Abstract
Induction of heme oxygenase -1 (HO-1) inhibits hepatitis C virus (HCV) replication. Of the products of the reaction catalyzed by HO-1 iron has been shown to inhibit HCV RNA polymerase, but little is known about the antiviral activity of biliverdin (BV). Herein, we report that BV inhibits viral replication and viral protein expression in a dose-dependent manner in replicons and cells harboring the infectious J6/JFH construct. Using the SensoLyte 620 HCV Protease Assay with a wide wavelength excitation/emission (591nm/622nm) fluorescence energy transfer peptide, we found that both recombinant and endogenous NS3/4A protease from replicon microsomes are potently inhibited by BV. Of the tetrapyrroles tested, BV was the strongest inhibitor of NS3/4A activity with an IC50 of 9 uM, similar to that of the commercial inhibitor, AnaSpec #25346 (IC50 5 uM). Lineweaver-Burk plots indicated mixed competitive and non-competitive inhibition of the protease by BV. In contrast, the effects of bilirubin (BR) on HCV replication and NS3/4A were much less potent. Because BV is rapidly converted to BR by biliverdin reductase (BVR) intracellularly, the effect of BVR knockdown on BV antiviral activity was assessed. After >80% silencing of BVR, inhibition of viral replication by BV was enhanced. BV also increased the antiviral activity of α-interferon in replicons.
Conclusion
BV is a potent inhibitor of HCV NS3/4A protease, which likely contributes to the antiviral activity of HO-1. These findings suggest that BV or its derivatives may be useful future drug therapies targeting the NS3/4A protease.
Keywords: Hepatitis C virus, biliverdin, bilirubin, NS3/4A protease
Introduction
Chronic hepatitis C virus (HCV) infection is an important cause of liver disease worldwide. A significant number of infected patients develop persistent viremia that leads to cirrhosis, end-stage liver disease, and hepatocellular carcinoma (1). Current standard treatment for chronic HCV infection, pegylated α-interferon and ribavirin, achieves viral eradication in only about half of patients treated (2). Structurally, the virus has a plus-stranded RNA genome with a single long open-reading frame containing 5’ and 3’ flanking non-translated nucleotide regions that are important for translation, replication, and immune recognition (3). The genome contains a serine-activated protease and an RNA dependent RNA polymerase (RdRp) that are important targets for development of new antiviral drugs. While anti-protease and anti-polymerase drugs promise to improve treatment outcomes, their efficacy may be limited by the rapid development of viral resistance (4).
Hepatocellular damage from HCV has been linked to oxidative stress (5). Consequently, we and others have been interested in the potential role of antioxidant enzymes as cytoprotective agents during HCV infection (6–9). Heme oxygenase-1 (HO-1) is an important cytoprotective enzyme, which is readily induced in response to a variety of stressors and cytotoxins. HO-1 oxidizes heme to equimolar concentrations of biliverdin (BV), carbon monoxide, and iron (10) (Fig. 1). Following heme oxidation, free BV is rapidly reduced to bilirubin (BR) by the enzyme biliverdin reductase (BVR) which is abundant in the hepatocyte.
Figure 1. Heme oxygenase and biliverdin reductase enzymatic reactions.
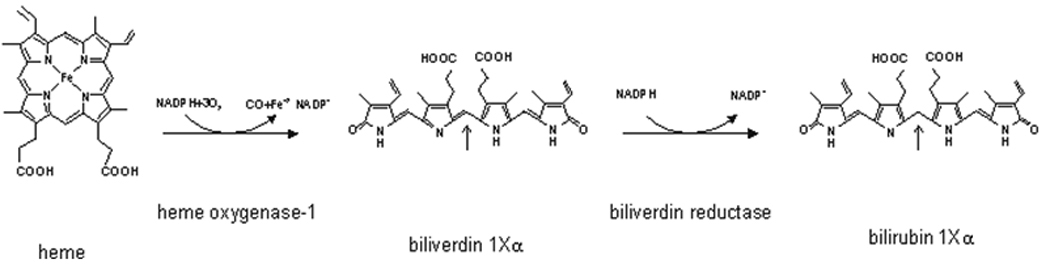
Heme is first oxidized by heme oxygenase-1 producing biliverdin, Carbon monoxide and iron. Biliverdin is then reduced at the central methene bridge position 10 (arrows) by biliverdin reductase to produce bilirubin.
We and others have previously shown that HO-1 induction or overexpression in replicons inhibits HCV replication (9, 11). Although the mechanism of this effect has not been clearly defined, it is reasonable to infer that one or more of the products of the reaction catalyzed by HO-1 may be responsible. Consistent with this prediction, iron has been demonstrated to inhibit the NS5B RdRp through inhibition of divalent cation binding (12). There are also accumulating data indicating that BV has antiviral activity (13), which has been linked to the induction of interferon response genes (13). However, these results do not exclude the possibility that BV has additional antiviral effects.
In this paper, we report that BV potently inhibits viral replication at concentrations remarkably similar to those achieved following induction of HO-1 by heme, suggesting that BV is a primary antiviral agent released during heme oxidation. Furthermore, BV, and to a much lesser extent the BV metabolite, bilirubin (BR) inhibits HCV NS3/4A protease in cell-free assays. These findings provide a plausible mechanism for the antiviral activity of HO-1 in addition to those reported previously and strongly suggest a potential role for BV or structural derivatives in future drug design targeting the HCV NS3/4A protease.
Materials and Methods
Materials
Taq DNA polymerase (Perkin-Elmer Cetus, Norwalk, CT), and Moloney murine leukemia virus reverse transcriptase (Gibco/BRL Life Technologies, Gaithersburg, MD) were used in these studies. Bile pigments were purchased from Frontier Scientific, Inc (Logan, UT) and included bilirubin-IX-α (#B584-9), biliverdin-IX-α hydrochloride (#B655-9) and mesobilirubin (B588-9). Bilirubin mixed isomers, (>99%) was purchased from Sigma Chemical Co (Saint Louis, MO). All preparations of tetrapyrroles were the purest form available (99% purity). The BR mixed isomer preparation contained 93% bilirubin IX-α, 3% bilirubin III-α, 3% bilirubin XIII-α and traces of β and γ isomers (MSDS information). BV was prepared by oxidation of highly purified α-bilirubin followed by final crystallization in ether (personal communication, Dr. Jerry Bommer, Echo Laboratories, Frontier Scientific, Salt Lake City, UT). All tetrapyrroles were dissolved in 0.2 N NaOH and added in small volumes to achieve the final concentration. Controls received an identical volume of diluted NaOH only. HCV protease assay kits [SensoLyte 620, Cat# 71146] and recombinant NS3/4A protease [(Ac-DEDif-EchaC), Cat #25346] were purchased from AnaSpec.
Antibodies
Antibody to human biliverdin reductase (BVR) and all secondary antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, CA) unless indicated otherwise.
