Abstract
Interleukin 6 (IL6) is a pleiotropic cytokine that not only affects the immune system, but also plays an active role in many physiological events in various organs. Notably, 35% of systemic IL6 originates from adipose tissues under noninflammatory conditions. Here, we describe a previously unknown function of melanocortins in regulating Il6 gene expression and production in 3T3-L1 adipocytes through membrane receptors which are called melanocortin receptors (MCRs). Of the five MCRs that have been cloned, MC2R and MC5R are expressed during adipocyte differentiation. α-Melanocyte-stimulating hormone (α-MSH) or ACTH treatment of 3T3-L1 adipocytes induces Il6 gene expression and production in a time- and concentration-dependent manner via various signaling pathways including the protein kinase A, p38 mitogen-activated protein kinase, cJun N-terminal kinase, and IκB kinase pathways. Specific inhibition of MC2R and MC5R expression with short interfering Mc2r and Mc5r RNAs significantly attenuated the α-MSH-induced increase of intracellular cAMP and both the level of Il6 mRNA and secretion of IL6 in 3T3-L1 adipocytes. Finally, when injected into mouse tail vein, α-MSH dramatically increased the Il6 transcript levels in epididymal fat pads. These results suggest that α-MSH in addition to ACTH may function as a regulator of inflammation by regulating cytokine production.
Introduction
Interleukin 6 (IL6) is a pleiotropic cytokine involved in the regulation of the immune response, acute-phase reaction, nervous and endocrine system function, bone metabolism, hematopoiesis, insulin resistance, and various human diseases (Kamimura et al. 2003). Notably, adipose tissue has been regarded as a major source of circulating IL6, particularly in obesity, because 35% of systemic IL6 originates from subcutaneous adipocytes (Mohamed-Ali et al. 1997, Fried et al. 1998). Recent studies have demonstrated that Il6 negatively affects insulin signaling in adipocytes by reducing expression of insulin receptor signaling components (Rotter et al. 2003), by inducing the suppressor of cytokine signaling 3 (a negative regulator of insulin signaling; Lagathu et al. 2003, Shi et al. 2004), and by decreasing adiponectin secretion (Fasshauer et al. 2003, Kristiansen & Mandrup-Poulsen 2005). Many IL6 inducers are known including IL1β, tumor necrosis factor-α, lysophosphatidic acid, prostaglandin E, thyroid-stimulating hormone, platelet-derived growth factor, catecholamine, palmitate, bacterial lipopolysaccharide (LPS), and viral infection and associated transforming growth factor-β (Verhasselt et al. 1997, Franchimont et al. 1999, Legrand-Poels et al. 2000, Fang et al. 2004, Ajuwon & Spurlock 2005, Liu et al. 2005, Tan et al. 2007, Antunes et al. 2008, Diya et al. 2008). This is the first time that melanocortins induce Il6 gene expression and production through the MCRs.
Melanocortins are generated by the proteolytic cleavage of the precursor molecule, proopiomelanocortin (POMC). Differential enzymatic cleavage of POMC by prohormone convertases results in the production of ACTH, α-melanocyte-stimulating hormone (α-MSH), β-MSH, γ-MSH, and other hormones. Even though these hormones are derived from different regions of the precursor POMC, because they share a core binding sequence His-Phe-Arg-Trp (HFRW), which is required for binding and activation of the receptors, ACTH can activate all five MCRs, whereas α-MSH can activate all MCRs except MC2R (Yang & Harmon 2003). α-MSH is one of the POMC derivatives, as N-terminal region of ACTH. It seems like α-MSH has a function that is similar to that of ACTH. Melanocortins are recognized as pivotal components of the hypothalamic–pituitary–adrenal (HPA) axis in mediating response to stress. It has been postulated that stress may be a causal factor in metabolic syndromes such as insulin resistance, type 2 diabetes, and hypertension via perturbation of the HPA axis (Gohil et al. 2001, Kaufman et al. 2007). The pituitary gland secretes critical hormones which affect many target tissues in our body. On the other hand, adipocyte tissue expresses receptors for many hormones including pituitary hormones, and secretes several factors which are called adipotropins (Schaffler et al. 2006). So, adipocyte tissue is not a passive organ any longer, and this is called hypothalamic–pituitary–adipose axis. To date, five MCRs have been described (MC1R–MC5R). All are coupled to stimulatory G-proteins (Gs), which after binding to melanocortins initiate the activation of adenylyl cyclase, ultimately resulting in the transcription of cAMP-responsive genes (Bohm et al. 2006). MC1R and MC2R have classically been considered to have a role in the skin pigmentation (Schaffler et al. 2006) and adrenal steroidogenesis respectively (Cone 2006). MC3R and MC4R are expressed primarily in the hypothalamus of the central nervous system (CNS). The best characterized effects of MC3R and MC4R are in the control of food intake, energy expenditure, and sexual function (Wikberg & Mutulis 2008), and disruption of Mc4r gene has been shown to cause obesity in mice (Butler et al. 2001). Among the five subtypes of MCRs, MC5R is a relatively ubiquitous receptor in peripheral tissues, suggesting a direct peripheral action for melanocortins. MC5R participates in the control of exocrine secretions in exocrine glands and fatty acid oxidation in skeletal muscle (Chen et al. 1997, An et al. 2007).
