Abstract
In eukaryotic cells, hormones and neurotransmitters that engage the phosphoinositide pathway evoke a biphasic increase in intracellular free Ca2+ concentration: an initial transient release of Ca2+ from intracellular stores is followed by a sustained phase of Ca2+ influx. This influx is generally store dependent. Most attention has focused on the link between the endoplasmic reticulum and store-operated Ca2+ channels in the plasma membrane. Here, we describe that respiring mitochondria are also essential for the activation of macroscopic store-operated Ca2+ currents under physiological conditions of weak intracellular Ca2+ buffering. We further show that Ca2+-dependent slow inactivation of Ca2+ influx, a widespread but poorly understood phenomenon, is regulated by mitochondrial buffering of cytosolic Ca2+. Thus, by enabling macroscopic store-operated Ca2+ current to activate, and then by controlling its extent and duration, mitochondria play a crucial role in all stages of store-operated Ca2+ influx. Store-operated Ca2+ entry reflects a dynamic interplay between endoplasmic reticulum, mitochondria and plasma membrane.
Keywords: Ca2+ buffering/mitochondria/store-operated Ca2+ entry
Introduction
Store-operated or capacitative Ca2+ entry, in which the process of emptying the inositol 1,4,5-trisphosphate (InsP3)-sensitive Ca2+ stores activates Ca2+ channels in the plasma membrane, is the major route for Ca2+ influx in non-excitable cells (Putney, 1986; Berridge, 1993). The best characterized and most widely distributed store-operated pathway is ICRAC (Hoth and Penner, 1992; Parekh and Penner, 1997). Ca2+ entry through CRAC channels is important for a host of functions, including refilling the stores, regulating the period of Ca2+ oscillations, driving exocytosis and controlling cell growth and proliferation (Parekh and Penner, 1997). The duration of ICRAC is, therefore, an important factor that determines the extent of activation of these Ca2+-dependent processes. In many cells, ICRAC is inactivated slowly following a global rise in intracellular Ca2+ (Ca2+-dependent slow inactivation; Foskett and Wong, 1994; Zweifach and Lewis, 1995a; Parekh, 1998). Ca2+-dependent slow inactivation is thought to contribute to the frequency of intracellular Ca2+ oscillations in various non-excitable cells as well as shaping the profile of the Ca2+ signal. The molecular mechanism underlying this slow inactivation is not known.
Under physiological conditions of weak intracellular Ca2+ buffering, InsP3 fails to activate any whole-cell (macroscopic) ICRAC (Broad et al., 1999; Fierro and Parekh, 2000; Glitsch and Parekh, 2000) despite substantial store emptying (Parekh et al., 1997). It was widely thought that Ca2+-inactivation of CRAC channels was the explanation for the absence of ICRAC. However, it has recently been established that this is not the case (Broad et al., 1999; Fierro and Parekh, 2000). Instead, it appears that the Ca2+ content of the stores needs to fall below a certain level in order for macroscopic ICRAC to activate (Fierro and Parekh, 2000). Understanding why InsP3 fails to activate ICRAC consistently in the presence of weak Ca2+ buffer is essential if we are to place the current in a physiological context.
Using the patch–clamp technique to record directly the store-operated Ca2+ current ICRAC, we find that mitochondria are a key determinant of the ability of ICRAC to activate in weak Ca2+ buffer. Furthermore, we find that Ca2+ buffering by respiring mitochondria can reduce the extent of Ca2+-dependent slow inactivation. We propose that, by determining activation and inactivation of macroscopic ICRAC, mitochondria are key orchestrators of intracellular Ca2+ signalling.
Results
Comparison of voltage ramps versus steps on ICRAC in strong Ca2+ buffer
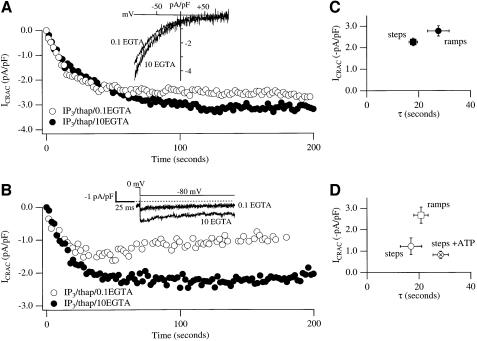
ICRAC was measured directly using the whole-cell patch– clamp technique. Stores were depleted rapidly and irreversibly by dialysing cells with a patch pipette solution containing a maximally effective concentration of the second messenger InsP3 (30 µM; Parekh et al., 1997) and the SERCA pump blocker thapsigargin. Figure 1A shows the time-course of development of ICRAC following dialysis with InsP3, thapsigargin and strong Ca2+ buffer (10 mM EGTA), a protocol that activates the current to its maximal extent (Glitsch and Parekh, 2000). ICRAC was monitored by applying voltage ramps (–100 to +100 mV in 50 ms) from a holding potential of 0 mV, and its amplitude was measured from the ramps at –80 mV. The inset in Figure 1A shows a typical ramp current (taken at 100 s), revealing the characteristics of ICRAC (voltage independent, inwardly rectifying, Erev >+50mV). The graph in Figure 1C plots the amplitude of ICRAC versus the time-constant (τ) of activation (n = 16). When ICRAC was measured instead by applying voltage steps to –80 mV for 250 ms, the current developed to reach an extent similar to those obtained with ramps (time-course of a recording is shown in Figure 1B, pooled data displayed in Figure 1C, n = 17). In strong buffer, all cells responded irrespective of whether the ramp or step protocol was used.
