Abstract
Background
Diabetes and congestive heart failure (CHF) are common comorbidities in hospitalized patients but the relationship between glycemic control, glycemic variability, and mortality in patients with both conditions is unclear.
Methods
We used administrative data to retrospectively identify patients with a diagnosis of CHF who underwent frequent glucose assessments. Time-weighted mean glucose (TWMG) was compared to other measures of glycemic control and a time-weighted measure of glycemic variability, the glycemic lability index (GLI). The outcome was hospital mortality.
Results
748 patients were included in the final analysis. TWMG was higher than unadjusted mean glucose (137+/−44.7 mg/dL vs. 167 +/−54.9, p<0.001), due in part to shorter sampling intervals at higher glucose levels. Hypoglycemia, defined as a glucose level <70 mg/dl, occurred during 6.3% of patient-days in survivors and 8.4% of patient-days among nonsurvivors (p=0.05). TWMG was similar (128 +/− 33.1 mg/dl vs. 138 +/− 45.1 mg/dl) in nonsurvivors vs. survivors, p=0.19). However, relatively few glucose readings were significantly elevated. Median GLI was higher in nonsurvivors compared to survivors (18.1 vs. 6.82, p=0.0003). Increasing GLI (OR 1.32, 95% CI 1.05-1.65), and hypoglycemia (OR 2.21, 95% CI 1.07-4.65), were independently associated with higher mortality in logistic regression analysis. Respiratory failure was associated with mortality, but not standard deviation of glucose.
Conclusions
Future studies analyzing glycemic control should control for variable sampling intervals. In this analysis, GLI was independently associated with increased mortality, independent of hypoglycemia. Prospective studies are needed to evaluate these findings.
INTRODUCTION
Congestive heart failure (CHF) and diabetes are common comorbidities in the hospital, with over 40% of patients with CHF having diabetes as a discharge diagnosis [1]. Several studies have shown that diabetes is an independent predictor of mortality in patients with CHF, but little is known about the relative importance of various metrics of glycemic control and CHF outcomes in the hospital [2,3]. Several investigators have shown that admission glucose in patients with CHF is independently associated with mortality [1,4,5], although this finding is not universal [6]. However, admission glucose does not provide a basis for intervention and more comprehensive assessments of glucose exposure may be useful for characterizing any association between mortality and glycemic control.
Previous studies have analyzed glycemic control using admission [1,4,5] (in CHF patients) or mean morning [7,8] (in the intensive care unit, ICU) glucose and different definitions of hypoglycemia. Of recent interest is the role of glycemic variability in outcomes. Rapid glucose swings are associated with more profound endothelial toxicity than are tonic glucose elevations in vitro [9]. In patients with diabetes, they are associated with higher levels of oxidative stress [10] and ischemic electrocardiogram changes [11]. Glycemic variability has also been independently associated with ICU mortality [12-16], but little is known about its role in hospitalized CHF patients and no mortality data have been reported outside of the ICU.
In the present study, we used a computerized data collection tool to analyze and compare measures of glycemic control in patients hospitalized with CHF. Furthermore, we investigated whether measures of glycemic control or glycemic variability are related to hospital mortality.
MATERIALS AND METHODS
Hospital admissions between January 1, 2005 and December 31, 2006 were searched using the Ohio State University’s Information Warehouse, a computerized administrative data analysis tool that validates, cleanses, and de-identifies patient information incorporated from multiple electronic sources. Patients with a primary discharge diagnosis of congestive heart failure (defined as a billing code of 428.0×) were identified. Patients having a hospital length of stay of less than 60 days who had mortality data and at least 2 point-of care-glucose values in a given day were included in the final analysis. This criterion would ensure that a minimum amount of data would be available for calculation of glycemic variability. Data collection and analysis from the Information Warehouse was approved by the Ohio State University’s Institutional Review Board.
The point-of-care glucose monitor employed at the study institution is the Accucheck Inform (Roche). In order to maintain homogeneity in glucose methodology, serum glucose values were excluded [17].
Glycemic Variables
The primary glucose variables of interest were total glycemic exposure, calculated as the time-weighted mean glucose (TWMG) using the trapezoidal rule divided by the total time in hours, and glucose lability index (GLI), also corrected for time. Time-weighting was performed in order to correct for nonuniform glucose sampling intervals and has been described previously [18].
GLI is a measure of glucose variability, determined by the sum of the square of the difference between successive glucose measurements divided by the difference in time between measurements [19].
Secondary glycemic variables included 3-day TWMG, unadjusted mean glucose, mean morning glucose (defined as that occurring between 4-8AM), standard deviation (SD), coefficient of variation (CV), and hypoglycemia. Hypoglycemia was defined as the percentage of patients with any blood glucose (BG) <70 mg/dL and as a percentage of total days on which a hypoglycemic event occurred. Hemoglobin A1c (HbA1c) was available for a subset of patients.
