Abstract
Polycaprolactone (PCL) was coated on porous tricalcium phosphate (TCP) scaffolds to achieve controlled protein delivery. Porous TCP scaffolds were fabricated using reticulated polyurethane foam as sacrificial scaffold with a porosity of 70–90 vol %. PCL was coated on sintered porous TCP scaffolds by dipping-drying process. The compressive strength of TCP scaffolds increased significantly after PCL coating. The highest strength of 2.41 MPa at a porosity of 70% was obtained for the TCP scaffold coated with 5% PCL solution. Model protein bovine serum albumin (BSA) was encapsulated efficiently within the PCL coating. The amount of BSA encapsulation was controlled by varying proteins’ composition in the PCL coating. The FTIR analysis confirmed that BSA retained its structural conformation and did not show significant denaturization during PCL coating. The release kinetics in phosphate buffer solution indicated that the protein release was controlled and sustained, and primarily dependant on protein concentration encapsulated in the PCL coating.
Keywords: porous tricalcium phosphate, polycaprolactone, coating, protein delivery
INTRODUCTION
A key component in tissue engineering for bone regeneration is the scaffold that serves as a template for cell interactions and the formation of bone-extracellular matrix to provide structural support to the newly formed tissue. 1 Scaffolds for bone regeneration should meet certain criteria to serve this function, including adequate mechanical stability to provide a suitable environment for new bone tissue formation, and biodegradability at a rate commensurate with remodeling. Additionally, scaffolds also can act as delivery vehicles for bone growth factors such as bone morphogenetic proteins (BMP) and transforming growth factors to induce bone tissue growth through the scaffolds 2, 3 Therefore, scaffolds should have high capacity for protein loading and should be able to maintain a controlled and sustained release.
Tricalcium phosphate (TCP) has been widely studied for their applications in orthopedics and tissue engineering. 4–8 TCP has excellent biocompatibility for bone tissue growth, and it is also biodegradable. However, in the porous form, the mechanical strength of TCP scaffolds are very low. 9 Moreover, protein loading on the surface of TCP is carried out by physical adsorption. The adsorption capacity is limited, which mainly depends on the surface area of the material. It is also difficult to control the amount of loaded proteins to fulfill various requirements. Furthermore, the protein release has been reported to be quite abrupt and only within the initial short period; instead of desired sustained and controlled release. 10
The hybridization of polymer with TCP is expected to improve mechanical property of these low-strength porous ceramics because of the flexibility of polymer. Some TCP or polymer composites have been developed with biopolymers such as chitosan, PLGA, PMMA, polypropylene, and polycaprolactone (PCL). 11–16 In most of those cases, TCP powders were mixed with polymers to make the composites. Similar to TCP, PCL has been widely studied for their applications in orthopedics and tissue engineering because of its excellent biocompatibility, and sustained biodegradability. 17–21 The hybridization of TCP and PCL can potentially enhance the mechanical properties of porous structures, and in vivo study indicated that TCP–PCL scaffolds helped to promote further bone healing. 22 However, previous reports on TCP–PCL composites were based on mixing of TCP powder with PCL polymers in which the ultimate composite has limited mechanical strength in the highly porous form because of the unsintered TCP phase. Particulate TCP–PCL composite scaffolds have been also developed as drug delivery systems for rhBMP-2.23 However, low loading efficiency and burst release behavior were reported.
We hypothesize here that highly porous sintered TCP scaffolds can offer pathways for bone tissue growth whereas controlled and sustained release of protein or growth factors from PCL coated struts can further induce osseo-integration. To verify our hypothesis, we have designed an experimental matrix to test if different amounts of protein can be loaded on highly porous sintered TCP followed by the protein release behavior, which can be controlled and sustained. To accomplish these goals, a PCL or protein composite coating design on porous TCP scaffold is obtained. Moreover, by coating with PCL layer, the brittleness of the porous TCP is decreased further. The amount of loaded protein is controlled easily by varying protein composition in PCL coating. Protein release from PCL coated TCP is primarily dependant on the degradation behavior of PCL. In this research, TCP scaffold is fabricated using polyurethane (PU) foam as a sacrificial template material and then TCP scaffold is sintered. PCL is coated onto sintered TCP that encapsulates a model protein bovine serum albumin (BSA). Therefore, during early stages of protein release, porous TCP maintains its mechanical properties and structure. The mechanical strength is investigated before and after coating with PCL. The release kinetics of protein from PCL coated porous TCP scaffold was studied in vitro.
