Abstract
Receptor-associating protein 46 (RAP46) is a cochaperone that regulates the transactivation function of several steroid receptors. It is transported into the nucleus by a liganded glucocorticoid receptor where it downregulates DNA binding and transactivation by this receptor. The N- and C-termini of RAP46 are both implicated in its negative regulatory function. In metabolic labelling experiments, we have shown that the N-terminus of RAP46 is modified by phosphorylation, but this does not contribute to the downregulation of glucocorticoid receptor activity. However, deletion of a sequence that binds 70 kDa heat shock protein (Hsp70) and the constitutive isoform of Hsp70 (Hsc70) at the C-terminus of RAP46 abrogated its negative regulatory action. Surface plasmon resonance studies showed that RAP46 binds the glucocorticoid receptor only when it has interacted with Hsp70/Hsc70, and confocal immunofluorescence analyses revealed a nuclear transport of Hsp70/Hsc70 by the liganded receptor. Together these findings demonstrate an important contribution of Hsp70/Hsc70 in the binding of RAP46 to the glucocorticoid receptor and suggest a role for this molecular chaperone in the RAP46-mediated downregulation of glucocorticoid receptor activity.
Keywords: BAG-1/cochaperone/glucocorticoid receptor/Hsp70/transactivation
Introduction
Glucocorticoid receptor (GR) and other members of the nuclear receptor family reside in the cytoplasm of target cells in an inactive complex with the molecular chaperones and cochaperones 90 kDa heat shock protein (Hsp90), Hsp70, p48 (Hip), p60/Hop, p23, FKBP52, FKBP54 and cyclophilin 40 (for review see Pratt and Toft, 1997). Molecular chaperones and cochaperones are defined as a group of unrelated proteins that mediate the correct assembly of other proteins but are not themselves components of the final functional structure (Anfinsen, 1973). These proteins are therefore thought to generate the correct folding for hormone binding through their association with steroid receptors (Bresnick et al., 1989; Smith and Toft, 1993; Bohen et al., 1995).
Molecular chaperones interact with the GR in an ordered fashion, involving an initial binding by Hsp70 and its cochaperones, followed by two molecules of Hsp90 (for reviews see Dittmar and Pratt, 1997; Frydman and Höhfeld, 1997; Buchner, 1999). An intermediate complex is formed, with the scaffold protein Hsp70 and Hsp90 organizing protein (Hop) holding Hsp70 and Hsp90 onto the receptor. Later, Hsp70 is reported to dissociate from the complex, allowing p23 and immunophilins to interact in order to generate a competent receptor for hormone binding and further translocation into the nucleus. While these processes are generally accepted as essential steps in the maturation of the GR, it is important to note that they were mainly deduced from in vitro assembly assays. Recently, the individual roles or requirements of the chaperone molecules in the maturation of steroid receptors have been questioned and the overall function of the molecular chaperones in the action of steroid receptors is being re-evaluated (Ylikomi et al., 1998; Morishima et al., 2000; Rajapandi et al., 2000).
Biochemical and genetic studies have clearly shown that Hsp90, p23 and cyclophilin 40 are required for hormone-dependent activation of the GR (Picard et al., 1990; Duina et al., 1996; Freeman et al., 2000). Further experiments have even revealed that in addition to their role in the maturation of steroid receptors in the cytoplasm, they may also regulate the action of these receptors distal to hormone binding (Diehl and Schmidt, 1993; Srinivasan et al., 1994; Kang et al., 1999). We have recently reported that the cochaperone RAP46 (also known as the M isoform of Bcl-2-associated athanogene 1, BAG-1M) is transported into the nucleus by the liganded GR, where it downregulates DNA binding by this receptor (Schneikert et al., 1999).
RAP46 was originally identified as a protein that associates with the GR and other steroid receptors (Zeiner and Gehring, 1995). It was found to be closely related to BAG-1, an interaction partner of the anti-apoptotic factor Bcl2 (Takayama et al., 1995). At present, this protein exists as a family of at least three members (BAG-1L, BAG-1M/RAP46 and BAG-1S) with divergent N-terminal but conserved C-terminal regions (Packham et al., 1997; Takayama et al., 1998; Yang et al., 1998). The BAG-1 proteins bind to the ATPase domain of Hsp70 and the constitutive isoform of Hsp70 (Hsc70) through their conserved C-terminal sequences (Zeiner et al., 1997; Bimston et al., 1998; Takayama et al., 1998) and modulate the chaperone activity of these proteins (Höhfeld and Jentsch, 1997; Takayama et al., 1997; Bimston et al., 1998; Nollen et al., 2000). It was therefore suggested that they would interfere with the hormone binding properties of steroid receptors; indeed effects of BAG-1 on hormone binding by the GR have been reported. BAG-1S at stoichiometric, but not at substoichiometric levels to Hsp70/Hsc70 inhibits hormone binding to the GR (Kanelakis et al., 1999). Other reported effects of BAG-1 proteins on nuclear receptors range from BAG-1S-mediated inhibition of transactivation and DNA binding by retinoic acid (RAR/RXR) heterodimeric receptors (Liu et al., 1998) to BAG-1L-mediated enhancement of the transactivation function of the androgen receptor (Froesch et al., 1998). The mechanisms involved in these regulatory pathways of BAG-1 have not yet been elucidated, but studies on the inhibition of GR action by RAP46 have identified both the N- and C-terminal sequences as essential regulatory domains (Schneikert et al., 1999).
Here we show that the N-terminus of RAP46 is a site for phosphorylation. Exchanging the N-terminal serine residues for non-phosphorylatable amino acids inhibited phosphorylation but did not affect the ability of RAP46 to downregulate DNA binding or transactivation by the GR. Deletion of the last C-47 amino acids with which RAP46 binds to Hsp70/Hsc70 had no effect on phosphorylation but abolished RAP46-mediated repression of the action of the GR. In surface plasmon resonance (SPR) studies, we showed that Hsp70 binds the GR in the absence as well as in the presence of hormone and that RAP46 interacts with the GR after the receptor had bound Hsp70/Hsc70. Together these results demonstrate a crucial role of the molecular chaperone Hsp70/Hsc70 in the negative regulation of GR activity by RAP46.
Results
The N-terminus of RAP46 is phosphorylated
The N-terminus of RAP46 has been shown to be important for downregulating DNA binding and subsequent transactivation functions of the GR (Schneikert et al., 1999). This region contains several serine and threonine residues as well as a putative consensus sequence for creatine kinase 2 (Takayama et al., 1998), implicating phosphorylation in the action of RAP46. In a metabolic labelling experiment with [32P]orthophosphate, we determined that RAP46 was mainly phosphorylated at its N-terminal sequence but phosphorylation was not required for the negative regulation of GR action. Inhibition of phosphorylation by olomoucine, a cyclin-dependent protein kinase inhibitor (Vesely et al., 1994), had no effect on RAP46-mediated inhibition of DNA binding by the GR (results not shown).
