treatment
Puromycin (Sigma, final concentration 10 µg/ml) and OA (Molecular Probes, final concentration 0.5 µM) were diluted in M2 medium.
Plasmid construction and in vitro synthesis of RNA
The expression vectors pKS:c-Raf and pKS:ΔRaf were kindly provided by I.Daar (Fabian et al., 1993). Xp42mapkD324N is subcloned at the BglII site of the pSP64(A) vector and allows in vitro transcription from the SP6 promoter (Umbhauer et al., 1995). pRN3MycERK2 was constructed by PCR amplification of the rat ERK2 from a pLexAERK2 plasmid (a gift of J.Cooper) using 5′-tgacgcggccgcggatccgtatggcggcgg and 3′-tgacgcggc cgcttaagatctgtatcctgg primers and then cloning at the NotI site of plasmid pRN3Myc (a gift from J.Moreau, IJM, Paris). pRN3HAMEK1(S218D/S222D) was constructed by PCR amplification of pECEHAMEK1(S218D/S222D) (Brunet et al., 1994; Pagès et al., 1994) using 5′-gatcgcggccgcatgtatgatgttcctgat and 5′-tacggcggccgctacgatgctagcggcat primers and then cloning at the NotI site of pRN3 (a gift from J.Moreau, IJM, Paris). The pRN3 vector allows in vitro transcription of polyadenylated mRNA from a T3 promoter. The pRN3Mos was constructed by RT–PCR amplification from mouse ovaries. Total RNA were extracted using an RNeasy mini-kit (Qiagen), and 500 ng of RNA were treated with 2 U of RQ1 DNase (Promega) for 20 min at 37°C, then heated for 5 min at 85°C. First strand cDNA synthesis was then performed with 50 U of MMuLV Superscript (Life Technologies) using 2.5 µM random hexamer (pdN6, Pharmacia), 1 mM dNTPs (Promega), in 10 mM Tris–HCl, pH 8.3, 5 mM MgCl2, 50 mM KCl for 1 h at 37°C, followed by 5 min at 95°C. The PCR amplification was then performed on 50 ng of cDNA using 5′-gatcagatctcaccatgccttcgcctctaagcc and 5′-gatcgaattctcagcctagtgcccctcggaaag primers. The 1 kb PCR product was then digested by BglII–EcoRI and cloned into pRN3.
In vitro synthesis of capped RNAs was performed using linearized plasmids with the mMessage mMachine kit (Ambion). The mRNAs were then purified on RNeasy columns (Qiagen) and eluted in injection buffer (10 mM Tris, 0.1 mM EDTA, pH 7.4) at a final concentration of 0.5 µg/µl. Aliquots of 4 µl were then stored at –80°C.
Microinjection
The in vitro synthesized mRNAs were microinjected into the cytoplasm of immature oocytes using an Eppendorf pressure microinjector and sterile pipettes. The oocytes were kept in M2 medium supplemented with dbcAMP during injection. They were then cultured at 37°C, in an atmosphere of 5% CO2 in air, for 5–12 h (depending on the experiment) in M2 medium supplemented with dbcAMP to allow overexpression of the exogenous proteins. The resumption of meiotic maturation was triggered by removal of the injected oocytes from the dbcAMP-containing medium and transfer into dbcAMP-free medium.
Immunoblotting
Oocytes at the appropriate stage of maturation were collected in sample buffer (Laemmli, 1970) and heated for 3 min at 100°C. Immunoblotting was then performed as described (Verlhac et al., 1996). The Raf1, MEK1 and MAP kinases were detected using the anti-Raf1 (Schultz et al., 1985), anti-MEK1 (a gift of Steven Pelech) and anti-ERK (no. 691, Santa Cruz Biotechnology, Inc.) polyclonal antibodies, respectively. The HA-tagged MEK* was detected using an anti-HA mouse monoclonal antibody (Boehringer Mannheim). The phosphorylated form of MAPK was detected using an anti-phospho-ERK (SC 7383, Santa Cruz Biotechnology). MycERK2 was detected using an anti-Myc antibody (SC 40, Santa Cruz Biotechnology). As a second layer, we used either an anti-rabbit or an anti-mouse conjugated to horseradish peroxidase (Amersham), and membranes were processed using the ECL detection system (Amersham).
