Abstract
Research increasingly suggests that changes in estrogen levels during aging may increase risk for Alzheimer disease, the most common type of dementia. This update reviews the newest information about estrogen and cognitive aging, including information regarding the role of bioavailable estrogen in older women and men, use of selective estrogen receptor modulators (SERMs) to improve cognition, and studies of genetic risk factors to elucidate the effects of endogenous estrogen on aging and cognition. Future trials are needed to determine whether alternate timing, dosage, formulation, or method of administration of hormone replacement can reduce risk of dementia.
Keywords: Alzheimer disease, dementia, estrogen, hormone replacement therapy, genetics
Introduction
Alzheimer disease (AD) is the most common cause of age-related dementia, accounting for as much as 50–60% of dementia cases. Clinically, AD is characterized by a progressive deterioration of cognitive and functional skills that begins during middle age or late in life, with variability in behavioral manifestations. Diagnosis of AD requires both the presence of dementia and a characteristic pattern of brain changes, including atrophy, neuronal loss, the presence of extracellular plaques containing beta amyloid peptides, intracellular neurofibrillary tangles, and granulovacuolar cytoplasmic changes in the neocortical association areas, hippocampus, and other brain regions. Estrogen is important in the normal maintenance of brain function in the nucleus basalis of Meynert and other regions typically affected by AD. Multiple lines of evidence suggest that loss of estrogens in the aging brain of both women and men may play a role in the cognitive declines associated with AD.
The Biological Roles of Estrogen in Neuroprotection
Estrogens are a group of steroid compounds that function as primary female sex hormones. They are produced primarily by developing follicles in the ovaries as well as by the corpus luteum and the placenta. Some estrogens are also produced in smaller amounts by other tissues such as the liver, adrenal glands, and the placenta. These secondary sources of estrogen are important sources of endogenous estrogen in postmenopausal women. Estrogen interacts with different types of receptors to exert its action, including ER-alpha, and ER-beta. Both receptors are highly expressed in the brain, although ER-alpha receptors are present in higher concentrations in the hippocampus and ER-beta receptors are present in higher concentrations in the basal forebrain and cerebral cortex.
In men, testosterone is converted to estradiol by aromatase enzyme which is located peripherally and throughout the male brain (See Figure 1). While studies suggest that the conversion rate of testosterone to estrogen is low, at about 0.2%, it is the primary source of plasma estradiol in men. Because testicular secretion of testosterone never completely stops, serum estrogen levels are higher in elderly males than in postmenopausal women.
Figure 1.
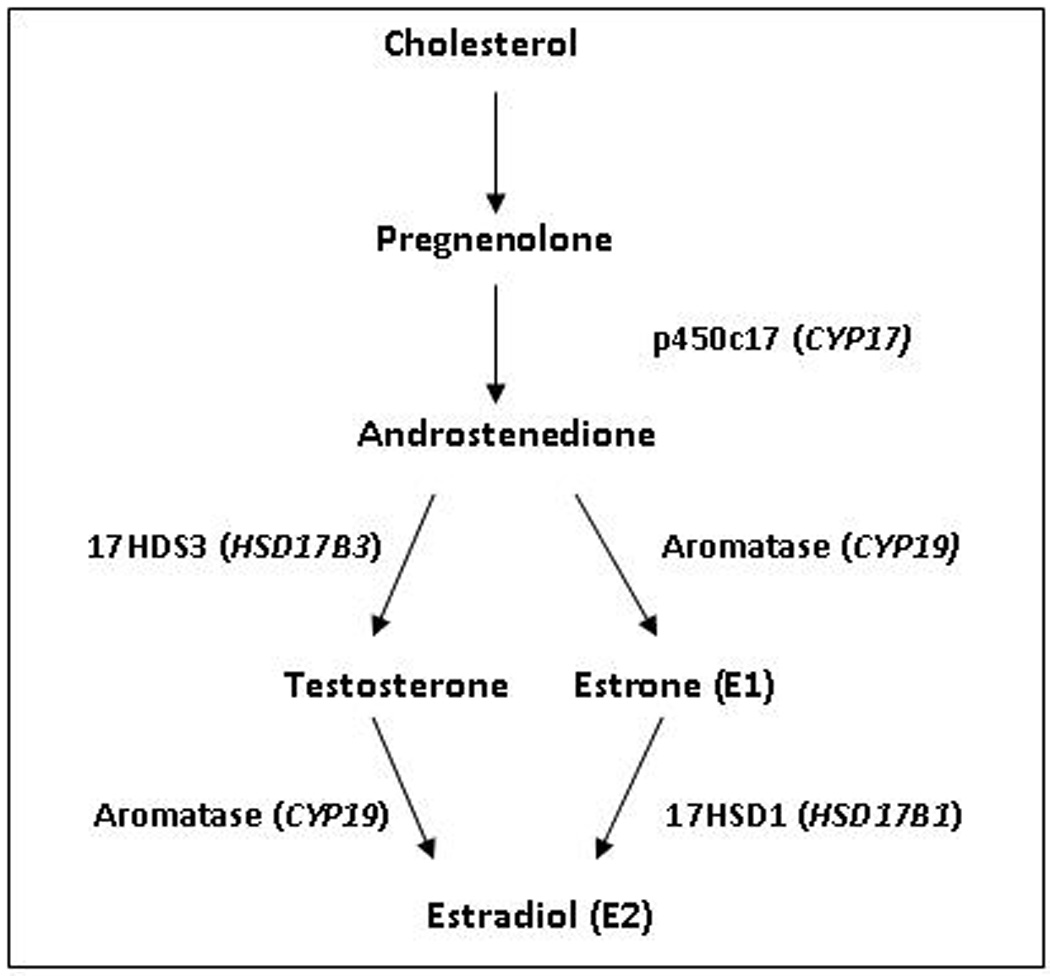
Biosynthesis of estradiol, including associated enzymes and genes (in parentheses)
Several hundred published papers have established that estrogen has beneficial effects on brain tissue and physiology in cell culture and animal models, including nonhuman primates; however, the mechanisms underlying estrogen neuroprotection have not been completely elucidated. Estrogen promotes the growth and survival of cholinergic neurons (1, 2), increases cholinergic activity (3), has antioxidant properties (4), and promotes the nonamyloidogenic metabolism of the amyloid precursor protein (5). None of these effects are exclusive and it is likely that many of the effects are interrelated.
