Abstract
Introduction
Danon disease is a rare X-linked myopathy that is characterized clinically by a triad of cardiomyopathy, skeletal myopathy and cognitive impairment. The purpose of this investigation was to quantify functional performance, muscle weakness and quadriceps activation in individuals with Danon disease as compared to healthy individuals.
Methods
Four males (ages 10–34) and four females (ages 16–50) with the genetic markers of Danon disease were compared to eight healthy males (ages 22–34) and eight healthy females (ages 23–41) and previously reported norms.
Results
Affected males and females had decreased functional performance, significant generalized muscle weakness and decreased quadriceps strength and activation when compared with healthy individuals. Affected males had larger deficits in function, strength and activation when compared with affected females.
Discussion
The results indicate that although the presentation of Danon disease is variable and is typically only described in males, muscle weakness patterns exist in both affected males and females.
Keywords: Danon disease, strength, functional performance, activation, skeletal myopathy
Introduction
Danon disease is a rare genetic cardiac and skeletal myopathy caused by a deficiency of the lysosome-associated membrane protein-2 (LAMP-2).1–3 The disease shows X-linked inheritance, and the original descriptions in boys noted cognitive impairment in addition to skeletal muscle weakness and cardiomyopathy.1,4,2–3 Skeletal and cardiac muscle biopsies reveal small, autophagic vacuoles in muscle cells,1 and additional manifestations include ophthalmic disease, abnormal electromyography and elevation of serum creatine kinase.1–6 Danon disease affects males earlier and more severely than females. Males present with skeletal muscle weakness and hypertrophic cardiomyopathy that often progresses to cardiac transplantation or death by heart failure in the second or third decade.4–6,2 In females, cardiac problems predominate, and a dilated cardiomyopathy phenotype is most common. It presents later in life and leads to death or transplantation by age 50.4–5
Although skeletal myopathy is commonly mentioned in the clinical presentation, cardiac problems have been the major focus in the literature. They outweigh skeletal muscle defects, which have generally been described in limited detail. In case reports of individual families, mild proximal limb7,3 and neck muscle weakness in males has been mentioned, and a few individuals even exhibit obvious muscle wasting.1,4–5 Muscle fatigue has been reported but not quantified. Some females have muscular complaints, but in general the myopathy appears to be mild, as patients remain ambulatory.1 Thus, at this time, the skeletal myopathy presentation in males and females has not been systematically described.
In other neuromuscular disorders, such as Duchene muscular dystrophy and motor and sensory neuropathies, muscle strength and functional performance testing are utilized to quantify functional limitations and impairments. Voluntary strength measures offer a simple, reliable and acceptable method to demonstrate change or progression of a disease.8 Hand-held dynamometry is a reliable measure to objectively quantify strength of specific muscle groups.9–10 Similarly, timed functional tests provide objective data on functional performance and the level of disability in clinical populations.11 Due to its rarity, no such quantitative skeletal muscle data have been reported for Danon disease, and thus the aim of our study was to quantify functional performance, muscle weakness and quadriceps activation in individuals with Danon disease as compared with healthy individuals.
Materials and Methods
Study design and participants
This investigation was a cross-sectional study of patients who were genetically confirmed to have Danon disease. They were compared with a previously collected convenience sample of data from healthy adults at our facility in addition to data previously described in the literature.12–13 Four males (ages10–34) and four females (ages16–50) from three unrelated families with Danon disease were studied (Table 1a). Two males were half-siblings, related through their mother who had died of cardiac failure in her fourth decade. The diagnosis of Danon disease was confirmed by DNA sequencing of the LAMP-2 gene at the University of Colorado Cardiovascular Institute laboratory as previously described14 (Table 1b), studies of reduced or absent LAMP-2 protein in fibroblasts, and supporting medical records that included muscle biopsies in other family members. Two of the males (unrelated) had undergone cardiac transplantation. All muscle testing occurred under a standard protocol at the University of Colorado Denver between July 2008 and December 2009. The Colorado Multiple Institutional Review Board approved the study, and informed consent was obtained from all patients. Comparative data on 16 healthy individuals [eight healthy males (ages 22–34) and eight healthy females (ages 23–41)] were available from muscle strength and functional performance validation (Table 1b).
Table 1a.
Demographic information for patients with Danon disease and healthy subjects
| Group | Number of Participants | Age (years) | Body Mass Index (kg/m2) |
|---|---|---|---|
| Healthy | |||
| Males | 8 | 26.9 ± 4.9 | 24.1 ± 3.2 |
| Females | 8 | 29.5 ± 7.5 | 22.5 ± 2.4 |
| Danon | |||
| Affected Males | 4 | 24.8 ± 10.5 | 23.0 ± 4.8 |
| Affected Females | 4 | 32.0 ± 17.0 | 22.7 ± 1.2 |
Values represented as mean ± standard deviation (sd)
Table 1b.