Cell lines and cell culture
The human hepatoma cell line (Huh5-15) with replicating sub-genomic HCV RNA (14) was a kind gift of Dr. Volker Lohmann (Institute for Virology, Johannes-Gutenberg University, Mainz, Germany), and cultivated as described (9). Huh7.5 cells harboring full length (Huh7.5FL) Con1 replicons (15) were a kind gift of Dr. Charles Rice (Rockefeller University, New York, NY). These cells were passed as recommended by their laboratory of origin (15). An infectious clone of HCV, J6/JFH, was inoculated into Huh7.5 cells and the cultures passed as previously described (16). Cells were incubated with BV, BR, or FeCl2 for 24–48 hours in DMEM containing 5% FBS.
Quantitative Real-time RT-PCR
Detailed procedure is described in Supplemental Methods available on line.
Immunocytochemical staining
Cells were fixed in absolute methanol, washed in PBS, and incubated with positive HCV genotype 2A polyvalent human serum. On western blots, this antiserum specifically recognized core, NS3, and NS5A at their appropriate mobility. Antibody binding was evaluated following labeling with anti-human secondary antibody-alkaline phosphatase conjugate and results recorded by photomicroscopy.
Western blot analysis
Western blots (WB) were performed as previously described using enhanced chemiluminescence for signal detection (ECLTM, Amersham) (17). Signal intensities were quantified using Image J software (NIH).
Biliverdin reductase (BVR) knockdown
Biliverdin reductase (BVR) siRNA and control (scrambled) siRNA were purchased from Santa Cruz Biotechnology (sc-44650 and sc-37007). BVR knockdown was performed as described previously (10). Efficiency of the knockdown was monitored by semiquantitative densitometry of BVR WB.
In vitro assay of HCV NS3/4A recombinant protease
Protease activity was determined fluorometrically with the SensoLyte 620 HCV Protease Assay (AnaSpec, Fremont, CA) using a wide wavelength excitation/emission (591 nm/622 nm respectively) fluorescence energy transfer peptide according to the manufacturer’s instructions. Control incubations with BV or metabolite only were performed to eliminate or correct for autofluorescence or quenching. A competitive inhibitor of the NS3/4A protease, AnaSpec #25346, was used as positive control.
For assays employing endogenous NS3/4A protease, detailed procedure is described in Supplemental Methods available on line.
Immunoprecipitation of NS5A
The detailed procedure is described in Supplemental Methods available on line.
Proliferation and cytotoxicity assays
These assays were performed as described in detail in Supplemental Methods available on line.
Statistical analysis
Data from individual experiments as well as combined data from separate experiments were expressed as mean +/− standard error of the mean. The significance between means was determined using Student’s t-test and when applicable, with ANOVA using pooled variances. P values less than 0.05 were considered significant. All experimental findings, whether performed singly or in parts were repeated at least three times.
Results
We have previously shown that induction of HO-1 with hemin results in decreased HCV replication in vitro (9), however, it was not known whether physiological concentrations of heme exert antiviral effects. Incubation of replicons with various amounts of hemin demonstrated a concentration-dependent antiviral effect of hemin, apparent at levels at as low as 5 µM (Table 1). These concentrations are well within the physiological range of heme in human circulation (10–16 µM) and, in the presence of HO-1, would be expected to yield equimolar quantities of BV, Fe and carbon monoxide.
Table 1.
Heme inhibition of HCV replication
| Replicon | Heme [uM] |
Relative [HCV] [ΔCΤ] |
*SEM |
|---|---|---|---|
| Huh5-15NS | 0 | 1.0 | 0.08 |
| 5 | 0.27 | 0.02 | |
| 10 | 0.09 | 0.003 | |
| 20 | 0.03 | 0.003 | |
| Huh7.5FL | 0 | 1.0 | 0.16 |
| 5 | 0.22 | 0.03 | |
| 10 | 0.08 | 0.02 | |
| 20 | 0.04 | 0.006 |
Log phase replicon cells were incubated with hemin or control vehicle overnight and the relative amount of HCV RNA then determined by the comparative cycle threshold level (ΔCΤ) as described in Methods. Each value is the mean of four determinations.
p < 0.01 all within group difference
Antiviral activity of BV and BR
We next tested BV and isomers of its metabolite BR for antiviral activity in HCV full-length and nonstructural replicons. In both replicon lines, BV showed significant antiviral activity at concentrations as low as 20 µM. In contrast, concentrations of BR-IX-α or BR mixed isomers required to suppress HCV replication were considerably higher (200 µM) (Fig. 2). For comparison, 20 µM of BV or BR corresponds to a circulating BR level of about 1.4 mg/dl. Western blots (Fig. 3A–B) confirmed decreased NS5A in both replicon lines following treatment with BV or BR. Levels of core protein were also reduced by BV or BR in full-length replicons, consistent with reduced replication of HCV. Treatment with BV dose-dependently decreased NS5A when assayed by WB (Fig. 3C) or immunoprecipitation using specific NS5A antibody (Fig. 3D). In accord with prior reports (12, 18), FeCl2 (100 µM) also decreased NS5A and core protein (Fig. 3A–B) as well as diminishing HCV RNA (not shown).
Figure 2. Biliverdin and bilirubin suppression of HCV replication.
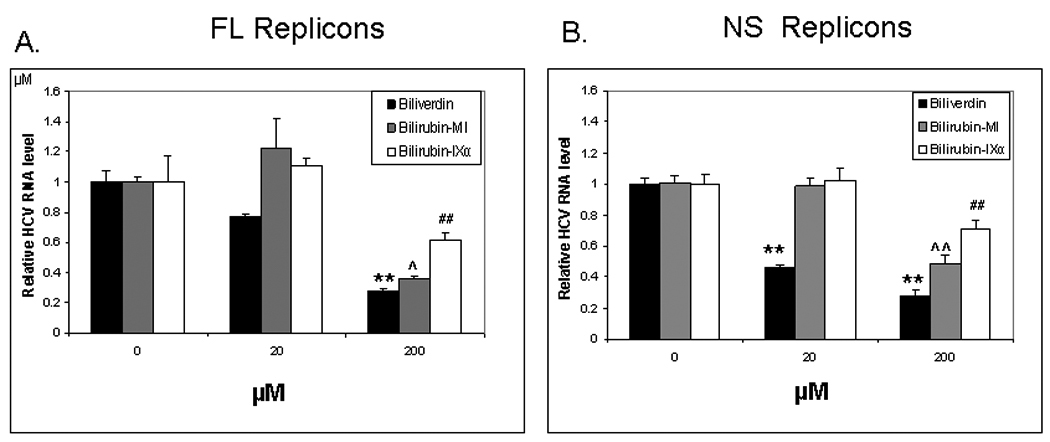
Log-phase full-length (FL) (A) or non-structural (NS) (B) replicon cells were treated 48 hours with indicated concentrations of BV or BR. HCV replication was determined by Real-Time RT-PCR using the Comparative Cycle Threshold (ΔCT) method as described in Supplemental methods available on line. Each value is the mean +/− the SEM of 5–6 determinations per treatment group. Biliverdin = >99% Biliverdin IX-α. Bilirubin-mixed isomers (MI) = 93% Bilirubin IX-α, and 6% associated Bilirubin isomers as described in Materials. Bilirubin IX-α = >99% Bilirubin IX-α.
[** or ## or ^^ p<0.01 from respective controls. ^ p < 0.05 from control.].