While significant neuroprotective actions of estrogen have been demonstrated in vitro and in animal studies, the evidence is less consistent in studies of aging women and men. Support for the role of estrogen in neuroprotection has come from epidemiologic studies of gender differences in risk for cognitive decline and dementia; from observational studies; from clinical trials of the effects of hormone replacement therapy; from studies of risk for cognitive decline and dementia associated with low levels of endogenous hormones, and from studies of the relation of variants in genes involved in estrogen biosynthesis and receptor activity on risk of AD.
Gender Differences in Cognitive Decline and Dementia
If estrogen plays a significant neuroprotective role in the pre-menopausal period, it would be anticipated that more elderly women than men would be affected by AD. However, evidence for sex differences in cognitive decline and dementia is mixed. Four population-based studies of sex differences in dementia published before 2000 reported a higher prevalence of AD in women; however, four others reported no difference. Inconsistencies might be due to earlier mortality and greater morbidity in men compared with women. Thus studies which look at the age-specific incidence of AD would best capture gender-specific differences. In the EURODEM Incidence Research Study, there was a higher incidence of AD in women compared with men (adjusted relative risk 1.54, 95% CI 1.21.-1.96) in all age groups combined (6). Likewise, a Swedish cohort demonstrated a higher incidence of AD in women than in men among all age groups, with the greatest difference seen in those over 90 years old (7). In contrast, the MoVIES study (8), the Rochester study (9), the Framingham study (10), the Baltimore Longitudinal Study of Aging (11), the East Boston Study (12) and the ACT cohort study (13) provided no support for a greater incidence of dementia or AD among women than men.
Observational Studies of Hormone Replacement Therapy
The hypothesis that hormone therapy might protect against cognitive aging arose from observational studies which demonstrated a lower risk of AD among women who had been treated with hormone therapy compared with those who had not. In observational studies, postmenopausal women who used estrogen-only or estrogen-progestogen hormonal replacement therapy (HRT) showed slower declines in cognitive function and decreased risk of AD (14–19), but not all studies found positive effects. Some inconsistencies may be related to the timing of HRT use. The Cache County Study found evidence that prior use of HRT (typically initiated early in menopause) protected against AD, while current use of HRT did not protect against AD unless initiated more than 10 years earlier (before age 63 on average) (19). The authors concluded that there was a limited window of time during which sustained HRT exposure appeared to reduce the risk of AD (19). Several articles since have raised the possibility that there is a critical period in which HRT exerts cognitive benefits, possibly early in the perimenopausal period or just after menopause (20*, 21). In addition, prior HRT use could have been associated with higher educational levels and better access to medical care which could themselves be the protective factors associated with lower risk. Finally, in earlier studies, the majority of women studied used estrogen-only therapy in the perimenopausal period and typically stopped use after age 60, factors which were not re-created in later evaluations.
Clinical Trials of Hormone Replacement Therapy
While clinical trials of HRT in women can correct for selection bias and confounding inherent in observational studies, they have demonstrated limited efficacy of HRT on maintenance of cognitive function, primarily assessed as verbal memory, or on risk for AD. Overall, they show a neutral effect of estrogen alone and a negative effect of combined estrogen-progestogen treatment on verbal memory. It is helpful to group study results by population and HRT formulation in order to evaluate result trends.
Several randomized, placebo-controlled trials of unopposed estrogen therapy on episodic verbal memory in women younger than 65 years old demonstrated improvement in test scores of verbal recall in women less than 65 years of age receiving estrogen only supplementation (22–24). Results were limited by sample sizes of fifty women or less and most or all of the women in these studies were surgically menopausal. Despite the small study samples and design limitations, the results of these studies support the hypothesis that estrogen alone either enhances or has no effect on verbal memory in younger women in short-term follow-up. Results of unopposed estrogen therapy in older women have been neutral. Randomized, placebo-controlled trials of estrogen and cognition in women with a mean age greater than 65 years old have each employed a different estradiol formulation(25–27).
While unopposed estrogen therapy has demonstrated positive effects on cognitive outcomes in younger women and neutral effects in older women, the results from combination estrogen-progestogen treatment trials are less encouraging in both groups. The largest study of any form of hormone therapy on verbal memory in women younger than age 65 was the Cognitive Complaints in Early Menopause Trial (28), which indicated a non-significant trend for conjugated equine estrogen/medroxyprogesterone acetate (CEE/MPA) to be associated with decline in both short- and long-delay free recall compared with placebo (p<0.07). A second study in younger women found that combined therapy with estrogen plus progestogen (dienogest) enhanced verbal memory compared with both placebo and estradiol (29). The two studies suggest that different forms of progestogens may have different effects on cognitive function in younger women, with negative effects of MPA on verbal memory and positive effects of dienogest, a progestogen with anti-androgenic effects.
A number of trials have evaluated combined estrogen/progestogen treatment in women over 65 years of age. Out of four trials evaluating combined therapy on cognition in postmenopausal women, three showed either a negative effect of HRT on verbal memory (30, 31), or a trend of negative effect on verbal memory (32) (p<0.06). These findings suggest a consistent, detrimental effect of combined hormone therapy on verbal memory. The largest trial to date is the Woman’s Health Initiative Memory Study (WHIMS) (33), which examined the impact of CEE in women with prior hysterectomy, and the impact of CEE/MPA in naturally post-menopausal women, on the incidence of dementia in women age 65 or older. In the combined therapy arm, CEE/MPA doubled the risk for all-cause dementia (HR=2.01; 95th % confidence interval= 1.21–3.48) (34). In the estrogen-alone arm there was no evidence that CEE changed the risk of all-cause dementia (HR= 1.49; 95% confidence interval =0.83–2.66) (35). However, the negative effects of the WHIMS trials may be related to the timing of treatment, the specific formulation of HRT provided and schedule used. Interestingly, analysis of prior HRT use in the perimenopausal period in the WHIMS population was associated with a lower rate of developing dementia regardless of the treatment arm. When AD was analyzed apart from other causes of dementia, prior HRT was associated with a 64% reduction in incidence. In sum, results of clinical trials of HRT vary depending of the type of therapy used and the age of initiation. The true effects of combination HRT on brain function are complicated by the finding that certain forms of progestogen, such as MPA, but not others, may antagonize the effects of estrogen on the hippocampus (36). This provides a motivation for exploring the impact of different estrogen/ progestogen combinations and dosages on verbal memory. Modes of HRT delivery also need to be evaluated. Transdermal HRT application, which bypasses first-pass metabolism, may have a different effect on hormone delivery and therefore on cognitive outcome. Additionally, the critical window hypothesis highlights the need to define the time period of effective intervention in order to optimize the timing of early hormone therapy on cognition. Finally, it should be determined how long HRT must be used to confer benefits. The answer to this question may be provided in part by the results of PREPARE (PREventing Post-menopausal Alzheimer’s Disease with Replacement Estrogens) an HRT trial in women 65 years of age or older with a family history of dementia in a first-degree relative, which was designed to determine if HRT delays AD or memory loss in women at increased risk for cognitive change (37*). While active study treatment was discontinued in response to the WHI Memory Study report, the study continues to follow participants for a total of five years blind to the original medication assignment. Future results will address whether there are lasting or delayed effects of HRT on cognition after treatment cessation.