Genetic testing and family structure of Danon patients
| Family Mutation* | Gender | Age (years) | Mutation Status | Cardiac Transplantation (age, years) |
|---|---|---|---|---|
| IVS1 +1G/A (intron 1) | ||||
| (son) | male | 10 | hemizygous | no |
| (mother) | female | 43 | heterozygous | no |
| Q83X (exon 3) | ||||
| (half-brother) | male | 25 | hemizygous | no |
| (half-brother) | male | 30 | hemizygous | yes (27) |
| c.delA1219 (exon 8) | ||||
| (daughter) | female | 16 | heterozygous | no |
| (daughter) | female | 18 | heterozygous | no |
| (son) | male | 34 | hemizygous | yes (23) |
| (mother) | female | 50 | heterozygous | no |
Mutation data based on NM_002294.1 genomic sequence for LAMP-2
Outcome measures
Functional performance was assessed using the timed-up-and-go (TUG) test, stair climbing test (SCT) and the number of completed sit-to-stands (STS) in 30 seconds. The TUG measures the time it takes a patient to rise from an arm chair (seat height of 46 cm), walk 3 m, turn and return to sitting in the same chair without physical assistance.15 This test has excellent inter-rater (ICC=0.99) and intra-rater reliability (ICC=0.99), as measured in a group of 60 functionally disabled older adults (mean age 80)16 and has also been validated in children with and without disabilities.17 The SCT measures the time it takes a patient to ascend and descend 12 stairs (17 cm high, 30 cm deep) without the use of the handrail; time UP and DOWN were recorded separately. The SCT places a high demand on the quadriceps and therefore measures a higher level of function while limiting the possibility of a ceiling effect compared to the TUG. This test has been shown to significantly correlate with the TUG.16 The STS requires subjects to perform as many sit to stands as possible from a standard chair (seat height of 46 cm) within a 30 second interval without the use of their upper extremities for assistance. Timing begins with the word “go,” the patient is instructed to stand up and sit down completely, and repetitions are counted each time the patient's bottom touches the chair. This test is used as an indicator of postural control, fall risk, lower-extremity strength, proprioception and disability.18 The ability to stand from a chair is a critical factor for independence in the community.18
Muscle strength was measured using a hand-held dynamometer (HHD)(Lafayette Manual Muscle Test System, Quebec Canada) in combination with isometric “make” and “break” tests in all individuals. The position of the dynamometer was held perpendicular to the limb segment toward which it was directed, and the plate of the dynamometer was placed in the same position on the tested limb each time. The tester manually stabilized the limb segment proximally and provided verbal and visual cues of the muscle contraction prior to the isometric test. Individuals were asked to maintain the maximum effort for 2–3 seconds, at which point the tester told them to stop. Strength for each muscle was tested until two maximal attempts were within 5% of each other, and the highest value, to the nearest tenth of a kg, was used in analysis. All measurements were obtained by one tester, and a description of each test position is provided in Supplementary Table. Strength data (kg) for each individual was grouped as generalized strength (average of all muscle groups), proximal strength (average of shoulder flexion, shoulder abduction, hip flexion, hip extension, hip abduction) and distal strength (average of wrist flexion, wrist extension, dorsiflexion, plantarflexion). Plantarflexion strength was assessed by the subject's ability to lift his/her heel off the ground up to 20 times while standing on one foot and balancing with light touch hand held assist for balance as necessary. Strength was normalized to BMI (kg/m2) for analysis.
Isometric quadriceps muscle strength was measured using an electromechanical dynamometer (Humac Norm; CSMI, Stoughton MA) as described in previous studies.13,19 Briefly, the patient was seated and stabilized with 85° hip flexion and 60° knee flexion. Up to three maximal voluntary isometric contractions (MVICs) were performed against the dynamometer's force transducer until two maximal attempts were within 5% of each other. The trial with the largest maximal volitional isometric force output was used for data analysis. Quadriceps muscle strength in individuals with Danon disease was compared to published norms (mean ± standard error of the mean (SEM)) that used almost identical methods13 (15 healthy males, age 22.9±1.4; 15 healthy females, age 22.3±2.0), because they were not available for the healthy adult controls tested in this study.
Quadriceps muscle activation testing was performed using a doublet interpolation test, as described previously.20–21 A value of 100% represents full voluntary muscle activation, and anything less than 100% represents incomplete motor unit recruitment or decreased motor unit discharge rates.20–22 Normalization of the torque from the superimposed doublet to the resting doublet allows for comparisons of quadriceps activation across individuals. We have not found sex-specific differences in muscle activation for healthy individuals (unpublished data), therefore, individuals with Danon disease were compared to previously reported values (mean±SEM) that included males and females (22 individuals; 11 males: mean age 24±2.2, 11 females: mean age 24.4±36).12 Due to stimulation intolerance, one of the affected females with Danon disease is not included in the activation data set (n=3). For both isometric strength and activation testing using an electromechanical dynamometer, reproducibility and consistency of testing methods is high compared to HHD testing.13,23–24,21,25 Therefore, comparisons to the existing literature were considered reasonable for the evaluation of muscle strength and activation in patients with Danon disease.