Fig. 1. ICRAC is smaller using hyperpolarizing steps than ramps in weak intracellular Ca2+ buffer. (A) Time-course of ICRAC, followed using voltage ramps, in strong and weak buffer (filled and open circles, respectively). Inset shows I–V relationships taken at 100 s. The current was measured at –80 mV from the ramps, and normalized for cell capacitance. (B) Time-course of ICRAC in strong and weak buffer following voltage steps to –80 mV (inset). The current was measured between 0.8 and 0.9 ms after the step (see Materials and methods). (C) A plot of ICRAC against activation time-constant in strong buffer using ramps or steps. (D) A similar plot to (C) but for low buffer. Note that inclusion of 2 mM Mg-ATP reduces the size of the current.
Comparison of voltage ramps versus steps on ICRAC in weak Ca2+ buffer
We then examined whether the size of ICRAC was affected by using the ramp versus step protocols in the presence of weak intracellular Ca2+ buffer. This better represents endogenous levels of Ca2+ buffer found within cells. Superimposed in Figure 1A (open circles) are recordings taken using ramps for cells dialysed with weak buffer (InsP3 + thapsigargin + 0.1 mM EGTA, n = 11; I–V relationship is shown in inset). The extent of the current as well as its rate of development were not significantly different from that seen in strong buffer (p = 0.6 each; Figure 1C and D). Only one cell failed to respond under these conditions. A strikingly different pattern was found, however, when ICRAC was monitored by applying voltage steps (instead of ramps) in weak buffer (Figure 1B and D). The current was now significantly smaller when compared with either strong buffer (also employing the step protocol) or weak buffer (using voltage ramps), (p = 0.018 and 0.014, respectively). Furthermore, three of 11 cells failed to produce any detectable current using the step protocol in weak buffer. Supplementing the weak Ca2+ buffer solution with 2 mM Mg-ATP tended to reduce further the size of ICRAC, when the current was measured using the voltage step protocol (but this was not significant, p = 0.29). In the presence of ATP, the rate of development of the current slowed slightly and, most dramatically, the probability that a cell would fail to generate detectable ICRAC increased substantially (12/30 cells now failed to respond, Figure 2E).
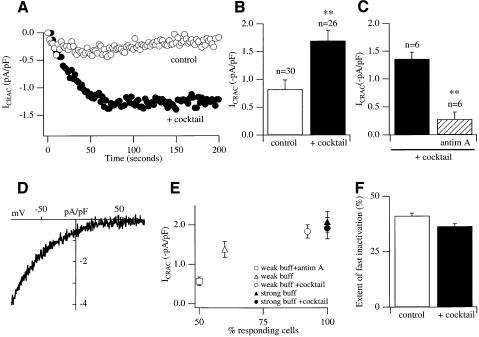
Fig. 2. Respiring mitochondria increase the size of ICRAC in weak but not strong buffer. (A) Time-course of ICRAC in weak buffer in the absence (control) and presence of the mitochondrial cocktail. (B) ICRAC is significantly bigger in the presence of cocktail compared with control. (C) Pre-treatment with antimycin A and oligomycin prevents the enhancing effect of cocktail. The experiments were carried out on cells from the same preparations using the same solutions. Cells were incubated for at least 20 min in 5 µg/ml antimycin A and 0.5 µg/ml oligomycin. (D) I–V relationship for a cell dialysed with cocktail when ICRAC had peaked. (E) A plot of current amplitude against % of responding cells in weak and strong buffer in the absence and presence of cocktail. (F) Extent of rapid inactivation in the absence (control) and presence of cocktail. For all panels, control refers to InsP3 + thapsigargin + 0.1 mM EGTA + ATP.
