Abstract
Introduction
The aims of this study were to test the hypothesis that Polarization Sensitive-Optical Coherence Tomography (PS-OCT) can be used to nondestructively measure and quantify the severity of the early demineralization of enamel on buccal and occlusal surfaces and assess the inhibitory effect of fluoride varnish in vivo.
Methods
A split mouth design was used to assess the effects of fluoride varnish on inhibiting dissolution on twenty test subjects. Orthodontic bands with a buccal window were cemented on the premolars and small incisions were produced on occlusal surfaces to serve as sites for plaque retention for enhanced demineralization. Bands were removed after 30 days and PS-OCT scans were acquired in vivo of occlusal and buccal areas. Teeth were extracted, sectioned and analyzed using polarized light microscopy (PLM) and transverse microradiography (TMR) for comparison with the PS-OCT images.
Results
High contrast PS-OCT images were acquired of both occlusal and buccal surfaces in vivo. Both occlusal and buccal surfaces, showed a significant difference (P < 0.05) in the integrated reflectivity (ΔR) between the “sound” and “carious” enamel groups. Although the mineral loss (ΔZ) and ΔR measured using TMR and PS-OCT were less for the fluoride varnish treated buccal surfaces the difference was not statistically significant (P>0.05).
Conclusions
Our results indicate that PS-OCT can non-destructively measure early enamel demineralization on the buccal and occulsal surfaces in vivo.
INTRODUCTION
Several in vitro studies have demonstrated the potential of optical coherence tomography (OCT) and polarization sensitive OCT (PS-OCT) for nondestructive measurement of the severity of early demineralization on enamel surfaces. (1–6) Demineralization is most often found in the pits and fissures of tooth occlusal surfaces, however to the best of our knowledge reliable “in vivo” demineralization models have not been developed for these surfaces. The most reliable and often used “in vivo” models of early demineralization that are used to investigate new methods for monitoring demineralization involve demineralization around orthodontic appliances.
Conventional optical coherence tomography (OCT) is a noninvasive technique for creating cross-sectional images of internal biological structures. (7) Colston et al. in 1998 was the first to image the soft and hard tissue structure of the oral cavity with OCT. (8) OCT directly measures the light reflected from each layer of the carious lesion, enabling the measurement of surface and sub-surface demineralization. Amaechi et al. (4,9) measured the severity of demineralization on artificial lesions on enamel and root surfaces using OCT by measuring loss of penetration depth in the images.
Polarization-sensitive OCT (PS-OCT) is capable of measuring the reflectivity of the reflected light in both orthogonal polarization states and it has significant advantages for quantitative measurements of lesion severity. Areas of demineralization can rapidly depolarize/scramble the polarization of the incident polarized light and provide improved contrast of carious lesions. (10) In addition, the confounding influence of the strong specular reflection from the tooth surface is reduced in the PS-OCT system. Baumgartner et al. were the first to present polarization resolved images of dental caries. (11)
One approach to quantifying the severity of the lesion is to integrate the reflectivity of the orthogonal polarization (⊥–axis) (10) or cross polarization (CP) image. In the CP image, the intensity of the strong reflection from the tooth surface and the reflectivity from sound enamel are reduced, therefore lesions appear with higher contrast with the surrounding sound enamel. The parallel (||)-axis image represents the linearly polarized light reflected in the original polarization incident on the tooth and it shows the strong specular reflection from the tooth surface and the reflectivity from sound enamel. The increase in the integrated reflectivity (ΔR) of the CP-OCT image has been correlated with the mineral loss (ΔZ) calculated from tranverse microradiography for enamel and dentin. (12) PS-OCT was used in this study to quantify in vivo the depth-resolved changes of light scattering in enamel that occurred upon demineralization.
Studies have shown that PS-OCT can be used to measure the inhibition of anti-caries agents in vitro including CO2 laser treatments (13) and fluoride treatments. (1,2,14) It has been successfully used for measuring the severity of natural caries lesions on smooth surfaces (6) and in the occlusal surfaces (12), for assessing the severity and extent of enamel developmental defects (15), and for measuring demineralization under restorations and sealants. (16) PS-OCT can also be used to monitor structural changes in lesions in enamel and dentin as they undergo remineralization. (17–20) This suggests that PS-OCT can be potentially used to assess the state of the carious lesion and determine whether it is active and progressing or whether it has been arrested and undergone remineralization.
During orthodontic treatment, patients are often at an elevated risk of developing areas of enamel decalcification, and subsequent dental caries. These areas of decalcification are commonly referred to as white spot lesions due to demineralization of the enamel by organic acids produced by cariogenic bacteria in plaque. (21) These unaesthetic areas of surface and subsurface demineralization around orthodontic appliances occur due to the inherent plaque retaining abilities of the bands and brackets and the additional obstacles for patients to maintain their oral hygiene around them. (22) (23) Demineralization underneath ill-fitting orthodontic bands is a very rapid process and has been shown to occur after only one month. (22),(24) The additional use of fluoride varnish in orthodontic treatment, over the buccal surfaces before cementation with glass ionomer cements was advocated by Ogaard et al.(25–27). It was demonstrated that the application of fluoride varnish on sound enamel reduced lesion development by 48% to 50% (28) compared to non-treated enamel and prevented further lesion progression. Schmidt et al. also showed fluoride varnish to be beneficial in reducing the depth of the demineralized lesion by 35% and demonstrated a greater efficacy when using resin-modified glass ionomers, with a 50% depth reduction of the demineralized area compared to conventional composite resins. (29)
Clinical validation of PS-OCT for the early detection of surface and subsurface demineralization will enable in vivo monitoring of early caries formation and its subsequent prevention by providing a means for the nondestructive testing of anti-caries agents. The areas of intended use include the high-risk tooth surfaces around orthodontic appliances and in the pits and fissures of the occlusal surfaces. With this in mind, the objective of this study was to demonstrate that polarization sensitive-optical coherence tomography (PS-OCT) can be used in vivo as a non-invasive optical device for clinical assessment of tooth demineralization and the efficacy of fluoride varnish in inhibiting demineralization.