Data analysis
Mann-Whitney rank sum tests were used to evaluate differences in demographics between patients and healthy controls. Comparisons between patients with Danon disease and healthy individuals were sex-matched, because significant differences in functional performance and strength by sex were present. That is, affected males were compared to healthy males, and affected females were compared to healthy females. Mann-Whitney rank sum tests were used to evaluate differences between groups for functional performance and strength measures. Statistical significance was set at an alpha level of 0.05. All data are represented as mean ± standard error of the mean (SEM) unless otherwise indicated. All statistical analyses were performed with SPSS, Version 16.0 (SPSS Inc, Chicago, IL).
Results
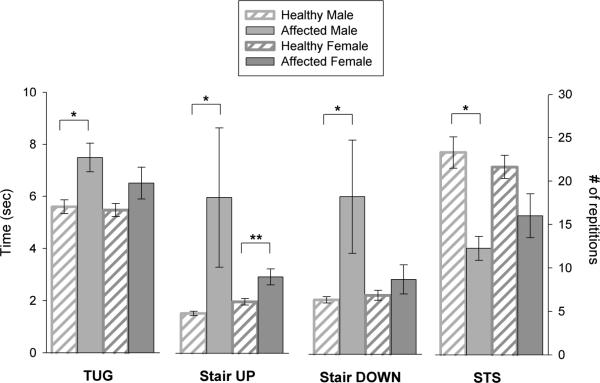
Patients with Danon disease were not statistically different from healthy individuals in terms of age (males: p=0.72; females: p=0.79) or BMI (males: p=0.68; females: p=0.83) (Table 1a). Affected males and females performed more poorly than the respective sex-matched, healthy individuals for functional performance measures (Figure 1). More specifically, affected males performed significantly worse than healthy males on all functional performance tests. When compared to healthy males, affected males were 33.9% slower (p=0.006) on the TUG, 392% slower (p=0.007) on stair climbing, 290% slower (p=0.007) stair descent, and performed 47.4% fewer (p=0.006) STS in 30 seconds (Table 2). Similarly, affected females performed more poorly than healthy females on all functional performance tests, yet they were only significantly different for SCT UP (48.3% slower; p=0.027). Although not significant, affected females performed 18.9% slower (p=0.124) on the TUG, 26.8% (p=0.234) slower while descending the stairs and 26% fewer (p=0.059) STS in 30 seconds when compared to healthy females.
Figure 1. Functional performance measures.
Affected males were compared to healthy males; affected females were compared to healthy females. Functional measures included the timed-up-and-go (TUG), ascent (SCT UP) and descent (SCT DOWN) of 12 stairs and the number of sit-to-stands (STS) completed in 30 seconds. Data (mean ± SEM) are represented in time (sec) for the TUG and SCT, and as number of repetitions for STS. Significant differences are indicated as *p≤0.01 and **p<0.05.
Table 2.
Average force (kg) of each muscle group across all individuals.
| Male | Female | |||||
|---|---|---|---|---|---|---|
| Healthy | Affected | % difference | Healthy | Affected | % difference | |
| Proximal | ||||||
| Shoulder Flexion | 0.50±0.02 | 0.19±0.03 | 62.0 | 0.35±0.03 | 0.23±0.03 | 34.3 |
| Shoulder Abduction | 0.49±0.02 | 0.19±0.03 | 61.2 | 0.35±0.03 | 0.22±0.03 | 37.1 |
| Hip Flexion | 1.10±0.04 | 0.54±0.12 | 50.9 | 0.99±0.06 | 0.73±0.13 | 26.3 |
| Hip Extension | 1.00±0.09 | 0.25±0.10 | 75.0 | 1.08±0.06 | 0.66±0.11 | 38.9 |
| Hip Abduction | 0.07±0.05 | 0.20±0.07 | 71.4 | 0.63±0.04 | 0.38±0.05 | 39.7 |
| Distal | ||||||
| Wrist Flexion | 0.74±0.05 | 0.28±0.04 | 62.2 | 0.53±0.03 | 0.23±0.02 | 56.6 |
| Wrist Extension | 0.84±0.03 | 0.24±0.05 | 71.4 | 0.64±0.04 | 0.38±0.01 | 40.6 |
| Plantarflexion | 0.84±0.03 | 0.17±0.07 | 79.8 | 0.90±0.03 | 0.81±0.06 | 10.0 |
| Dorsiflexion | 1.06±0.06 | 0.42±0.17 | 60.4 | 1.04±0.06 | 0.66±0.04 | 36.5 |
| Other | ||||||
| Neck Flexion | 0.59±0.02 | 0.26±0.02 | 55.9 | 0.39±0.04 | 0.33±0.04 | 15.4 |
| Neck Extension | 0.78±0.05 | 0.37±0.05 | 52.6 | 0.56±0.06 | 0.49±0.07 | 12.5 |
| Elbow Flexion | 1.15±0.05 | 0.44±0.05 | 61.7 | 0.90±0.05 | 0.55±0.05 | 38.9 |
| Elbow Extension | 0.93±0.05 | 0.35±0.03 | 62.4 | 0.57±0.03 | 0.46±0.08 | 19.3 |
| Knee Flexion | 1.10±0.09 | 0.56±0.06 | 49.1 | 1.00±0.05 | 0.68±0.13 | 32.0 |
| Knee Extension | 1.14±0.11 | 0.68±0.10 | 40.4 | 1.05±0.08 | 0.70±0.15 | 33.3 |
Muscles are grouped as proximal, distal and other. All values are represented as mean±SEM. Percent difference is calculated as [1-(Affected/Healthy)* 100] for both males and females.