During the voltage ramps, very little Ca2+ actually enters the cell because first, the ramp duration is so short (50 ms) and secondly, cells are at those negative potentials that favour Ca2+ entry only briefly. Since the amplitude of ICRAC was similar using steps or ramps in strong buffer, the discrepancy in amplitudes between ramps and steps in weak buffer does not reflect the voltage protocols per se. Instead, in weak buffer, cytosolic Ca2+ would increase substantially during the step protocol. The elevated Ca2+ would be maintained after each voltage step because the SERCA pumps have been blocked with thapsigargin to prevent store refilling, and these pumps are very active in RBL cells (Fierro and Parekh, 1999a). In strong buffer on the other hand, the incoming Ca2+ during the long hyperpolarizing steps is effectively captured by the large amounts of free EGTA present in the cytosol.
The small ICRAC seen in weak buffer using the step protocol represents Ca2+-dependent slow inactivation, a well-documented feature of ICRAC that operates over a time-frame of tens of seconds (Foskett and Wong, 1994; Zweifach and Lewis, 1995a; Parekh, 1998). Because thapsigargin was present throughout, the small current does not reflect Ca2+-dependent deactivation of ICRAC due to store refilling. Slow Ca2+-dependent inactivation is thought to contribute to the frequency of intracellular Ca2+ oscillations in various non-excitable cells as well as shaping the profile of the Ca2+ signal (Foskett and Wong, 1994; Zweifach and Lewis, 1995a; Parekh and Penner, 1997). The molecular mechanism underlying this slow inactivation is not known but, in RBL cells, it does not seem to involve a protein kinase, phosphatase or GTP-binding protein (Parekh, 1998).
Respiring mitochondria reduce Ca2+-dependent slow inactivation
Because mitochondria can take up significant amounts of Ca2+ following Ca2+ elevations in the cytosol (Rizzuto et al., 1993; Babcock et al., 1997; Duchen, 1999; Tinel et al., 1999) and can buffer Ca2+ that has entered through the store-operated pathway (Lawrie et al., 1996; Hoth et al., 1997; Hartmann and Verkhratsky, 1998), we investigated whether mitochondrial Ca2+ uptake affected the size of ICRAC using the step protocol (InsP3 + thapsigargin + 0.1 mM EGTA + ATP, henceforth control). We dialysed cells with a cocktail (malate, pyruvate, NaH2PO4, cAMP and GTP) known to be important for sustaining respiring mitochondria in the whole-cell recording configuration (Gunter and Pfeiffer, 1990; Villalba et al., 1994; Herrington et al., 1996). The effects were dramatic. Figure 2A compares the time-course of development of ICRAC for cells dialysed in the absence (control) and presence of cocktail. Inclusion of cocktail significantly increased the size of the current almost 2-fold (Figure 2B, p <0.01). This potentiation was due to a specific increase in ICRAC, as opposed to another current, because the typical I–V relationship was preserved (Figure 2D). Furthermore, whereas only 60% of cells responded to control solution (using the step protocol), ∼90% of cells responded in the presence of cocktail (Figure 2E). Inclusion of cocktail, therefore, increases the probability of obtaining macroscopic ICRAC. The size of ICRAC in responding cells in weak buffer was smaller than in the presence of cocktail, although this was not significant (p = 0.10).
Antimycin A accelerates Ca2+-dependent slow inactivation
The effects of the cocktail appeared to be due entirely to an action on mitochondria because pre-treating cells with antimycin A, an inhibitor of complex III in the respiratory chain that results in mitochondrial depolarization (Duchen, 1999), together with oligomycin (to prevent the ATP synthase from operating in reverse), suppressed the enhancing effect of cocktail on ICRAC (Figure 2C). The fraction of responding cells in the presence of antimycin A + cocktail, as well as the amplitude, was even less than control recordings made in the absence of cocktail (Figure 2E). This suggests that a little mitochondrial Ca2+ uptake is taking place even when cocktail is not present. Antimycin A + oligomycin have no effect on the extent of ICRAC in strong buffer, ruling out effects on the CRAC channels themselves or their mechanism of activation (data not shown).
Effects of mitochondrial cocktail are Ca2+ dependent
The effects of the mitochondrial cocktail solution were Ca2+ dependent because no enhancing effect on ICRAC was seen in strong Ca2+ buffer using either the ramp or step protocols [ramps: –2.13 ± 0.15 and –2.25 ± 0.35 for control and cocktail, respectively, (n = 6 for both, p = 0.31), steps: –2.07 ± 0.26 and –1.91 ± 0.27 (n = 6 for both, p = 0.67)]. Strong buffer prevents a rise in intracellular Ca2+, and so mitochondrial Ca2+ uptake will be substantially reduced.