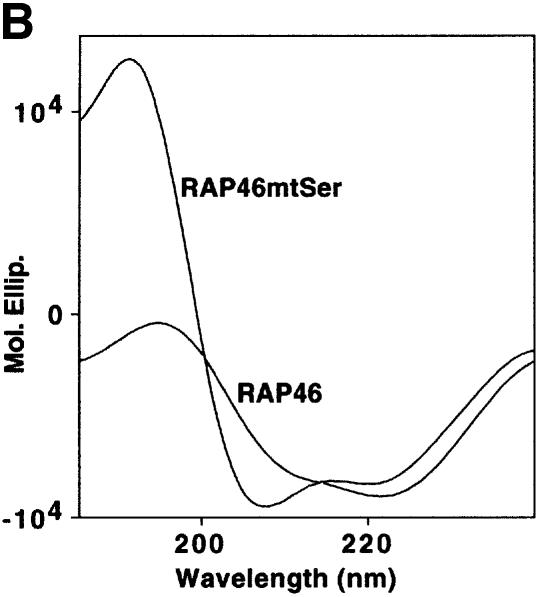
In a more direct experiment, all nine phosphorylatable serine residues in the first 70 amino acids of RAP46 were mutated into amino acids that can not be modified (e.g. alanine, leucine, lysine and asparagine) (Figure 1A). These non-phosphorylatable amino acids were chosen arbitrarily to avoid cross-hybridization of the oligonucleotides used in the mutagenesis with the highly repetitive N-terminal region of the RAP46 gene. This massive mutation drastically altered the structure of the protein as determined by circular dichroism measurements in which bacterially purified glutathione S-transferase (GST)– RAP46mtSer and GST–RAP46 (Figure 1B) were compared. Application of the standard Chou–Fasman or Robson folding algorithms to critical areas of the proteins showed that the mutations increased α-helical tendency and disrupted favourable turn configurations within the wild-type protein. The results of the secondary structure are consistent with this prediction (Table I). Note that α-helices seemed to be produced in the mutant protein at the expense of turns and β-sheets. The extent of helix increase is therefore greater than would be expected from the folding algorithm and would thus have far-reaching effects on the protein.
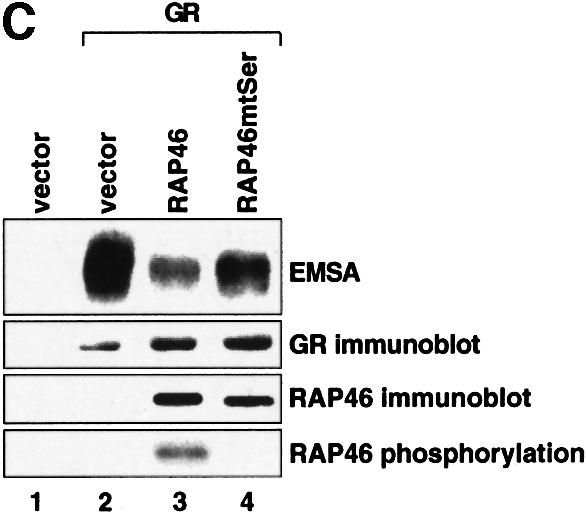
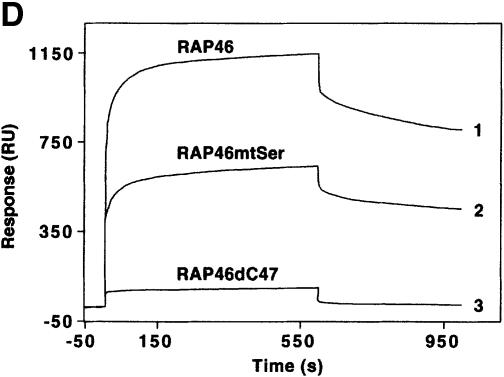
Fig. 1. Mutation of the serine residues at the N-terminus of RAP46 does not abolish the inhibitory effect of RAP46 on DNA binding by the GR. (A) The sequences of the first 70 amino acids of the wild-type and the N-terminal serine mutant of RAP46 are illustrated. The amino acids exchanged are indicated by boxes. (B) Mutation of the serine residues at the N-terminus of RAP46 alters the secondary structure of the protein. Purified, bacterially produced fusion proteins GST–RAP46 and GST–RAP46mtSer were subjected to circular dichroism measurements. (C) Mutation of the N-terminal serine residues does not alter the negative effect of RAP46 on DNA binding by the GR. A hundred thousand COS-7 cells were transiently transfected with 0.9 µg of either an empty expression vector (vector) or an expression vector encoding glucocorticoid receptor (GR) and 0.9 µg of expression vectors encoding HA-tagged RAP46 or the mutant RAP46mtSer. One half of the transfected cells was used for EMSA and the other half was metabolically labelled with [32P]orthophosphate for 4 h and immunoprecipitated with an anti-HA antibody. The immunoprecipitated proteins were resolved by SDS–PAGE and exposed to autoradiography (RAP46 phosphorylation) or immunoblotted using antibodies recognizing either the GR (GR immunoblot) or RAP46 as well as RAP46mtSer (RAP46 immunoblot). (D) Surfaces with an average of 2000 resonance units (RU) immobilized GST–RAP46 fusion proteins were obtained by covalent immobilization of an anti-GST antibody with the help of a standard amine coupling protocol, followed by capture of the different fusion proteins through antibody–antigen interaction. Injection of Hsp70 over the surface resulted in a strong interaction signal by 100 ng GST–RAP46 (1), whereas a decrease in the interaction signal was obtained when 100 ng GST–RAP46mtSer (2) were used for capture. No interaction was detected with 100 ng GST–RAP46dC47 (3).
Table I. Average secondary structure content (%) of wild-type RAP46 and the multiple serine-substituted mutant (RAP46mutSer) as calculated from the far-UV CD spectra (see Materials and methods).
| Secondary structure estimation | Wild-type RAP46 | RAP46mtSer |
|---|---|---|
| α-helix | 21 | 34 |
| β-sheet | 27 | 9 |
| Turn | 9 | 32 |
| Polyproline helix | 15 | 15 |
| Other structures | 8 | 19 |
The mutations were, however, effective in destroying the site(s) of phosphorylation, as demonstrated in an in vivo phosphorylation assay (Figure 1C, compare lanes 3 and 4 in RAP46 phosphorylation panel), but they did not destroy the ability of RAP46mtSer to inhibit DNA binding by the GR, although this effect was slightly reduced [Figure 1C, compare lanes 3 and 4 in the electrophoretic mobility shift assay (EMSA) panel]. The mutations also did not destroy the binding of RAP46 to Hsp70. In SPR studies in which GST–RAP46, GST–RAP46mtSer and GST–RAP46dC47 were captured onto a carboxymethyl (CM) sensor chip by an anti-GST antibody and Hsp70 was passed over the chip, Hsp70 bound RAP46 and RAP46mtSer, although its affinity to RAP46Ser was slightly reduced. As a control, RAP46dC47 lacking the Hsp70/Hsc70 binding site did not interact with Hsp70 (Figure 1D). Together, these findings demonstrate that inhibition of N-terminal phosphorylation of RAP46 does not abolish the ability of this protein to downregulate DNA binding by the GR or to bind Hsp70.
As deletion of the Hsp70/Hsc70 binding site inhibited the interaction of RAP46 with Hsp70, we investigated how this would affect the action of the GR.
The C-terminus of RAP46 is required for downregulating GR action
In cotransfection experiments, RAP46 inhibited DNA binding by the GR, as we have previously reported (Schneikert et al., 1999), but not the mutant lacking the last 47 C-terminal amino acids (Figure 2, EMSA panel, compare lanes 3 and 4). DNA binding by this mutant receptor even seemed enhanced, most probably due to the high level of receptor in the cellular extract used for the assay. The inability of the C-terminal mutant of RAP46 to inhibit DNA binding by the receptor was also reflected in its inability to repress transactivation (Figure 2, transactivation panel, compare lanes 3 and 4). However both the wild-type and mutant RAP46 were phosphorylated to an identical extent, which provides further support to the notion that events at the C-terminus and not phosphorylation at the N-terminus contribute to the downregulation of GR activity by RAP46.
