Abstract
Sphingolipids are important components of cell membranes that may also serve as cell signaling molecules; ceramide plays a central role in sphingolipid metabolism. The aim of this study was to examine the effect of 5 weeks of aerobic training on key enzymes and intermediates of ceramide metabolism in skeletal muscles. The experiments were carried out on rats divided into two groups: (1) sedentary and (2) trained for 5 weeks (on a treadmill). The activity of serine palmitoyltransferase (SPT), neutral and acid sphingomyelinase (nSMase and aSMase), neutral and alkaline ceramidases (nCDase and alCDase) and the content of sphingolipids was determined in three types of skeletal muscle. We also measured the fasting plasma insulin and glucose concentration for calculating HOMA-IR (homeostasis model assessment) for estimating insulin resistance. We found that the activities of aSMase and SPT increase in muscle in the trained group. These changes were followed by elevation in the content of sphinganine. The activities of both isoforms of ceramidase were reduced in muscle in the trained group. Although the activities of SPT and SMases increased and the activity of CDases decreased, the ceramide content did not change in any of the studied muscle. Although ceramide level did not change, we noticed increased insulin sensitivity in trained animals. It is concluded that training affects the activity of key enzymes of ceramide metabolism but also activates other metabolic pathways which affect ceramide metabolism in skeletal muscles.
Keywords: Serine palmitoyltransferase, Sphingomyelinase, Ceramidase, Sphingolipid intermediates, Skeletal muscle, Training
Introduction
Sphingolipids are an important lipid class that is present in all higher organisms. Ceramide (Cer) is the key compound on the crossroads of sphingolipid metabolism. Ceramide mediates a number of biological processes, including apoptosis, proliferation, differentiation, growth arrest, inflammation and heat stress response [1–4]. The variety of cellular effects of ceramide can be attributed to its ability to alter the activity of kinases, phosphatases and transcription factors [5–7]. Other sphingolipid intermediates, such as sphingosine (Sph), sphingosine-1-phosphate (S1P), ceramide-1-phosphate are also important second messengers [8–12]. The cellular content of ceramide is determined by a balance between the rate of its formation and degradation. There are two major types of ceramides production. One of them is hydrolysis of sphingomyelin and the other one is de novo biosynthesis. Sphingomyelin is located in the plasma membrane, in lysosomes, and in endosomes. Its hydrolysis is catalyzed by the neutral and acid sphingomyelinase (n- and aSMase). De novo synthesis is initiated by condensation of serine with palmitoyl-CoA to generate 3-ketosphinganine [13]. This, the rate-limiting step in de novo sphingolipid biosynthesis is catalyzed by the enzyme serine-palmitoyltransferase (SPT). 3-Ketosphingosine is rapidly reduced to sphinganine (SPA) by the action of the enzyme 3-ketosphinganine reductase. Next, SPA is acylated to form dihydroceramide by the action of dihydroceramide synthase. The last step of ceramide synthesis is conversion of dihydroceramide to ceramide by insertion of a 4,5-trans-double bond into dihydroceramide. This reaction is catalyzed by the enzyme dihydroceramide desaturase [13]. Ceramide is hydrolyzed by the enzyme ceramidase (CDase) to yield a free fatty acid and sphingosine. There are three isoforms of ceramidase: acid (aCDase), neutral (nCDase) and alkaline (alCDase). By the use of enzymatic assay and Northern blot analysis almost no activity or mRNA of aCDase was found in skeletal muscle [14].
Ceramide has been shown to be present in skeletal muscle [15–17]. Its content in the muscles depends on the muscle type. The higher content of ceramide was observed in muscle composed mostly of oxidative fibers than in muscle composed mostly of glycolytic fibers [16]. Skeletal muscles are responsible for 70–80% of whole body insulin-stimulated glucose uptake [18, 19]. Recent evidence suggests that the accumulation of intramuscular lipids is involved in the induction of insulin-resistance [20, 21]. One candidate for this action is ceramide [17]. The intracellular level of ceramide is increased in the muscles of obese, insulin-resistant Zucker rats [22] and obese insulin-resistant humans [17]. Adams et al. reported that ceramide content increased nearly twofold in skeletal muscles of obese humans. It is also well known that trained subjects are more insulin sensitive than sedentary subjects [23, 24].
A previous study showed that endurance training decreases the total content of sphingomyelin and ceramides, increases the content of sphinganine, does not affect the sphingosine content, and increases nSMase activity [25]. Moreover, sphingolipid content and the activity of enzymes of ceramide metabolism both depend on the duration of exercise and muscle type [26]. In the mentioned study, the ceramide level decreased after 30 min of running but increased after exhaustive exercise [26]. Data obtained from human skeletal muscle showed that training did not change either ceramide content or nSMase activity [27]. There was no data on the effect of training on the activity of SPT, acid sphingomyelinase and ceramidases in rat skeletal muscles. Therefore the aim of the present study was to examine the effect of training on the activity of key enzymes of ceramide metabolism and selected sphingolipid intermediates in skeletal muscle.
Materials and Methods
Animals and Study Design
The investigation was approved by the Ethical Committee for Animal Experiments at the Medical University of Bialystok. The experiments were carried out on male Wistar rats (200–250 g) fed ad libitum on commercial food pellets for rodents. Animals were housed in standard conditions (21 ± 2 °C, 12 h light/12 h dark cycle) with free access to tap water and food pellets. The animals were randomly divided into two groups (N = 8 in each group): (1) sedentary (control) (2) trained for 5 weeks on an electrically driven treadmill according to the following protocol [28]: 1st week 1 h daily at a speed of 960 m/h. The same running time was applied during successive weeks but the running speed was increased as follows: 2nd week 1,200 m/h, 3rd week 1,440 m/h, 4th–6th week 1,680 m/h. This type of training was previously found to affect ceramide metabolism in both cardiac and skeletal muscle of the rat [25, 29]. Twenty four hours after the last exercise bout in the training program, the rats were anaesthetized along with the controls with pentobarbital sodium administered intraperitoneally at a dose of 80 mg/kg. The soleus and the red and white sections of the gastrocnemius were excised, cleaned of any visible adipose tissue, nerves, and fascias and frozen in liquid nitrogen. These muscles are composed predominantly of slow-twitch oxidative, fast-twitch oxidative-glycolytic, and fast-twitch glycolytic fibers, respectively [30, 31]. We used a homeostasis model assessment for calculating insulin resistance (HOMA-IR) in both groups.
