Periodontitis, a biofilm-associated inflammatory disease of the periodontium, is a major cause of tooth loss in the world. This disease appears to have multiple etiologies, the most studied of which are microbial and immunological. The primary microbial factor contributing to disease is a shift in the content of the oral microflora, while the primary immunological factor is the destructive host inflammatory response. Several techniques have been used clinically to treat periodontitis, but the most successful ones appear to address both the bacterial and inflammatory components of the condition. Therefore, this review will detail the microbial and molecular nature of periodontitis, and it will also compare the efficacy of traditional and emerging technologies for treating this costly disease.
THE MICROBIOLOGY OF BIOFILMS
Traditionally, the field of microbiology has focused on studying bacteria in planktonic culture—that is, in test tubes. However, in the environment, bacteria grow in complex polymicrobial associations known as biofilms. Research has shown that these biofilms exhibit exquisite structural and functional heterogeneity that is not observed when these same bacteria are grown in planktonic culture. Therefore, appreciating the fundamental biology of biofilms is vital to understanding the pathogenesis of biofilm-associated diseases such as periodontitis.
Biofilm Structure
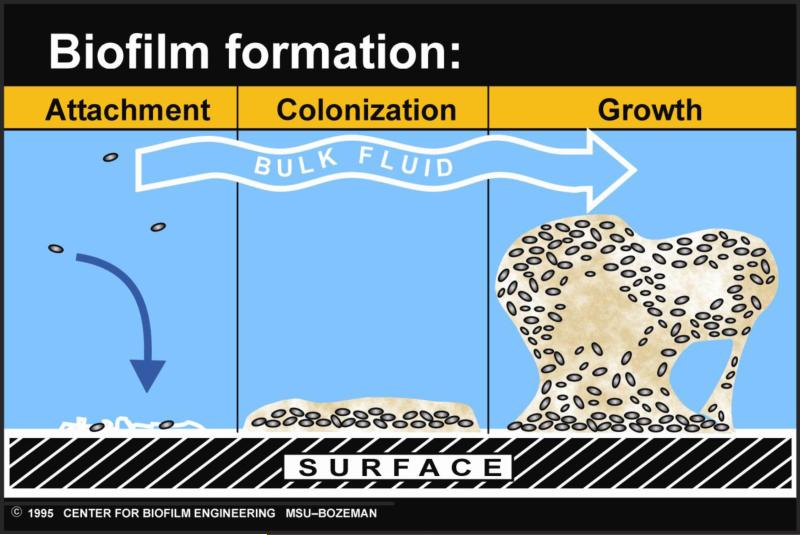
In natural settings, biofilms generally take the form of polymicrobial communities attached to biotic or abiotic surfaces. As a surface becomes colonized with individual cells, the bacteria form microcolonies which then secrete a sticky extracellular polymeric substance (18). The extracellular polymeric substance consists of polysaccharides, proteins, lipids, nucleic acids, and other polymers, and it helps the bacteria adhere to the surface, as well as to each other (42). Upon secretion of the extracellular polymeric substance, the biofilm matures by becoming larger and taking on a distinctive architecture (18). (This process is illustrated in Fig. 1.) Usually, this structure includes separate regions of fast- and slow-growing cells (4), the presence of water channels which circulate metabolites (18), and the establishment of nutrient gradients (28). Such complex structural organization allows the biofilm to exhibit functional heterogeneity.
Fig. 1.
The process of biofilm formation. Initially, individual bacterial cells attach to a surface. These cells then produce a sticky extracellular polymeric substance, which aids in attachment and allows the biofilm to grow larger. As it matures, the biofilm takes on a distinctive architecture, including water channels and nutrient gradients. The diagram is courtesy of P. Dirckx at the MSU Center for Biofilm Engineering.
Biofilm Function
Before the study of biofilms, it was generally thought that only eukaryotic tissues were capable of differentiated function. However, research has shown that subpopulations of bacteria within biofilms are also able to exhibit functional heterogeneity. This phenomenon appears to be due to several different factors.
First, biofilms in the natural environment are polymicrobial. For instance, it is estimated that over 700 bacterial species reside in the oral cavity (1). Interestingly, Streptococcus gordonii, an early colonizer in the oral cavity, actively recruits other bacteria, such as Porphyromonas gingivalis, to join the biofilm community via several genetic mechanisms. For example, genes in S. gordonii which are vital to biofilm formation include: intercellular or intracellular signaling genes (cbe and spxB); genes involved in cell wall integrity and maintenance of adhesive proteins (murE, msrA and atf); extracellular capsule biosynthesis genes (pgsA and atf); and genes involved in physiology (gdhA, ccmA and ntpB) (37). This recruitment of metabolically diverse bacteria, therefore, contributes to the overall functional heterogeneity of the biofilm. Second, subpopulations of the same bacterial species have exhibited functional differentiation. For example, subpopulations of Pseudomonas aeruginosa display varying susceptibilities to antimicrobial agents, with the most resistant cells capping the mushroom-shaped biofilm and the most susceptible cells residing near the attachment surface (4, 24). Finally, cell-to-cell communication, which often takes the form of quorum sensing in bacteria, can be used to regulate community behavior. Several different species of oral bacteria, for instance, are able to produce and respond to the quorum sensing molecule autoinducer-2 (35).
Such structural and functional heterogeneity allows biofilms to demonstrate tremendous metabolic and phenotypic flexibility. Obviously, this confers several new characteristics and advantages on the biofilm. One such characteristic is an increased ability to attach to surfaces, which is brought about by regulation of genes involved in attachment (e.g., pili) and by production of the extracellular polymeric substance (18). Another advantage is metabolic cooperation (18), wherein the waste product of one bacterial species serves as the food source for another. In the dental plaque biofilm, streptococci ferment carbohydrates to lactic acid, which is itself degraded to propionate and acetate by Veillonella spp. (45). Such metabolic cooperation also allows bacterial communities to utilize food sources that otherwise would be energetically impossible for any one species to utilize alone (18). Additionally, and perhaps most relevant clinically, biofilms often exhibit resistance to antibiotics that easily kill bacteria growing in planktonic culture. For example, minimal inhibitory concentrations can be 20- to 100-fold higher for bacteria found in biofilms compared to planktonic cultures (12). This could be because antibiotics have trouble penetrating the sticky extracellular polymeric substance, or it could be due to the fact that slow-growing subpopulations of bacteria found in specialized niches within the biofilm are often less susceptible to antibiotics (28). Yet another advantage is the ability of biofilms to avoid the host immune system. Antibodies are unable to perforate the matrix, and phagocytes often have difficulty engulfing large biofilm fragments (23). Interestingly, recent research suggests that evasion of host innate immunity can be induced when Aggregatibacter actinomycetemcomitans—a pathogen associated with an aggressive form of periodontitis—is co-cultured with S. gordonii (54). For example, it has been shown that hydrogen peroxide released by S. gordonii as part of its normal metabolism is “sensed” by A. actinomycetemcomitans, which in turn responds by inducing the complement resistance protein ApiA, subsequently becoming serum resistant (54).
Research has now firmly established that dental plaque should be thought of as a biofilm (16, 45, 68), and that periodontitis should be considered a biofilm-associated disease (59). Because of the numerous advantages biofilms possess over planktonic bacteria, this can make treatment of infections difficult. These complications must be kept in mind when treating periodontitis and other biofilm-associated diseases.
BIOFILMS AND HUMAN HEALTH
From the oral cavity to the intestinal tract, the human body constantly interacts with the biofilms that constitute our normal microbial flora. Research has shown that the composition of this microflora is closely tied to the health status of the host.