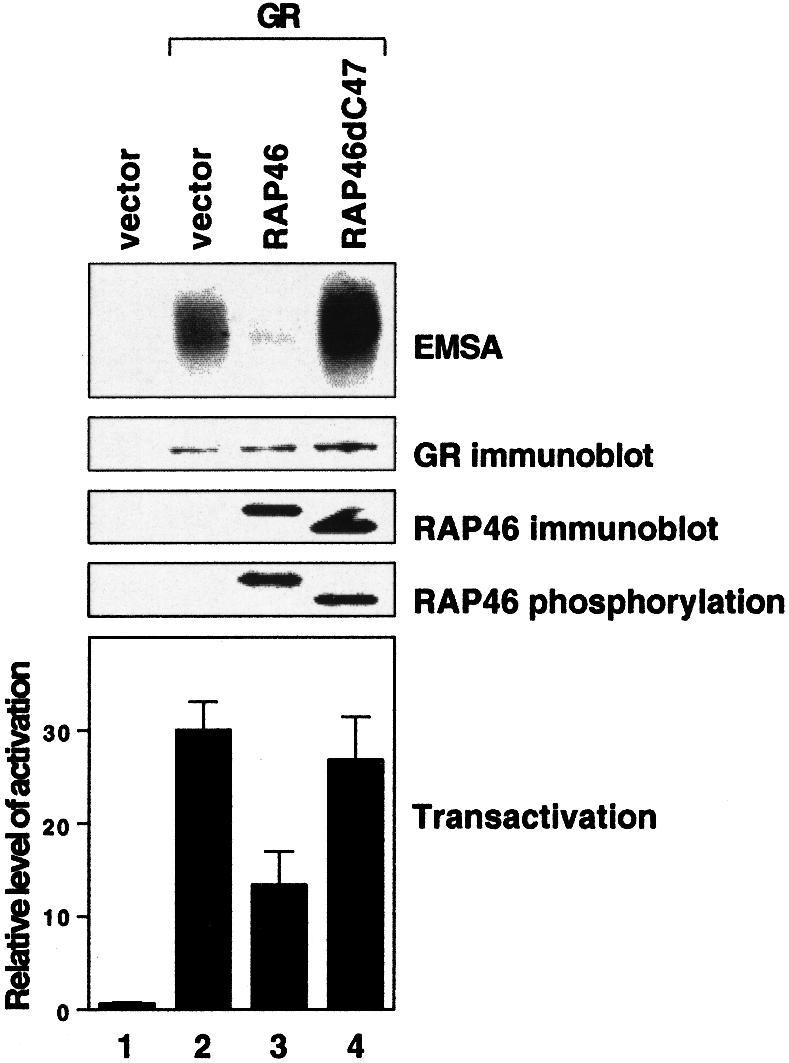
Fig. 2. The Hsp70 binding domain is essential for RAP46-mediated downregulation of DNA binding by the GR. COS-7 cells were transiently transfected with 0.2 µg of GR-responsive MMTV-luciferase (pGL3MMTV) indicator gene and 10 ng of an internal control encoding the renilla luciferase under the control of the ubiquitin promoter. In addition, 0.9 µg of either an empty expression vector (vector) or an expression vector encoding glucocorticoid receptor (GR), or the mutant RAP46dC47 were cotransfected. Both RAP46 proteins were tagged with HA. The transfected cells were either left untreated or were metabolically labelled with [32P]orthophosphate for 4 h. Whole-cell extracts were prepared for luciferase activity measurements (transactivation) or for EMSA and SDS–PAGE. In the latter case, the gels were immunoblotted using antibodies recognizing either the GR (GR immunoblot) or the HA tag on RAP46 or RAP46dC47 (RAP46 immunoblot). Immunoprecipitation with an anti-HA antibody was carried out on the cellular extracts from the metabolically labelled cells, followed by SDS–PAGE and autoradiography. The relative level of activation of the luciferase indicator plasmid in the transactivation assay was derived from the ratios of firefly over renilla luciferase activities obtained in three different experiments. For transactivation assays, dexamethasone (100 nM) was added to the cells immediately after transfection. For EMSA, the hormone was added to the reaction mixture before incubation of the cellular extracts with the radioactive DNA probe.
Since the last 47 C-terminal amino acids of RAP46 have already been described as necessary for interaction with Hsp70/Hsc70 (Takayama et al., 1997; also our results in Figure 1D), our finding that its deletion prevented RAP46 from inhibiting DNA binding by the GR suggests an additional function for this region of RAP46.
Hsp70: an adaptor protein for the interaction of RAP46 with the GR
To determine a possible link between Hsp70/Hsc70 binding and inhibition of GR activity by RAP46, we investigated whether Hsp70/Hsc70 is required for RAP46 to bind to the GR.
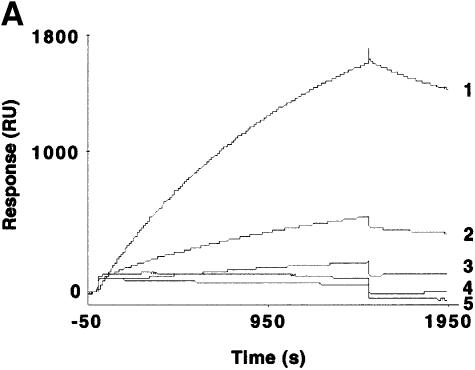
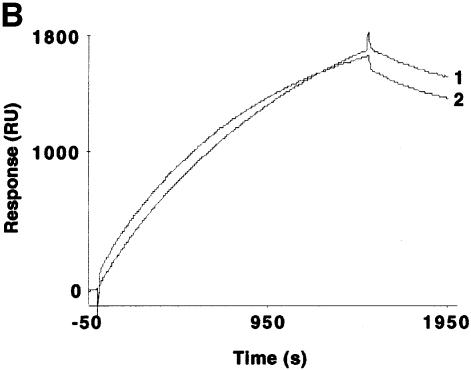
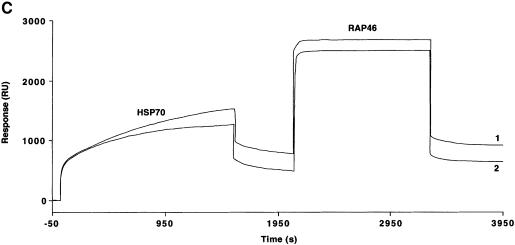
With the use of SPR measurements, we evaluated the interaction between recombinant purified Hsp70 and bacterially purified GST–RAP46 with human GR prepared from crude baculovirus extracts. We immobilized GR tagged with His6 residues at its N-terminus onto the surface of a nitrilotriacetic acid (NTA) sensor chip in the absence and presence of hormone, and passed Hsp70 or GST–RAP46 over it. A typical sensogram with increasing concentrations of Hsp70 and the chip-coupled GR unambiguously demonstrated a dose-dependent hGR–Hsp70 interaction (Figure 3A), but no binding was detected with extracts from mock-transfected crude baculovirus extracts (data not shown). We could also show that the interaction kinetic is not drastically affected by hormone, although our receptor preparation bound hormone with Kd = 11.4 ± 5.5 nM and possessed an active receptor content of 0.30 ± 0.15 pmol/mg protein (Figure 3B). Interestingly, GST alone or the bacterially purified GST–RAP46 did not bind the GR (results not shown). Binding of RAP46 to the GR only occurred when Hsp70 was first allowed to interact with the receptor, followed by the addition of RAP46 in a sequential order (Figure 3C). When RAP46 was first added to the immobilized GR followed by Hsp70, only the binding of Hsp70 to the GR was observed (results not shown). This indicates that Hsp70 serves as an adaptor for the interaction of RAP46 with the GR.
Fig. 3. Hsp70 binds to the GR in the absence or presence of hormone and its binding is required for the interaction of RAP46 with GR in SPR. (A) Sensograms showing injections of Hsp70 at 100 (1), 50 (2), 25 (3), 12.5 (4) and 5 ng/µl (5) with 1500 RU His-tagged GR produced in baculovirus coupled to the surface of a NTA chip. A dose-dependent increase in resonance units (Response, RU) can be seen. (B) SPR signals obtained when 100 ng of Hsp70 were injected in the absence (1) or presence (2) of 10–7 M dexamethasone and 1500 RU His-tagged GR coupled to the NTA chip. A minor decrease in the interaction is observed in the presence of dexamethasone. (C) The consecutive addition of 100 ng RAP46 to a preformed GR–Hsp70 complex in the absence (1) or presence (2) of 10–7 M dexamethasone resulted in an additional increase in RU, indicative of RAP46 binding. The values of the apparent rate constants for the Hsp70–GR interaction deduced from the experiments described in (A) were Ka = 5.4 × 102 M/s and Kd = 8.23 × 10–4/s. From these data the apparent dissociation equilibrium constant was calculated to be Kd = 1.5 × 10–6 M. All calculations were based on the assumption of monomolecular 1:1 binding characteristics, where A + B = AB. However, the apparent rate and dissociation equilibrium constants have to be seen as first approximations since a steady drift in the baseline (i.e. a time-dependent decline in GR binding; not shown) was observed. This is a well known phenomenon when working with non-covalent capturing of His-tagged proteins to the surface of a NTA sensor chip.