Figure 3. Effect of BV, BR, and FeCl2 treatment on HCV proteins.
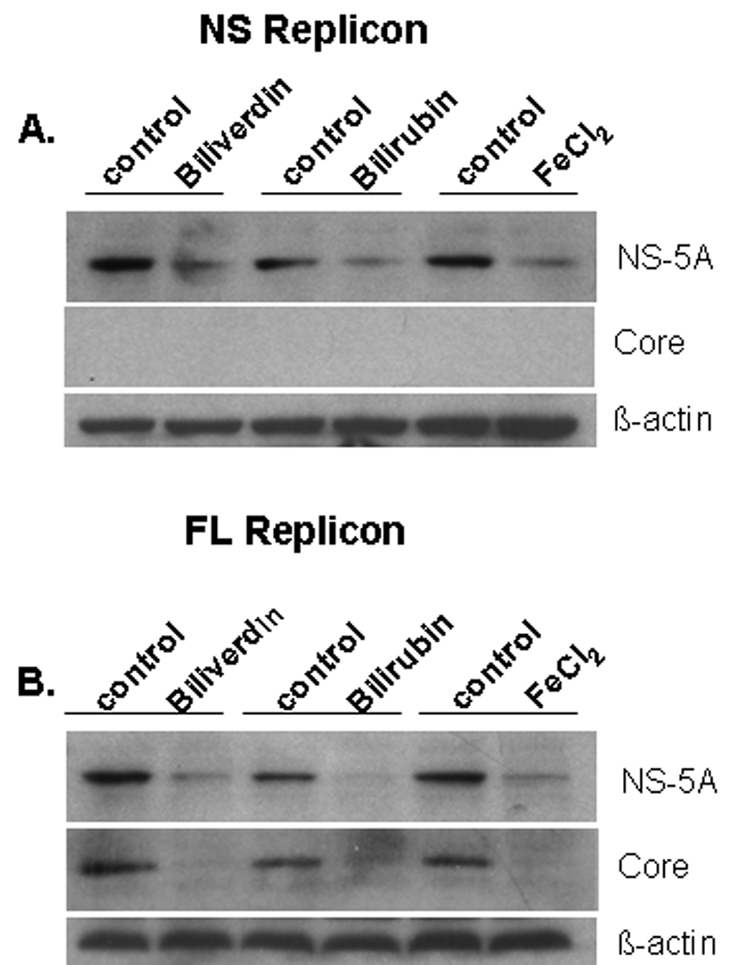
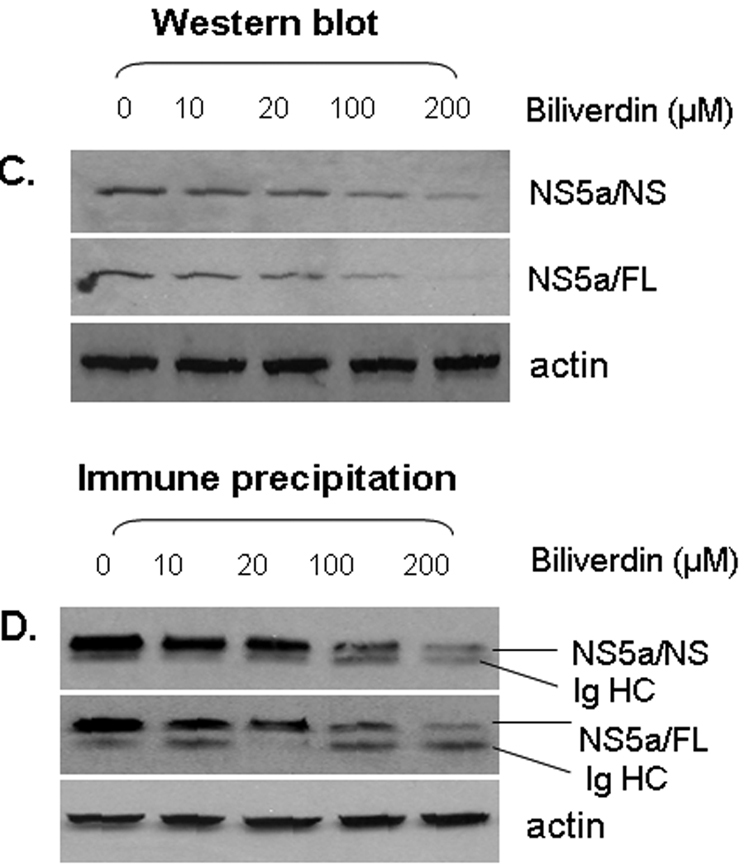
(A, B) Log-phase NS or FL replicons were treated with biliverdin (20 µM), BR (200 µM) or FeCl2 (100 µM) for 24 h. Cells were then lysed and protein expression evaluated by WB. (C, D) Full-length replicons were treated overnight with various concentrations of BV and assayed by WB (C) or immunoprecipitation for NS5A (D). In D, upper band was identified as NS5A and lower band was immunoglobulin heavy chain (Ig HC).
Cellular proliferation and toxicity profoundly affect replicon expression of HCV RNA and protein (19). Consequently, we evaluated whether BV influenced cell growth or was toxic under the present assay conditions. Presentation and description of these experiments are in the Supplemental supporting data available online. We observed no effect of BV or BR on cellular proliferation or toxicity when cells were incubated with tetrapyrrole in medium containing 5 or 10% FBS, the conditions used for incubation of cells throughout the manuscript.
We next tested the effects of BV (20–200 µM) on HCV infection of Huh7.5 cells with J6/JFH infectious HCV construct (16). BV markedly decreased Huh7.5 cell infection with J6/JFH, based on immunoreactivity of HCV polyvalent sera (Fig. 4A–C) and measurement of HCV RNA (Fig. 4D).
Figure 4. Biliverdin inhibition of J6/JFH HCV replication in Huh7.5 cells.