Rapid Ca2+-dependent inactivation of ICRAC is not affected by mitochondrial Ca2+ uptake
Rapid Ca2+-dependent inactivation of CRAC channels operates on a millisecond time-scale and reflects the build-up of a microdomain of Ca2+ in the vicinity of each open channel (Zweifach and Lewis, 1995b; Fierro and Parekh, 1999b). The Ca2+ binding site(s) are thought to lie <6 nm from the channel mouth (Zweifach and Lewis, 1995b). If mitochondria were physically very close to CRAC channels, then one might expect fast inactivation to be substantially reduced in the presence of cocktail. However, this was not the case (Figure 2F). Hence, mitochondria are located at least several nanometres away from the CRAC channel mouth.
Effects of ruthenium red
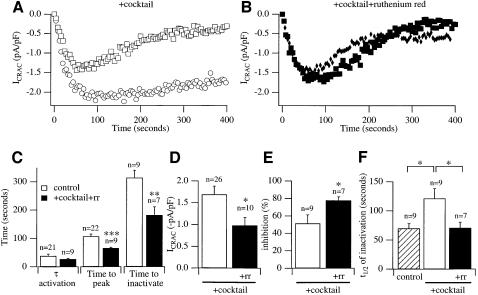
Mitochondrial Ca2+ uptake occurs through a uniporter that is blocked by ruthenium red (Duchen, 1999). To probe further the role of mitochondria on the properties of ICRAC, we examined whether ruthenium red interfered with the effects of the cocktail. Figure 3A shows relatively long recordings from two cells dialysed with cocktail together with InsP3 + thapsigargin + ATP in weak buffer. The current was followed using the voltage step protocol. Analysis of various features of the current is presented in Figure 3C–F (open bars). ICRAC inactivated slowly over this time-frame but the extent of this was quite variable. Inclusion of ruthenium red in the pipette solution prevented the effects of the mitochondrial cocktail. The time-course of the current is shown in Figure 3B for two cells and averaged data are summarized in Figure 3C–F (filled bars). Although ruthenium red did not significantly alter τ activation (Figure 3C, p = 0.25), it significantly reduced (i) the time to peak of the current as well as the time to inactivate (Figure 3C), and (ii) the amplitude of ICRAC such that the size was now similar to that seen without cocktail (Figure 3D). Furthermore, in the presence of ruthenium red, the extent of inactivation increased (Figure 3E) and slow inactivation developed more quickly (Figure 3F). The shorter time to peak in ruthenium red presumably reflects the faster onset of slow inactivation. Ruthenium red had no effect on the properties of ICRAC in strong buffer, arguing against a direct action on CRAC channels themselves (data not shown). Ruthenium red also did not alter the extent of fast inactivation when compared with control or cocktail in weak buffer (data not shown).
Fig. 3. Inhibition of Ca2+ uptake into respiring mitochondria reduces the extent of ICRAC and accelerates slow inactivation. (A) Time-course of ICRAC in the presence of the mitochondrial cocktail for two cells showing the range of slow inactivation. One showed only modest inactivation (circles), whereas the other inactivated substantially (squares). (B) Time-course of ICRAC in two cells dialysed with 100 µM ruthenium red together with cocktail, showing substantial inactivation. We used this concentration in order to achieve high intracellular levels shortly after breaking into the cells. (C–F) Compare various properties of ICRAC for cocktail (open bars) with cocktail + ruthenium red (filled bars). Apart from τ-activation, ruthenium red significantly reduced all parameters compared with cocktail alone. Time to inactivate was measured from the time at which ICRAC had peaked to the time where inactivation had reached a steady state. The difference between peak current and steady-state current was taken as the inactivation % after normalization to peak amplitude.