To determine further how Hsp70 contributes to the action of RAP46, we developed a cell-free assay that allowed us to examine the effect of Hsp70 on RAP46-mediated inhibition of DNA binding by the GR. The assay used consisted of an EMSA with extracts from COS-7 cells transfected with the GR. Addition of GST–RAP46 inhibited DNA binding by the receptor, possibly by making use of endogenous Hsc70 in the extract (Figure 4A, lane 4). This inhibitory effect of RAP46 was specific as it was not observed with GST alone (lane 3) or with the C-terminal deletion mutant of RAP46 lacking the Hsp70/Hsc70 binding site (Figure 4A, lane 5).
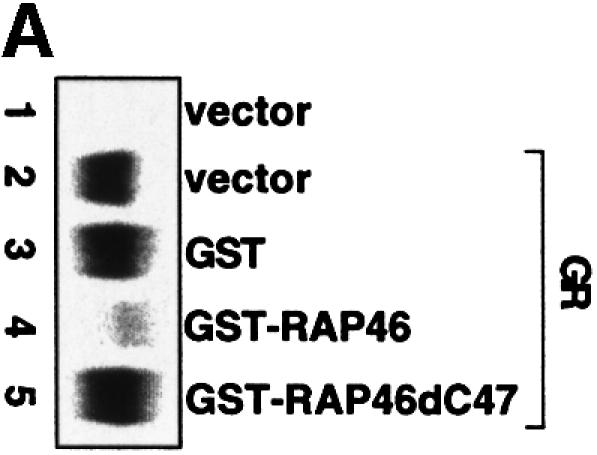
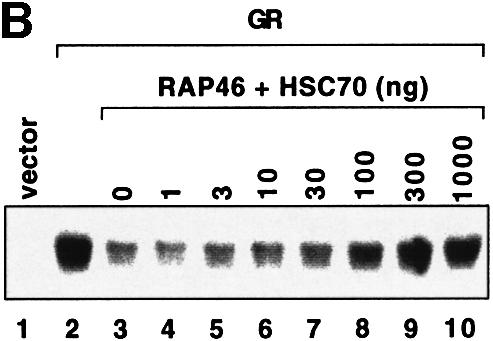
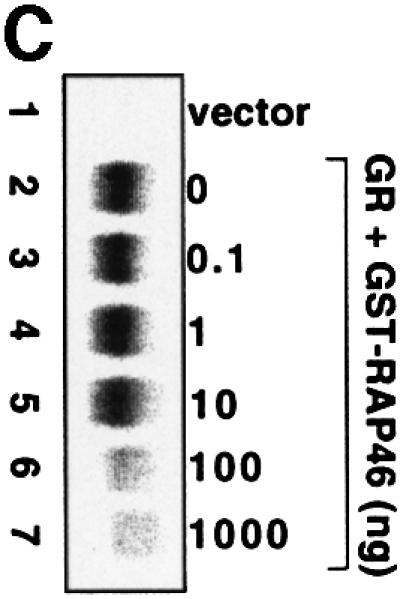
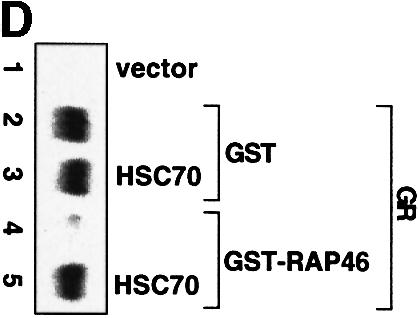
Fig. 4. In vitro cell-free system for studying the effect of RAP46 on DNA binding by the GR. One hundred thousand COS-7 cells in 3.4 cm culture dishes were transiently transfected by the Fugene transfection procedure with 0.9 µg empty expression vector or with an expression vector encoding the GR. Whole-cell extracts were prepared and subjected to an EMSA using radioactively labelled GR-response element as a probe. Where indicated, different amounts of bacterially purified GST or GST fusion proteins were added to the cell extracts. The fusion proteins consisted of GST alone or GST fused to either the wild-type RAP46 sequence (GST–RAP46) or RAP46 lacking the last 47 C-terminal amino acids (GST–RAP46dC47). (A) Inhibition of DNA binding by the GR is mediated by RAP46 and not RAP46C47. EMSA was carried out with cell extracts containing the GR and 1 µg GST, GST–RAP46 or GST–RAP46dC47. (B) Hsc70 relieves RAP46-mediated inhibition of DNA binding by the GR. COS-7 cells were transiently transfected with an empty expression vector (vector, lane 1), or an expression vector encoding either the GR (lanes 2–10) or an HA-tagged RAP46 (lanes 3–10). Whole-cell extracts were prepared and subjected to an EMSA using radioactively labelled GR response element as a probe. Where indicated, different amounts of purified Hsc70 were added to the whole-cell extracts. (C) Increased concentration of RAP46 downregulated DNA binding by the GR. Concentrations of GST–RAP46 ranging from 0.1 to 1000 ng was added to the cellular extract before the DNA binding reaction were carried out. (D) A RAP46–Hsc70 complex does not affect DNA binding by the GR. COS-7 cells were transiently transfected with either an empty expression vector (vector, lane 1) or an expression vector encoding the GR (lanes 2–5). Whole-cell extracts were prepared and subjected to EMSA. One microgram of bacterially produced GST (lanes 2 and 3) or GST–RAP46 fusion protein (lanes 4 and 5) were added to the cell extracts before providing the hormone and the probe. Where indicated, GST and GST–RAP46 were premixed with 2 µg of purified Hsc70 before addition to the cell extracts (lanes 3 and 5). In (A–D), dexamethasone (100 nM) was added to the EMSA reaction mixture prior to incubation with the radioactive probe.
The effective concentration of Hsp70/Hsc70 for the action of RAP46 was quite crucial. We estimated by western blot analyses the ratio of Hsc70 to RAP46 in the extract to be ∼10:1 (results not shown). If this ratio was drastically altered by further addition of exogenous Hsc70, repression of DNA binding by the GR was abrogated (Figure 4B, compare lanes 8–10 with lane 3). At the highest concentration, Hsc70 alone did not have any significant effect on DNA binding by the GR (results not shown). The vast excess of Hsc70 to RAP46 possibly sequestered all the available RAP46 needed for interaction with the GR–Hsc70 complex in the inhibition of DNA binding by the GR. Note the slightly higher affinity of Hsc70 for RAP46 (Kd = 500 nM; Stuart et al., 1998) compared with that of Hsp70 for the GR (Kd = 1.5 µM; see the results of our SPR studies in Figure 3A). If the concentration of GST–RAP46 was drastically increased and Hsc70 was kept constant, RAP46 still inhibited DNA binding by the GR (Figure 4C, compare lanes 5–7 with lane 2) due to the disproportionately high level of Hsc70 in the extract. If, on the other hand, RAP46 was first allowed to complex with Hsc70 before addition to the cellular extract containing the GR, no inhibition of DNA binding by the GR was observed (Figure 4D). All these findings suggest that Hsp70/Hsc70 needs to bind to the GR before RAP46 can exert its inhibitory function.