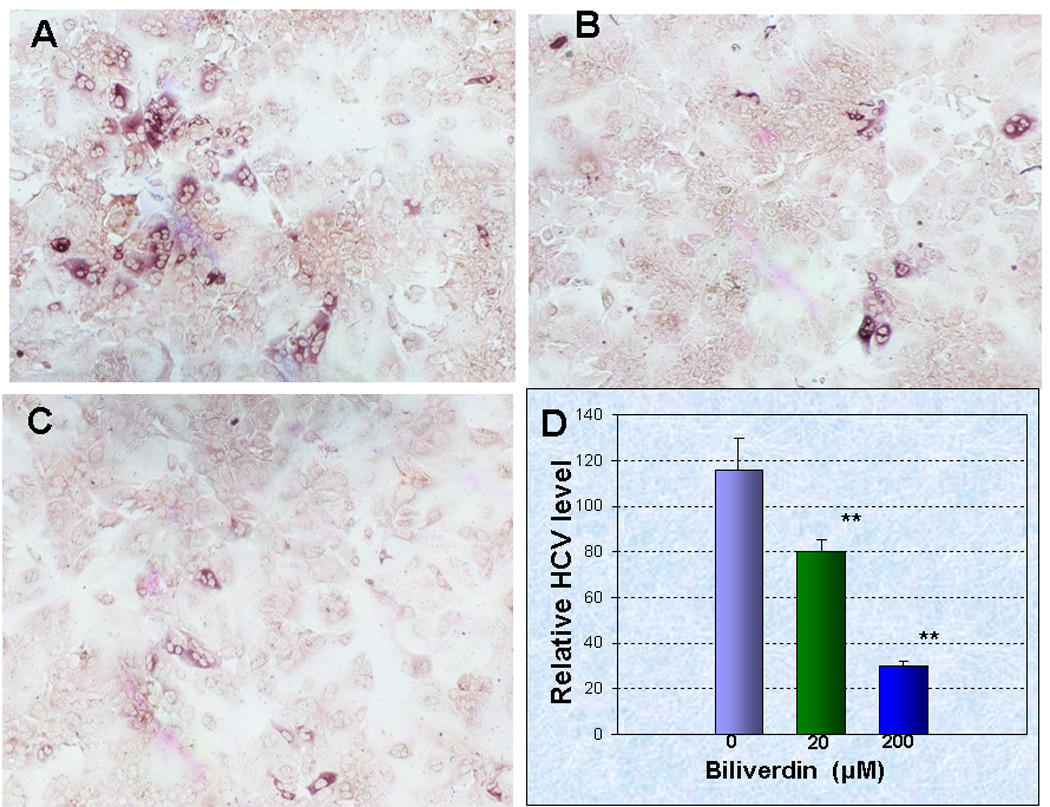
(A–C) J6/JFH infected Huh7.5 cells were treated with different concentrations of BV (0, 20, 200 µM, A–C respectively) for 72 h. Cultures were then fixed and stained immunocytochemically with HCV genotype 2A polyvalent human serum. (D) HCV RNA was quantified using ΔCT assay with 3–5 determinations per treatment group. Each bar represents the mean +/− SEM for each treatment group. **HCV RNA in BV-treated cells versus control (p < 0.01).
Biliverdin inhibits NS3/4A protease
De-conjugated bile pigments are known to inhibit serine-activated pancreatic proteases such as chymotrypsin and trypsin (20). This led us to evaluate the effects of BV and other tetrapyrroles on the HCV NS3/4A protease (Fig. 5A–C). These assays were conducted with wide wavelength excitation/emission (591 nm / 622 nm, respectively) transfer peptides. Preliminary experiments established that shorter fluorescence wavelength transfer peptides (340 nm / 490 nm or 490 nm / 520 nm, excitation / emission, respectively) could not be employed because BV, BR, and other tetrapyrroles showed unacceptable autofluorescence and/or quenching at the shorter wavelengths.
Figure 5. Inhibition of NS3/4A protease.
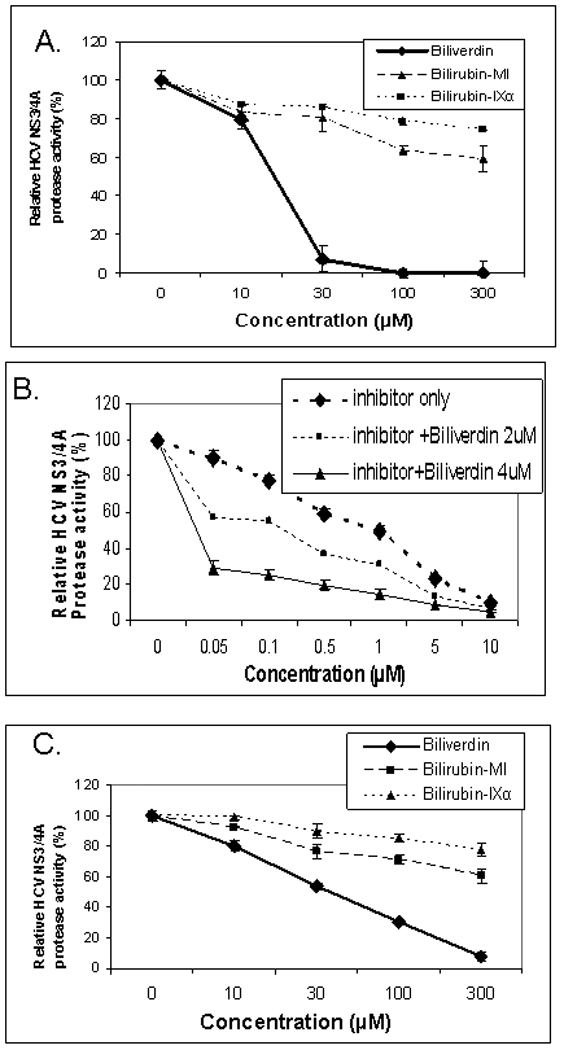
(A–B) Protease activity was determined fluorometrically using recombinant NS3/4A enzyme as described in Methods. (C) Endogenous NS3/4A protease activity in microsomes of replicons was measured using the same assay with partially purified NS3/4A protease from replicon cells as described in Supplemental methods available online. Each point is the mean+/− SEM of 3–5 determinations per point
Inhibitor refers to the commercial NS3/4A protease competitive inhibitor, AnaSpec #25346. Biliverdin = >99% Biliverdin IX-α. Bilirubin-mixed isomers (MI) = 93% Bilirubin IX-α, and 6% associated Bilirubin isomers as described in Materials. Bilirubin IX-α = >99% bilirubin IX-α.
In an assay utilizing a recombinant protease, BV was a markedly more potent inhibitor than BR (either highly purified BR-IXα or BR mixed isomers) (Fig. 5A). BV also displayed the highest IC50 (9.3 µM) of any tetrapyrrole tested (Table 2), which was similar to that of the commercial NS3/4A inhibitor, AnaSpec #25346. Notably, the IC50 value for the commercial inhibitor in our hands (4.9 µM) was indistinguishable from the value reported by the manufacturer (5 µM), supporting the accuracy of our assay. Assays conducted in the presence of both BV and #25346 showed an additive effect (Fig. 5B), indicating a mixed inhibitory mechanism of BV on the NS3/4A protease as described below (Fig. 6). A modification of the fluorescence protease assay was also performed in which freshly prepared protease from replicons was used in place of recombinant protease, as described by Yu et al (21) (Fig. 5D). The results of these experiments were similar to those with the recombinant enzyme, although inhibition of the endogenous protease required slightly higher concentrations of BV than the recombinant enzyme, possibly due to conversion of BV to BR by endogenous BVR in the microsomes.
Table 2.
Activity of biliverdin and derivatives for Inhibition of HCV NS3/4A protease as compared to known competitive inhibitor AnaSpec #25346
| Test Compound | IC50 (uM) |
|---|---|
| AnaSpec #25346 | 4.9 |
| Biliverdin | 9.3 |
| Bilirubin (mixed isomers) | >300 |
| Bilirubin IXα | >300 |
| Mesobilirubin | >300 |
| Biliverdin dimethylester | >300 |
Protease activity was determined fluorometrically with the SensoLyte 620 HCV Protease Assay using a wide wavelength excitation/emission (591nm/622nm) fluorescence energy transfer peptide. Concentrations of inhibitor required for 50% inhibition (IC50) were determined by regression analysis.
Figure 6. Kinetics of BV inhibition of NS3/4A protease.