Biofilms Associated with Oral Health
With the understanding that many microorganisms associated with the human body are uncultivable, the National Institutes of Health funded a new initiative known as the Human Microbiome Project. The project's primary aim is metagenomics, that is, the global genetic analysis of entire microbial communities associated with five regions of the human body: oral cavity, nasal passages, skin, gastrointestinal tract, and urogenital tract (50). By identifying members of the microflora and sequencing portions of their genomes, the project hopes to reveal which microbial communities are associated with health and disease.
In the case of the oral cavity, attempts to characterize the normal microbial flora have met with challenges. First, over 700 species have been detected in the oral cavity, over half of which have never been cultivated (1). Second, there is substantial diversity in the content of the microflora between individuals (48) and between different oral sites within the same individual (1, 5). Third, research has indicated that dietary changes combined with poor hygiene can cause a shift in the composition of the oral microflora (2, 5). Finally, some evidence suggests that the oral microbiome changes as humans age (33). Such variation makes it difficult to identify a “typical” oral microbiome for a healthy individual.
However, despite these obstacles, progress has been made. Some studies have identified bacteria that appear to be associated with oral health. For instance, a positive association has been observed between oral health and the presence of Veillonella (39) or Capnocytophaga ochracea (57). Additionally, Streptococcus salivarius tends to inhabit the dorsal surface of the tongue of healthy patients who lack halitosis (34). An ambitious study which attempted to define the normal oral microbiome found that species such as Streptococcus mitis, Gemella haemolysans, and Granulicatella adiacens were common in healthy subjects (1). While agreement on exactly which species could be used as markers of oral health is yet to be achieved, an overall picture of what types of bacteria are commonly found in healthy individuals is starting to emerge. Despite what is still unknown, it is perfectly clear that the health of the host is inextricably tied to the nature of the oral microflora.
Microbial Shift and Disease-Associated Biofilms
Just as entire microbial communities can be associated with health, current research also points to the conclusion that entire microbial communities can be associated with disease. Because more than one bacterial species may be associated with a particular disease, the traditional concept of “one germ, one disease” may need modification. Perhaps even more revolutionizing is the idea that the lack of a beneficial organism from a biofilm may be just as important as the presence of a pathogen in the contribution to disease (66). Because of these revelations, a hypothesis formed linking certain diseases to a shift in the membership of the local microbiota.
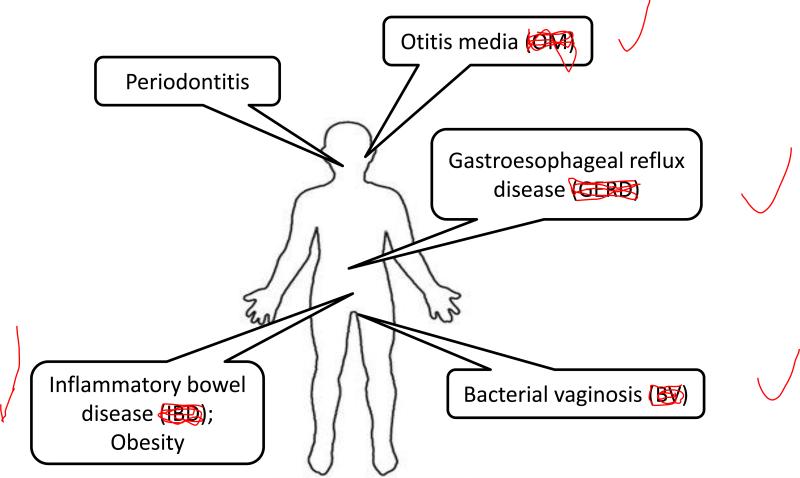
Instead of being associated with one particular etiologic agent, many chronic diseases appear to follow the “microbial shift” hypothesis. Microbial shift, more commonly known as dysbiosis, refers to the concept that some diseases are due to a decrease in the number of beneficial symbionts and/or an increase in the number of pathogens (Fig. 2). For example, intestinal dysbiosis is thought to be the cause of inflammatory bowel disease. Etiologic agents for Crohn's disease and ulcerative colitis, the two major types of inflammatory bowel disease, have never been discovered. However, there is an association between inflammatory bowel disease and a decrease in the overall numbers of intestinal bacteria, in particular members of the phyla Bacteroidetes and Firmicutes (22). Because of findings like this, probiotics (living microorganisms able to survive in the host and which are able to induce beneficial results) and prebiotics (indigestible carbohydrates which stimulate the growth of particular species of the host microflora) currently are being investigated for the treatment of inflammatory bowel disease (15).
Fig. 2.
Examples of human diseases associated with a shift in the content of the microflora.
Other conditions which are linked to a shift in the composition of the microflora include bacterial vaginosis and gastroesophageal reflux disease. In the case of bacterial vaginosis, bacteria of the phyla Actinobacteria and Bacteroidetes appear to be associated with disease (52). For gastroesophageal reflux disease, evidence suggests that a shift in the esophageal microbiota from gram-positive aerobes to gram-negative anaerobes is linked with disease (78), an association that has also been hypothesized for the development of periodontitis (44). Additionally, research indicates otitis media is associated with dysbiosis (73). Interestingly, even conditions that were never thought to be associated with bacteria may end up being caused, at least in part, by dysbiosis. Recent research involving the gut microbiome links obesity with an increase in bacteria of the phylum Firmicutes and a decrease in bacteria of the phylum Bacteroidetes (41).
Microbial Shift Leading to Periodontitis
Similar to the diseases discussed above, recent research indicates that dysbiosis in the oral cavity can lead to periodontitis. The long-standing paradigm is that as periodontitis develops, the oral microbiota shifts from one consisting primarily of gram-positive aerobes to one consisting primarily of gram-negative anaerobes (44). The development of oral dysbiosis is likely to occur over an extended period of time, gradually changing the symbiotic host-microbe relationship to a pathogenic one. During this metamorphosis, the oral health of the host deteriorates until a state of clinical disease develops. Simultaneously, a succession of distinguishable microbial complexes occurs. The first such complex that has been associated with disease is the so-called “orange complex,” which consists of gram-negative, anaerobic species such as Prevotella intermedia and Fusobacterium nucleatum (64, 66). As the disease worsens, the microbiota shift to the so-called “red complex,” which consists of the periodontal pathogens Porphyromonas gingivalis, Tannerella forsythia, and Treponema denticola (64, 66).
However, recent research has challenged this paradigm. For instance, Riep et al. (57) discovered that periodontal pathogens such as P. gingivalis and T. forsythia could also be frequently isolated from healthy controls. Kumar et al. (39) directly contradicted the existing pattern when they observed that the gram-negative bacterium Veillonella was associated with periodontal health, while the gram-positive anaerobe Filifactor alocis was associated with disease. Even more daunting is the likelihood that other pathogens associated with periodontitis have never been isolated. By cloning and sequencing 16S rRNA genes, an investigation showed that uncultivated clones from the phyla Deferribacteres, Bacteroidetes, OP11, and TM7 were associated with chronic periodontitis (38). To complicate matters even further, it has been proposed that two different herpesvirus species, Epstein-Barr virus and human cytomegalovirus, act synergistically with bacteria in the pathogenesis of periodontitis (63).
Adding yet another layer of complexity in identifying the etiologic agents is the recent discovery that periodontitis can spontaneously develop in several different kinds of immunocompromised mice. For example, spontaneous disease develops in P/E-selectin knockout mice (49), as well as in mice lacking the anti-inflammatory molecule interleukin-10 (3). Perhaps most interestingly, mice overproducing interleukin-1α also develop spontaneous periodontitis, even with the continuous administration of systemic antibiotics (19). Thus, these findings demonstrate that in addition to a bacterial etiology, genetic and immunological factors also likely contribute to periodontitis. Concomitantly, these revelations make choosing an appropriate treatment for periodontitis much more difficult.