Macroscopic ICRAC activates in weak Ca2+ buffer provided mitochondria are active
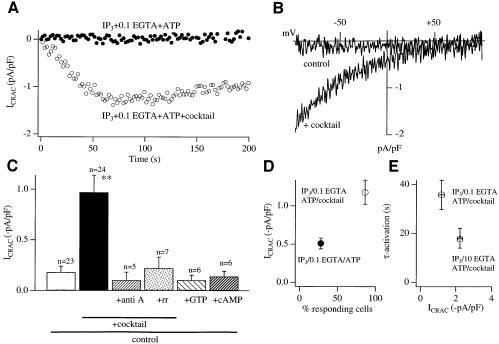
In weak intracellular Ca2+ buffer, InsP3 usually fails to activate any macroscopic ICRAC unless SERCA pumps are blocked with thapsigargin (Fierro and Parekh, 2000). InsP3 nevertheless reduces the Ca2+ content of the stores (Parekh et al., 1997). Activation of macroscopic ICRAC, therefore, seems to occur only when the Ca2+ content of the stores falls below a certain level, and the ability to reach this level depends on the prevalent SERCA pump activity. Any factor that regulates SERCA pump-mediated Ca2+ uptake is likely to have quite profound effects on Ca2+ entry under physiological levels of intracellular Ca2+ buffering. We hypothesized that mitochondrial Ca2+ uptake might be able to compete with the SERCA pumps for removing cytosolic Ca2+ following InsP3-mediated Ca2+ release, as well as reducing possible Ca2+-inactivation of InsP3 receptors, especially since mitochondria can be spatially very close to the sites of Ca2+ release from the endoplasmic reticulum (Jouvaille et al., 1995; Rizzuto et al., 1998; Hajnoczky et al., 1999). Respiring mitochondria might, therefore, enable InsP3 to deplete the stores more effectively such that the threshold for macroscopic activation of ICRAC is reached. To test this, we dialysed cells with InsP3 and weak buffer in the absence and presence of cocktail. Importantly, thapsigargin was not included in the pipette solution so SERCA pumps were active. InsP3 in the absence of cocktail usually failed to activate any detectable ICRAC (Figure 4A and B), and, in the small fraction of cells that responded, the current was very small (Figure 4C). Inclusion of cocktail had quite pronounced effects. A robust ICRAC could now be activated (Figure 4A and B). Furthermore, the majority of cells now responded (Figure 4D), although the size of the current was smaller than in strong buffer and activated more slowly (Figure 4E). The effects of the cocktail were prevented by pre-treatment with the combination of antimycin A and oligomycin, or by including ruthenium red in the pipette together with InsP3 and cocktail (Figure 4C). On the other hand, dialysis with only cAMP or GTP (two constituents of the cocktail) failed to enhance the current (Figure 4C).
Fig. 4. ICRAC activates to InsP3 alone in weak buffer provided mitochondria are active. (A) Time-course of ICRAC for a cell dialysed with InsP3 + 0.1 mM EGTA + ATP (control) and then for one supplemented with cocktail. No thapsigargin was present. (B) I–V relationship from voltage ramps is shown (taken when after 60 s). (C) Bar chart depicting the enhancing effect of cocktail, and that the mitochondrial inhibitors antimycin A (+ oligomycin) and ruthenium red prevent the effects of cocktail. Control + cAMP or GTP did not mimic the effects of cocktail. Again, thapsigargin was not present. (D) Plot of amplitude of ICRAC versus % responding cells for control and cocktail-treated cells (no thapsigargin present). Only the amplitudes of responding cells were analysed. (E) τ-activation versus amplitude of ICRAC is plotted for cocktail-treated cells in weak buffer (no thapsigargin) and strong buffer (InsP3 + thapsigargin + 10 mM EGTA + ATP). Note that in weak buffer without thapsigargin, ICRAC was still sub-maximal in spite of cocktail. This reflects some SERCA-dependent refilling, because the current was larger in weak buffer when thapsigargin was included.
Discussion
Our new findings demonstrate that Ca2+ uptake into mitochondria is essential for the normal functioning of the store-operated Ca2+ current under the physiological conditions of weak intracellular Ca2+ buffering. Our results provide a novel explanation for a long-standing problem in the calcium signalling field, namely why the Ca2+-mobilizing second messenger InsP3 fails to activate macroscopic ICRAC in weak Ca2+ buffer. It was generally believed that this inability of InsP3 reflected Ca2+-inactivation of CRAC channels, but we and others have recently shown that this is not so (Broad et al., 1999; Fierro and Parekh, 2000). Instead, it seems that substantial depletion of stores is required for macroscopic ICRAC to activate, and InsP3 is not able to exceed this apparent threshold. We now find that Ca2+ uptake by respiring mitochondria is essential for ICRAC to activate under physiological conditions. We propose that removal of cytosolic Ca2+ by mitochondria competes effectively with store refilling by SERCA pumps, and may also reduce Ca2+-inactivation of InsP3 receptors. Combined, this would enable InsP3 to deplete the stores sufficiently for macroscopic ICRAC to activate (see Figure 5 and legend for a cartoon depicting this model). Detailed studies have established that mitochondria can be physically close to endoplasmic reticulum (Rizzuto et al., 1998) and sense Ca2+ microdomains generated by open InsP3 receptors (Jouvaille et al., 1995; Hajnoczky et al., 1999). Our results provide a functional explanation for this close apposition and indicate that cross-talk between the two organelles determines whether macroscopic ICRAC activates under the conditions of weak intracellular buffer. In strong buffer, mitochondrial uptake will be severely attenuated, SERCA pumps will be much less active and InsP3 routinely activates ICRAC.