The melanocortin peptides ACTH, α-MSH, and β-lipotropin have long been recognized to have different degrees of lipolytic activity in the adipocytes of various mammalian species (Boston 1999). ACTH has recently been shown to inhibit leptin production in 3T3-L1 adipocytes (Norman et al. 2003) and to promote a pro-inflammatory adipocytokine profile (Iwen et al. 2008). However, the peripheral effects of α-MSH and MCRs in adipocytes have not been extensively investigated. In this study, we demonstrate for the first time that melanocortins are inducers of Il6 in 3T3-L1 adipocytes. We show that MC5R mediates the α-MSH-induced increase in Il6 gene expression, and that MC2R mediates the ACTH-induced increase in Il6 gene expression via the activation of the protein kinase A (PKA), p38 mitogen-activated protein kinase (MAPK), cJun N-terminal kinase (JNK), and IκB kinase (IKK) signaling pathways.
Materials and methods
3T3-L1 cell cultures
3T3-L1 cells were grown in DMEM containing 1% penicillin/streptomycin (P/S) and 10% fetal bovine serum (FBS) until they reach confluency. They were then differentiated with the differentiation mixture (DMEM containing 1% P/S and 10% FBS, 500 μM 3-isobutyl-1-methylxanthine, 10 μg/ml insulin, and 1 μM dexamethasone) for 2 days as described (Jun et al. 2006). Insulin was present for an additional 4 days, and then, the culture medium was changed with only DMEM (1% P/S and 10% FBS) for 2 days. Each medium was changed every 2 days.
RNA extraction and real-time quantitative reverse transcription-PCR
Total RNA was extracted from differentiated adipocytes using TRIzol reagent (Invitrogen) according to the manufacturer's protocol. For real-time quantitative reverse transcription (RT)-PCR, total RNA was reversely transcribed using an ImProm-II RT system (Promega) according to the manufacturer's instructions. For detection and quantification, a MyiQ real-time PCR detection system (Bio-Rad) was used. PCRs were performed using a SYBR Premix Ex Taq II (Takara, Seoul, Republic of Korea). PCRs were carried out in a final volume of 20 μl using 0·5 μM of each primer, cDNA, and 10 μl of the supplied enzyme mixture containing the DNA double-strand-specific SYBR Green I dye for detection of PCR products. PCRs were performed with a 3-min preincubation at 95 °C followed by 40 cycles of 15 s at 95 °C and 30 s at 60 °C. PCR products were verified by melting curve analysis, agarose gel electrophoresis, and DNA sequencing.
The following primers were used:
Arbp (forward, 5′-AAAACTCCGGTCTGGATTTATTTAG; reverse, 5′-TAATTCACACCTGGAAAATCTTTGT),
Gapdh (forward, 5′-GCCATCAATGACCCCTTCATT; reverse, 5′-GCTCCTGGAAGATGGTGATGG),
Add1 (forward, 5′-CATCTGTTGTAAGGTGTATTTGCTG; reverse, 5′-AGATGACTAGGGAACTGTGTGTGTT),
PPARγ (forward, 5′-TTGCTGAACGTGAAGCCCATCGAGG; reverse, 5′-GTCCTTGTAGATCTCCTGGAGCAG),
C/EBPa (forward, 5′-CCATTTTATTTGGTCTTTTGTTTTG; reverse, 5′-CTACATACACCCTTGGACAACTAGG),
Pref1 (forward, 5′-CATGAAAGAGCTCAACAAGAGTACC; reverse, 5′-GTTATACTGCAACAGGAGGTTCTTC),
Pai1 (forward, 5′-CCTGGTCAACCACCTTAGTTAGATA; reverse, 5′-AAATCAGAGAGAAAGAGGGAGAGAG),
aP2 (forward, 5′-ACAATAAAGAGAAAACGAGATGGTG; reverse, 5′-TGCTTGCTTATTAGTGGAAAATCAT),
Mc1r (forward, 5′-GATTTGGGAATTAGACAAGACCTTT; reverse, 5′-GGACAAAGAAGTGTTCAGTACCAGT),
Mc2r (forward, 5′-TAAAGGGACCAAATAACACATCAGT; reverse, 5′-CTTTCCTGTTTAGCACAACATTTC),
Mc3r (forward, 5′-ATATTCTGTGGGAGATTGAGTGAAG; reverse, 5′-CCAACAATAATAACAACCATGACAA),
Mc4r (forward, 5′-TAAGTTTGTGACTTTTGACATGGAA; reverse, 5′-TGGAACCTTGATAAATAACAGGAAA),
Mc5r (forward, 5′-GTAAACAGAAGATTCAACTCCCAGA; reverse, 5′-CGTTCAGGGTAAGATTCAATACAGT),
Rbp4 (forward, 5′-GACAGCTACTCCTTTGTGTTTTCTC; reverse 5′-AGAAATCTTCAAACTTCACATCCT),
Igf1 (forward, 5′-GGAAAGGAAGTACATTTGAAGAACA; reverse, 5′-TTATTTGGTAGGTGTTTCGATGTTT),
Igf2 (forward, 5′-AAAAACAATTGGCAAAATCAAATAA; reverse, 5′-TTACACTAAAGGTGCTTGGATAAGG),
Il6 (forward, 5′-AGGCTTAATTACACATGTTCTCTGG; reverse, 5′-TTATATCCAGTTTGGTAGCATCCAT),
adiponectin (forward, 5′-GTTCTCTTCACCTACGACCAGTATC; reverse, 5′-AAAGCCAGTAAATGTAGAGTCGTTG),
resistin (forward, 5′-ACTGACAAGAAGATCAAACAAGACT; reverse, 5′-AGTGACACACTTTTTCTTCACGAAT), and
Tnfα (forward, 5′-GATTTGCTATCTCATACCAGGAGAA; reverse, 5′-AAGTCTAAGTACTTGGGCAGATTGA).
