Abstract
The brain is the most lipid-rich organ in the body and, owing to the impermeable nature of the blood–brain barrier, lipid and lipoprotein metabolism within this organ is distinct from the rest of the body. Apolipoproteins play a well-established role in the transport and metabolism of lipids within the CNS; however, evidence is emerging that they also fulfill a number of functions that extend beyond lipid transport and are critical for healthy brain function. The importance of apolipoproteins in brain physiology is highlighted by genetic studies, where apolipoprotein gene polymorphisms have been identified as risk factors for several neurological diseases. Furthermore, the expression of brain apolipoproteins is significantly altered in several brain disorders. The purpose of this article is to provide an up-to-date assessment of the major apolipoproteins found in the brain (ApoE, ApoJ, ApoD and ApoA-I), covering their proposed roles and the factors influencing their level of expression. Particular emphasis is placed on associations with neurological and psychiatric disorders.
Keywords: apolipoprotein, brain, CNS, lipid, neurological disease
Structure & function of apolipoproteins
The definition of ‘apolipoprotein’ can be some-what misleading. Textbooks and medical dictionaries tend to define apolipoproteins as “Any of various proteins that combine with a lipid to form a lipoprotein, such as HDL and LDL” [301], or “The protein components of lipoproteins, which remain after the lipids to which the proteins are bound have been removed” [302]. However, there are apolipoproteins, such as ApoD and Apo(a), that are not capable of forming even a nascent lipoprotein particle on their own, but rather associate with lipoproteins via hydrophobic surface features or through disulfide linkage to another ‘integral’ apolipoprotein [1,2]. For example, ApoD is structurally related to the lipocalin family, and its function as a lipid-transport protein and its identity as a component of either peripheral or CNS lipoproteins is still not completely resolved. However, it is clear that a hydrophobic ligand-binding pocket is a prominent feature of ApoD, which is important for high-affinity binding of arachidonic acid and certain steroid hormones [3]. It may be argued that if we are to consider ApoD as an ‘apolipoprotein’, then possibly other lipid-binding proteins, such as α-synuclein (fatty acid binding) and the Niemann Pick type C1 protein (lysosomal cholesterol transport), could be similarly considered to have ‘apolipoprotein functions’ [4,5]. For the purpose of this article, we have not discussed all proteins that may bind/transport lipid, rather, we have focused on those proteins that have both been historically referred to as apolipoproteins and have been clearly detected in the brain or cerebrospinal fluid (CSF) at the protein level.
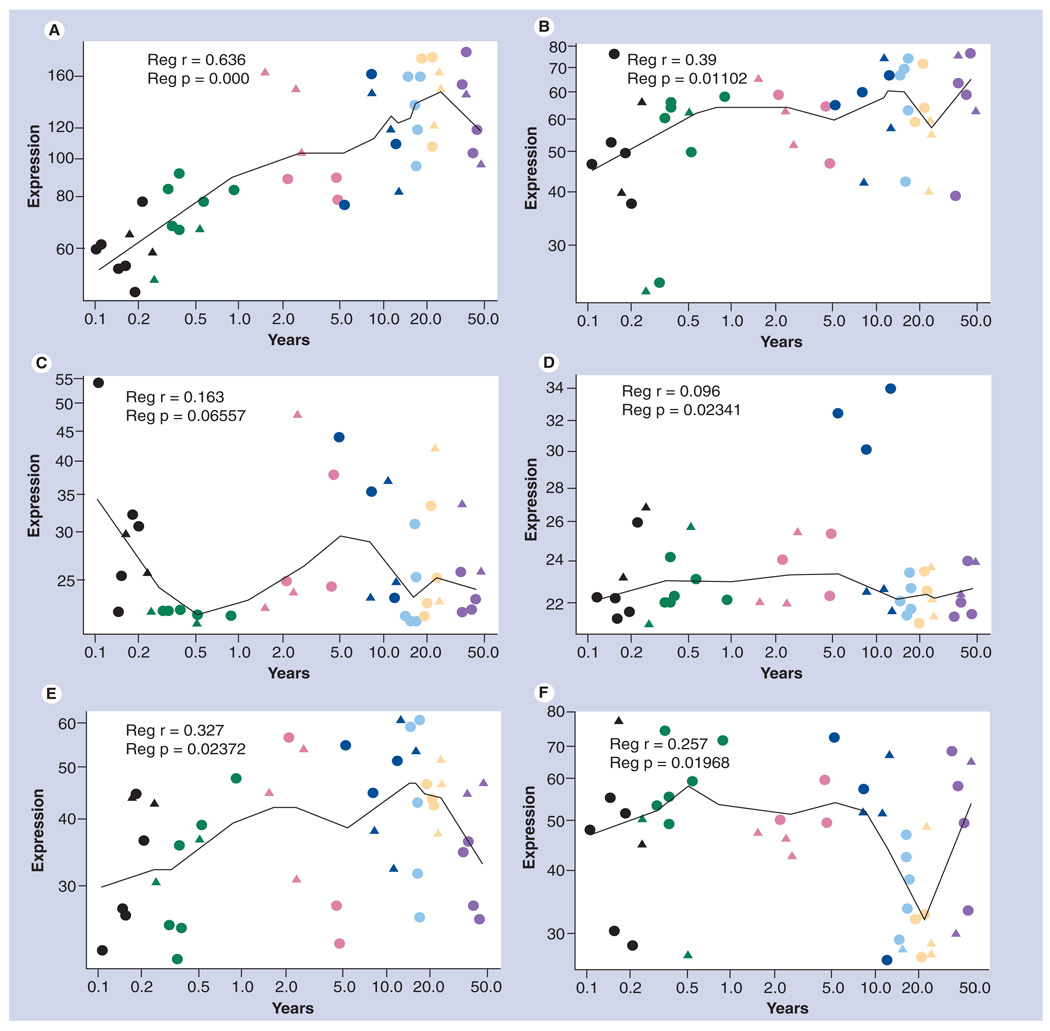
We should also acknowledge that a great volume of data related to apolipoprotein mRNA or protein expression, especially when the human brain is considered, may only be relevant for a specific brain region at a specific time in development or aging. Related to this, we have assembled apolipoprotein gene microarray data from a study, first described by Weickert et al. in 2009 [6], accessible at the National Center for Biotechnology Information Gene Expression Omnibus data-base [7,8,303], which highlights the dramatic changes in the expression of the quantitatively major apolipoproteins expressed in the human brain (Figures 1–5). These data indicate that, of the 22 apolipoprotein genes analyzed in the prefrontal cortex, only three (APOD [Figure 3C], APOE [Figure 3D] and CLU [Figure 4C]) are expressed at high levels. Furthermore, the expression of all three of these genes is dramatically affected by age, with APOD and CLU increasing five- to ten-fold from neonatal to adult ages, and APOE levels dropping by approximately 50% over the same period (Figures 3 & 4). These changes have also been confirmed previously at the protein level [9,10]. Four other genes are expressed at levels above background (~60 arbitrary expression units). We found that APOC1 mRNA levels (Figure 2C), while quite variable, are higher within the first 5 years of life, gradually dropping to adult levels. By contrast, APOC2 (Figure 2D) is at the limit of detection in most babies, but increases during the school-age period, suggesting that while APOC1 is being downregulated APOC2 may be upregulated. APOL2 mRNA (Figure 5A) increases gradually within the first decade of life, while APOL3 mRNA (Figure 5B) is expressed at lower levels, but follows a similar trend. Furthermore, it must be considered that just because a result may be at or below the limit of detection by microarray, it does not conclusively mean that a gene is not being expressed, albeit at lower levels. With these caveats in mind, we focus our attention to what is known about the major apolipoproteins in the brain regarding their role in neurological disorders.
Figure 1. Expression of apolipoproteins APO-A1, -A2, -A4 and -A5 during development of the human prefrontal cortex.
(A) Expression of APOA1. (B) Expression of APOA2. (C) Expression of APOA4. (D) Expression of APOA5. Data are derived from an Affymetrix GeneChip (HG-U133_Plus_2) microarray study conducted by Harris et al. [8] and accessible at the National Center for Biotechnology Information Gene Expression Omnibus database [7,303]. Fresh-frozen post-mortem prefrontal cortex tissue (Brodman area 46) was obtained from 44 individuals varying from 0 to 49 years of age. RNA was extracted from these samples and hybridized to HG133plus2.0 GeneChips. The data were used to examine patterns of gene expression over the course of human postnatal developmental and aging. Seven developmental periods are defined as neonate (<3 months: black), infant (3 months to <1 year: green), toddler (1 year to <5 years: red), school age (5 years to <13 years: dark blue), teenage (13 years to <20 years: light blue), young adult (20 years to <26 years: yellow) and adult (35 years to <50 years: purple). Data are plotted for both sexes (males: circles; females: triangles). Gene expression values that are less than 60 are below the limit of reliable detection, and genes with expression values in this range are, therefore, considered not to be expressed in the brain. The p-values shown were calculated as follows. Affymetrix Microarray Suite (MAS5.0) was used for image processing and data acquisition. The Bioconductor package was used to compute normalized expression values from the Affymetrix.cel files. Statistical analysis was performed using R and Bioconductor soft ware (Free Software Foundation, Boston, MA, USA). Probe sets that met the criteria of being 50% present in at least one of the age subgroups were retained in the analysis (33,210 probe sets retained; 61% of total number). Differential gene expression across chronological age was analyzed by linear regression comparing age (log scale) with gene expression (log scale) as the dependent variable. Statistical models in Supplementary Affymetrix Microarray Suite (MAS 5.0) was used for image processing and data acquisition.
Figure 5. Expression of apolipoproteins APO-L2, -L3, -L4, -L5, -L6 and -M during development of the human prefrontal cortex.
(A) Expression of APOL2. (B) Expression of APOL3. (C) Expression of APOL4. (D) Expression of APOL5. (E) Expression of APOL5. (F) Expression of APOM. See caption from Figure 1 for details of how these graphs were obtained.
Figure 3. Expression of apolipoproteins APO-C3, -C4, -D and -E during development of the human prefrontal cortex.
(A) Expression of APOC3. (B) Expression of APOC4. (C) Expression of APOD. (D) Expression of APOE. See caption from Figure 1 for details of how these graphs were obtained.
Figure 4. Expression of apolipoproteins CLU and APO-F, -H and -L1 during development of the human prefrontal cortex.
(A) Expression of APOF. (B) Expression of APOH. (C) Expression of CLU. (D) Expression of APOL1. See caption from Figure 1 for details of how these graphs were obtained.
CLU: Clusterin.
Figure 2. Expression of apolipoproteins LPA and APO-B, -CI and -C2 during development of the human prefrontal cortex.
(A) Expression of LPA. (B) Expression of APOB. (C) Expression of APOC1. (D) Expression of APOC2. See caption from Figure 1 for details of how these graphs were obtained.
The apolipoprotein family of proteins play an essential role in physiology by facilitating the transport of lipids and lipophilic substrates throughout the aqueous fluids of the body. In general, a key feature of apolipoproteins (aside from ApoD and Apo[a]) is that they possess highly conserved amphipathic α-helices, which are responsible for their lipid-binding capability [11,12]. Most apolipoproteins also contain specialized functional domains, such as a signal peptide (that directs newly translated peptides towards the secretory pathway) and a receptor binding domain (RBD). The major binding partner for the apolipoprotein RBD in the CNS is the LDL receptor (LDLR) family of receptors (which includes LDLR, ApoER2, VLDLR, MEGF7, LRP1, LRP1b and LRP2) [13,14], and this interaction facilitates the tightly regulated delivery of lipids and substrates to specific cells in the brain, as well as regulateing signal transduction pathways [15]. It is now also recognized that apolipoproteins are involved in several functions beyond lipid transport (discussed in detail later) [12].
Unique features of CNS lipid/cholesterol & apolipoprotein metabolism
The brain is the most cholesterol-rich organ in the body. While the brain is only 2% of total body mass, it contains 25% of total unesterified cholesterol in the body [16]. Virtually all cholesterol produced in the brain (>99.5%) remains unesterified, and the majority of this is present within myelin sheaths and, to a lesser degree, plasma membranes of glial and neuronal cells [17]. Lipid metabolism in the CNS is unique in that there is virtually no exchange of cholesterol and lipoproteins from the peripheral circulation, owing mostly to the impermeable nature of the BBB [18,19]. Thus, cholesterol in the CNS exists in a compartment distinct from the rest of the body. This has been clearly demonstrated in numerous studies, where radiolabelled cholesterol injected into the periphery cannot be detected in the CNS [20–22]. Thus, practically all cholesterol present in the CNS is synthesized de novo in brain cells, and is efficiently recycled within the CNS, having a half-life of between 1 and 5 years in comparison with a few hours in the periphery [23,24].
Distinct differences also exist in the composition of lipoprotein particles and the apolipoproteins present in the CNS compared with the plasma and peripheral tissues. The transport of lipids throughout the CNS is carried out predominantly by specialized HDL-like lipoproteins, unlike the rest of the body, where a broad range of lipoprotein densities are utilized [25–27]. The most abundant apolipoproteins in the CSF are ApoE (0.3 ± 0.2 mg/dl) and ApoA-I (0.37 ± 0.08 mg/dl) with lower quantities of ApoJ, ApoD, ApoA-II and ApoA-IV [27,28]. The apolipoproteins ApoA-I, ApoE and ApoJ are predominantly located on distinctly independent lipoprotein particles within the CSF. ApoJ lipoproteins are typically smaller, and contain less lipid than ApoA-I and ApoE lipoproteins [29]. Astrocytes are the major source of ApoE and ApoJ in the CSF, and in vitro studies, examining the profile of lipoproteins secreted by murine astrocytes, have corroborated these in vivo observations [30]. A review focusing on lipoprotein biogenesis in the brain compared with the plasma compartment provides information on similarities and differences regarding lipid-transfer proteins and other factors [31].
Of the 22 known apolipoproteins, nine have been detected at the protein level, to varying degrees, in the CNS, and evidence of significant mRNA expression has only been found for eight (APOC1, APOC2, APOD, APOE, CLU, APOL2 and APOL3 [Figures 2C, 3C, 3D, 4C, 5A & 5B] and text regarding APOA4). It is possible that some brain apolipoproteins were actually synthesized outside the CNS; thus, we would not expect their encoding mRNA to be found in brain cells. For example, the transit of peripherally derived apolipoproteins into the CNS has been suggested for ApoA-I and ApoA-II, as they may enter the CSF via the choroid plexus [26].