MATERIAL AND METHODS
Twenty test subjects were recruited from the UCSF Orthodontic Clinic according to a protocol approved by the Committee on Human Research. Eleven test subjects (55%) were male, and 9 (45%) were female. The age range was 10 to 33 years (mean 19.1± 6.7 years). All participants received oral hygiene instructions and were advised to use a fluoride dentifrice.
The inclusion criteria for our subjects were that they must be adolescents between the ages of 10–18 years old or adults; be able to give informed consent in either English or Spanish; reside in locales with community fluoridation (to eliminate water fluoridation as a confounding variable); be ready for orthodontic fixed treatment; be treatment planned for bilateral premolar extractions in the same arch; and to have all restorative needs met and teeth cleaned prior to bonding. The exclusion criteria for our subjects were patients suffering from systemic diseases or conditions that can effect their oral health (i.e. diabetes, HIV, heart conditions requiring antibiotic prophylaxis); patients taking medications that effect oral flora or saliva (ie. antibiotics taken in the last 3 months); patients with a drug or alcohol addiction; patients who have taken in-office fluoride treatment within the past 3 months, and teeth with visible defects and demineralization on the buccal surfaces.
The study used 20 pairs of sound premolars scheduled for extraction due to orthodontic reasons. Tooth surfaces were visually examined for existing caries, sealants and developmental defects. During the initial bonding appointment of these premolars, modified orthodontic bands that were developed by Ogaard et al. (24) where placed on the premolars. Two metal posts (0.8mm thick) were welded to the inner surface of the buccal part of the bands to mimic a space for plaque accumulation, which can occur with ill-fitting bands. Conservative superficial preparations (incisions) of 2-mm in length, 600-μm in width, and 500-μm in depth were made with a small dental bur on the occusal surfaces in the central fissure of each premolar. These small lesions served as an adequate site for plaque retention and subsequent acid dissolution.
Before cementation of the bands, one premolar in each patient was randomly selected to be pretreated with a fluoride varnish containing 5% NaF in a natural colophonium resin, Cavity ShieldR from 3M Espe Omni Preventive Care, Inc. (Bentonville, AR) on their buccal surface according to the manufacturer instructions. The control contralateral premolar did not receive any additional fluoride treatment (split mouth model). The specially designed orthodontic bands were then cemented with a glass ionomer cement, Ultra Band-Lok from Reliance Orthodontic Products (Itasca, IL), to the mesial, distal and lingual surfaces of both the premolars treatment planned for extractions. The occlusal surfaces of the premolars were imaged with PS-OCT at the initial banding appointment since it is difficult to decay in the occlusal surfaces with conventional examination. Since defects and demineralization are more obvious on the smooth buccal surfaces we did not think pre-scans were needed for those surfaces. After a mean time of 34 days (range 27–43 days; SD 4.8 days) the bands were removed and the teeth were imaged a second time with the PS-OCT on both the buccal and occlusal surfaces. The teeth were then extracted the same day as the second scan for 18 of the 20 patients. Of the two patients who received their extractions on another day, one waited 3 days, and the other waited 24 days. The extracted teeth were collected from the oral surgeon, labeled, and stored in a thymol solution.
The PS-OCT system that was used for in vivo imaging employed polarization maintaining (PM) fiber and a optical coherence domain reflectometer (OCDR) system employing high-efficiency piezoelectric fiber-stretchers, and an InGaAs receiver fabricated by Optiphase, Inc. (Van Nuys, CA). This OCDR system was coupled with a broadband high-power superluminescent diode (SLD) from Denselight (Jessup, Md) with an output power of 10 mW and a bandwidth of 83 nm for a spatial resolution of 12 μm in air and 7.5 μm in enamel. To enable the acquisition of in vivo, high-resolution b-scan images, a low profile scanning stage was integrated with an optical-fiber probe, Fig. 1. Each a-scan, representing a single axial scan of reflectivity vs. depth was 2000 points over a range of 5.7-mm in air and 3.6 mm in enamel. A MM-3M-F-05 mini-stage from National Aperture Inc (Salem, MA), was used with a MS-4CA Servo Amplifier system and the National Instruments (Austin, TX) PCI-7344 motion controller. This stage has a lateral scan range of 12.7-mm, sufficient to scan across the entire tooth with a speed of 6 mm/sec to avoid motion artifacts. The PM fiber probe consisted of a fiber optic collimator connected to a 15-mm focal length aspheric lens to provide a spot diameter of 20 μm. The rate of the a-scans was 150 scan/sec. The PS-OCT system was controlled using Labview software from National Instruments.
Figure 1.