Relative value of gene expression was analyzed using the 2C(t) method (Livak & Schmittgen 2001).
Western blot analysis
3T3-L1 adipocytes were plated in 60-mm tissue culture dishes and treated with melanocortins for various time periods. After treatment, the cells were washed twice with cold PBS and then lysed with lysis buffer (250 mM Tris–Cl (pH 6·5), 2% SDS, 4% β-mercaptoethanol, 0·02% bromophenol blue, and 10% glycerol). Equal amounts of whole cell lysates were resolved by 12·5% SDS-PAGE and transferred to a polyvinylidene difluoride membrane. Membranes were blocked using Tris-buffered saline with Tween 20 (TTBS, 150 mM NaCl, 10 mM Tris–HCl, pH 8·0, and 0·05% Tween 20) containing 5% skimmed milk for 30 min, and were then incubated overnight with the indicated primary antibody. After washing three times with TTBS, the membranes were probed with HRP-conjugated secondary antibody to allow for the detection of the appropriate bands using an ECL detection system (Neuronex Co., Daegu, Republic of Korea).
Measurement of [3H] cAMP
Intracellular cAMP generation was determined by [3H] cAMP competition assay in binding to cAMP-binding protein as described previously by Jun et al. (2006) with some modifications. To determine the cAMP production induced by melanocortins, the 3T3-L1 adipocytes were stimulated with agonists for 20 min in the presence of the phosphodiesterase inhibitor Ro 20–1724 (5 μM), and the reaction was quickly terminated by three repeated cycles of freezing and thawing. The samples were then centrifuged at 12 000 g for 5 min at 4 °C. The cAMP assay is based on the competition between [3H]-labeled cAMP and unlabeled cAMP present in the sample for binding to a crude cAMP-binding protein prepared from bovine adrenal cortex according to the method of Brown et al. (1971). Bound [3H] cAMP in the supernatant was then determined by liquid scintillation counting. Each sample was incubated with 50 μl [3H]-labeled cAMP (5 μCi) and 100 μl binding protein for 2 h at 4 °C. Separation of protein-bound cAMP from unbound cAMP was achieved by absorption of free cAMP onto charcoal (100 μl), followed by centrifugation at 12 000 g at 4 °C. The 200-μl supernatant was then placed into an Eppendorf tube containing 1·2 ml scintillation cocktail to measure radioactivity. The cAMP concentration in the sample was determined based on a standard curve and expressed as picomoles per microgram of protein.
ELISA
Medium from differentiated 3T3-L1 cells cultured in 24-well plates was analyzed for IL6 using a kit (R&D systems, Minneapolis, MN, USA).
RNA interference and transfection
The siRNA SMART pool containing 50 nmol of a mixture of four oligonucleotides which target mouse Mc2r and Mc5r mRNA destruction by RISCs was purchased from Dharmacon, Inc (Lafayette, CO, USA). Transfection of the 3T3-L1 adipocytes with the siRNA SMART pool oligonucleotides was carried out with a microporator (Digital Biotechnology, Daegu, Republic of Korea) according to the protocol provided by the company.
Animal experiment
Eight-week-old C57BL/6J male mice were obtained from the Orient Bio (Sungnam, South Korea). All the mouse experiments were performed in the animal facility under POSTECH institutional guidelines. The mice were kept in individual cages in a room in which lighting was controlled (12 h light:12 h darkness), and the temperature was maintained at 23 °C. Mice were sacrificed by cervical dislocation 0, 1, 3, and 6 h after injecting vehicle or 1 mg/kg of α-MSH i.v. Immediately after the mice were sacrificed, epididymal fat pads were removed. Total RNA was extracted from epididymal fat pads using TRIzol reagent (Invitrogen) according to the manufacturer's protocol, and then Il6 transcript levels were measured using a real-time quantitative RT-PCR.