Altered cholesterol metabolism and polymorphisms within the apolipoprotein genes have been associated with several neurological and psychiatric conditions, thus emphasizing the important role these factors play in CNS physiology. The purpose of this article is to provide an up-to-date account of the most abundant apolipoproteins present within the CNS.
ApoE
ApoE is highly expressed in the CNS, and the brain is the second largest site of ApoE synthesis in the body [32]. There is no exchange of brain-derived and peripheral ApoE; therefore, the CNS represents a distinct compartment in terms of ApoE metabolism [19]. Astrocytes are the primary source of ApoE in the brain; however, ApoE is also expressed by oligodendrocytes, ependymal layer cells, microglia and, under certain physiological circumstances, in neurons [33–41]. The upstream promoter region of the APOE gene contains several regulatory regions that can bind a variety of different transcription factors (TFs) [42–49]. One of the best characterized mechanisms of APOE upregulation is regulation by the liver X receptors (LXRs) and retinoid X receptors (RXRs), usually in response to increased levels of cellular cholesterol and oxy-sterols [44]. LXR and RXR also activate expression of the membrane-bound lipid-transport proteins ABCA1 and ABCG1, which are involved in transferring lipids to ApoE in the brain [50–52]. Importantly, a lack of these transport proteins results in poorly lipidated ApoE, which is turned over more rapidly in the brain [53].
ApoE plays a major role in cholesterol and phospholipid transport in the CNS. However, APOE−/− mice display only a mild phenotype, and there is evidence to suggest that ApoD may play a compensatory role, as this protein is increased 50-fold in these animals [54,55]. Briefly, ApoE is secreted by astrocytes as a nascent lipoprotein [56–58], cholesterol is then transferred to ApoE, predominantly via ABCA1, which is expressed on astrocytes, microglia and neurons [50,52]. ABCG1 may also play a role in transferring lipids to ApoE, as it matures into a lipidated lipoprotein particle [52], and there is evidence that ABCG1 can regulate brain lipid homeostasis [51,59–61]. It has been proposed that, in astroglial cells, endogenously synthesized ApoE-containing lipoproteins, and partially lipidated ApoA-I-containing lipoproteins formed via the action of ABCA1, accept additional lipids via ABCG1, which promotes maturation of a larger lipoprotein particle [51]. However, in vivo studies in mice suggest that ABCG1 may not play the major role in ApoE lipidation [60].
The transport of cholesterol to cells within the CNS is then mediated by the interaction of ApoE with cell surface receptors, which are abundant in the brain (including LDLR, LDL receptor-related protein [LRP1], VLDL receptor [VLDLR], LR8/ApoE receptor-2 and LRP2), followed by endocytosis (reviewed in [62]).
In humans, ApoE exists as three major isoforms – ApoE2, ApoE3 and ApoE4 – which differ in their Cys/Arg composition at positions 112 and 158. ApoE2 contains Cys112, Cys158; ApoE3 contains Cys112, Arg158; and ApoE4 contains Arg112, Arg158 [63]. Inheritance of ApoE4 results in a dose-dependent increase in the risk of developing late-onset Alzheimer’s disease (AD), and is the most important genetic risk factor known for this illness, whereas ApoE2 is associated with decreased risk [64,65]. This finding has been replicated in many studies, including two recent genome-wide association studies [66,67]. Additionally, possession of ApoE4 is associated with adverse outcomes in several CNS pathological states, including traumatic brain injury (TBI), spinal cord injury (SCI) and stroke [68–70]. Interestingly, a meta-analysis study of ApoE and Parkinson’s disease (PD) found that the APOE ε2 allele is associated with a higher disease risk [71]. These findings highlight the importance of ApoE in neurobiology. The precise mechanism by which differences in ApoE isoform increase risk in these circumstances is not completely understood; however, ApoE is known to play a variety of roles in the brain, in addition to lipid transport, which may be of relevance.
ApoE is found to colocalize with amyloid-β (Aβ) in the AD brain, and this occurs primarily through interaction with the lipid-binding amphipathic α-helical region of ApoE [65,72–75]. ApoE plays a role in enhancing the clearance and degradation of Aβ in the brain. The binding of ApoE to Aβ forms a complex that can interact with ApoE receptors, resulting in endocytosis of Aβ, and either removal across the BBB or intracellular degradation in microglia and astrocytes [76–84]. In vitro studies using lipid-associated ApoE (which is physiologically more relevant than lipid-free ApoE) have found that ApoE3 binds Aβ with approximately 20-fold greater avidity than ApoE4, and this is hypothesized to result in ApoE4 being less efficient at Aβ clearance [85]. In accordance with this, total Aβ levels are increased in the brain tissue of AD and TBI patients who carry ApoE4 [86,87].
In transgenic mouse models of AD, expression of mutant human amyloid precursor protein (hAPP) results in intense Aβ deposition, which, interestingly, is reduced on an APOE−/− background [88]. This indicates that murine ApoE facilitates Aβ plaque formation. In stark contrast, the addition of human ApoE isoforms to this mutant hAPP/APOE−/− model results in a further delay in Aβ deposition (ApoE2 > ApoE3 > ApoE4) [89–91]. The cellular mechanism(s) underlying these effects and the observed interspecies difference are not entirely understood; however, these findings highlight the influential role ApoE plays in modulating Aβ deposition.
Following peripheral nerve injury, secretion of ApoE by resident macrophages is dramatically increased, resulting in a 100–200-fold increase in ApoE levels at the site of injury, where it plays an important role in the redistribution of lipids to regenerating neurites [32,36,92]. ApoE is increased following TBI, and experimentally induced brain lesions and CNS neurons are capable of synthesizing ApoE in response to stressful stimuli, such as cerebral infarction and hypoxia [41,93–97]. Several lines of evidence indicate this increase may be conferring a neuroprotective and regenerative effect in the CNS [95,98,99]. Of potential relevance to this, the regulatory region of the APOE gene contains binding sites for several inflammatory response-associated TFs (reviewed in [46]), most notably nuclear factor κB (NF-κB) [47]. NF-κB regulates the expression of over 200 genes, thus enabling it to coordinate the cellular response to a wide range of stressful stimuli. The regulatory pathways of NF-κB are elaborate, and this TF can play a major role in several responses, including cell survival, inflammation and immune regulation [100]. Several studies have searched for differences in ApoE expression associated with AD status or ApoE isoform with varying results [101–112]. However, overall, it appears that the ApoE isoform is the more influential regulator of ApoE protein level, with lower total ApoE levels associated with the ε4 allele [110,113].
ApoE is proposed to be involved in several other functions, including immunomodulation, oxidative stress, stabilization of neuronal microtubules, synaptic plasticity and apoptosis [114–119]. The contributions that these ApoE-related functions may make toward normal physiology and AD remain to be established.
ApoJ/clusterin
ApoJ, also known as clusterin (encoded by the CLU gene), is a predominantly secreted 75–80-kDa disulfide-linked heterodimeric glycoprotein, which serves as both a lipid-transport protein (with the ability to induce cholesterol efflux) and a molecular chaperone in the cellular stress response [120–122]. In addition, lower mass versions of ApoJ (~49–60 kDa) are observed in the cytosol and nucleus, particularly during cellular stress (reviewed in [123]). The promoter region of the CLU gene contains several response elements for transcription factors involved in the response to cellular stress [124–127]. ApoJ is dramatically upregulated during cellular stress, and is able to bind and stabilize a diverse range of misfolded proteins, including several involved in neurodegenerative conditions, such as Aβ, Lewy bodies and prion deposits [128–130]. In the extracellular space, ApoJ binds these substrates, inhibiting an inflammatory response and promoting endocytosis by phagocytic cells via interaction with the megalin/LRP-2 receptor [131,132]. ApoJ also promotes the phagocytosis of apoptotic debris by this same mechanism [133,134].
ApoJ is produced in several regions of the brain, primarily by astrocytes, but also in pyramidal neurons of the hippocampus and Purkinje neurons in the cerebellum [135,136]. Neuronal levels of ApoJ protein can increase significantly following stressful conditions [137,138]. Furthermore, increased expression of brain ApoJ is observed in several pathological conditions; AD, multiple sclerosis, TBI, SCI, gliomas, ischemia, epilepsy, chemically induced lesions and aging [94,95,139–142]. It seems likely that ApoJ is playing a protective role in these injurious states, as APOJ−/− mice display significantly impaired recovery to cerebral ischemic insult [143].
In AD, ApoJ levels are particularly increased in regions most afflicted with AD pathology: the hippocampus and entorhinal cortex [144,145]. ApoJ can bind Aβ fibrils, and is associated with Aβ plaques, neuropil threads and cerebrovascular amyloid deposits [144,146,147]. In vitro and experimental animal models have demonstrated that ApoJ is able to inhibit Aβ aggregation and facilitate its clearance across the BBB [148,149]. There is evidence that some of these processes are regulated in a cooperative manner with ApoE [150]. Interestingly, APOJ−/− on a mutant hAPP mouse background results in decreased levels of Aβ deposition, analogous to the result observed with APOE−/− mice (see previously). By contrast, double knockout of both APOE and APOJ on this hAPP background results in dramatically increased Aβ deposition [150]. The mechanism(s) responsible for this paradoxical observation is not entirely understood. The importance of ApoJ in AD pathogenesis is further underlined by two recent genome-wide association studies linking polymorphism within the CLU gene with a significantly increased risk of AD [66,67]. In fact, after APOE ε4, this polymorphism is the second most influential genetic risk factor for late-onset AD.
In addition, there is emerging evidence to suggest that ApoJ may play a role in neuritic outgrowth. Similar to ApoE and ApoD, ApoJ can escape the secretory pathway and enter the cytosol under certain stressful conditions [151–154], and experiments by Kang et al. [155], have reported that cytosolic ApoJ can bind with the microtubule-destabilizing protein superior cervical ganglia neural specific 10-like protein SCLIP, resulting in enhanced neurite outgrowth. This is of interest when it is considered that neuritic dysfunction is a major part of many neurodegenerative illnesses [156,157]. Therefore, this finding may represent another potential mechanism by which ApoJ may exert a neuroprotective effect.
ApoD
ApoD is a 27–33-kDa glycoprotein that does not share significant degrees of homology with other apolipoproteins but, instead, is structurally more similar to the lipocalin family (which includes ApoM). Thus, ApoD cannot support the synthesis of nascent lipoprotein particles [3,158,159]. Early studies suggested that ApoD binds lipids, including cholesterol, arachidonic acid and a variety of steroids such as pregnenolone, dihydrotestosterone, testosterone, dehydroepiandrosterone and estradiol [160–163]. The ApoD crystal structure reveals an eight-stranded, antiparallel β-barrel flanked by an α-helix [3]. ApoD contains a binding pocket that is highly specific for certain lipids (progesterone and arachidonic acid). However, other lipids may interact via an alternate mechanism [164]. ApoD has a number of ‘exposed’ hydrophobic residues residing in three out of its four extended loops [3]. This generates a region of surface hydrophobicity close to the open end of the binding pocket, which facilitates ApoD association with HDL particles, and also permits insertion of ApoD into cellular lipid membranes [3]. These exposed hydrophobic residues may also permit low-affinity binding with many of the lipids mentioned previously.
ApoD is expressed in several regions of the brain in both cortex and cerebellum. Various human and mammalian studies, utilizing northern and western blotting analyses, have demonstrated that the majority of ApoD is produced in white matter and the subarachnoid space [165,166], but also in the dorsolateral prefrontal cortex, occipital cortex, substantia nigra, hippocampus and cerebellum [9,167]. Brain ApoD is synthesized and excreted predominantly by oligodendrocytes and astrocytes. Under normal circumstances, neuronal ApoD protein, but not mRNA, is also observed in Purkinje neurons and, to a lesser degree, in cortical neurons; however, this may be increased under pathological conditions [168].
Interestingly, ApoD expression is altered in several disease states and normal aging. ApoD expression is increased in the dorsolateral prefrontal cortex during normal aging and in patients with schizophrenia [9,167]. The dorsolateral prefrontal cortex is a region that plays a critical role in attention and working memory and is implicated in psychiatric disorders, such as schizophrenia, autism and depression [169,170]. In AD, ApoD is increased in the CSF and affected brain regions (including hippocampus, frontal cortex and temporal cortex) [103,171,172], plus an increased incidence of neuronal ApoD immunostaining, is observed in cortical neurons, and appears to correlate with neurofibrillary tangle density (a hallmark of AD pathology) [168,173]. ApoD is also increased in the substantia nigra of PD patients, and in the CSF of patients with meningoencaphalitis, stroke, dementia and motor neurone disease [171,174]. Furthermore, animal models have demonstrated an increase in ApoD expression following TBI, stroke, kainic acid injection and an entorhinal cortex lesion, and an approximately 30-fold increase in murine models of the human cholesterol-storage disorder, Niemann–Pick disease [55,175–179].
The increased expression of ApoD observed in such a diverse range of injurious/pathological conditions may reflect a general role of ApoD in the stress response and neuroprotection. In accordance with this theory, the promoter region of the APOD gene contains several consensus sites for factors involved in the stress response, including NF-κB and AP1 (for reviews see [154,180]).
Increased oxidative stress is a key feature in both normal aging and many neurological disorders [9,181–183], and recent studies strongly support an antioxidant role of ApoD in the brain that is correlated with its ability to prevent lipid peroxidation [184]. Knockout of APOD in mice, and glial lazarillo, its homolog in Drosophila, results in increased peroxidation of brain lipids, decreased life expectancy and impaired performance in tests of behavior and learning [184,185]. Conversely, overexpression of human ApoD in these animal models has been demonstrated to decrease lipid peroxidation, plus enhance neuroprotection and life expectancy in response to experimentally induced stressors [184,186–188].