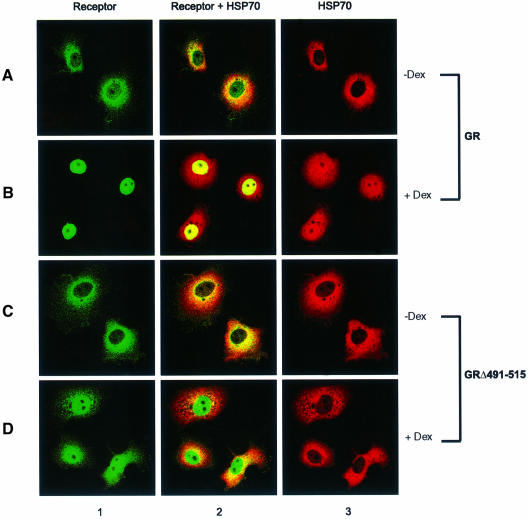
As RAP46 is transported into the nucleus by the liganded GR (Schneikert et al., 1999), we would also expect Hsp70/Hsc70 to be transported into the nucleus by the liganded receptor if Hsp70/Hsc70 is necessary for RAP46 to bind the GR. In laser confocal immunofluorescence studies, the cellular localization of Hsp70 protein containing an immunotag was determined after coexpression with a green fluorescent protein (GFP)-tagged GR in COS-7 cells. In the absence of hormone, both proteins were detected in the cytoplasm (Figure 5, panel A2). In the presence of hormone, the GR was not only translocated into the nucleus, as we have previously reported (Schneikert et al., 1999), but Hsp70 was also cotransported into the nucleus (Figure 5, panel B2). In cells transfected only with Hsp70, this protein remained in the cytoplasm in the presence as well as in the absence of dexamethasone (results not shown). Note that the entire cytoplasmic Hsp70 was not recruited into the nucleus. This is possibly due to the high amount of this protein in the transfected COS-7 cells made up of endogenous Hsc70 and Hsp70, as well as the exogenously added Hsp70.
Fig. 5. Confocal immunofluorescence analysis of the intracellular localization of GR and Hsp70. Five hundred thousand COS-7 cells in 10 cm culture dishes were transiently transfected by the Fugene procedure with 4 µg of expression vectors encoding GR–GFP or GR(Δ491–515)–GFP and Hsp70. The Hsp70 was tagged with a decapeptide from human testis lactate dehydrogenase. Twenty-four hours after the transfection, the cells were treated for 16 h with vehicle alone (0.1% ethanol) (–Dex) or vehicle containing 0.1 µM dexamethasone (+ Dex) before harvesting, processing and visualization with laser confocal microscopy. The green fluorescence arises from the GFP-tagged receptors, whereas the red fluorescence comes from staining of Hsp70 with a rat antibody against the lactate dehydrogenase tag followed by an anti-rat antibody labelled with rhodamine. The yellow to orange colour indicates colocalization of the receptor and Hsp70.
The translocation of Hsp70 into the nucleus by the GR was, however, specific since a mutant GR lacking the hinge region was unable to perform this function. In cotransfection experiments with this mutant GR and the tagged Hsp70, the two proteins were detected in the cytoplasm in the absence of hormone (Figure 5, panel C2). In the presence of hormone, while the mutant GR was transported into the nucleus, Hsp70 remained in the cytoplasm (Figure 5, panel D2). As the hinge region of the GR is also needed for the nuclear localization of RAP46 (Schneikert et al., 1999), these results together demonstrate that Hsp70 interacts with the liganded GR and that this interaction may be necessary for the nuclear localization and the negative regulatory action of the cochaperone RAP46.
Discussion
In this work, we have analyzed features of RAP46 necessary for the negative regulation of DNA binding and, as a result, the transactivation function of the GR. We have shown that the N-terminus, which contributes to the regulatory action of this protein on GR action, is a target for phosphorylation. This N-terminal phosphorylation is, however, not required for the regulation of GR activity. It is sensitive to olomoucine, an inhibitor of cyclin-dependent protein kinase (Vesely et al., 1994), suggesting that phosphorylation of RAP46 may occur during cell-cycle progression and may contribute to the reported cell-cycle effects of this protein (Kullmann et al., 1998). We also found out that N-terminal amino acid residues reported as necessary for RAP46 to bind directly to DNA (Zeiner et al., 1999) are not involved in the inhibition of DNA binding by the GR (J.Schneikert and A.C.B.Cato, unpublished data). Instead, deletion of the Hsp70/Hsc70 binding site at the C-terminal end of RAP46 abrogated the negative regulatory function of this protein on GR action.
In a previous study we have shown that deletion of the Hsp70/Hsc70 binding site of RAP46 prevented nuclear transport of RAP46 by the liganded GR (Schneikert et al., 1999), suggesting that Hsp70/Hsc70, which binds to this region, may act as a transporter protein for the GR. In our present study we have identified the same binding site as a necessary domain for the inhibition of GR action by RAP46, possibly employing Hsp70/Hsc70 as a bridging molecule for the interaction of RAP46 with the GR. These two functions are not mutually exclusive since a transporter protein could still play the role of a bridging molecule.
While we have not yet managed in a nuclear import assay to demonstrate a contribution of Hsp70/Hsc70 in the nuclear transport of RAP46 by the GR, we can clearly show its requirement for the association of RAP46 with the GR. In SPR measurements we demonstrated that RAP46 did not interact with the GR unless Hsp70 was bound to the receptor. It is important to note that the association and dissociation kinetics of the Hsp70–GR interaction were only slightly impaired by the presence of hormone, indicating that Hsp70 bound both the non-activated as well as the hormone-activated receptor. This is at variance with well entrenched notions that the chaperone molecules completely dissociate from the GR when it is activated by hormone. Our results, however, agree with a number of observations that Hsp70/Hsc70 can still associate with the GR in its activated state (Diehl and Schmidt, 1993; Srinivasan et al., 1994) and further describe a novel function of this molecular chaperone in GR action.
We confirmed the SPR results on the association of Hsp70 with the activated GR with laser confocal immunofluorescence data, by which we demonstrated nuclear colocalization of Hsp70 and the GR in the presence of hormone in COS-7 cells cotransfected with tagged GR and Hsp70 constructs. However, Hsp70 was not observed in the nuclei of cells cotransfected with a mutant GR lacking the hinge region. This identified the hinge region as an interaction surface between Hsp70 and the GR, with a probable role in the transport of Hsp70 into the nucleus by the GR. Interestingly, the hinge region of the vitamin D3 receptor has also been described as an interaction site of DnaK and Hsp70 (Swamy et al., 1999). An algorithm that allows the prediction of DnaK/Hsp70 binding sites on protein sequences (Rüdiger et al., 1997) helped us identify the N-terminus in the hinge region of the GR as a likely target of interaction of Hsp70 (W.-G.Thies and A.C.B.Cato, unpublished data). This region contains the right half of the bipartite nuclear localization sequence of the GR and may define the contact point for the nuclear transport of both the GR and Hsp70/Hsp70. Specific amino acid exchanges in this region will reveal how the interaction of the GR with Hsp70 is related to the hormone-dependent nuclear transport of the receptor. In our present study, we have shown that a GR with a hinge region deletion still entered the nucleus in a hormone-dependent manner, albeit less efficiently. This indicates that other molecular chaperones interacting with the hormone binding domain of the receptor (e.g. Hsp90; Giannoukos et al., 1999) may suffice in a cellular environment to allow the receptor to reach the correct state for ligand binding and nuclear transport. This raises questions about the previously described role of Hsp70/Hsc70 in the in vitro maturation process of the receptor (Dittmar and Pratt, 1997; Frydman and Höhfeld, 1997; Buchner, 1999), which will need to be answered in future experimentation.