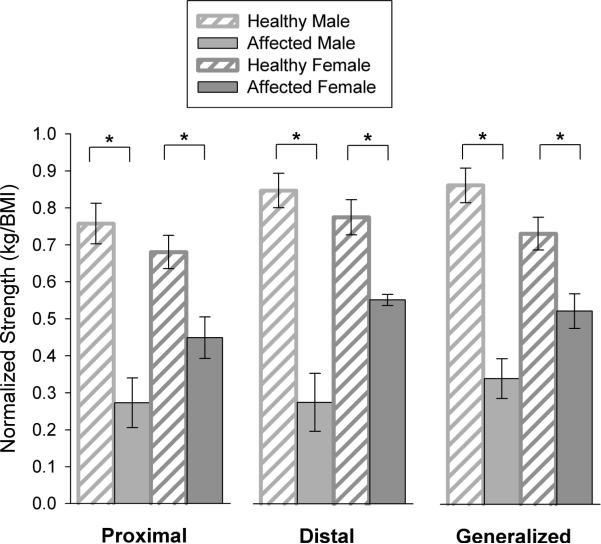
Strength, as assessed using the HHD, followed the same trend as the functional performance tests. Affected males and females demonstrated greater weakness than healthy, sex-matched individuals (Figure 2). When compared to healthy males, affected males demonstrated significantly greater weakness in proximal (p=0.007), distal (p=0.007) and generalized (p=0.007) muscle groups. Similarly, affected females demonstrated significantly weaker proximal (p=0.011), distal (p=0.007) and generalized (p=0.011) muscle strength compared to healthy females. While significant weakness was present in both affected males and females, the magnitude of weakness was greater in affected males than females. Overall, for generalized strength, males were 60.0±5% weaker than healthy males, while affected females were only 30.5±5% weaker than healthy females.
Figure 2. Normalized hand-held dynamometry (HHD) strength.
Affected males were compared to healthy males; affected females were compared to healthy females. Muscle strength (mean ± SEM) is represented as kg/BMI and is presented for the following: 1) average of shoulder flexion, shoulder abduction, hip flexion, hip extension and hip abduction (proximal); 2) average of wrist flexion, wrist extension, plantarflexion and dorsiflexion (distal); and 3) average across all muscle groups (generalized). Significant differences are indicated as *p<0.01.
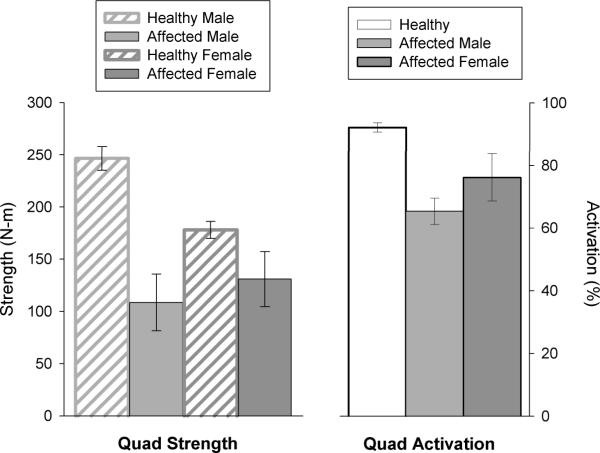
Quadriceps strength, measured using an electromechanical dynamometer in patients with Danon disease, compared to previously reported values13, showed a similar pattern of weakness as that found with HHD measurements (Figure 3). That is, average quadriceps strength in affected males was 108.5±27.1 N-m compared to 246.6±11.5 N-m previously reported for healthy males.13 Similarly, the average quadriceps strength for affected females (130.9±26.3 N-m) was less than previously reported values for healthy females (177.9±8.2 N-m).13 Overall, when compared to respective healthy individuals, affected males demonstrated a larger deficit in strength (56.0%) than females (26.4%).
Figure 3. Quadriceps strength and activation.
Affected males (n=4) and females (n=3) were compared to previously reported results for healthy individuals, and strength comparisons were available by gender.13 Activation values were compared to a healthy cohort of combined males and females, because it was not possible to separate these data by gender.12 Quadriceps strength is represented as N-m and quadriceps activation as a percentage, with 100% indicating complete voluntary activation. All data represented as mean ± SEM.
Both affected males and females demonstrated worse quadriceps activation than that previously reported for healthy males and females (Figure 3).12 Quadriceps activation in the affected males and females was 65.4±8.4% and 76.2±15.1% respectively compared to previous reports of 92.2±1.4% for healthy males and females.12 Although data were not available for quadriceps activation in healthy individuals by gender, muscle activation has not been shown to differ by gender; therefore, comparisons for affected males and females were made using a combined activation value for healthy men and women reported in the literature (Figure 3).12 Compared to previously reported quadriceps activation values for healthy individuals, affected males and females had 29.1% and 17.4% less activation, respectively.
