Abstract
Objective
The aim of this study was to elucidate the molecular signaling mechanisms by which substance P (SP) modulates lymphatic muscle contraction and to determine whether SP stimulates both contractile as well as inflammatory pathways in the lymphatics.
Methods
A rat mesenteric lymphatic muscle cell culture model (RMLMCs) and known specific pharmacological inhibitors were utilized to delineate SP mediated signaling pathways in lymphatics.
Results
We detected expression of neurokinin receptor 1 (NK1R) and neurokinin receptor 3 (NK3R) in RMLMCs. SP stimulation increased phosphorylation of myosin light chain 20 (MLC20) as well as p38 mitogen associated protein kinase (p38-MAPK) and extracellular signal regulated kinase (ERK1/2) indicating activation of both a contractile and a pro-inflammatory MAPK pathway. Pharmacological inhibition of both NK1R and NK3R significantly affected the downstream SP signaling. We further examined whether there was any crosstalk between the two pathways upon SP stimulation. Inhibition of ERK1/2 decreased levels of p-MLC20 after SP activation, in a PKC dependent manner, indicating a potential crosstalk between these two pathways.
Conclusions
These data provide the first evidence that SP mediated crosstalk between pro-inflammatory and contractile signaling mechanisms exists in the lymphatic system and may be an important bridge between lymphatic function modulation and inflammation.
Keywords: Lymphatic muscle cells, Substance P, Neurokinin receptors, Myosin light chain 20 phosphorylation, MAP kinase pathways
Introduction
The lymphatic system is intimately involved in the maintenance of body fluid balance, protein homeostasis and immune function. A dysfunctional lymphatic circulation can result in a wide range of clinical disorders including edema, altered lymphocyte circulation, depressed immune function and impaired lipid metabolism. However, due to the relatively poor understanding of the mechanisms of contractile activity of lymphatic muscle cells that are essential for lymph flow, only very few therapies and almost no pharmacological treatment options are present for lymphatic dysfunction [33, 53].
One of the modulators of lymphatic vessel function and lymph flow is Substance P (SP)[4, 10]. SP is an 11 amino acid mammalian neuropeptide that belongs to a family of structurally related peptides called tachykinins, which include neurokinin A and neurokinin B. It is a major mediator of inflammatory responses and affects multiple aspects of immune cell function [28]. In the lymphatic system SP is thought to be released from C sensory fibres around lymphatics [3]. Some of the biological responses of SP include motor control, contraction of smooth muscle, endothelial dependent vasodilation, plasma protein extravasation, nociception, sensory perception and proinflammatory functions [22]. SP is known to mediate its effects through three neurokinin receptors (NK-1R, NK2R, NK3R). These receptors are seven transmembrane G-protein coupled receptors that have preferred binding affinities for SP, NKA and NKB respectively [26]. SP can bind to all three receptors, albeit with varying affinities [38]. A positive chronotropic effect of SP on the lymphatic pump had been shown both in vivo or in vitro mesenteric lymphatic preparations from various animal models [12, 32] [4]. We have recently shown that in rat mesenteric lymphatics, SP acts directly on lymphatic muscle cells and has substantial positive ionotropic effects that improve pump efficiency [10].
SP plays a major role in inflammation and many inflammatory mediators alter lymphatic contractility and lymph pump activity [28, 47]. During acute or chronic inflammation, SP-mediated signaling has been shown to activate the pro-inflammatory extracellular signal regulated kinases (ERK1/2) and p38 Mitogen Activated Protein Kinases (p-38 MAPK) pathway in pancreatic acinar cells or macrophages, respectively [31, 40]. Furthermore, activation of the MAPK pathway by SP has been demonstrated to induce the synthesis of proinflammatory cytokines and chemokines [11]. The MAPKs are essentially a family of serine threonine kinases that include ERK1, ERK2, p38 kinase and Jun NH2-terminal kinase [9]. ERK1/2 and p38 MAPK have been previously reported to have a role in the contraction of both vascular and intestinal smooth muscle and to modulate upstream regulators of myosin light chain 20 (MLC20) phosphorylation [7]. In smooth muscle, agonist-induced contraction is also accompanied by activation and phosphorylation of key components in the MAPK cascade [35]. We have also recently shown that SP increases MLC20 phosphorylation in rat mesenteric lymphatics [49]. Taken together, we hypothesized that SP activates both contractile as well as pro-inflammatory pathways in lymphatic muscle cells through the neurokinin receptors. To test this hypothesis we investigated the expression of NKRs and MAPKs in lymphatic muscle cells and used pharmacological inhibitors to test the activation of downstream signaling pathways by SP. Our results indicate for the first time that both NK1R and NK3R are involved in SP-mediated activation of MLC20, p38 MAPK and ERK1/2 pathways. In addition, the data suggest that a crosstalk mechanism exists between these different pathways that may play a role in modulating the inflammatory response as well as lymphatic muscle contraction.
Materials and methods
Materials
Phospho MLC20 (Ser 19), phospho-p38, p-38, ERK1/2 and phospho-ERK1/2 antibodies were purchased from Cell Signaling technology (Beverly, MA). Phospho-MYPT1 and MYPT1 antibodies were from Millipore (Billerica, MA). NK1R antibody was purchased from Santacruz Biotechnology (Santa Cruz, CA) and NK3R was obtained from Novus Biologicals (Littleton, CA). Total MLC20, horseradish-peroxidase (HRP)-conjugated anti-mouse IgG and anti-rabbit IgG secondary antibodies, NK1R inhibitor (L703606), NK3R inhibitor (SB222200), PKC inhibitor (GF109203X), protease inhibitor cocktail and DMSO were obtained from Sigma-Aldrich (St. Louis, MO). p38 MAPK inhibitor (SB203580) and ERK1/2 inhibitor (PD-98059) were purchased from Calbiochem (San Diego, CA). Dulbecco’s Modified Eagle Medium (DMEM), fetal bovine serum (FBS), trypsin and triple antibiotics were from Invitrogen (Gaithersburg, MD). All other chemicals and reagents were from Sigma (St. Louis, MO).
Cell culture
The primary rat thoracic duct lymphatic muscle cell line (RTLMC) and rat mesenteric lymphatic muscle cell line (RMLMC) were established from rat thoracic and mesenteric tissue explants respectively, as we have previously described [27]. The phenotypes of these cell lines have also been routinely verified by immunofluorescence or western blots using various contractile proteins, namely α–vascular smooth muscle actin, γ-enteric actin, smooth muscle myosin heavy chain, caldesmon, calponin and SM22α ([27] data not shown). Cells were cultured in DMEM supplemented with 10% FBS and 1% triple antibiotics and maintained at 37°C in a 10% CO2 incubator.
Agonist and inhibitor treatment
RMLMC were plated on 24 well plates and the cells were then grown to around 80% confluence. The cells were serum-deprived for 24hrs before treatment. They were then stimulated with SP (1μM) for various time points ranging from 0, 15s, 30s, 1min, 3mins and 5mins. (For some experiments two additional time points of 15mins and 30mins were included as it has been shown in other studies that SP activates components of the MAPK pathway until around 30 mins, reaching peak activation between 15–20 mins [11, 40]. For the inhibitor treatments, NK1R, NK3R, p38, pERK and PKC inhibitors (L703606, SB222200, PD98059, SB203580 and GF109203X respectively) were dissolved in DMSO to make stock solutions. They were then diluted in serum free DMEM before addition to the cells. Concentrations of inhibitors used for the treatment were 500pM, 10nM, 100nM, 1μM, 10μM and 100μM. The cells were incubated with the respective inhibitors for an hour prior to stimulation with SP (1μM) for 3mins. DMSO vehicle controls were used to ensure that the effect of the inhibitors on the proteins that were analyzed was specific and not due to the 0.1% DMSO, which was present in the inhibitor treated samples (data not shown). No cytotoxicity was evident after DMSO or any of the agonist or inhibitor treatments.