There is also evidence suggesting that ApoD may play a role analogous to ApoE in the redistribution of cholesterol and phospholipids during nerve regeneration and remyelination. Nerve-crush injury to the rat sciatic nerve results in an approximate 500-fold increase in ApoD protein and an approximate 40-fold increase in mRNA [189,190]. Similarly, following stroke, ApoD is increased in pyramidal neurons and oligodendrocytes within the infarct area [176]. In fact, there are several lines of evidence indicating a similar or complimentary/overlapping function of ApoD with ApoE. These are that ApoD can also bind with Aβ plaques [191], ApoD protein is increased approximately 50-fold in the frontal cortex of APOE−/− mice [55], both ApoD and ApoE are increased in the hippocampus following an entorhinal cortex lesion [55] and kainic acid exposure [177], and ApoE isoform may influence the concentration of ApoD in the hippocampus and CSF [171]. It has been speculated that colocalization with compact Aβ plaques may indicate a role for ApoD in the aggregation and/or removal of Aβ; however, this is yet to be demonstrated [191].
The importance of ApoD in AD pathophysiology is reinforced by genetic studies [192–194]. Interestingly, an intronic polymorphism within the APOD gene, found exclusively in people of African heritage, is significantly associated with increased AD risk [194], but only when the APOE ε4 allele is also present. Since this sequence change linked to AD risk is not in a coding region, it would be interesting to determine if this polymorphism can alter APOD mRNA splicing and/or gene regulation.
In addition to the association of ApoD with neurodegenerative disease, the region on chromosome 3 encoding APOD has been tightly linked with schizophrenia in a genome-scan meta-analysis [195], and an APOD polymorphism is associated with long-term clinical outcome [196]. Interestingly, the atypical antipsychotic drug, clozapine, has been demonstrated to increase ApoD levels in the rodent brain, and it remains possible that this increase plays an underlying therapeutic role in this drugs mechanism of action [197,198].
ApoA-I
ApoA-I is a 27-kDa protein, which is one of the most abundant apolipoproteins in the CSF, and ApoA-I protein, but not mRNA, has been detected in brain tissue [25,26,199–201]. In support of this, we find that APOA1 mRNA is at very low/absent levels throughout human development and adulthood (Figure 1A). ApoA-I in the CNS is believed to be plasma derived, crossing the BBB at the choroid plexus [25,26]. There is also evidence to suggest brain endothelial cells are able to synthesize ApoA-I and contribute to the CNS pool [202,203].
Decreased levels of ApoA-I have been reported in the brain tissue and CSF of schizophrenic patients [201]. Decreased levels were also observed, on average, in the liver, red blood cells and serum; however, there was no correlation between peripheral and CNS levels within individual patients. This indicates that, although brain ApoA-I originates in the periphery, the mechanisms regulating ApoA-I concentration and metabolism in CNS are clearly independent.
Interestingly, a polymorphism in the promoter region of the APOA1 gene (−75 A/G) has been linked with an earlier age of AD onset, whereby individuals homozygous for the A allele (at this location) develop AD, on average, 8 years earlier than heterozygotes [204]. This allele is associated with a modest increase in plasma ApoA-I and, depending on the in vitro model used, an increased rate of ApoA-I expression [205–208]. Altered levels of ApoA-I in the AD brain have not yet been clearly established. One study has observed an increase of ApoA-I in the CSF of AD and dementia patients [209], while another study found no difference [200]. We have found that ApoA-I protein is significantly decreased in cortical homogenates from AD patients (n = 15), compared with controls (n = 8) [elliott da & garner b, unpublished data].
The function(s) of brain ApoA-I are not entirely clear. ApoA-I plays a major role in peripheral cholesterol transport and it seems probable that ApoA-I plays an analogous role in the CNS [199]. In support of this, ApoA-I is an important lecithin-cholesterol acyltranferase (LCAT) activator and, as LCAT is expressed in the brain [166], this provides a plausible mechanism for CNS cholesterol transport. ApoA-I has also been demonstrated to bind with the extra-cellular domain of APP (which can be cleaved to produce Aβ [210]), and has been found to co-localize with occasional Aβ plaques in the cortex of AD patients [200,211]. This raises the possibility that ApoA-I could influence APP cleavage and Aβ aggregation and/or clearance; however, this is yet to be clarified. A greater understanding of both the functions ApoA-I fulfills in the brain, and the mechanisms regulating ApoA-I metabolism in the CNS, should yield insights into its association with schizophrenia and AD.
Less-abundant brain apolipoproteins
Other apolipoproteins have been detected in the brain, albeit at much lower levels than those discussed. Of particular interest, ApoC-I mRNA and protein has been detected in brain astrocytes and polymorphisms of the APOC1 gene are associated with altered transcription and increased incidence of AD [212–217]. Interestingly, ApoC-I is known to block ApoE receptor interaction, and expression of APOC1 decreases with age (Figure 2C) [218–220].
ApoA-IV is synthesized in the hypothalamus and colocalizes predominantly with neurons and, to a lesser extent, glia in this brain region [221,222] (of note, data presented in Figure 1C, where APOA4 expression is negligible, is from the prefrontal cortex [Brodmann area 46], not hypothalamus). Expression of ApoA-IV is increased following a high-fat meal, and ApoA-IV has been demonstrated to play a centrally acting role in satiety [223–225]. There is also evidence to suggest that a polymorphic version of ApoA-IV, which is more efficient at activating LCAT, may be associated with increased AD risk [226].
APOL2 is expressed in the brain (Figure 5A), with the level of expression being increased in schizophrenia [227]. In addition, polymorphisms in this gene have been linked to schizophrenia risk [228]. However, the biological function of ApoL-II in the brain is currently unclear [229].
ApoA-II, ApoC-II, ApoC-III and ApoH proteins have all been detected in the CNS [25,27,28,200,230–232]. However, the regulation and possible roles of these apolipoproteins in the CNS is poorly understood [222,226,233].
Future perspective
Our current understanding of the major apoipoproteins in the brain strongly suggests that ApoD, ApoE and ApoJ exert beneficial effects in several neurological conditions. Thus, it is tempting to speculate that a therapeutic intervention aimed at increasing expression of these apolipoproteins may be of clinical benefit. Brain ApoE levels in mice are increased upon administration of the LXR agonist, TO-901317, and in transgenic models of AD, this is associated with decreased Aβ production [234–236]. As discussed previously, the antipsychotic drug clozapine is able to increase brain ApoD levels, and investigations are ongoing to see whether this clozapine-induced ApoD increase may be of benefit in a broader range of neurological conditions [237].
A greater understanding of brain apolipoprotein biology and the mechanisms responsible for polymorphism-associated disease risk, should provide fresh insight into the underlying mechanisms of neurological disease and uncover novel therapeutic targets. More specifically, further elucidation of apolipoprotein interactions with the LDLR family of receptors, and the role this plays in neurological and psychiatric conditions, may lead to therapeutics that directly target these receptors. Of relevance to this, it has been found that mimetic peptides, derived solely from the receptor-binding domain of ApoE, are able to exert strong bioactive effects [238–240]. These peptides have been demonstrated to confer a neuroprotective effect in animal models of TBI, multiple sclerosis, cerebral hemorrhage and AD [241–246]. Furthermore, these studies highlight that the entire holoprotein of an apolipoprotein does not necessarily need to be present in order to confer a beneficial effect, and it is conceivable that future research may identify small functional peptides derived from ApoD and ApoJ [247]. In addition, technology is currently being developed that aims to promote the transport of therapeutic peptides across the BBB and make them more resistant to degradation [247–250].
Acknowledgments
Brett Garner is supported by a Research Fellowship from the Australian Research Council (ARC Grant No. FT0991986). David A Elliott is supported by a Viertel Postdoctoral Fellowship awarded by Alzheimer’s Australia. Cyndi Shannon Weickert is supported by the Schizophrenia Research Institute, New South Wales Health, Macquarie Group Foundation, Prince of Wales Medical Research Institute and the University of New South Wales. The authors would like to thank their collaborator Maree J Webster for her valuable contribution to the construction of the developmental microarray results. This work was supported by funding from the NIH National Institute of Mental Health Intramural Research Program.
Footnotes
Financial & competing interests disclosure
The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Bibliography
Papers of special note have been highlighted as:
▪ of interest
▪▪ of considerable interest
- 1.Gaubatz JW, Heideman C, Gotto AM, Jr, Morrisett JD, Dahlen GH. Human plasma lipoprotein [a]. Structural properties. J. Biol. Chem. 1983;258(7):4582–4589. [PubMed] [Google Scholar]
- 2.Weech PK, Provost P, Tremblay NM, et al. Apolipoprotein D – an atypical apolipoprotein. Prog. Lipid Res. 1991;30(2–3):259–266. doi: 10.1016/0163-7827(91)90023-x. [DOI] [PubMed] [Google Scholar]
- 3.Eichinger A, Nasreen A, Kim HJ, Skerra A. Structural insight into the dual ligand specificity and mode of high density lipoprotein association of apolipoprotein D. J. Biol. Chem. 2007;282(42):31068–31075. doi: 10.1074/jbc.M703552200. [DOI] [PubMed] [Google Scholar]
- 4.Ben Gedalya T, Loeb V, Israeli E, Altschuler Y, Selkoe DJ, Sharon R. α-synuclein and polyunsaturated fatty acids promote clathrin-mediated endocytosis and synaptic vesicle recycling. Traffic. 2009;10(2):218–234. doi: 10.1111/j.1600-0854.2008.00853.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kwon HJ, Abi-Mosleh L, Wang ML, et al. Structure of N-terminal domain of NPC1 reveals distinct subdomains for binding and transfer of cholesterol. Cell. 2009;137(7):1213–1224. doi: 10.1016/j.cell.2009.03.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Weickert CS, Elashoff M, Richards AB, et al. Transcriptome analysis of male–female differences in prefrontal cortical development. Mol. Psychiatry. 2009;14(6):558–561. doi: 10.1038/mp.2009.5. [DOI] [PubMed] [Google Scholar]
- 7.Edgar R, Domrachev M, Lash AE. Gene expression omnibus: NCBI gene expression and hybridization array data repository. Nucleic Acids Res. 2002;30(1):207–210. doi: 10.1093/nar/30.1.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Harris LW, Lockstone HE, Khaitovich P, Weickert CS, Webster MJ, Bahn S. Gene expression in the prefrontal cortex during adolescence: implications for the onset of schizophrenia. BMC Med. Genomics. 2009;2:28. doi: 10.1186/1755-8794-2-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim WS, Wong J, Weickert CS, Webster MJ, Bahn S, Garner B. Apolipoprotein-D expression is increased during development and maturation of the human prefrontal cortex. J. Neurochem. 2009;109(4):1053–1066. doi: 10.1111/j.1471-4159.2009.06031.x. [DOI] [PubMed] [Google Scholar]
- 10.Charnay Y, Imhof A, Vallet PG, et al. Clusterin expression during fetal and postnatal CNS development in mouse. Neuroscience. 2008;155(3):714–724. doi: 10.1016/j.neuroscience.2008.06.022. [DOI] [PubMed] [Google Scholar]
- 11.Li WH, Tanimura M, Luo CC, Datta S, Chan L. The apolipoprotein multigene family: biosynthesis, structure, structure–function relationships, and evolution. J. Lipid Res. 1988;29(3):245–271. [PubMed] [Google Scholar]
- 12.Hoofnagle AN, Heinecke JW. Lipoproteomics: using mass spectrometry-based proteomics to explore the assembly, structure, and function of lipoproteins. J. Lipid Res. 2009;50(10):1967–1975. doi: 10.1194/jlr.R900015-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Beffert U, Stolt PC, Herz J. Functions of lipoprotein receptors in neurons. J. Lipid Res. 2004;45(3):403–409. doi: 10.1194/jlr.R300017-JLR200. [DOI] [PubMed] [Google Scholar]
- 14.Qiu S, Korwek KM, Weeber EJ. A fresh look at an ancient receptor family: emerging roles for low density lipoprotein receptors in synaptic plasticity and memory formation. Neurobiol. Learn Mem. 2006;85(1):16–29. doi: 10.1016/j.nlm.2005.08.009. [DOI] [PubMed] [Google Scholar]
- 15.Li Y, Cam J, Bu G. Low-density lipoprotein receptor family: endocytosis and signal transduction. Mol. Neurobiol. 2001;23(1):53–67. doi: 10.1385/MN:23:1:53. [DOI] [PubMed] [Google Scholar]
- 16.Dietschy JM, Turley SD. Thematic review series: brain lipids. Cholesterol metabolism in the central nervous system during early development and in the mature animal. J. Lipid Res. 2004;45(8):1375–1397. doi: 10.1194/jlr.R400004-JLR200. [DOI] [PubMed] [Google Scholar]
- 17.Snipes GJ, Suter U. Cholesterol and myelin. Subcell. Biochem. 1997;28:173–204. doi: 10.1007/978-1-4615-5901-6_7. [DOI] [PubMed] [Google Scholar]
- 18.Bjorkhem I, Meaney S. Brain cholesterol: long secret life behind a barrier. Arterioscler. Thromb. Vasc. Biol. 2004;24(5):806–815. doi: 10.1161/01.ATV.0000120374.59826.1b. [DOI] [PubMed] [Google Scholar]
- 19.Linton MF, Gish R, Hubl ST, et al. Phenotypes of apolipoprotein B and apolipoprotein E after liver transplantation. J. Clin. Invest. 1991;88(1):270–281. doi: 10.1172/JCI115288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chobanian AV, Hollander W. Body cholesterol metabolism in man. I. The equilibration of serum and tissue cholesterol. J. Clin. Invest. 1962;41:1732–1737. doi: 10.1172/JCI104631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Meaney S, Hassan M, Sakinis A, et al. Evidence that the major oxysterols in human circulation originate from distinct pools of cholesterol: a stable isotope study. J. Lipid Res. 2001;42(1):70–78. [PubMed] [Google Scholar]
- 22.Plotz EJ, Kabara JJ, Davis ME, LeRoy GV, Gould RG. Studies on the synthesis of cholesterol in the brain of the human fetus. Am. J. Obstet. Gynecol. 1968;101(4):534–538. doi: 10.1016/0002-9378(68)90565-6. [DOI] [PubMed] [Google Scholar]
- 23.Andersson M, Elmberger PG, Edlund C, Kristensson K, Dallner G. Rates of cholesterol, ubiquinone, dolichol and dolichyl-P biosynthesis in rat brain slices. FEBS Lett. 1990;269(1):15–18. doi: 10.1016/0014-5793(90)81107-y. [DOI] [PubMed] [Google Scholar]
- 24.Bjorkhem I, Diczfalusy U, Lutjohann D. Removal of cholesterol from extrahepatic sources by oxidative mechanisms. Curr. Opin. Lipidol. 1999;10(2):161–165. doi: 10.1097/00041433-199904000-00010. [DOI] [PubMed] [Google Scholar]
- 25. Roheim PS, Carey M, Forte T, Vega GL. Apolipoproteins in human cerebrospinal fluid. Proc. Natl Acad. Sci. USA. 1979;76(9):4646–4649. doi: 10.1073/pnas.76.9.4646.. ▪ Seminal study that identifies apolipoproteins in the cerebrospinal fluid (CSF).