Discussion
Danon disease is rare and therefore, the myopathy of Danon disease has been described in a limited, non-quantitative fashion. Our study is the first to provide quantitative muscle strength and functional performance data for the myopathic phenotype of Danon disease in males and females. The data indicated that both affected males and females performed less well on functional performance, strength and activation tests compared to healthy individuals. Affected males demonstrated a greater loss of function, strength and muscle activation than affected females. Distinct from other genetic neuromuscular diseases featuring either proximal or distal muscle weakness,26–28 the skeletal myopathy associated with Danon disease involves generalized muscle weakness. Functional performance on timed tests, such as the SCT, TUG, and STS, can identify individuals at risk for losing functional independence.29 The battery of tests chosen for this investigation has been shown to relate to strength,30 functional mobility,31 risk of falls31 and oxygen consumption (VO2 max)32. Affected males consistently demonstrated significantly poorer performance on all functional measures compared to healthy males. Fewer limitations were found in affected females, yet deficits in functional performance were still observed.
In animal models of Duchenne muscular dystrophy, eccentric muscle contractions result in increased damage to muscle tissue with progression of the disease.33–34 Therefore, we evaluated functional performance with eccentric muscle activity (SCT DOWN) separately from concentric activity (SCT UP). We found that both affected males and females had significantly increased difficulty with SCT UP, while only affected males performed significantly worse than healthy males on SCT DOWN. Although concentric muscle strength appears to be more affected than eccentric strength in this study, evaluation of additional individuals is necessary to definitively confirm this finding. While the performance of affected males was significantly reduced in comparison with healthy males on all functional tasks, the functional limitations noted in these patients were not severely disabling. Each affected male in this study was able to attend school and/or maintain a job but required more time to complete tasks than healthy males.
In our study, we found that people with Danon disease had more generalized muscle weakness in males and females rather than the prominent proximal findings that were described in males on the basis of non-quantitative evaluations.7,3 More specifically in affected males, ankle plantarflexors, hip abductors and hip extensors exhibited the greatest weakness when compared to the healthy males (Table 2). Weakness in these muscle groups may serve as an early indicator of disease progression. Muscle weakness of reduced magnitude in affected females followed a similar pattern to that of affected males. Additionally, one third of the affected males and females reported signs of excessive muscle fatigue such as “burning when walking up a flight of stairs.” Finally, while only some individuals reported muscle weakness, all affected individuals revealed some muscle weakness when compared to the respective healthy individuals.
Both affected males and females demonstrated less quadriceps strength when compared to healthy individuals. Quadriceps weakness has been shown to strongly relate to poorer functional performance measures such as decreased gait speed, imbalance, impaired stair-climbing ability and difficulty getting up from a seated position and may explain much of the decreased functional performance observed in our patients.35–42 Although we did not measure muscle atrophy, our results of decreased quadriceps muscle activation suggest that central activation deficits (i.e. deficits in the neural drive to the muscle) are present in patients with Danon disease to explain some of the muscle weakness present. Such central activation deficits suggest that rehabilitation strategies that optimize muscle activation, in conjunction with volitional muscle strengthening exercises, may be justified.
The principal limitation of this investigation was the small sample size of patients with Danon disease and the single time point used to assess phenotypes. A minor limitation of this study was the comparison of quadriceps strength and activation in patients with Danon disease to previously published results using similar methods. Yet, methods for quadriceps muscle strength and activation testing are reproducible measures,23–24,21 and therefore, such comparisons were justified to provide general comparisons between patients and healthy controls. We considered the possibility of concomitant cardiac failure confounding the muscle strength findings. None of our patients had clinical findings of heart failure, and our findings were present also in the two males who were clinically stable after cardiac transplantation. Thus, the data appear to be largely driven by skeletal muscle weakness and are not complicated by cardiac disease. Lastly, our study was cross-sectional in design, and no conclusions can be offered regarding the rate of progression of muscular defects in males and females with Danon disease.
Our study is the first to provide quantitative data to researchers and clinicians on the myopathy and functional performance phenotypes in Danon disease. These data illustrate that both affected males and females had decreased functional performance, decreased muscle strength, and decreased quadriceps muscle activation when compared to healthy individuals, and males exhibited larger deficits than females. Skeletal muscle disease in males is less severe than in Duchenne muscular dystrophy, another X-linked skeletal and cardiac myopathy; however, in our study all women had skeletal muscle abnormalities which were greater than the approximately 20% of females with Duchenne genetic defects.43 Future studies may investigate the progression of disease and the role of rehabilitation in mitigating the functional deficits noted in this patient population. As this group of patients frequently undergoes cardiac transplantation, cardiac rehabilitation teams should be alert for the presence of underlying proximal and distal muscle weakness in both males and females that may lead to a more protracted recovery in the post-transplantation period.