MATERIALS AND METHODS
Fabrication of Porous TCP Scaffolds
Porous TCP scaffolds were fabricated using PU foam as a template. PU foam (E.N. Murray CO., Denver, CO) with porosity of 12 pores/cm was used as reticulated template. The TCP ceramic slurry was prepared using commercial high-purity TCP powder from Berkeley Advanced Biomaterials (Berkeley, CA). About 26 g of TCP powder and 30 ml distilled water were mixed in a ball mill for 6 h with 0.6 g dispersant of D-3021 (Rohm and Haas, Philadelphia, PA). About 0.75 g 1-butanol (Fisher Chemical, Fair Lawn, NJ) and 2.6 g polyvinyl alcohol (PVA, Alfa Aesar, Ward Hill, MA) were added as antifoaming agent and binder, respectively. The PU foam was completely immersed into the slurry and compressed in order to disperse the slurry uniformly throughout the porous foam. Then the excess slurry was squeezed out to keep the pores open. The green porous body was dried for 15 h in air at room temperature and then heat-treated in a muffle furnace to burn out the PU foam and binder at 600°C for 90 min with a slow heating rate of 1°C/min. Finally, the porous TCP scaffold was obtained via sintering at 1200°C for 2 h.
PCL Coating
Polycaprolactone pellets (PCL, MW 10,000, Aldrich, St. Louis, MO) were dissolved in dichloromethane (DCM, CH2Cl2, J.T. Baker, Phillipsburg, NJ) with the concentration of 2.5%, 3.75%, and 5% w/v, respectively. Sintered TCP scaffolds were dipped into the composite solutions for 5 min and the excess solution was removed to form a uniform coating. The coated scaffolds were then dried for 24 h at room temperature.
Scaffold Characterization
The microstructures of the scaffolds were observed using a scanning electron microscope (SEM, Hitachi s-570, Japan). Pore size was measured by analyzing SEM images, and porosity of the scaffolds was determined by measuring the physical dimensions and mass of the samples. An X-ray diffractometer (PW 3040/00 X’pert MPD, Philips, Eindhoven, the Netherlands) was used to analyze the phase composition. The infrared spectra of PCL coatings were recorded by an infrared Fourier transform spectrometer (FTIR, Nicolet 6700, ThermoFisher, Madison, WI). The compressive strengths of porous TCP scaffolds before and after PCL coating were tested using a screw driven Instron machine with a loading rate of 0.5 mm/min.
Protein Encapsulation
Bovine serum albumin (BSA, A7030, Sigma, Saint Louis, MO) was used as model protein. To prepare 5%, 10%, and 15% (BSA/PCL, w/w) of PCL+BSA composition coating, preset amounts of BSA aqueous solution (50 mg/ml) was added in PCL solution, and dispersed uniformly by stirring vigorously for 4 h. Then, the same coating process was followed. For control, pure TCP and the scaffold with only PCL coating were also tested for BSA loading capacity. The loading was obtained by immersion in 0.5 mg/ml BSA aqueous solution for 2 h.
Protein Release In Vitro
BSA-loaded TCP scaffolds were immersed in 20 ml phosphate buffer solution (PBS) under 37°C at pH 7.4. At predetermined periods of time, BSA release amount was measured by Micro BCA™ protein assay kit (Pierce, Rockford, IL) and followed the manufacturer’s instruction. The medium was refreshed at each test period.