- 26. Pitas RE, Boyles JK, Lee SH, Hui D, Weisgraber KH. Lipoproteins and their receptors in the central nervous system. Characterization of the lipoproteins in cerebrospinal fluid and identification of apolipoprotein B,E(LDL) receptors in the brain. J. Biol. Chem. 1987;262(29):14352–14360.. ▪ Comprehensive investigation that characterizes the major lipoproteins present in the CSF and the key receptor pathways involved in lipoprotein metabolism.
- 27.Borghini I, Barja F, Pometta D, James RW. Characterization of subpopulations of lipoprotein particles isolated from human cerebrospinal fluid. Biochim. Biophys. Acta. 1995;1255(2):192–200. doi: 10.1016/0005-2760(94)00232-n. [DOI] [PubMed] [Google Scholar]
- 28.Comprehensive analysis of the major lipoprotein subclasses present in the CSF Koch S, Donarski N, Goetze K, et al. Characterization of four lipoprotein classes in human cerebrospinal fluid. J. Lipid Res. 2001;42(7):1143–1151.. ▪ Comprehensive analysis of the major lipoprotein subclasses present in the CSF.
- 29.Suzuki T, Tozuka M, Kazuyoshi Y, et al. Predominant apolipoprotein J exists as lipid-poor mixtures in cerebrospinal fluid. Ann. Clin. Lab. Sci. 2002;32(4):369–376. [PubMed] [Google Scholar]
- 30.DeMattos RB, Brendza RP, Heuser JE, et al. Purification and characterization of astrocyte-secreted apolipoprotein E, J-containing lipoproteins from wild-type and human apoE transgenic mice. Neurochem. Int. 2001;39(5–6):415–425. doi: 10.1016/s0197-0186(01)00049-3. [DOI] [PubMed] [Google Scholar]
- 31.LaDu M-J, Yu C, Youmans KL. Proposed mechanism for lipoprotein remodeling in the brain. Biochim. Biophys. Acta. 2010;1801(8):819–823. doi: 10.1016/j.bbalip.2010.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mahley RW, Apolipoprotein E. cholesterol transport protein with expanding role in cell biology. Science. 1988;240(4852):622–630. doi: 10.1126/science.3283935. [DOI] [PubMed] [Google Scholar]
- 33.Bao F, Arai H, Matsushita S, Higuchi S, Sasaki H. Expression of apolipoprotein E in normal and diverse neurodegenerative disease brain. Neuroreport. 1996;7(11):1733–1739. doi: 10.1097/00001756-199607290-00008. [DOI] [PubMed] [Google Scholar]
- 34.Diedrich JF, Minnigan H, Carp RI, et al. Neuropathological changes in scrapie, Alzheimer’s disease are associated with increased expression of apolipoprotein E and cathepsin D in astrocytes. J. Virol. 1991;65(9):4759–4768. doi: 10.1128/jvi.65.9.4759-4768.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Han SH, Hulette C, Saunders AM, et al. Apolipoprotein E is present in hippocampal neurons without neurofibrillary tangles in Alzheimer’s disease and in age-matched controls. Exp. Neurol. 1994;128(1):13–26. doi: 10.1006/exnr.1994.1108. [DOI] [PubMed] [Google Scholar]
- 36. Ignatius MJ, Gebicke-Harter PJ, Skene JH, et al. Expression of apolipoprotein E during nerve degeneration and regeneration. Proc. Natl Acad. Sci. USA. 1986;83(4):1125–1129. doi: 10.1073/pnas.83.4.1125.. ▪▪ Seminal paper demonstrating the major role ApoE plays in nerve regeneration.
- 37.Metzger RE, LaDu MJ, Pan JB, Getz GS, Frail DE, Falduto MT. Neurons of the human frontal cortex display apolipoprotein E immunoreactivity: implications for Alzheimer’s disease. J Neuropathol. Exp. Neurol. 1996;55(3):372–380. doi: 10.1097/00005072-199603000-00013. [DOI] [PubMed] [Google Scholar]
- 38.Nakai M, Kawamata T, Taniguchi T, Maeda K, Tanaka C. Expression of apolipoprotein E mRNA in rat microglia. Neurosci. Lett. 1996;211(1):41–44. doi: 10.1016/0304-3940(96)12716-6. [DOI] [PubMed] [Google Scholar]
- 39.Nathan BP, Bellosta S, Sanan DA, Weisgraber KH, Mahley RW, Pitas RE. Differential effects of apolipoproteins E3, E4 on neuronal growth in vitro. Science. 1994;264(5160):850–852. doi: 10.1126/science.8171342. [DOI] [PubMed] [Google Scholar]
- 40.Poirier J, Hess M, May PC, Finch CE. Astrocytic apolipoprotein E mRNA, GFAP mRNA in hippocampus after entorhinal cortex lesioning. Brain Res. Mol. Brain Res. 1991;11(2):97–106. doi: 10.1016/0169-328x(91)90111-a. [DOI] [PubMed] [Google Scholar]
- 41.Xu Q, Bernardo A, Walker D, Kanegawa T, Mahley RW, Huang Y. Profile and regulation of apolipoprotein E (APOE) expression in the CNS in mice with targeting of green fluorescent protein gene to the APOE locus. J. Neurosci. 2006;26(19):4985–4994. doi: 10.1523/JNEUROSCI.5476-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chang DJ, Paik YK, Leren TP, Walker DW, Howlett GJ, Taylor JM. Characterization of a human apolipoprotein E gene enhancer element and its associated protein factors. J. Biol. Chem. 1990;265(16):9496–9504. [PubMed] [Google Scholar]
- 43.Adroer R, Lopez-Acedo C, Oliva R. Conserved elements in the 5′ regulatory region of the amyloid precursor protein gene in primates. Neurosci. Lett. 1997;226(3):203–206. doi: 10.1016/s0304-3940(97)00283-8. [DOI] [PubMed] [Google Scholar]
- 44.Laffitte BA, Repa JJ, Joseph SB, et al. LXRs control lipid-inducible expression of the apolipoprotein E gene in macrophages and adipocytes. Proc. Natl Acad. Sci. USA. 2001;98(2):507–512. doi: 10.1073/pnas.021488798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Salero E, Gimenez C, Zafra F. Identification of a non-canonical E-box motif as a regulatory element in the proximal promoter region of the apolipoprotein E gene. Biochem. J. 2003;370(Pt 3):979–986. doi: 10.1042/BJ20021142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lahiri DK. Apolipoprotein E as a target for developing new therapeutics for Alzheimer’s disease based on studies from protein, RNA, and regulatory region of the gene. J. Mol. Neurosci. 2004;23(3):225–233. doi: 10.1385/JMN:23:3:225. [DOI] [PubMed] [Google Scholar]
- 47.Du Y, Chen X, Wei X, et al. NF-κB mediates amyloid β peptide-stimulated activity of the human apolipoprotein E gene promoter in human astroglial cells. Brain Res. Mol. Brain Res. 2005;136(1–2):177–188. doi: 10.1016/j.molbrainres.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 48.Naukkarinen J, Gentile M, Soro-Paavonen A, et al. USF1 and dyslipidemias: converging evidence for a functional intronic variant. Hum. Mol. Genet. 2005;14(17):2595–2605. doi: 10.1093/hmg/ddi294. [DOI] [PubMed] [Google Scholar]
- 49.Ramos MC, Matias S, Artiga MJ, et al. Neuronal specific regulatory elements in apolipoprotein E gene proximal promoter. Neuroreport. 2005;16(9):1027–1030. doi: 10.1097/00001756-200506210-00029. [DOI] [PubMed] [Google Scholar]
- 50. Wahrle SE, Jiang H, Parsadanian M, et al. ABCA1 is required for normal central nervous system ApoE levels and for lipidation of astrocyte-secreted ApoE. J. Biol. Chem. 2004;279(39):40987–40993. doi: 10.1074/jbc.M407963200.. ▪ Identifies the important role of ABCA1 in ApoE lipidation in the CNS.
- 51.Karten B, Campenot RB, Vance DE, Vance JE. Expression of ABCG1, but not ABCA1, correlates with cholesterol release by cerebellar astroglia. J. Biol. Chem. 2006;281(7):4049–4057. doi: 10.1074/jbc.M508915200. [DOI] [PubMed] [Google Scholar]
- 52.Kim WS, Rahmanto AS, Kamili A, et al. Role of ABCG1, ABCA1 in regulation of neuronal cholesterol efflux to apolipoprotein E discs and suppression of amyloid-β peptide generation. J. Biol. Chem. 2007;282(5):2851–2861. doi: 10.1074/jbc.M607831200. [DOI] [PubMed] [Google Scholar]
- 53.Hirsch-Reinshagen V, Wellington CL. Cholesterol metabolism, apolipoprotein E, adenosine triphosphate-binding cassette transporters, Alzheimer’s disease. Curr. Opin. Lipidol. 2007;18(3):325–332. doi: 10.1097/MOL.0b013e32813aeabf. [DOI] [PubMed] [Google Scholar]
- 54.Jansen PJ, Lutjohann D, Thelen KM, et al. Absence of ApoE upregulates murine brain ApoD, ABCA1 levels, but does not affect brain sterol levels, while human ApoE3 and human ApoE4 upregulate brain cholesterol precursor levels. J. Alzheimers Dis. 2009;18(2):319–329. doi: 10.3233/JAD-2009-1150. [DOI] [PubMed] [Google Scholar]
- 55.Terrisse L, Seguin D, Bertrand P, Poirier J, Milne R, Rassart E. Modulation of apolipoprotein D and apolipoprotein E expression in rat hippocampus after entorhinal cortex lesion. Brain Res. Mol. Brain Res. 1999;70(1):26–35. doi: 10.1016/s0169-328x(99)00123-0. [DOI] [PubMed] [Google Scholar]
- 56.Elshourbagy NA, Liao WS, Mahley RW, Taylor JM. Apolipoprotein E mRNA is abundant in the brain and adrenals, as well as in the liver, and is present in other peripheral tissues of rats and marmosets. Proc. Natl Acad. Sci. USA. 1985;82(1):203–207. doi: 10.1073/pnas.82.1.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Pitas RE, Boyles JK, Lee SH, Foss D, Mahley RW. Astrocytes synthesize apolipoprotein E and metabolize apolipoprotein E-containing lipoproteins. Biochim. Biophys. Acta. 1987;917(1):148–161. doi: 10.1016/0005-2760(87)90295-5.. ▪▪ Identifies the fundamental role astrocytes play in regulating the synthesis and metabolism of ApoE-containing lipoproteins in the CNS.
- 58.Fagan AM, Holtzman DM, Munson G, et al. Unique lipoproteins secreted by primary astrocytes from wild type, apoE−/−, and human apoE transgenic mice. J. Biol. Chem. 1999;274(42):30001–30007. doi: 10.1074/jbc.274.42.30001. [DOI] [PubMed] [Google Scholar]
- 59.Tarr PT, Edwards PA. ABCG1, ABCG4 are coexpressed in neurons and astrocytes of the CNS and regulate cholesterol homeostasis through SREBP-2. J. Lipid Res. 2008;49(1):169–182. doi: 10.1194/jlr.M700364-JLR200. [DOI] [PubMed] [Google Scholar]
- 60.Burgess BL, Parkinson PF, Racke MM, et al. ABCG1 influences the brain cholesterol biosynthetic pathway but does not affect amyloid precursor protein or apolipoprotein E metabolism in vivo. J. Lipid Res. 2008;49(6):1254–1267. doi: 10.1194/jlr.M700481-JLR200. [DOI] [PubMed] [Google Scholar]
- 61.Wang N, Yvan-Charvet L, Lutjohann D, et al. ATP-binding cassette transporters G1, G4 mediate cholesterol and desmosterol efflux to HDL and regulate sterol accumulation in the brain. FASEB J. 2008;22(4):1073–1082. doi: 10.1096/fj.07-9944com. [DOI] [PubMed] [Google Scholar]
- 62.Rebeck GW, LaDu MJ, Estus S, Bu G, Weeber EJ. The generation and function of soluble apoE receptors in the CNS. Mol. Neurodegener. 2006;1:15. doi: 10.1186/1750-1326-1-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rall SC, Jr, Weisgraber KH, Innerarity TL, Mahley RW. Structural basis for receptor binding heterogeneity of apolipoprotein E from type III hyperlipoproteinemic subjects. Proc. Natl Acad. Sci. USA. 1982;79(15):4696–4700. doi: 10.1073/pnas.79.15.4696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Corder EH, Saunders AM, Strittmatter WJ, et al. Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer’s disease in late onset families. Science. 1993;261(5123):921–923. doi: 10.1126/science.8346443.. ▪▪ Landmark study identifying ApoE4 as a major risk factor for late-onset alzheimer’s disease.
- 65.Strittmatter WJ, Saunders AM, Schmechel D, et al. Apolipoprotein E: high-avidity binding to β-amyloid and increased frequency of type 4 allele in late-onset familial Alzheimer disease. Proc. Natl Acad. Sci. USA. 1993;90(5):1977–1981. doi: 10.1073/pnas.90.5.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Harold D, Abraham R, Hollingworth P, et al. Genome-wide association study identifies variants at CLU, PICALM associated with Alzheimer’s disease. Nat. Genet. 2009;41(10):1088–1093. doi: 10.1038/ng.440.. ▪ Landmark genetic study that associates APOJ (CLU) polymorphism as a risk factor for Alzheimer’s disease.
- 67. Lambert JC, Heath S, Even G, et al. Genome-wide association study identifies variants at CLU, CR1 associated with Alzheimer’s disease. Nat. Genet. 2009;41(10):1094–1099. doi: 10.1038/ng.439.. ▪ Landmark genetic study that associates ApoJ (CLU) polymorphism as a risk factor for Alzheimer’s disease.