The Content of Sphingosine, Sphinganine and Sphingosine-1-phosphate
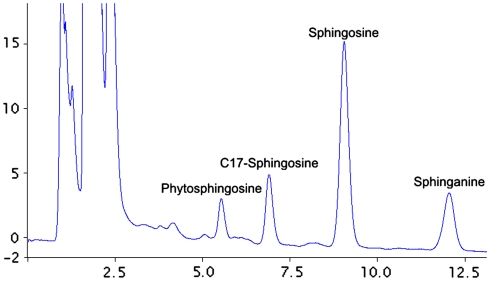
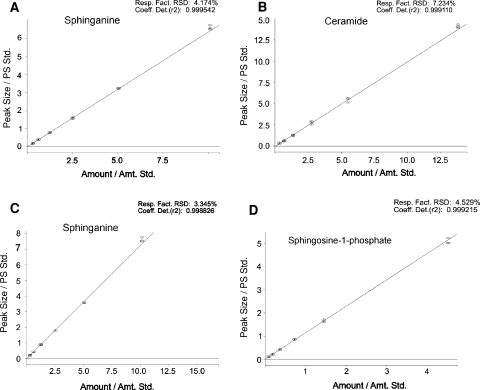
The content of Sph, SPA and S1P was measured using the method previously described by Min et al. [32]. Internal standards (C17-sphingosine and C17-S1P, Avanti Polar Lipids) were added to the samples before homogenization and ultrasonication. The dried lipid residues were redissolved in ethanol and sphingoid bases were converted to their o-phthalaldehyde derivatives and analyzed on an HPLC system (ProStar, Varian Inc.) equipped with a fluorescence detector and C18 reversed-phase column (Varian Inc. OmniSpher 5, 4.6 × 150 mm). The isocratic eluent composition of acetonitrile (Merck): water (9:1 v/v) and a flow rate of 1 ml/min were used. The column temperature was maintained at 33 °C. Figure 1 presents a chromatogram showing the separation of sphingolipids. Figure 2 shows the standard curve of SPA, Cer, Sph and S1P.
Fig. 1.
HPLC chromatography separation of sphingolipids
Fig. 2.
Standard curve of sphinganine (a), ceramides (b), sphingosine (c), sphingosine-1-phosphate (d)
The Content of Ceramide and Dihydroceramide
A small volume of the chloroform phase containing lipids extracted as described above was transferred to a fresh tube containing 31 pmol of C17-sphingosine as an internal standard. The samples were evaporated under a nitrogen stream, redissolved in 1 M KOH in 90% methanol and heated at 90 °C for 60 min to convert ceramide into sphingosine. This digestion procedure does not convert complex sphingolipids, such as sphingomyelin, galactosylceramide or glucosylceramide, into free sphingoid bases [33]. Samples were then partitioned by the addition of chloroform and water. The upper phase was discarded and the lower phase was evaporated under nitrogen. The content of free sphingosine liberated from ceramide was then analyzed by means of HPLC as described above. The calibration curves were prepared using N-palmitoylsphingosine and N-palmitoylsphinganine (Avanti Polar Lipids, Alabaster, AL, USA) as standards. The chloroform extract used for the analysis of Cer and dhCer levels also contains small amounts of free sphingoid bases. Therefore, the content of ceramide and dihydroceramide was corrected for the level of free sphingosine and sphinganine, respectively, as determined in the same sample.
The Activity of Sphingomyelinases
The activity of n- and aSMase was determined according to Liu and Hannun [34]. The activity of both sphingomyelinases was measured with the use of radiolabeled substrate [N-methyl-14C]-sphingomyelin (Perkin-Elmer Life Sciences). The product of reaction—14C-choline phosphate—was extracted with CHCl3:methanol (2:1, v/v), transferred to scintillation vials and counted using a Packard TRI-CARB 1900 TR scintillation counter.
The Activity of Ceramidases
The activity of alCDase and nCDase was measured by the method of Nikolova-Karakashian and Merrill [35]. The activity of the enzymes was determined with the use of radiolabeled [N-palmitoyl-1-14C]-sphingosine (Moravek Biochemicals) as a substrate. Unreacted ceramide and liberated 14C-palmitate were separated with basic Dole solution (isopropanol:heptane:1 N NaOH, 40:10:1, v/v/v). Radioactivity of the 14C-palmitate was measured by scintillation counting.
The Activity of Serine Palmitoyltransferase
The activity of SPT was examined as described by Merrill [36] with the use of a radiolabeled substrate, [3H]-l-serine (Moravek Biochemicals). Briefly, rat skeletal muscle microsomal fraction was obtained by ultracentrifugation at 150,000g for 40 min. Microsomes were incubated for 10 min at 37 °C in the reaction buffer (100 mM HEPES (pH 8.3), 5 mM DTT (dithiothreitol), 2.5 mM EDTA (pH 7.0), 50 μM pyridoxal phosphate, 200 μM palmitoyl-CoA and 2 mM l-serine, 44,000 dpm/nmol). The labeled lipid product 3-ketosphinganine was extracted with CHCl3:methanol (1:2, v/v) and the radioactivity was measured by scintillation counting.