Two large clinical trials may provide important information on the effect of HRT on cognitive function in younger and older postmenopausal women. The Kronos Early Estrogen Prevention Study (KEEPS) is a five year, multicenter clinical trial that will enroll women who are within 36 months of their final menstrual period. Participants will be randomized to receive oral CEE plus micronized progesterone, transdermal estradiol plus micronized progesterone, or placebo. The trial was designed to address the dual concerns that the older mean age of the WHI population may have affected response to HRT, and that the mode of HRT administration may impact health outcome (38**). Cognitive outcome tracking will be part of a secondary analysis, and women included in the study must have an MMSE>=23. The Early versus Late Intervention Trial with Estradiol (ELITE) at UCLA is a single site clinical trial that will enroll a total of 504 post-menopausal women who are less than six years from menopause (early) versus 10 years or more from menopause (late) to receive either oral estradiol or placebo (39). Both KEEPS and ELITE will examine verbal memory as a primary outcome and focus on naturally menopausal women.
Endogenous Estrogen and Cognition
The ability of current HRT trials to address whether estrogen protects against AD is limited by different patterns of use and different formulations. Studies of endogenous estrogen could potentially address the role of estrogen in the pathogenesis of AD more directly; however, results of these studies have also been inconsistent. In the Study of Osteoporotic Fractures, women with the highest estrone levels had significantly poorer performance in some cognitive test scores over five years (40). In contrast, a large cross-sectional study from the Netherlands (41) found that women in the highest quintile of either estradiol or estrone were 40% less likely to be cognitively impaired than women in the lowest quintile. Among postmenopausal women with Down Syndrome (DS), women with early onset of menopause (<= 46 years) had earlier onset and increased risk of dementia compared with women with onset of menopause after 46 years (42). This suggests that lower lifetime endogenous estrogen exposure increases the risk of dementia in this population.
In evaluating the impact of endogenous estrogen, it is important to assess bioavailable hormone levels, which may differ from total estrogen levels. An estimated 37% of estradiol in older women circulates bound to sex hormone binding globulin (SHBG) and only the non-SHBG bound fraction (e.g. bioavailable estradiol) is thought to cross the blood brain barrier. Several observational studies have found an association between elevated SHBG and cognitive decline or AD (18, 42, 43). In the Study of Osteoporotic Fractures, women with high concentrations of free and bioavailable estradiol were less like to develop cognitive impairment after six years than were women with low concentrations (18). Among postmenopausal women with DS, those with lower levels of bioavailable E2 at baseline were four times as likely to develop AD (HR=4.1, 95% CI= 1.2–13.9)(44). These studies suggest that low bioavailable estrogen levels associated with high SHBG after menopause may accelerate the development of AD. Again, however, the literature is conflicting, as both the Rancho Bernardo Study and the Rotterdam Study either failed to identify consistent association total or bioavailable estradiol on cognitive test outcome (14) or demonstrated that women with higher calculated bioavailable estradiol levels had significantly poorer results in delayed recall (45). These inconsistencies may be related to variable hormone measurement procedures or may be due to the difficulty of measuring circulating estradiol in older women who have been postmenopausal for many years.
Estrogen in Men
Studies of the relationship between endogenous estrogen and testosterone levels and cognition in men are conflicting. A limited number of studies have demonstrated that older men with high serum estradiol levels performed better or declined more slowly on tests of verbal memory (46), working memory (47, 48), visual memory (49), spatial scan (50) and global cognitive functioning (51). Other studies have been unable to demonstrate a positive relationship between estradiol levels and verbal memory in men (49, 50, 52–54), or have found that higher endogenous estrogen levels increased the risk of AD (55).
Due to aromatization, both testosterone and estradiol levels increase significantly in men following testosterone supplementation (56). As a result, some studies of testosterone supplementation in older men can be used to indirectly evaluate the effects of exogenous estrogen on cognition. Higher estradiol concentrations resulting from testosterone supplementation have been associated with better executive function and verbal memory (56, 57); no effect on executive function or verbal memory (58, 59), 2004); and worsening of executive function, verbal fluency, spatial cognition and working memory (48, 56, 60). In another study, when testosterone was given to older men with or without an aromatase inhibitor, improvements in verbal memory were apparent only in the group treated with testosterone alone (61), suggesting that estradiol, aromatized from testosterone, was responsible for the beneficial effects on verbal memory. There have also been a limited number of trials with direct estradiol supplementation in older men. Trials of micronized estradiol add-back therapy in men with prostate cancer who previously underwent androgen deprivation demonstrated both improvement (57) and no change in verbal recall (54). One small prospective, controlled estrogen replacement study in healthy elderly men with mild memory loss demonstrated either a statistically significant improvement or a trend toward significance in some but not all tests of verbal recall in men with mild cognitive impairment following twelve weeks of estradiol treatment compared to baseline scores (62*). These results only occurred in one group of men tested and need to be replicated with a larger population. A few studies examined the association between bioavailable estradiol and a series of cognitive measures including executive function, verbal fluency, and verbal and spatial memory (14, 63), but no relationship was demonstrated.