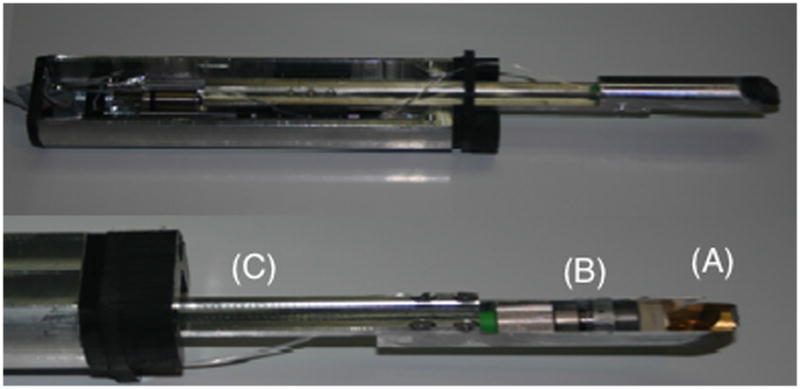
The scanning hand-piece that was used to acquire the in vivo images. An autoclavable sleeve composed of heat resistant Delrin was placed over the arm of the OCT probe. A 45° mirror (A) delivers the light from the lens, collimator and optical fiber (B) mounted on the probe arm (C) that retracts to acquire each b-scan.
After scanning, teeth were extracted, sterilized with gamma irradiation, and then mounted in acrylic cubes to facilitate the handling of the samples. Each of the 40 premolar samples (20 pairs), were photographed at a magnification of 12X. The teeth were serial sectioned to a thickness of 200-μm thick slices using an Isomet 5000 saw from Buehler (Lake Bluff, IL) for polarized light microscopy (PLM) and transverse microradiography (TMR). PLM was carried out using a Meiji Techno RZT microscope from Meiji Techno Co., Ltd. (Saitama, Japan) with an integrated digital camera, EOS Digital Rebel XT from Canon Inc. (Tokyo, Japan). The sample sections were imbibed in water and examined in the bright field mode with crossed polarizers and a red I plate with 500-nm retardation.
A custom built digital microradiography (TMR) system was used to measure the volume percent mineral content in the areas of demineralization on the tooth sections. (30) High-resolution microradiographs were taken using Cu Ka radiation from a Philips 3100 x-ray generator and a Photonics Science FDI x-ray digital imager, Microphotonics, (Allentown, PA). The x-ray digital imager consists of a 1392 × 1040 pixel interline CCD directly bonded to a coherent micro fiber-optic coupler that transfers the light from an optimized gadolinium oxysulphide scintillator to the CCD sensor. The pixel resolution is 2.1 μm, and the images can be acquired in real time at a frame rate of 10 fps. A high-speed motion control system with Newport (Irvine, CA) UTM150 and 850G stages and an ESP 300 controller coupled to a video microscopy and laser targeting system was used for precise positioning of the tooth sample in the field of view of the imaging system.
The PS-OCT b-scans, arrays of a-scans forming “optical” thin sections, were matched to the corresponding PLM and TMR images of the “physical” thin sections for comparison. A line profile was drawn through the same area of demineralization in all three images, and calculations were taken at these points. The depth of the demineralized areas were compared with the depths from the PLM and PS-OCT images. The intensity of backscattered light (ΔR) was also measured in the PS-OCT images, as a function of depth within the tissue. The integrated light reflectivity (ΔR) in the PS-OCT CP images (⊥-axis image) was compared with the relative mineral loss (ΔZ) measured in the TMR images. The integrated mineral loss (ΔZ) was calculated by subtracting the mineral profile in the lesion area from the sound profile. The lesion was defined as areas of birefringence loss (dark areas) in the PLM image and the depth was measured using image analysis software.
Those samples that manifested integrated mineral loss values (ΔZ) exceeding 100 were designated as “carious” and those that were less than 100 were designated as “sound”. According to optical attenuation measurements in demineralized enamel at 1310-nm as a function of mineral loss (31), a 5% loss in volume percent mineral yields a 20 fold increase in light scattering while a loss of 10–15% gives the maximum increase in light scattering, roughly a 50 times increase. Therefore, OCT is anticipated to be more sensitive than TMR and we chose a fairly low ΔZ value of 100 as a demarcation between sound and carious areas. This represents a 5% mineral loss over a depth of 20-μm which is anticipated to produce a measurable increase in reflectivity.
The unpaired t-test was used to test for statistical differences in ΔR and ΔZ values. Unpaired t-tests were also used to test if there was a significant difference, at p < 0.05, in the lesion depth between sound and carious enamel groups calculated with both PLM and PS-OCT. Differences in mineral loss values or lesion depths between the test group (fluoride-releasing varnish) and the control teeth were analyzed by a paired t-test using Instat from Graphpad Software (La Jolla, CA) and the level of significance was set at p < 0.05. The correlation between ΔR and ΔZ measured using PS-OCT and TMR and for the lesion depth measured using PLM and PS-OCT were determined for the buccal and occlusal surfaces using the Pearson correlation coefficient (rP).
RESULTS
High quality PS-OCT images were acquired of lesions on both buccal and occlusal surfaces. In most of the images the dentinal enamel junction (DEJ) is visible indicating that the optical penetration is sufficient to acquire images through the full thickness of enamel. Figure 2 shows buccal scans from a “sound” premolar that was not banded and subject to demineralization. The DEJ is visible from the cementum enamel junction (CEJ) to the crown. There are subsurface bands that are complementary in the two polarization states (|| and ⊥-axis images),.i.e., intensity maxima in one state matches the position of the minima in the other state. The banding visible in the crown is due to a combination of birefringence and the structure of the incremental growth lines in the tooth. The surface reflection near the CEJ is very strong which can cause artifacts to appear above and below the surface.
Figure 2.