Plasma Insulin and Glucose Concentration
The plasma insulin concentration was determined using an ELISA kit (Mercodia Insulin ELISA kit). The plasma glucose concentration was measured using the Glucose Ox Liquid Kit (Pointe Scientific).
Plasma Free Fatty Acids (FFA) Concentration
The FFA concentration was determined using the Wako NEFA C kit (Wako Chemicals).
Statistical Analysis
All data are presented as means ± SD. Data were analyzed by one-way analysis of variance (ANOVA), followed by the Newman–Keuls post hoc test. p values <0.05 were taken to indicate statistical significance.
Results
Plasma FFA, Glucose and Insulin Concentration and HOMA-IR
The cellular ceramide level depends on the availability of plasma FFA. Circulating FFA must be converted to their active form—fatty acyl coenzyme A—after entering the cell in order to participate in further metabolic processes. Acyl-CoA is used as a substrate in the de novo sphingolipids biosynthesis. In our study the plasma FFA concentration decreased from 260 ± 17 nmol × ml−1 (control group) to 111.06 nmol × ml−1 (p < 0.01) (training group) (Table 1). To estimate the insulin sensitivity we measured fasting plasma insulin and glucose concentration for HOMA-IR calculation. The equation for calculating HOMA-IR was as follows: HOMA-IR = (fasting plasma glucose × fasting plasma insulin)/2,430, where fasting plasma glucose was in mg/dl and fasting plasma insulin in μU/ml [37]. Both plasma glucose and insulin concentration were significantly lower in the trained group compared to the sedentary group (Table 1). We found that HOMA-IR values were lower in the trained group (HOMA-IRtrained = 0.54) than in the sedentary group (HOMA-IRsedentary = 1.13) which means that exercised animals were more insulin sensitive than sedentary rats.
Table 1.
Effect of training on plasma free fatty acid, glucose and insulin concentration
| Control | Training | |
|---|---|---|
| Plasma FFA concentration (nmol × ml−1) | 260 ± 17 | 111.06 ± 15b |
| Plasma glucose concentration (mg/dl) | 140.76 ± 16.6 | 90.18 ± 11.8a |
| Plasma insulin concentration (µU/ml) | 19.6 ± 2.4 | 14.6 ± 2.1a |
| HOMA-IR | 1.13 ± 0.10 | 0.54 ± 0.04b |
Values are means ± SD (n = 8 in each group). The rats were either sedentary (control) or trained on the electrically driven treadmill as described in the methods
HOMA-IR = [fasting glucose (mg/dl) × fasting insulin (µU/ml)]/2,430
FFA free fatty acids
a p < 0.01, b p < 0.001 versus the control group
The Content of Skeletal Muscle Sphingolipids (Table 2)
Table 2.
Effect of training on the content of sphingolipids in rat skeletal muscle
| Sphingosine | Sphinganine | S1P | Ceramide | SPA1P | dhCer | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Control | Training | Control | Training | Control | Training | Control | Training | Control | Training | Control | Training | |
| Soleus | 1.00 ± 0.17 | 1.17 ± 0.21 | 0.39 ± 0.06 | 0.59 ± 0.11a | 0.22 ± 0.04 | 0.27 ± 0.04 | 24.57 ± 3.53 | 28.70 ± 3.59 | 0.224 ± 0.07 | 0.202 ± 0.05 | 0.836 ± 0.19 | 0.891 ± 0.15 |
| RG | 1.00 ± 0.24 | 1.20 ± 0.26 | 0.38 ± 0.08 | 0.54 ± 0.09a | 0.25 ± 0.06 | 0.28 ± 0.03 | 24.57 ± 3.63 | 24.60 ± 3.13 | 0.167 ± 0.06 | 0.269 ± 0.06a | 0.990 ± 0.28 | 1.003 ± 0.24 |
| WG | 0.51 ± 0.08 | 0.59 ± 0.06 | 0.21 ± 0.02 | 0.27 ± 0.05 | 0.09 ± 0.01 | 0.11 ± 0.04 | 22.05 ± 3.97 | 23.32 ± 3.40 | 0.093 ± 0.04 | 0.337 ± 0.04b | 1.059 ± 0.33 | 0.956 ± 0.26 |
Values are means ± SD (n = 8 in each group). The rats were either sedentary (control) or trained on the electrically driven treadmill as described in the methods
RG red section of the gastrocnemius, WG white section of the gastrocnemius, S1P sphingosine-1-phosphate, SPA1P sphinganine-1-phosphate, dhCer dihydroceramide
a p < 0.01, b p < 0.001 versus the control group
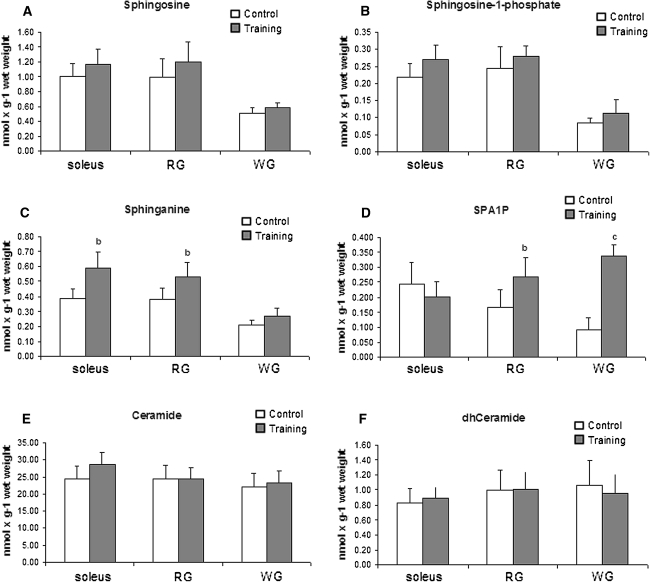
Sphingolipids, especially ceramides, are very active biologically and mediate a number of biological processes. It seems that ceramides play an important role in the induction of different diseases states such as insulin resistance [38, 39]. Data from our previous study showed that ceramide metabolism changes with the duration of single-bout exercise [26]. In the present study we wanted to examine how training affects sphingolipids content and enzymes activities implicated in ceramide metabolism in skeletal muscle. Therefore we measured the content of the following sphingolipids: sphingosine, sphingosine-1-phosphate, sphinganine, sphinganine-1-phosphate (SPA1P), ceramides and dihydroceramide (dhCer). The content of Sph (Fig. 3a) and S1P (Fig. 3b) did not change in any studied muscle after endurance training. SPA level increased by 52% (p < 0.01) and 41% (p < 0.01) in the soleus and red section of the gastrocnemius, respectively, compared to the control group. There was no difference in the SPA content in the white section of the gastrocnemius between trained and sedentary groups (Fig. 3c). The content of sphinganine-1-phosphate increased in both sections of the gastrocnemius in trained animals. In the red section of the gastrocnemius the SPA1P level was 50% (p < 0.01) higher than in the same muscle in the control group. In the white section of the gastrocnemius the SPA1P level was almost four times higher (p < 0.001) then in the same muscle from the sedentary group (Fig. 3d). The content of ceramide and dhCer did not change in any studied muscle from the trained group (Fig. 3e, f).