Epidemiology of Periodontitis: Rethinking Koch's Postulates
With the revelation that genetic, immunological, environmental, and microbial factors could all plausibly be associated with chronic conditions such as periodontitis, the necessity to reevaluate Koch's postulates has been brought to light. Even if it is to be assumed that the underlying cause of periodontitis is strictly microbial, determining the precise bacterial etiology still faces difficulties. Now that there is a wide consensus that periodontitis is a biofilm-associated disease (14, 59), the primary goal is determining which of the 700 species or more found in the oral cavity is/are responsible. As discussed above, this goal has proven to be quite elusive. While Koch's postulates served medical microbiologists well for determining the causation of many human diseases, their limitations have been brought to light in the study of chronic infections. However, two different concepts may help resolve this issue.
The first is the concept of a “pathogenic microbial community” (78). This concept was exquisitely explained in a review by Siqueira and Rôças (62). Essentially, the authors suggest that since enormous variation in the composition of the oral microflora has been observed—even between patients with the same disease—it is best to approach the etiology of periodontitis from a “community-as-pathogen” model, as opposed to the traditional single-pathogen model. This approach could be supported with the use of functional gene arrays (79). Environmental microbiology, just like oral microbiology, must cope with the presence of uncultivable bacteria. To sidestep this issue, it is common practice in the field to assess the presence of genes involved in biogeochemical processes from environmental samples, instead of assessing the presence of individual bacterial species or taxa. For instance, one study determined if genes for carbon, nitrogen, phosphorus, and sulfur cycling were present in sediment samples taken from the Gulf of Mexico (76). If this technology was adapted to monitor the presence of genes involved in pathogenesis, it may prove useful for medical microbiology. In the case of oral microbiology, bacterial communities from healthy and diseased periodontal samples could be screened for “pathogenic genes” using functional gene arrays, and correlations between the presence of pathogenic genes and periodontitis could be established.
The second concept is Hill's criteria of causality. (See Table 1) Because of the rigid nature of Koch's postulates, it is difficult or impossible to satisfy them for many chronic conditions. The causal link between infection with Helicobacter pylori and peptic ulcer disease is almost universally accepted not because it fulfills Koch's postulates, but because it fulfills Hill's criteria of causality (43). In order for causation to be established, Hill's criteria requires that most of the following be fulfilled: biological plausibility, dose response, strength of association, specificity of association, consistency, and temporality (43). Thus, given the current obstacles, it appears that the etiology of periodontitis might be more readily established if current research combines the pathogenic microbial community concept with Hill's criteria of causality.
Table 1.
Hill's criteria of causality applied to periodontitis*.
| Questions | Examples of Questions Specific to Periodontitis | |
|---|---|---|
| Biological Plausibility | Does a hypothesized effect make sense in the context of current biological knowledge? | Can dysbiosis (microbial shift) be associated with chronic human disease? |
| Dose Response | ||
| Natural | Does disease occur more in individuals closer to the source? | Are higher levels of pathogenic bacteria associated with periodontitis? |
| Interventional | Does disease recede with antimicrobial treatment? | Does therapy reduce the number of suspected agents and improve the oral health of the patient? |
| Strength of Association | What is the risk of disease after infection? | Do most patients who have these pathogens develop periodontitis? Are most patients with periodontitis colonized with the same bacterial pathogens (e.g., “red complex” bacteria)? |
| Specificity of Association | Is the agent associated with only one clinical syndrome? | Do “red complex” bacteria cause diseases other than periodontitis? |
| Consistency | Do studies by different groups consistently arrive at the same findings? | Do most laboratories agree upon which bacterial species are associated with periodontitis? |
| Temporality | Does infection precede disease? | Does infection with the suspected pathogens precede development of periodontitis? Can these pathogens induce periodontitis in animal models? |
Table adapted from Lowe et al. (43).
Healthy vs. Diseased Periodontal Tissue
Despite the difficulty in defining the precise etiology of periodontitis, one thing that is certain is the striking difference in the immune status of periodontal tissue between healthy and diseased patients. Details of the many immunological differences were recently discussed in a review by Darveau (17). Essentially, clinically healthy periodontal tissue maintains a highly ordered, mild state of inflammation. For example, E-selectin expression (47) and an established interleukin-8 gradient (71) constantly guide neutrophils toward the junctional epithelium that borders the normal oral microflora, which is thought to provide the stimulus for this mild inflammatory response (17). However, clinically diseased periodontal tissue exhibits a marked histopathology. For instance, the expression of inflammatory molecules normally present in small amounts (such as Toll-like receptor-2) is greatly increased (56); different inflammatory molecules (such as Toll-like receptor-4) are expressed (17, 56); and the highly ordered state of mild inflammation is replaced by a disordered state of severe inflammation (17, 70). Thus, it is proposed that the shift from a symbiotic microflora to a dysbiotic, pathogenic community triggers the potent host inflammatory response which contributes to the tissue destruction and alveolar bone loss characteristic of periodontitis (17).
FIGHTING ORAL BIOFILMS: ADJUNCTIVE TREATMENTS FOR PERIODONTITIS
Because multiple etiologies factor into the development of periodontitis, choosing appropriate treatment options can be quite difficult. Is scaling and root planing alone sufficient? If more aggressive therapy is chosen, which one is to be used? If antibiotics are to be used, which bacterial species should be targeted? Does the patient have an underlying genetic or immunological problem that needs to be addressed? Which treatments are most cost-effective? Questions such as these must be taken into consideration when choosing an appropriate therapy.
Scaling and root planing is the primary therapy of choice for most clinicians, and it is widely considered the “gold standard” for treating periodontitis. However, scaling and root planing alone often does not produce the clinical outcomes desired in severe cases. For instance, recolonization of pathogens and recurrence of disease are quite common. Because of the underlying microbial basis of periodontitis, it is becoming more conventional to use antimicrobial therapy adjunctively with scaling and root planing, especially when treating more difficult cases of the disease. Additionally, because the host inflammatory response also plays a major role in disease progression, treatments aimed at suppressing inflammation (so-called “host modulation therapy”) can be used. The remainder of this review will focus on traditional and emerging antimicrobial therapies and host modulation therapy, all of which can be used in combination with scaling and root planing in the treatment of periodontitis.
Antibiotics
Antibiotics are often used adjunctively with scaling and root planing, and they can be applied locally or administered systemically. Dozens of studies on the efficacy of local antibiotic therapy have been conducted. A meta-analysis of the literature determined that local, sustained-release minocycline significantly improved patient outcomes when compared to scaling and root planing alone (29). Another meta-analysis confirmed this result, and it found that minocycline and tetracycline were the most effective local adjunctive therapies as measured by probing depth reduction and clinical attachment level gain (9).
Meta-analyses have also been performed to analyze the efficacy of systemically administered antibiotics. One such study concluded that adjunctive use of spiramycin and amoxicillin/metronidazole conferred statistically significant benefits for probing depth reduction and clinical attachment level gain, respectively, over scaling and root planing alone (31). In agreement with this, a recent clinical study showed that systemic administration of amoxicillin/metronidazole significantly improved clinical outcomes six months after full-mouth periodontal debridement (13). Another meta-analysis determined that systemic use of antibiotics, in some cases, could double the clinical attachment level gain when compared to mechanical therapy alone (25). However, the same study concluded that insufficient evidence existed to recommend the usage of any particular antibiotic, but that administration of tetracycline, metronidazole, and amoxicillin/metronidazole showed positive clinical effects (25). A separate review echoed the uncertainty in the literature in regard to the efficacy of systemic antibiotics, but firmly concluded that systemic antibiotics should only be used adjunctively with debridement and never as a standalone treatment (32).