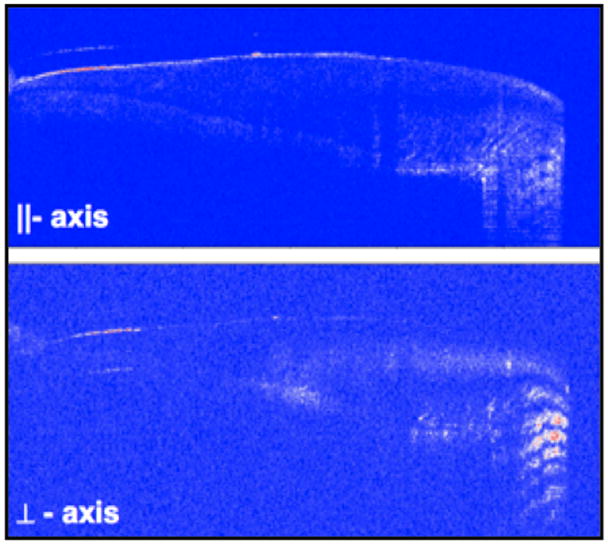
PS-OCT b-scans (|| and ⊥–axis images) taken of the buccal surface of a sound premolar. The dentin-enamel junction is clearly visible from the root to the crown.
Figure 3 shows a buccal scan from a test subject after removal of the orthodontic band. An area of demineralization can be seen near the center of the scan. The demineralization causes an increase in reflectivity and a loss of intensity from lower layers in the tooth, i.e., the DEJ can no longer be seen below the area of demineralization. The lesion contrast is higher in the cross polarization (CP) image (⊥-axis). There is also increased reflectivity in the CP image below the surface near the CEJ, and comparison of the two polarization images shows that the increase does not occur in the original polarization state (||-axis) indicating that it is caused by the enamel birefringence and not demineralization which causes increased reflectivity in the same locations of the images representing both polarization states.
Figure 3.

PS-OCT b-scans (|| and ⊥–axis images) taken of the buccal surface of a test subject after removal of the band after 30-days. The lesion area (white box) exhibits increased reflectivity and reduced optical penetration.
Images of two lesions that were produced on occlusal surfaces are shown in Figs. 4 and 5. PS-OCT polarization images of the occlusal surface of one of the samples after 30 days exposure is shown in Fig. 4 along with a PLM image of the thin section best matching the OCT scan position. Two areas of demineralization are visible, one area at the base of the incision and a larger area to the right of the occlusal pit. Those areas are demarcated by the white boxes in the CP (⊥-axis) image. The lesion to the right of the occlusal pit is well outside the incision area and it is in an unusual location for demineralization and may be a developmental defect, even though it lacks the distinct surface zone characteristic of such lesions. Only the lesion at the base of the incision was used for analysis.
Figure 4.
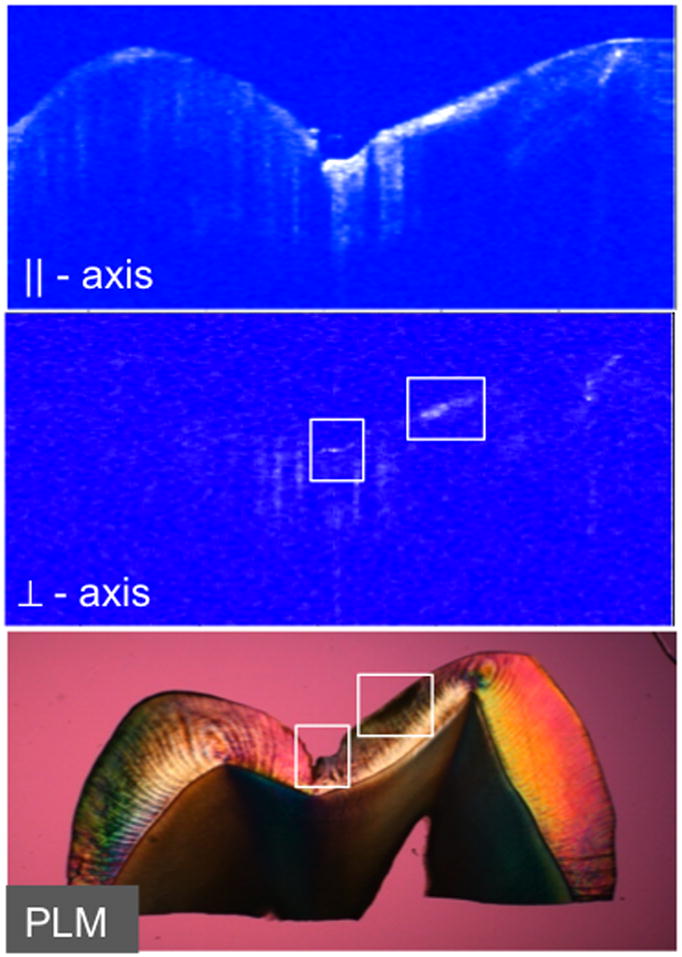
PLM and PS-OCT images of the occlusal surface of the premolar of one premolar after 30-days centered on the incision. Areas of demineralization are shown in the white boxes in the CP PS-OCT (⊥–axis ) and PLM images.
Figure 5.

The best matched TMR and CP PS-OCT images of the occlusal surface of one sample. A line profile (black-line) was drawn through the area of demineralization at the base of the incision as shown and ΔR and ΔZ were calculated at that position for comparison.
Figure 5 shows a comparison of the corresponding TMR image of a thin section and the CP (⊥-axis) PS-OCT image. They match reasonably well but are not perfect since the TMR image is of a sample 200-μm thick while the “optical” slice taken with OCT is 1/10 the thickness, 20-μm. After sectioning, we had to reject 4 occlusal samples because they had sealants which we had missed during the initial examination of the occlusal surfaces.