Fig. 3.
Effect of training on the content of sphingosine (a), sphingosine-1-phosphate (b), sphinganine (c), sphinganine-1-phosphate (d), ceramide (e) and dihydroceramide (f) in three types of rat skeletal muscles. Values are means ± SD (n = 8 in each group). The rats were either sedentary (control) or trained on an electrically driven treadmill as described in the methods. b p < 0.01, c p < 0.001 versus the control group. RG, red section of the gastrocnemius; WG, white section of the gastrocnemius
The Enzymes of Ceramide Metabolism (Table 3)
Table 3.
Effect of training on the activity of enzymes implicated in sphingolipids metabolism in rat skeletal muscle
| SPT | nSMase | aSMase | nCDase | alCDase | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Control | Training | Control | Training | Control | Training | Control | Training | Control | Training | |
| Soleus | 9.79 ± 0.92 | 18.81 ± 2.29c | 13.54 ± 1.35 | 12.71 ± 2.41 | 23.37 ± 3.13 | 35.12 ± 6.71c | 1.19 ± 0.11 | 0.97 ± 0.14a | 1.48 ± 0.22 | 1.16 ± 0.14a |
| Gastrocnemius | 11.10 ± 1.40 | 36.51 ± 5.63c | 16.54 ± 1.17 | 21.57 ± 3.73b | 20.80 ± 3.75 | 52.47 ± 8.19c | 1.17 ± 0.19 | 1.11 ± 0.14 | 1.53 ± 0.22 | 1.16 ± 0.20a |
| White section of the Gastrocnemius | 10.73 ± 1.28 | 42.42 ± 5.98c | 11.63 ± 1.23 | 16.38 ± 3.06c | 9.13 ± 0.61 | 20.81 ± 3.90c | 1.66 ± 0.19 | 1.15 ± 0.19a | 1.90 ± 0.18 | 1.26 ± 0.15b |
Values are means ± SD (n = 8 in each group). The rats were either sedentary (control) or trained on the electrically driven treadmill as described in the methods
a p < 0.05, b p < 0.01, c p < 0.001 versus the control group
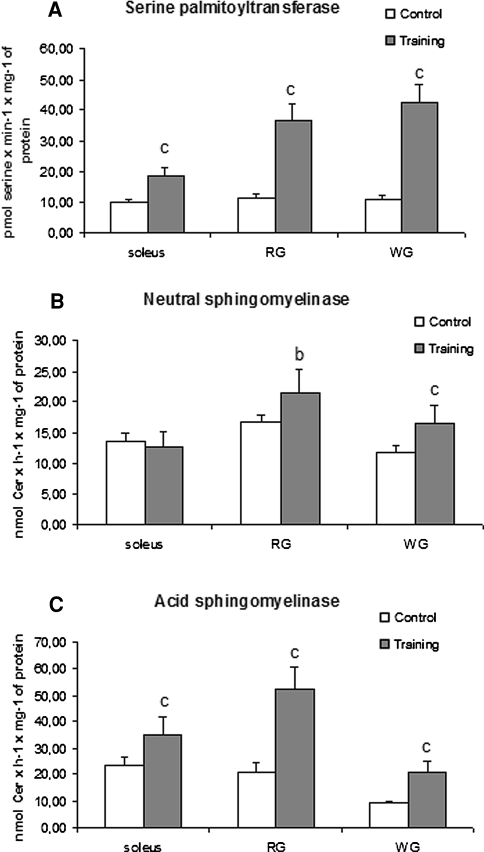
The activities of the key enzymes implicated in ceramides metabolism: SPT, nSMase, aSMase (enzymes responsible for ceramides generation) and nCDase and alCDase (enzymes responsible for ceramides degradation) were measured. There are limited data about the activity of the above-mentioned enzymes in skeletal muscle. The only available data on the activity of all the enzymes in muscle refer to the enzymes’ activity at rest, after a single-bout of exercise [26], from diabetic and healthy animals with increased plasma FFA concentration [40] and from animals fed high fat diets [41]. In the present study the activity of SPT increased in each studied muscle in the trained group. The enzyme activity was almost two times (p < 0.001), three times (p < 0.001) and almost four times (p < 0.001) higher in the soleus, red, and white section of the gastrocnemius, respectively, compared to the control group (Fig. 4a).
Fig. 4.