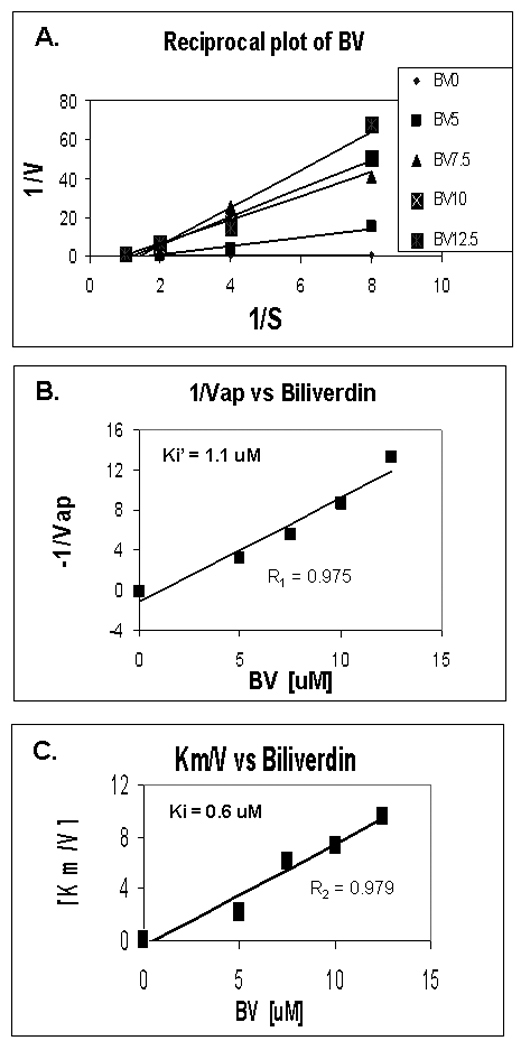
(A) Reciprocal (Lineweaver-Burk) plot of substrate concentration versus enzyme activity. Recombinant protease activity was determined fluorometrically as described in Fig. 5 and Methods. Each point is the mean +/− SEM of 3–5 determinations per point. (B,C) Secondary plots of 1/Vap (y-intercepts) or Km/V (slopes) versus BV concentrations to estimate Ki’ and Ki of BV inhibition non-competitive and competition inhibition respectively. Plot of [BV] vs either 1/Vap or Km/V showed highly significant linearity, (r = 0.975 and r = 0.979 respectively, P< 0.005) suggesting mixed inhibition of NS3/4A protease by BV (Ki’ = 1.1 and Ki = 0.6 µM, respectively).
The kinetics of BV inhibition of NS3/4A protease was assessed on Lineweaver-Burk Plots (Fig. 6A). These data indicated that BV competitively inhibits NS3/4A protease, based on the characteristic increase in slope with higher concentrations of inhibitor. Slopes (Km/V) and y intercepts (1/Vmax) of the primary reciprocal plots were then used to make secondary plots (Fig. 6B–C) to estimate Ki and Ki’, respectively, as general indices of competitive and noncompetitive inhibition. Note that plots of BV vs either 1/Vap or Km/V showed highly significant linearity, (r1 = 0.975 and r2 = 0.979 respectively, P< 0.005) suggesting that BV has both noncompetitive and competitive inhibitor activity for NS3/4A protease (Ki’ = 1.1 and Ki = 0.6 uM, respectively).
BVR knockdown
BV is rapidly reduced to BR by the soluble enzyme biliverdin reductase (BVR) (Fig. 1). We hypothesized that knockdown of BVR expression would result in increased antiviral activity for BV by diminishing its conversion to the less potent BR. Preliminary WB showed that knock down of BVR was highly efficient and led to > 80% reduction of BVR expression in both replicon lines (Fig. 7A). The antiviral activity of BV was significantly enhanced by BVR knockdown compared to control (scramble) RNA knockdown (Fig. 7B, left panel, p < 0.01). In contrast, knockdown of BVR prior to incubation of replicons with BR had no significant effect on the relatively modest antiviral activity of BR (Fig. 7B, right panel). Taken together, these data support the concept that BVR knockdown augments the antiviral activity of BV by arresting its conversion to BR and thereby maintaining higher intracellular levels of BV.
Fig. 7. Effect of biliverdin reductase (BVR) knockdown on antiviral activity.
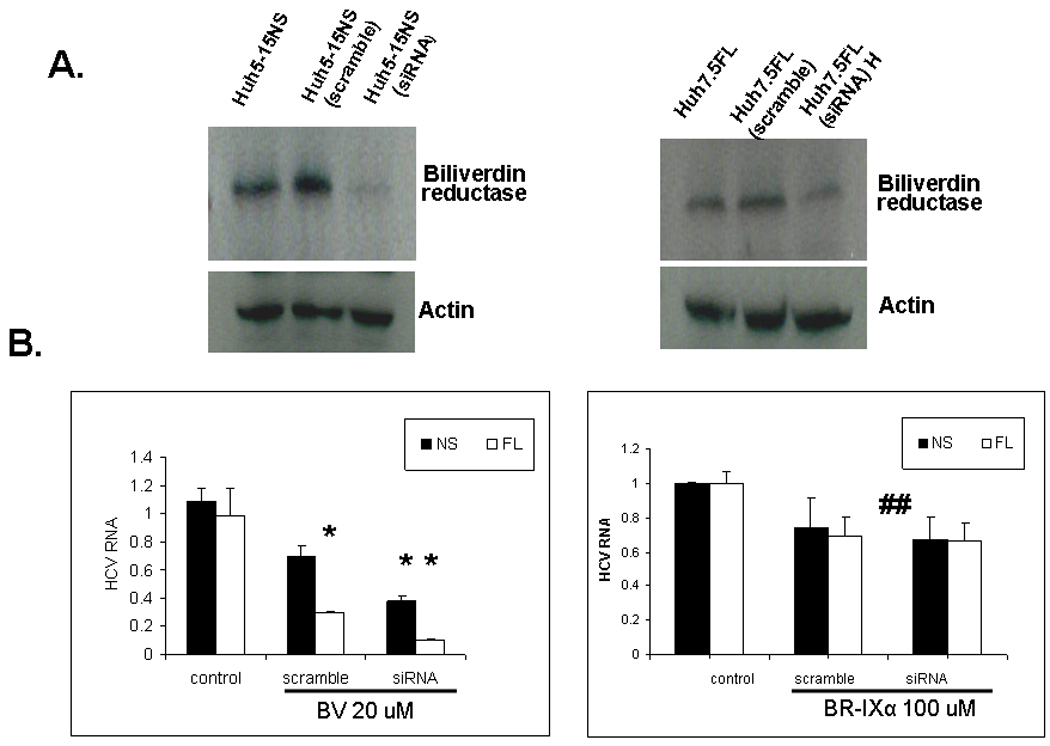
(A) The efficiency of BVR knockdown was determined by WB after transfection of BVR siRNA or scrambled controlled RNA into NS (left panel) or FL (right panel) replicons. Real-time RT-PCR measurements for HCV RNA were performed after control vehicle or BV (20 µM) overnight incubation in both replicon lines (B left panel). Note that the antiviral activity of BV was significantly enhanced (P < 0.01) when BVR was knocked down. In B (right panel) HCV RNA was quantified after control vehicle or BR (100 µM) overnight incubation in both replicon lines. BVR knockdown had no effect on the antiviral activity of BR. Each graph is the mean +/− SEM of 5–6 determinations for each treatment. (## not significant), (* p < 0.01, ** p < 0.005).