Tissue samples
Male Sprague-Dawley rats weighing 290–330 grams were used for these experiments. The rats were housed in an environmentally controlled vivarium approved by the American Association for Accreditation of Laboratory Animal Care. All animal protocols in this study were approved by Texas A & M University Laboratory Animal Care Committee. The animal was fasted for 24 hours before each experiment, with water available ad libitum. The rats were anaesthetized with Innovar-Vet (0.3 ml kg−1 I.M.), which is a combination of a droperidol-fentanyl solution (droperidol 20 mg ml−1, fentanyl 0.4 mg ml−1) and diazepam (2.5 mg kg−1 IM). To isolate a rat mesenteric lymphatic vessel, a 6–7 cm long loop of small intestine was exteriorized through a midline laparotomy. A section of the mesentery was gently positioned over a semicircular viewing pedestal on a vessel preparation board. A mesenteric lymphatic vessel was centered over an optical window on the preparation board. The exteriorized tissues were continuously suffused with a Dulbecco’s phosphate-buffered saline (Invitrogen Corporation, Carlsbad, CA). The lymphatic vessel (80–120 μm in diameter) was isolated under a dissecting microscope using extreme caution not to damage the vessel. After isolation, the vessels were rapidly snap frozen in liquid nitrogen and then stored at −80° till they were used for protein studies.
RNA analyses
RNA was isolated from RTLMC and RMLMC cultures using TRIzol reagent. RNA was also isolated from rat brain tissue that was used as a control. DNAse treatment was done to remove any contaminating genomic DNA. RNA quality was checked by agarose gel electrophoresis. Forward and reverse primers for NK-1R, NK-2R and NK-3R were designed for gene specific RT-PCR to test for expression of NK receptors in lymphatic muscle cells. The primer sequences used were as follows:
NK1R forward 5′-CTCAGCCACGGCTACCAAAG-3′
NK1R reverse 5′-TGGTCTCTGTGGTGGAGTAGT-3′
NK2R forward 5′-AGCAATGCCACGGGTGTTAC-3′
NK2R reverse 5′-GCCAGAATGATCCAGATGACT-3′
NK3R forward 5′-CATCTATGGTCTTCACAGCGAGT-3′
NK3R reverse 5′-AGTCTGGGTTTCAACGGATCAATA-3′
RNA was converted to first strand cDNA using reverse transcriptase with a 3′ gene specific primer. An annealing temperature of 60° C and a 2.0 mM MgCl2 concentration were ideal conditions for NK3R PCR results. For NK1R, PCR amplification was carried out at 57° C. PCR expression of NK2R in brain tissue was standardized at 55° C and 2.0 mM MgCl2. Actin PCR was run as a control at 60° C and 1.5 MgCl2. PCR amplification of the 128bp NK2R band in rat brain tissue was taken as positive control.
Western blot analyses
After agonist and/or inhibitor treatments RMLMC cells were lyzed in 1X SDS buffer supplemented with protease and phosphatase inhibitors. RTLMC as well as rat brain, aorta, and lymphatic tissues from mesentery, thoracic and cervical regions were sonicated in the protein-solubilizing buffer and run on a 4–20% precast gradient SDS-polyacrylamide gel (Bio-Rad Laboratories). The proteins were transferred to a nitrocellulose membrane with a Bio-Rad transblot apparatus. The transfer was verified with Ponceau-S staining. The membrane was blocked in 5% milk in TBS/TBST and then incubated with a primary antibody followed by incubation with the corresponding HRP-conjugated secondary antibody. The following dilutions of the primary antibodies in TBS were used: p- MLC20 (1:500), MLC20 and GAPDH (1:5000), phospho-MYPT1, MYPT1, phospho-p38 MAPK, p-38 MAPK, ERK1/2, phospho-ERK1/2, NK1R and NK3R (1:1000) and actin (1:10000). The immunoreactive bands were visualized using the Pierce detection system (SuperSignal West Dura Extended Duration Substrate, Pierce). Densitometric analyses on the resulting bands were performed using Quantity One Multi-Analyst Software (BioRad). The membranes were stripped using ImmunoPure® IgG Elution Buffer (Pierce) and then re-probed. GAPDH was used as the loading control.
Statistical analysis
Data are expressed as mean±SEM that was calculated and plotted using GraphPad Prism (GraphPad Software Inc, La Jolla, CA). Statistical analysis was carried out for all the experiments using one-way Analysis of Variance (ANOVA) followed by Fisher’s least-significant difference (PLSD) posthoc test with the StatView Software (SAS Institute Inc, Cary, NC) [52]. A value of p<0.05 was considered to be significant.
Results
SP enhances phosphorylation of MLC20 in a time dependent manner
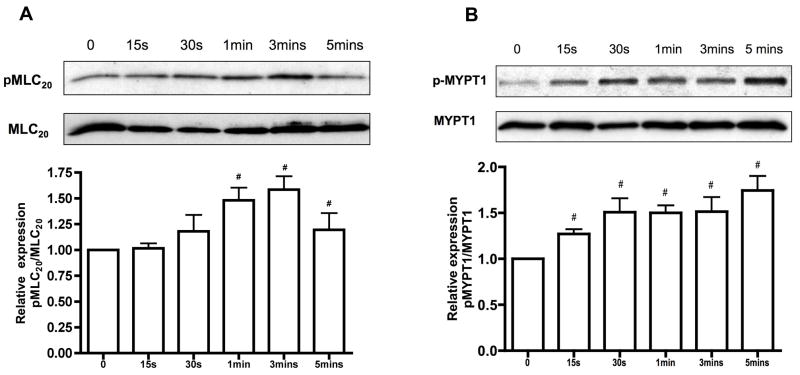
To investigate the role of SP in stimulating MLC20 phosphorylation in lymphatics, mesenteric lymphatic muscle cells were treated with SP (1μM) for various time points (Figure 1A). SP treatment showed a significant increase in the levels of MLC20 phosphorylation in a time-dependent manner, with a maximum effect observed at 3 mins. Furthermore, activation of MLC20 by SP was found to correlate with SP-mediated phosphorylation of p-MYPT1, a known regulator of MLC20 phosphorylation (Figure 1B).
Figure 1.
A) Effect of SP treatment on phosphorylation of MLC20. Top panel: representative blots from RMLMC cells treated with SP (1μM) for varying time points as indicated (0–5mins) and probed with phospho MLC20 or total MLC20. Bottom panel: relative expressions of pMLC20/MLC20 were calculated from three blots and the respective means ± SEM were plotted. p<0.05 was considered significant. B) Effect of SP treatment on MLCP. Top panel: representative blots from RMLMC cells treated with SP as described earlier and then probed for phospho MYPT1 or total MYPT1. Bottom panel: relative expression ratios of p-MYPT1/MYPT1 were calculated from three blots and the respective means ± SEM were plotted. # indicates significant compared with control (no treatment) and p<0.05 was considered significant.
NK1R and NK3R are expressed in lymphatic muscle cells and tissues
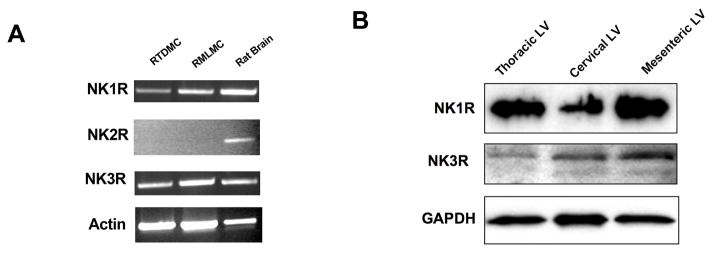
It is not clear which neurokinin receptors are expressed in the lymphatic system and there is no clear documented evidence as to which specific receptor is important for SP-mediated signaling in the lymphatics. Hence, expression of the NK1R, NK2R and NK3R was examined in the RTLMC and RMLMC cell lines. RT-PCR analysis of the receptor mRNA expression was carried out for all three receptors using semi-quantitative RT-PCR analyses. Total RNA isolated from rat brain tissue was used as a control. Expression of the NK1R and NK3R was readily detected in both the cell lines. However there was no detectable expression of NK2R in the two lymphatic muscle cell lines analyzed, although NK2R expression was found in the brain tissue used as a positive control (Fig 2A.). Expression of the NK1R and NK3R was then examined at the protein level and expression was found in the various rat lymphatic tissues including thoracic, cervical and mesenteric lymphatics (Figure 2B).