As in women, inconsistency in these studies may be attributed to small sample size, inability to consistently measure estradiol in a standardized manner, or failure to measure bioavailable estradiol. Finally, estradiol levels in men are low at baseline and decrease as men age, which may limit sensitivity in evaluating the effect of small changes on cognitive outcomes. It has been suggested that studies of endogenous estradiol (49) demonstrate a clearer positive relationship between estrogen and cognition. Thus, there may be a critical window period for the cognitive effects of estrogen in men as well as in women.
SERMs
Selective estrogen-receptor modulators (SERMs) do not have the basic steroid structure of endogenous estrogens but exert tissue-specific estrogen-like effects both peripherally and in the brain, although the SERMs which are currently FDA-approved were not specifically designed to act in the brain. Raloxifene, approved by the FDA for osteoporosis prevention, and tamoxifen, used in breast cancer prevention, are both SERMs. The mechanisms of action of raloxifene and tamoxifen differ within different areas of the brain as well as from one another. In secondary analyses of a large randomized placebo-controlled trial examining postmenopausal women with osteoporosis, high-dose raloxifene demonstrated a lower risk of mild cognitive impairment and a trend towards a lower risk of Alzheimer disease (64). In an ancillary study which examined cognitive outcomes in women assigned to treatment with either tamoxifen or raloxifene for breast cancer, (CoSTAR), women assigned to the two agents had similar cognitive profiles over time (65). Because a placebo arm was not included in the original STAR trial, these results did not address how raloxifene and tamoxifen may affect cognition compared with nontreatment. A secondary analysis which compared global cognition function outcomes between women receiving CEE in WHIMS and women treated with tamoxifen and raloxifene in CoSTAR demonstrated that each active therapy as associated with a small mean relative deficit in scores of <= 0.5 units when compared to placebo. Although of small magnitude, these findings raise the possibility that tamoxifen and raloxifene may adversely affect cognition in older women; however, the effects of long-term treatment are unknown (66**). Future studies evaluating SERMs which are specific to the brain need to be performed.
Genetic Factors
Genes involved in estrogen biosynthesis and estrogen receptor activity are also potential contributors to risk of AD. Variants of these genes could influence age at onset or risk of AD by affecting the neuroprotective activity of estrogen or by altering estrogen levels over long periods of time. Additionally, examination of polymorphisms in estrogen genes may serve as a marker of hormone status in cohorts of elder women where measurement of hormone levels may not be informative. Five candidate genes ER-alpha, ER-beta, CYP17, CYP19, and HSD17B1 are likely to be contributors. Although variants in these genes have been associated with differences in estrogen levels and with increased risk for estrogen-related disorders such as osteoporosis, breast or endometrial cancer and some studies of risk for AD, their contribution to risk of AD has not yet been systematically examined, with all genes assessed in the same population.
As noted above, two estrogen receptors, ER-α and ER-β, have been identified in the brain and have been found in regions affected in AD, including the hippocampus, basal forebrain and amygdala. Two closely linked restriction fragment length polymorphisms (RFLPs), PvuII and XbaI, located in the first intron of ESR1, the gene for ER-α, have been reported to influence estrogen receptor expression and, in turn, may influence risk for AD. In several case-control studies, the XX or PP genotypes and the XXPP haplotype of the ESR1 gene have been associated with increased risk for AD in Asian and European populations and in women with DS (67–71). However, not all studies have found positive associations and risk alleles have been inconsistent across studies. Additionally, the association between the XX and PP genotypes or the XXPP haplotype and risk for AD may vary by the presence of the APOE ɛ4 allele and by gender in some, but not all, studies. These findings, while not conclusive, suggest that ESR1 genotype may influence risk for AD by affecting estrogen receptor expression. Additionally, variants in ESR2, the gene for ER-β, have been associated with increased risk for AD in women, but not men (72). One study found a “CA” repeat in ESR2 that was more strongly protective in men than in women (73). The Health ABC study reported that two single nucleotide polymorphisms on ESR2 were associated with development of cognitive impairment among both non-demented community dwelling men and women (74*).
Two key genes, CYP17 and CYP19, are involved in the peripheral synthesis of estrogens (See Figure 1). Polymorphisms in CYP17 and CYP19 have been repeatedly associated with variation in hormone levels, age at onset of menopause and increased risk of estrogen-related diseases such as bone mass, fracture risk, breast cancer and osteoporosis. Several studies have found associations between single nucleotide polymorphisms (SNPs) and haplotype blocks in CYP19 and risk for AD in both men and women (75, 76), while others have found associations with AD primarily among women (77, 78). The HSD17B1 gene encodes the enzyme 17β-hydroxysteroid dehydrogenase 1 which catalyzes the conversion of estrone to estradiol. The Ser312/Gly coding common variant of HSD17B1, htSNP rs#605059 in Exon 6, is of particular interest as it has been associated with risk for breast cancer tumors and endometriosis. Other common variants of HSD17B1 are also candidate genetic susceptibility factors for AD, but no study to date has examined their relation to age at onset or risk of AD. Existing studies have examined only a few SNPs and several genes of interest have not been examined. Further study of polymorphisms in these genes and in other genes that are involved in estrogen pathways may clarify biological mechanisms relating variation in estrogen to risk of AD.
Conclusion
While individual study results are inconsistent, overall data from epidemiologic studies, observational studies and clinical trials of hormone replacement therapy, studies of endogenous hormones and evaluations of genetic variants involved in estrogen biosynthesis and receptor activity indicate that estrogen plays an important role in the pathogenesis of cognitive decline and risk for AD in both men and women. Looking ahead, genetic studies may help to clarify some of the ambiguity in the existing data. Additionally, while the association between hormones and cognitive function has attracted much attention in women, this relationship is just beginning to be explored in men and needs to be more fully examined in both populations using bioavailable hormone levels. Finally, the concept of a critical window period for hormone supplementation needs to be evaluated in large groups of younger individuals whose cognitive outcomes are then followed for an extended period of time. This is a particularly valuable area of study as it provides the potential for effective intervention and treatment before clinical or pathological features of dementia have begun.
Acknowledgments
This work was supported by grants NIH R01AG014673 and IIRG-08-90655 to Nicole Schupf and by funds from the Charles L and Ann Lee Saunders Brown Fellowship fund and NIH 5T32 MH2004 Clinical Research Training in Geriatric Neuropsychiatry to Sarah Janicki.
Contributor Information
Sarah C. Janicki, G.H. Sergievsky Center, Columbia University Medical Center, New York, NY 10032.