Effect of training on the activity of a serine palmitoyltransferase, b neutral sphingomyelinase, c acid sphingomyelinase in three types of rat skeletal muscles. Values are means ± SD (n = 8 in each group). The rats were either sedentary (control) or trained on an electrically driven treadmill as described in the methods. b p < 0.01, c p < 0.001 versus the control group. RG, red section of the gastrocnemius; WG, white section of the gastrocnemius
In the soleus, there was no difference in nSMase activity between the control and trained groups. In the red and white sections of the gastrocnemius, the activity of nSMase increased by 30% (p < 0.01) and by 41% (p < 0.001), respectively, compared to the control group (Fig. 4b).
The activity of the aSMase increased in each studied muscle in the trained group. The enzyme activity was 1.5 (p < 0.001), 2.5 (p < 0.001) and 2.3 times (p < 0.001) higher in the soleus, red and white sections of the gastrocnemius respectively, compared to the control group (Fig. 4c).
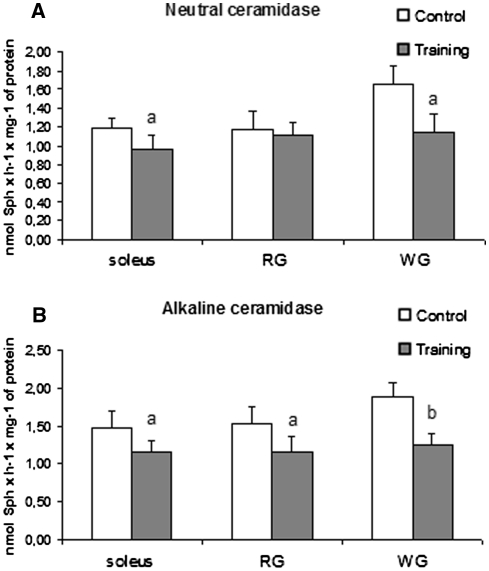
The activity of nCDase decreased by 19% (p < 0.05) and by 30% (p < 0.05) in the soleus and white section of the gastrocnemius respectively, compared to the control group. The enzyme activity did not change in red section of the gastrocnemius (Fig. 5a).
Fig. 5.
Effect of training on the activity of neutral (a) and alkaline (b) ceramidase in rat skeletal muscle. Values are means ± SD (n = 8 in each group). The rats were either sedentary (control) or trained on the electrically driven treadmill as described in the methods. a p < 0.05, b p < 0.01, c p < 0.001 versus the control group. RG, red section of the gastrocnemius; WG, white section of the gastrocnemius
The activity of alkaline ceramidase (alCDase) decreased by 21% (p < 0.05), 24% (p < 0.05) and 34% (p < 0.01) in the soleus, red and white sections of the gastrocnemius respectively, compared to the control group (Fig. 5b).
Discussion
Endurance training induces several changes in lipid metabolism in skeletal muscles. The major change is an increased capacity of the muscles to utilize free fatty acids as a source of energy. Certain changes in phospholipid content and composition and the content of triacylglycerols have been also described [42, 43]. Recent research has provided information on the role of sphingolipids in induction of insulin resistance. Therefore, knowledge of how the ceramide metabolism can be changed seems to be very important for the invention of new treatments to prevent ceramide accumulation. Ceramide metabolism is changed in muscle in rats fed a high fat diet [41], diabetic and healthy animals with increased plasma FFA concentration [40], and also after a single-bout of exercise [26]. There are very few data in the literature concerning the effect of training on the activity of the key enzymes of ceramide metabolism in skeletal muscle. Our results suggest that the regulation of ceramide metabolism depends on the type of exercise. We found that the short-time exercise caused a reduction in ceramide content but exhaustive exercise led to an elevated ceramide content in muscle [26]. Data from the current study show that training affects the ceramide metabolism in a different way to single-bout exercise. The ceramide level does not change in the trained group although the activities of the key enzymes of ceramide metabolism changed. The data regarding the content of ceramide are in agreement with the results reported previously, where 8 week training did not affect the ceramide content in rat gastrocnemius muscle [44]. In human skeletal muscle, training did not change the total ceramide content [27]. However, there are also conflicting data on the ceramide content in trained muscle. Experiments performed on obese subjects showed that endurance training reduces ceramide content and improves glucose tolerance [45, 46]. It must be mentioned that in these cases, obese people were examined and the ceramide metabolism in obesity can be different than in lean subjects. However, in the literature, there are also data showing that ceramide content decreases in muscle from trained rats [25]. The possible explanation of this discrepancy could be different analytical techniques used for measurement of the ceramide level in these two studies. In the mentioned work, ceramide content was measured by means of gas–liquid chromatography (GLC) after transmethylation of the ceramide fatty acids. Ceramide was purified by means of thin layer chromatography (TLC). The total content of different ceramides was measured including species containing: sphingosine, sphinganine, phytosphingosine, and homologs as a sphingoid base.
In our work, we have demonstrated that ceramide generation increased (increased activity of SPT, nSMase and aSMase) and decreased ceramides degradation (decreased activity of both CDases isoforms). Because of increased activity of enzymes responsible for ceramide production and decreased activity of enzymes responsible for ceramides degradation, we would expect to have an elevated ceramide content in the muscle, but we did not notice any changes in the ceramides level. We also did not observed any changes in muscle dihydroceramide content between trained and sedentary groups. This suggests that in the trained muscle other enzymes responsible for ceramides degradation/conversion are activated. We suspect that ceramide kinase, sphingomyelin synthase or glucosylceramide synthase are activated. Contribution of these pathways to regulation of ceramide content has not been recognized, so far.
Presently, we reported increased activity of SPT (enzyme catalyzing the reaction of condensation of serine and palmitoyl-CoA) and elevated content of sphinganine (main intermediate in sphingolipids de novo biosynthesis) in trained muscle. The elevation in the activity of SPT and the SPA content during exercise strongly indicates that physical exercise augments de novo sphingolipids synthesis in the muscles. The accumulation of SPA in the skeletal muscles of trained rats was observed also in the former study [25]. In our work we have observed an increased content of sphinganine-1-phosphate. It seems that sphinganine is directed to SPA1P instead of dhCer in the de novo synthesis pathway. Unfortunately, we have been unable to compare results from SPT activity and SPA1P content with any others because there is no information about SPT activity and SPA1P content in trained muscle.