Figure 2.
Expression of neurokinin receptors in cultured lymphatic muscle cells. A) RT-PCR analysis of NKRs in RTDMC and RMLMC. Rat brain is used as a positive control. B) Western blot analysis of NK1R and NK3R in rat thoracic, cervical and mesenteric lymphatic vessels (LV). Data are representative of three independent experiments.
SP activates the p38 MAPK and ERK1/2 pathways in lymphatic muscle cells
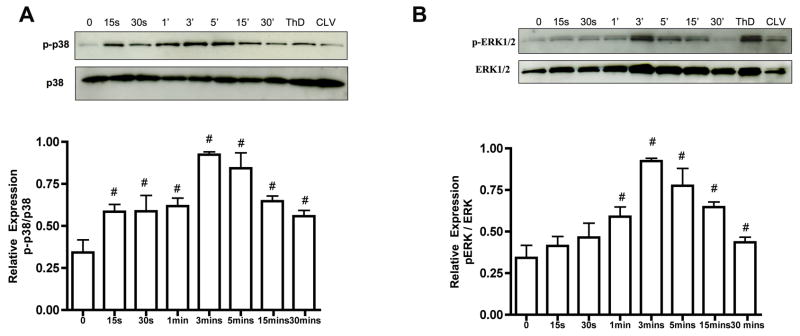
In order to determine whether components of the MAPK pathway are expressed in the lymphatic system and if SP activates this pathway, we analyzed the expression of p38 MAPK and ERK1/2 in lymphatic muscle cells. SP stimulated the increase in phosphorylation of p38 MAPK in a time-dependent manner. The expression of p-p38 MAPK was found to increase within 15 seconds of stimulation with SP. Expression peaked at around 3 mins and then gradually declined by 30mins. The levels of p-38 MAPK remained unchanged (Figure 3A). Phosphorylated ERK1/2 also showed a significant increase at 3mins and expression levels declined almost to basal levels after 30mins of SP treatment (Figure 3B). Phosphorylated forms of both p38 MAPK and ERK1/2 were also detected in cervical and thoracic lymphatic tissues (Figure 3).
Figure 3.
Activation of p38 MAPK and ERK1/2 pathways in the lymphatic system after SP stimulation. A) Top panel: representative blot showing the expression of phospho p38 (p-p38) and total p38 in SP-treated RMLMCs at different time points. Bottom panel: the relative expression ratios of p-p38/total p38 from three blots were calculated and the respective means ± SEM were plotted. B) Top panel: representative blot showing the expression of phospho ERK1/2 (p-ERK1/2) and total ERK1/2 in SP-treated RMLMCs at different time points. Bottom panel: the relative expression ratios of p-ERK1/2/total ERK1/2 from three blots were calculated and the respective means ± SEM were plotted. # indicates significant difference compared with control (no treatment) and p<0.05 was considered significant in both A and B. Thoracic duct (ThD) and cervical lymphatic vessel (CLV) also showed expression of these proteins.
SP signals through a combination of NK1R and NK3R and their inhibition suppresses downstream signaling pathways activated by SP
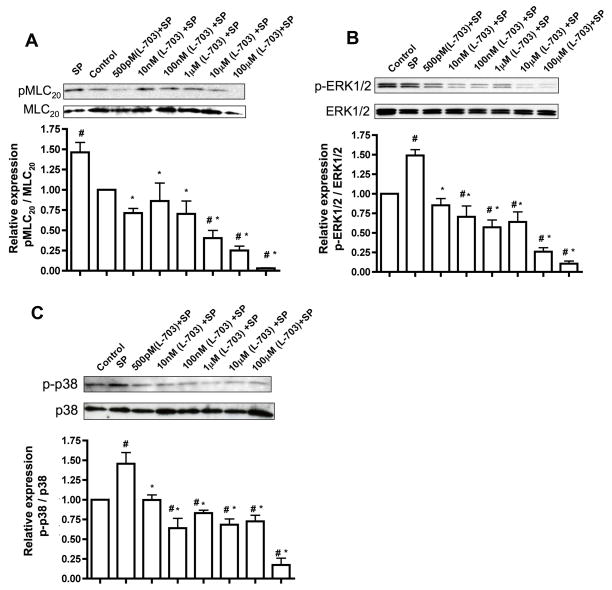
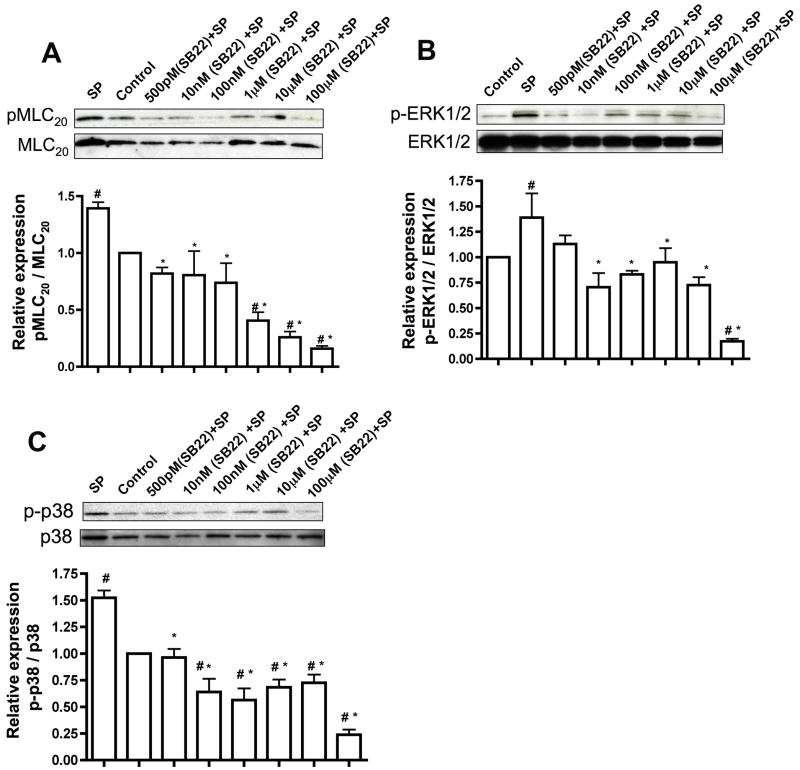
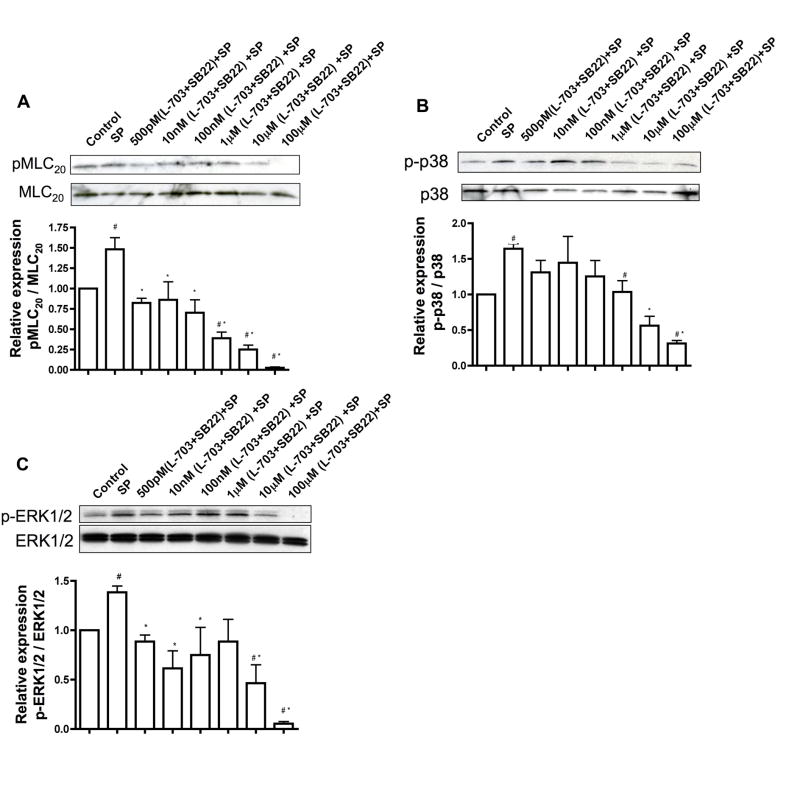
In order to determine which of the two neurokinin receptors were essential for SP signaling in the lymphatics, we used inhibitors specific for neurokinin receptors and assayed for the downstream signaling effectors of SP. RMLMC cells were treated with varying concentrations (500pM to 100μM) of NK1R inhibitor (L-703606) or NK3R inhibitor (SB222200) or a combination of both inhibitors together for one hour and then stimulated with SP for 3 mins. Pre-incubation of RMLMC cells with NK1R or NK3R inhibitors significantly decreased SP-induced phosphorylated forms of MLC20, p-38 and ERK1/2 in an inhibitor concentration dependent manner (Figures 4 and 5, respectively). At higher concentrations of L-703606 (10μM and100μM) inhibition of NK1R showed a more potent decrease in the phosphorylated forms of MLC20, p-38 and ERK1/2 as compared to the inhibition of NK3R, indicating that NK1R may be the more active receptor for SP signaling in the lymphatics. When the cells were treated with a combination of both inhibitors and then stimulated with SP, almost complete inhibition of p-MLC20, p-p38 MAPK and p-ERK was observed at higher concentrations of the inhibitors (Figure 6).