Nicole Schupf, G.H. Sergievsky Center, Columbia University Medical Center, New York, NY 10032, The Taub Institute for Research on Alzheimer’s Disease and the Aging Brain, Columbia University Medical Center, New York NY 10032
References
- 1.Goodman Y, Bruce AJ, Cheng B, Mattson MP. Estrogens attenuate and corticosterone exacerbates excitotoxicity, oxidative injury, and amyloid beta-peptide toxicity in hippocampal neurons. J Neurochem. 1996;66:1836–1844. doi: 10.1046/j.1471-4159.1996.66051836.x. [DOI] [PubMed] [Google Scholar]
- 2.Toran-Allerand CD, Miranda RC, Bentham WD, et al. Estrogen receptors colocalize with low-affinity nerve growth factor receptors in cholinergic neurons of the basal forebrain. Proc Natl Acad Sci U S A. 1992;89:4668–4672. doi: 10.1073/pnas.89.10.4668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Luine VN. Estradiol increases choline acetyltransferase activity in specific basal forebrain nuclei and projection areas of female rats. Exp Neurol. 1985;89:484–490. doi: 10.1016/0014-4886(85)90108-6. [DOI] [PubMed] [Google Scholar]
- 4.Behl C, Widmann M, Trapp T, Holsboer F. 17-beta estradiol protects neurons from oxidative stress-induced cell death in vitro. Biochem Biophys Res Commun. 1995;216:473–482. doi: 10.1006/bbrc.1995.2647. [DOI] [PubMed] [Google Scholar]
- 5.Jaffe AB, Toran-Allerand CD, Greengard P, Gandy SE. Estrogen regulates metabolism of Alzheimer amyloid beta precursor protein. J Biol Chem. 1994;269:13065–13068. [PubMed] [Google Scholar]
- 6.Andersen K, Launer LJ, Dewey ME, et al. Gender differences in the incidence of AD and vascular dementia: The EURODEM Studies. EURODEM Incidence Research Group. Neurology. 1999;53:1992–1997. doi: 10.1212/wnl.53.9.1992. [DOI] [PubMed] [Google Scholar]
- 7.Fratiglioni L, Viitanen M, von Strauss E, Tontodonati V, Herlitz A, Winblad B. Very old women at highest risk of dementia and Alzheimer's disease: incidence data from the Kungsholmen Project, Stockholm. Neurology. 1997;48:132–138. doi: 10.1212/wnl.48.1.132. [DOI] [PubMed] [Google Scholar]
- 8.Ganguli M, Dodge HH, Chen P, Belle S, DeKosky ST. Ten-year incidence of dementia in a rural elderly US community population: the MoVIES Project. Neurology. 2000;54:1109–1116. doi: 10.1212/wnl.54.5.1109. [DOI] [PubMed] [Google Scholar]
- 9.Rocca WA, Cha RH, Waring SC, Kokmen E. Incidence of dementia and Alzheimer's disease: a reanalysis of data from Rochester, Minnesota, 1975–1984. Am J Epidemiol. 1998;148:51–62. doi: 10.1093/oxfordjournals.aje.a009560. [DOI] [PubMed] [Google Scholar]
- 10.Bachman DL, Wolf PA, Linn RT, et al. Incidence of dementia and probable Alzheimer's disease in a general population: the Framingham Study. Neurology. 1993;43:515–519. doi: 10.1212/wnl.43.3_part_1.515. [DOI] [PubMed] [Google Scholar]
- 11.Kawas C, Gray S, Brookmeyer R, Fozard J, Zonderman A. Age-specific incidence rates of Alzheimer's disease: the Baltimore Longitudinal Study of Aging. Neurology. 2000;54:2072–2077. doi: 10.1212/wnl.54.11.2072. [DOI] [PubMed] [Google Scholar]
- 12.Hebert LE, Scherr PA, Beckett LA, et al. Age-specific incidence of Alzheimer's disease in a community population. JAMA. 1995;273:1354–1359. [PubMed] [Google Scholar]
- 13.Kukull WA, Higdon R, Bowen JD, et al. Dementia and Alzheimer disease incidence: a prospective cohort study. Arch Neurol. 2002;59:1737–1746. doi: 10.1001/archneur.59.11.1737. [DOI] [PubMed] [Google Scholar]
- 14.Barrett-Connor E, von Muhlen D, Laughlin GA, Kripke A. Endogenous levels of dehydroepiandrosterone sulfate, but not other sex hormones, are associated with depressed mood in older women: the Rancho Bernardo Study. J Am Geriatr Soc. 1999;47:685–691. doi: 10.1111/j.1532-5415.1999.tb01590.x. [DOI] [PubMed] [Google Scholar]
- 15.Jacobs DM, Tang MX, Stern Y, et al. Cognitive function in nondemented older women who took estrogen after menopause. Neurology. 1998;50:368–373. doi: 10.1212/wnl.50.2.368. [DOI] [PubMed] [Google Scholar]
- 16.Paganini-Hill A, Henderson VW. Estrogen deficiency and risk of Alzheimer's disease in women. Am J Epidemiol. 1994;140:256–261. doi: 10.1093/oxfordjournals.aje.a117244. [DOI] [PubMed] [Google Scholar]
- 17.Tang MX, Jacobs D, Stern Y, et al. Effect of oestrogen during menopause on risk and age at onset of Alzheimer's disease. Lancet. 1996;348:429–432. doi: 10.1016/S0140-6736(96)03356-9. [DOI] [PubMed] [Google Scholar]
- 18.Yaffe K, Lui LY, Grady D, Cauley J, Kramer J, Cummings SR. Cognitive decline in women in relation to non-protein-bound oestradiol concentrations. Lancet. 2000;356:708–712. doi: 10.1016/S0140-6736(00)02628-3. [DOI] [PubMed] [Google Scholar]
- 19.Zandi PP, Carlson MC, Plassman BL, et al. Hormone replacement therapy and incidence of Alzheimer disease in older women: the Cache County Study. JAMA. 2002;288:2123–2129. doi: 10.1001/jama.288.17.2123. [DOI] [PubMed] [Google Scholar]
- 20. Henderson VW. Aging, estrogens and episodic memory in women. Cogn Behav Neurol. 2009;22:205–214. doi: 10.1097/WNN.0b013e3181a74ce7. A useful review of the relations between estrogen exposures and memory in women, with a discussion of the critical window hypothesis in estrogen supplementation.