Because interferon remains a cornerstone of HCV therapy, we examined the effects of BV on the antiviral activity of α-interferon. As shown in Fig. 8, BV had a clear additive effect when exposed to cells in the presence of interferon. These findings indicate that BV does not appear to compromise the action of interferon, but rather to enhance it. They also raise the possibility that the BV or stable derivatives could be used as antiprotease agents in combination with interferon.
Figure 8. Additive effect of BV on α-interferon antiviral activity.
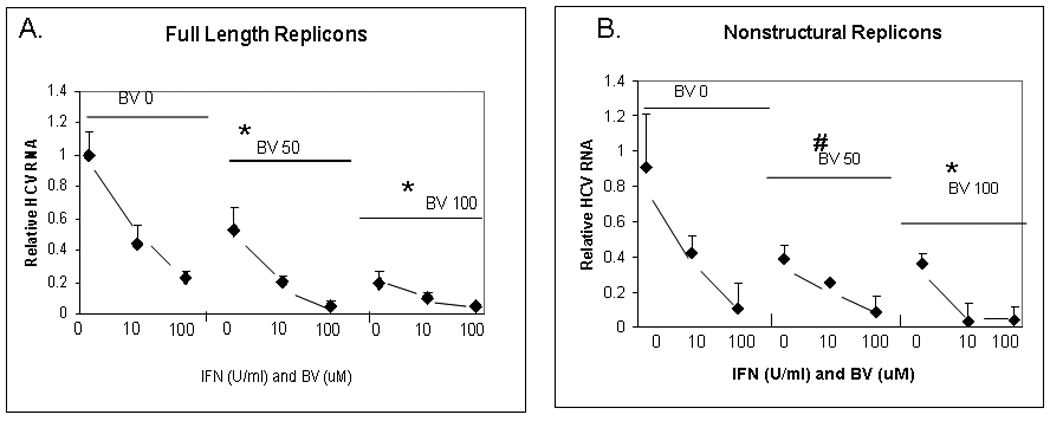
FL or NS replicons were treated with indicated amounts of α-interferon alone or in the presence of 50 or 100 µM BV overnight. HCV replication was determined by real-time RT-PCR using the ΔCT method. Each value is the mean +/− SEM of 3 determinations per point.
(*) p < 0.01 vs control
(#) p < 0.005 vs control
Discussion
Heme oxygenase catalyzes the breakdown of heme to equimolar quantities of BV, iron and carbon monoxide. Expression of the inducible isoenzyme HO-1, also known as heat shock protein 32, is readily upregulated in response to stressors such as hypoxia, heat shock, heavy metals, and oxidants (22). Along with other investigators, we have shown that HO-1 expression is down-regulated in HCV-infected human liver and highly modulated in some in vitro models of HCV (6, 9, 17, 23–25). Furthermore, in cell culture models of HCV, HO-1 modulates both oxidative stress and HCV replication (9, 11).
In order to identify the mechanisms by which exogenous heme or HO-1 overexpression inhibits HCV replication in culture, (9, 11, 26), we studied the antiviral activities of heme oxidation products. Two reports have addressed the ability of iron to inhibit HCV replication (12, 18), however, little attention has been directed at the other heme degradation products, BV and carbon monoxide. Our data demonstrate that BV has potent antiviral activity against HCV in two separate replicon lines and also inhibits replication in J6/JFH construct-infected Huh7.5 cells. Most importantly, our findings provide evidence that BV is a potent inhibitor of the HCV NS3/4A protease. In addition to our preliminary data (27, 28), Lehman et al (13) recently reported that BV has antiviral activity in replicon cells and noted that antiviral activity was accompanied by a rise in specific interferon stimulated gene (ISG) products. These observations are consistent with our data showing that BV inhibits NS3/4A protease. We propose that the rise in ISGs is a direct result of NS3/4A inactivation by BV, which prevents cleavage of the adapter molecules TRIF and IPS-1, thereby restoring TLR3 and RIG-I signaling for innate interferon production (29, 30). Work is currently underway to explore this possibility further.
Iron has also been shown to inhibit HCV replication through prevention of divalent cation binding to RdRp (12, 18). Our results showing that FeCl2 inhibits replication (Fig. 3) support these data. Thus, the identification of BV as a strong antiviral agent with activity against the NS3/4A protease demonstrates that heme oxidation by HO-1 liberates at least two antiviral agents, iron and BV. These potent antiviral effects may explain the downregulation of HO-1 by HCV in infected human liver, in contrast to other liver diseases where HO-1 is frequently upregulated (6). Importantly, the antiviral activity of heme is apparent at physiological serum concentrations, raising the possibility that heme and/or BV could be used as specifically targeted antiviral compounds (STAT-C). Heme (as hemin) is already commercially available for treatment of the porphyrias. Although antiviral activities of BV have not been formally addressed in vivo, the compound appears safe and has been shown to prevent hepatic reperfusion injury and vascular injury-induced intimal hyperplasia in rodent models (31).
Since the discovery of HCV, the NS3/4A protease has been an attractive target for antiviral therapies. Structurally, the enzyme is a typical β-barrel serine-activated protease with a canonical Asp-His-Ser catalytic triad (32, 33). Both boceprevir and telaprevir, two promising antiviral agents currently in phase III trials, utilize an α-ketoamide functional group as a “serine trap” to bind and slowly dissociate from the catalytic serine in the enzyme’s active site. However, BV does not contain an α-ketoamide moiety and work is currently underway to determine the chemical structure(s) important for its interaction with the protease. Inhibition appears complex since kinetic studies showed a mixed competitive and non-competitive mechanism. Consequently, in addition to competitive binding to the substrate active site, BV may exert allosteric effects on enzyme activity, possibly through the known antioxidant or solvent effects of tetrapyrroles (34).
The HO reaction releases nearly exclusively BV-IX-α (35), which is then reduced to BR-IX-α (36), the predominant BR isomer produced by adult mammalian liver. The fact that highly purified BR-IX-α and mixed isomers of BR, are much weaker inhibitors of NS3/4A protease than BV suggests that BR is unlikely to exert antiviral activity in vivo at normal BR blood levels. Interestingly, BV differs from BR by a lone carbon-carbon double bond at position 10 (Fig. 1, arrow). It is intriguing that this single difference causes such a profound difference in the IC50 values of the two compounds (9 µM vs > 300 µM, respectively) (Table 2). We speculate that the fixed planar double bond at position 10 may be crucial for active site binding and we are pursuing this further with structure-function studies of BV.
The inhibition of NS3/4A protease by BV, and to a lesser extent BR and other tetrapyrroles is not without precedence. In the bowel, unconjugated BR, but not BV, inhibits chymotrypsin and trypsin in a dose-related fashion at similar concentrations to those reported here for antiviral activity (20). In contrast, BV and BR inhibit HIV protease with nearly equivalent potency (37), while BV has been shown to decrease viral activity of herpesvirus 6 in vitro (38).