Figure 4.
Inhibition of NK1R suppresses SP-mediated downstream signaling. RMLMC cells were serum-starved and treated with the NK1R inhibitor L-703606 at concentrations of 500pM, 10nM, 100nM, 1μM, 10μM and 100μM and then subsequently treated with SP for 3′ as described in the Methods section. A, B and C. Top panels: Representative blots showing expression of pMLC20 & total MLC20, p-ERK & total ERK and phospho-p-38 (p-p-38) & total p38, respectively were presented; bottom panels: the relative expression ratios of pMLC20/MLC20, p-ERK1/2/total ERK1/2 and p-p-38/total p38 were calculated from three blots and the respective means± SEM were plotted. # indicates P<0.05 compared with control (no treatment) and * indicates P<0.05 compared with SP, that was considered significant.
Figure 5.
Inhibition of NK3R suppresses SP-mediated downstream signaling. RMLMC cells were serum-starved and treated with the NK3R inhibitor SB222200 at concentrations 500pM, 10nM, 100nM, 1μM, 10μM and 100μM and then subsequently treated with SP for 3′. A, B and C. Top panels: Representative blots showing expression of pMLC20 & total MLC20, p-ERK & total ERK and phospho-p-38 (p-p-38) & total p38, respectively were presented; bottom panels: the relative expression ratios of pMLC20/MLC20, p-ERK1/2/total ERK1/2, and p-p-38/total p38 were calculated from three blots and the respective means± SEM were plotted. # indicates P<0.05 compared with control (no treatment) and * indicates P<0.05 compared with SP, that was considered significant.
Figure 6.
SP signals through a combination of NK1R and NK3R in the lymphatic system. Serum starved RMLMC cells were treated with a combination of NK1R inhibitor (L-703606) and NK3R inhibitor (SB222200) in concentrations ranging from 500pM to 100μM and then subsequently stimulated with SP for 3mins. A, B and C. Top panels: Representative blots showing expression of pMLC20 & total MLC20, phospho-p-38 (p-p-38) & total p38, p-ERK & total ERK, respectively were presented; bottom panels: the relative expression ratios of pMLC20/MLC20, p-p-38/total p38 and p-ERK1/2/total ERK1/2, respectively was calculated from three blots and the respective means± SEM were plotted. # indicates P<0.05 compared with control (no treatment) and * indicates P<0.05 compared with SP, that was considered significant.
Inhibition of p-ERK and PKC decreases MLC20 phosphorylation while p-38 MAPK inhibition does not
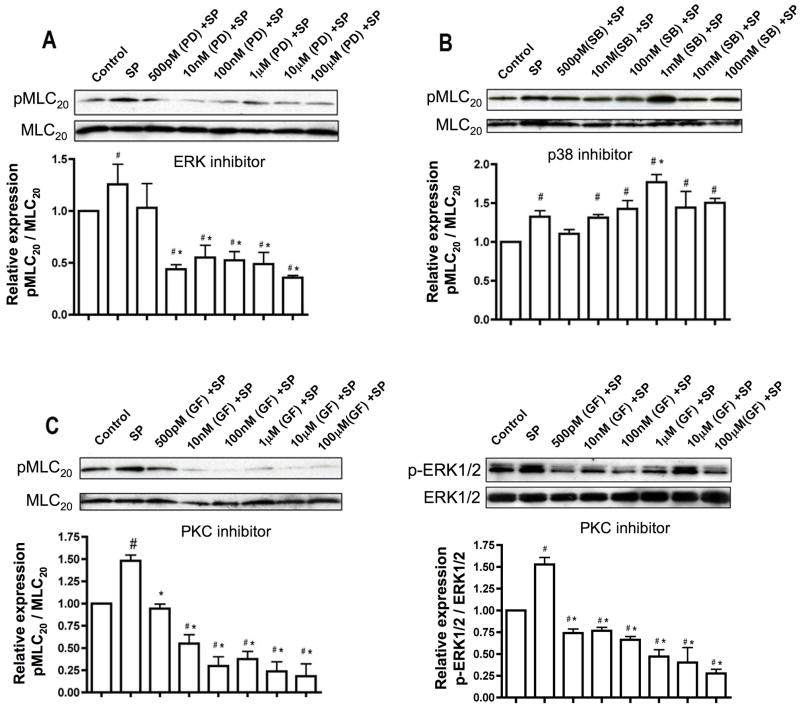
Since increased phosphorylation of p-p38 MAPK and p-ERK, as well as MLC20, occurs in the lymphatics on stimulation with SP, and both p-p38 MAPK and p-ERK1/2 have been implicated in muscle contraction [7], we investigated crosstalk between these two pathways. To explore the role of activated MAPKs in SP stimulated MLC20 phosphorylation, we treated the cells with a specific inhibitor of p38 MAPK (SB 203580) or p-ERK1/2 (PD 98059) at different concentrations and then stimulated the cells with SP. Treatment of the cells with the p-ERK1/2 inhibitor significantly reduced the levels of phosphorylation of MLC20 in a concentration-dependent manner (Figure 7A). Conversely, inhibition of p38 MAPK had almost no effect (or a slight increase at 1μM) on levels of phosphorylated MLC20 (Figure 7B). As PKC is a known regulator of p-ERK, we wanted to examine whether it was involved in mediating the effects of SP on MLC20 phosphorylation. PKC inhibition with GF109203X and subsequent SP stimulation showed a decrease in MLC20 phosphorylation levels (Figure 7C). Furthermore PKC inhibition decreased the levels of p-ERK1/2 (Figure 7D), suggesting that PKC plays an intermediary role in SP induced and p-ERK mediated activation of MLC20.
Figure 7.