It is perhaps not surprising that antibiotics yield only modest results clinically. It must be kept in mind that periodontitis is a biofilm-associated disease, and biofilms are notoriously difficult to treat with antibiotics. One complicating factor is that the identity of many oral bacteria is still unknown, and even if antibiotics could successfully target a particular known pathogen, there remains the possibility that other unidentified pathogens—which possibly serve a functionally or ecologically equivalent role as the known pathogen—would be unaffected by the treatment. Unfortunately, using multiple antibiotics simultaneously leaves a patient susceptible to developing an oral yeast infection or experiencing other severe systemic adverse effects. Yet another complication is the fact that even commensal bacteria can trigger an immune response if they reach critically high numbers or are in an inappropriate habitat within the host. Thus, the clinical spectrum of an antibiotic may be more important than previously thought. Additionally, antibiotics pose an allergy risk or produce side effects in some patients. Perhaps most importantly, the majority of antibiotics do not directly suppress the host inflammatory response which is largely responsible for the tissue destruction characteristic of periodontitis. Therefore, although antibiotics have been clearly demonstrated to be of benefit, there remains a need for more effective anti-infective therapies.
Antiseptics
Adjunctive application of antiseptics such as chlorhexidine, bleach (sodium hypochlorite), povidone-iodine, and amine fluoride can be used as an alternative to antibiotics. As is the case for antibiotics, many studies already have been conducted to determine the efficacy of antiseptics in treating periodontitis. For instance, one such study concluded that chlorhexidine was more effective than minocycline at destroying P. gingivalis biofilms in vitro (51). Another in vitro biofilm study showed that P. gingivalis was completely eradicated after 30 minutes of exposure to chlorhexidine, povidone-iodine, or Listerine (7).
However, despite the success of in vitro studies, research involving patients has been controversial. Although one meta-analysis of the literature showed a significant increase in clinical attachment level gain when compared to scaling and root planing alone for sustained-release chlorhexidine chips (29), a different meta-analysis showed that chlorhexidine was not as effective as minocycline or tetracycline in probing depth reduction or clinical attachment level gain (9). Furthermore, a meta-analysis comparing the use of chlorhexidine and other antiseptics in full-mouth disinfection procedures to conventional staged debridement concluded that full-mouth disinfection conferred no clinically relevant advantage over conventional staged debridement (40). However, the same study did show that full-mouth disinfection conferred a slight benefit over conventional staged debridement in probing depth reduction for both moderate pockets (mean difference of 0.2 mm) and deep pockets (mean difference of 0.5 mm) (40). Finally, although one group showed that chlorhexidine and bleach were able to substantially reduce the overall number of bacteria in endodontic patients (61), a meta-analysis determined that the same two antiseptics exhibited low efficacy in their ability to eliminate Enterococcus faecalis from endodontic patients (20).
It appears, therefore, that antiseptics may have substantial drawbacks when used as adjunctive therapy. Besides exhibiting only slight improvements in probing depth reduction or clinical attachment level gain, antiseptics also fail to address the issue of host inflammation. Thus, as is the case with antibiotics, there are opportunities for further research in this area.
Host Modulation Therapy
A promising approach is host modulation therapy, which as the name suggests, aims to modulate the host by suppressing the inflammatory response. As discussed above, periodontitis is characterized by detrimental inflammatory processes which destroy periodontal tissue. Matrix metalloproteinases, many of which are produced by infiltrating neutrophils, mediate this tissue destruction by degrading plasma membrane proteins and extracellular matrix proteins such as collagen (53). Since matrix metalloproteinases also promote bone resorption, it is thought that these enzymes significantly contribute to the tissue destruction and alveolar bone loss that defines periodontitis (53). Additionally, it has been long known that members of the tetracycline family of antibiotics possess the ability to inhibit matrix metalloproteinases independent of their anti-microbial activities. Doxycycline was found to be the most potent inhibitor of matrix metalloproteinases (53), and subsequently, a meta-analysis determined that adjunctive administration of subantimicrobial doses of doxycycline conferred a statistically significant benefit over scaling and root planing alone (55). Specifically, subantimicrobial doses of doxycycline used adjunctively with scaling and root planing showed a clinical attachment level gain that was 0.3-0.4 mm greater than when scaling and root planing was used alone (53). By comparison, these results are similar to those seen with the adjunctive use of locally administered antimicrobial therapies (29).
Besides doxycycline, other host modulating drugs have been investigated. A meta-analysis found that non-steroidal anti-inflammatory drugs and bone-sparing agents (such as bisphosphonates) could have potential in the treatment of periodontal disease (55). Another intriguing possibility is the use of “pro-resolving agents”—drugs that promote the resolution of inflammation, as opposed to merely blocking it (8). Resolution of inflammation involves host biochemical pathways that restore homeostasis to the periodontal tissue, and some research indicates that periodontitis results from a failure in these resolution pathways (75). Indeed, the pro-resolving agent resolvin E1, a derivative of omega-3 eicosapentaenoic acid, was shown to regenerate lost tissue and bone in a P. gingivalis-induced model of periodontitis in rabbits (30). Other known pro-resolving agents with potential therapeutic uses include lipoxins and protectins (74).
Photodynamic Therapy
Another promising approach is the use of antimicrobial photodynamic therapy. This technique uses long-wavelength visible light (red light) to activate photosensitizing agents (photosensitizers) which produce reactive oxygen species, such as free radicals and singlet oxygen (36). These toxic oxygen derivatives then react with essential cellular components such as DNA, proteins, and lipids, leading to cell death. Clinically, most photosensitizers used include dyes (such as methylene blue, acridine orange, and toluidine blue O), porphyrins, chlorins, and furocoumarins (36). Conveniently, antimicrobial photodynamic therapy can be highly localized by using certain dyes that preferentially stain bacteria, thus limiting any unintended oxidative damage in the surrounding tissue (67).
Recent research has demonstrated a potential use for antimicrobial photodynamic therapy in the treatment of periodontitis. An in vitro study showed that not only does antimicrobial photodynamic therapy destroy P. gingivalis, it also inactivates a virulence-associated protease, as well as the destructive host inflammatory mediators tumor necrosis factor-α and interleukin-1β (11). Another in vitro study suggested that antimicrobial photodynamic therapy was more effective than antibiotics at killing bacterial cells in biofilms (21). Thus, in vitro research involving antimicrobial photodynamic therapy has been encouraging, and more studies examining its long-term efficacy should be conducted. Clinical trials with antimicrobial photodynamic therapy are the subject of another review in this issue and therefore will not be discussed here. However, because this treatment appears to simultaneously destroy bacterial pathogens and suppress the destructive host inflammatory response, there has been a strong interest in the therapeutic potential of antimicrobial photodynamic therapy. (See Chapter XX.)
Probiotic Therapy
The use of probiotics to treat diseases associated with a shift in the microflora, such as inflammatory bowel disease, is already being investigated. Therefore, probiotic therapy has also been proposed for the treatment of periodontitis. Indeed, recent proof-of-concept research has shown that when a mixture of streptococcal species was applied to the teeth of canines as an adjunctive therapy following root planing, there was a delay in the recolonization of periodontal pathogens and a reduction in inflammation (69). Also, patient studies have shown that use of a mouth rinse containing Bacillus subtilis (72) or oral administration of tablets containing Lactobacillus salivarius (46) is able to reduce the number of periodontal pathogens. While this approach seems promising, it is still a relatively new concept, and more research needs to be conducted to determine its clinical efficacy.