Materials
α-, β-, and γ-MSH, SB203580, SP600125, Bay11-7085, H89, 6-Bnz-cAMP, forskolin, isoproterenol, and LPS were purchased from the Sigma–Aldrich Inc. ACTH was purchased from Phoenix Pharmaceuticals Company Inc. (Burlingame, CA, USA).
Statistical analysis
All numerical values are given as mean±s.d. Significance of differences between mean values of two groups was evaluated using Student's t-test for unpaired data as appropriate. A probability of P<0·001 or P<0·05 was considered significant.
Results
MCR subtypes are expressed in metabolic tissues and 3T3-L1 adipocytes
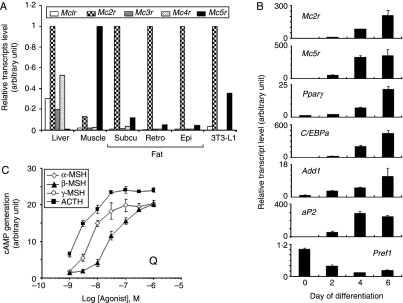
To investigate the expression of the five MCR subtypes in mouse metabolic tissues and 3T3-L1 adipocytes, real-time quantitative RT-PCR was carried out using primers specific for each subtype. Both MC2R and MC5R were the two most abundant MCRs in all adipose tissues (subcutaneous, retroperitoneal, and epididymal fat), and the patterns of MCR expression in 3T3-L1 adipocytes were similar to those of adipose tissues (Fig. 1A). Next, we investigated whether the levels of Mc2r and Mc5r were changed during adipocyte differentiation. Levels of individual transcripts were analyzed by real-time quantitative RT-PCR during the time course of 3T3-L1 differentiation up to 6 days post induction. The kinetics of Mcr mRNA expression were compared to those of several markers of adipogenesis, including the transcription factors peroxisome proliferator-activated receptor γ (PPARγ), CAAT/enhancer-binding protein α (C/EBPa), and adipocyte determination differentiation factor 1 (Add1), as well as adipose lipid-binding protein (aP2). We also assayed the kinetics of the expression of the preadipocyte marker, preadipocyte factor 1 (Pref1; Fig. 1B). These data indicate that adipogenesis is accompanied by the upregulation of Mc2r and Mc5r mRNAs. MCRs are G-protein-coupled receptors and are coupled to adenylyl cyclase via Gs (Cone 2006). Because mRNA expression is not necessarily correlated with protein expression or activity, the capacities of the melanocortins to stimulate cAMP production were compared in order to characterize their pharmacological properties in 3T3-L1 adipocytes. As shown in Fig. 1C, ACTH was most active in elevating the level of intracellular cAMP followed by α-MSH, β-MSH, and γ-MSH. This is consistent with the properties of the MC2R subtype, which is selective for ACTH, and of the MC5R subtype, which binds preferentially to α-MSH, with equal affinity to β-MSH and ACTH, and with lowest affinity to γ-MSH (Cone 2006). So, α-MSH is the most potent activator of MC5R. These data suggest that MC2R and MC5R are functionally active in 3T3-L1 adipocytes.
Figure 1.
Functional expression of melanocortin receptor subtypes in mouse tissues and 3T3-L1 adipocytes. (A) Real-time quantitative RT-PCR analysis of melanocortin receptor subtypes in mouse metabolic tissues and 3T3-L1 adipocytes. Detection of mRNA corresponding to all five Mcrs in liver, muscle, subcutaneous fat, retroperitoneal fat, epididymal fat, and 3T3-L1 adipocytes. (B) Real-time quantitative RT-PCR analysis of Mc2r and Mc5r during adipogenesis. The cDNAs derived from the 3T3-L1 cells which had undergone differentiation for various time periods were amplified using specific primers. For comparison, five differentiation-specific genes were also analyzed: PPARγ, C/EBPa, Add1, aP2, and Pref1. The relative expression of the transcripts was normalized to acidic ribosomal phosphoprotein P0 (Arbp) mRNA levels, and values represent the mean±s.d. (C) Concentration-dependent effects of melanocortins on cAMP generation. 3T3-L1 adipocytes were treated with various concentrations of α-MSH, β-MSH, γ-MSH, or ACTH for 20 min, and then cAMP production was measured. The data present the means±s.d. of triplicate samples. Each experiment was performed independently at least three times.