Inhibition of p-p38MAPK has almost no effect on phosphorylation of MLC20 while inhibition of p-ERK and PKC significantly decrease pMLC20. A and B. Top panels: Representative blots showing the expression of pMLC20 and total MLC20 in RMLC cells treated with p-ERK1/2 inhibitor (PD09859) and p-38MAPK inhibitor (SB203580), respectively, at concentrations ranging from 500pM to 100μM and then subsequently treated with SP for 3′; bottom panels: the relative expression of pMLC20/MLC20 was calculated from three blots and the respective means± SEM were plotted. (C and D) Top panels: Representative blots showing the expression of pMLC20 & total MLC20 and p-ERK1/2 and total ERK1/2 in RMLC cells treated with the PKC inhibitor (GF109203X) in same concentrations as in (A) and then subsequently treated with SP for 3′; bottom panels: the relative expression ratios of pMLC20/total MLC20 and pERK1/2/total ERK1/2 were calculated from three blots and the respective means± SEM were plotted. # indicates P<0.05 compared with control (no treatment) and * indicates P<0.05 compared with SP, that was considered significant in A, B, C and D.
Discussion
The data presented in this study demonstrate that SP activates p38 MAPK/ERK1/2 and MLC20 phosphorylation, indicating that SP modulates both pro-inflammatory and contractile pathways in LMCs, through NK1 and NK3 receptors. In addition, the results show that the SP mediated inflammatory and contractile regulatory pathways are intertwined in the lymphatics.
Several studies have shown that classical inflammatory mediators, such as prostanoids [8, 13, 18, 24, 45, 55] histamine [2, 16, 21, 23, 43] or nitric oxide [25] modulate lymphatic pumping and drainage. In addition, neuromediators that are important in immune and inflammatory responses, such as SP, calcitonin gene related peptide (CGRP), neuropeptide Y or vasoactive intestinal polypeptide, are reported to strongly affect lymphatic vessel contractility [1, 4, 10, 17, 29, 49]. Thus there is substantial evidence that the lymphatic system is intimately involved in and highly altered during inflammatory disease. Release of inflammatory mediators, in addition to increasing vascular permeability during inflammation is thought to play a pivotal role in modulating lymphatic vessel function [47]. However, the signaling mechanisms of these inflammatory mediators have not been addressed in lymphatics. In this study, we have addressed the SP-mediated signaling pathways in lymphatic muscle cells.
Previous functional studies have shown that SP stimulates contraction of rat mesenteric lymphatic vessels [4, 10, 49]. At low concentrations SP enhances lymphatic contractility and pump efficiency. In quiescent vessels, this stimulation of lymphatic contraction frequency causes an active pumping state, resulting in an increased lymph flow [4]. It is proposed that SP release is graded and is thus able to modify lymphatic vessel function and hence lymph flow in a concentration-dependent manner [10]. Several in vivo and in vitro lymphatic contractile measurement studies [4, 12, 32] showed that the lymphatic pumping or contractile activity reached maximum at 1.0 μM SP concentration. Furthermore, we have recently shown that 1.0 μM SP increased both the tonic contraction strength and phosphorylation of MLC20 in rat mesenteric lymphatics [49]. Hence we used 1.0 μM SP concentration in this study and addressed the SP-mediated downstream signaling pathways that are involved in the regulation of MLC20 phosphorylation.
The molecular analyses data (Figure 2) demonstrate that both NK1R and NK3R are expressed in lymphatics, whereas the NK2R expression was not detected. In guinea pig mesenteric lymphatics, the nonspecific NK receptor blocker spantide and the more selective NK1 receptor antagonist WIN51708 impaired SP-induced alterations of lymphatic pumping, indicating that SP actions in the lymphatics were mediated by NK1 receptors [32]. In contrast, in rat mesenteric lymphatics the effects of SP on lymphatic pumping activity were only partially mediated through NK1R and other NK receptors were possibly involved [10]. The authors further proposed that SP exerts its effects on the rat mesenteric lymphatics by acting on the lymphatic muscle and not the endothelium [10]. In this study we have found that in rat mesenteric lymphatic muscle cells both NK1R and NK3R are necessary for SP mediated signaling. It has been recently shown that the functional NK2R is absent in the mouse macrophage and its main ligand NKA signals through the NK1R [40]. We also failed to detect the expression of NK2R in lymphatic muscle cell cultures by RT-PCR, although positive expression of NK2R was detected in the rat brain, indicating that SP possibly mediates its functions primarily through the NK1R and/or NK3R in lymphatic muscle cells. However, further experiments, such as RT/PCR and western blots, are warranted to completely rule out the possibility of detectable NK2R expression in the intact lymphatic tissues.
As shown in figure 2C, double bands of the NK1R were observed in cell culture samples treated with SP. It has been reported that after exposure to high concentrations of agonists (>10 nM SP), the NK1R is extensively phosphorylated, which may account for the second higher-sized band that we observe [34]. The signaling pathways of NK receptors can affect each other by intracellular crosstalk and it has been shown in case of G-protein coupled receptors (GPCRs) that a single agonist can regulate a cell through activation of different receptor subtypes [30] [36]. It has been proposed that when receptor desensitization and internalization of one neurokinin receptor occurs, cells may still respond to the agonists by retention of another neurokinin receptor at the cell surface [36]. In keeping with this hypothesis, our data suggest that SP signals through a combination of both NK1R and NK3R. Upon inhibition of the NK1R and NK3R, individually, there is substantial reduction of phosphorylated MLC20, p-ERK and p-p38 MAPK, and the inhibition is almost complete when both the NK receptors are inactivated, suggesting the involvement of both receptors in SP-mediated signaling in the lymphatic system (Figures 4–6). Furthermore, our data suggest that NK1R inhibition causes the more potent effect, considerably decreasing SP mediated downstream signaling at higher inhibitor concentrations, indicating that NK1R maybe the main functional receptor for SP signaling in lymphatics.
SP has been shown to activate members of the MAPK cascade including ERK1/2 and p38 MAPK [50]. Mammalian MAPKs form a complex network of signal transduction pathways. p38 MAP kinases are mainly activated by pro-inflammatory cytokines and environmental stress. Whereas, the ERK group of MAP kinases are activated in response to extracellular stimuli [44]. During chronic inflammation, SP has been shown to enhance chemokine responses in macrophages by activation of the p38 MAPK and ERK1/2 pathways [42]. Stimulation of SP receptors in astrocytoma cells leads to an increase in ERK1/2 phosphorylation and this activation pathway is calcium-dependent [51]. p38 MAPK has been shown to be activated by proinflammatory cytokines such as TNF-α and IL-1. SP-activated p38 MAPK functions as a signal transduction component that mediates SP-induced IL-6 expression [11]. This study shows for the first time that SP activates p38 MAPK and ERK1/2, in the lymphatic system. Both SP and the lymphatic system are intrinsically involved in mediating inflammatory responses as well as immune function, and functions of the lymphatic system are dependent on its contractile activity [4] Hence in this study we also evaluated if there was an overlap between the MAPK and MLC20 signaling pathways in the lymphatic system.
MAPK pathways as the p38 MAPK and p-ERK1/2 have been implicated as having a role in MLC20 phosphorylation, as well as being closely linked to the contraction process in vascular smooth muscle [15]. ERK1/2 has been shown to directly phosphorylate MLC kinase, resulting in enhanced phosphorylation of MLC20 [19]. Our results correlate well with these findings and demonstrate that inhibition of ERK1/2 phosphorylation decreases phosphorylation of MLC20 (Figure 7A). Several studies have also reported a connection between PKC and p-ERK1/2 activation as well as PKC activation by SP stimulation [41]. PKC is a major modulator of contraction, which is regulated by Ca2+-dependent and -independent mechanisms in vascular smooth muscle [48]. In colonic smooth muscle it has been shown that MAPK is activated during PKC-mediated contraction [14]. Hence, we investigated the role of PKC in mediating SP-induced activation of p-ERK1/2 and its subsequent stimulatory effects on phosphorylation of MLC20. Our results suggest that PKC may be playing an intermediary role in this pathway and that PKC inhibition reduces activation of both p-ERK and MLC20. Fiebich et al., [11] have shown that p38 MAPK pathways induced by SP are independent from SP-mediated ERK1/2 activation. Interestingly, in the present study we found that inhibition of p38 did not decrease MLC20 phosphorylation, suggesting that ERK1/2 and p38 activation by SP may also mediate different functions in the lymphatics.