Clinical Variables
The primary clinical outcome was hospital mortality. An assessment of left ventricular ejection fraction was available for a subset of patients. Diabetes was defined for the purpose of the study as any billing code for diabetes or a HbA1c >6.5%.
Our institution implemented hospital-wide inpatient glycemic control initiatives, beginning in January 2006. These included updated clinical practice guidelines, implementation of computerized diabetes order sets and streamlining of computerized diabetes related orders, administration of insulin based on carbohydrate counting techniques, and hospital-wide education initiatives. A universal (ICU and non-ICU beds), nursing-run insulin drip protocol (target glucose 100-150 mg/dl) was instituted based upon the Yale protocol [20]. Intravenous insulin is ordered at the discretion of the provider, but is generally recommended in hyperglycemic critically ill patients (definition varies by unit) and in other patients with 3 consecutive glucose values exceeding 200 mg/dl despite intervention. Glucose monitoring is recommended hourly in patients who are receiving an insulin infusion and with meals and bedtime otherwise. As it is possible that this program had an effect on glycemic control in hospitalized heart failure patients, we further evaluated results by year of admission.
Analysis
Continuous variables are reported as mean +/− standard deviation, with the exception of GLI, which was reported as median and interquartile range due to nonnormal distribution. Dichotomous variables are reported as sums and percentages. Continuous variables were compared with a t-test (except length of stay, GLI, SD, and CV, for which Wilcoxon rank-sum was employed). Fisher’s exact test was used for comparing dichotomous variables and Pearson Chi-square was used for multi-category comparisons. Multivariable logistic regression was performed with mortality as the dependent variable and single predictors (p-value <0.2) as dependent variables. Single predictors included log GLI, log TWMG, log-SD, hypoglycemia (modeled as a binary variable), age, gender, race, year of admission, and hospital billing codes for acute respiratory failure (billing codes 518.8, 799.02), renal disease (billing codes 250.4, 403.×, 404.×, 584.×, 585.×, V45.1), atrial arrhythmia (427.×) and hypertension (403.×). Acute respiratory failure was chosen for analysis since it was found to be highly significantly associated with mortality as a single predictor (p<0.0001, data not shown) and renal failure, atrial arrhythmias and hypertension were included as the most commonly coded comorbidities. TWMG, GLI, CV, and SD were log-transformed for correlations and for logistic regression analyses in order to provide better dispersion of the data and improved prediction. Analyses were performed using JMP 6.0 software.
RESULTS
A summary of demographics and clinical information is detailed in Table 1. 1144 patients with a primary discharge diagnosis of CHF were identified. Of these, two patients with a length of stay >60 days and 4 patients without mortality data were excluded. Of the remainder, 748 patients met the glucose testing frequency criterion and 9,236 glucose measurements were included in the final analysis. Eighty-three (11%) of patients had a billing code for diabetes but when HbA1c >6.5% was added as a criterion for identification, the number of patients with diabetes increased to 270 (36%). However, HbA1c was only available in 334 patients (45%). Overall, patients with diabetes were younger and less likely to have a diagnosis code for respiratory failure.
Table 1.
Summary of Patient Characteristics and Mortality
| Total (748) |
Survivors (N=713) |
Nonsurvivors (N=35) |
P-value | DM (N=270) |
No DM (N=478) |
P-value | |
|---|---|---|---|---|---|---|---|
| Race | |||||||
| African American | 295 (39.4%) | 281 (39%) | 14 (40%) | >0.99 | 112 (42.1%) | 183 (39.2%) | 0.48 |
| White American | 438 (58.6%) | 418 (59%) | 20 (57%) | >0.99 | 154 (57.9%) | 284 60.8%) | |
| Male | 426 (57.0%) | 408 (57%) | 18 (51%) | 0.49 | 152 (56.3%) | 274 (57.4%) | 0.82 |
| Age | 65.8 +/−13.8 | 65.5 +/− 13.6 | 70.0 +/− 16.3 | 0.06 | 63 +/− 13 | 67 +/− 14 | <0.0001 |
| Ejection Fraction N=336 |
31.7 +/− 17.3 | 31.8+/− 17.2 | 30.6 +/− 18.7 | 0.77 | 32 +/− 18 | 31 +/− 17 | 0.70 |
| Admission in 2005 | 365 (49%) | 348 (95%) | 17 (4.7%) | NA | 228 (47.7%) | 137 (50.7%) | 0.45 |
| Respiratory failure | 35 (4.7%) | 23 (3.2%) | 12 (34.3%) | <0.0001 | 4 (1.5%) | 31 (6.5%) | 0.002 |
| Renal disease | 197 (26.3%) | 190 (26.7%) | 7 (20.0%) | 0.44 | 77 (28.5%) | 120 (25.1%) | 0.34 |
| Atrial arrhythmia | 134 (17.9%) | 125 (17.5%) | 9 (25.7%) | 0.26 | 48 (17.8%) | 86 (18.0%) | >0.99 |
| Hypertension | 109 (14.6%) | 106 (14.9%) | 3 (8.57%) | 0.46 | 43 (15.9%) | 66 (13.8%) | 0.45 |
| Valvular disorder | 75 (10.0%) | 69 (9.68%) | 6 (17.1%) | 0.15 | 25 (9.3%) | 50 (10.5%) | 0.70 |
Values are mean +/− standard deviation or number (percentage), NA=not applicable, DM=diabetes mellitus.