In summary we have evaluated the antiviral activity of BV, the primary tetrapyrrole product of heme oxidation. Our findings demonstrate that BV is a potent antiviral agent, likely as a consequence of its ability to inhibit the NS3/4A protease. These findings suggest that heme, BV, or related derivatives may be useful for future drug therapies targeting the NS3/4A protease.
Supplementary Material
Acknowledgments
Research Support
Supported in part by Merit Review grants from the Veterans Administration (KEB), the NIH R21 DK068453-01A1 and VA Merit Review (WNS), RO1 DK058597-4 (BAL)and the University of Iowa Carver Trust Foundation (WNS and APM) and the American Cancer Society Seed Award (ZZ).
List of abbreviations
- HCV
hepatitis C virus
- HO
heme oxygenase
- RT-PCR
Reverse-Transcriptase Polymerase Chain Reaction
- BV
biliverdin
- BR
bilirubin
- BVR
biliverdin reductase
- FL
full-length
- NS
nonstructural
- GAPDH
glyceraldehyde-3-phosphate dehydrogenase
- DMEM
Dulbecco’s modified minimal essential medium
- WB
Western blot
- CV
coefficient of variation
- ANOVA
analysis of variance
- FBS
Fetal Bovine Serum
- MTT
[3-(4,5-dimethylthiazol-2-yl)-2,5-dephenyl-2H-tetrazoliumbromide]
Footnotes
Presented in part at the American Association for the Study of Liver Diseases, 50th Annual Meeting, San Francisco, CA, 2008 and 2009, abstracts # 1905 and 791 respectively.
Conflict of Interest.
None of the authors who participated in this study have commercial or other associations that might pose a conflict of interest.
Reference List
- 1.Firpi RJ, Nelson DR. Current and future hepatitis C therapies. Archives of Medical Research. 2007 Aug;38(6):678–690. doi: 10.1016/j.arcmed.2006.09.002. [DOI] [PubMed] [Google Scholar]
- 2.Manns MP, Wedemeyer H, Cornberg M. Treating viral hepatitis C: Efficacy, side effects, and complications. Gut. 2006 Sep;55(9):1350–1359. doi: 10.1136/gut.2005.076646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lindenbach BD, Rice CM. Unravelling hepatitis C virus replication from genome to function. Nature. 2005 Aug 18;436(7053):933–938. doi: 10.1038/nature04077. [DOI] [PubMed] [Google Scholar]
- 4.Soriano V, Peters MG, Zeuzem S. New Therapies for Hepatitis C Virus Infection. Clinical Infectious Diseases. 2009 Feb 1;48(3):313–320. doi: 10.1086/595848. [DOI] [PubMed] [Google Scholar]
- 5.Choi J, Ou JHJ. Mechanisms of liver injury. III. Oxidative stress in the pathogenesis of hepatitis C virus. American Journal of Physiology-Gastrointestinal and Liver Physiology. 2006 May;290(5):G847–G851. doi: 10.1152/ajpgi.00522.2005. [DOI] [PubMed] [Google Scholar]
- 6.Abdalla MY, Britigan BE, Wen F, Icardi M, McCormick ML, LaBrecque DR, et al. Down-regulation of heme oxygenase-1 by hepatitis C virus infection in vivo and by the in vitro expression of hepatitis C core protein. Journal of Infectious Diseases. 2004 Sep 15;190(6):1109–1118. doi: 10.1086/423488. [DOI] [PubMed] [Google Scholar]
- 7.Okuda M, Li K, Beard MR, Showalter LA, Scholle F, Lemon SM, et al. Mitochondrial injury, oxidative stress, and antioxidant gene expression are induced by hepatitis C virus core protein. Gastroenterology. 2002 Feb;122(2):366–375. doi: 10.1053/gast.2002.30983. [DOI] [PubMed] [Google Scholar]
- 8.Qadri I, Iwahashi M, Capasso JM, Hopken MW, Flores S, Schaack J, et al. Induced oxidative stress and activated expression of manganese superoxide dismutase during hepatitis C virus replication: role of JNK, p38 MAR and AP-1. Biochemical Journal. 2004 Mar 15;378:919–928. doi: 10.1042/BJ20031587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhu ZW, Wilson AT, Mathahs MM, Wen F, Brown KE, Luxon BA, et al. Heme Oxygenase-1 Suppresses Hepatitis C Virus Replication and Increases Resistance of Hepatocytes to Oxidant Injury. Hepatology. 2008 Nov;48(5):1430–1439. doi: 10.1002/hep.22491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ryter SW, Morse D, Choi AMK. Carbon monoxide and bilirubin - Potential therapies for pulmonary/vascular injury and disease. American Journal of Respiratory Cell and Molecular Biology. 2007 Feb;36(2):175–182. doi: 10.1165/rcmb.2006-0333TR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shan Y, Zheng JY, Lambrecht RW, Bonkovsky HL. Reciprocal effects of Micro-RNA-122 on expression of heme oxygenase-1 and hepatitis C virus genes in human hepatocytes. Gastroenterology. 2007 Oct;133(4):1166–1174. doi: 10.1053/j.gastro.2007.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fillebeen C, Rivas-Estilla AM, Bisaillon M, Ponka P, Muckenthaler M, Hentze MW, et al. Iron inactivates the RNA polymerase NS5B and suppresses subgenomic replication of hepatitis C virus. Journal of Biological Chemistry. 2005 Mar 11;280(10):9049–9057. doi: 10.1074/jbc.M412687200. [DOI] [PubMed] [Google Scholar]
- 13.Lehmann E, EL-Tantawy WH, Ocker M, Bartenschlager R, Lohmann V, Hashemolhosseini S, et al. The Heme Oxygenase 1 Product Biliverdin Interferes with Hepatitis C Virus Replication by Increasing Antiviral Interferon Response. Hepatology. 2010 Feb;51(2):398–404. doi: 10.1002/hep.23339. [DOI] [PubMed] [Google Scholar]
- 14.Lohmann V, Korner F, Koch JO, Herian U, Theilmann L, Bartenschlager R. Replication of subgenomic hepatitis C virus RNAs in a hepatoma cell line. Science. 1999 Jul 2;285(5424):110–113. doi: 10.1126/science.285.5424.110. [DOI] [PubMed] [Google Scholar]
- 15.Blight KJ, McKeating JA, Rice CM. Highly permissive cell lines for subgenomic and genomic hepatitis C virus RNA replication. Journal of Virology. 2002 Dec;76(24):13001–13014. doi: 10.1128/JVI.76.24.13001-13014.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lindenbach BD, Evans MJ, Syder AJ, Wolk B, Tellinghuisen TL, Liu CC, et al. Complete replication of hepatitis C virus in cell culture. Science. 2005 Jul 22;309(5734):623–626. doi: 10.1126/science.1114016. [DOI] [PubMed] [Google Scholar]