- 21.Resnick SM, Henderson VW. Hormone therapy and risk of Alzheimer disease: a critical time. JAMA. 2002;288:2170–2172. doi: 10.1001/jama.288.17.2170. [DOI] [PubMed] [Google Scholar]
- 22.Joffe H, Hall JE, Gruber S, et al. Estrogen therapy selectively enhances prefrontal cognitive processes: a randomized, double-blind, placebo-controlled study with functional magnetic resonance imaging in perimenopausal and recently postmenopausal women. Menopause. 2006;13:411–422. doi: 10.1097/01.gme.0000189618.48774.7b. [DOI] [PubMed] [Google Scholar]
- 23.Phillips SM, Sherwin BB. Effects of estrogen on memory function in surgically menopausal women. Psychoneuroendocrinology. 1992;17:485–495. doi: 10.1016/0306-4530(92)90007-t. [DOI] [PubMed] [Google Scholar]
- 24.Sherwin BB. Estrogen and/or androgen replacement therapy and cognitive functioning in surgically menopausal women. Psychoneuroendocrinology. 1988;13:345–357. doi: 10.1016/0306-4530(88)90060-1. [DOI] [PubMed] [Google Scholar]
- 25.Almeida OP, Lautenschlager NT, Vasikaran S, Leedman P, Gelavis A, Flicker L. A 20-week randomized controlled trial of estradiol replacement therapy for women aged 70 years and older: effect on mood, cognition and quality of life. Neurobiol Aging. 2006;27:141–149. doi: 10.1016/j.neurobiolaging.2004.12.012. [DOI] [PubMed] [Google Scholar]
- 26.Schiff R, Bulpitt CJ, Wesnes KA, Rajkumar C. Short-term transdermal estradiol therapy, cognition and depressive symptoms in healthy older women. A randomised placebo controlled pilot cross-over study. Psychoneuroendocrinology. 2005;30:309–315. doi: 10.1016/j.psyneuen.2004.08.007. [DOI] [PubMed] [Google Scholar]
- 27.Wolf OT, Kudielka BM, Hellhammer DH, Torber S, McEwen BS, Kirschbaum C. Two weeks of transdermal estradiol treatment in postmenopausal elderly women and its effect on memory and mood: verbal memory changes are associated with the treatment induced estradiol levels. Psychoneuroendocrinology. 1999;24:727–741. doi: 10.1016/s0306-4530(99)00025-6. [DOI] [PubMed] [Google Scholar]
- 28.Maki PM, Gast MJ, Vieweg AJ, Burriss SW, Yaffe K. Hormone therapy in menopausal women with cognitive complaints: a randomized, double-blind trial. Neurology. 2007;69:1322–1330. doi: 10.1212/01.wnl.0000277275.42504.93. [DOI] [PubMed] [Google Scholar]
- 29.Linzmayer L, Semlitsch HV, Saletu B, et al. Double-blind, placebo-controlled psychometric studies on the effects of a combined estrogen-progestin regimen versus estrogen alone on performance, mood and personality of menopausal syndrome patients. Arzneimittelforschung. 2001;51:238–245. doi: 10.1055/s-0031-1300030. [DOI] [PubMed] [Google Scholar]
- 30.Pefanco MA, Kenny AM, Kaplan RF, et al. The effect of 3-year treatment with 0.25 mg/day of micronized 17beta-estradiol on cognitive function in older postmenopausal women. J Am Geriatr Soc. 2007;55:426–431. doi: 10.1111/j.1532-5415.2007.01085.x. [DOI] [PubMed] [Google Scholar]
- 31.Resnick SM, Maki PM, Rapp SR, et al. Effects of combination estrogen plus progestin hormone treatment on cognition and affect. J Clin Endocrinol Metab. 2006;91:1802–1810. doi: 10.1210/jc.2005-2097. [DOI] [PubMed] [Google Scholar]
- 32.Grady D, Yaffe K, Kristof M, Lin F, Richards C, Barrett-Connor E. Effect of postmenopausal hormone therapy on cognitive function: the Heart and Estrogen/Progestin Replacement Study. Am J Med. 2002;113:543–548. doi: 10.1016/s0002-9343(02)01270-6. [DOI] [PubMed] [Google Scholar]
- 33.Shumaker SA, Reboussin BA, Espeland MA, et al. The Women's Health Initiative Memory Study (WHIMS): a trial of the effect of estrogen therapy in preventing and slowing the progression of dementia. Control Clin Trials. 1998;19:604–621. doi: 10.1016/s0197-2456(98)00038-5. [DOI] [PubMed] [Google Scholar]
- 34.Shumaker SA, Legault C, Rapp SR, et al. Estrogen plus progestin and the incidence of dementia and mild cognitive impairment in postmenopausal women: the Women's Health Initiative Memory Study: a randomized controlled trial. JAMA. 2003;289:2651–2662. doi: 10.1001/jama.289.20.2651. [DOI] [PubMed] [Google Scholar]
- 35.Shumaker SA, Legault C, Kuller L, et al. Conjugated equine estrogens and incidence of probable dementia and mild cognitive impairment in postmenopausal women: Women's Health Initiative Memory Study. JAMA. 2004;291:2947–2958. doi: 10.1001/jama.291.24.2947. [DOI] [PubMed] [Google Scholar]
- 36.Nilsen J, Brinton RD. Divergent impact of progesterone and medroxyprogesterone acetate (Provera) on nuclear mitogen-activated protein kinase signaling. Proc Natl Acad Sci U S A. 2003;100:10506–10511. doi: 10.1073/pnas.1334098100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Sano M, Jacobs D, Andrews H, et al. A multi-center, randomized, double blind placebo-controlled trial of estrogens to prevent Alzheimer's disease and loss of memory in women: design and baseline characteristics. Clin Trials. 2008;5:523–533. doi: 10.1177/1740774508096313. A design description of the PREPARE (PREventing Post-menopausal Alzheimer’s Disease with Replacement Estrogens) trial. Though study results are not yet published, it is anticipated that they will address whether there are lasting or delayed effects of HRT on cognition after treatment cessation.