Comparison of Measures of Glycemia
Measures of glycemic control are compared in Table 2; 4.1% of glucose measures were <70 mg/dl, 85% were 71-200 mg/dl, and 10.8% were >200 mg/dl.
Table 2.
Glycemic Parameters
| Total (748) |
Survivor (713) |
Nonsurvivor (35) |
P-value | Diabetes (270) |
No Diabetes (478) |
P-value | 2005 (365) |
2006 (383) |
P-value | |
|---|---|---|---|---|---|---|---|---|---|---|
| TWMG (mg/dl) | 137 (44.7) | 138 (45.1) | 128 (33.1) | 0.19 | 151.5 (53.1) | 129.3 (36.8) | <0.0001 | 142 (47) | 133 (42) | 0.004 |
| 3-day TWMG (mg/dl) | 142.0 (51.3) | 142 (51.5) | 130 (44.6) | 0.15 | 157.0 (60.0) | 133.2 (43.3) | <0.0001 | 145.9 (53.9) | 137.9 (48.3) | 0.03 |
| *GLI (mg/dl)2/hr*day−1 | 7.15 (2.39-19.1) |
6.82 (2.29-18.4) |
18.1 (8.76-44.2) |
0.0003 | 8.6 (3.25-23.1) |
6.0 (1.89-17.6) |
0.0009 | 34.4 (101) | 29.1 (105) | 0.48 |
| *SD | 3.88 (3.47-4.22) |
3.89 (3.49-4.24) |
3.76 (3.35-4.05) |
0.11 | 4.06 (3.73-4.36) |
3.78 (3.37-4.13) |
<0.0001 | 3.93 (3.56-4.29) |
3.83 (3.40-4.19) |
0.013 |
| *CV | 0.30 (0.23-0.39) |
0.30 (0.23-0.39) |
0.29 (0.21-0.36) |
0.61 | 0.34 (0.26-0.41) |
0.29 (0.21-0.38) |
<0.0001 | 0.31 (0.24-0.40) |
0.30 (0.22-0.38) |
0.18 |
| HbA1c (%, N=334) | 7.58 (1.96) | 7.61 (1.97) | 6.47 (1.03) | 0.13 | 8.40 (1.92) | 5.97 (0.48) | * | 7.71 (1.95) | 7.48 (1.97) | 0.29 |
| Hypoglycemia (% of patient-days) |
6.5 | 6.3 | 8.4 | 0.05 | 8.7 | 5.3 | 0.008 | 6.0 | 7.0 | 0.36 |
| Hypoglycemia (N, %) | 258 (34.2%) | 239 (33.5%) | 19 (54.3%) | 0.012 | 100 (37.0%) | 158 (33.1%) | 0. 30 | 124 (34.0%) | 134 (35.0%) | 0.82 |
| >70 mg/dl | 490 (65.5%) | 474 (66.5%) | 16 (45.7%) | |||||||
| 60-69 mg/dl | 90 (12.0%) | 82 (11.5%) | 8 (22.9%) | 0.07# | ||||||
| 40-59 mg/dl | 124 (16.6%) | 116 (16.3%) | 8 (22.9%) | |||||||
| <40 mg/dl | 44 (5.9%) | 41 (5.8%) | 3 (8.6%) |
Median (IQR), and analyzed using Wilcoxon rank sum. All other continuous variables reported as mean (SD) and analyzed with student’s t-test.
Pearson chi square test for overall association across 2 groups × 4 categories. TWMG=time-weighted mean glucose, GLI=time-adjusted glycemic lability index. Hypoglycemia is defined as any glucose <70 mg/dl except as indicated.