Some interesting questions that arise from this study and still remain to be answered are what is the functional consequence of SP “inducing” inflammation and the subsequent modulation in contraction? Another question is whether these functions are linked in order to heal or does SP activation/contraction lead to lymphatic dysfunction? From the literature it is evident that SP has been extensively described to be an important mediator of inflammatory responses and pathological conditions associated with inflammation, including asthma, inflammatory bowel disease, rheumatoid arthritis and pancreatitis [6, 37, 39, 54]. SP is released from C-sensory fibers that have been shown to be involved in neurogenic inflammation and SP has been implicated as one of the agents responsible for vasodilation and plasma extravasation under these conditions [4, 20].
We have previously shown that SP causes an increase in lymphatic contractility both in vivo and in vitro [4, 10]. Lymph flow has been generally found to increase during inflammatory reactions, due to increases in vascular permeability and resultant increases in interstitial fluid; and the function of lymphatic vessels to adapt their pumping activity to changes in fluid load is particularly important during inflammatory reactions [46, 47]. It has been shown that mesenteric lymphatic pumping increases during edemagenic stress caused by dilution of plasma in rats in vivo [5]. The multitude of inflammatory mediators, which gain entry into the lymphatic vessels or present in the vessel environment, could also regulate lymph flow, by directly affecting lymphatic contractility [46]. In vivo however, it is difficult to determine whether a lymphatic response is due to a direct stimulation by inflammatory mediators, or is a secondary consequence of other aspects of the inflammatory response, such as edema and subsequent vessel filling [47].
From the present study it is evident that SP induces both inflammatory pathways and contractile pathways in the lymphatic muscle cells. It is also evident that the key inflammatory pathway mediator, p38 MAPK is activated very rapidly (within 15 seconds) upon SP stimulation while the contractile regulatory molecule, p-ERK is activated relatively slower following SP application. Taken together, we propose that SP could be an important determinant in response to pathological conditions to modulate the lymphatic contractile activity. Our notion is that different concentrations of SP found during inflammatory conditions may activate parallel pathways, which crosstalk with each other, and that a graded release of SP in such conditions would maintain a fine balance between modulation of contractility and progression of inflammation. Future experiments are warranted to test this hypothesis.
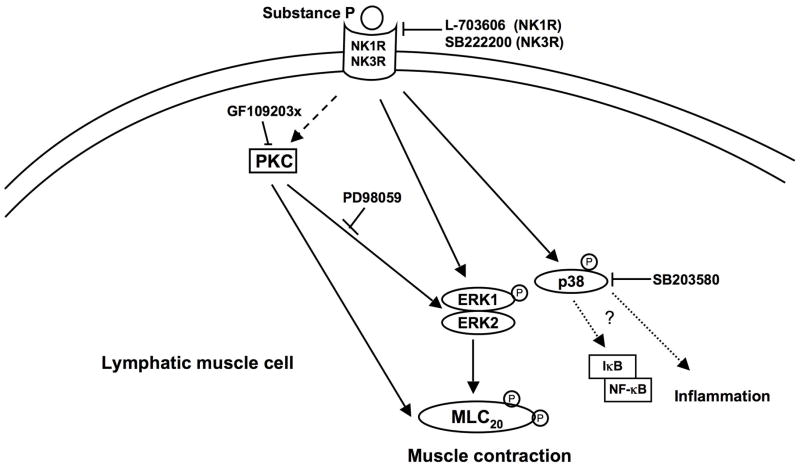
In summary, the results presented in this study demonstrate for the first time that SP mediated signaling in the lymphatic muscle occurs either singly or through a combination of NK1R and NK3R, and that SP activates the proinflammatory p38 MAPK and ERK1/2 pathways, and contractile ERK1/2 and MLC20 pathways (Figure 8). Our data suggest that there is crosstalk between the MAPK and MLC20 pathways although SP may be activating parallel contractile and inflammatory pathways through its effects on ERK1/2 and p38 MAPK. Further studies are warranted to delineate the other molecular players involved in this crosstalk and their roles in inflammation and lymphatic muscle contraction need to be evaluated. This study thus provides the first evidence that SP mediated signaling in the lymphatic system may be an important bridge between the lymphatic functions of flow modulation, muscle contraction and inflammation.
Figure 8.
Schematic representation of SP-mediated signaling and activation of the MAPK and MLC20 pathways in lymphatic muscle cells. SP binding to NK1R and/or NK3R activates the MAPK pathway, specifically ERK1/2 and p38, causing an increase in their phosphorylated forms. SP-mediated downstream activation can be blocked by the neurokinin receptor inhibitor NK1R (L703606) and/or NK3R (SB222200) are shown. Activation of p-ERK increases MLC20 phosphorylation, which is diminished upon inhibition of ERK1/2 with PD-98059. Inhibition of p38 phosphorylation does not decrease pMLC20 and may play a role in activation of inflammatory cytokines and chemokines by inflammatory mediators as NF-κB and I-κB. PKC possibly plays an intermediary role in this SP-mediated downstream signaling by activation of ERK-mediated MLC20 phosphorylation, which is abrogated on inhibition with GF109203X. SP signaling may thus be activating parallel contractile and inflammatory pathways by activation of p-ERK and p-38MAPK, respectively. Dashed arrows represent components that have not been directly assessed in this study.
Acknowledgments
Grant Support: This work was supported by AHA 09POST2280005 to SC, R01 HL089784 to MJD, RO1HL070308 to DCZ and NIH RO1 HL080526 and KO2 HL086650 to MM.