Acknowledgments
Acknowledgements
We thank Ira Daar for the gift of the Raf-1 and ΔRaf clones, J.Cooper for the gift of the pLexA-ERK2 plasmid, Gilles Pagès and Jacques Pouysségur for the gift of the MEK* plasmid, and Richard Scharzmann for his expert photographic work. This work was supported by grants from CNRS and FRM to B.M., and ARC 9913 to M.H.V. C.L. is the recipient of a MERT fellowship.
References
- Abrieu A., Fisher,D., Simon,M.-N., Dorée,M. and Picard,A. (1997) MAPK inactivation is required for the G2 to M-phase transition of the first mitotic cell cycle. EMBO J., 16, 6407–6413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alessi D.R., Smythe,C. and Keyse,S.M. (1993) The human CL100 gene encodes a Tyr/Thr-protein phosphatase which potently and specifically inactivates MAP kinase and suppresses its activation by oncogenic ras in Xenopus oocyte extracts. Oncogene, 8, 2015–2020. [PubMed] [Google Scholar]
- Anderson N.G., Maller,J.L., Tonk,N.K. and Sturgill,T.W. (1990) Requirement for integration of signals from two distincts pathways for activation of MAP kinase. Nature, 343, 651–653. [DOI] [PubMed] [Google Scholar]
- Bitangcol J.C., Chau,A.S., Stadnick,E., Lohka,M.J., Dicken,B. and Shibuya,E.K. (1998) Activation of the p42 mitogen-activated protein kinase pathway inhibits Cdc2 activation and entry into M-phase in cycling Xenopus egg extracts. Mol. Biol. Cell, 9, 451–467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunet A., Pagès,G. and Pouysségur,J. (1994) Constitutively active mutants of MAP kinase kinase (MEK1) induce growth factor-relaxation and oncogenicity when expressed in fibroblasts. Oncogene, 9, 3379–3387. [PubMed] [Google Scholar]
- Brunner D., Oellers,N., Szabad,J., Biggs,W.H.,III, Zipursky,S.L. and Hafen,E. (1994) A gain-of-function mutation in Drosophila MAP kinase activates multiple receptor tyrosine kinase signaling pathway. Cell, 76, 875–888. [DOI] [PubMed] [Google Scholar]
- Carnero A., Jimenez,B. and Lacal,J.C. (1994) Progesterone but not ras requires MPF for in vivo activation of MAPK and S6KII: MAPK is an essential connection point of both signaling pathways. J. Cell. Biochem., 55, 465–476. [DOI] [PubMed] [Google Scholar]
- Chiri S., De Nadai,C. and Ciapa,B. (1998) Evidence for MAP kinase activation during mitotic division. J. Cell Sci., 111, 2519–2527. [DOI] [PubMed] [Google Scholar]
- Choi T.S., Fukasawa,K., Zhou,R.P., Tessarollo,L., Borror,K., Resau,J. and Vandewoude,G.F. (1996) The Mos/mitogen-activated protein kinase (MAPK) pathway regulates the size and degradation of the first polar body in maturing mouse oocytes. Proc. Natl Acad. Sci. USA, 93, 7032–7035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu Y., Solski,P.A., Khosravi-Far,R., Der,C.J. and Kelly,K. (1996) The mitogen-activated protein kinase phosphatases PAC1, MKP-1 and MKP-2 have unique substrate specificities and reduced activity in vivo toward the ERK2 sevenmaker mutation. J. Biol. Chem., 271, 6497–6501. [DOI] [PubMed] [Google Scholar]
- Colledge W.H., Carlton,M.B.L., Udy,G.B. and Evans,M.J. (1994) Disruption of c-mos causes parthenogenetic development of unfertilized mouse eggs. Nature, 370, 65–68. [DOI] [PubMed] [Google Scholar]