Mean Glucose
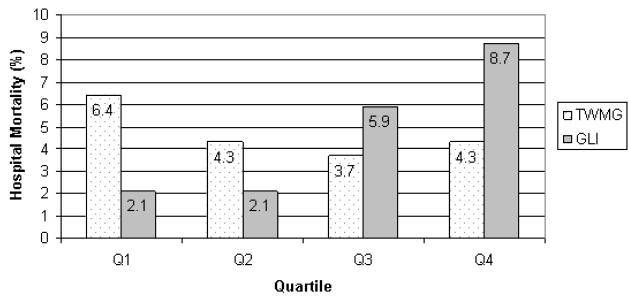
TWMG was 137 +/−44.7 mg/dL, and was significantly lower than unadjusted mean glucose (167 +/− 54.9), but higher than mean AM BG (101 +/− 33.3 mg/dl, p<0.0001 for both comparisons, adjusted for multiple comparisons using Tukey-Kramer method). As the glucose level increased, the time to subsequent glucose measurement was slightly shorter (r −0.10, p<0.0001). Patients with study-defined diabetes had significantly higher TWMG than did those without diabetes. There was no difference in TWMG (calculated over the entire hospitalization or at 3 days) or HbA1c between survivors and nonsurvivors. There was no association between mortality and quartile of TWMG (p=0.64) although there was a suggestion of a U-shaped relationship (Figure 1). TWMG decreased significantly from 2005 to 2006 but there was no change in mortality or hospital length of stay.
Figure 1.
Hypoglycemia
Patients with study-defined diabetes had a greater percentage of patient-days with hypoglycemia than did those without diabetes, but the percentage of patients with any hypoglycemia was unaffected by diabetes status. The percentage of patients with any hypoglycemia was significantly greater in nonsurvivors compared to survivors, and there was a trend for a difference in the percentage of patient days with hypoglycemia. There was no difference in hypoglycemia over time.
Glycemic Variability
Among patients with study-defined diabetes, GLI, SD, and CV were significantly higher than for those patients without diabetes (table 2, p<0.001 for all comparisons). The median GLI was greater in nonsurvivors compared to survivors, but the standard deviation and coefficient of variability were not significantly different. Increasing quartile of GLI was associated with a stepwise increase in mortality from quartiles 2 through 4, while quartiles 1 and 2 were similar (p=0.005 for overall association using Chi square test, figure 1). Log-transformed values of TWMG and GLI were correlated overall (r 0.30, p<0.0001). In patients without hypoglycemia, a simple logistic regression model demonstrated that GLI was associated with a trend for higher mortality (p=0.05), whereas in patients with hypoglycemia, the association was statistically significant (p=0.03). There was a significant decrease in SD, but not GLI or CV, from 2005 to 2006.
Outcomes
Univariable Outcomes
In univariate analysis, study-defined diabetes was associated with shorter length of stay, lower rates of respiratory failure diagnoses, and lower mortality (Table 3). Because there were only 3 deaths among patients with study-defined diabetes, statistical analyses determining whether relationships between glycemic parameters and mortality differed in patients with or without diabetes were not performed. Hospital length of stay did not change from 2005 to 2006.
Table 3.
Outcomes Data Stratified by Diabetes
| Total | DM | No DM | P-value | |
|---|---|---|---|---|
| Hospital length of stay * | 6.0 (3.0-10) | 5.0 (3.0-8.0) | 6.0 (3.8-11) | <0.0001 |
| Respiratory Failure | 35 (4.7%) | 4 (1.5%) | 31 (6.5%) | 0.0017 |
| Mortality | 35 (4.7%) | 3 (1.1%) | 32 (6.7%) | 0.0002 |
Values are median (IQR, analyzed using Wilcoxon rank sum) or or number (percentage).
Multivariable Models
Multivariable logistic regression analysis revealed that log-transformed GLI and respiratory failure were independently associated with mortality in the final model (Table 4). Adding diabetes (defined as the presence of a diagnosis code or HbA1c >6.5%) did not substantially change the model. Neither SD nor CV were significant when substituted for GLI in the final model. A model containing hypoglycemia stratified by severity demonstrated that log transformed GLI remained an independent predictor (p=0.008), and mild (lowest measured glucose 60-69 mg/dl, p=0.015), but not moderate (lowest measured glucose 40-59 mg/dl, p=0.48) or severe (lowest glucose < 40 mg/dl, p=0.46) hypoglycemia was associated with increased mortality. When GLI was analyzed by quartile, hypoglycemia was no longer significant and only the highest quartile of GLI was significantly associated with mortality (p=0.04, data not shown). Finally, when patients with any (<70 mg/dl, N=258) or moderate (40-59 mg/dl, N=124) hypoglycemia were excluded from the model, log GLI was no longer significantly associated with mortality. When patients with only severe hypoglycemia (<40 mg/dl, N=44) were excluded, GLI remained significant (p=0.03), but hypoglycemia was no longer significant (p=0.07, data not shown). There was no significant interaction between GLI and hypoglycemia and age was not significant in any of the models.
Table 4.