- 68.Jordan BD. Genetic influences on outcome following traumatic brain injury. Neurochem. Res. 2007;32(4–5):905–915. doi: 10.1007/s11064-006-9251-3. [DOI] [PubMed] [Google Scholar]
- 69.Jha A, Lammertse DP, Coll JR, et al. Apolipoprotein E ε4 allele and outcomes of traumatic spinal cord injury. J. Spinal Cord Med. 2008;31(2):171–176. doi: 10.1080/10790268.2008.11760708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Slooter AJ, Tang MX, van Duijn CM, et al. Apolipoprotein E ε4 and the risk of dementia with stroke. A population-based investigation. JAMA. 1997;277(10):818–821. doi: 10.1001/jama.277.10.818. [DOI] [PubMed] [Google Scholar]
- 71.Huang X, Chen PC, Poole C. APOE-ε2 allele associated with higher prevalence of sporadic Parkinson disease. Neurology. 2004;62(12):2198–2202. doi: 10.1212/01.wnl.0000130159.28215.6a. [DOI] [PubMed] [Google Scholar]
- 72.Beyreuther K, Masters CL. Amyloid precursor protein (APP) and β A4 amyloid in the etiology of Alzheimer’s disease: precursor–product relationships in the derangement of neuronal function. Brain Pathol. 1991;1(4):241–251. doi: 10.1111/j.1750-3639.1991.tb00667.x. [DOI] [PubMed] [Google Scholar]
- 73.Namba Y, Tomonaga M, Kawasaki H, Otomo E, Ikeda K. Apolipoprotein E immunoreactivity in cerebral amyloid deposits and neurofibrillary tangles in Alzheimer’s disease and kuru plaque amyloid in Creutzfeldt–Jakob disease. Brain Res. 1991;541(1):163–166. doi: 10.1016/0006-8993(91)91092-f. [DOI] [PubMed] [Google Scholar]
- 74.Wisniewski T, Frangione B. Apolipoprotein E: a pathological chaperone protein in patients with cerebral and systemic amyloid. Neurosci. Lett. 1992;135(2):235–238. doi: 10.1016/0304-3940(92)90444-c. [DOI] [PubMed] [Google Scholar]
- 75.Strittmatter WJ, Weisgraber KH, Huang DY, et al. Binding of human apolipoprotein E to synthetic amyloid β peptide: isoform-specific effects and implications for late-onset alzheimer disease. Proc. Natl Acad. Sci. USA. 1993;90(17):8098–8102. doi: 10.1073/pnas.90.17.8098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Jiang Q, Lee CY, Mandrekar S, et al. ApoE promotes the proteolytic degradation of Aβ. Neuron. 2008;58(5):681–693. doi: 10.1016/j.neuron.2008.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Gylys KH, Fein JA, Tan AM, Cole GM. Apolipoprotein E enhances uptake of soluble but not aggregated amyloid-β protein into synaptic terminals. J. Neurochem. 2003;84(6):1442–1451. doi: 10.1046/j.1471-4159.2003.01643.x. [DOI] [PubMed] [Google Scholar]
- 78.Rebeck GW, Reiter JS, Strickland DK, Hyman BT. Apolipoprotein E in sporadic Alzheimer’s disease: allelic variation and receptor interactions. Neuron. 1993;11(4):575–580. doi: 10.1016/0896-6273(93)90070-8. [DOI] [PubMed] [Google Scholar]
- 79.Urmoneit B, Prikulis I, Wihl G, et al. Cerebrovascular smooth muscle cells internalize Alzheimer amyloid β protein via a lipoprotein pathway: implications for cerebral amyloid angiopathy. Lab. Invest. 1997;77(2):157–166. [PubMed] [Google Scholar]
- 80.Beffert U, Aumont N, Dea D, Lussier-Cacan S, Davignon J, Poirier J. Apolipoprotein E isoform-specific reduction of extracellular amyloid in neuronal cultures. Brain Res. Mol. Brain Res. 1999;68(1–2):181–185. doi: 10.1016/s0169-328x(99)00073-x. [DOI] [PubMed] [Google Scholar]
- 81.Cole GM, Ard MD. Influence of lipoproteins on microglial degradation of Alzheimer’s amyloid β-protein. Microsc. Res. Tech. 2000;50(4):316–324. doi: 10.1002/1097-0029(20000815)50:4<316::AID-JEMT11>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 82. Yang DS, Small DH, Seydel U, et al. Apolipoprotein E promotes the binding and uptake of β-amyloid into Chinese hamster ovary cells in an isoform-specific manner. Neuroscience. 1999;90(4):1217–1226. doi: 10.1016/s0306-4522(98)00561-2.. ▪ Clear evidence establishing an isoform-specific difference in ApoE-mediated β-amyloid uptake.
- 83.Koistinaho M, Lin S, Wu X, et al. Apolipoprotein E promotes astrocyte colocalization and degradation of deposited amyloid-β peptides. Nat. Med. 2004;10(7):719–726. doi: 10.1038/nm1058. [DOI] [PubMed] [Google Scholar]
- 84.Deane R, Sagare A, Hamm K, et al. apoE isoform-specific disruption of amyloid β peptide clearance from mouse brain. J. Clin. Invest. 2008;118(12):4002–4013. doi: 10.1172/JCI36663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. LaDu MJ, Falduto MT, Manelli AM, Reardon CA, Getz GS, Frail DE. Isoform-specific binding of apolipoprotein E to β-amyloid. J. Biol. Chem. 1994;269(38):23403–23406.. ▪ Presents evidence of a dramatic isoform-specific difference in ApoE binding with β-amyloid.
- 86.Schmechel DE, Saunders AM, Strittmatter WJ, et al. Increased amyloid β-peptide deposition in cerebral cortex as a consequence of apolipoprotein E genotype in late-onset alzheimer disease. Proc. Natl Acad. Sci. USA. 1993;90(20):9649–9653. doi: 10.1073/pnas.90.20.9649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Nicoll JA, Roberts GW, Graham DI. Apolipoprotein E ε 4 allele is associated with deposition of amyloid β-protein following head injury. Nat. Med. 1995;1(2):135–137. doi: 10.1038/nm0295-135. [DOI] [PubMed] [Google Scholar]
- 88.Bales KR, Verina T, Cummins DJ, et al. Apolipoprotein E is essential for amyloid deposition in the APP(V717F) transgenic mouse model of Alzheimer’s disease. Proc. Natl Acad. Sci. USA. 1999;96(26):15233–15238. doi: 10.1073/pnas.96.26.15233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Holtzman DM, Bales KR, Wu S, et al. Expression of human apolipoprotein E reduces amyloid-β deposition in a mouse model of Alzheimer’s disease. J. Clin. Invest. 1999;103(6):R1–R21. doi: 10.1172/JCI6179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Holtzman DM, Bales KR, Tenkova T, et al. Apolipoprotein E isoform-dependent amyloid deposition and neuritic degeneration in a mouse model of Alzheimer’s disease. Proc. Natl Acad. Sci. USA. 2000;97(6):2892–2897. doi: 10.1073/pnas.050004797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Fagan AM, Watson M, Parsadanian M, Bales KR, Paul SM, Holtzman DM. Human and murine ApoE markedly alters A β metabolism before and after plaque formation in a mouse model of Alzheimer’s disease. Neurobiol. Dis. 2002;9(3):305–318. doi: 10.1006/nbdi.2002.0483. [DOI] [PubMed] [Google Scholar]
- 92.Snipes GJ, McGuire CB, Norden JJ, Freeman JA. Nerve injury stimulates the secretion of apolipoprotein E by nonneuronal cells. Proc. Natl Acad. Sci. USA. 1986;83(4):1130–1134. doi: 10.1073/pnas.83.4.1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Horsburgh K, Fitzpatrick M, Nilsen M, Nicoll JA. Marked alterations in the cellular localisation and levels of apolipoprotein E following acute subdural haematoma in rat. Brain Res. 1997;763(1):103–110. doi: 10.1016/s0006-8993(97)00411-3. [DOI] [PubMed] [Google Scholar]
- 94.Iwata A, Browne KD, Chen XH, Yuguchi T, Smith DH. Traumatic brain injury induces biphasic upregulation of ApoE, ApoJ protein in rats. J. Neurosci. Res. 2005;82(1):103–114. doi: 10.1002/jnr.20607. [DOI] [PubMed] [Google Scholar]
- 95.White F, Nicoll JA, Horsburgh K. Alterations in ApoE, ApoJ in relation to degeneration and regeneration in a mouse model of entorhinal cortex lesion. Exp. Neurol. 2001;169(2):307–318. doi: 10.1006/exnr.2001.7655. [DOI] [PubMed] [Google Scholar]
- 96.Aoki K, Uchihara T, Sanjo N, et al. Increased expression of neuronal apolipoprotein E in human brain with cerebral infarction. Stroke. 2003;34(4):875–880. doi: 10.1161/01.STR.0000064320.73388.C6. [DOI] [PubMed] [Google Scholar]
- 97.Boschert U, Merlo-Pich E, Higgins G, Roses AD, Catsicas S. Apolipoprotein E expression by neurons surviving excitotoxic stress. Neurobiol. Dis. 1999;6(6):508–514. doi: 10.1006/nbdi.1999.0251. [DOI] [PubMed] [Google Scholar]
- 98.Fagan AM, Murphy BA, Patel SN, et al. Evidence for normal aging of the septohippocampal cholinergic system in apoE−/− mice but impaired clearance of axonal degeneration products following injury. Exp. Neurol. 1998;151(2):314–325. doi: 10.1006/exnr.1998.6818. [DOI] [PubMed] [Google Scholar]
- 99.Chen Y, Lomnitski L, Michaelson DM, Shohami E. Motor and cognitive deficits in apolipoprotein E-deficient mice after closed head injury. Neuroscience. 1997;80(4):1255–1262. doi: 10.1016/s0306-4522(97)00007-9. [DOI] [PubMed] [Google Scholar]
- 100.Pereira SG, Oakley F. Nuclear factor-κB1: regulation and function. Int. J. Biochem. Cell Biol. 2008;40(8):1425–1430. doi: 10.1016/j.biocel.2007.05.004. [DOI] [PubMed] [Google Scholar]
- 101.Yamada T, Kondo A, Takamatsu J, Tateishi J, Goto I. Apolipoprotein E mRNA in the brains of patients with Alzheimer’s disease. J. Neurol. Sci. 1995;129(1):56–61. doi: 10.1016/0022-510x(94)00249-n. [DOI] [PubMed] [Google Scholar]
- 102.Yamagata K, Urakami K, Ikeda K, et al. High expression of apolipoprotein E mRNA in the brains with sporadic Alzheimer’s disease. Dement. Geriatr. Cogn. Disord. 2001;12(2):57–62. doi: 10.1159/000051236. [DOI] [PubMed] [Google Scholar]
- 103.Thomas EA, Laws SM, Sutcliffe JG, et al. Apolipoprotein D levels are elevated in prefrontal cortex of subjects with Alzheimer’s disease: no relation to apolipoprotein E expression or genotype. Biol. Psychiatry. 2003;54(2):136–141. doi: 10.1016/s0006-3223(02)01976-5. [DOI] [PubMed] [Google Scholar]
- 104.Laws SM, Hone E, Taddei K, et al. Variation at the APOE-491 promoter locus is associated with altered brain levels of apolipoprotein E. Mol. Psychiatry. 2002;7(8):886–890. doi: 10.1038/sj.mp.4001097. [DOI] [PubMed] [Google Scholar]
- 105.Fukumoto H, Ingelsson M, Garevik N, et al. APOE ε 3/ε4 heterozygotes have an elevated proportion of apolipoprotein E4 in cerebrospinal fluid relative to plasma, independent of Alzheimer’s disease diagnosis. Exp. Neurol. 2003;183(1):249–253. doi: 10.1016/s0014-4886(03)00088-8. [DOI] [PubMed] [Google Scholar]
- 106.Bertrand P, Poirier J, Oda T, Finch CE, Pasinetti GM. Association of apolipoprotein E genotype with brain levels of apolipoprotein E and apolipoprotein J (clusterin) in Alzheimer disease. Brain Res. Mol. Brain Res. 1995;33(1):174–178. doi: 10.1016/0169-328x(95)00097-c. [DOI] [PubMed] [Google Scholar]
- 107.Beffert U, Cohn JS, Petit-Turcotte C, et al. Apolipoprotein E and β-amyloid levels in the hippocampus and frontal cortex of Alzheimer’s disease subjects are disease-related and apolipoprotein E genotype dependent. Brain Res. 1999;843(1–2):87–94. doi: 10.1016/s0006-8993(99)01894-6. [DOI] [PubMed] [Google Scholar]
- 108.Glockner F, Meske V, Ohm TG. Genotype-related differences of hippocampal apolipoprotein E levels only in early stages of neuropathological changes in Alzheimer’s disease. Neuroscience. 2002;114(4):1103–1114. doi: 10.1016/s0306-4522(02)00178-1. [DOI] [PubMed] [Google Scholar]
- 109.Ramaswamy G, Xu Q, Huang Y, Weisgraber KH. Effect of domain interaction on apolipoprotein E levels in mouse brain. J. Neurosci. 2005;25(46):10658–10663. doi: 10.1523/JNEUROSCI.1922-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Riddell DR, Zhou H, Atchison K, et al. Impact of apolipoprotein E (ApoE) polymorphism on brain ApoE levels. J. Neurosci. 2008;28(45):11445–11453. doi: 10.1523/JNEUROSCI.1972-08.2008.. ▪ Demonstrates that the ApoE4 isoform is associated with decreased ApoE protein levels in the mouse brain.