- Daar I., Nebreda,A.R., Yew,N., Sass,P., Paules,R., Santos,E., Wigler,M. and Vande Woude,G.F. (1991) The ras oncoprotein and M-phase activity. Science, 253, 74–76. [DOI] [PubMed] [Google Scholar]
- Dent P., Haser,W., Haystead,T.A.J., Vincent,L.A., Roberts,T.M. and Sturgill,T.W. (1992) Activation of mitogen-activated protein kinase kinase by v-Raf in NIH 3T3 cells and in vitro. Science, 257, 1404–1407. [DOI] [PubMed] [Google Scholar]
- Fabian J.R., Morrison,D.K. and Daar,I.O. (1993) Requirement for Raf and MAP kinase function during the meiotic maturation of Xenopus oocytes. J. Cell Biol., 122, 645–652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faure S., Morin,N. and Dorée,M. (1998) Inactivation of protein kinase A is not required for c-mos translation during meiotic maturation of Xenopus oocytes. Oncogene, 17, 1215–1221. [DOI] [PubMed] [Google Scholar]
- Fisher D.L., Brassac,T., Galas,S. and Dorée,M. (1999) Dissociation of MAP kinase activation and MPF activation in hormone-stimulated maturation of Xenopus oocytes. Development, 126, 4537–4546. [DOI] [PubMed] [Google Scholar]
- Fukuda M., Gotoh,Y., Kosako,H., Hattori,S. and Nishida,E. (1994) Analysis of the Ras p21/mitogen-activated protein kinase signaling in vitro and in Xenopus oocytes. J. Biol. Chem., 269, 33097–33101. [PubMed] [Google Scholar]
- Gavin A.C., Vassalli,J.D., Cavadore,J.C. and Schorderet-Slatkine,S. (1992) Okadaic acid and p13suc1 modulate the reinitiation of meiosis in mouse oocytes. Mol. Reprod. Dev., 33, 287–296. [DOI] [PubMed] [Google Scholar]
- Gavin A.C., Cavadore,J.C. and Schorderet-Slatkine,S. (1994) Histone H1 kinase activity, germinal vesicle breakdown and M phase entry in mouse oocytes. J. Cell Sci., 107, 275–283. [DOI] [PubMed] [Google Scholar]
- Gross S.D., Schwab,M.S., Taieb,F.E., Lewellyn,A.L., Qian,Y.-W. and Maller,J.L. (2000) The critical role of the MAP kinase pathway in meiosis II in Xenopus oocytes is mediated by p90rsk. Curr. Biol., 10, 430–438. [DOI] [PubMed] [Google Scholar]
- Haccard O., Sarcevic,B., Lewellyn,A., Hartley,R., Roy,L., Izumi,T., Erikson,E. and Maller,J.L. (1993) Induction of metaphase arrest in cleaving Xenopus embryos by MAP kinase. Science, 262, 1262–1265. [DOI] [PubMed] [Google Scholar]
- Haccard O., Lewellyn,A., Hartley,R.S., Erikson,E. and Maller,J.L. (1995) Induction of Xenopus oocyte meiotic maturation by MAP kinase. Dev. Biol., 168, 677–682. [DOI] [PubMed] [Google Scholar]
- Hashimoto N. (1996) Role of c-mos proto-oncogene product in the regulation of mouse oocyte maturation. Horm. Res., 46, 11–14. [DOI] [PubMed] [Google Scholar]
- Hashimoto N. et al. (1994) Parthenogenetic activation of oocytes in c-mos-deficient mice. Nature, 370, 68–71. [DOI] [PubMed] [Google Scholar]
- Howe L.R., Leevers,S.J., Gomez,N., Nakielny,S., Cohen,P. and Marshall,C.J. (1992) Activation of the MAP kinase pathway by the protein kinase raf. Cell, 71, 335–342. [DOI] [PubMed] [Google Scholar]
- Keyse S.M. (1998) Protein phosphatases and the regulation of MAP kinase activity. Semin. Cell. Dev. Biol., 9, 143–152. [DOI] [PubMed] [Google Scholar]
- Kosako H., Gotoh,Y. and Nishida,E. (1994a) Mitogen-activated protein kinase kinase is required for the mos-induced metaphase arrest. J. Biol. Chem., 269, 28354–28358. [PubMed] [Google Scholar]