The integrated mineral loss (ΔZ) was measured for the buccal and occlusal surfaces of each sample using TMR, the gold standard, for comparison with the integrated reflectivity from the lesion area in the PS-OCT CP (⊥-axis) image, which we call ΔR. Moreover, the ΔZ values were used to divide all our samples into two resultant groups: the “sound” enamel and the “carious” enamel groups. The ΔZ and ΔR values for the sound and carious buccal and occlusal groups are listed in Table I. There was a statistically significant difference between the sound and carious enamel groups in both the occlusal and buccal surfaces, p < 0.05 for ΔZ and ΔR. There was also a positive correlation between the ΔR and ΔZ values measured for both the occlusal surfaces (rP= 0.50, P < 0.01, n=36) and the buccal surfaces (rP= 0.52, P < 0.001, n=40).
Table I.
Measured values of the lesion depth and severity measured using OCT, TMR and PLM.
| Lesion Type | Number Samples | PLM Depth (μm) | OCT Depth (μm) | TMR (ΔZ) | OCT (ΔR) | |
|---|---|---|---|---|---|---|
| Overall - | Sound | 27 | 58(54) | 61(31) | 3(35) | 70(72) |
| Carious | 49 | 150(108) | 118(84) | 1228(1556) | 214(202) | |
| Occlusal - | Sound | 11 | 28(31) | 61(36) | 4(35) | 70(34) |
| Carious | 25 | 154(127) | 121(70) | 1603(2014) | 225(214) | |
| Buccal - | Sound | 16 | 78(58) | 65(59)* | 2(37) | 81(89) |
| Carious | 24 | 146(86) | 115(98)* | 838(711) | 203(193) | |
| Buccal – | Fluoride | 20 | 104(75)* | 89(109)* | 395(579)* | 116(158)* |
| No Fluoride | 20 | 128(69)* | 101(60)* | 572(732)* | 210(198)* | |
groups were not significantly different P>0.05
The lesion depth of the demineralized areas was measured in the same area of each sample using both PLM and PS-OCT. The lesion depths for the buccal and occlusal lesions are also tabulated in Table I. There was a statistically significant difference between the sound and carious enamel groups in both the PLM and PS-OCT lesion depth measurements, p < 0.05. However, for the buccal surface, there was only a significant difference between the two enamel groups, when the lesion depth was calculated with PLM. When the lesion depths on the buccal surfaces were measured in the PS-OCT images, there was no significant difference between the sound and carious enamel groups, p > 0.05. There was also a positive correlation between the depths measured using PLM and PS-OCT for both the occlusal surfaces (rP= 0.64, P < 0.0001, n=36) and the buccal surfaces (rP= 0.53, P < 0.001, n=40).
Fluoride varnish was used to treat only the buccal surfaces and the mean ΔZ and ΔR values for the buccal surfaces treated with fluoride varnish are lower than for the untreated surfaces suggesting that there was less demineralization, however the differences were not large enough to be statistically significant. The mean lesion depths measured using PLM and OCT were also lower for the varnish treated surfaces but they did not break statistically.
DISCUSSION
In this study our principal objectives were show that PS-OCT could be used to acquire high quality images of demineralization in vivo and demonstrate that it could be used to measure the severity of demineralization non-destructively. Those objectives were successful on both buccal and occlusal surfaces. Our primary goal was not to develop new in vivo models of demineralization or prove that fluoride works under orthodontic bands. Although, we feel that we chose the most likely model to produce lesions in 30-days, the performance of the model for demineralization around orthodontic bands was disappointing since the mean DZ value was only 572 for non-fluoride groups which is less than desired and much less than that observed in previous studies around orthodontic appliances. Apparently, it was not sufficient to measure a significance difference between the fluoride and non-fluoride groups using the gold standard, TMR or PS-OCT. However, enough demineralization did occur which was confirmed with TMR to demonstrate that PS-OCT can be used to measure differences in the severity of early demineralization on buccal surfaces. This has greater significance since PS-OCT can now be used for future studies in which teeth do not require extraction for comparison with TMR. Such studies can focus on the most important tooth surfaces, such as around brackets and are unrestricted in the periods of demineralization and the severity of acid challenge.
The previously untested occlusal model was more effective in generating lesions than the buccal model, although in a couple of cases there were sealants present that were initially undetected. Since most new caries lesions are found in the pits and fissures of the occlusal surfaces, arguably those are the most important areas to image. The topography of the occlusal surface also makes them the most challenging surfaces to image. There is no well proven model that can be used to produce early lesions in occlusal surfaces, i.e., there are no existing models analogous to the orthodontic band/brackets models that have been used successfully on buccal surfaces which can be used to produce early lesions on occlusal surfaces. Since the small incisions were quite effective in producing demineralization on these surfaces this model may be an effective model for other in vivo studies that require tooth extraction. The performance of OCT appeared to be better for the occlusal surfaces. The convoluted occusal topography can be an advantage for OCT, since flat parallel surfaces are more likely to produce strong specular reflection from the sample surface generating artifacts making it more difficult to assess shallow demineralization.
OCT, PLM and TMR failed to show a statistically significant difference in the amount of demineralization between the premolars treated with fluoride varnish and the control groups, p > 0.05. Therefore, the application of fluoride varnish was not particularly effective as it was applied in this study. In the study by Gorton and Featherstone in 2003 (32), the cariostatic effect of fluoride-releasing glass ionomer cements around the orthodontic appliances, was demonstrated after a four week period. It is most likely that improved patient compliance since then has reduced the rate of development of white spot lesions.