Our results together with other published data add to the accumulating evidence that in addition to well established roles in protein synthesis and trafficking (Ellis, 1997; Gottesman et al., 1997), molecular chaperones and cochaperones also participate directly in transcriptional regulation. For example, the bacterial chaperones Dnak and DnaJ are regulators of the prokaryotic transcription factor σ32 (Gamer et al., 1992). The chaperonin GroEL has been shown to facilitate DNA binding to the bacterial transcription factor NodD (Ogawa and Long, 1995), and recently a human nuclear localized chaperone that regulates DNA binding and transcriptional activity of bZIP proteins has been described (Virbasius et al., 1999). On the basis of the results presented here, we would add RAP46 to the family of cochaperones that directly influence the DNA binding activity of transcription factors. However, other functions of the RAP46 (BAG-1) family of proteins have been proposed. At equivalent stoichiometric levels to Hsp70/Hsc70, one of the smaller isoforms of this family, BAG-1S, is reported to inhibit hormone binding by the GR (Kanelakis et al., 1999). We have shown previously that RAP46 (BAG-1M) and a mutant lacking the first 70 amino acids (equivalent to BAG-1S) do not affect hormone binding by the GR (Schneikert et al., 1999). We have now demonstrated in this work that extracts from cells we have transfected with BAG-1 constructs contain substoichiometric levels of these proteins to Hsc70. The difference in our results and those of Kanelakis et al. (1999) with respect to hormone binding by the GR in the presence of the BAG-1 proteins may therefore be due to differences in the two experiments in the ratios of the BAG-1 proteins to Hsp70/Hsc70.
From our results on the RAP46-mediated regulation of DNA binding by the GR, we propose the following model. At stoichiometric levels of BAG-1 proteins to Hsp70/Hsc70, the hormone binding activity of the GR is abrogated in a process that is unclear at the moment. At substoichiometric levels of BAG-1, particularly RAP46/BAG-1M to Hsp70/Hsc70, Hsp70/Hsc70 binds to the C-terminal region of RAP46 and to a domain encompassing the hinge region of the GR. The liganded GR transports both Hsp70/Hsc70 and RAP46 into the nucleus, where RAP46 uses its N-terminal sequence in a process that is not fully understood to downregulate transactivation by the GR. However, this process does not require N-terminal phosphorylation of RAP46. The first step in the negative regulatory action of RAP46 on DNA binding by the GR is thus the interaction of this protein with GR bound to Hsp70/Hsc70. Our findings therefore demonstrate a regulatory action of a chaperone–cochaperone complex in DNA binding by the GR.
Materials and methods
Cell culture
Simian kidney COS-7 cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal calf serum (FCS), 100 U/ml penicillin and 100 µg/ml streptomycin at 37°C in an atmosphere of 5% CO2.
Plasmid constructs
The expression vector containing the wild-type human GR has been described by Giguère et al. (1986). The plasmids pT2RAP46, pGL3MMTV and GRΔ491–515GFP have all been described by Schneikert et al. (1999). The control plasmid for transfection, pTKRenilla Luc, was obtained from Promega (Mannheim) and the expression vector GR-GFP has been described by Carey et al. (1996). The construct pSV-Hsp70-tag containing the entire human Hsp70 cDNA modified by the addition of a sequence encoding human testis-specific lactate dehydrogenase immunotag at the C-terminus has already been described (Jäättelä et al., 1992). Mutagenesis of the N-terminus of RAP46 was carried out by using six oligonucleotides containing the appropriate mutations to exchange nine serine residues into non-phosphorylatable amino acids within SacII and BstEII restriction sites. A pair of the following oligonucleotides was used at a time. TCGCAGGCCGCGGATGAAGAAAAAGACTAGGCGCCGGTTAACTAGAAAAG and AGCTAGCGTCAATTCTTCAGCACGTGTTAGCTCCTCTTTTCTAGTTAACC were annealed to each other followed by a fill-in reaction with the Klenow fragment of DNA polymerase I in the presence of [α-32P]dCTP to generate product 1; GCTGAAGAATTGACGCTAGCTGAAGAGGCAACGTGGCTTGAGGAAGCGACCAGAAT and CAGTCTATTCATTTCTTCGCCTTGCGTGGCCTCTTCATTCTGAGTGCTTCCTCAAG were then used in a similar reaction to generate product 2; as well as GAAATGAATAGACTGCAAGAAGTCACTCGAGATGAAGAGTTAACAAGGCTAG and CTCTCTGGTCACCTCCTCTAGCCTTGTTAAC to yield product 3. The three products were purified on polyacrylamide gel and eluted. Product 2 was hybridized to either product 1 or product 3 followed by a second round of two fill-in reactions. The resulting products were finally annealed in a third and final fill-in reaction. The fragment generated was digested with both SacII and BstEII and cloned between the respective sites of pT2RAP46, to create the plasmid pT2RAP46mtSer. The mutations in this plasmid were confirmed by DNA sequencing. The construct pT2RAP46dC47 was created by replacing the SacII–XbaI fragment of pT2RAP46 with the SacII–XbaI fragment of pcDNA3RAP46ΔC47 (Zeiner et al., 1999). The constructs GST–RAP46 and GST–RAP46mtSer were obtained by inserting NarI fragments derived from pT2RAP46 and pT2RAP46mtSer into an EcoRI site of pGEX2T (Pharmacia) by blunt-end ligation. The recombinant GST–RAP46dC47 was constructed by inserting a 997 bp BglII–PstI fragment of pGEX2THap46ΔC47 (Zeiner et al., 1999) into the BglII–PstI sites of pGEX2T-RAP46. The human GR with His6 residues at its N-terminal region was cloned into the polylinker region of the baculovirus transfer vector pBB249.
Transient transfections
Unless otherwise stated, transient transfection was carried out with the Fugene transfection reagent (Pharmacia) on 100 000 COS-7 cells in 3.4-cm-diameter culture dishes. The ratios of the transfected plasmids were 0.2 µg reporter plasmid, 10 ng pTKRenilla Luc and 0.9 µg expression vector. The transfected cells were harvested 36 h later and luciferase assays were performed with the Dual Luciferase Assay System from Promega according to the manufacturer’s instructions.
In vivo phosphorylation
Thirty-six hours after transfection, the COS-7 cells were washed in medium lacking both FCS and phosphate, and incubated in 750 µl of this medium for 1 h. [32P]orthophosphate, 750 µCi (5000 Ci/mmol) were added for 4 h and the cells were washed in ice-cold phosphate-buffered saline (PBS), harvested and lysed in 1 ml HKMEN buffer (10 mM HEPES pH 7.2, 140 mM KCl, 5 mM MgCl2, 2 mM EGTA, 0.2% Nonidet P-40, protease inhibitors) containing phosphatase inhibitors (1 mM NaVO4, 20 mM NaF, 25 mM β-glycerophosphate). The lysates were passed three times through a 27 gauge needle and cleared by centrifugation for 5 min at 14 000 r.p.m. in an Eppendorf centrifuge. Immunoprecipitations were performed with either mouse monoclonal anti-GFP antibodies (Dianova, Hamburg) or 12CA5 antibody directed against the haemagglutinin (HA) tag for 3 h at 4°C. Immune complexes were recovered with 40 µl of 50% slurry of protein A–Sepharose (Pharmacia) in PBS, washed five times with HKMEN buffer and analysed by SDS–PAGE followed by either autoradiography or an immunoblot with either the C16 anti-BAG-1 antibody (Santa Cruz) or mouse monoclonal anti-GFP antibody.
Immunofluorescence
Immunofluorescence experiments were performed as previously described by Schneikert et al. (1999). The exogenous Hsp70-tagged protein was detected by a rat polyclonal anti-LDH antibody specific for the lactate dehydrogenase immunotag (Wissing and Jäättelä, 1996).
EMSAs and immunoblots
EMSAs and immunoblots with extracts of COS-7 cells transfected with the GR and/or RAP46 were performed as described previously by Gast et al. (1995). In the cell-free repression assay, extracts of COS-7 cells transfected with the GR were mixed with bacterially purified GST–RAP46, or mutants thereof, before being subjected to the EMSA.
Expression and purification of GR
His6-tagged human GR was expressed in baculovirus and processed as previously described by Whiting et al. (1995) and Srinivasan et al. (1994).