- 111.Sullivan PM, Mace BE, Maeda N, Schmechel DE. Marked regional differences of brain human apolipoprotein E expression in targeted replacement mice. Neuroscience. 2004;124(4):725–733. doi: 10.1016/j.neuroscience.2003.10.011. [DOI] [PubMed] [Google Scholar]
- 112.Fryer JD, Simmons K, Parsadanian M, et al. Human apolipoprotein E4 alters the amyloid-β 40:42 ratio and promotes the formation of cerebral amyloid angiopathy in an amyloid precursor protein transgenic model. J. Neurosci. 2005;25(11):2803–2810. doi: 10.1523/JNEUROSCI.5170-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Elliott DA, Tsoi K, Holinkova S, et al. Isoform-specific proteolysis of apolipoprotein-E in the brain. Neurobiol. Aging. 2009 doi: 10.1016/j.neurobiolaging.2009.02.006. (Epub ahead of print). [DOI] [PubMed] [Google Scholar]
- 114.Arai H, Kashiwagi S, Nagasaka Y, Uchida K, Hoshii Y, Nakamura K. Oxidative modification of apolipoprotein E in human very-low-density lipoprotein and its inhibition by glycosaminoglycans. Arch. Biochem. Biophys. 1999;367(1):1–8. doi: 10.1006/abbi.1999.1222. [DOI] [PubMed] [Google Scholar]
- 115.Elliott DA, Kim WS, Jans DA, Garner B. Apoptosis induces neuronal apolipoprotein-E synthesis and localization in apoptotic bodies. Neurosci. Lett. 2007;416(2):206–210. doi: 10.1016/j.neulet.2007.02.014. [DOI] [PubMed] [Google Scholar]
- 116.Mahley RW, Nathan BP, Pitas RE, Apolipoprotein E. Structure, function, and possible roles in Alzheimer’s disease. Ann NY Acad. Sci. 1996;777:139–145. doi: 10.1111/j.1749-6632.1996.tb34412.x. [DOI] [PubMed] [Google Scholar]
- 117.Mahley RW, Rall SC., Jr Apolipoprotein E: far more than a lipid transport protein. Annu. Rev. Genomics. Hum. Genet. 2000;1:507–537. doi: 10.1146/annurev.genom.1.1.507. [DOI] [PubMed] [Google Scholar]
- 118.Miyata M, Smith JD. Apolipoprotein E allele-specific antioxidant activity and effects on cytotoxicity by oxidative insults and β-amyloid peptides. Nat. Genet. 1996;14(1):55–61. doi: 10.1038/ng0996-55. [DOI] [PubMed] [Google Scholar]
- 119.Strittmatter WJ, Saunders AM, Goedert M, et al. Isoform-specific interactions of apolipoprotein E with microtubule-associated protein tau: implications for Alzheimer disease. Proc. Natl Acad. Sci. USA. 1994;91(23):11183–11186. doi: 10.1073/pnas.91.23.11183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Gelissen IC, Hochgrebe T, Wilson MR, et al. Apolipoprotein J (clusterin) induces cholesterol export from macrophage-foam cells: a potential anti-atherogenic function? Biochem. J. 1998;331(Pt 1):231–237. doi: 10.1042/bj3310231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Jones SE, Jomary C. Clusterin. Int. J. Biochem. Cell Biol. 2002;34(5):427–431. doi: 10.1016/s1357-2725(01)00155-8. [DOI] [PubMed] [Google Scholar]
- 122.Wyatt AR, Yerbury JJ, Wilson MR. Structural characterization of clusterin-chaperone client protein complexes. J. Biol. Chem. 2009;284(33):21920–21927. doi: 10.1074/jbc.M109.033688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Rizzi F, Bettuzzi S. The clusterin paradigm in prostate and breast carcinogenesis. Endocr. Relat. Cancer. 2010;17(1):R1–R17. doi: 10.1677/ERC-09-0140. [DOI] [PubMed] [Google Scholar]
- 124.Wong P, Pineault J, Lakins J, et al. Genomic organization and expression of the rat TRPM-2 (clusterin) gene, a gene implicated in apoptosis. J. Biol. Chem. 1993;268(7):5021–5031. [PubMed] [Google Scholar]
- 125.Rosemblit N, Chen CL. Regulators for the rat clusterin gene: DNA methylation and cis-acting regulatory elements. J. Mol. Endocrinol. 1994;13(1):69–76. doi: 10.1677/jme.0.0130069. [DOI] [PubMed] [Google Scholar]
- 126. Michel D, Chatelain G, North S, Brun G. Stress-induced transcription of the clusterin/apoJ gene. Biochem. J. 1997;328(Pt 1):45–50. doi: 10.1042/bj3280045.. ▪ Identifies key pathways mediating stress-induced APOJ upregulation.
- 127.Loison F, Debure L, Nizard P, le Goff P, Michel D, le Drean Y. Up-regulation of the clusterin gene after proteotoxic stress: implication of HSF1–HSF2 heterocomplexes. Biochem. J. 2006;395(1):223–231. doi: 10.1042/BJ20051190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Ghiso J, Matsubara E, Koudinov A, et al. The cerebrospinal-fluid soluble form of Alzheimer’s amyloid β is complexed to SP-40,40 (apolipoprotein J), an inhibitor of the complement membrane-attack complex. Biochem. J. 1993;293(Pt 1):27–30. doi: 10.1042/bj2930027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Sasaki K, Doh-ura K, Ironside JW, Iwaki T. Increased clusterin (apolipoprotein J) expression in human and mouse brains infected with transmissible spongiform encephalopathies. Acta Neuropathol. 2002;103(3):199–208. doi: 10.1007/s004010100456. [DOI] [PubMed] [Google Scholar]
- 130.Freixes M, Puig B, Rodriguez A, Torrejon-Escribano B, Blanco R, Ferrer I. Clusterin solubility and aggregation in Creutzfeldt–Jakob disease. Acta Neuropathol. 2004;108(4):295–301. doi: 10.1007/s00401-004-0891-6. [DOI] [PubMed] [Google Scholar]
- 131.Kounnas MZ, Loukinova EB, Stefansson S, et al. Identification of glycoprotein 330 as an endocytic receptor for apolipoprotein J/clusterin. J. Biol. Chem. 1995;270(22):13070–13075. doi: 10.1074/jbc.270.22.13070. [DOI] [PubMed] [Google Scholar]
- 132.Zlokovic BV, Martel CL, Matsubara E, et al. Glycoprotein 330/megalin: probable role in receptor-mediated transport of apolipoprotein J alone and in a complex with Alzheimer disease amyloid β at the blood–brain and blood–cerebrospinal fluid barriers. Proc. Natl Acad. Sci. USA. 1996;93(9):4229–4234. doi: 10.1073/pnas.93.9.4229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Bartl MM, Luckenbach T, Bergner O, Ullrich O, Koch-Brandt C. Multiple receptors mediate apoJ-dependent clearance of cellular debris into nonprofessional phagocytes. Exp. Cell Res. 2001;271(1):130–141. doi: 10.1006/excr.2001.5358. [DOI] [PubMed] [Google Scholar]
- 134.Gardai SJ, McPhillips KA, Frasch SC, et al. Cell-surface calreticulin initiates clearance of viable or apoptotic cells through transactivation of LRP on the phagocyte. Cell. 2005;123(2):321–334. doi: 10.1016/j.cell.2005.08.032. [DOI] [PubMed] [Google Scholar]
- 135.Pasinetti GM, Johnson SA, Oda T, Rozovsky I, Finch CE. Clusterin (SGP-2): a multifunctional glycoprotein with regional expression in astrocytes and neurons of the adult rat brain. J. Comp. Neurol. 1994;339(3):387–400. doi: 10.1002/cne.903390307. [DOI] [PubMed] [Google Scholar]
- 136.Garden GA, Bothwell M, Rubel EW. Lack of correspondence between mRNA expression for a putative cell death molecule (SGP-2) and neuronal cell death in the central nervous system. J. Neurobiol. 1991;22(6):590–604. doi: 10.1002/neu.480220605. [DOI] [PubMed] [Google Scholar]
- 137.Dragunow M, Preston K, Dodd J, Young D, Lawlor P, Christie D. Clusterin accumulates in dying neurons following status epilepticus. Brain Res. Mol. Brain Res. 1995;32(2):279–290. doi: 10.1016/0169-328x(95)00088-a. [DOI] [PubMed] [Google Scholar]
- 138.Yasuhara O, Aimi Y, Yamada T, Matsuo A, McGeer EG, McGeer PL. Clusterin as a marker for ischaemic Purkinje cells in human brain. Neurodegeneration. 1994;3(4):325–329. [PubMed] [Google Scholar]
- 139.May PC, Lampert-Etchells M, Johnson SA, Poirier J, Masters JN, Finch CE. Dynamics of gene expression for a hippocampal glycoprotein elevated in Alzheimer’s disease and in response to experimental lesions in rat. Neuron. 1990;5(6):831–839. doi: 10.1016/0896-6273(90)90342-d. [DOI] [PubMed] [Google Scholar]
- 140.Danik M, Chabot JG, Mercier C, et al. Human gliomas and epileptic foci express high levels of a mRNA related to rat testicular sulfated glycoprotein 2, a purported marker of cell death. Proc. Natl Acad. Sci. USA. 1991;88(19):8577–8581. doi: 10.1073/pnas.88.19.8577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Wu E, Brosnan CF, Raine CS. SP-40,40 immunoreactivity in inflammatory CNS lesions displaying astrocyte/oligodendrocyte interactions. J. Neuropathol. Exp. Neurol. 1993;52(2):129–134. doi: 10.1097/00005072-199303000-00005. [DOI] [PubMed] [Google Scholar]
- 142.Klimaschewski L, Obermuller N, Witzgall R. Regulation of clusterin expression following spinal cord injury. Cell Tissue Res. 2001;306(2):209–216. doi: 10.1007/s004410100431. [DOI] [PubMed] [Google Scholar]
- 143.Imhof A, Charnay Y, Vallet PG, et al. Sustained astrocytic clusterin expression improves remodeling after brain ischemia. Neurobiol. Dis. 2006;22(2):274–283. doi: 10.1016/j.nbd.2005.11.009. [DOI] [PubMed] [Google Scholar]
- 144.Giannakopoulos P, Kovari E, French LE, Viard I, Hof PR, Bouras C. Possible neuroprotective role of clusterin in Alzheimer’s disease: a quantitative immunocytochemical study. Acta Neuropathol. 1998;95(4):387–394. doi: 10.1007/s004010050815. [DOI] [PubMed] [Google Scholar]
- 145.Lidstrom AM, Bogdanovic N, Hesse C, Volkman I, Davidsson P, Blennow K. Clusterin (apolipoprotein J) protein levels are increased in hippocampus and in frontal cortex in Alzheimer’s disease. Exp. Neurol. 1998;154(2):511–521. doi: 10.1006/exnr.1998.6892. [DOI] [PubMed] [Google Scholar]
- 146.Kida E, Choi-Miura NH, Wisniewski KE. Deposition of apolipoproteins E, J in senile plaques is topographically determined in both Alzheimer’s disease, Down’s syndrome brain. Brain Res. 1995;685(1–2):211–216. doi: 10.1016/0006-8993(95)00482-6. [DOI] [PubMed] [Google Scholar]
- 147.Calero M, Rostagno A, Matsubara E, Zlokovic B, Frangione B, Ghiso J. Apolipoprotein J (clusterin), Alzheimer’s disease. Microsc. Res. Tech. 2000;50(4):305–315. doi: 10.1002/1097-0029(20000815)50:4<305::AID-JEMT10>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 148.Wilson MR, Yerbury JJ, Poon S. Potential roles of abundant extracellular chaperones in the control of amyloid formation and toxicity. Mol. Biosyst. 2008;4(1):42–52. doi: 10.1039/b712728f. [DOI] [PubMed] [Google Scholar]
- 149.Bell RD, Sagare AP, Friedman AE, et al. Transport pathways for clearance of human Alzheimer’s amyloid β-peptide and apolipoproteins E, J in the mouse central nervous system. J. Cereb. Blood Flow Metab. 2007;27(5):909–918. doi: 10.1038/sj.jcbfm.9600419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.DeMattos RB, Cirrito JR, Parsadanian M, et al. ApoE and clusterin cooperatively suppress Aβ levels and deposition: evidence that ApoE regulates extracellular Aβ metabolism in vivo. Neuron. 2004;41(2):193–202. doi: 10.1016/s0896-6273(03)00850-x. [DOI] [PubMed] [Google Scholar]
- 151.Nizard P, Tetley S, le Drean Y, et al. Stress-induced retrotranslocation of clusterin/ApoJ into the cytosol. Traffic. 2007;8(5):554–565. doi: 10.1111/j.1600-0854.2007.00549.x. [DOI] [PubMed] [Google Scholar]
- 152.Quinn CM, Kagedal K, Terman A, et al. Induction of fibroblast apolipoprotein E expression during apoptosis, starvation-induced growth arrest and mitosis. Biochem. J. 2004;378(Pt 3):753–761. doi: 10.1042/BJ20031352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Kim WS, Elliott DA, Kockx M, et al. Analysis of apolipoprotein E nuclear localization using green fluorescent protein and biotinylation approaches. Biochem. J. 2008;409(3):701–709. doi: 10.1042/BJ20071261. [DOI] [PubMed] [Google Scholar]
- 154.Do Carmo S, Levros LC, Jr, Rassart E. Modulation of apolipoprotein D expression and translocation under specific stress conditions. Biochim. Biophys. Acta. 2007;1773(6):954–969. doi: 10.1016/j.bbamcr.2007.03.007. [DOI] [PubMed] [Google Scholar]
- 155.Kang SW, Shin YJ, Shim YJ, Jeong SY, Park IS, Min BH. Clusterin interacts with SCLIP (SCG10-like protein) and promotes neurite outgrowth of PC12 cells. Exp. Cell Res. 2005;309(2):305–315. doi: 10.1016/j.yexcr.2005.06.012. [DOI] [PubMed] [Google Scholar]
- 156.Hashimoto M, Masliah E. Cycles of aberrant synaptic sprouting and neurodegeneration in Alzheimer’s and dementia with Lewy bodies. Neurochem. Res. 2003;28(11):1743–1756. doi: 10.1023/a:1026073324672. [DOI] [PubMed] [Google Scholar]
- 157.Klucken J, McLean PJ, Gomez-Tortosa E, Ingelsson M, Hyman BT. Neuritic alterations and neural system dysfunction in Alzheimer’s disease and dementia with Lewy bodies. Neurochem. Res. 2003;28(11):1683–1691. doi: 10.1023/a:1026061021946. [DOI] [PubMed] [Google Scholar]