Logistic Regression Model--Mortality
| OR (95% CI) | P-value | |
|---|---|---|
| Model Containing GLI | ||
| Respiratory Failure | 13.7 (5.82-31.3) | <0.0001 |
| Hypoglycemia | 2.21 (1.07-4.65) | 0.03 |
| GLI* | 1.32 (1.05-1.65) | 0.02 |
| Model with Hypoglycemia Severity | ||
| Respiratory Failure | 14.40 (6.22-33.3) | <0.0001 |
| GLI* | 1.36 (1.08-1.70) | 0.008 |
| BG 60-70 mg/dl | 3.19 (1.25-8.11) | 0.015 |
| BG 40-69 mg/dl | 1.42 (0.55-3.67) | 0.48 |
| BG <40 mg/dl | 1.67 (0.43-6.45) | 0.46 |
| Model Adding Diabetes | ||
| Respiratory Failure | 10.5 (4.48-24.7) | <0.0001 |
| Hypoglycemia | 2.48 (1.18-5.20) | 0.016 |
| GLI* | 1.37 (1.09-1.71) | 0.006 |
| Diabetes | 0.16 (0.047-0.55) | 0.004 |
| Model Containing SD | ||
| Respiratory Failure | 13.7 (5.93-31.7) | <0.0001 |
| Hypoglycemia | 2.95 (1.35-6.45) | 0.007 |
| SD* | 0.67 (0.36-1.22) | 0.19 |
| Model Containing CV | ||
| Respiratory Failure | 14.4 (6.25-33.1) | <0.0001 |
| Hypoglycemia | 2.83 (1.23-6.52) | 0.015 |
| CV* | 0.75 (0.55-2.94) | 0.49 |
Log-transformed values; TWMG=time weighted mean glucose, AA=African American, GLI=time-adjusted glycemic lability index. Variables that were not significant included age, gender, race, year of admission, and diagnosis codes for renal disease, atrial arrhythmia, hypertension, valvular disorder.
DISCUSSION
The salient finding of this investigation is that in a cohort of patients admitted to the hospital with congestive heart failure, glycemic variability, as reflected by GLI, had an independent effect on the risk of mortality whereas measures of mean glucose level during hospitalization did not. This association was confirmed in a sample of patients that by other glycemic measures may be regarded as relatively well controlled. Furthermore, since SD and CV were not significantly associated with mortality, the association appears to be dependent upon the rapidity of glucose swings. The calculation of GLI takes into account the rate of change in glucose, whereas SD and CV do not. To our knowledge, this is the first study to identify such an association in patients with CHF.
Compared to other methods for calculating glycemic variability, GLI was chosen for this study due to its ease of use, ability to correct for heterogeneity in timing of glucose measurements, and previous superior association with hospital outcomes compared to other methods of capturing glucose variability [21]. It is unlikely that this study was able to capture true glycemic variability, for which continuous or near-continuous glucose monitoring is desirable. Therefore, we cannot exclude the possibility that glycemic variability is predicting unrecognized hypoglycemia, which itself was independently associated with mortality, even with mild events. The association was not significant when patients without documented hypoglycemia were excluded, but the greatly reduced sample size of this subgroup limits our ability to detect such a relationship. We did not find a significant interaction between hypoglycemia and GLI in logistic regression analysis. Furthermore, the association between glycemic variability and mortality is consistent with mounting ICU data [12-16]. It is worth noting that log-transformed values were not used in comparison studies and that other studies found an association between SD and mortality (12,14-16), whereas we did not. The mechanism for the association is not fully understood, but preliminary studies suggest a role of glycemic fluctuations in promoting endothelial toxicity [9], oxidative stress [10], and ischemia [11]. The importance of this finding lies in the potential application of interventions that target glycemic variability. Clinical trials are needed to evaluate methods of reducing glycemic variability in the inpatient setting. However, in the outpatient setting, a physiologic insulin regimen that incorporates both basal and prandial insulin components is associated with fewer glycemic fluctuations [22]. In contrast, sliding scale monotherapy, which is reactive, rather than anticipatory in nature, is considered nonphysiologic [23].
The inclusion of only patients with bedside glucose monitoring allowed for more robust calculation of glycemic variability than is possible with once daily plasma glucose measurements in the typical hospitalized patient without diabetes. Furthermore, inclusion of such patients is more relevant since it is more likely to identify patients who were receiving therapies that could modify measures of glycemic control. In a recent report, spontaneous, but not iatrogenic, hypoglycemia was associated with adverse outcomes in hospitalized patients with acute myocardial infarction [24]. Unfortunately, we do not have information related to treatment of hyperglycemia. An ICU study reported that glycemic variability, measured using coefficient of variation (which is not time-dependent), is associated with mortality in patients without known diabetes, but not in patients with diabetes [25]. Future studies should address whether the association between glycemic variability and mortality is also modified by treatment modality.