Supplementary Material
Acknowledgements
We wish to thank John Kvale, DPT and Cara Wells for their contributions to this investigation. We would also like to acknowledge the following funding sources: MO1-RR00069, General Clinical Research Centers Program, NCRR, NIH Muscular Dystrophy Association (MDA67944)
Abbreviations
- BMI
body mass index
- EMG
electromyography
- HHD
hand-held dynamometer
- Kg
kilograms
- LAMP-2
lysosome-associated membrane protein-2
- MVIC
maximal voluntary isometric contraction
- N-m
Newton Meters
- SCT
stair climbing test
- SCT DOWN
time to descend the stairs
- SCT UP
time to ascend the stairs
- Sd
standard deviation
- Sec
seconds
- SEM
standard error of the mean
- STS
sit-to-stands
- TUG
timed-up-and-go
- QUAD
quadriceps
- VO2 max
maximal oxygen consumption
References
- 1.Sugie K, Yamamoto A, Murayama K, Oh SJ, Takahashi M, Mora M, Riggs JE, Colomer J, Iturriaga C, Meloni A, Lamperti C, Saitoh S, Byrne E, DiMauro S, Nonaka I, Hirano M, Nishino I. Clinicopathological features of genetically confirmed Danon disease. Neurology. 2002;58(12):1773–1778. doi: 10.1212/wnl.58.12.1773. [DOI] [PubMed] [Google Scholar]
- 2.Yang Z, McMahon CJ, Smith LR, Bersola J, Adesina AM, Breinholt JP, Kearney DL, Dreyer WJ, Denfield SW, Price JF, Grenier M, Kertesz NJ, Clunie SK, Fernbach SD, Southern JF, Berger S, Towbin JA, Bowles KR, Bowles NE. Danon disease as an underrecognized cause of hypertrophic cardiomyopathy in children. Circulation. 2005;112(11):1612–1617. doi: 10.1161/CIRCULATIONAHA.105.546481. [DOI] [PubMed] [Google Scholar]
- 3.Prall FR, Drack A, Taylor M, Ku L, Olson JL, Gregory D, Mestroni L, Mandava N. Ophthalmic manifestations of Danon disease. Ophthalmology. 2006;113(6):1010–1013. doi: 10.1016/j.ophtha.2006.02.030. [DOI] [PubMed] [Google Scholar]
- 4.August DA. Anesthetic implications of Danon disease. Paediatr Anaesth. 2009;19(10):1026–1028. doi: 10.1111/j.1460-9592.2009.03125.x. [DOI] [PubMed] [Google Scholar]
- 5.Dworzak F, Casazza F, Mora M, De Maria R, Gronda E, Baroldi G, Rimoldi M, Morandi L, Cornelio F. Lysosomal glycogen storage with normal acid maltase: a familial study with successful heart transplant. Neuromuscul Disord. 1994;4(3):243–247. doi: 10.1016/0960-8966(94)90025-6. [DOI] [PubMed] [Google Scholar]
- 6.Maron BJ, Roberts WC, Arad M, Haas TS, Spirito P, Wright GB, Almquist AK, Baffa JM, Saul JP, Ho CY, Seidman J, Seidman CE. Clinical outcome and phenotypic expression in LAMP2 cardiomyopathy. JAMA. 2009;301(12):1253–1259. doi: 10.1001/jama.2009.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Morisawa Y, Fujieda M, Murakami N, Naruse K, Okada T, Morita H, Sawada K, Miyazaki J, Kurashige T, Nonaka I. Lysosomal glycogen storage disease with normal acid maltase with early fatal outcome. J Neurol Sci. 1998;160(2):175–179. doi: 10.1016/s0022-510x(98)00242-1. [DOI] [PubMed] [Google Scholar]
- 8.Wiles CM, Karni Y. The measurement of muscle strength in patients with peripheral neuromuscular disorders. J Neurol Neurosurg Psychiatry. 1983;46(11):1006–1013. doi: 10.1136/jnnp.46.11.1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bohannon RW. Test-retest reliability of hand-held dynamometry during a single session of strength assessment. Phys Ther. 1986;66(2):206–209. doi: 10.1093/ptj/66.2.206. [DOI] [PubMed] [Google Scholar]
- 10.Hayes K, Walton JR, Szomor ZL, Murrell GA. Reliability of 3 methods for assessing shoulder strength. J Shoulder Elbow Surg. 2002;11(1):33–39. doi: 10.1067/mse.2002.119852. [DOI] [PubMed] [Google Scholar]
- 11.Carter GT, Abresch RT, Fowler WM, Jr., Johnson ER, Kilmer DD, McDonald CM. Profiles of neuromuscular diseases. Spinal muscular atrophy. Am J Phys Med Rehabil. 1995;74(5 Suppl):S150–159. doi: 10.1097/00002060-199509001-00009. [DOI] [PubMed] [Google Scholar]