RESULTS
Scaffolds Microstructure and Phases
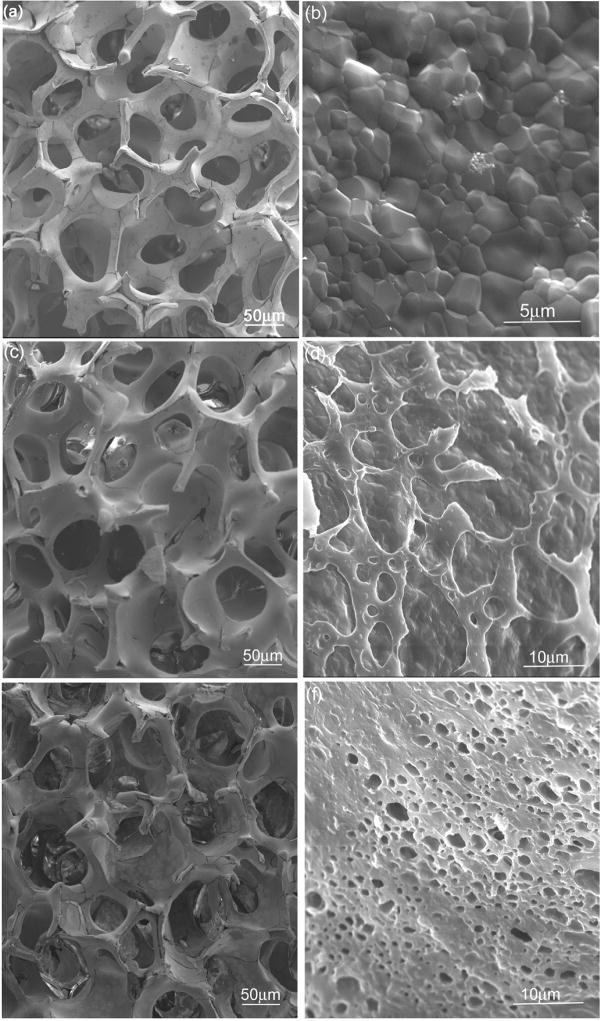
Figure 1a shows a typical SEM morphology of porous TCP scaffold obtained by a PU foam reticulate method. The scaffold exhibits an interconnected porous structure with open macropores varied from 300 to 800 μm. The bulk porosity of TCP scaffold obtained by this method can be varied from 70% to 90%. From the high magnification stem of a sintered porous TCP [Figure 1(b)], a dense microstructure can be observed that indicates good sintering of TCP scaffold with typical grain size between 0.5 and 2 μm.
Figure 1.
SEM morphologies of porous TCP scaffold (a, b) and with PCL coating at various concentrations: (c, d) 2.5% and (e, f) 5%.
SEM morphologies of TCP scaffolds coated with PCL at various concentrations are presented in Figure 1(c–f). When coated with PCL at low concentration of 2.5% [Figure 1(c)], the scaffold is covered partially with PCL [Figure 1(d)], and maintains the initial framework structure. With the increase of PCL concentration, more surface of TCP scaffold gets covered with PCL coating, the stems becomes thicker and some pores can partially be clogged, as shown in Figure 1(e–f).
Mechanical Strength
The compressive strength of TCP scaffolds with and without PCL coating is presented in Figure 3. The scaffold with higher porosity shows lower compressive strength. Compared with pure TCP, PCL coated scaffolds exhibits higher compressive strength. The compressive strength increases with higher PCL concentration. The highest strength of 2.41 MPa for 70% porosity sample was obtained for the scaffold coated with 5% PCL solution.
Figure 3.