Assessing the Efficacy of Treatment
Essentially, the parameters used to assess the efficacy of treatment fall within two broad categories: biological and clinical. Biologically, it is important to determine if treatment altered the content of the microflora and if treatment helped resolve the host inflammatory response. Several methods have been employed to address the former issue.
A quick, accurate, and inexpensive approach for identifying the members of a complex microbial biofilm is the DNA-DNA checkerboard (66). The technique relies upon the hybridization of labeled probe DNA to the genomic DNA isolated from bacterial cells in patient plaque samples. This technique has been used routinely to identify and quantify bacteria using up to 40 different species-specific DNA probes at a time (66). With this technology, researchers have been able to successfully characterize the microflora associated with health and disease in subgingival (64) and supragingival (26) plaque. More importantly, this technique has been used to determine the nature of the microbial biofilm before and after treatment of periodontitis. For instance, one study showed a reduction in the number of red and orange complex bacteria, as well as a reduction in the number of sites colonized by these pathogenic bacteria, following various forms of treatment (65). Another study showed that following scaling and root planing and weekly supragingival plaque removal for three months, the microbial profile of the treated patients was similar to those of periodontally healthy people (77). Thus, the DNA-DNA checkerboard technique has been vital to both researchers and clinicians in characterizing the nature of the biofilms associated with periodontal health and disease.
Despite its success, alternative procedures have been sought which are easier to perform or more suitable to the preferences of particular researchers and clinicians. Among these is a procedure which relies on multiplex PCR to identify bacteria in plaque samples. The efficacy of this procedure, commercially available in a kit known as micro-IDent (add company name and address), was comparable to that of the DNA-DNA checkerboard (27). Other techniques which have shown promise in monitoring the microflora during the course of periodontal treatment include terminal restriction fragment length polymorphism analysis (58) and real-time PCR (10).
Because suppression and resolution of host inflammation is also important in treating periodontitis, a novel method to assess the efficacy of treatment is to examine the host response directly. A recent study used microarrays and real-time PCR to demonstrate that inflammatory genes were down-regulated in periodontitis patients following therapy (6). In addition to analyzing the effectiveness of treatment, this method may also help reveal which host genes are most responsible for the development and persistence of periodontitis.
The clinical parameters used to assess the efficacy of treatment are arguably the most important because they directly measure the health of the patient. When it comes to patient care, improving oral (and overall) health and the patient's quality of life are of paramount concern. Biological considerations are meaningful from a scientific and academic viewpoint, but they are of secondary importance. The clinical parameters typically measured—probing depth reduction, clinical attachment level gain, bleeding-on-probing reduction, and prevention of tooth loss—are both the most common and most clinically meaningful measures of the efficacy of treatment.
CONCLUDING REMARKS
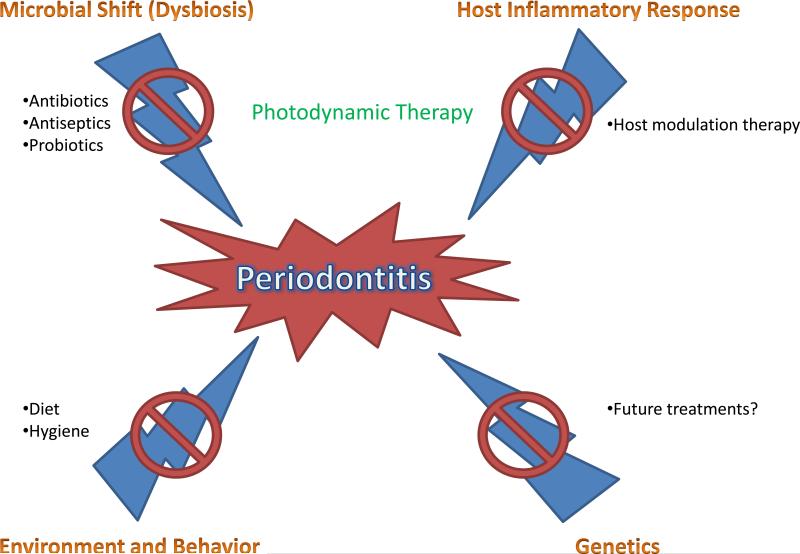
The literature as it currently stands appears to indicate that oral dysbiosis, or a shift from beneficial symbiotic bacteria to pathogenic bacteria, is at least partially responsible for the development of periodontitis. However, despite great advances in our knowledge of the underlying microbial basis of this disease, the fact still remains that periodontitis has multiple etiologies which have yet to be fully understood. Thus, while a microbial shift is known to play a significant role in the development of periodontitis, genetic, immunologic, and environmental factors must also be investigated in order for clinicians and researchers to fully understand disease progression (Fig. 3).
Fig. 3.
The various causes and treatments of periodontitis. Antibiotics, antiseptics, and probiotics have been used to control the microbial nature of periodontitis. Host modulation therapy has been used to reduce the destructive inflammatory response underlying periodontitis. Photodynamic therapy, a unique and promising technology, has been shown to address both the microbial and immunological basis of the disease. Proper diet and hygiene have been used to treat the environmental and behavioral aspects of periodontitis. As of yet, no treatment exists to address any potential underlying genetic predisposition to periodontitis.
Because of the various risk factors that contribute to periodontitis, it is possible that there will be no “magic bullet” treatment. It is also likely true that the underlying cause of periodontitis is different in different patients. For instance, one patient's periodontitis may be due to a shift in the oral microflora due to poor hygiene, while another patient's periodontitis may be due to an underlying genetic abnormality that leads to a destructive immune response. In light of this, periodontitis is perhaps better described not as a disease but as a symptom of an underlying condition. For successful treatment, it is imperative that this underlying cause be identified and addressed. Indeed, the complexity of periodontitis emphasizes the necessity of “individualized medicine” and implementing a treatment that is highly tailored to the specific needs of the patient.
Despite these complications, recent advances show tremendous potential to help patients suffering from periodontitis. Host modulation therapy, photodynamic therapy, and probiotic therapy may provide advantages not observed when antibiotics or antiseptics are used. However, much research still needs to be conducted on these new alternatives. Most importantly, well-designed and large-scale randomized clinical trials need to be performed comparing the “gold standard” of scaling and root planing to the new therapies used alone or adjunctively with scaling and root planing. Additionally, the future development of the “$1000 genome” (60) may help clinicians identify mutations in their patients’ DNA which might predispose them to aberrant immune responses.
Overall, the goal for both researchers and clinicians is to find the best treatment. From a biological perspective, the most successful treatments will likely need to attack the integrity of the periodontal biofilm and suppress the destructive host inflammatory response. From a clinical perspective, the best treatments are those that are simple, affordable, and able to confer a clinically relevant benefit to the patient.
ACKNOWLEDGMENTS
We would like to thank Camille Bretz for reviewing the manuscript.