- 12.Krishnan C, Williams GN. Evoked tetanic torque and activation level explain strength differences by side. Eur J Appl Physiol. 2009;106(5):769–774. doi: 10.1007/s00421-009-1057-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Krishnan C, Williams GN. Variability in antagonist muscle activity and peak torque during isometric knee strength testing. Iowa Orthop J. 2009;29:149–158. [PMC free article] [PubMed] [Google Scholar]
- 14.Taylor MR, Ku L, Slavov D, Cavanaugh J, Boucek M, Zhu X, Graw S, Carniel E, Barnes C, Quan D, Prall R, Lovell MA, Mierau G, Ruegg P, Mandava N, Bristow MR, Towbin JA, Mestroni L. Danon disease presenting with dilated cardiomyopathy and a complex phenotype. J Hum Genet. 2007;52(10):830–835. doi: 10.1007/s10038-007-0184-8. [DOI] [PubMed] [Google Scholar]
- 15.Steffen TM, Hacker TA, Mollinger L. Age- and gender-related test performance in community-dwelling elderly people: Six-Minute Walk Test, Berg Balance Scale, Timed Up & Go Test, and gait speeds. Phys Ther. 2002;82(2):128–137. doi: 10.1093/ptj/82.2.128. [DOI] [PubMed] [Google Scholar]
- 16.Podsiadlo D, Richardson S. The timed “;Up & Go”: a test of basic functional mobility for frail elderly persons. J Am Geriatr Soc. 1991;39(2):142–148. doi: 10.1111/j.1532-5415.1991.tb01616.x. [DOI] [PubMed] [Google Scholar]
- 17.Williams EN, Carroll SG, Reddihough DS, Phillips BA, Galea MP. Investigation of the timed `up & go' test in children. Dev Med Child Neurol. 2005;47(8):518–524. doi: 10.1017/s0012162205001027. [DOI] [PubMed] [Google Scholar]
- 18.Whitney SL, Wrisley DM, Marchetti GF, Gee MA, Redfern MS, Furman JM. Clinical measurement of sit-to-stand performance in people with balance disorders: validity of data for the Five-Times-Sit-to-Stand Test. Phys Ther. 2005;85(10):1034–1045. [PubMed] [Google Scholar]
- 19.Mintken PE, Carpenter KJ, Eckhoff D, Kohrt WM, Stevens JE. Early neuromuscular electrical stimulation to optimize quadriceps muscle function following total knee arthroplasty: a case report. J Orthop Sports Phys Ther. 2007;37(7):364–371. doi: 10.2519/jospt.2007.2541. [DOI] [PubMed] [Google Scholar]
- 20.Behm D, Power K, Drinkwater E. Comparison of interpolation and central activation ratios as measures of muscle inactivation. Muscle Nerve. 2001;24(7):925–934. doi: 10.1002/mus.1090. [DOI] [PubMed] [Google Scholar]
- 21.Behm DG, St-Pierre DM, Perez D. Muscle inactivation: assessment of interpolated twitch technique. J Appl Physiol. 1996;81(5):2267–2273. doi: 10.1152/jappl.1996.81.5.2267. [DOI] [PubMed] [Google Scholar]
- 22.Stevens JE, Pathare NC, Tillman SM, Scarborough MT, Gibbs CP, Shah P, Jayaraman A, Walter GA, Vandenborne K. Relative contributions of muscle activation and muscle size to plantarflexor torque during rehabilitation after immobilization. J Orthop Res. 2006;24(8):1729–1736. doi: 10.1002/jor.20153. [DOI] [PubMed] [Google Scholar]
- 23.Colombo R, Mazzini L, Mora G, Parenzan R, Creola G, Pirali I, Minuco G. Measurement of isometric muscle strength: a reproducibility study of maximal voluntary contraction in normal subjects and amyotrophic lateral sclerosis patients. Med Eng Phys. 2000;22(3):167–174. doi: 10.1016/s1350-4533(00)00024-2. [DOI] [PubMed] [Google Scholar]
- 24.Snyder-Mackler L, De Luca PF, Williams PR, Eastlack ME, Bartolozzi AR., 3rd Reflex inhibition of the quadriceps femoris muscle after injury or reconstruction of the anterior cruciate ligament. J Bone Joint Surg Am. 1994;76(4):555–560. doi: 10.2106/00004623-199404000-00010. [DOI] [PubMed] [Google Scholar]
- 25.Martin HJ, Yule V, Syddall HE, Dennison EM, Cooper C, Aihie Sayer A. Is hand-held dynamometry useful for the measurement of quadriceps strength in older people? A comparison with the gold standard Bodex dynamometry. Gerontology. 2006;52(3):154–159. doi: 10.1159/000091824. [DOI] [PubMed] [Google Scholar]
- 26.Mathews KD, Moore SA. Limb-girdle muscular dystrophy. Curr Neurol Neurosci Rep. 2003;3(1):78–85. doi: 10.1007/s11910-003-0042-9. [DOI] [PubMed] [Google Scholar]
- 27.Chance PF, Pleasure D. Charcot-Marie-Tooth syndrome. Arch Neurol. 1993;50(11):1180–1184. doi: 10.1001/archneur.1993.00540110060006. [DOI] [PubMed] [Google Scholar]