- 17.Abdalla MY, Ahmad IM, Spitz DR, Schmidt WN, Britigan BE. Hepatitis C virus-core and non structural proteins lead to different effects on cellular antioxidant defenses. Journal of Medical Virology. 2005 Aug;76(4):489–497. doi: 10.1002/jmv.20388. [DOI] [PubMed] [Google Scholar]
- 18.Yuasa K, Naganuma A, Sato K, Ikeda M, Kato N, Takagi H, et al. Zinc is a negative regulator of hepatitis C virus RNA replication. Liver International. 2006 Nov;26(9):1111–1118. doi: 10.1111/j.1478-3231.2006.01352.x. [DOI] [PubMed] [Google Scholar]
- 19.Pietschmann T, Lohmann V, Rutter G, Kurpanek K, Bartenschlager R. Characterization of cell lines carrying self-replicating hepatitis C virus RNAs. Journal of Virology. 2001 Feb;75(3):1252–1264. doi: 10.1128/JVI.75.3.1252-1264.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Qin XF. Inactivation of digestive proteases by deconjugated bilirubin: the possible evolutionary driving force for bilirubin or biliverdin predominance in animals. Gut. 2007 Nov;56(11):1641–1642. doi: 10.1136/gut.2007.132076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yu XM, Sainz B, Uprichard SL. Development of a Cell-Based Hepatitis C Virus Infection Fluorescent Resonance Energy Transfer Assay for High-Throughput Antiviral Compound Screening. Antimicrobial Agents and Chemotherapy. 2009 Oct;53(10):4311–4319. doi: 10.1128/AAC.00495-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ryter SW, Alam J, Choi AMK. Heme oxygenase-1/carbon monoxide: From basic science to therapeutic applications. Physiological Reviews. 2006 Apr;86(2):583–650. doi: 10.1152/physrev.00011.2005. [DOI] [PubMed] [Google Scholar]
- 23.Ghaziani T, Shan Y, Lambrecht RW, Donohue SE, Pietschmann T, Bartenschlager R, et al. HCV proteins increase expression of heme oxygenase-1 (HO-1) and decrease expression of Bach1 in human hepatoma cells. Journal of Hepatology. 2006 Jul;45(1):5–12. doi: 10.1016/j.jhep.2005.12.020. [DOI] [PubMed] [Google Scholar]
- 24.Shan Y, Zheng JY, Moschenross D, Lambrecht RW, Bonkovsky HL. An antagomir of mir-122 down-regulates hepatitis c virus infection and up-regulates herne oxygenase-1 expression in human hepatocytes. Gastroenterology. 2007 Apr;132(4):A824. [Google Scholar]
- 25.Wen F, Brown KE, Britigan BE, Schmidt WN. Hepatitis C core protein inhibits induction of heme oxygenase-1 and sensitizes hepatocytes to cytotoxicity. Cell Biology and Toxicology. 2008 Apr;24(2):175–188. doi: 10.1007/s10565-007-9027-9. [DOI] [PubMed] [Google Scholar]
- 26.Fillebeen C, Muckenthaler M, Andricipoulos B, Bisaillon M, Mounir Z, Hentze MW, et al. Expression of the subgenomic hepatitis C virus replicon alters iron homeostasis in Huh7 cells. Journal of Hepatology. 2007 Jul;47(1):12–22. doi: 10.1016/j.jhep.2007.01.035. [DOI] [PubMed] [Google Scholar]
- 27.Zhu ZW, Wilson AT, Brown K, Luxon BA, Schmidt WN. Heme Oxygenase-1 Reaction Products Suppress Hepatitis C Virus Replication in Full Length and Non-Structural Replicon Cell Lines. Hepatology. 2008 Oct;48(4):1162A–1163A. [Google Scholar]
- 28.Zhu ZW, Zhao Y, Brown KE, Luxon BA, Schmidt WN. Antiviral Effects of Heme Oxygenase-1: Inhibition of Hcv Ns3/4A Protease by Biliverdin and Its Metabolites. Hepatology. 2009 Oct;50(4):675A. [Google Scholar]
- 29.Foy E, Li K, Wang CF, Sumpter R, Ikeda M, Lemon SM, et al. Regulation of interferon regulatory factor-3 by the hepatitis C virus serine protease. Science. 2003 May 16;300(5622):1145–1148. doi: 10.1126/science.1082604. [DOI] [PubMed] [Google Scholar]
- 30.Foy E, Li K, Sumpter R, Loo YM, Johnson CL, Wang CF, et al. Control of antiviral defenses through hepatitis C virus disruption of retinoic acid-inducible gene-I signaling. Proceedings of the National Academy of Sciences of the United States of America. 2005 Feb 22;102(8):2986–2991. doi: 10.1073/pnas.0408707102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nakao A, Murase N, Ho C, Toyokawa H, Billiar TR, Kanno S. Biliverdin administration prevents the formation of intimal hyperplasia induced by vascular injury. Circulation. 2005 Jul 26;112(4):587–591. doi: 10.1161/CIRCULATIONAHA.104.509778. [DOI] [PubMed] [Google Scholar]
- 32.Love RA, Parge HE, Wickersham JA, Hostomsky Z, Habuka N, Moomaw EW, et al. The crystal structure of hepatitis C virus NS3 proteinase reveals a trypsin-like fold and a structural zinc binding site. Cell. 1996 Oct 18;87(2):331–342. doi: 10.1016/s0092-8674(00)81350-1. [DOI] [PubMed] [Google Scholar]
- 33.Yan YW, Li Y, Munshi S, Sardana V, Cole JL, Sardana M, et al. Complex of NS3 protease and NS4A peptide of BK strain hepatitis C virus: A 2.2 angstrom resolution structure in a hexagonal crystal form. Protein Science. 1998 Apr;7(4):837–847. doi: 10.1002/pro.5560070402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McDonagh AF. Turning green to gold. Nature Structural Biology. 2001 Mar;8(3):198–200. doi: 10.1038/84915. [DOI] [PubMed] [Google Scholar]
- 35.Noguchi M, Yoshida T, Kikuchi G. Specific Requirement of Nadph-Cytochrome-C Reductase for the Microsomal Heme Oxygenase Reaction Yielding Biliverdin-Ix-Alpha. Febs Letters. 1979;98(2):281–284. doi: 10.1016/0014-5793(79)80200-8. [DOI] [PubMed] [Google Scholar]
- 36.Tenhunen R, Ross ME, Marver HS, Schmid R. Reduced Nicotinamide-Adenine Dinucleotide Phosphate Dependent Biliverdin Reductase. Partial Purification and Characterization. Biochemistry. 1970;9(2):298. doi: 10.1021/bi00804a016. [DOI] [PubMed] [Google Scholar]
- 37.McPhee F, Caldera PS, Bemis GW, McDonagh AF, Kuntz ID, Craik CS. Bile pigments as HIV-1 protease inhibitors and their effects on HIV-1 viral maturation and infectivity in vitro. Biochemical Journal. 1996 Dec 1;320:681–686. doi: 10.1042/bj3200681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nakagami T, Taji S, Takahashi M, Yamanishi K. Antiviral Activity of A Bile Pigment, Biliverdin, Against Human Herpesvirus-6 (Hhv-6) Invitro. Microbiology and Immunology. 1992;36(4):381–390. doi: 10.1111/j.1348-0421.1992.tb02037.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