Footnotes
Conflict of Interest: None declared
References
- 1.Akopian VA, Balashov NV, Khanipov GF. Effect of calcitonin gene-related peptide on lymphatic vessels. Zh Evol Biokhim Fiziol. 1998;34:675–682. [PubMed] [Google Scholar]
- 2.Amelang E, Prasad CM, Raymond RM, Grega GJ. Interactions among inflammatory mediators on edema formation in the canine forelimb. Circ Res. 1981;49:298–306. doi: 10.1161/01.res.49.2.298. [DOI] [PubMed] [Google Scholar]
- 3.Amerini S, Mantelli L, Ledda F. Nitric oxide is not involved in the effects induced by non-adrenergic non-cholinergic stimulation and calcitonin gene-related peptide in the rat mesenteric vascular bed. Neuropeptides. 1993;25:51–55. doi: 10.1016/0143-4179(93)90068-l. [DOI] [PubMed] [Google Scholar]
- 4.Amerini S, Ziche M, Greiner ST, Zawieja DC. Effects of substance P on mesenteric lymphatic contractility in the rat. Lymphat Res Biol. 2004;2:2–10. doi: 10.1089/1539685041690409. [DOI] [PubMed] [Google Scholar]
- 5.Benoit JN, Zawieja DC, Goodman AH, Granger HJ. Characterization of intact mesenteric lymphatic pump and its responsiveness to acute edemagenic stress. American Journal of Physiology. 1989;257:H2059–2069. doi: 10.1152/ajpheart.1989.257.6.H2059. [DOI] [PubMed] [Google Scholar]
- 6.Broccardo M, Linari G, Agostini S, Amadoro G, Carpino F, Ciotti MT, Petrella C, Petrozza V, Severini C, Improta G. Expression of NK-1 and NK-3 tachykinin receptors in pancreatic acinar cells after acute experimental pancreatitis in rats. Am J Physiol Gastrointest Liver Physiol. 2006;291:G518–524. doi: 10.1152/ajpgi.00505.2005. [DOI] [PubMed] [Google Scholar]
- 7.Cao W, Sohn UD, Bitar KN, Behar J, Biancani P, Harnett KM. MAPK mediates PKC-dependent contraction of cat esophageal and lower esophageal sphincter circular smooth muscle. Am J Physiol Gastrointest Liver Physiol. 2003;285:G86–95. doi: 10.1152/ajpgi.00156.2002. [DOI] [PubMed] [Google Scholar]
- 8.Chan AK, Vergnolle N, Hollenberg MD, von der Weid PY. Proteinase-activated receptor 2 activation modulates guinea-pig mesenteric lymphatic vessel pacemaker potential and contractile activity. J Physiol. 2004;560:563–576. doi: 10.1113/jphysiol.2004.071399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cobb MH, Goldsmith EJ. How MAP kinases are regulated. J Biol Chem. 1995;270:14843–14846. doi: 10.1074/jbc.270.25.14843. [DOI] [PubMed] [Google Scholar]
- 10.Davis MJ, Lane MM, Davis AM, Durtschi D, Zawieja DC, Muthuchamy M, Gashev AA. Modulation of lymphatic muscle contractility by the neuropeptide substance P. Am J Physiol Heart Circ Physiol. 2008;295:H587–597. doi: 10.1152/ajpheart.01029.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fiebich BL, Schleicher S, Butcher RD, Craig A, Lieb K. The neuropeptide substance P activates p38 mitogen-activated protein kinase resulting in IL-6 expression independently from NF-kappa B. J Immunol. 2000;165:5606–5611. doi: 10.4049/jimmunol.165.10.5606. [DOI] [PubMed] [Google Scholar]
- 12.Foy WL, Allen JM, McKillop JM, Goldsmith JP, Johnston CF, Buchanan KD. Substance P and gastrin releasing peptide in bovine mesenteric lymphatic vessels: chemical characterization and action. Peptides. 1989;10:533–537. doi: 10.1016/0196-9781(89)90138-1. [DOI] [PubMed] [Google Scholar]
- 13.Gao J, Zhao J, Rayner SE, Van Helden DF. Evidence that the ATP-induced increase in vasomotion of guinea-pig mesenteric lymphatics involves an endothelium-dependent release of thromboxane A2. Br J Pharmacol. 1999;127:1597–1602. doi: 10.1038/sj.bjp.0702710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gerthoffer WT, Yamboliev IA, Shearer M, Pohl J, Haynes R, Dang S, Sato K, Sellers JR. Activation of MAP kinases and phosphorylation of caldesmon in canine colonic smooth muscle. J Physiol. 1996;495 (Pt 3):597–609. doi: 10.1113/jphysiol.1996.sp021619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goldberg PL, MacNaughton DE, Clements RT, Minnear FL, Vincent PA. p38 MAPK activation by TGF-beta1 increases MLC phosphorylation and endothelial monolayer permeability. Am J Physiol Lung Cell Mol Physiol. 2002;282:L146–154. doi: 10.1152/ajplung.2002.282.1.L146. [DOI] [PubMed] [Google Scholar]
- 16.Haddy FJ, Scott JB, Grega GJ. Effects of histamine on lymph protein concentration and flow in the dog forelimb. Am J Physiol. 1972;223:1172–1177. doi: 10.1152/ajplegacy.1972.223.5.1172. [DOI] [PubMed] [Google Scholar]
- 17.Hosaka K, Rayner SE, von der Weid PY, Zhao J, Imtiaz MS, van Helden DF. Calcitonin gene-related peptide activates different signaling pathways in mesenteric lymphatics of guinea pigs. Am J Physiol Heart Circ Physiol. 2006;290:H813–822. doi: 10.1152/ajpheart.00543.2005. [DOI] [PubMed] [Google Scholar]
- 18.Johnston MG, Gordon JL. Regulation of lymphatic contractility by arachidonate metabolites. Nature. 1981;293:294–297. doi: 10.1038/293294a0. [DOI] [PubMed] [Google Scholar]
- 19.Klemke RL, Cai S, Giannini AL, Gallagher PJ, de Lanerolle P, Cheresh DA. Regulation of cell motility by mitogen-activated protein kinase. J Cell Biol. 1997;137:481–492. doi: 10.1083/jcb.137.2.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lembeck F, Holzer P. Substance P as neurogenic mediator of antidromic vasodilation and neurogenic plasma extravasation. Naunyn Schmiedebergs Arch Pharmacol. 1979;310:175–183. doi: 10.1007/BF00500282. [DOI] [PubMed] [Google Scholar]
- 21.Lewis GP, Winsey NJ. The action of pharmacologically active substances on the flow and composition of cat hind limb lymph. Br J Pharmacol. 1970;40:446–460. [PMC free article] [PubMed] [Google Scholar]
- 22.Maggi DA, Patacchini R, Rovero P, Giachetti A. Tachykinin receptors and tachykinin receptor antagonists. J Auton Pharmacolog. 1993;13:1–70. doi: 10.1111/j.1474-8673.1993.tb00396.x. [DOI] [PubMed] [Google Scholar]
- 23.McNamee JE. Histamine decreases selectivity of sheep lung blood-lymph barrier. J Appl Physiol. 1983;54:914–918. doi: 10.1152/jappl.1983.54.4.914. [DOI] [PubMed] [Google Scholar]
- 24.Mizuno R, Koller A, Kaley G. Regulation of the vasomotor activity of lymph microvessels by nitric oxide and prostaglandins. American Journal of Physiology. 1998;274:R790–796. doi: 10.1152/ajpregu.1998.274.3.R790. [DOI] [PubMed] [Google Scholar]
- 25.Mizuno R, Koller A, Kaley G. Regulation of the vasomotor activity of lymph microvessels by nitric oxide and prostaglandins. Am J Physiol. 1998;274:R790–796. doi: 10.1152/ajpregu.1998.274.3.R790. [DOI] [PubMed] [Google Scholar]
- 26.Mizuta K, Gallos G, Zhu D, Mizuta F, Goubaeva F, Xu D, Panettieri RA, Jr, Yang J, Emala CW., Sr Expression and coupling of neurokinin receptor subtypes to inositol phosphate and calcium signaling pathways in human airway smooth muscle cells. Am J Physiol Lung Cell Mol Physiol. 2008;294:L523–534. doi: 10.1152/ajplung.00328.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Muthuchamy M, Gashev A, Boswell N, Dawson N, Zawieja D. Molecular and functional analyses of the contractile apparatus in lymphatic muscle. Faseb J. 2003;17:920–922. doi: 10.1096/fj.02-0626fje. [DOI] [PubMed] [Google Scholar]