Melanocortins induce Il6 expression in 3T3-L1 adipocytes
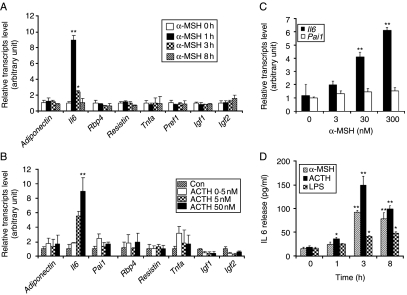
We examined the effect of α-MSH and ACTH on the level of adipocytokines in 3T3-L1 adipocytes. Of the adipocytokines tested, only IL6 was induced by treatment with α-MSH (Fig. 2A) or ACTH (Fig. 2B). Levels of Il6 mRNA were elevated within 1 h of α-MSH treatment and returned to baseline level by 3 h, as determined by real-time quantitative RT-PCR (Fig. 2A). Dramatic increase of Il6 mRNA was observed when 50 nM ACTH was administered (Fig. 2B). α-MSH at a concentration of 3–300 nM induced Il6 gene expression in a concentration-dependent manner, but it did not induce the expression of Pai1 (negative control; Fig. 2C). In agreement with this finding, the level of IL6 protein secreted into the culture medium of 3T3-L1 adipocytes increased steadily after α-MSH or ACTH addition, and peaked at ∼3 h as determined by IL6 ELISA (Fig. 2D). ACTH increased Il6 gene expression to higher levels than seen for α-MSH, but the profile of the time course was similar to that of α-MSH. LPS was included in the assay as a positive control. No further increase in the level of IL6 was detected when the experiment was extended to 24 h (data not shown), indicating that maximal IL6 protein production was attained by 3 h. Taken together, these data suggest that melanocortins function as inducers of Il6 expression in 3T3-L1 adipocytes.
Figure 2.
Melanocortins increase Il6 gene expression and secretion. (A) Differentiated 3T3-L1 adipocytes were incubated with α-MSH (300 nM) for 0, 1, 3, and 8 h, and various adipocytokines including adiponectin, Il6, Rbp4, resistin, Tnfa, Pref1, Igf1, and Igf2 mRNA levels were analyzed with real-time quantitative RT-PCR using Gapdh as a reference. Values represent the mean±s.d. of triplicate samples. (B) Differentiated 3T3-L1 adipocytes were incubated with various concentrations of ACTH. After 1 h, the cells were harvested, and various adipocytokines including adiponectin, Il6, Pai1, Rbp4, resistin, Tnfa, Igf1, and Igf2 mRNA levels were analyzed with real-time quantitative RT-PCR using Gapdh as a reference. (C) Differentiated 3T3-L1 adipocytes were stimulated with various concentrations of α-MSH. After 1 h, the cells were harvested, and Il6 and Pai1 mRNA levels were determined using real-time quantitative RT-PCR. (D) 3T3-L1 adipocytes were stimulated with α-MSH (300 nM), ACTH (50 nM), and LPS (1 μg/ml) for 1, 3, and 8 h. The supernatants were collected, and IL6 concentrations were determined by ELISA. Values represent the means±s.d. of triplicate samples. *P<0·05; **P<0·001 compared with the zero time point. Each experiment was performed independently at least three times.
PKA, p38 MAPK, JNK, and IKK signaling pathways are involved in the melanocortin-mediated increase in Il6 gene expression and secretion
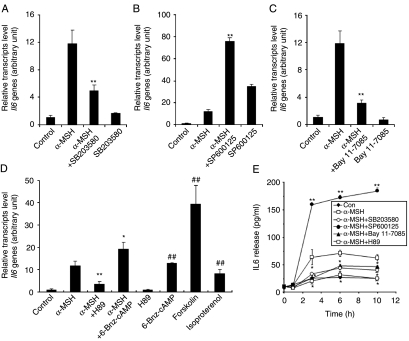
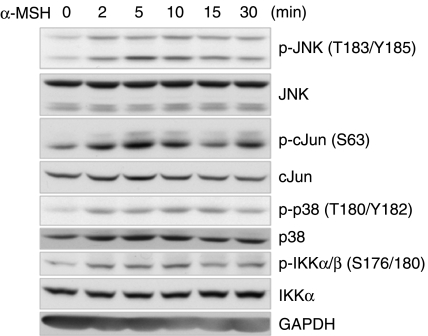
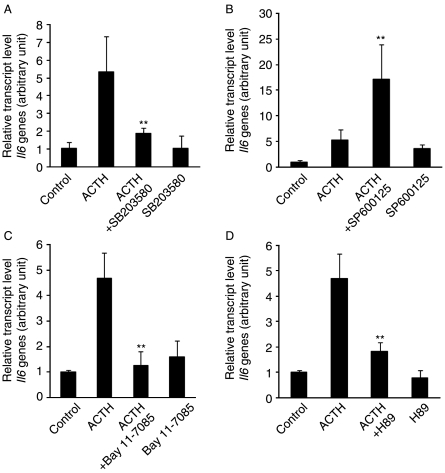
It has been reported that several stimuli including proinflammatory cytokines, β2-adrenergic receptor agonists, and adiponectin induce IL6 production through the p38 MAPK, PKA, and NFκB signaling pathways in various tissues and cells (Legrand-Poels et al. 2000, Tang et al. 2007). Therefore, we investigated the possibility that these mediators are also involved in the signal transduction pathway leading to increased IL6 production in α-MSH-treated 3T3-L1 adipocytes. Pretreatment of cells with either the p38-specific inhibitor SB203580 or the IKK inhibitor Bay11-7082 for 30 min prior to α-MSH treatment attenuated α-MSH-induced Il6 production, whereas the JNK inhibitor SP600125 markedly potentiated α-MSH-induced Il6 production (Fig. 3A–C). The results suggest that p38 MAPK and IKK signaling pathways activate α-MSH-induced Il6 gene expression, but JNK signaling pathway inhibits α-MSH-induced Il6 gene expression. Western blot analysis revealed that α-MSH stimulation of 3T3-L1 adipocytes immediately activated JNK, p38 MAPK, and IKK