- 28.Sussman M. Duchenne muscular dystrophy. J Am Acad Orthop Surg. 2002;10(2):138–151. doi: 10.5435/00124635-200203000-00009. [DOI] [PubMed] [Google Scholar]
- 29.Gill TM, Williams CS, Tinetti ME. Assessing risk for the onset of functional dependence among older adults: the role of physical performance. J Am Geriatr Soc. 1995;43(6):603–609. doi: 10.1111/j.1532-5415.1995.tb07192.x. [DOI] [PubMed] [Google Scholar]
- 30.Takai Y, Ohta M, Akagi R, Kanehisa H, Kawakami Y, Fukunaga T. Sit-to-stand test to evaluate knee extensor muscle size and strength in the elderly: a novel approach. J Physiol Anthropol. 2009;28(3):123–128. doi: 10.2114/jpa2.28.123. [DOI] [PubMed] [Google Scholar]
- 31.Shumway-Cook A, Brauer S, Woollacott M. Predicting the probability for falls in community-dwelling older adults using the Timed Up & Go Test. Phys Ther. 2000;80(9):896–903. [PubMed] [Google Scholar]
- 32.Cataneo DC, Cataneo AJ. Accuracy of the stair climbing test using maximal oxygen uptake as the gold standard. J Bras Pneumol. 2007;33(2):128–133. doi: 10.1590/s1806-37132007000200005. [DOI] [PubMed] [Google Scholar]
- 33.Petrof BJ, Shrager JB, Stedman HH, Kelly AM, Sweeney HL. Dystrophin protects the sarcolemma from stresses developed during muscle contraction. Proc Natl Acad Sci U S A. 1993;90(8):3710–3714. doi: 10.1073/pnas.90.8.3710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vilquin JT, Brussee V, Asselin I, Kinoshita I, Gingras M, Tremblay JP. Evidence of mdx mouse skeletal muscle fragility in vivo by eccentric running exercise. Muscle Nerve. 1998;21(5):567–576. doi: 10.1002/(sici)1097-4598(199805)21:5<567::aid-mus2>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 35.Rantanen T, Guralnik JM, Izmirlian G, Williamson JD, Simonsick EM, Ferrucci L, Fried LP. Association of muscle strength with maximum walking speed in disabled older women. Am J Phys Med Rehabil. 1998;77(4):299–305. doi: 10.1097/00002060-199807000-00008. [DOI] [PubMed] [Google Scholar]
- 36.Skelton DA, Greig CA, Davies JM, Young A. Strength, power and related functional ability of healthy people aged 65–89 years. Age Ageing. 1994;23(5):371–377. doi: 10.1093/ageing/23.5.371. [DOI] [PubMed] [Google Scholar]
- 37.Moreland JD, Richardson JA, Goldsmith CH, Clase CM. Muscle weakness and falls in older adults: a systematic review and meta-analysis. J Am Geriatr Soc. 2004;52(7):1121–1129. doi: 10.1111/j.1532-5415.2004.52310.x. [DOI] [PubMed] [Google Scholar]
- 38.Brown M, Sinacore DR, Host HH. The relationship of strength to function in the older adult. J Gerontol A Biol Sci Med Sci. 1995;50(Spec No):55–59. doi: 10.1093/gerona/50a.special_issue.55. [DOI] [PubMed] [Google Scholar]
- 39.Connelly DM, Vandervoort AA. Effects of detraining on knee extensor strength and functional mobility in a group of elderly women. J Orthop Sports Phys Ther. 1997;26(6):340–346. doi: 10.2519/jospt.1997.26.6.340. [DOI] [PubMed] [Google Scholar]
- 40.Moxley Scarborough D, Krebs DE, Harris BA. Quadriceps muscle strength and dynamic stability in elderly persons. Gait Posture. 1999;10(1):10–20. doi: 10.1016/s0966-6362(99)00018-1. [DOI] [PubMed] [Google Scholar]
- 41.Mizner RL, Petterson SC, Snyder-Mackler L. Quadriceps strength and the time course of functional recovery after total knee arthroplasty. J Orthop Sports Phys Ther. 2005;35(7):424–436. doi: 10.2519/jospt.2005.35.7.424. [DOI] [PubMed] [Google Scholar]
- 42.Brown M, Sinacore DR, Host HH. The relationship of strength to function in the older adult. J Gerontol A Biol Sci Med Sci. 1995;50(Spec No):55–59. doi: 10.1093/gerona/50a.special_issue.55. [DOI] [PubMed] [Google Scholar]
- 43.Hoogerwaard EM, Bakker E, Ippel PF, Oosterwijk JC, Majoor-Krakauer DF, Leschot NJ, Van Essen AJ, Brunner HG, van der Wouw PA, Wilde AA, de Visser M. Signs and symptoms of Duchenne muscular dystrophy and Becker muscular dystrophy among carriers in The Netherlands: a cohort study. Lancet. 1999;353(9170):2116–2119. doi: 10.1016/s0140-6736(98)10028-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.