- 28.O’Connor TM, O’Connell J, O’Brien DI, Goode T, Bredin CP, Shanahan F. The role of substance P in inflammatory disease. J Cell Physiol. 2004;201:167–180. doi: 10.1002/jcp.20061. [DOI] [PubMed] [Google Scholar]
- 29.Ohhashi T, Olschowka JA, Jacobowitz DM. Vasoactive intestinal peptide inhibitory innervation in bovine mesenteric lymphatics. A histochemical and pharmacological study. Circ Res. 1983;53:535–538. doi: 10.1161/01.res.53.4.535. [DOI] [PubMed] [Google Scholar]
- 30.Rameshwar P. Implication of possible therapies targeted for the tachykinergic system with the biology of neurokinin receptors and emerging related proteins. Recent Pat CNS Drug Discov. 2007;2:79–84. doi: 10.2174/157488907779561781. [DOI] [PubMed] [Google Scholar]
- 31.Ramnath RD, Sun J, Adhikari S, Zhi L, Bhatia M. Role of PKC-delta on substance P-induced chemokine synthesis in pancreatic acinar cells. Am J Physiol Cell Physiol. 2008;294:C683–692. doi: 10.1152/ajpcell.00360.2007. [DOI] [PubMed] [Google Scholar]
- 32.Rayner SE, Van Helden DF. Evidence that the substance P-induced enhancement of pacemaking in lymphatics of the guinea-pig mesentery occurs through endothelial release of thromboxane A2. Br J Pharmacol. 1997;121:1589–1596. doi: 10.1038/sj.bjp.0701306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rockson SG. Lymphedema. Curr Treat Options Cardiovasc Med. 2000;2:237–242. doi: 10.1007/s11936-000-0018-x. [DOI] [PubMed] [Google Scholar]
- 34.Roosterman D, Cottrell GS, Schmidlin F, Steinhoff M, Bunnett NW. Recycling and resensitization of the neurokinin 1 receptor. Influence of agonist concentration and Rab GTPases. J Biol Chem. 2004;279:30670–30679. doi: 10.1074/jbc.M402479200. [DOI] [PubMed] [Google Scholar]
- 35.Sbrissa D, Yamada H, Hajra A, Bitar KN. Bombesin-stimulated ceramide production and MAP kinase activation in rabbit rectosigmoid smooth muscle cells. Am J Physiol. 1997;272:G1615–1625. doi: 10.1152/ajpgi.1997.272.6.G1615. [DOI] [PubMed] [Google Scholar]
- 36.Schmidlin F, Dery O, Bunnett NW, Grady EF. Heterologous regulation of trafficking and signaling of G protein-coupled receptors: beta-arrestin-dependent interactions between neurokinin receptors. Proc Natl Acad Sci U S A. 2002;99:3324–3329. doi: 10.1073/pnas.052161299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Scholzen TE, Steinhoff M, Bonaccorsi P, Klein R, Amadesi S, Geppetti P, Lu B, Gerard NP, Olerud JE, Luger TA, Bunnett NW, Grady EF, Armstrong CA, Ansel JC. Neutral endopeptidase terminates substance P-induced inflammation in allergic contact dermatitis. J Immunol. 2001;166:1285–1291. doi: 10.4049/jimmunol.166.2.1285. [DOI] [PubMed] [Google Scholar]
- 38.Severini C, Improta G, Falconieri-Erspamer G, Salvadori S, Erspamer V. The tachykinin peptide family. Pharmacol Rev. 2002;54:285–322. doi: 10.1124/pr.54.2.285. [DOI] [PubMed] [Google Scholar]
- 39.Sun J, Ramnath RD, Bhatia M. Neuropeptide substance P upregulates chemokine and chemokine receptor expression in primary mouse neutrophils. Am J Physiol Cell Physiol. 2007;293:C696–704. doi: 10.1152/ajpcell.00060.2007. [DOI] [PubMed] [Google Scholar]
- 40.Sun J, Ramnath RD, Tamizhselvi R, Bhatia M. Neurokinin A engages neurokinin-1 receptor to induce NF-kappaB-dependent gene expression in murine macrophages: implications of ERK1/2 and PI 3-kinase/Akt pathways. Am J Physiol Cell Physiol. 2008;295:C679–691. doi: 10.1152/ajpcell.00042.2008. [DOI] [PubMed] [Google Scholar]
- 41.Sun J, Ramnath RD, Tamizhselvi R, Bhatia M. Role of protein kinase C and phosphoinositide 3-kinase-Akt in substance P-induced proinflammatory pathways in mouse macrophages. Faseb J. 2009;23:997–1010. doi: 10.1096/fj.08-121756. [DOI] [PubMed] [Google Scholar]
- 42.Sun J, Ramnath RD, Zhi L, Tamizhselvi R, Bhatia M. Substance P enhances NF-kappaB transactivation and chemokine response in murine macrophages via ERK1/2 and p38 MAPK signaling pathways. Am J Physiol Cell Physiol. 2008;294:C1586–1596. doi: 10.1152/ajpcell.00129.2008. [DOI] [PubMed] [Google Scholar]
- 43.Svensjo E, Adamski SW, Su K, Grega GJ. Quantitative physiological and morphological aspects of microvascular permeability changes induced by histamine and inhibited by terbutaline. Acta Physiol Scand. 1982;116:265–273. doi: 10.1111/j.1748-1716.1982.tb07140.x. [DOI] [PubMed] [Google Scholar]
- 44.Thalhamer T, McGrath MA, Harnett MM. MAPKs and their relevance to arthritis and inflammation. Rheumatology (Oxford) 2008;47:409–414. doi: 10.1093/rheumatology/kem297. [DOI] [PubMed] [Google Scholar]
- 45.Vergnolle N, Wallace JL, Bunnett NW, Hollenberg MD. Protease-activated receptors in inflammation, neuronal signaling and pain. Trends Pharmacol Sci. 2001;22:146–152. doi: 10.1016/s0165-6147(00)01634-5. [DOI] [PubMed] [Google Scholar]
- 46.von der Weid P. Review article: lymphatic vessel pumping and inflammation--the role of spontaneous constrictions and underlying electrical pacemaker potentials. Aliment Pharmacol Ther. 2001;15:1115–1129. doi: 10.1046/j.1365-2036.2001.01037.x. [DOI] [PubMed] [Google Scholar]
- 47.von der Weid PY, Muthuchamy M. Regulatory mechanisms in lymphatic vessel contraction under normal and inflammatory conditions. Pathophysiology. 2009;17:263–276. doi: 10.1016/j.pathophys.2009.10.005. [DOI] [PubMed] [Google Scholar]
- 48.Walsh MP, Horowitz A, Clement-Chomienne O, Andrea JE, Allen BG, Morgan KG. Protein kinase C mediation of Ca(2+)-independent contractions of vascular smooth muscle. Biochem Cell Biol. 1996;74:485–502. doi: 10.1139/o96-053. [DOI] [PubMed] [Google Scholar]
- 49.Wang W, Nepiyushchikh Z, Zawieja DC, Chakraborty S, Zawieja SD, Gashev AA, Davis MJ, Muthuchamy M. Inhibition of myosin light chain phosphorylation decreases rat mesenteric lymphatic contractile activity. Am J Physiol Heart Circ Physiol. 2009;297:H726–734. doi: 10.1152/ajpheart.00312.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Xia Z, Dickens M, Raingeaud J, Davis RJ, Greenberg ME. Opposing effects of ERK and JNK-p38 MAP kinases on apoptosis. Science. 1995;270:1326–1331. doi: 10.1126/science.270.5240.1326. [DOI] [PubMed] [Google Scholar]
- 51.Yamaguchi K, Kugimiya T, Miyazaki T. Substance P receptor in U373 MG human astrocytoma cells activates mitogen-activated protein kinases ERK1/2 through Src. Brain Tumor Pathol. 2005;22:1–8. doi: 10.1007/s10014-005-0178-1. [DOI] [PubMed] [Google Scholar]
- 52.Yu XY, Undem BJ, Spannhake EW. Protective effect of substance P on permeability of airway epithelial cells in culture. Am J Physiol. 1996;271:L889–895. doi: 10.1152/ajplung.1996.271.6.L889. [DOI] [PubMed] [Google Scholar]
- 53.Zawieja D. Lymphatic biology and the microcirculation: past, present and future. Microcirculation. 2005;12:141–150. doi: 10.1080/10739680590900003. [DOI] [PubMed] [Google Scholar]
- 54.Zhang Y, Berger A, Milne CD, Paige CJ. Tachykinins in the immune system. Curr Drug Targets. 2006;7:1011–1020. doi: 10.2174/138945006778019363. [DOI] [PubMed] [Google Scholar]
- 55.Zhao J, van Helden DF. ATP-induced endothelium-independent enhancement of lymphatic vasomotion in guinea-pig mesentery involves P2X and P2Y receptors. Br J Pharmacol. 2002;137:477–487. doi: 10.1038/sj.bjp.0704899. [DOI] [PMC free article] [PubMed] [Google Scholar]