Moreover, we also noticed increased activity of both isoforms of sphingomyelinases. We assume that increased nSMase activity is the effect of an increased content of TNF-α. During exercise TNF-α concentration increases and this agent is well known as an SMase activator [47]. Increased activity of nSMase was also previously observed in trained rat muscles [25]. In the literature there are also conflicting results related to nSMase activity. Data from trained muscle in human subjects showed that training does not affect nSMase activity [27].
Besides increased activities of enzymes responsible for ceramide production (SPT, n- and aSMase), we have simultaneously observed decreased activity of both isoforms of CDases. We cannot compare this result with any others studies because this is the only study when the enzymes activities were measured in trained muscles. In our work we did not observe any changes in Sph content and this result is in line with the results obtained previously [25].
It is well known that accumulation of ceramides, diacylglycerols and LCACoA in skeletal muscle is responsible for induction of insulin resistance. In our work, although ceramide content did not change in trained muscle, increased insulin sensitivity was observed—HOMA-IR values were significantly lower in trained animals compared to sedentary rats (Table 1).
In summary, ceramide content does not change in the trained group although the animals were more insulin sensitive, Moreover, the activity of enzymes responsible for ceramide production (SPT, nSMase, aSMase) increased and the activity of enzymes responsible for ceramide degradation (nCDase, alCDase) decreased. These results suggest that in trained muscle other metabolic pathways are activated and have an effect on the ceramide content.
Acknowledgments
This work was supported by the Ministry of Scientific Research and Information Technology, Grant: 2P05A 011 28 and Medical University of Bialystok, grant 3-18578, 3-18631 and 3-18610.
Conflict of interest
There is no conflict of interest for this study.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Abbreviations
- Cer
Ceramide
- dhCer
Dihydroceramide
- Sph
Sphingosine
- SPA
Sphinganine
- S1P
Sphingosine-1-phosphate
- SPA1P
Sphinganine-1-phosphate
- SPT
Serine palmitoyltransferase
- nCDase
Neutral ceramidase
- alCDase
Alkaline ceramidase
- nSMase
Neutral sphingomyelinase
- aSMase
Acidic sphingomyelinase
References
- 1.Ohanian J, Ohanian V. Sphingolipids in mammalian cell signalling. Cell Mol Life Sci. 2001;58:2053–2068. doi: 10.1007/PL00000836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kolesnick R, Fuks Z. Radiation and ceramide-induced apoptosis. Oncogene. 2003;22:5897–5906. doi: 10.1038/sj.onc.1206702. [DOI] [PubMed] [Google Scholar]
- 3.Oh HL, Seok JY, Kwon CH, Kang SK, Kim YK. Role of MAPK in ceramide-induced cell death in primary cultured astrocytes from mouse embryonic brain. Neurotoxicology. 2006;27:31–38. doi: 10.1016/j.neuro.2005.05.008. [DOI] [PubMed] [Google Scholar]
- 4.MacRae VE, Burdon T, Ahmed SF, Farquharson C. Ceramide inhibition of chondrocyte proliferation and bone growth is IGF-I independent. J Endocrinol. 2006;191:369–377. doi: 10.1677/joe.1.06958. [DOI] [PubMed] [Google Scholar]
- 5.Verheij M, Bose R, Lin XH, Yao B, Jarvis WD, Grant S, Birrer MJ, Szabo E, Zon LI, Kyriakis JM, Haimovitz-Friedman A, Fuks Z, Kolesnick RN. Requirement for ceramide-initiated SAPK/JNK signalling in stress-induced apoptosis. Nature. 1996;380:75–79. doi: 10.1038/380075a0. [DOI] [PubMed] [Google Scholar]
- 6.Wang YM, Seibenhener ML, Vandenplas ML, Wooten MW. Atypical PKC zeta is activated by ceramide, resulting in coactivation of NF-kappaB/JNK kinase and cell survival. J Neurosci Res. 1999;55:293–302. doi: 10.1002/(SICI)1097-4547(19990201)55:3<293::AID-JNR4>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 7.Schubert KM, Scheid MP, Duronio V. Ceramide inhibits protein kinase B/Akt by promoting dephosphorylation of serine 473. J Biol Chem. 2000;275:13330–13335. doi: 10.1074/jbc.275.18.13330. [DOI] [PubMed] [Google Scholar]
- 8.Spiegel S, Olivera A, Zhang H, Thompson EW, Su Y, Berger A. Sphingosine-1-phosphate, a novel second messenger involved in cell growth regulation and signal transduction, affects growth and invasiveness of human breast cancer cells. Breast Cancer Res Treat. 1994;31:337–348. doi: 10.1007/BF00666166. [DOI] [PubMed] [Google Scholar]