proteins in a time-dependent manner (Fig. 4). To determine whether elevation of intracellular cAMP is also necessary for Il6 induction by α-MSH, we used a pharmacological inhibitor of the cAMP signaling cascade. Pretreatment of 3T3-L1 adipocytes for 30 min with the PKA inhibitor H89 led to a reduction of the α-MSH-induced increase in the level of Il6 mRNA (Fig. 3D). Because H89 may also inhibit kinases other than PKA in the concentration range used here, we confirmed the involvement of PKA using the specific PKA activator 6-Bnz-cAMP, as well as other cAMP-elevating agents including forskolin, an activator of adenylyl cyclase, and isoproterenol, a β-adrenergic receptor agonist. Figure 3D shows that 6-Bnz-cAMP, forskolin, and isoproterenol mimicked the effect of α-MSH on Il6 expression in 3T3-L1 adipocytes. Finally, we also observed these inhibitory effects on the IL6 release in a time-dependent manner (Fig. 3E). The same effects were observed in the ACTH-mediated Il6 gene expression pattern (Fig. 5A–D). Taken together, these data indicate that PKA, p38 MAPK, JNK, and IKK mediated Il6 production induced by α-MSH or ACTH in 3T3-L1 adipocytes.
Figure 3.
PKA, p38 MAPK, JNK, and IKK are involved in α-MSH-induced Il6 gene expression and secretion. Differentiated 3T3-L1 adipocytes were pretreated with A, SB203580 (10 μM); B, SP600125 (10 μM); C, Bay 11-7085 (10 μM); and D, H89 (1 μM) and 6-Bnz-cAMP (50 μM) for 30 min prior to treatment with α-MSH (300 nM) for 1 h. (D) 3T3-L1 adipocytes were also treated with forskolin (3 μM), and isoproterenol (3 μM) alone for 1 h. (E) In the presence of each inhibitor, cells were treated with α-MSH for indicated time intervals, and culture medium was collected to measure secreted IL6. Data represent the means±s.d. of triplicate samples. *P<0·05; **P<0·001 compared with the α-MSH-treated group. #P<0·001 compared with the control group. Each experiment was performed independently at least three times.
Figure 4.
Phosphorylation of JNK, p38 MAPK, and IKK in response to α-MSH. Serum-starved (12 h) 3T3-L1 adipocytes were treated with α-MSH (300 nM) for the indicated time periods. Whole-cell extracts were analyzed by western blots of phospho-JNK, phospho-cJUN, phospho-p38 kinases, and phospho-IKK protein. Equal loading of protein was checked by glyceraldehyde-3-phosphate dehydrogenase (GAPDH) expression. Each experiment was performed independently at least three times.
Figure 5.
PKA, p38 MAPK, JNK, and IKK are involved in ACTH-induced Il6 gene expression. Differentiated 3T3-L1 adipocytes were pretreated with A, SB203580 (10 μM); B, SP600125 (10 μM); C, Bay 11-7085 (10 μM); and D, H89 (1 μM) for 30 min prior to treatment with ACTH (50 nM) for 1 h. Data represent the means±s.d. of triplicate samples. *P<0·05; **P<0·001 compared with the ACTH-treated group. Each experiment was performed independently at least three times.
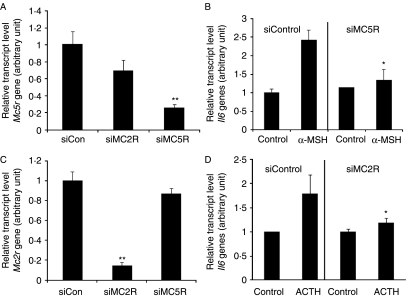
Involvement of the MC5R in the α-MSH-induced increase in Il6 gene expression and MC2R in the ACTH-induced increase in Il6 gene expression
To investigate the role of the MC5R subtype in the α-MSH-mediated increase in Il6 production and of MC2R subtype in the ACTH-mediated increase in Il6 production, we specifically inhibited MC5R expression by RNAi using a short interfering Mc5r RNA (siMC5R) and Mc2r RNA (siMC2R) in 3T3-L1 adipocytes (Fig. 6A and C). Suppression of MC5R expression significantly attenuated the α-MSH-induced increase in Il6 mRNA level (Fig. 6B), and suppression of MC2R expression also significantly attenuated the ACTH-induced increase in Il6 mRNA level (Fig. 6D) in 3T3-L1 adipocytes. However, suppression of MC5R expression could not attenuate the ACTH-induced increase in Il6 mRNA level, and suppression of MC2R expression could not attenuate α-MSH-induced increase in Il6 mRNA level (data not shown). These results suggest that MC5R is involved in the α-MSH-induced Il6 expression, and that MC2R is involved in the ACTH-induced Il6 expression in 3T3-L1 adipocytes.