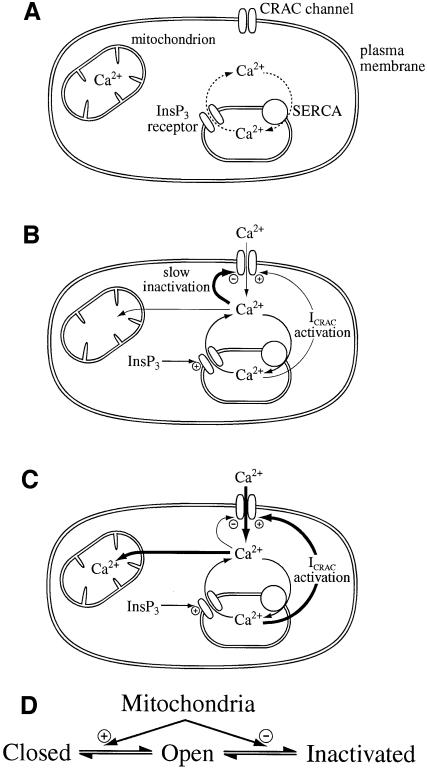
Fig. 5. Cartoon summary of mitochondrial role in ICRAC in weak (physiological) intracellular Ca2+ buffer. (A) Shows the resting state, where ICRAC is not functioning. Stores are full and any Ca2+ that leaks from the stores is re-sequestrated by the SERCA pumps. (B) Following an increase in the levels of the second messenger InsP3 in the absence of active mitochondrial Ca2+ uptake, Ca2+ is released from the stores. However, the SERCA pumps are able to re-sequestrate sufficient Ca2+ to prevent the threshold for macroscopic activation of ICRAC from being reached. Only a very small fraction of CRAC channels are activated (undetectable in whole-cell mode). Furthermore, the rise in cytosolic Ca2+ results in strong inactivation of ICRAC through Ca2+-dependent slow inactivation. (C) In the presence of respiring mitochondria, InsP3 activates macroscopic ICRAC. Ca2+ released from the stores by InsP3 is taken up by mitochondria through a ruthenium red-sensitive uniporter. This reduces the amount of Ca2+ available to the SERCA pumps and in the vicinity of open InsP3 receptors, such that the stores are depleted sufficiently for macroscopic ICRAC to activate (less refilling by SERCA pumps and less inactivation of InsP3 receptors). ICRAC now activates. Some refilling does occur because inclusion of thapsigargin enhances the size of the current. Furthermore, mitochondrial Ca2+ buffering reduces the rate and extent of Ca2+-dependent slow inactivation, thereby increasing the size and duration of the current. (D) A simplified gating scheme for CRAC channels summarizing the role of mitochondrial Ca2+ buffering. Mitochondria facilitate opening (Closed to Open transition) whilst simultaneously reducing inactivation (Open to Inactivated transition). In this way, mitochondria have a much larger impact on ICRAC than through either transition alone.
Could mitochondria be directly involved in the activation mechanism of ICRAC through a process independent of Ca2+ buffering? In pancreatic β cells, mitochondria release a diffusible factor (glutamate) that promotes Ca2+-independent secretion of insulin (Maechler and Wollheim, 1999). However, we do not think that mitochondria release the activating signal for ICRAC because pre-treating cells with antimycin/oligomycin does not prevent ICRAC from developing in strong Ca2+ buffer.
Finally, our results provide new insight into the important, but elusive mechanism underlying slow inactivation of ICRAC. Increasing Ca2+ uptake into mitochondria delays the onset of slow inactivation and reduces its extent, whereas inactivation develops more quickly and to a greater extent when mitochondrial uptake is compromised. It is particularly striking that the same mechanism, Ca2+ buffering by mitochondria, should have profound effects on both the activation and inactivation of ICRAC. By facilitating activation (through ensuring more store depletion) and, at the same time, reducing slow inactivation, mitochondria will increase ICRAC to a much larger extent than through an action on activation or inactivation alone. A gating scheme depicting this dual action of mitochondria on ICRAC is shown in Figure 5D.
Under physiological conditions, mitochondria therefore play a pivotal role in all stages of store-operated Ca2+ entry, determining whether macroscopic ICRAC activates, to what extent, and then for how long Ca2+ entry stays operational.
Materials and methods
Cell culture
Rat basophilic leukaemia cells (RBL-1) cells, which were bought from Cell Bank at the Sir William Dunn School of Pathology, Oxford University, were cultured as previously described (Fierro and Parekh, 2000).