- 38. Miller VM, Black DM, Brinton EA, et al. Using Basic Science to Design a Clinical Trial: Baseline Characteristics of Women Enrolled in the Kronos Early Estrogen Prevention Study (KEEPS) J Cardiovasc Transl Res. 2009;2:228–239. doi: 10.1007/s12265-009-9104-y. Discusses the design and rationale of KEEPS (Kronos Early Estrogen Prevention Study), which will investigate the critical timing hypothesis of estrogen supplementation by evaluating the effects of HRT on a younger female population.
- 39.Hodis H. [Date accessed.3.20.2010];Early vs Late Intervention Trial with Estradiol (ELITE) [online]. Available. [Google Scholar]
- 40.Yaffe K, Grady D, Pressman A, Cummings S. Serum estrogen levels, cognitive performance, and risk of cognitive decline in older community women. J Am Geriatr Soc. 1998;46:816–821. doi: 10.1111/j.1532-5415.1998.tb02713.x. [DOI] [PubMed] [Google Scholar]
- 41.Lebrun CE, van der Schouw YT, de Jong FH, Pols HA, Grobbee DE, Lamberts SW. Endogenous oestrogens are related to cognition in healthy elderly women. Clin Endocrinol (Oxf) 2005;63:50–55. doi: 10.1111/j.1365-2265.2005.02297.x. [DOI] [PubMed] [Google Scholar]
- 42.Schupf N, Pang D, Patel BN, et al. Onset of dementia is associated with age at menopause in women with Down's syndrome. Ann Neurol. 2003;54:433–438. doi: 10.1002/ana.10677. [DOI] [PubMed] [Google Scholar]
- 43.Hoskin EK, Tang MX, Manly JJ, Mayeux R. Elevated sex-hormone binding globulin in elderly women with Alzheimer's disease. Neurobiol Aging. 2004;25:141–147. doi: 10.1016/s0197-4580(03)00046-0. [DOI] [PubMed] [Google Scholar]
- 44.Schupf N, Winsten S, Patel B, et al. Bioavailable estradiol and age at onset of Alzheimer's disease in postmenopausal women with Down syndrome. Neurosci Lett. 2006;406:298–302. doi: 10.1016/j.neulet.2006.07.062. [DOI] [PubMed] [Google Scholar]
- 45.Geerlings MI, Launer LJ, de Jong FH, et al. Endogenous estradiol and risk of dementia in women and men: the Rotterdam Study. Ann Neurol. 2003;53:607–615. doi: 10.1002/ana.10521. [DOI] [PubMed] [Google Scholar]
- 46.Yaffe K, Barnes D, Lindquist K, et al. Endogenous sex hormone levels and risk of cognitive decline in an older biracial cohort. Neurobiol Aging. 2007;28:171–178. doi: 10.1016/j.neurobiolaging.2006.10.004. [DOI] [PubMed] [Google Scholar]
- 47.Carlson LE, Sherwin BB. Higher levels of plasma estradiol and testosterone in healthy elderly men compared with age-matched women may protect aspects of explicit memory. Menopause. 2000;7:168–177. doi: 10.1097/00042192-200007030-00007. [DOI] [PubMed] [Google Scholar]
- 48.Young LA, Neiss MB, Samuels MH, Roselli CE, Janowsky JS. Cognition is not modified by large but temporary changes in sex hormones in men. J Clin Endocrinol Metab. 2010;95:280–288. doi: 10.1210/jc.2009-1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kampen DL, Sherwin BB. Estradiol is related to visual memory in healthy young men. Behav Neurosci. 1996;110:613–617. doi: 10.1037//0735-7044.110.3.613. [DOI] [PubMed] [Google Scholar]
- 50.Hogervorst E, De Jager C, Budge M, Smith AD. Serum levels of estradiol and testosterone and performance in different cognitive domains in healthy elderly men and women. Psychoneuroendocrinology. 2004;29:405–421. doi: 10.1016/s0306-4530(03)00053-2. [DOI] [PubMed] [Google Scholar]
- 51.Senanarong V, Vannasaeng S, Poungvarin N, et al. Endogenous estradiol in elderly individuals: cognitive and noncognitive associations. Arch Neurol. 2002;59:385–389. doi: 10.1001/archneur.59.3.385. [DOI] [PubMed] [Google Scholar]
- 52.Wolf OT, Kirschbaum C. Endogenous estradiol and testosterone levels are associated with cognitive performance in older women and men. Horm Behav. 2002;41:259–266. doi: 10.1006/hbeh.2002.1770. [DOI] [PubMed] [Google Scholar]
- 53.Lessov-Schlaggar CN, Reed T, Swan GE, et al. Association of sex steroid hormones with brain morphology and cognition in healthy elderly men. Neurology. 2005;65:1591–1596. doi: 10.1212/01.wnl.0000184512.08249.48. [DOI] [PubMed] [Google Scholar]
- 54.Matousek RH, Sherwin BB. A randomized controlled trial of add-back estrogen or placebo on cognition in men with prostate cancer receiving an antiandrogen and a gonadotropin-releasing hormone analog. Psychoneuroendocrinology. 2010;35:215–225. doi: 10.1016/j.psyneuen.2009.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Geerlings MI, Strozyk D, Masaki K, et al. Endogenous sex hormones, cognitive decline, and future dementia in old men. Ann Neurol. 2006;60:346–355. doi: 10.1002/ana.20918. [DOI] [PubMed] [Google Scholar]
- 56.Cherrier MM, Matsumoto AM, Amory JK, et al. Characterization of verbal and spatial memory changes from moderate to supraphysiological increases in serum testosterone in healthy older men. Psychoneuroendocrinology. 2007;32:72–79. doi: 10.1016/j.psyneuen.2006.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Beer TM, Bland LB, Bussiere JR, et al. Testosterone loss and estradiol administration modify memory in men. J Urol. 2006;175:130–135. doi: 10.1016/S0022-5347(05)00049-2. [DOI] [PubMed] [Google Scholar]
- 58.Kenny AM, Bellantonio S, Gruman CA, Acosta RD, Prestwood KM. Effects of transdermal testosterone on cognitive function and health perception in older men with low bioavailable testosterone levels. J Gerontol A Biol Sci Med Sci. 2002;57:M321–M325. doi: 10.1093/gerona/57.5.m321. [DOI] [PubMed] [Google Scholar]