- 9.Pyne S, Pyne NJ. Sphingosine 1-phosphate signalling in mammalian cells. Biochem J. 2000;349:385–402. doi: 10.1042/0264-6021:3490385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liang H, Yao N, Song JT, Luo S, Lu H, Greenberg JT. Ceramides modulate programmed cell death in plants. Genes Dev. 2003;17:2636–2641. doi: 10.1101/gad.1140503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pettus BJ, Bielawska A, Subramanian P, Wijesinghe DS, Maceyka M, Leslie CC, Evans JH, Freiberg J, Roddy P, Hannun YA, Chalfant CE. Ceramide 1-phosphate is a direct activator of cytosolic phospholipase A2. J Biol Chem. 2004;279:11320–11326. doi: 10.1074/jbc.M309262200. [DOI] [PubMed] [Google Scholar]
- 12.Phillips DC, Martin S, Doyle BT, Houghton JA. Sphingosine-induced apoptosis in rhabdomyosarcoma cell lines is dependent on pre-mitochondrial Bax activation and post-mitochondrial caspases. Cancer Res. 2007;67:756–764. doi: 10.1158/0008-5472.CAN-06-2374. [DOI] [PubMed] [Google Scholar]
- 13.Merrill AH., Jr De novo sphingolipid biosynthesis: a necessary, but dangerous, pathway. J Biol Chem. 2002;277:25843–25846. doi: 10.1074/jbc.R200009200. [DOI] [PubMed] [Google Scholar]
- 14.Li CM, Hong SB, Kopal G, He X, Linke T, Hou WS, Koch J, Gatt S, Sandhoff K, Schuchman EH. Cloning and characterization of the full-length cDNA and genomic sequences encoding murine acid ceramidase. Genomics. 1998;50:267–274. doi: 10.1006/geno.1998.5334. [DOI] [PubMed] [Google Scholar]
- 15.Turinsky J, Bayly BP, O’Sullivan DM. 1, 2-Diacylglycerol and ceramide levels in rat skeletal muscle and liver in vivo. Studies with insulin, exercise, muscle denervation, and vasopressin. J Biol Chem. 1990;265:7933–7938. [PubMed] [Google Scholar]
- 16.Dobrzyn A, Gorski J. Ceramides and sphingomyelins in skeletal muscles of the rat: content and composition. Effect of prolonged exercise. Am J Physiol Endocrinol Metab. 2002;282:E277–E285. doi: 10.1152/ajpendo.00151.2001. [DOI] [PubMed] [Google Scholar]
- 17.Adams JM, 2nd, Pratipanawatr T, Berria R, Wang E, DeFronzo RA, Sullards MC, Mandarino LJ. Ceramide content is increased in skeletal muscle from obese insulin-resistant humans. Diabetes. 2004;53:25–31. doi: 10.2337/diabetes.53.1.25. [DOI] [PubMed] [Google Scholar]
- 18.Kruszynska YT, Olefsky JM. Cellular and molecular mechanisms of non-insulin dependent diabetes mellitus. J Investig Med. 1996;44:413–428. [PubMed] [Google Scholar]
- 19.DeFronzo RA, Ferrannini E, Sato Y, Felig P, Wahren J. Synergistic interaction between exercise and insulin on peripheral glucose uptake. J Clin Invest. 1981;68:1468–1474. doi: 10.1172/JCI110399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Krssak M, Falk Petersen K, Dresner A, DiPietro L, Vogel SM, Rothman DL, Roden M, Shulman GI. Intramyocellular lipid concentrations are correlated with insulin sensitivity in humans: a 1H NMR spectroscopy study. Diabetologia. 1999;42:113–116. doi: 10.1007/s001250051123. [DOI] [PubMed] [Google Scholar]
- 21.Phillips DI, Caddy S, Ilic V, Fielding BA, Frayn KN, Borthwick AC, Taylor R. Intramuscular triglyceride and muscle insulin sensitivity: evidence for a relationship in nondiabetic subjects. Metabolism. 1996;45:947–950. doi: 10.1016/S0026-0495(96)90260-7. [DOI] [PubMed] [Google Scholar]
- 22.Turinsky J, O’Sullivan DM, Bayly BP. 1,2-Diacylglycerol and ceramide levels in insulin-resistant tissues of the rat in vivo. J Biol Chem. 1990;265:16880–16885. [PubMed] [Google Scholar]
- 23.Stallknecht B, Larsen JJ, Mikines KJ, Simonsen L, Bulow J, Galbo H. Effect of training on insulin sensitivity of glucose uptake and lipolysis in human adipose tissue. Am J Physiol Endocrinol Metab. 2000;279:E376–E385. doi: 10.1152/ajpendo.2000.279.2.E376. [DOI] [PubMed] [Google Scholar]
- 24.Teran-Garcia M, Rankinen T, Koza RA, Rao DC, Bouchard C. Endurance training-induced changes in insulin sensitivity and gene expression. Am J Physiol Endocrinol Metab. 2005;288:E1168–E1178. doi: 10.1152/ajpendo.00467.2004. [DOI] [PubMed] [Google Scholar]
- 25.Dobrzyn A, Zendzian-Piotrowska M, Gorski J. Effect of endurance training on the sphingomyelin-signalling pathway activity in the skeletal muscles of the rat. J Physiol Pharmacol. 2004;55:305–313. [PubMed] [Google Scholar]
- 26.Blachnio-Zabielska A, Baranowski M, Zabielski P, Gorski J. Effect of exercise duration on the key pathways of ceramide metabolism in rat skeletal muscles. J Cell Biochem. 2008;105:776–784. doi: 10.1002/jcb.21877. [DOI] [PubMed] [Google Scholar]
- 27.Helge JW, Dobrzyn A, Saltin B, Gorski J. Exercise and training effects on ceramide metabolism in human skeletal muscle. Exp Physiol. 2004;89:119–127. doi: 10.1113/expphysiol.2003.002605. [DOI] [PubMed] [Google Scholar]
- 28.Langfort J, Czarnowski D, Pilis W, Wojcik B, Gorski J. Effect of various types of exercise training on 5′-nucleotidase and adenosine deaminase activities in rat heart: influence of a single bout of endurance exercise. Biochem Mol Med. 1996;59:28–32. doi: 10.1006/bmme.1996.0060. [DOI] [PubMed] [Google Scholar]