Figure 6.
α-MSH increases Il6 gene expression through MC5R, and ACTH increases Il6 gene expression through MC2R. 3T3-L1 adipocytes were transfected for 48 h with siRNAs targeted to the MC5Rs (siMC5Rs), or scrambled control siRNAs (siControl), and transfected for 24 h with siRNAs targeted to the MC2Rs (siMC2Rs), or scrambled control siRNA (siControl). (A) The MC5R-specific siRNA reduced endogenous Mc5r expression as detected by real-time quantitative RT-PCR. Gapdh served as an internal control. (B) 3T3-L1 adipocytes in which MC5R had been knocked down were stimulated with α-MSH for 1 h. Il6 gene expression was evaluated by real-time quantitative RT-PCR. (C) The MC2R-specific siRNA reduced endogenous Mc2r expression as detected by real-time quantitative RT-PCR. Gapdh served as an internal control. (D) 3T3-L1 adipocytes in which MC2R had been knocked down were stimulated with ACTH for 1 h. Il6 gene expression was evaluated by real-time quantitative RT-PCR. Values represent the means±s.d. of triplicate samples. *P<0·05; **P<0·001 compared with the siControl-transfected group. Each experiment was performed independently at least three times.
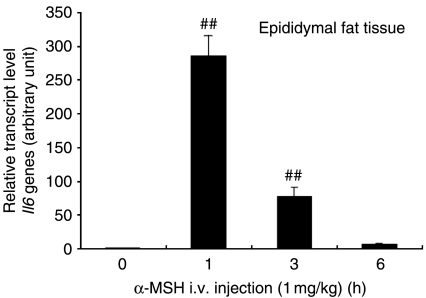
Effect of α-MSH on Il6 expression in primary fat tissue
To investigate whether α-MSH regulates Il6 expression in primary fat tissue, the level of Il6 mRNA in epididymal fat pads was measured. Mice were i.v. injected with α-MSH (1 mg/kg) in the tail vein, and Il6 mRNA level in fat pads was measured at 0, 1, 3, and 6 h after α-MSH injection. Il6 transcript level in fat pads peaked 1 h after injection and returned rapidly to the baseline by 6 h (Fig. 7), which correlated well with the profile of α-MSH-induced Il6 gene expression in 3T3-L1 adipocytes. These results suggest that MCRs function to trigger Il6 expression in response to α-MSH in fat tissue.
Figure 7.
I.v. injection of α-MSH induces Il6 gene expression in mouse epididymal fat tissue. Mice were i.v. injected via tail vein with 200 μl sterile PBS containing 1 mg/kg α-MSH. Epididymal fat tissue was collected at 1, 3, and 6 h after α-MSH injection. Il6 transcript levels were determined by real-time quantitative RT-PCR. Values represent the means±s.d., n=3 for each time point. ##P<0·001 compared with the control group. Each experiment was performed independently at least three times.
Discussion
In contrast to the abundant data available on the classical roles of melanocortins in processes such as skin pigmentation, steroidogenesis, energy expenditure, and appetite regulation in the CNS (Cone 2006), little is known about the peripheral functions of melanocortins, particularly in the adipose tissues and in melanocortin interaction with adipocytokines. It has been reported that adipose tissues contribute as much as 35% of the basal circulating IL6. Moreover, there is a positive correlation between elevated IL6 and obesity and insulin resistance (Fernandez-Real & Ricart 2003). Here, we further characterized IL6 as a newly described protein target of the melanocortin signaling pathway in adipocytes. Recently, other groups (Hoch et al. 2008, Iwen et al. 2008) reported that melanocortin induced Il6 gene expression, which was revealed by real-time quantitative RT-PCR analysis. In this study, we have demonstrated that melanocortins increase Il6 gene expression and secretion through MCRs in 3T3-L1 adipocytes and mouse epididymal fat tissue. In addition, we have investigated knockdown effects on each MCR. These results suggest that α-MSH-induced increase in Il6 production is mainly mediated by MC5R, and that ACTH-induced increase in Il6 production is mediated by MC2R. PKA, p38 MAPK, JNK, and IKK signaling pathways played important roles in the melanocortin-mediated Il6 production.