A curious finding was that patients with study-defined diabetes (HbA1c >6.5% or a billing code for diabetes) had a lower risk of mortality despite greater measures of glycemic variability. Absence of an effect of diabetes status has been reported previously in the ICU literature [26]. However, it may also represent underreporting (underbilling for diabetes) in sicker patients rather than a true relationship. Diagnosis codes for diabetes are likely to be underrepresented in the inpatient setting due to competing priorities in the billing process [27]. Although the number of patients with a second or third diagnosis code for diabetes was low, the actual number in this sample is likely to be much higher, because only patients with fingerstick glucose values were included in the analysis. In addition, we were also unable to determine whether the relationship between TWMG, GLI, or hypoglycemia was modified by diabetes status since there were only 3 deaths in patients with confirmed diabetes. In contrast, inaccuracies of using CHF as a diagnosis code are primarily due to underestimation, not overestimation of the true prevalence [28]. Thus, although some patients with CHF may have been missed, the selection criteria employed were likely to identify only patients who are hospitalized with heart failure.
The data do not suggest that hyperglycemia is associated with mortality, but few patients were significantly hyperglycemic, precluding definitive conclusions regarding any such link. As with previous studies, our data suggest a U-shaped relationship between mean glucose and mortality [29,30]. Unlike previous studies [31,32], we did not find any difference in HbA1C in patients who died versus those who did not die, but HbA1c was only available in a subset of patients.
Previous studies have analyzed hospital glycemic control on the basis of admission [1,4,5] or mean morning [7,8] glucose. However, these may not adequately capture true glycemic exposure. In the time-weighted assessment, glucose measures that are obtained close together carry less overall influence on the mean than similar values that are farther apart in time. Since hyperglycemic values were more frequent than hypoglycemic values, the net effect is the impression of higher glucose exposure in unadjusted mean glucose analysis compared to TWMG. Others have reported glucose by time-weighting [18]. The current data support this approach for analyzing mean glucose and further support its use for capturing glycemic variability. The observation carries ramifications for choosing glycemic targets for various patient populations and for comparing studies, since past guidelines [33] and design of multi-center prospective trials [18,34] have been based upon studies that report morning glucose values.
The major limitations of this study relate to its retrospective nature and are noted in the discussion above. Furthermore, a heterogeneous population with various etiology of CHF was included. We are unable to determine ICU status because the majority of patients with CHF as a primary diagnosis are admitted to the study institution’s heart hospital, where beds are interchangeable between ICU and non-ICU status. In particular, we were unable to report requirements for mechanical ventilation, a more objective variable than the diagnosis code for respiratory failure that was included in the mortality models. Thus, the results may not apply to specific patients with CHF. Strengths of this study include the largest patient sample to date examining in-hospital glycemic control of hospitalized CHF patients, inclusion of all evaluable bedside glucose values, and the time-weighted analyses for both mean glucose and glucose variability, which account for heterogeneity in glucose sampling intervals.
The data show that time-weighting of glucose values for studies assessing glycemic control is indicated. Furthermore, increased glycemic variability is associated with higher mortality, independent of hypoglycemia. The data should support the need for prospective studies to determine if patients with CHF would benefit from interventions that improved glycemic variability. In the meantime, efforts to control hyperglycemia should minimize fluctuations in glucose among patients with CHF.
Acknowledgements
The authors wish to thank the Ohio State University Information Warehouse, the Ohio State University Clinical Research Center (supported by Award Number UL1RR025755 from the National Center for Research Resources) for assistance with data collection and analysis. This research was also supported by NIH grant number 1K23DK080891-02.
List of Abbreviations
- CHF
congestive heart failure
- GLI
Glycemic lability index
- TWMG
Time-weighted mean glucose
- SD
standard deviation
- CV
coefficient of variation
- BG
blood glucose
- ICU
intensive care unit
- HbA1c
hemoglobin A1c
- EF
Ejection fraction
Footnotes
There are no relevant conflicts of interest to report. Parts of the data were presented in poster form at the 68th American Diabetes Association Scientific Sessions in 2008.