Electrophysiology
Patch–clamp experiments were conducted in the tight-seal whole-cell configuration at room temperature (20–25°C) as previously described (Parekh, 1998; Fierro and Parekh, 2000). Sylgard-coated fire-polished pipettes had d.c. resistances of 2.9–4 MΩ when filled with standard internal solution that contained: 145 mM caesium glutamate, 8 mM NaCl, 1 mM MgCl2, 0.03 mM InsP3, 10 mM HEPES pH 7.2 with CsOH. EGTA was included in the pipette solution at concentrations of either 0.1 mM (weak buffer) or 10 mM (strong buffer), as indicated in the text. Thapsigargin (2 µM) was always included in the pipette solution, except for the experiments of Figure 4. A correction of +10 mV was applied for the subsequent liquid junction potential that arose from this glutamate-based internal solution. Mitochondrial cocktail contained: 2 mM pyruvic acid, 2 mM malic acid, 1 mM NaH2PO4, 0.5 mM cAMP, 2 mM Mg2+-ATP, 0.5 mM GTP. Extracellular solution contained: 145 mM NaCl, 2.8 mM KCl, 10 mM CaCl2, 2 mM MgCl2, 10 mM CsCl, 10 mM d-glucose, 10 mM HEPES pH 7.4 with NaOH. ICRAC was measured by applying either voltage ramps (–100 to +100 mV in 50 ms) or hyperpolarizing voltage steps (to –80 mV, 250 ms duration) at 0.5 Hz from a holding potential of 0 mV as previously described. Currents were filtered using an 8-pole Bessel filter at 2.5 kHz and digitized at 100 µs. Currents were normalized by dividing the amplitudes (measured from the voltage ramps at –80 mV or averaged between 0.8 and 0.9 ms after the onset of the hyperpolarizing step) by the cell capacitance. The settling time of the clamp was ∼100 µs (typical series resistance of 9 MΩ and cell capacitance of 15 pF). Peak currents were therefore measured at 0.8–0.9 ms after the step to minimize potential contributions from uncompensated capacitative currents. Furthermore, ICRAC inactivates partially (40%) during a hyperpolarizing step to –80 mV under the present conditions, with time-constants of ∼10 and 120 ms (Fierro and Parekh, 1999b). By measuring the current very shortly after the onset of the step, we obtained a better estimate of the true peak. Capacitative currents were compensated before each ramp by using the automatic compensation of the EPC 9 and EPC 9-2 amplifiers. All leak currents were subtracted by averaging the first ramp current (or two), and then subtracting this from all subsequent currents. Data are presented as mean ± SEM, and statistical evaluation was carried out using both Student’s t and Mann–Whitney non-parametric tests.
Thapsigargin was purchased from Alomone laboratories. All other chemicals were purchased from Sigma.
Acknowledgments
Acknowledgements
The work was supported by a Wellcome Trust grant (to A.B.P.). J.A.G. has a Marie Curie EU Postdoctoral Fellowship. A.B.P. is a Wellcome Trust Career Development Fellow and holds the Amersham Medical Fellowship in Cell Biology at Keble College, Oxford.
References
- Babcock D.F., Herrington,J., Goodwin,P.C., Park,Y.B. and Hille,B. (1997) Mitochondrial participation in the intracellular Ca2+ network. J. Cell Biol., 136, 833–844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge M.J. (1993) Inositol trisphosphate and calcium signalling. Nature, 361, 315–325. [DOI] [PubMed] [Google Scholar]
- Broad L.M., Armstrong,D.L. and Putney,J.W. (1999) Role of the inositol 1,4,5-trisphosphate receptor in Ca2+ feedback inhibition of calcium release-activated calcium current (ICRAC). J. Biol. Chem., 274, 32881–32888. [DOI] [PubMed] [Google Scholar]
- Duchen M.R. (1999) Contributions of mitochondria to animal physiology: from homeostatic sensor to calcium signalling and cell death. J. Physiol., 516, 1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fierro L. and Parekh,A.B. (1999a) On the characterisation of the mechanism underlying passive activation of the Ca2+ release-activated Ca2+ current ICRAC. J. Physiol., 520, 407–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fierro L. and Parekh,A.B. (1999b) Fast calcium-dependent inactivation of calcium release-activated calcium current (CRAC) in RBL-1 cells. J. Membr. Biol., 168, 9–17. [DOI] [PubMed] [Google Scholar]
- Fierro L. and Parekh,A.B. (2000) Substantial depletion of the intracellular calcium stores is required for macroscopic activation of ICRAC in RBL-1 cells. J. Physiol., 522, 247–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foskett J.K. and Wong,D.C.P. (1994) [Ca2+]i inhibition of Ca2+ release-activated Ca2+ influx underlies agonist- and thapsigargin-induced [Ca2+]i oscillations in salivary acinar cells. J. Biol. Chem., 269, 31525–31532. [PubMed] [Google Scholar]