- 158.Rassart E, Bedirian A, Do Carmo S, et al. Apolipoprotein D. Biochim. Biophys. Acta. 2000;1482(1–2):185–198. doi: 10.1016/s0167-4838(00)00162-x. [DOI] [PubMed] [Google Scholar]
- 159.Flower DR, North AC, Sansom CE. The lipocalin protein family: structural and sequence overview. Biochim. Biophys. Acta. 2000;1482(1–2):9–24. doi: 10.1016/s0167-4838(00)00148-5. [DOI] [PubMed] [Google Scholar]
- 160.Pearlman WH, Gueriguian JL, Sawyer ME. A specific progesterone-binding component of human breast cyst fluid. J. Biol. Chem. 1973;248(16):5736–5741. [PubMed] [Google Scholar]
- 161.Lea OA. Binding properties of progesterone-binding Cyst protein, PBCP. Steroids. 1988;52(4):337–338. doi: 10.1016/0039-128x(88)90135-3. [DOI] [PubMed] [Google Scholar]
- 162.Dilley WG, Haagensen DE, Cox CE, Wells SA., Jr Immunologic and steroid binding properties of the GCDFP-24 protein isolated from human breast gross cystic disease fluid. Breast Cancer Res. Treat. 1990;16(3):253–260. doi: 10.1007/BF01806333. [DOI] [PubMed] [Google Scholar]
- 163.Morais Cabral JH, Atkins GL, Sanchez LM, Lopez-Boado YS, Lopez-Otin C, Sawyer L. Arachidonic acid binds to apolipoprotein D: implications for the protein’s function. FEBS Lett. 1995;366(1):53–56. doi: 10.1016/0014-5793(95)00484-q. [DOI] [PubMed] [Google Scholar]
- 164.Vogt M, Skerra A. Bacterially produced apolipoprotein D binds progesterone and arachidonic acid, but not bilirubin or E-3M2H. J. Mol. Recognit. 2001;14(1):79–86. doi: 10.1002/1099-1352(200101/02)14:1<79::AID-JMR521>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 165.Provost PR, Villeneuve L, Weech PK, Milne RW, Marcel YL, Rassart E. Localization of the major sites of rabbit apolipoprotein D gene transcription by in situ hybridization. J. Lipid Res. 1991;32(12):1959–1970. [PubMed] [Google Scholar]
- 166.Smith KM, Lawn RM, Wilcox JN. Cellular localization of apolipoprotein D and lecithin:cholesterol acyltransferase mRNA in rhesus monkey tissues by in situ hybridization. J. Lipid Res. 1990;31(6):995–1004. [PubMed] [Google Scholar]
- 167.Thomas EA, Dean B, Pavey G, Sutcliffe JG. Increased CNS levels of apolipoprotein D in schizophrenic and bipolar subjects: implications for the pathophysiology of psychiatric disorders. Proc. Natl Acad. Sci USA. 2001;98(7):4066–4071. doi: 10.1073/pnas.071056198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Belloir B, Kovari E, Surini-Demiri M, Savioz A. Altered apolipoprotein D expression in the brain of patients with Alzheimer disease. J. Neurosci. Res. 2001;64(1):61–69. doi: 10.1002/jnr.1054. [DOI] [PubMed] [Google Scholar]
- 169.Lewis DA, Gonzalez-Burgos G. Pathophysiologically based treatment interventions in schizophrenia. Nat. Med. 2006;12(9):1016–1022. doi: 10.1038/nm1478. [DOI] [PubMed] [Google Scholar]
- 170.Koenigs M, Grafman J. The functional neuroanatomy of depression: distinct roles for ventromedial and dorsolateral prefrontal cortex. Behav. Brain Res. 2009;201(2):239–243. doi: 10.1016/j.bbr.2009.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Terrisse L, Poirier J, Bertrand P, et al. Increased levels of apolipoprotein D in cerebrospinal fluid and hippocampus of Alzheimer’s patients. J. Neurochem. 1998;71(4):1643–1650. doi: 10.1046/j.1471-4159.1998.71041643.x. [DOI] [PubMed] [Google Scholar]
- 172.Kalman J, McConathy W, Araoz C, Kasa P, Lacko AG. Apolipoprotein D in the aging brain and in Alzheimer’s dementia. Neurol. Res. 2000;22(4):330–336. doi: 10.1080/01616412.2000.11740678. [DOI] [PubMed] [Google Scholar]
- 173.Glockner F, Ohm TG. Hippocampal apolipoprotein D level depends on Braak stage, APOE genotype. Neuroscience. 2003;122(1):103–110. doi: 10.1016/s0306-4522(03)00529-3. [DOI] [PubMed] [Google Scholar]
- 174.Ordonez C, Navarro A, Perez C, Astudillo A, Martinez E, Tolivia J. Apolipoprotein D expression in substantia nigra of Parkinson disease. Histol. Histopathol. 2006;21(4):361–366. doi: 10.14670/HH-21.361. [DOI] [PubMed] [Google Scholar]
- 175.Franz G, Reindl M, Patel SC, et al. Increased expression of apolipoprotein D following experimental traumatic brain injury. J. Neurochem. 1999;73(4):1615–1625. doi: 10.1046/j.1471-4159.1999.0731615.x. [DOI] [PubMed] [Google Scholar]
- 176.Rickhag M, Deierborg T, Patel S, Ruscher K, Wieloch T. Apolipoprotein D is elevated in oligodendrocytes in the peri-infarct region after experimental stroke: influence of enriched environment. J. Cereb. Blood Flow Metab. 2008;28(3):551–562. doi: 10.1038/sj.jcbfm.9600552. [DOI] [PubMed] [Google Scholar]
- 177.Ong WY, He Y, Suresh S, Patel SC. Differential expression of apolipoprotein D and apolipoprotein E in the kainic acid-lesioned rat hippocampus. Neuroscience. 1997;79(2):359–367. doi: 10.1016/s0306-4522(96)00608-2. [DOI] [PubMed] [Google Scholar]
- 178.Ong WY, Hu CY, Patel SC. Apolipoprotein D in the Niemann-Pick type C disease mouse brain: an ultrastructural immunocytochemical analysis. J. Neurocytol. 2002;31(2):121–129. doi: 10.1023/a:1023993405851. [DOI] [PubMed] [Google Scholar]
- 179.Suresh S, Yan Z, Patel RC, Patel YC, Patel SC. Cellular cholesterol storage in the Niemann–Pick disease type C mouse is associated with increased expression and defective processing of apolipoprotein D. J. Neurochem. 1998;70(1):242–251. doi: 10.1046/j.1471-4159.1998.70010242.x. [DOI] [PubMed] [Google Scholar]
- 180.Do Carmo S, Seguin D, Milne R, Rassart E. Modulation of apolipoprotein D and apolipoprotein E mRNA expression by growth arrest and identification of key elements in the promoter. J. Biol. Chem. 2002;277(7):5514–5523. doi: 10.1074/jbc.M105057200. [DOI] [PubMed] [Google Scholar]
- 181.Markesbery WR. Oxidative stress hypothesis in Alzheimer's disease. Free Radic. Biol. Med. 1997;23(1):134–147. doi: 10.1016/s0891-5849(96)00629-6. [DOI] [PubMed] [Google Scholar]
- 182.Prabakaran S, Swatton JE, Ryan MM, et al. Mitochondrial dysfunction in schizophrenia: evidence for compromised brain metabolism and oxidative stress. Mol. Psychiatry. 2004;9(7):684–697. 643. doi: 10.1038/sj.mp.4001511. [DOI] [PubMed] [Google Scholar]
- 183.Onyango IG, Khan SM. Oxidative stress, mitochondrial dysfunction, and stress signaling in Alzheimer’s disease. Curr. Alzheimer Res. 2006;3(4):339–349. doi: 10.2174/156720506778249489. [DOI] [PubMed] [Google Scholar]
- 184. Ganfornina MD, Do Carmo S, Lora JM, et al. Apolipoprotein D is involved in the mechanisms regulating protection from oxidative stress. Aging Cell. 2008;7(4):506–515. doi: 10.1111/j.1474-9726.2008.00395.x.. ▪▪ Presents conclusive in vivo evidence demonstrating the major role ApoD plays in protecting from oxidative stress in the CNS.
- 185.Sanchez D, Lopez-Arias B, Torroja L, et al. Loss of glial lazarillo, a homolog of apolipoprotein D, reduces lifespan and stress resistance in Drosophila. Curr. Biol. 2006;16(7):680–686. doi: 10.1016/j.cub.2006.03.024. [DOI] [PubMed] [Google Scholar]
- 186.Walker DW, Muffat J, Rundel C, Benzer S. Overexpression of a Drosophila homolog of apolipoprotein D leads to increased stress resistance and extended lifespan. Curr. Biol. 2006;16(7):674–679. doi: 10.1016/j.cub.2006.01.057. [DOI] [PubMed] [Google Scholar]
- 187.Do Carmo S, Jacomy H, Talbot PJ, Rassart E. Neuroprotective effect of apolipoprotein D against human coronavirus OC43-induced encephalitis in mice. J. Neurosci. 2008;28(41):10330–10338. doi: 10.1523/JNEUROSCI.2644-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Muffat J, Walker DW, Benzer S. Human ApoD, an apolipoprotein up-regulated in neurodegenerative diseases, extends lifespan and increases stress resistance in Drosophila. Proc. Natl Acad. Sci. USA. 2008;105(19):7088–7093. doi: 10.1073/pnas.0800896105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Boyles JK, Notterpek LM, Anderson LJ. Accumulation of apolipoproteins in the regenerating and remyelinating mammalian peripheral nerve. Identification of apolipoprotein D, apolipoprotein A-IV, apolipoprotein E, and apolipoprotein A-I. J. Biol. Chem. 1990;265(29):17805–17815. [PubMed] [Google Scholar]
- 190.Seguin D, Desforges M, Rassart E. Molecular characterization and differential mRNA tissue distribution of mouse apolipoprotein D. Brain Res. Mol. Brain Res. 1995;30(2):242–250. doi: 10.1016/0169-328x(95)00008-g. [DOI] [PubMed] [Google Scholar]
- 191.Desai PP, Ikonomovic MD, Abrahamson EE, et al. Apolipoprotein D is a component of compact but not diffuse amyloid-β plaques in Alzheimer’s disease temporal cortex. Neurobiol. Dis. 2005;20(2):574–582. doi: 10.1016/j.nbd.2005.04.012. [DOI] [PubMed] [Google Scholar]
- 192.Price DL, Tanzi RE, Borchelt DR, Sisodia SS. Alzheimer’s disease: genetic studies and transgenic models. Annu. Rev. Genet. 1998;32:461–493. doi: 10.1146/annurev.genet.32.1.461. [DOI] [PubMed] [Google Scholar]
- 193.Hiltunen M, Mannermaa A, Thompson D, et al. Genome-wide linkage disequilibrium mapping of late-onset Alzheimer’s disease in Finland. Neurology. 2001;57(9):1663–1668. doi: 10.1212/wnl.57.9.1663. [DOI] [PubMed] [Google Scholar]
- 194.Desai PP, Hendrie HC, Evans RM, Murrell JR, DeKosky ST, Kamboh MI. Genetic variation in apolipoprotein D affects the risk of Alzheimer disease in African–Americans. Am. J. Med. Genet. B Neuropsychiatr. Genet. 2003;116B(1):98–101. doi: 10.1002/ajmg.b.10798. [DOI] [PubMed] [Google Scholar]
- 195.Lewis CM, Levinson DF, Wise LH, et al. Genome scan meta-analysis of schizophrenia and bipolar disorder, part II: schizophrenia. Am. J. Hum. Genet. 2003;73(1):34–48. doi: 10.1086/376549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Hansen T, Hemmingsen RP, Wang AG, et al. Apolipoprotein D is associated with long-term outcome in patients with schizophrenia. Pharmacogenomics J. 2006;6(2):120–125. doi: 10.1038/sj.tpj.6500350. [DOI] [PubMed] [Google Scholar]
- 197.Thomas EA, Danielson PE, Nelson PA, et al. Clozapine increases apolipoprotein D expression in rodent brain: towards a mechanism for neuroleptic pharmacotherapy. J. Neurochem. 2001;76(3):789–796. doi: 10.1046/j.1471-4159.2001.00027.x. [DOI] [PubMed] [Google Scholar]
- 198.Thomas EA, George RC, Danielson PE, et al. Antipsychotic drug treatment alters expression of mRNAs encoding lipid metabolism-related proteins. Mol. Psychiatry. 2003;8(12):983–993. 950. doi: 10.1038/sj.mp.4001425. [DOI] [PubMed] [Google Scholar]
- 199.Mahley RW, Innerarity TL, Rall SC, Jr, Weisgraber KH. Plasma lipoproteins: apolipoprotein structure and function. J. Lipid Res. 1984;25(12):1277–1294. [PubMed] [Google Scholar]
- 200.Harr SD, Uint L, Hollister R, Hyman BT, Mendez AJ. Brain expression of apolipoproteins E, J, A-I in Alzheimer’s disease. J. Neurochem. 1996;66(6):2429–2435. doi: 10.1046/j.1471-4159.1996.66062429.x. [DOI] [PubMed] [Google Scholar]
- 201.Huang JT, Wang L, Prabakaran S, et al. Independent protein-profiling studies show a decrease in apolipoprotein A1 levels in schizophrenia CSF, brain and peripheral tissues. Mol. Psychiatry. 2008;13(12):1118–1128. doi: 10.1038/sj.mp.4002108. [DOI] [PubMed] [Google Scholar]
- 202.Weiler-Guttler H, Sommerfeldt M, Papandrikopoulou A, et al. Synthesis of apolipoprotein A-1 in pig brain microvascular endothelial cells. J. Neurochem. 1990;54(2):444–450. doi: 10.1111/j.1471-4159.1990.tb01892.x. [DOI] [PubMed] [Google Scholar]
- 203.Mockel B, Zinke H, Flach R, Weiss B, Weiler-Guttler H, Gassen HG. Expression of apolipoprotein A-I in porcine brain endothelium in vitro. J. Neurochem. 1994;62(2):788–798. doi: 10.1046/j.1471-4159.1994.62020788.x. [DOI] [PubMed] [Google Scholar]
- 204.Vollbach H, Heun R, Morris CM, et al. APOA1 polymorphism influences risk for early-onset nonfamiliar AD. Ann. Neurol. 2005;58(3):436–441. doi: 10.1002/ana.20593. [DOI] [PubMed] [Google Scholar]
- 205.Tuteja R, Tuteja N, Melo C, Casari G, Baralle FE. Transcription efficiency of human apolipoprotein A-I promoter varies with naturally occurring A to G transition. FEBS Lett. 1992;304(1):98–101. doi: 10.1016/0014-5793(92)80597-a. [DOI] [PubMed] [Google Scholar]