It was beneficial to have the patients receive their extractions on the same day as their second PS-OCT scans, to ensure that the remineralizing effects of saliva did not occur. Eighteen out of the 20 patients received their extractions the same day, however one received their extractions 3 days later and another received their extractions 24 days later. Ogaard et al. in 1994 showed that lesion regression by saliva was a speedy process complete within a few weeks in nearly all cases. (33) This is of great importance in the orthodontic practice since white spot lesions that developed rapidly between one appointment and the next (eg. 4 weeks), may regress rapidly by removing a loose band for some weeks. In our study however, it was advantageous to have our patients receive their extractions the same day as their debonding appointment, since lesion regression by saliva was not our objective. Remineralization of the lesions would reduce the amount of measureable demineralization. Despite the fact that two patients did not receive their extractions on the same day, when our results are recalculated to eliminate the data from these two patients, our results remain unchanged.
There was a positive correlation between ΔZ and ΔR for buccal and occlusal surfaces however the correlation was weaker compared to our previous in vitro comparisons using artificial lesions rP = 0.76 (12) and natural lesions, rP ~0.7 for smooth surface lesions (6) and natural occlusal lesions. (34) The lower correlation is most likely due to mismatch of the OCT scans to the histological sections. We had particular difficultly in matching the PS-OCT b-scans to the histological thin sections used for TMR and PLM. The lesions produced on the buccal and occlusal surfaces varied markedly in position and severity which compounded the problem. The acquisition of entire 3D tomographic images of the entire tooth surface can address this problem and we plan to take that approach in future studies. Newly developed Fourier domain systems (FD-OCT) using spectrometers and rapidly swept laser sources can acquire real-time high-resolution three dimensional (3-D) images in a second. (35,36) Moreover, since lesions are highly variable in position and depth the ability to acquire volumetric 3-D images of the entire lesion will greatly facilitate these types of studies.
In addition, TMR is not an ideal gold standard for comparison since it is not very sensitive to small changes in mineral density (< 5%). This was particularly evident in this study. Many of the tooth surfaces that were designated as sound in the TMR images appeared to have some demineralization in the OCT and PLM images. Small changes in mineral density can cause large changes in light scattering. Therefore, OCT and PLM are much more sensitive than TMR and this explains why many of the “sound” tooth surfaces yielded higher reflectivity. In a recent study we calculated the correlation between PLM and TMR for measuring the lesion depth of artificial lesions on tooth sections with shallow lesions (40–100-μm deep) and it was 0.88. This correlation may appear high, however when you consider the measurements were on the same tooth sections with highly uniform lesions with steep mineral gradients, the agreement is much less than anticipated. The use of thin sections for analysis also causes problems due to the influence of tooth curvature. The outer tooth curvature can contribute to a sizable mineral loss in TMR measurements for shallow lesions. One can correct for curvature by subtracting the lesion cross sectional area from a sound cross sectional area but it may be difficult to identify such sound areas on natural tooth surfaces. The curvature can also cause errors with PLM since high curvature causes the tooth edge to appear black in a similar fashion to demineralization due to the high angle of incidence. We used tooth sections that were 200-μm thick to ensure that we did not destroy a large number of samples during sectioning and the thicker sections compounded this problem. These problems with the use of thin sections underscores the importance of nondestructive methods such as OCT for in vitro measurements in addition to the more obvious in vivo use.
Since PS-OCT can be used to measure the depth and severity of demineralization it’s greatest potential utility for use in orthodontics is to provide a non-destructive method for assessing the severity of white spot lesions around brackets. Not only can PS-OCT be potentially used to assess the severity of demineralization for diagnosis but other in vitro studies suggest that it can also be used to determine whether intervention is effective by measuring the formation of a highly mineralized low reflectivity layer on the surface of the lesion indicative of remineralization or it can be used to measure the existence of a similar layer prior to any treatment indicative of a developmental defect which could be left alone. (17,18,37,38) Although, we used a model involving the orthodontic bands there is no reason that PS-OCT cannot be used around brackets in fact, PS-OCT can be used to measure decay under sealants and composite adhesives so that demineralization can even be assessed under the flash around the bracket if there is leakage. (1,39)
This first clinical study employing PS-OCT to monitor early caries lesions indicates that there is considerable potential for this approach. We were able to acquire high quality PS-OCT images of early caries lesions, differentiate between sound and demineralized areas and show a correlation between integrated reflectivity and mineral loss. Although, we anticipated higher correlation between ΔZ and ΔR based on our previous in vitro studies. Moreover, the 30-day orthodontic band model failed to produce significant differences in demineralization between the fluoride and non- fluoride groups in this test subject population and more extended periods of demineralization are likely required. We believe the ability to acquire complete 3D images and monitor the lesion development over longer periods of time will greatly facilitate these studies and we plan future studies along these lines.
Acknowledgments
This work was supported by NIH/NIDCR Grant R01-DE17869. The authors would also like to acknowledge the contributions of John D. B. Featherstone, Gerald Nelson, Peter Trinh, Arthur Miller, Janice Lee and the residents of the UCSF Division of Orthodontics.
This work was supported by NIH/NIDCR Grant# R01-DE17869.