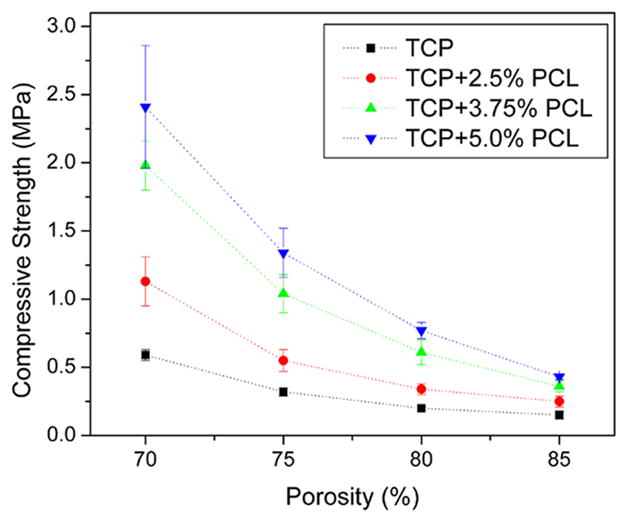
Compressive strength of porous TCP scaffolds with and without PCL coating. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
BSA Encapsulation
Table I shows the summary of the amount of BSA encapsulated on TCP scaffolds with and without PCL coating. The amount of loaded protein on pure TCP scaffold is limited, which is about 1.40 mg BSA/g TCP. After coating with PCL, the scaffolds displayed higher capacity to encapsulate BSA. The amount of 2.45, 4.73, and 6.62 mg BSA/g TCP were obtained for the samples encapsulated with 5%, 10%, and 15% of BSA, respectively. Moreover, the amount of BSA encapsulation could be controlled by varying protein concentration in the PCL coating.
Table 1.
The amount of BSA loaded on TCP scaffolds with and without PCL coating
| Samples | BSA encapsulation (mg) | Normalized to TCP scaffold (mg BSA/g TCP) |
|---|---|---|
| TCP+PCL+ 5% BSA | 1.5 ± 0.06 | 2.45 ± 0.07 |
| TCP+PCL+ 10% BSA | 2.8 ± 0.23 | 4.73 ± 0.43 |
| TCP+PCL+ 15% BSA | 4.1 ± 0.25 | 6.62 ± 0.40 |
| TCP* | 0.80 ± 0.05 | 1.40 ± 0.09 |
The loading amounts are obtained by immersion in 0.5mg/ml BSA aqueous solution for 2 h.
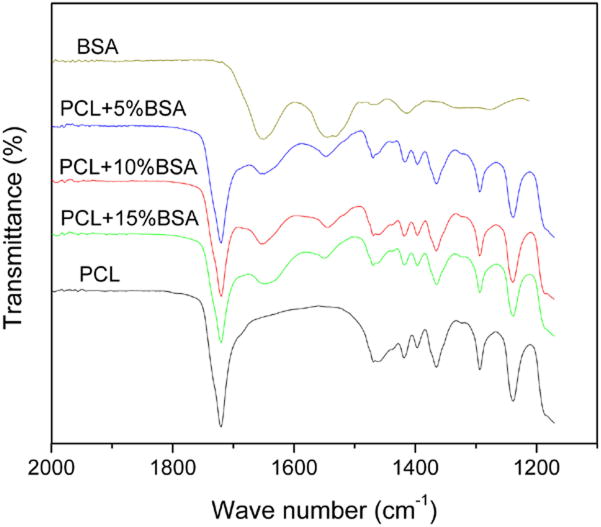
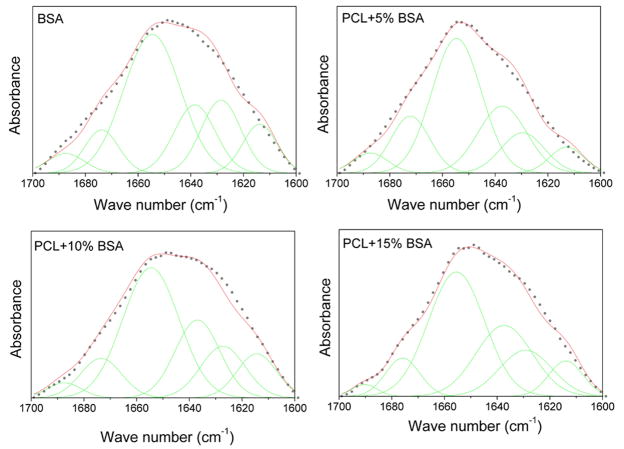
FTIR spectra of native and encapsulated BSA in PCL coating are shown in Figure 4. The amide band I (1600–1700 cm−1) is because of the C=O stretching mode, and the amide II band (1500–1600 cm−1) is attributed to the bending and the stretching mode of N-H and C-N vibrations. 24 After encapsulation in PCL coating, the amide I and amide II bands can be identified clearly. Amide I band is analyzed to figure out the secondary structures of BSA protein. The band can be disintegrated into its fragments by Curve-Fitting method, as shown in Figure 5. The band at 1654 cm−1 is assigned to α-helix, and the bands at 1628 and 1638 cm−1 are β-sheet conformation, whereas the other subbands are unordered secondary structures. 25 The changes in BSA secondary structural content are summarized in Table II. It can be seen that BSA retained most of its structures and there was no significant decomposition of BSA after encapsulation in PCL coating. Compared with native BSA, only minor decrease in α-helix content can be seen.