- 29.Dobrzyn A, Knapp M, Gorski J. Effect of acute exercise and training on metabolism of ceramide in the heart muscle of the rat. Acta Physiol Scand. 2004;181:313–319. doi: 10.1111/j.1365-201X.2004.01295.x. [DOI] [PubMed] [Google Scholar]
- 30.Sullivan TE, Armstrong RB. Rat locomotory muscle fiber activity during trotting and galloping. J Appl Physiol. 1978;44:358–363. doi: 10.1152/jappl.1978.44.3.358. [DOI] [PubMed] [Google Scholar]
- 31.Dyck DJ, Peters SJ, Glatz J, Gorski J, Keizer H, Kiens B, Liu S, Richter EA, Spriet LL, van der Vusse GJ, Bonen A. Functional differences in lipid metabolism in resting skeletal muscle of various fiber types. Am J Physiol. 1997;272:E340–E351. doi: 10.1152/ajpendo.1997.272.3.E340. [DOI] [PubMed] [Google Scholar]
- 32.Min JK, Yoo HS, Lee EY, Lee WJ, Lee YM. Simultaneous quantitative analysis of sphingoid base 1-phosphates in biological samples by o-phthalaldehyde precolumn derivatization after dephosphorylation with alkaline phosphatase. Anal Biochem. 2002;303:167–175. doi: 10.1006/abio.2002.5579. [DOI] [PubMed] [Google Scholar]
- 33.Bose R, Chen P, Loconti A, Grullich C, Abrams JM, Kolesnick RN. Ceramide generation by the reaper protein is not blocked by the caspase inhibitor, p35. J Biol Chem. 1998;273:28852–28859. doi: 10.1074/jbc.273.44.28852. [DOI] [PubMed] [Google Scholar]
- 34.Liu B, Hannun YA. Sphingomyelinase assay using radiolabeled substrate. Methods Enzymol. 2000;311:164–167. doi: 10.1016/S0076-6879(00)11077-8. [DOI] [PubMed] [Google Scholar]
- 35.Nikolova-Karakashian M, Merrill AH., Jr Ceramidases. Methods Enzymol. 2000;311:194–201. doi: 10.1016/S0076-6879(00)11081-X. [DOI] [PubMed] [Google Scholar]
- 36.Merrill AH., Jr Characterization of serine palmitoyltransferase activity in Chinese hamster ovary cells. Biochim Biophys Acta. 1983;754:284–291. doi: 10.1016/0005-2760(83)90144-3. [DOI] [PubMed] [Google Scholar]
- 37.Cacho J, Sevillano J, de Castro J, Herrera E, Ramos MP. Validation of simple indexes to assess insulin sensitivity during pregnancy in Wistar and Sprague-Dawley rats. Am J Physiol Endocrinol Metab. 2008;295:E1269–E1276. doi: 10.1152/ajpendo.90207.2008. [DOI] [PubMed] [Google Scholar]
- 38.Pickersgill L, Litherland GJ, Greenberg AS, Walker M, Yeaman SJ. Key role for ceramides in mediating insulin resistance in human muscle cells. J Biol Chem. 2007;282:12583–12589. doi: 10.1074/jbc.M611157200. [DOI] [PubMed] [Google Scholar]
- 39.Teruel T, Hernandez R, Lorenzo M. Ceramide mediates insulin resistance by tumor necrosis factor-alpha in brown adipocytes by maintaining Akt in an inactive dephosphorylated state. Diabetes. 2001;50:2563–2571. doi: 10.2337/diabetes.50.11.2563. [DOI] [PubMed] [Google Scholar]
- 40.Blachnio-Zabielska A, Zabielski P, Baranowski M, Gorski J. Effects of streptozotocin-induced diabetes and elevation of plasma FFA on ceramide metabolism in rat skeletal muscle. Horm Metab Res. 2010;42:1–7. doi: 10.1055/s-0029-1238322. [DOI] [PubMed] [Google Scholar]
- 41.Blachnio-Zabielska A, Baranowski M, Zabielski P, Gorski J. Effect of high fat diet enriched with unsaturated and diet rich in saturated fatty acids on sphingolipid metabolism in rat skeletal muscle. J Cell Physiol. 2010;225:786–791. doi: 10.1002/jcp.22283. [DOI] [PubMed] [Google Scholar]
- 42.Gorski J. Muscle triglyceride metabolism during exercise. Can J Physiol Pharmacol. 1992;70:123–131. doi: 10.1139/y92-019. [DOI] [PubMed] [Google Scholar]
- 43.Gorski J, Zendzian-Piotrowska M, de Jong YF, Niklinska W, Glatz JF. Effect of endurance training on the phospholipid content of skeletal muscles in the rat. Eur J Appl Physiol Occup Physiol. 1999;79:421–425. doi: 10.1007/s004210050532. [DOI] [PubMed] [Google Scholar]
- 44.Tsalouhidou S, Petridou A, Mougios V. Effect of chronic exercise on DNA fragmentation and on lipid profiles in rat skeletal muscle. Exp Physiol. 2009;94:362–370. doi: 10.1113/expphysiol.2008.045732. [DOI] [PubMed] [Google Scholar]
- 45.Bruce CR, Thrush AB, Mertz VA, Bezaire V, Chabowski A, Heigenhauser GJ, Dyck DJ. Endurance training in obese humans improves glucose tolerance and mitochondrial fatty acid oxidation and alters muscle lipid content. Am J Physiol Endocrinol Metab. 2006;291:E99–E107. doi: 10.1152/ajpendo.00587.2005. [DOI] [PubMed] [Google Scholar]
- 46.Dube JJ, Amati F, Stefanovic-Racic M, Toledo FG, Sauers SE, Goodpaster BH. Exercise-induced alterations in intramyocellular lipids and insulin resistance: the athlete’s paradox revisited. Am J Physiol Endocrinol Metab. 2008;294:E882–E888. doi: 10.1152/ajpendo.00769.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mathias S, Pena LA, Kolesnick RN. Signal transduction of stress via ceramide. Biochem J. 1998;335(Pt 3):465–480. doi: 10.1042/bj3350465. [DOI] [PMC free article] [PubMed] [Google Scholar]