References
- 1.Chong SL, Darling CL, Fried D. Nondestructive measurement of the inhibition of demineralization on smooth surfaces using polarization-sensitive optical coherence tomography. Lasers Surg Med. 2007;39(5):422–427. doi: 10.1002/lsm.20506. [DOI] [PubMed] [Google Scholar]
- 2.Fried D, Xie J, Shafi S, Featherstone JDB, Breunig T, Lee CQ. Early detection of dental caries and lesion progression with polarization sensitive optical coherence tomography. J Biomed Optics. 2002;7(4):618–627. doi: 10.1117/1.1509752. [DOI] [PubMed] [Google Scholar]
- 3.Jones RS, Darling CL, Featherstone JDB, Fried D. Imaging artificial caries on occlusal surfaces with polarization sensitive optical coherence tomography. Caries Res. 2006;40(2):81–89. doi: 10.1159/000091052. [DOI] [PubMed] [Google Scholar]
- 4.Amaechi BT, Higham SM, Podoleanu Ag, Rodgers JA, Jackson DA. Use of Optical Coherence Tomography for Assessment of Dental caries. J Oral Rehab. 2001;28(12):1092–1093. doi: 10.1046/j.1365-2842.2001.00840.x. [DOI] [PubMed] [Google Scholar]
- 5.Amaechi BT, Podoleanu A, Higham SM, Jackson DA. Correlation of quantitative light-induced fluorescence and optical coherence tomography applied for detection and quantification of early dental caries. J Biomed Opt. 2003;8(4):642–647. doi: 10.1117/1.1606685. [DOI] [PubMed] [Google Scholar]
- 6.Ngaotheppitak P, Darling CL, Fried D. Polarization Optical Coherence Tomography for the Measuring the Severity of Caries Lesions. Lasers Surg Med. 2005;37(1):78–88. doi: 10.1002/lsm.20169. [DOI] [PubMed] [Google Scholar]
- 7.Bouma BE, GJT . In: Handbook of Optical Coherence Tomography. Dekker M, editor. New York: 2002. [Google Scholar]
- 8.Colston BW, Jr, Everett MJ, Da Silva LB, Otis LL, Stroeve P, Nathel H. Imaging of hard- and soft-tissue structure in the oral cavity by optical coherence tomography. Appl Opt. 1998;37(16):3582–3585. doi: 10.1364/ao.37.003582. [DOI] [PubMed] [Google Scholar]
- 9.Amaechi BT, Podoleanu AG, Komarov G, Higham SM, Jackson DA. Quantification of root caries using optical coherence tomography and microradiography: a correlational study. Oral Health Prev Dent. 2004;2(4):377–382. [PubMed] [Google Scholar]
- 10.Fried D, Xie J, Shafi S, Featherstone JD, Breunig TM, Le C. Imaging caries lesions and lesion progression with polarization sensitive optical coherence tomography. J Biomed Opt. 2002;7(4):618–627. doi: 10.1117/1.1509752. [DOI] [PubMed] [Google Scholar]
- 11.Baumgartner A, Dichtl S, Hitzenberger CK, Sattmann H, Robl B, Moritz A, Fercher AF, Sperr W. Polarization-sensitive optical coherence tomography of dental structures. Caries Res. 2000;34(1):59–69. doi: 10.1159/000016571. [DOI] [PubMed] [Google Scholar]
- 12.Jones RS, Darling CL, Featherstone JD, Fried D. Imaging artificial caries on the occlusal surfaces with polarization-sensitive optical coherence tomography. Caries Res. 2006;40(2):81–89. doi: 10.1159/000091052. [DOI] [PubMed] [Google Scholar]
- 13.Hsu DJ, Darling CL, Lachica MM, Fried D. Nondestructive assessment of the inhibition of enamel demineralization by CO2 laser treatment using polarization sensitive optical coherence tomography. J Biomed Opt. 2008;13(5):054027. doi: 10.1117/1.2976113. [DOI] [PubMed] [Google Scholar]
- 14.Fried D, Featherstone JDB, Darling CL, Jones RS, Ngaotheppitak P, Buehler CM. In: Early Caries Imaging and Monitoring with Near-IR Light in Incipient and Hidden Caries. Boston DW, editor. Philadelphia: W. B Saunders Company; 2005. pp. 771–794. [DOI] [PubMed] [Google Scholar]
- 15.Hirasuna K, Fried D, Darling CL. Near-infrared imaging of developmental defects in dental enamel. J Biomed Opt. 2008;13(4):044011, 1–7. doi: 10.1117/1.2956374. [DOI] [PubMed] [Google Scholar]
- 16.Jones RS, Staninec M, Fried D. Imaging artificial caries under composite sealants and restorations. J Biomed Opt. 2004;9(6):1297–1304. doi: 10.1117/1.1805562. [DOI] [PubMed] [Google Scholar]
- 17.Can AM, Darling CL, Fried D. High-resolution PS-OCT of enamel remineralization. Lasers in Dentistry XIV; San Jose, CA, USA. SPIE; 2008. pp. 68430Tpp. 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jones RS, Darling CL, Featherstone JDB, Fried D. Remineralization of in vitro Dental Caries Assessed with Polarization Sensitive Optical Coherence Tomography. J Biomed Opt. 2006;11(1):014016, 1–9. doi: 10.1117/1.2161192. [DOI] [PubMed] [Google Scholar]
- 19.Jones RS, Fried D. Quantifying the remineralization of artificial caries lesions using PS-OCT. Lasers in Dentistry XII; SPIE; 2006. pp. 613701pp. 1–8. [Google Scholar]
- 20.Manesh SK, Darling CL, Fried D. Polarization sensitive optical coherence tomography for the nondestructive assessment of the remineralization of dentin. J Biomed Optics. 2009;14(4):044002, 1–6. doi: 10.1117/1.3158995. [DOI] [PubMed] [Google Scholar]