REFERENCES
- 1.Aas JA, Paster BJ, Stokes LN, Olsen I, Dewhirst FE. Defining the normal bacterial flora of the oral cavity. J Clin Microbiol. 2005;43:5721–5732. doi: 10.1128/JCM.43.11.5721-5732.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Al-Ahmad A, Roth D, Wolkewitz M, Wiedmann-Al-Ahmad M, Follo M, Ratka-Kruger P, Deimling D, Hellwig E, Hannig C. Change in diet and oral hygiene over an 8-week period: effects on oral health and oral biofilm. Clin Oral Investig. 2009 doi: 10.1007/s00784-009-0318-9. [DOI] [PubMed] [Google Scholar]
- 3.Al-Rasheed A, Scheerens H, Rennick DM, Fletcher HM, Tatakis DN. Accelerated alveolar bone loss in mice lacking interleukin-10. J Dent Res. 2003;82:632–635. doi: 10.1177/154405910308200812. [DOI] [PubMed] [Google Scholar]
- 4.An D, Parsek MR. The promise and peril of transcriptional profiling in biofilm communities. Curr Opin Microbiol. 2007;10:292–296. doi: 10.1016/j.mib.2007.05.011. [DOI] [PubMed] [Google Scholar]
- 5.Avila M, Ojcius DM, Yilmaz O. The oral microbiota: living with a permanent guest. DNA Cell Biol. 2009;28:405–411. doi: 10.1089/dna.2009.0874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Beikler T, Peters U, Prior K, Eisenacher M, Flemmig TF. Gene expression in periodontal tissues following treatment. BMC Med Genomics. 2008;1:30. doi: 10.1186/1755-8794-1-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bercy P, Lasserre J. Susceptibility to various oral antiseptics of Porphyromonas gingivalis W83 within a biofilm. Adv Ther. 2007;24:1181–1191. doi: 10.1007/BF02877764. [DOI] [PubMed] [Google Scholar]
- 8.Bhatavadekar NB, Williams RC. New directions in host modulation for the management of periodontal disease. J Clin Periodontol. 2009;36:124–126. doi: 10.1111/j.1600-051X.2008.01354.x. [DOI] [PubMed] [Google Scholar]
- 9.Bonito AJ, Lux L, Lohr KN. Impact of local adjuncts to scaling and root planing in periodontal disease therapy: a systematic review. J Periodontol. 2005;76:1227–1236. doi: 10.1902/jop.2005.76.8.1227. [DOI] [PubMed] [Google Scholar]
- 10.Boutaga K, Savelkoul PH, Winkel EG, van Winkelhoff AJ. Comparison of subgingival bacterial sampling with oral lavage for detection and quantification of periodontal pathogens by real-time polymerase chain reaction. J Periodontol. 2007;78:79–86. doi: 10.1902/jop.2007.060078. [DOI] [PubMed] [Google Scholar]
- 11.Braham P, Herron C, Street C, Darveau R. Antimicrobial photodynamic therapy may promote periodontal healing through multiple mechanisms. J Periodontol. doi: 10.1902/jop.2009.090214. in press. [DOI] [PubMed] [Google Scholar]
- 12.Brown MR, Allison DG, Gilbert P. Resistance of bacterial biofilms to antibiotics: a growth-rate related effect? J Antimicrob Chemother. 1988;22:777–780. doi: 10.1093/jac/22.6.777. [DOI] [PubMed] [Google Scholar]
- 13.Cionca N, Giannopoulou C, Ugolotti G, Mombelli A. Amoxicillin and metronidazole as an adjunct to full-mouth scaling and root planing of chronic periodontitis. J Periodontol. 2009;80:364–371. doi: 10.1902/jop.2009.080540. [DOI] [PubMed] [Google Scholar]
- 14.Costerton JW, Stewart PS, Greenberg EP. Bacterial biofilms: a common cause of persistent infections. Science. 1999;284:1318–1322. doi: 10.1126/science.284.5418.1318. [DOI] [PubMed] [Google Scholar]
- 15.Damaskos D, Kolios G. Probiotics and prebiotics in inflammatory bowel disease: microflora 'on the scope'. Br J Clin Pharmacol. 2008;65:453–467. doi: 10.1111/j.1365-2125.2008.03096.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Darveau RP, Tanner A, Page RC. The microbial challenge in periodontitis. Periodontol 2000. 1997;14:12–32. doi: 10.1111/j.1600-0757.1997.tb00190.x. [DOI] [PubMed] [Google Scholar]
- 17.Darveau RP. The oral microbial consortium's interaction with the periodontal innate defense system. DNA Cell Biol. 2009;28:389–395. doi: 10.1089/dna.2009.0864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Davey ME, O'Toole GA. Microbial biofilms: from ecology to molecular genetics. Microbiol Mol Biol Rev. 2000;64:847–867. doi: 10.1128/mmbr.64.4.847-867.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dayan S, Stashenko P, Niederman R, Kupper TS. Oral epithelial overexpression of IL-1alpha causes periodontal disease. J Dent Res. 2004;83:786–790. doi: 10.1177/154405910408301010. [DOI] [PubMed] [Google Scholar]
- 20.Estrela C, Silva JA, de Alencar AH, Leles CR, Decurcio DA. Efficacy of sodium hypochlorite and chlorhexidine against Enterococcus faecalis--a systematic review. J Appl Oral Sci. 2008;16:364–368. doi: 10.1590/S1678-77572008000600002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fontana CR, Abernethy AD, Som S, Ruggiero K, Doucette S, Marcantonio RC, Boussios CI, Kent R, Goodson JM, Tanner AC, Soukos NS. The antibacterial effect of photodynamic therapy in dental plaque-derived biofilms. J Periodontal Res. 2009 doi: 10.1111/j.1600-0765.2008.01187.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Frank DN, St Amand AL, Feldman RA, Boedeker EC, Harpaz N, Pace NR. Molecular-phylogenetic characterization of microbial community imbalances in human inflammatory bowel diseases. Proc Natl Acad Sci U S A. 2007;104:13780–13785. doi: 10.1073/pnas.0706625104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fux CA, Costerton JW, Stewart PS, Stoodley P. Survival strategies of infectious biofilms. Trends Microbiol. 2005;13:34–40. doi: 10.1016/j.tim.2004.11.010. [DOI] [PubMed] [Google Scholar]
- 24.Haagensen JA, Klausen M, Ernst RK, Miller SI, Folkesson A, Tolker-Nielsen T, Molin S. Differentiation and distribution of colistin- and sodium dodecyl sulfate-tolerant cells in Pseudomonas aeruginosa biofilms. J Bacteriol. 2007;189:28–37. doi: 10.1128/JB.00720-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Haffajee AD, Socransky SS, Gunsolley JC. Systemic anti-infective periodontal therapy. A systematic review. Ann Periodontol. 2003;8:115–181. doi: 10.1902/annals.2003.8.1.115. [DOI] [PubMed] [Google Scholar]
- 26.Haffajee AD, Socransky SS, Patel MR, Song X. Microbial complexes in supragingival plaque. Oral Microbiol Immunol. 2008;23:196–205. doi: 10.1111/j.1399-302X.2007.00411.x. [DOI] [PubMed] [Google Scholar]