Figure 4.
FTIR spectra of native BSA and encapsulated in PCL coatings. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
Figure 5.
Infrared spectra of native and encapsulated BSA in the amide I region and their Gaussian curve-fitting. The black broken lines are amide I FTIR spectra overlaid with the result of the Gaussian curve-fitting (red solid lines); the individual Gaussian bands (green lines) are shown as symmetrical peaks underneath the IR spectra. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
Table 2.
Secondary structural content of native and encapsulated BSA in PCL coatings. (Determined by Gaussian curve-fitting of the FTIR spectra)
| α-helix | β-sheet | other | |
|---|---|---|---|
| BSA | 46.2 | 31.8 | 22 |
| PCL+5%BSA | 41.1 | 32.4 | 26.4 |
| PCL+10%BSA | 42.3 | 33 | 22.7 |
| PCL+15%BSA | 39.1 | 37.5 | 23.4 |
BSA Release
The BSA release in PBS is shown in Figure 6(a). BSA was released from TCP scaffolds with PCL+BSA composition coating by a two-step release pattern including an initial burst release followed by a relatively slow sustained release. An initial burst in the first 6 h was around 45, 71, and 98 μg/ml for the samples encapsulated with 5%, 10%, and 15% of BSA, respectively. Then the protein release was sustained during the next 15 days. The average release amount was 4.6, 7.4, and 11.1 μg/ml/day, respectively. The release amount was related to the loading concentration, that is, larger release amounts are for higher initial loading concentrations of BSA. The BSA release was normalized to the initial protein encapsulated in the coatings, and are presented in Figure 6(b). The result showed that the protein release was at almost the same rate, about 3% of BSA/day, for all scaffolds during 15 days. In contrast, BSA release from pure TCP and the scaffolds with only PCL coating were significantly different and burst in nature. About 80–90% of loaded BSA was released within the first 24 h [Figure 6(c)].
Figure 6.
BSA cumulative release kinetics: (a) TCP scaffolds with PCL+BSA composition coating, (b) normalized to the initial encapsulated protein in the coatings, and (c) pure TCP and the scaffolds with only PCL coating. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
DISCUSSION
In this study, porous beta-TCP scaffold was developed and coated with PCL for their use in bone tissue engineering. Porous TCP scaffold can provide enough space for bone tissue in growth and blood circulation. However, high porosity also decreases the mechanical properties of scaffold, which can limit its applications.9 The major issue when considering porous TCP for tissue engineering lies with its poor mechanical strength, which lead to the difficulty even in handling. The presence of fragments and debris also could cause adverse effects after implanted in vivo.
The PCL coating is expected to improve mechanical property of these fragile TCP scaffolds because PCL adds significantly higher strain to failure to the brittle system. The results of mechanical test indicate that the strength of TCP scaffolds increase after coating with PCL, and the increase is more significant with increasing PCL concentration. The PCL coating results in thicker stems and lower porosity, which might affect the mechanical properties of the scaffold. However, at similar porosity, PCL-coated scaffolds exhibit higher mechanical strength than uncoated scaffold. This can be attributed to the decrease of the brittleness of the scaffold after coated with PCL. The presence of flaws in TCP framework also influences the mechanical strength of scaffold, which might initiate fracture. The PCL coatings fill or blunt some of those flaws, as shown in Figure 7. This contributes to higher mechanical strength of the scaffolds after PCL coating.