- 21.Featherstone JD. The science and practice of caries prevention. J Am Dent Assoc. 2000;131(7):887–899. doi: 10.14219/jada.archive.2000.0307. [DOI] [PubMed] [Google Scholar]
- 22.O’Reilly MM, Featherstone JD. Demineralization and remineralization around orthodontic appliances: an in vivo study. Am J Orthod Dentofacial Orthop. 1987;92(1):33–40. doi: 10.1016/0889-5406(87)90293-9. [DOI] [PubMed] [Google Scholar]
- 23.Bishara SE, Ostby AW. White Spot Lesions: Formation, Prevention, and Treatment. Seminars in Orthodontics. 2008;14(3):174–182. [Google Scholar]
- 24.Ogaard B, Rolla G, Arends J. Orthodontic appliances and enamel demineralization. Part 1. Lesion development. Am J Orthod Dentofacial Orthop. 1988;94(1):68–73. doi: 10.1016/0889-5406(88)90453-2. [DOI] [PubMed] [Google Scholar]
- 25.Ogaard B, Duschner H, Ruben J, Arends J. Microradiography and confocal laser scanning microscopy applied to enamel lesions formed in vivo with and without fluoride varnish treatment. Eur J Oral Sci. 1996;104(4 Pt 1):378–383. doi: 10.1111/j.1600-0722.1996.tb00095.x. [DOI] [PubMed] [Google Scholar]
- 26.Ogaard B. Effects of fluoride on caries development and progression in vivo. J Dent Res. 1990;69(Spec No):813–819. doi: 10.1177/00220345900690S155. Discussion 820–813. [DOI] [PubMed] [Google Scholar]
- 27.Ogaard B, Rolla G, Helgeland K. Fluoride retention in sound and demineralized enamel in vivo after treatment with a fluoride varnish (Duraphat) Scand J Dent Res. 1984;92(3):190–197. doi: 10.1111/j.1600-0722.1984.tb00878.x. [DOI] [PubMed] [Google Scholar]
- 28.Todd MA, Staley RN, Kanellis MJ, Donly KJ, Wefel JS. Effect of a fluoride varnish on demineralization adjacent to orthodontic brackets. Am J Orthod Dentofacial Orthop. 1999;116(2):159–167. doi: 10.1016/s0889-5406(99)70213-1. [DOI] [PubMed] [Google Scholar]
- 29.Schmit JL, Staley RN, Wefel JS, Kanellis M, Jakobsen JR, Keenan PJ. Effect of fluoride varnish on demineralization adjacent to brackets bonded with RMGI cement. Am J Orthod Dentofacial Orthop. 2002;122(2):125–134. doi: 10.1067/mod.2002.126595. [DOI] [PubMed] [Google Scholar]
- 30.Darling CL, Featherstone JDB, Le CQ, Fried D. An automated digital microradiography system for assessing tooth demineralization. Lasers in Dentistry VX; San Jose. SPIE; 2009. pp. 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Darling CL, Huynh GD, Fried D. Light Scattering Properties of Natural and Artificially Demineralized Dental Enamel at 1310-nm. J Biomed Optics. 2006;11(3):034023, 1–11. doi: 10.1117/1.2204603. [DOI] [PubMed] [Google Scholar]
- 32.Gorton J, Featherstone JD. In vivo inhibition of demineralization around orthodontic brackets. Am J Orthod Dentofacial Orthop. 2003;123(1):10–14. doi: 10.1067/mod.2003.47. [DOI] [PubMed] [Google Scholar]
- 33.Ogaard B, Ten Bosch JJ. Regression of white spot enamel lesions. A new optical method for quantitative longitudinal evaluation in vivo. Am J Orthod Dentofacial Orthop. 1994;106(3):238–242. doi: 10.1016/S0889-5406(94)70042-7. [DOI] [PubMed] [Google Scholar]
- 34.Ngaotheppitak P, Darling CL, Fried D, Bush J, Bell S. PS-OCT of Occlusal and Interproximal Caries Lesions viewed from Occlusal Surfaces. Lasers in Dentistry XII; SPIE; 2006. pp. 61370Lpp. 1–9. [Google Scholar]
- 35.Madjarova VD, Yasuno Y, Makita S, Hori Y, Voeffray JB, Itoh M, Yatagai T, Tamura M, Nanbu T. Investigations of soft and hard tissues in oral cavity by spectral domain optical coherence tomography. Coherence Domain Optical Methods and Optical Coherence Tomography in Biomedicine X; SPIE; 2006. pp. 60790Npp. 1–7. [Google Scholar]
- 36.Furukawa H, Hiro-Oka H, Amano T, ChoiTakeo D, Miyazawa T, Yoshimura R, Shimizu K, Ohbayashi K. Reconstruction of three-dimensional structure of an extracted tooth by OFDR-OCT. Coherence Domain Optical Methods and Optical Coherence Tomography in Biomedicine X; SPIE; 2006. pp. 60790Tpp. 1–7. 6079. [Google Scholar]
- 37.Manesh SK, Darling CL, Fried D. Polarization-sensitive optical coherence tomography for the nondestructive assessment of the remineralization of dentin. J Biomed Opt. 2009;14(4):044002, 1–9. doi: 10.1117/1.3158995. [DOI] [PubMed] [Google Scholar]
- 38.Jones RS, Fried D. Remineralization of Enamel Caries Can Decrease Optical Reflectivity. J Dent Res. 2006;85(9):804–808. doi: 10.1177/154405910608500905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jones RS, Staninec M, Fried D. Imaging artificial caries under composite sealants and restorations. J Biomed Opt. 2004;9(6):1297–1304. doi: 10.1117/1.1805562. [DOI] [PubMed] [Google Scholar]