- 27.Haffajee AD, Yaskell T, Torresyap G, Teles R, Socransky SS. Comparison between polymerase chain reaction-based and checkerboard DNA hybridization techniques for microbial assessment of subgingival plaque samples. J Clin Periodontol. 2009;36:642–649. doi: 10.1111/j.1600-051X.2009.01434.x. [DOI] [PubMed] [Google Scholar]
- 28.Hall-Stoodley L, Stoodley P. Evolving concepts in biofilm infections. Cell Microbiol. 2009;11:1034–1043. doi: 10.1111/j.1462-5822.2009.01323.x. [DOI] [PubMed] [Google Scholar]
- 29.Hanes PJ, Purvis JP. Local anti-infective therapy: pharmacological agents. A systematic review. Ann Periodontol. 2003;8:79–98. doi: 10.1902/annals.2003.8.1.79. [DOI] [PubMed] [Google Scholar]
- 30.Hasturk H, Kantarci A, Goguet-Surmenian E, Blackwood A, Andry C, Serhan CN, Van Dyke TE. Resolvin E1 regulates inflammation at the cellular and tissue level and restores tissue homeostasis in vivo. J Immunol. 2007;179:7021–7029. doi: 10.4049/jimmunol.179.10.7021. [DOI] [PubMed] [Google Scholar]
- 31.Herrera D, Sanz M, Jepsen S, Needleman I, Roldan S. A systematic review on the effect of systemic antimicrobials as an adjunct to scaling and root planing in periodontitis patients. J Clin Periodontol. 2002;29(Suppl 3):136–159. doi: 10.1034/j.1600-051x.29.s3.8.x. discussion 160-132. [DOI] [PubMed] [Google Scholar]
- 32.Herrera D, Alonso B, Leon R, Roldan S, Sanz M. Antimicrobial therapy in periodontitis: the use of systemic antimicrobials against the subgingival biofilm. J Clin Periodontol. 2008;35:45–66. doi: 10.1111/j.1600-051X.2008.01260.x. [DOI] [PubMed] [Google Scholar]
- 33.Kang JG, Kim SH, Ahn TY. Bacterial diversity in the human saliva from different ages. J Microbiol. 2006;44:572–576. [PubMed] [Google Scholar]
- 34.Kazor CE, Mitchell PM, Lee AM, Stokes LN, Loesche WJ, Dewhirst FE, Paster BJ. Diversity of bacterial populations on the tongue dorsa of patients with halitosis and healthy patients. J Clin Microbiol. 2003;41:558–563. doi: 10.1128/JCM.41.2.558-563.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kolenbrander PE, Andersen RN, Blehert DS, Egland PG, Foster JS, Palmer RJ., Jr Communication among oral bacteria. Microbiol Mol Biol Rev. 2002;66:486–505. doi: 10.1128/MMBR.66.3.486-505.2002. table of contents. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Konopka K, Goslinski T. Photodynamic therapy in dentistry. J Dent Res. 2007;86:694–707. doi: 10.1177/154405910708600803. [DOI] [PubMed] [Google Scholar]
- 37.Kuboniwa M, Tribble GD, James CE, Kilic AO, Tao L, Herzberg MC, Shizukuishi S, Lamont RJ. Streptococcus gordonii utilizes several distinct gene functions to recruit Porphyromonas gingivalis into a mixed community. Mol Microbiol. 2006;60:121–139. doi: 10.1111/j.1365-2958.2006.05099.x. [DOI] [PubMed] [Google Scholar]
- 38.Kumar PS, Griffen AL, Barton JA, Paster BJ, Moeschberger ML, Leys EJ. New bacterial species associated with chronic periodontitis. J Dent Res. 2003;82:338–344. doi: 10.1177/154405910308200503. [DOI] [PubMed] [Google Scholar]
- 39.Kumar PS, Leys EJ, Bryk JM, Martinez FJ, Moeschberger ML, Griffen AL. Changes in periodontal health status are associated with bacterial community shifts as assessed by quantitative 16S cloning and sequencing. J Clin Microbiol. 2006;44:3665–3673. doi: 10.1128/JCM.00317-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lang NP, Tan WC, Krahenmann MA, Zwahlen M. A systematic review of the effects of full-mouth debridement with and without antiseptics in patients with chronic periodontitis. J Clin Periodontol. 2008;35:8–21. doi: 10.1111/j.1600-051X.2008.01257.x. [DOI] [PubMed] [Google Scholar]
- 41.Ley RE, Turnbaugh PJ, Klein S, Gordon JI. Microbial ecology: human gut microbes associated with obesity. Nature. 2006;444:1022–1023. doi: 10.1038/4441022a. [DOI] [PubMed] [Google Scholar]
- 42.Liu YQ, Liu Y, Tay JH. The effects of extracellular polymeric substances on the formation and stability of biogranules. Appl Microbiol Biotechnol. 2004;65:143–148. doi: 10.1007/s00253-004-1657-8. [DOI] [PubMed] [Google Scholar]
- 43.Lowe AM, Yansouni CP, Behr MA. Causality and gastrointestinal infections: Koch, Hill, and Crohn's. Lancet Infect Dis. 2008;8:720–726. doi: 10.1016/S1473-3099(08)70257-3. [DOI] [PubMed] [Google Scholar]
- 44.Marsh PD. Microbial ecology of dental plaque and its significance in health and disease. Adv Dent Res. 1994;8:263–271. doi: 10.1177/08959374940080022001. [DOI] [PubMed] [Google Scholar]
- 45.Marsh PD. Dental plaque: biological significance of a biofilm and community life-style. J Clin Periodontol. 2005;32(Suppl 6):7–15. doi: 10.1111/j.1600-051X.2005.00790.x. [DOI] [PubMed] [Google Scholar]
- 46.Mayanagi G, Kimura M, Nakaya S, Hirata H, Sakamoto M, Benno Y, Shimauchi H. Probiotic effects of orally administered Lactobacillus salivarius WB21-containing tablets on periodontopathic bacteria: a double-blinded, placebo-controlled, randomized clinical trial. J Clin Periodontol. 2009;36:506–513. doi: 10.1111/j.1600-051X.2009.01392.x. [DOI] [PubMed] [Google Scholar]
- 47.Moughal NA, Adonogianaki E, Thornhill MH, Kinane DF. Endothelial cell leukocyte adhesion molecule-1 (ELAM-1) and intercellular adhesion molecule-1 (ICAM-1) expression in gingival tissue during health and experimentally-induced gingivitis. J Periodontal Res. 1992;27:623–630. doi: 10.1111/j.1600-0765.1992.tb01746.x. [DOI] [PubMed] [Google Scholar]
- 48.Nasidze I, Li J, Quinque D, Tang K, Stoneking M. Global diversity in the human salivary microbiome. Genome Res. 2009;19:636–643. doi: 10.1101/gr.084616.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Niederman R, Westernoff T, Lee C, Mark LL, Kawashima N, Ullman-Culler M, Dewhirst FE, Paster BJ, Wagner DD, Mayadas T, Hynes RO, Stashenko P. Infection-mediated early-onset periodontal disease in P/E-selectin-deficient mice. J Clin Periodontol. 2001;28:569–575. doi: 10.1034/j.1600-051x.2001.028006569.x. [DOI] [PubMed] [Google Scholar]
- 50.National Institutes of Health [August 10, 2009];Human Microbiome Project. Available at: http://nihroadmap.nih.gov/hmp/.