Figure 7.
SEM morphology of PCL coated TCP scaffold, showing the flaw covered by PCL coating.
As a scaffold for bone tissue engineering, porous TCP is required to be loaded with some bone-related growth factors that can be released in a controlled and sustained manner. The control of loading amount is the prerequisite for the release behavior. The protein loading on pure TCP is obtained by physical adsorption. The adsorption capacity is limited, and is also difficult to control. For the scaffold with PCL + BSA composition coating, protein loading is facilitated by encapsulation of protein in PCL coating. Thus, more protein can be encapsulated and the amount of protein encapsulation can be controlled efficiently by changing the protein concentration in PCL coating.
Retaining structural conformation and bioactivity of loaded proteins are very important for a protein delivery. From the FTIR spectra, amide I and amide II bands can be observed after encapsulation in PCL coating. Amide I and amide II bands are the main bands for BSA, and generally employed to study the protein structure. 25–27 The analysis of protein secondary structures indicates that BSA retains its main secondary structures when encapsulated in PCL coating. Compare to native BSA, no obvious change can be seen for the amount of α-helix content, which is a preferred indicator of the protein’s structural integrity. 25 The results of FTIR analysis confirm that the encapsulation of BSA in PCL coating does not lead to any significant denaturization of BSA.
In addition to having high capacity of protein encapsulation, scaffolds are also required to have appropriate release kinetics. Ideally, protein should be released continually during the healing or bone tissue in growth process. For pure TCP and the scaffold with only PCL coating, protein adsorption on the material surface are obtained by weak physical force, that is, van der Waals and electrostatic interactions. 24 These bonding forces are weak, thus the proteins release is quite abrupt within the initial several hours. For scaffold with PCL+BSA coating, the protein is encapsulated in PCL coating layer. With the degradation of PCL coating, protein is released in sustained manner. Furthermore, the release amount is also controlled, which mainly depends on the protein concentration encapsulated within the PCL coating. Overall, our experimental results confirm a new design for porous TCP scaffold via coating with PCL. Results clearly show that not only higher mechanical strength, but also PCL-coated scaffold allow us to load more protein, and realize controlled and sustained release of the model protein, BSA.
CONCLUSIONS
To achieve sustained and controlled protein delivery and improve mechanical strength, porous beta-TCP scaffolds were coated with PCL. Model protein BSA was encapsulated efficiently within the PCL coating without significant denaturization. The amount of BSA encapsulation can be controlled by varying proteins composition in PCL coating. The release kinetics in PBS indicated that BSA protein release was sustained, and the release amount was controlled. The compressive strength of beta-TCP scaffolds also increased after coating with PCL. This work has demonstrated the potential of PCL-coated porous beta-TCP can be used as a bone tissue engineering scaffold and should be investigated further in vivo.
Figure 2.
XRD analysis of TCP from sintered scaffolds. The primary phase is beta TCP.
Figure 2 shows the X-ray diffraction plot of the ground powders from the sintered TCP scaffolds made from the commercial starting powders (Berkeley Advanced Materials, CA). It can be seen that the sintered powder, made by grinding sintered scaffold, are primarily of beta-TCP phase as labeled based on the JCPDS file # 09-0169.
Acknowledgments
The authors thank The National Institutes of Health (Grant # NIH-R01-EB-007351) and The National Science Foundation (NSF) for financial support under the Presidential CAREER Award for Scientists and Engineers (RECASE) grant to Dr. S. Bose (CTS # 0134476).