SPR experiments
The BIAcore 2000 system, NTA and CM-5 sensor chips, amine coupling kit containing N-hydroxysuccinimide, N-ethyl-N′-(3-diethylamino propyl)carbodiimide and ethanolamine hydrochloride as well as anti-GST antibody were all obtained from Biacore AB (Freiburg, Germany). The buffer used for the experiments with the CM-5-chip was 10 mM HEPES, 150 mM NaCl, 3.4 mM EDTA, 0.05% Tween-20 pH 7.4 (HBS). The buffer used for the NTA chip experiments was 10 mM HEPES, 150 mM NaCl, 50 µM EDTA and 0.005% Tween-20 pH 7.4 (eluent buffer). Recombinant human Hsp70 and bovine Hsc70 were obtained from StressGen Biotechnologies Corp. (Victoria BC, Canada). Dexamethasone was purchased from Sigma.
Circular dichroism spectroscopy
Circular dichroism spectra of GST–RAP46 and GST–RAP46mtSer fusion proteins were obtained using a Jasco J-710 circular polarizing spectrometer with Peltier temperature control. β-androsterone (0.05%) in spectral grade dioxane was employed to calibrate signal intensity. Spectra were measured over a wavelength range of 190–240 nm with the band width set at 1.0 nm, 0.1 nm resolution, scanning speed at 5 nm/min and with a 4 s response time at a sensitivity of 10 m°. Typically, a sample containing 100 µg/ml protein in a 1 mm dichroically neutral quartz cuvette was measured as the sum of four transients. A similarly signal-averaged baseline spectrum was subtracted and signal-to-noise ratio further enhanced by a Fourier transform procedure to filter out high frequency/narrow band width components. The spectrum was then converted to mean residue ellipticity values (Θmrw) for purposes of secondary structure estimation. The time-averaged secondary structure content of the two proteins was calculated from the far-UV CD spectra according to the self-consistent method of Sreerama and Woody (1993, 1994) using a sample set of 18 proteins and closure criteria set at 30 iterations or less.
Acknowledgments
Acknowledgements
This work was supported by the German Science Foundation and in part by Boehringer Ingelheim. We thank Hartwig Hennekes, Berlin, for stimulating discussions and Alex Dimerski, Berlin, for excellent technical assistance.
References
- Anfinsen C.B. (1973) Principles that govern the folding of protein chains. Science, 181, 223–230. [DOI] [PubMed] [Google Scholar]
- Bimston D., Song,J., Winchester,D., Takayama,S., Reed,J.C. and Morimoto,R.I. (1998) BAG-1, a negative regulator of Hsp70 chaperone activity, uncouples nucleotide hydrolysis from substrate release. EMBO J., 17, 6871–6878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohen S.P., Kralli,A. and Yamamoto,K.R. (1995) Hold’em and fold’em: chaperones and signal transduction. Science, 268, 1303–1304. [DOI] [PubMed] [Google Scholar]
- Bresnick E.H., Dalman,F.C., Sanchez,E.R. and Pratt,W.B. (1989) Evidence that the 90-kDa heat shock protein is necessary for the steroid binding conformation of the L cell glucocorticoid receptor. J. Biol. Chem., 264, 4992–4997. [PubMed] [Google Scholar]
- Buchner J. (1999) Hsp90 & Co.—a holding for folding. Trends Biochem. Sci., 24, 136–141. [DOI] [PubMed] [Google Scholar]
- Carey K.L., Richards,K.M., Lounsbury,K.M. and Macara,I.G. (1996) Evidence using a green fluorescence protein–glucocorticoid receptor chimera that the RAN/TC4 GTPase mediates an essential function independent of nuclear protein import. J. Cell Biol., 133, 987–996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diehl E.E. and Schmidt,T.J. (1993) Heat shock protein 70 is associated in substoichiometric amounts with the rat hepatic glucocorticoid receptor. Biochemistry, 32, 13510–13515. [DOI] [PubMed] [Google Scholar]
- Dittmar K.D. and Pratt,W.B. (1997) Folding of the glucocorticoid receptor by the reconstituted hsp90-based chaperone machinery. J. Biol. Chem., 272, 13047–13054. [DOI] [PubMed] [Google Scholar]
- Duina A.A., Chang,H.-C.J., Marsh,J.A., Lindquist,S. and Gaber,R.F. (1996) A cyclophilin function in Hsp90-dependent signal trans duction. Science, 274, 1713–1715. [DOI] [PubMed] [Google Scholar]
- Ellis R.J. (1997) Molecular chaperones: avoiding the crowd. Curr. Biol., 7, R531–R533. [DOI] [PubMed] [Google Scholar]
- Freeman B.C., Felts,S.J., Toft,D.O. and Yamamoto,K.R. (2000) The p23 molecular chaperones act at a late step in intracellular receptor action to differentially affect ligand efficacies. Genes Dev., 14, 422–434. [PMC free article] [PubMed] [Google Scholar]
- Froesch B.A., Takayama,S. and Reed,J.C. (1998) BAG-1L protein enhances androgen receptor function. J. Biol. Chem., 273, 11660–11666. [DOI] [PubMed] [Google Scholar]
- Frydman J. and Höhfeld,J. (1997) Chaperones get in touch: the Hip–Hop connection. Trends Biochem. Sci., 22, 87–92. [DOI] [PubMed] [Google Scholar]
- Gamer J., Bujard,H. and Bukau B. (1992) Physical interaction between heat shock proteins DnaK, DnaJ and GrpE and the bacterial heat shock transcription factor σ32. Cell, 69, 833–842. [DOI] [PubMed] [Google Scholar]
- Gast A., Neuschmid-Kaspar,F., Klocker,H and Cato,A.C.B. (1995) A single amino acid exchange abolishes dimerization of androgen receptor and causes Reifenstein syndrome. Mol. Cell. Endocrinol., 111, 93–98. [DOI] [PubMed] [Google Scholar]
- Giannoukos G., Silverstein,A.M., Pratt,W.B. and Simons,S.S.,Jr (1999) The seven amino acids (547–553) of the rat glucocorticoid receptor required for steroid and hsp90 binding contain a functionally independent LXXLL motif that is critical for steroid binding. J. Biol. Chem., 274, 36527–36536. [DOI] [PubMed] [Google Scholar]
- Giguère V., Hollenberg,S.M., Rosenfeld.,M.G. and Evans,R.M. (1986) Functional domains of the human glucocorticoid receptor. Cell, 46, 645–652. [DOI] [PubMed] [Google Scholar]
- Gottesman S., Wickner,S. and Maurizi,M.R. (1997) Protein quality control: triage by chaperones and proteases. Genes Dev., 11, 815–823. [DOI] [PubMed] [Google Scholar]
- Höhfeld J. and Jentsch,S. (1997) GrpE-like regulation of the Hsp70 chaperone by the anti-apoptotic protein BAG-1. EMBO J., 16, 6209–6216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jäättelä M., Wissing,D., Bauer,P.A. and Li,G.C. (1992) Major heat shock protein hsp70 protects tumor cells from tumor necrosis factor cytotoxicity. EMBO J., 11, 3507–3512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanelakis K.C., Morishima,Y., Dittmar,K.D., Galigniana,M.D., Takayama,S., Reed,J.C. and Pratt,W.B. (1999) Differential effects of the hsp70-binding protein BAG-1 on glucocorticoid receptor folding by the hsp90-based chaperone machinery. J. Biol. Chem., 274, 34134–34140. [DOI] [PubMed] [Google Scholar]