- 206.Angotti E, Mele E, Costanzo F, Avvedimento EV. A polymorphism (G->A transition) in the -78 position of the apolipoprotein A-I promoter increases transcription efficiency. J. Biol. Chem. 1994;269(26):17371–17374. [PubMed] [Google Scholar]
- 207.Jeenah M, Kessling A, Miller N, Humphries S. G to A substitution in the promoter region of the apolipoprotein AI gene is associated with elevated serum apolipoprotein AI and high density lipoprotein cholesterol concentrations. Mol. Biol. Med. 1990;7(3):233–241. [PubMed] [Google Scholar]
- 208.Juo SH, Wyszynski DF, Beaty TH, Huang HY, Bailey-Wilson JE. Mild association between the A/G polymorphism in the promoter of the apolipoprotein A-I gene and apolipoprotein A-I levels: a meta-analysis. Am. J. Med. Genet. 1999;82(3):235–241. [PubMed] [Google Scholar]
- 209.Demeester N, Castro G, Desrumaux C, et al. Characterization and functional studies of lipoproteins, lipid transfer proteins, and lecithin:cholesterol acyltransferase in CSF of normal individuals and patients with Alzheimer’s disease. J. Lipid Res. 2000;41(6):963–974. [PubMed] [Google Scholar]
- 210.Kang J, Lemaire HG, Unterbeck A, et al. The precursor of Alzheimer’s disease amyloid A4 protein resembles a cell-surface receptor. Nature. 1987;325(6106):733–736. doi: 10.1038/325733a0. [DOI] [PubMed] [Google Scholar]
- 211.Koldamova RP, Lefterov IM, Lefterova MI, Lazo JS. Apolipoprotein A-I directly interacts with amyloid precursor protein and inhibits A β aggregation and toxicity. Biochemistry. 2001;40(12):3553–3560. doi: 10.1021/bi002186k. [DOI] [PubMed] [Google Scholar]
- 212.Petit-Turcotte C, Stohl SM, Beffert U, et al. Apolipoprotein C-I expression in the brain in Alzheimer’s disease. Neurobiol. Dis. 2001;8(6):953–963. doi: 10.1006/nbdi.2001.0441. [DOI] [PubMed] [Google Scholar]
- 213.Lauer SJ, Walker D, Elshourbagy NA, Reardon CA, Levy-Wilson B, Taylor JM. Two copies of the human apolipoprotein C-I gene are linked closely to the apolipoprotein E gene. J. Biol. Chem. 1988;263(15):7277–7286. [PubMed] [Google Scholar]
- 214.Shen P, Howlett GJ. Two coding regions closely linked to the rat apolipoprotein E gene: nucleotide sequences of rat apolipoprotein C-I, ECL cDNA. Arch. Biochem. Biophys. 1992;297(2):345–353. doi: 10.1016/0003-9861(92)90683-n. [DOI] [PubMed] [Google Scholar]
- 215.Tysoe C, Galinsky D, Robinson D, et al. APO E, APO CI loci are associated with dementia in younger but not older late-onset cases. Dement. Geriatr. Cogn. Disord. 1998;9(4):191–198. doi: 10.1159/000017046. [DOI] [PubMed] [Google Scholar]
- 216.Poduslo SE, Neal M, Herring K, Shelly J. The apolipoprotein CI A allele as a risk factor for Alzheimer’s disease. Neurochem. Res. 1998;23(3):361–367. doi: 10.1023/a:1022409617539. [DOI] [PubMed] [Google Scholar]
- 217.Drigalenko E, Poduslo S, Elston R. Interaction of the apolipoprotein E, CI loci in predisposing to late-onset Alzheimer’s disease. Neurology. 1998;51(1):131–135. doi: 10.1212/wnl.51.1.131. [DOI] [PubMed] [Google Scholar]
- 218.Weisgraber KH, Mahley RW, Kowal RC, Herz J, Goldstein JL, Brown MS. Apolipoprotein C-I modulates the interaction of apolipoprotein E with β-migrating very low density lipoproteins (β-VLDL) and inhibits binding of β-VLDL to low density lipoprotein receptor-related protein. J. Biol. Chem. 1990;265(36):22453–22459. [PubMed] [Google Scholar]
- 219.Kowal RC, Herz J, Weisgraber KH, Mahley RW, Brown MS, Goldstein JL. Opposing effects of apolipoproteins E, C on lipoprotein binding to low density lipoprotein receptor-related protein. J. Biol. Chem. 1990;265(18):10771–10779. [PubMed] [Google Scholar]
- 220.Sehayek E, Eisenberg S. Mechanisms of inhibition by apolipoprotein C of apolipoprotein E-dependent cellular metabolism of human triglyceride-rich lipoproteins through the low density lipoprotein receptor pathway. J. Biol. Chem. 1991;266(27):18259–18267. [PubMed] [Google Scholar]
- 221.Liu M, Doi T, Shen L, et al. Intestinal satiety protein apolipoprotein AIV is synthesized and regulated in rat hypothalamus. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2001;280(5):R1382–R1387. doi: 10.1152/ajpregu.2001.280.5.R1382. [DOI] [PubMed] [Google Scholar]
- 222.Shen L, Pearson KJ, Xiong Y, et al. Characterization of apolipoprotein A-IV in brain areas involved in energy homeostasis. Physiol. Behav. 2008;95(1–2):161–167. doi: 10.1016/j.physbeh.2008.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Okumura T, Fukagawa K, Tso P, Taylor IL, Pappas TN. Intracisternal injection of apolipoprotein A-IV inhibits gastric secretion in pylorus-ligated conscious rats. Gastroenterology. 1994;107(6):1861–1864. doi: 10.1016/0016-5085(94)90833-8. [DOI] [PubMed] [Google Scholar]
- 224.Tso P, Chen Q, Fujimoto K, Fukagawa K, Sakata T. Apolipoprotein A-IV: a circulating satiety signal produced by the small intestine. Obes. Res. 1995;3 Suppl. 5:689S–695S. doi: 10.1002/j.1550-8528.1995.tb00487.x. [DOI] [PubMed] [Google Scholar]
- 225.Tso P, Liu M, Kalogeris TJ. The role of apolipoprotein A-IV in food intake regulation. J. Nutr. 1999;129(8):1503–1506. doi: 10.1093/jn/129.8.1503. [DOI] [PubMed] [Google Scholar]
- 226.Csaszar A, Kalman J, Szalai C, Janka Z, Romics L. Association of the apolipoprotein A-IV codon 360 mutation in patients with Alzheimer’s disease. Neurosci. Lett. 1997;230(3):151–154. doi: 10.1016/s0304-3940(97)00500-4. [DOI] [PubMed] [Google Scholar]
- 227. Mimmack ML, Ryan M, Baba H, et al. Gene expression analysis in schizophrenia: reproducible up-regulation of several members of the apolipoprotein L family located in a high-susceptibility locus for schizophrenia on chromosome 22. Proc. Natl Acad. Sci. USA. 2002;99(7):4680–4685. doi: 10.1073/pnas.032069099.. ▪ Identifies the upregulation of APOL family genes in schizophrenia.
- 228.Takahashi S, Cui YH, Han YH, et al. Association of SNPs and haplotypes in APOL1, 2 and 4 with schizophrenia. Schizophr. Res. 2008;104(1–3):153–164. doi: 10.1016/j.schres.2008.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Vanhollebeke B, Pays E. The function of apolipoproteins L. :Cell Mol. Life Sci. 2006;63(17):1937–1944. doi: 10.1007/s00018-006-6091-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Rebeck GW, Alonzo NC, Berezovska O, et al. Structure and functions of human cerebrospinal fluid lipoproteins from individuals of different APOE genotypes. Exp. Neurol. 1998;149(1):175–182. doi: 10.1006/exnr.1997.6710. [DOI] [PubMed] [Google Scholar]
- 231.Montine KS, Bassett CN, Ou JJ, Markesbery WR, Swift LL, Montine TJ. Apolipoprotein E allelic influence on human cerebrospinal fluid apolipoproteins. J. Lipid Res. 1998;39(12):2443–2451. [PubMed] [Google Scholar]
- 232.Caronti B, Calderaro C, Alessandri C, et al. β2-glycoprotein I (β2-GPI) mRNA is expressed by several cell types involved in anti-phospholipid syndrome-related tissue damage. Clin. Exp. Immunol. 1999;115(1):214–219. doi: 10.1046/j.1365-2249.1999.00770.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Fujimoto K, Fukagawa K, Sakata T, Tso P. Suppression of food intake by apolipoprotein A-IV is mediated through the central nervous system in rats. J. Clin. Invest. 1993;91(4):1830–1833. doi: 10.1172/JCI116395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Eckert GP, Vardanian L, Rebeck GW, Burns MP. Regulation of central nervous system cholesterol homeostasis by the liver X receptor agonist TO-901317. Neurosci. Lett. 2007;423(1):47–52. doi: 10.1016/j.neulet.2007.05.063. [DOI] [PubMed] [Google Scholar]
- 235.Koldamova RP, Lefterov IM, Staufenbiel M, et al. The liver X receptor ligand T0901317 decreases amyloid β production in vitro and in a mouse model of Alzheimer’s disease. J. Biol. Chem. 2005;280(6):4079–4088. doi: 10.1074/jbc.M411420200. [DOI] [PubMed] [Google Scholar]
- 236.Sun Y, Yao J, Kim TW, Tall AR. Expression of liver X receptor target genes decreases cellular amyloid β peptide secretion. J. Biol. Chem. 2003;278(30):27688–27694. doi: 10.1074/jbc.M300760200. [DOI] [PubMed] [Google Scholar]
- 237.Ruscher K, Erickson A, Kuric E, Inacio AR, Wieloch T. Effects of chronic clozapine administration on apolipoprotein D levels and on functional recovery following experimental stroke. Brain Res. 2010;1321:152–163. doi: 10.1016/j.brainres.2010.01.024. [DOI] [PubMed] [Google Scholar]
- 238.Aono M, Bennett ER, Kim KS, et al. Protective effect of apolipoprotein E-mimetic peptides on N-methyl-d-aspartate excitotoxicity in primary rat neuronal-glial cell cultures. Neuroscience. 2003;116(2):437–445. doi: 10.1016/s0306-4522(02)00709-1. [DOI] [PubMed] [Google Scholar]
- 239.Laskowitz DT, Fillit H, Yeung N, Toku K, Vitek MP. Apolipoprotein E-derived peptides reduce CNS inflammation: implications for therapy of neurological disease. Acta Neurol. Scand. Suppl. 2006;185:15–20. doi: 10.1111/j.1600-0404.2006.00680.x. [DOI] [PubMed] [Google Scholar]
- 240.Laskowitz DT, Thekdi AD, Thekdi SD, et al. Downregulation of microglial activation by apolipoprotein E and apoE-mimetic peptides. Exp. Neurol. 2001;167(1):74–85. doi: 10.1006/exnr.2001.7541. [DOI] [PubMed] [Google Scholar]
- 241.Laskowitz DT, McKenna SE, Song P, et al. COG1410, a novel apolipoprotein E-based peptide, improves functional recovery in a murine model of traumatic brain injury. J. Neurotrauma. 2007;24(7):1093–1107. doi: 10.1089/neu.2006.0192. [DOI] [PubMed] [Google Scholar]
- 242.Wang H, Durham L, Dawson H, et al. An apolipoprotein E-based therapeutic improves outcome and reduces Alzheimer’s disease pathology following closed head injury: evidence of pharmacogenomic interaction. Neuroscience. 2007;144(4):1324–1333. doi: 10.1016/j.neuroscience.2006.11.017. [DOI] [PubMed] [Google Scholar]
- 243.Li FQ, Sempowski GD, McKenna SE, Laskowitz DT, Colton CA, Vitek MP. Apolipoprotein E-derived peptides ameliorate clinical disability and inflammatory infiltrates into the spinal cord in a murine model of multiple sclerosis. J. Pharmacol. Exp. Ther. 2006;318(3):956–965. doi: 10.1124/jpet.106.103671. [DOI] [PubMed] [Google Scholar]
- 244.Gao J, Wang H, Sheng H, et al. A novel apoE-derived therapeutic reduces vasospasm and improves outcome in a murine model of subarachnoid hemorrhage. Neurocrit. Care. 2006;4(1):25–31. doi: 10.1385/NCC:4:1:025. [DOI] [PubMed] [Google Scholar]
- 245.James ML, Sullivan PM, Lascola CD, Vitek MP, Laskowitz DT. Pharmacogenomic effects of apolipoprotein E on intracerebral hemorrhage. Stroke. 2009;40(2):632–639. doi: 10.1161/STROKEAHA.108.530402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Sarantseva S, Timoshenko S, Bolshakova O, et al. Apolipoprotein E-mimetics inhibit neurodegeneration and restore cognitive functions in a transgenic Drosophila model of Alzheimer’s disease. PLoS One. 2009;4(12):E8191. doi: 10.1371/journal.pone.0008191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Navab M, Ruchala P, Waring AJ, et al. A novel method for oral delivery of apolipoprotein mimetic peptides synthesized from all l-amino acids. J. Lipid Res. 2009;50(8):1538–1547. doi: 10.1194/jlr.M800539-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Kratzer I, Wernig K, Panzenboeck U, et al. Apolipoprotein A-I coating of protamine-oligonucleotide nanoparticles increases particle uptake and transcytosis in an in vitro model of the blood–brain barrier. J. Control Release. 2007;117(3):301–311. doi: 10.1016/j.jconrel.2006.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Brasnjevic I, Steinbusch HW, Schmitz C, Martinez-Martinez P. Delivery of peptide and protein drugs over the blood–brain barrier. Prog. Neurobiol. 2009;87(4):212–251. doi: 10.1016/j.pneurobio.2008.12.002. [DOI] [PubMed] [Google Scholar]
- 250.Sarantseva SV, Bol’shakova OI, Timoshenko SI, Kolobov AA, Vitek MP, Shvartsman AL. [Protein transduction domain peptide mediates delivery to the brain via the blood–brain barrier in Drosophila] Biomed. Khim. 2009;55(1):41–49. [PubMed] [Google Scholar]
Websites
- 301.Medline Plus. Online dictionary. www.nlm.nih.gov/medlineplus/mplusdictionary.html.
- 302.Medical Online Dictionary. www.online-medical-dictionary.org/
- 303.National Center for Biotechnology Information. Gene expression omnibus. www.ncbi.nlm.nih.gov/geo/query/acc. cgi?acc=GSE13564.