- Glitsch M.D. and Parekh,A.B. (2000) Ca2+ store dynamics determines the pattern of activation of the store-operated Ca2+ current ICRAC in response to InsP3 in rat basophilic leukemia cells. J. Physiol., 523, 283–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunter T.E. and Pfeiffer,D.R. (1990) Mechanisms by which mitochondria transport calcium. Am. J. Physiol., 258, C755–C786. [DOI] [PubMed] [Google Scholar]
- Hajnoczky G., Hager,R. and Thomas,A.P. (1999) Mitochondria suppress local feedback activation of inositol 1,4,5-trisphosphate-receptors by Ca2+. J. Biol. Chem., 274, 14157–14162. [DOI] [PubMed] [Google Scholar]
- Hartmann J. and Verkhratsky,A. (1998) Relations between intracellular Ca2+ stores and store-operated Ca2+ entry in primary cultured human glioblastoma cells. J. Physiol., 513, 411–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrington J., Park,Y.B., Babcock,D.F. and Hille,B. (1996) Dominant role of mitochondria in clearance of large Ca2+ loads from rat adrenal chromaffin cells. Neuron, 16, 219–228. [DOI] [PubMed] [Google Scholar]
- Hoth M. and Penner,R. (1992) Depletion of intracellular calcium stores activates a calcium current in mast cells. Nature, 355, 353–356. [DOI] [PubMed] [Google Scholar]
- Hoth M., Fanger,C.M. and Lewis,R.S. (1997) Mitochondrial regulation of store-operated calcium signaling in T lymphocytes. J. Cell Biol., 137, 633–648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jouvaille L.S., Ichas,F., Holmuhamedov,E.L., Camacho,P. and Lechleiter, J.D. (1995) Synchronization of calcium waves by mitochondrial substrates in Xenopus laevis oocytes. Nature, 377, 438–411. [DOI] [PubMed] [Google Scholar]
- Lawrie A.M., Rizzuto,R., Pozzan,T. and Simpson,A.W.M. (1996) A role for calcium influx in the regulation of mitochondrial calcium in endothelial cells. J. Biol. Chem., 271, 10753–10759. [DOI] [PubMed] [Google Scholar]
- Maechler P. and Wollheim,C.B. (1999) Mitochondrial glutamate acts as a messenger in glucose-induced insulin exocytosis. Nature, 402, 685–689. [DOI] [PubMed] [Google Scholar]
- Parekh A.B. (1998) Slow feedback inhibition of calcium release-activated calcium current (CRAC) by calcium entry. J. Biol. Chem., 273, 14925–14932. [DOI] [PubMed] [Google Scholar]
- Parekh A.B. and Penner,R. (1997) Store depletion and calcium influx. Physiol. Rev., 77, 901–930. [DOI] [PubMed] [Google Scholar]
- Parekh A.B., Fleig,A. and Penner,R. (1997) The store-operated calcium current ICRAC: Nonlinear activation by InsP3 and dissociation from calcium release. Cell, 89, 973–980. [DOI] [PubMed] [Google Scholar]
- Putney J.W. (1986) A model for receptor-regulated calcium entry. Cell Calcium, 7, 1–12. [DOI] [PubMed] [Google Scholar]
- Rizzuto R., Brini,M., Murgia,M. and Pozzan,T. (1993) Microdomains with strong Ca2+ close to IP3-sensitive channels that are sensed by neighbouring mitochondria. Science, 262, 744–747. [DOI] [PubMed] [Google Scholar]
- Rizzuto R., Pinton,P., Carrington,W., Fay,F.S., Fogarty,K.E., Lifshitz, L.S., Tuft,R.A. and Pozzan,T. (1998) Close contacts with the endoplasmic reticulum as determinants of mitochondrial Ca2+ responses. Science, 280, 1763–1766. [DOI] [PubMed] [Google Scholar]
- Tinel H., Cancela,J.M., Mogami,H., Gerasimenko,J.V., Gerasimenko, O.V., Tepikin,A.V. and Petersen,O.H. (1999). Active mitochondria surrounding the pancreatic acinar granule region prevent spreading of inositol trisphosphate-evoked local cytosolic Ca2+ signals. EMBO J., 18, 4999–5008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villalba M. et al. (1994) The role of pyruvate in neuronal calcium homeostasis: effects on intracellular calcium pools. J. Biol. Chem., 269, 2468–2476. [PubMed] [Google Scholar]
- Zweifach A. and Lewis,R.S. (1995a) Slow calcium-dependent inactivation of depletion-activated calcium current. Store-dependent and independent mechanisms. J. Biol. Chem., 270, 14445–14451. [DOI] [PubMed] [Google Scholar]
- Zweifach A. and Lewis,R.S. (1995b) Rapid inactivation of depletion-activated calcium current (ICRAC) due to local calcium feedback. J. Gen. Physiol., 105, 209–226. [DOI] [PMC free article] [PubMed] [Google Scholar]