- 59.Taxel P, Stevens MC, Trahiotis M, Zimmerman J, Kaplan RF. The effect of short-term estradiol therapy on cognitive function in older men receiving hormonal suppression therapy for prostate cancer. J Am Geriatr Soc. 2004;52:269–273. doi: 10.1111/j.1532-5415.2004.52067.x. [DOI] [PubMed] [Google Scholar]
- 60.Janowsky JS, Chavez B, Orwoll E. Sex steroids modify working memory. J Cogn Neurosci. 2000;12:407–414. doi: 10.1162/089892900562228. [DOI] [PubMed] [Google Scholar]
- 61.Cherrier MM, Matsumoto AM, Amory JK, et al. The role of aromatization in testosterone supplementation: effects on cognition in older men. Neurology. 2005;64:290–296. doi: 10.1212/01.WNL.0000149639.25136.CA. [DOI] [PubMed] [Google Scholar]
- 62.Sherwin BB, Chertkow H, Schipper H, Nasreddine Z. A randomized controlled trial of estrogen treatment in men with mild cognitive impairment. Neurobiol Aging. 2009 doi: 10.1016/j.neurobiolaging.2009.11.002. (in press) [DOI] [PubMed] [Google Scholar]
- 63.Yaffe K, Lui LY, Zmuda J, Cauley J. Sex hormones and cognitive function in older men. J Am Geriatr Soc. 2002;50:707–712. doi: 10.1046/j.1532-5415.2002.50166.x. [DOI] [PubMed] [Google Scholar]
- 64.Yaffe K, Krueger K, Cummings SR, et al. Effect of raloxifene on prevention of dementia and cognitive impairment in older women: the Multiple Outcomes of Raloxifene Evaluation (MORE) randomized trial. Am J Psychiatry. 2005;162:683–690. doi: 10.1176/appi.ajp.162.4.683. [DOI] [PubMed] [Google Scholar]
- 65.Legault C, Maki PM, Resnick SM, et al. Effects of tamoxifen and raloxifene on memory and other cognitive abilities: cognition in the study of tamoxifen and raloxifene. J Clin Oncol. 2009;27:5144–5152. doi: 10.1200/JCO.2008.21.0716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Espeland MA, Shumaker SA, Limacher M, et al. Relative effects of tamoxifen, raloxifene, and conjugated equine estrogens on cognition. J Womens Health (Larchmt) 2010;19:371–379. doi: 10.1089/jwh.2009.1605. This secondary analysis study raises the possibility that both tamoxifen and raloxifene adversely affect cognitive function in older women; however, the magnitude of the effect is small, and the long-term consequences are unknown.
- 67.Brandi ML, Becherini L, Gennari L, et al. Association of the estrogen receptor alpha gene polymorphisms with sporadic Alzheimer's disease. Biochem Biophys Res Commun. 1999;265:335–338. doi: 10.1006/bbrc.1999.1665. [DOI] [PubMed] [Google Scholar]
- 68.Corbo RM, Gambina G, Ruggeri M, Scacchi R. Association of estrogen receptor alpha (ESR1) PvuII and XbaI polymorphisms with sporadic Alzheimer's disease and their effect on apolipoprotein E concentrations. Dement Geriatr Cogn Disord. 2006;22:67–72. doi: 10.1159/000093315. [DOI] [PubMed] [Google Scholar]
- 69.Isoe-Wada K, Maeda M, Yong J, et al. Positive association between an estrogen receptor gene polymorphism and Parkinson’s disease with dementia. Eur J Neurol. 1999;6:431–435. doi: 10.1046/j.1468-1331.1999.640431.x. [DOI] [PubMed] [Google Scholar]
- 70.Ji Y, Urakami K, Wada-Isoe K, Adachi Y, Nakashima K. Estrogen receptor gene polymorphisms in patients with Alzheimer's disease, vascular dementia and alcohol-associated dementia. Dement Geriatr Cogn Disord. 2000;11:119–122. doi: 10.1159/000017224. [DOI] [PubMed] [Google Scholar]
- 71.Schupf N, Lee JH, Wei M, et al. Estrogen Receptor- alpha variants increase risk of Alzheimer Disease in women with Down syndrome. Dement Geriatr Cogn Disord. 2008;25:476–482. doi: 10.1159/000126495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Pirskanen M, Hiltunen M, Mannermaa A, et al. Estrogen receptor beta gene variants are associated with increased risk of Alzheimer's disease in women. Eur J Hum Genet. 2005;13:1000–1006. doi: 10.1038/sj.ejhg.5201447. [DOI] [PubMed] [Google Scholar]
- 73.Forsell C, Enmark E, Axelman K, et al. Investigations of a CA repeat in the oestrogen receptor beta gene in patients with Alzheimer's disease. Eur J Hum Genet. 2001;9:802–804. doi: 10.1038/sj.ejhg.5200714. [DOI] [PubMed] [Google Scholar]
- 74. Yaffe K, Lindquist K, Sen S, et al. Estrogen receptor genotype and risk of cognitive impairment in elders: findings from the Health ABC study. Neurobiol Aging. 2009;30:607–614. doi: 10.1016/j.neurobiolaging.2007.08.003. This study’s findings that several SNPs in the ESR1 and ESR2 genes were associated with riskof developing cognitive impairment suggest that estrogen receptor genetic variants may play a role in cognitive aging.
- 75.Huang R, Poduslo SE. CYP19 haplotypes increase risk for Alzheimer's disease. J Med Genet. 2006;43:e42. doi: 10.1136/jmg.2005.039461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Iivonen S, Corder E, Lehtovirta M, et al. Polymorphisms in the CYP19 gene confer increased risk for Alzheimer disease. Neurology. 2004;62:1170–1176. doi: 10.1212/01.wnl.0000118208.16939.60. [DOI] [PubMed] [Google Scholar]
- 77.Butler HT, Warden DR, Hogervorst E, Ragoussis J, Smith AD, Lehmann DJ. Association of the aromatase gene with Alzheimer's disease in women. Neurosci Lett. 2010;468:202–206. doi: 10.1016/j.neulet.2009.10.089. [DOI] [PubMed] [Google Scholar]
- 78.Corbo RM, Gambina G, Ulizzi L, Moretto G, Scacchi R. Genetic variation of CYP19 (aromatase) gene influences age at onset of Alzheimer's disease in women. Dement Geriatr Cogn Disord. 2009;27:513–518. doi: 10.1159/000221832. [DOI] [PubMed] [Google Scholar]