- Kosako H., Gotoh,Y. and Nishida,E. (1994b) Requirement for the MAP kinase kinase/MAP kinase cascade in Xenopus oocyte maturation. EMBO J., 13, 2131–2138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuang J. and Ashorn,C.L. (1993) At least two kinases phosphorylate the MPM-2 epitope during Xenopus oocyte maturation. J. Cell Biol., 123, 859–868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuang J., Penkala,J.E., Ashorn,C.L., Wright,D.A., Saunders,G.F. and Rao,P.N. (1991) Multiple forms of maturation-promoting factor in unfertilized Xenopus eggs. Proc. Natl Acad. Sci. USA, 88, 11530–11534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U.K. (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature, 227, 680–685. [DOI] [PubMed] [Google Scholar]
- Leevers S.J. and Marshall,C.J. (1992) Activation of extracellular signal-regulated kinase, ERK2, by p21ras oncoprotein. EMBO J., 11, 569–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda S., Kosako,H., Takenaka,K., Moriyama,K., Sakai,H., Akiyama,T., Gotoh,Y. and Nishida,E. (1992) Xenopus MAP kinase activator: identification and function as a key intermediate in the phosphorylation cascade. EMBO J., 11, 973–982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minshull J., Sun,H., Tonks,N.K. and Murray,A.W. (1994) A MAP kinase-dependent spindle assembly checkpoint in Xenopus egg extracts. Cell, 79, 475–486. [DOI] [PubMed] [Google Scholar]
- Mordret G. (1993) Map kinase kinase—a node connecting multiple pathways. Biol.Cell, 79, 193–207. [DOI] [PubMed] [Google Scholar]
- Muslin A.J., Macnicol,A.M. and Williams,L.T. (1993) Raf-1 protein kinase is important for progesterone-induced Xenopus oocyte maturation and acts downstream of mos. Mol. Cell. Biol., 13, 4197–4202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nebreda A.R. and Hunt,T. (1993) The c-mos proto-oncogene protein kinase turns on and maintains the activity of MAP kinase, but not MPF, in cell-free extract of Xenopus oocytes and eggs. EMBO J., 12, 1979–1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagès G., Brunet,A., L’Allemain,G. and Pouysségur,J. (1994) Constitutive mutant and putative regulatory serine phosphorylation site of mammalian MAP kinase kinase (MEK1). EMBO J., 13, 3003–3010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philipova R. and Whitaker,M. (1998) MAP kinase activity increases during mitosis in early sea urchin embryos [published erratum appears in J. Cell Sci. 1998 Nov 18; 111 (Pt 24): following 3703]. J. Cell Sci., 111, 2497–2505. [DOI] [PubMed] [Google Scholar]
- Polanski Z. (1986) In vivo and in vitro maturation rate of oocytes from two strains of mice. J. Reprod. Fertil., 78, 103–109. [DOI] [PubMed] [Google Scholar]
- Polanski Z., Ledan,E., Brunet,S., Louvet,S., Kubiak,J.Z., Verlhac,M.-H. and Maro,B. (1998) Cyclin synthesis controls the progression of meiotic maturation in mouse oocytes. Development, 125, 4989–4997. [DOI] [PubMed] [Google Scholar]