REFERENCES
- 1.Adams KF, Jr, Fonarow GC, Emerman CL, et al. ADHERE Scientific Advisory Committee and Investigators Characteristics and outcomes of patients hospitalized for heart failure in the United States: rationale, design, and preliminary observations from the first 100,000 cases in the Acute Decompensated Heart Failure National Registry (ADHERE) Am Heart J. 2005;149:209–16. doi: 10.1016/j.ahj.2004.08.005. [DOI] [PubMed] [Google Scholar]
- 2.Dries DL, Sweitzer NK, Drazner MH, Stevenson LW, Gersh BJ. Prognostic impact of diabetes mellitus in patients with heart failure according to the etiology of left ventricular systolic dysfunction. J Am Coll Cardiol. 2001;38:421–80. doi: 10.1016/s0735-1097(01)01408-5. [DOI] [PubMed] [Google Scholar]
- 3.Gustafsson I, Brendorp B, Seibaek M, Køber L, et al. Danish Investigators of Arrhythmia and Mortality on Dofetilde Study Group Influence of diabetes and diabetes-gender interaction on the risk of death in patients hospitalized with congestive heart failure. J Am Coll Cardiol. 2004;43:771–7. doi: 10.1016/j.jacc.2003.11.024. [DOI] [PubMed] [Google Scholar]
- 4.Gorelik O, Almoznino-Sarafian D, Alon I, et al. Heart failure in diabetes mellitus: clinical features and prognostic implications. Cardiology. 2005;103:161–6. doi: 10.1159/000084587. [DOI] [PubMed] [Google Scholar]
- 5.Barsheshet A, Garty M, Grossman E, et al. Admission blood glucose level and mortality among hospitalized nondiabetic patients with heart failure. Arch Intern Med. 2006;166:1613–19. doi: 10.1001/archinte.166.15.1613. [DOI] [PubMed] [Google Scholar]
- 6.Kosiborod M, Inzucchi SE, Spertus JA, et al. Elevated admission glucose and mortality in elderly patients hospitalized with heart failure. Circulation. 2009;119:1800–7. doi: 10.1161/CIRCULATIONAHA.108.821843. [DOI] [PubMed] [Google Scholar]
- 7.Van den Berghe G, Wouters P, Weekers F, et al. Intensive insulin therapy in critically ill patients. N Engl J Med. 2001;345:1359–67. doi: 10.1056/NEJMoa011300. [DOI] [PubMed] [Google Scholar]
- 8.Van den Berghe G, Wilmer A, Hermans G, et al. Intensive insulin therapy in the medical ICU. N Engl J Med. 2001;354:449–518. doi: 10.1056/NEJMoa052521. [DOI] [PubMed] [Google Scholar]
- 9.Quagliaro L, Piconi L, Assaloni R, Martinelli L, Motz E, Ceriello A. Intermittent high glucose enhances apoptosis related to oxidative stress in human umbilical vein endothelial cells: the role of protein kinase C and NAD(P)H-oxidase activation. Diabetes. 2003;52:2795–804. doi: 10.2337/diabetes.52.11.2795. [DOI] [PubMed] [Google Scholar]
- 10.Monnier L, Mas E, Ginet C, et al. Activation of oxidative stress by acute glucose fluctuations compared with sustained chronic hyperglycemia in patients with type 2 diabetes. JAMA. 2006;295:1681–7. doi: 10.1001/jama.295.14.1681. [DOI] [PubMed] [Google Scholar]
- 11.Desouza C, Salazar H, Cheong B, Murgo J, Fonseca V. Association of hypoglycemia and cardiac ischemia: a study based on continuous monitoring. Diabetes Care. 2003;26:1485–9. doi: 10.2337/diacare.26.5.1485. [DOI] [PubMed] [Google Scholar]
- 12.Egi M, Bellomo R, Stachowski E, French CJ, Hart G. Variability of blood glucose concentration and short-term mortality in critically ill patients. Anesthesiology. 2006;105:244–52. doi: 10.1097/00000542-200608000-00006. [DOI] [PubMed] [Google Scholar]
- 13.Vogelzang M, van der Horst IC, Nijsten MW. Hyperglycaemic index as a tool to assess glucose control: a retrospective study. Crit Care. 2004;8:R122–7. doi: 10.1186/cc2840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hermanides J, Vriesendorp TM, Bosman RJ. Glucose variability is associated with intensive care unit mortality. Crit Care Med. 2010;38:838–42. doi: 10.1097/CCM.0b013e3181cc4be9. [DOI] [PubMed] [Google Scholar]
- 15.Krinsley JS. Glycemic variability: a strong independent predictor of mortality in critically ill patients. Crit Care Med. 2008;36:3008–13. doi: 10.1097/CCM.0b013e31818b38d2. [DOI] [PubMed] [Google Scholar]
- 16.Meyfroidt G, Keenan DM, Wang X, Wouters PJ, Veldhuis JD, Van den Berghe G. Dynamic characteristics of blood glucose time series during the course of critical illness: effects of intensive insulin therapy and relative association with mortality. Crit Care Med. 2010;38:1021–1029. doi: 10.1097/CCM.0b013e3181cf710e. [DOI] [PubMed] [Google Scholar]
- 17.Dungan K, Chapman J, Braithwaite SS, Buse JB. Glucose measurement: confounding issues in setting targets for inpatient management. Diabetes Care. 2007;30:403–9. doi: 10.2337/dc06-1679. [DOI] [PubMed] [Google Scholar]