- 51.Noiri Y, Okami Y, Narimatsu M, Takahashi Y, Kawahara T, Ebisu S. Effects of chlorhexidine, minocycline, and metronidazole on Porphyromonas gingivalis strain 381 in biofilms. J Periodontol. 2003;74:1647–1651. doi: 10.1902/jop.2003.74.11.1647. [DOI] [PubMed] [Google Scholar]
- 52.Oakley BB, Fiedler TL, Marrazzo JM, Fredricks DN. Diversity of human vaginal bacterial communities and associations with clinically defined bacterial vaginosis. Appl Environ Microbiol. 2008;74:4898–4909. doi: 10.1128/AEM.02884-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Preshaw PM, Hefti AF, Jepsen S, Etienne D, Walker C, Bradshaw MH. Subantimicrobial dose doxycycline as adjunctive treatment for periodontitis. A review. J Clin Periodontol. 2004;31:697–707. doi: 10.1111/j.1600-051X.2004.00558.x. [DOI] [PubMed] [Google Scholar]
- 54.Ramsey MM, Whiteley M. Polymicrobial interactions stimulate resistance to host innate immunity through metabolite perception. Proc Natl Acad Sci U S A. 2009;106:1578–1583. doi: 10.1073/pnas.0809533106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Reddy MS, Geurs NC, Gunsolley JC. Periodontal host modulation with antiproteinase, anti-inflammatory, and bone-sparing agents. A systematic review. Ann Periodontol. 2003;8:12–37. doi: 10.1902/annals.2003.8.1.12. [DOI] [PubMed] [Google Scholar]
- 56.Ren L, Leung WK, Darveau RP, Jin L. The expression profile of lipopolysaccharide-binding protein, membrane-bound CD14, and toll-like receptors 2 and 4 in chronic periodontitis. J Periodontol. 2005;76:1950–1959. doi: 10.1902/jop.2005.76.11.1950. [DOI] [PubMed] [Google Scholar]
- 57.Riep B, Edesi-Neuss L, Claessen F, Skarabis H, Ehmke B, Flemmig TF, Bernimoulin JP, Gobel UB, Moter A. Are putative periodontal pathogens reliable diagnostic markers? J Clin Microbiol. 2009;47:1705–1711. doi: 10.1128/JCM.01387-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sakamoto M, Huang Y, Ohnishi M, Umeda M, Ishikawa I, Benno Y. Changes in oral microbial profiles after periodontal treatment as determined by molecular analysis of 16S rRNA genes. J Med Microbiol. 2004;53:563–571. doi: 10.1099/jmm.0.45576-0. [DOI] [PubMed] [Google Scholar]
- 59.Schaudinn C, Gorur A, Keller D, Sedghizadeh PP, Costerton JW. Periodontitis: an archetypical biofilm disease. J Am Dent Assoc. 2009;140:978–986. doi: 10.14219/jada.archive.2009.0307. [DOI] [PubMed] [Google Scholar]
- 60.Shendure J, Mitra RD, Varma C, Church GM. Advanced sequencing technologies: methods and goals. Nat Rev Genet. 2004;5:335–344. doi: 10.1038/nrg1325. [DOI] [PubMed] [Google Scholar]
- 61.Siqueira JF, Jr., Rocas IN, Paiva SS, Guimaraes-Pinto T, Magalhaes KM, Lima KC. Bacteriologic investigation of the effects of sodium hypochlorite and chlorhexidine during the endodontic treatment of teeth with apical periodontitis. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2007;104:122–130. doi: 10.1016/j.tripleo.2007.01.027. [DOI] [PubMed] [Google Scholar]
- 62.Siqueira JF, Jr., Rocas IN. Community as the unit of pathogenicity: an emerging concept as to the microbial pathogenesis of apical periodontitis. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2009;107:870–878. doi: 10.1016/j.tripleo.2009.01.044. [DOI] [PubMed] [Google Scholar]
- 63.Slots J. Herpesviral–bacterial interactions in periodontal diseases. Periodontol 2000. 2010;52:117–140. doi: 10.1111/j.1600-0757.2009.00308.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Socransky SS, Haffajee AD, Cugini MA, Smith C, Kent RL., Jr Microbial complexes in subgingival plaque. J Clin Periodontol. 1998;25:134–144. doi: 10.1111/j.1600-051x.1998.tb02419.x. [DOI] [PubMed] [Google Scholar]
- 65.Socransky SS, Haffajee AD, Smith C, Martin L, Haffajee JA, Uzel NG, Goodson JM. Use of checkerboard DNA-DNA hybridization to study complex microbial ecosystems. Oral Microbiol Immunol. 2004;19:352–362. doi: 10.1111/j.1399-302x.2004.00168.x. [DOI] [PubMed] [Google Scholar]
- 66.Socransky SS, Haffajee AD. Periodontal microbial ecology. Periodontol 2000. 2005;38:135–187. doi: 10.1111/j.1600-0757.2005.00107.x. [DOI] [PubMed] [Google Scholar]
- 67.Street CN, Andersen R, Loebel NG. Periowave demonstrates bactericidal activity against periopathogens and leads to improved clinical outcomes in the treatment of adult periodontitis. In: Kessel DH, editor. Optical Methods for Tumor Treatment and Detection: Mechanisms and Techniques in Photodynamic Therapy XVIII. Vol. 7164. SPIE; San Jose, CA: 2009. [Google Scholar]
- 68.ten Cate JM. Biofilms, a new approach to the microbiology of dental plaque. Odontology. 2006;94:1–9. doi: 10.1007/s10266-006-0063-3. [DOI] [PubMed] [Google Scholar]
- 69.Teughels W, Newman MG, Coucke W, Haffajee AD, Van Der Mei HC, Haake SK, Schepers E, Cassiman JJ, Van Eldere J, van Steenberghe D, Quirynen M. Guiding periodontal pocket recolonization: a proof of concept. J Dent Res. 2007;86:1078–1082. doi: 10.1177/154405910708601111. [DOI] [PubMed] [Google Scholar]
- 70.Tonetti MS, Imboden MA, Gerber L, Lang NP, Laissue J, Mueller C. Localized expression of mRNA for phagocyte-specific chemotactic cytokines in human periodontal infections. Infect Immun. 1994;62:4005–4014. doi: 10.1128/iai.62.9.4005-4014.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Tonetti MS, Imboden MA, Lang NP. Neutrophil migration into the gingival sulcus is associated with transepithelial gradients of interleukin-8 and ICAM-1. J Periodontol. 1998;69:1139–1147. doi: 10.1902/jop.1998.69.10.1139. [DOI] [PubMed] [Google Scholar]
- 72.Tsubura S, Mizunuma H, Ishikawa S, Oyake I, Okabayashi M, Katoh K, Shibata M, Iizuka T, Toda T. The effect of Bacillus subtilis mouth rinsing in patients with periodontitis. Eur J Clin Microbiol Infect Dis. 2009 doi: 10.1007/s10096-009-0790-9. [DOI] [PubMed] [Google Scholar]
- 73.Usviatsov B, Parshuta LI, Dolgov VA. [Microbial biocenosis in the mucous membranes of the nose and the middle ear in patients with purulent otitis]. Zh Mikrobiol Epidemiol Immunobiol. 2000:85–88. [PubMed] [Google Scholar]
- 74.Van Dyke TE. Control of inflammation and periodontitis. Periodontol 2000. 2007;45:158–166. doi: 10.1111/j.1600-0757.2007.00229.x. [DOI] [PubMed] [Google Scholar]
- 75.Van Dyke TE. The management of inflammation in periodontal disease. J Periodontol. 2008;79:1601–1608. doi: 10.1902/jop.2008.080173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wu L, Kellogg L, Devol AH, Tiedje JM, Zhou J. Microarray-based characterization of microbial community functional structure and heterogeneity in marine sediments from the Gulf of Mexico. Appl Environ Microbiol. 2008;74:4516–4529. doi: 10.1128/AEM.02751-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ximenez-Fyvie LA, Haffajee AD, Som S, Thompson M, Torresyap G, Socransky SS. The effect of repeated professional supragingival plaque removal on the composition of the supra- and subgingival microbiota. J Clin Periodontol. 2000;27:637–647. doi: 10.1034/j.1600-051x.2000.027009637.x. [DOI] [PubMed] [Google Scholar]
- 78.Yang L, Lu X, Nossa CW, Francois F, Peek RM, Pei Z. Inflammation and intestinal metaplasia of the distal esophagus are associated with alterations in the microbiome. Gastroenterology. 2009;137:588–597. doi: 10.1053/j.gastro.2009.04.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Zhou J. Microarrays for bacterial detection and microbial community analysis. Curr Opin Microbiol. 2003;6:288–294. doi: 10.1016/s1369-5274(03)00052-3. [DOI] [PubMed] [Google Scholar]