- Posada J., Yew,N., Ahn,N.G., Vande Woude,G.F. and Cooper,J. (1993) Mos stimulates MAP kinase in Xenopus oocytes and activates a MAP kinase kinase in vitro. Mol. Cell. Biol., 13, 2546–2553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rime H., Talbi,N., Popoff,M.R., Suziedelis,K., Jessus,C. and Ozon,R. (1998) Inhibition of small G proteins by Clostridium sordellii lethal toxin activates cdc2 and MAP kinase in Xenopus oocytes. Dev. Biol., 204, 592–602. [DOI] [PubMed] [Google Scholar]
- Sagata N., Oskarsson,M., Copeland,T., Brumbaugh,J. and Van de Woude,G.F. (1988) Function of c-mos proto-oncogene product in meiotic maturation in Xenopus oocytes. Nature, 335, 519–525. [DOI] [PubMed] [Google Scholar]
- Sagata N., Daar,I., Oskarsson,M., Showalter,S.D. and Vande Woude,G.F. (1989) The product of the mos proto-oncogene as a candidate initiator for oocyte maturation. Science, 245, 643–646. [DOI] [PubMed] [Google Scholar]
- Schultz A.M., Copeland,T.D., Mark,G.E., Rapp,U.R. and Oroszlan,S. (1985) Detection of myristylated gag-raf transforming protein with raf-specific antipeptide sera. Virology, 146, 78–89. [DOI] [PubMed] [Google Scholar]
- Shibuya E.K., Polverino,A.J., Chang,E., Wigler,M. and Ruderman,J.V. (1992) Oncogenic ras triggers the activation of 42-kDa mitogen-activated protein kinase in extracts of quiescent Xenopus oocytes. Proc. Natl Acad. Sci. USA, 89, 9831–9835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibuya E.K. and Ruderman,J.V. (1993) Mos induces the in vitro activation of mitogen-activated protein kinases in lysates of frog oocytes and mammalian somatic cells. Mol. Biol. Cell, 4, 781–790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohaskey M.L. and Ferrell,J.L. (1999) Distinct, constitutively active MAPK phosphatases function in Xenopus oocytes: implications for p42 MAPK regulation in vivo. Mol. Biol. Cell, 10, 3729–3743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sturgill T.W., Ray,L.B., Erikson,E. and Maller,J. (1988) Insulin-stimulated MAP2 kinase phosphorylates and activates ribosomal protein S6 kinase II. Nature, 334, 715–718. [DOI] [PubMed] [Google Scholar]
- Sun H., Charles,C.H., Lau,L.F. and Tonks,N.K. (1993) MKP-1 (3CH134), an immediate early gene product, is a dual specificity phosphatase that dephosphorylates MAP kinase in vivo. Cell, 75, 487–493. [DOI] [PubMed] [Google Scholar]
- Tachibana K., Machida,T., Nomura,Y. and Kishimoto,T. (1997) MAP kinase links the fertilization signal transduction pathway to the G1/S-phase transition in starfish eggs. EMBO J., 16, 4333–4339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umbhauer M., Marshall,C.J., Mason,C.S., Old,R.W. and Smith,J.C. (1995) Mesoderm induction in Xenopus caused by activation of MAP kinase. Nature, 376, 58–62. [DOI] [PubMed] [Google Scholar]
- Verlhac M.H., de Pennart,H., Maro,B., Cobb,M.H. and Clarke,H.J. (1993) MAP kinase becomes stably activated at metaphase and is associated with microtubule-organizing centers during meiotic maturation of mouse oocytes. Dev. Biol., 158, 330–340. [DOI] [PubMed] [Google Scholar]
- Verlhac M.H., Kubiak,J.Z., Weber,M., Géraud,G., Colledge,W.H., Evans,M.J. and Maro,B. (1996) Mos is required for MAP kinase activation and is involved in microtubule organization during meiotic maturation in the mouse. Development, 122, 815–822. [DOI] [PubMed] [Google Scholar]