- Kang K.I., Meng,X., Devin-Leclerc,J., Bouhouche,I., Chadli,A., Cadepond,F., Baulieu,E.-E. and Catelli,M.-G. (1999) The molecular chaperone Hsp90 can negatively regulate the activity of a glucocorticosteroid-dependent promoter. Proc. Natl Acad. Sci. USA, 96, 1439–1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kullmann M., Schneikert,J., Moll,J., Heck,S., Zeiner,M., Gehring,U. and Cato,A.C.B. (1998) RAP46 is a negative regulator of glucocorticoid receptor action and hormone-induced apoptosis. J. Biol. Chem., 273, 14620–14625. [DOI] [PubMed] [Google Scholar]
- Liu R., Takayama,S., Zheng,Y., Froesch,B. Chen,G.-Q., Zhang,X., Reed,J.C. and Zhang,X.-K. (1998) Interaction of BAG-1 with retinoic acid receptor and its inhibition of retinoic acid-induced apoptosis in cancer cells. J. Biol. Chem., 273, 16985–16992. [DOI] [PubMed] [Google Scholar]
- Morishima Y., Kanelakis,K.C., Silverstein,A.M., Dittmar,K.D., Estrada,L. and Pratt,W.B. (2000) The Hsp organizer protein hop enhances the rate of, but is not essential for, glucocorticoid receptor folding by the multiprotein Hsp90-based chaperone system. J. Biol. Chem., 275, 6894–6900. [DOI] [PubMed] [Google Scholar]
- Nollen E.A.A., Brunsting,J.F., Song,J., Kampinga,H.H. and Morimoto,R.I. (2000) Bag1 functions in vivo as a negative regulator of Hsp70 chaperone activity. Mol. Cell. Biol., 20, 1083–1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa J. and Long,S.R. (1995) The Rhizobium meliloti groELc locus is required for regulation of early nod genes by the transcription activator NodD. Genes Dev., 9, 714–729. [DOI] [PubMed] [Google Scholar]
- Packham G., Brimmell,M. and Cleveland,J.L. (1997) Mammalian cells express two differently localized Bag-1 isoforms generated by alternative translation. Biochem. J., 328, 807–813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picard D., Khursheed,B., Garabedian,M.J., Fortin,M.G., Lindquist,S. and Yamamoto,K.R. (1990) Reduced levels of hsp90 compromise steroid receptor action in vivo. Nature, 348, 166–168. [DOI] [PubMed] [Google Scholar]
- Pratt W.B. and Toft,D.O. (1997) Steroid receptor interactions with heat shock protein and immunophilin chaperones. Endocrinol. Rev., 18, 306–360. [DOI] [PubMed] [Google Scholar]
- Rajapandi T., Greene,L.E. and Eisenberg,E. (2000) The molecular chaperones Hsp90 and Hsp70 are both necessary and sufficient to activate hormone binding by glucocorticoid receptor. J. Biol. Chem., 275, 22597–22604. [DOI] [PubMed] [Google Scholar]
- Rüdiger S., Germeroth,L., Schneider-Mergener,J. and Bukau,B. (1997) Substrate specificity of the DnaK chaperone determined by screening cellulose-bound peptide libraries. EMBO J., 16, 1501–1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneikert J., Hübner,E., Martin,E. and Cato,A.C.B. (1999) A nuclear action of the eukaryotic cochaperone RAP46 in downregulation of glucocorticoid receptor activity. J. Cell Biol., 146, 929–940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith D.F. and Toft,D.O. (1993) Steroid receptors and their associated proteins. Mol. Endocrinol., 7, 4–11. [DOI] [PubMed] [Google Scholar]
- Sreerama N. and Woody,R.W. (1993) A self-consistent method for the analysis of protein secondary structure from circular dichroism. Anal. Biochem., 209, 32–44. [DOI] [PubMed] [Google Scholar]
- Sreerama N. and Woody,R.W. (1994) Poly (pro) II helices in globular proteins: identification and circular dichroic analysis. Biochemistry, 33, 10022–10025. [DOI] [PubMed] [Google Scholar]
- Srinivasan G., Patel,N.T. and Thompson,E.B. (1994) Heat shock protein is tightly associated with the recombinant human glucocorticoid receptor:glucocorticoid response element complex. Mol. Endocrinol., 8, 189–196. [DOI] [PubMed] [Google Scholar]
- Stuart J.K., Myszka,D.G., Joss,L., Mitchell,R.S., McDonald,S.M., Xie,Z., Takayama,S., Reed,J.C. and Ely,K.R. (1998) Character ization of interactions between the anti-apoptotic protein BAG-1 and Hsc70 molecular chaperones. J. Biol. Chem., 273, 22506–22514. [DOI] [PubMed] [Google Scholar]
- Swamy N., Mohr,S.C., Xu,W. and Ray,R. (1999) Vitamin D receptor interacts with DnaK/heat shock protein 70: identification of DnaK interaction site on vitamin D receptor. Arch. Biochem. Biophys., 363, 219–226. [DOI] [PubMed] [Google Scholar]
- Takayama S., Sato,T., Krajewski,S., Kochel,K., Irie,S., Millan,J.A. and Reed,J.C. (1995) Cloning and functional analysis of BAG-1: a novel Bcl-2-binding protein with anti-cell death activity. Cell, 80, 279–284. [DOI] [PubMed] [Google Scholar]
- Takayama S., Bimston,D.N., Matsuzawa,S.-I., Freeman,B.C., Aime-Sempe,C., Xie,Z., Morimoto,R.I. and Reed,J.C. (1997) BAG-1 modulates the chaperone activity of Hsp70/Hsc70. EMBO J., 16, 4887–4896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takayama S. et al. (1998) Expression and localization of Hsp70/Hsc-binding anti-apoptotic protein BAG-1 and its variants in normal tissue and tumor cell lines. Cancer Res., 58, 3116–3131. [PubMed] [Google Scholar]
- Vesely J. et al. (1994) Inhibition of cyclin-dependent kinases by purine analogues. Eur. J. Biochem., 224, 771–786. [DOI] [PubMed] [Google Scholar]
- Virbasius C.-M.A., Wagner,S. and Green,M.R. (1999) A human nuclear-localized chaperone that regulates dimerization, DNA binding and transcriptional activity of bZIP proteins. Mol. Cell, 4, 219–228. [DOI] [PubMed] [Google Scholar]
- Whiting J.P., Wafford,K.A., Pribilla,I. and Petri,T. (1995) Channel cloning, mutagenesis and expression. In Ashley,R.H. (ed.), Ion Channels: A Practical Approach. Oxford University Press, Oxford, pp. 160–169. [Google Scholar]
- Wissing D. and Jäättelä,M. (1996) Hsp27 and Hsp70 increase the survival of WEHI-S cells exposed to hyperthermia. Int. J. Hyperthermia, 12, 125–138. [DOI] [PubMed] [Google Scholar]
- Yang X., Chernenko,G., Hao,Y., Ding,Z., Pater,M.M., Pater,A. and Tang,S.-C. (1998) Human BAG-1/RAP46 protein is generated as four isoforms by alternative translation initiation and overexpressed in cancer cells. Oncogene, 17, 981–989. [DOI] [PubMed] [Google Scholar]
- Ylikomi T., Wurtz,J.-M., Syvälä,H., Passinen,S., Pekki,A., Haverinen,M., Bläuer,M., Tuohimaa,P. and Gronemeyer,H. (1998) Reappraisal of the role of heat shock proteins as regulators of steroid receptor activity. Crit. Rev. Biochem. Mol. Biol., 33, 437–466. [DOI] [PubMed] [Google Scholar]
- Zeiner M. and Gehring,U. (1995) A protein that interacts with members of the nuclear hormone receptor family: identification and cDNA cloning. Proc. Natl Acad. Sci. USA, 92, 11465–11469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeiner M., Gebauer,M. and Gehring,U. (1997) Mammalian protein RAP46: an interaction partner and modulator of 70 kDa heat shock proteins. EMBO J., 16, 5483–5490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeiner M., Niyaz,Y. and Gehring,U. (1999) The hsp70-associating protein Hap46 binds to DNA and stimulates transcription. Proc. Natl Acad. Sci. USA, 96, 10194–10199. [DOI] [PMC free article] [PubMed] [Google Scholar]