References
- 1.Karageorgiou V, Kaplan D. Porosity of 3D biomaterial scaffolds and osteogenesis. Biomaterials. 2005;26:5474–5491. doi: 10.1016/j.biomaterials.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 2.Matsushita N, Terai H, Okada T, Nozaki K, Inoue H, Miyamoto S, Takaoka K. Accelerated repair of a bone defect with a synthetic biodegradable bone-inducing implant. J Orthop Sci. 2006;11:505–511. doi: 10.1007/s00776-006-1048-3. [DOI] [PubMed] [Google Scholar]
- 3.Ma BK, Clarke SA, Brooks RA, Rushton N. The effect of simvastatin on bone formation and ceramic resorption in a peri-implant defect model. Acta Biomater. 2008;4:149–155. doi: 10.1016/j.actbio.2007.05.004. [DOI] [PubMed] [Google Scholar]
- 4.Bandyopadhyay A, Bernard S, Xue W, Bose S. Feature article: calcium phosphate based resorbable ceramics: influence of MgO, ZnO and SiO2 dopants. J Am Cer Soc. 2006;89(9):2675–2688. [Google Scholar]
- 5.Seeley Z, Bandyopadhyay A, Bose S. Influence of TiO2 and Ag2O addition on mechanical properties of tricalcium phosphate. J Biomed Mater Res: Part A. 2007;82A(1):113–121. doi: 10.1002/jbm.a.31077. [DOI] [PubMed] [Google Scholar]
- 6.Chu TMG, Warden SJ, Turner CH, Stewart RL. Segmental bone regeneration using a load-bearing biodegradable carrier of bone morphogenetic protein-2. Biomaterials. 2007;28:459–467. doi: 10.1016/j.biomaterials.2006.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yuan J, Cui L, Zhang WJ, Liu W, Cao YL. Repair of canine mandibular bone defects with bone marrow stromal cells and porous beta-tricalcium phosphate. Biomaterials. 2007;28:1005–1013. doi: 10.1016/j.biomaterials.2006.10.015. [DOI] [PubMed] [Google Scholar]
- 8.Seeley Z, Bandyopadhyay A, Bose S. Tricalcium phosphate based resorbable ceramics: influence of NaF and CaO addition. Mater Sci Eng C. 2008;28(1):11–17. [Google Scholar]
- 9.Bose S, Darsell J, Kintner M, Hosick H, Bandyopadhyay A. Pore size and pore volume effects on calcium phosphate based ceramics. Mater Sci Eng C. 2003;23:479–486. [Google Scholar]
- 10.Ziegler J, Mayr-Wohlfart U, Kessler S, Breitig D, Gunther KP. Adsorption and release properties of growth factors from biodegradable implants. J Biomed Mater Res. 2002;59:422–428. doi: 10.1002/jbm.1258. [DOI] [PubMed] [Google Scholar]
- 11.Fini M, Giavaresi G, Aldini NN, Torricelli P, Botter R, Beruto D, Giardino R. A bone substitute composed of polymethylmethacrylate and alpha-tricalcium phosphate: results in terms of osteoblast function and bone tissue formation. Biomaterials. 2002;23:4523–4531. doi: 10.1016/s0142-9612(02)00196-5. [DOI] [PubMed] [Google Scholar]
- 12.Soriano I, Evora C. Formulation of calcium phosphates/poly (d, l-lactide) blends containing gentamicin for bone implantation. J Control Release. 2000;68:121–134. doi: 10.1016/s0168-3659(00)00251-0. [DOI] [PubMed] [Google Scholar]
- 13.Zhang Y, Zhang MQ. Three-dimensional macroporous calcium phosphate bioceramics with nested chitosan sponges for load-bearing bone implants. J Biomed Mater Res. 2002;61:1–8. doi: 10.1002/jbm.10176. [DOI] [PubMed] [Google Scholar]
- 14.Kalita SJ, Bose S, Hosick HL, Bandyopadhyay A. Development of controlled porosity polymer-ceramic composite scaffolds via fused deposition modeling. Mat Sci Eng C. 2003;23:611–620. [Google Scholar]