- 18.NICE-SUGAR Study Investigators. Finfer S, Chittock DR, Su SY, et al. Intensive versus conventional glucose control in critically ill patients. N Engl J Med. 2009;360:283–97. doi: 10.1056/NEJMoa0810625. [DOI] [PubMed] [Google Scholar]
- 19.Ryan EA, Shandro T, Green K, et al. Assessment of the severity of hypoglycemia and glycemic lability in type 1 diabetic subjects undergoing islet transplantation. Diabetes. 2004;53:955–62. doi: 10.2337/diabetes.53.4.955. [DOI] [PubMed] [Google Scholar]
- 20.Goldberg PA. Memoirs of a root canal salesman: the successful implementation of a hospital-wide intravenous insulin infusion protocol. Endocr Pract. 2006;12(Suppl 3):79–85. doi: 10.4158/EP.12.S3.79. [DOI] [PubMed] [Google Scholar]
- 21.Ali NA, O’Brien JM, Dungan K, et al. Glucose variability and mortality in patients with sepsis. Crit Care Med. 2008;36:2316–21. doi: 10.1097/CCM.0b013e3181810378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kudva YC, Basu A, Jenkins GD, et al. Glycemic variation and hypoglycemia in patients with well-controlled type 1 diabetes on a multiple daily insulin injection program with use of glargine and ultralente as basal insulin. Endocr Pract. 2007;13:244–50. doi: 10.4158/EP.13.3.244. [DOI] [PubMed] [Google Scholar]
- 23.Umpierrez GE, Palacio A, Smiley D. Sliding scale insulin use: myth or insanity? Am J Med. 2007;120:563–7. doi: 10.1016/j.amjmed.2006.05.070. [DOI] [PubMed] [Google Scholar]
- 24.Kosiborod M, Inzucchi SE, Goyal A, et al. Relationship between spontaneous and iatrogenic hypoglycemia and mortality in patients hospitalized with acute myocardial infarction. JAMA. 2009;301:1556–64. doi: 10.1001/jama.2009.496. [DOI] [PubMed] [Google Scholar]
- 25.Krinsley JS. Glycemic variability and mortality in critically ill patients: the impact of diabetes. J Diabetes Sci Technol. 2009;3:1292–301. doi: 10.1177/193229680900300609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Graham BB, Keniston A, Gajic O, Alvarez CA Trillo, Medvedev S, Douglas IS. Diabetes mellitus does not adversely affect outcomes from a critical illness. Crit Care Med. 2010;38:16–24. doi: 10.1097/CCM.0b013e3181b9eaa5. [DOI] [PubMed] [Google Scholar]
- 27.Robbins JM. Diagnosing diabetes and preventing rehospitalizations: the urban diabetes study. Med Care. 2006;44:292–296. doi: 10.1097/01.mlr.0000199639.20342.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fonseca C, Sarmento PM, Marques F, Ceia F. Validity of a discharge diagnosis of heart failure: implications of misdiagnosing. Congest Heart Fail. 2008;14:187–91. doi: 10.1111/j.1751-7133.2008.07752.x. [DOI] [PubMed] [Google Scholar]
- 29.Ishihara M, Kojima S, Sakamoto T, Japanese Acute Coronary Syndrome Study (JACSS) Investigators Comparison of blood glucose values on admission for acute myocardial infarction in patients with versus without diabetes mellitus. Am J Cardiol. 2009;104:769–74. doi: 10.1016/j.amjcard.2009.04.055. [DOI] [PubMed] [Google Scholar]
- 30.Kosiborod M, Inzucchi SE, Krumholz HM. Glucometrics in patients hospitalized with acute myocardial infarction: defining the optimal outcomes-based measure of risk. Circulation. 2008;117:1018–27. doi: 10.1161/CIRCULATIONAHA.107.740498. [DOI] [PubMed] [Google Scholar]
- 31.Eshaghian S, Horwich TB, Fonarow GC. An unexpected inverse relationship between HbA1c levels and mortality in patients with diabetes and advanced systolic heart failure. Am Heart J. 2006;151:91.e1–91.e6. doi: 10.1016/j.ahj.2005.10.008. [DOI] [PubMed] [Google Scholar]
- 32.Gerstein HC, Swedberg K, Carlsson J, et al. CHARM Program Investigators The hemoglobin A1c level as a progressive risk factor for cardiovascular death, hospitalization for heart failure, or death in patients with chronic heart failure: an analysis of the Candesartan in Heart failure: Assessment of Reduction in Mortality and Morbidity (CHARM) program. Arch Intern Med. 2008;168:1699–1704. doi: 10.1001/archinte.168.15.1699. [DOI] [PubMed] [Google Scholar]
- 33.Garber AJ, Moghissi ES, Bransome ED, Jr, et al. The American College of Endocrinology Task Force on Inpatient Diabetes Metabolic Control: American College of Endocrinology position statement on inpatient diabetes and metabolic control. Endocr Pract. 2004;10(Suppl. 2):4–9. doi: 10.4158/EP.10.S2.4. [DOI] [PubMed] [Google Scholar]
- 34.Preiser JC, Devos P, Ruiz-Santana S, et al. A prospective randomised multi-centre controlled trial on tight glucose control by intensive insulin therapy in adult intensive care units: the Glucontrol study. Intensive Care Med. 2009;35:1738–48. doi: 10.1007/s00134-009-1585-2. [DOI] [PubMed] [Google Scholar]