- 15.Yeo A, Rai B, Sju E, Cheong JJ, Teoh SH. The degradation profile of novel, bioresorbable PCL-TCP scaffolds: an in vitro and in vivo study. J Biomed Mater Res A. 2008;84A:208–214. doi: 10.1002/jbm.a.31454. [DOI] [PubMed] [Google Scholar]
- 16.Verma D, Katti K, Katti D. Bioactivity in in situ hydroxyapatite-polycaprolactone composites. J Biomed Mater Res A. 2006;78A:772–780. doi: 10.1002/jbm.a.30774. [DOI] [PubMed] [Google Scholar]
- 17.Venugopal J, Low S, Choon AT, Kumar AB, Ramakrishna S. Electrospun-modified nanofibrous scaffolds for the mineralization of osteoblast cells. J Biomed Mater Res A. 2008;85A:408–417. doi: 10.1002/jbm.a.31538. [DOI] [PubMed] [Google Scholar]
- 18.Izquierdo R, Garcia-Giralt N, Rodriguez MT, Caceres E, Garcia SJ, Ribelles JLG, Monleon M, Monllau JC, Suay J. Biodegradable PCL scaffolds with an interconnected spherical pore network for tissue engineering. J Biomed Mater Res A. 2008;85A:25–35. doi: 10.1002/jbm.a.31396. [DOI] [PubMed] [Google Scholar]
- 19.Chang HI, Lau YC, Yan C, Coombes AGA. Controlled release of an antibiotic, gentamicin sulphate, from gravity spun polycaprolactone fibers. J Biomed Mater Res A. 2008;84A:230–237. doi: 10.1002/jbm.a.31476. [DOI] [PubMed] [Google Scholar]
- 20.Rai B, Oest ME, Dupont KM, Ho KH, Teoh SH, Guldberg RE. Combination of platelet-rich plasma with polycaprolactone-tricalcium phosphate scaffolds for segmental bone defect repair. J Biomed Mater Res A. 2007;81A:888–899. doi: 10.1002/jbm.a.31142. [DOI] [PubMed] [Google Scholar]
- 21.Li WJ, Danielson KG, Alexander PG, Tuan RS. Biological response of chondrocytes cultured in three-dimensional nanofibrous poly(epsilon-caprolactone) scaffolds. J Biomed Mater Res A. 2003;67A:1105–1114. doi: 10.1002/jbm.a.10101. [DOI] [PubMed] [Google Scholar]
- 22.Swieszkowski W, Tuan BHS, Kurzydlowski KJ, Hutmacher DW. Repair and regeneration of osteochondral defects in the articular joints. Biomol Eng. 2007;24:489–495. doi: 10.1016/j.bioeng.2007.07.014. [DOI] [PubMed] [Google Scholar]
- 23.Rai B, Teoh SH, Hutmacher DW, Cao T, Ho KH. Novel PCL-based honeycomb scaffolds as drug delivery systems for rhBMP-2. Biomaterials. 2005;26:3739–3748. doi: 10.1016/j.biomaterials.2004.09.052. [DOI] [PubMed] [Google Scholar]
- 24.Hartmann M. Ordered mesoporous materials for bioadsorption and biocatalysis. Chem Mater. 2005;17:4577–4593. [Google Scholar]
- 25.Fua K, Griebenowb K, Hsiehc L, Klibanovd AM, Langera R. FTIR characterization of the secondary structure of proteins encapsulated within PLGA microspheres. J Control Release. 1999;58:357–366. doi: 10.1016/s0168-3659(98)00192-8. [DOI] [PubMed] [Google Scholar]
- 26.Carrasquillo KG, Stanley AM, Aponte-Carro JC, De Je’susa P, Costantinob HR, Bosquesc CJ, Griebenow K. Non-aqueous encapsulation of excipient-stabilized spray-freeze dried BSA into poly(lactide-co-glycolide) microspheres results in release of native protein. J Control Release. 2001;76:199–208. doi: 10.1016/s0168-3659(01)00430-8. [DOI] [PubMed] [Google Scholar]
- 27.Wang F, Yang Z, Zhou Y, Weng S, Zhang L, Wu J. Influence of metal ions on phosphatidylcholine-bovine serum albumin model membrane, an FTIR study. J Mol Struct. 2006;794:1–11. [Google Scholar]