We have demonstrated that the patterns of MCR expression in the 3T3-L1 adipocytes are in agreement with those of retroperitoneal, epididymal, and subcutaneous fat tissues. The differential expression profiles of five MCRs in 3T3-L1 cells have been reported (Norman et al. 2003). The expression pattern of MCRs has been analyzed with RT-PCR. We also performed RT-PCR and real-time quantitative RT-PCR. The analysis that was applied for the profile of MCRs was applied to mouse tissues. We found and confirmed that they expressed all types of MCRs by sequencing RT-PCR products, but of the five MCRs subtypes, MC2R and MC5R are expressed at the highest level in all adipose tissues. In addition to its role in the induction of IL6, described in this study, α-MSH has a direct effect on lipid metabolism and leptin regulation (Hoggard et al. 2004, Harmer et al. 2008). The observed effects of ACTH and α-MSH on IL6 expression and secretion occur at concentrations similar to those that induce lipolysis and inhibit leptin expression in adipocytes. We have assumed that ACTH and α-MSH act primarily on the MC2R and MC5R respectively (Cone 2006), since α-MSH does not bind to MC2R and the hierarchical arrangement of melanocortins with regard to elevating intracellular cAMP level is evidenced for the functional expression of MC5R in 3T3-L1 adipocytes (Fig. 1C). Finally, the MC5R knockdown experiment confirmed the involvement of MC5R in α-MSH-induced Il6 production (Fig. 6B), and MC2R in ACTH-induced Il6 production (Fig. 6D).
Widely considered a primary regulator of inflammation, p38 integrates inflammatory responses by regulating several aspects of target gene transcription and translation. p38 enhances both the transcriptional activity of NFκB via acetylation of p65 (Saha et al. 2007) and the translation of inflammatory cytokines, including IL6, via stabilization of their mRNAs by phosphorylation of AU-rich element-binding proteins (Zhao et al. 2008). Because MCRs are coupled to adenylyl cyclase and several cAMP-elevating GPCR ligands such as prostaglandin E2, thyroid-stimulating hormone, and catecholamine have been shown to upregulate Il6 mRNA expression in different cell lines (Mohamed-Ali et al. 2001, Liu et al. 2005, Antunes et al. 2006), it is reasonable to hypothesize that melanocortins activate Il6 production in adipocytes. The cAMP signal transduction pathways can activate the exchange protein directly activated by cAMP (EPAC), which acts independently of PKA (Holz et al. 2006). However, a specific activator of EPAC (8-CPT-2′OMe-cAMP) did not induce IL6 secretion in adipocytes (data not shown), whereas a specific activator of PKA (6-Bnz-cAMP) potentiated α-MSH-induced Il6 expression (Fig. 3D). These data suggest that the PKA, but not the EPAC, pathway is involved in the melanocortin-induced Il6 production in 3T3-L1 adipocytes.
In this study, we have described a novel pro-inflammatory role for MCRs in adipocytes. IKK/NFκB activation and JNK pathway are critical regulators of inflammation, and have been reported to be necessary for IL6 induction in many cells (Libermann & Baltimore 1990, Tuyt et al. 1999, An et al. 2003). The induction of IL6 has been reported. The classical pathway of NFκB activation is controlled by the IKK complex. Activated IKK phosphorylates IκB, which is then ubiquitinated and rapidly degraded, allowing NFκB to translocate from the cytoplasm to the nucleus, where it activates gene transcription (Niederberger & Geisslinger 2008). While the activation of the MCRs expressed in immune cells has been reported to have an anti-inflammatory effect, inhibiting NFκB activation and cytokine production induced by pro-inflammatory stimuli such as LPS (Yoon et al. 2003), our results nonetheless demonstrated that the activation of MCRs in the absence of another pro-inflammatory stimulus leads to the upregulation of Il6 mRNA and protein levels in adipocytes. JNK activation is also involved in the expression of IL6 in human monocytes (Tuyt et al. 1999), but α-MSH-mediated JNK activation suppresses Il6 production in adipocytes as shown in Figs 3–5. It seems likely that adipocytes do not share the same regulatory pathways that have been identified in other cell types, such as immune cells. Moreover, recent studies on the IL6 promoter have demonstrated that induction of IL6 by several transcription factors including C/EBPα, AP1, CREB, and NFκB occurs in a highly stimulus- or cell-specific manner (Fasshauer et al. 2003, Persson et al. 2005, Chen et al. 2006).
Since adipose tissue has recently been regarded as a fast-acting endocrine organ within the hierarchy of the hypothalamus and pituitary gland and because adipose tissue expresses specific receptors for pituitary hormones and hypothalamic releasing factors at both mRNA and protein levels (Schaffler et al. 2006), our findings provide further evidence of a significant interaction between adipocytokines and neuropeptides such as α-MSH and ACTH. Our findings provide evidence for the existence of a hypothalamic–pituitary–adipose axis, and for the concept of ‘adipotropins’, which describe the roles of pituitary and hypothalamic hormones or releasing factors which directly target adipocytes via their specific receptors (Schaffler et al. 2006).
We have measured Il6 expression level after injection of 1 mg/kg of α-MSH. The concentration which we used is about 5 μM per mouse approximately based on the blood volume which is about 6–8% of body weight. In most cases, the range from 200 μg/kg to 10 mg/kg for the concentration of α-MSH is commonly used. The i.v. injection of α-MSH increased Il6 production as shown in Fig. 7. To confirm this, we administered an abdominal injection of α-MSH of 1 mg/kg, and showed similar results (data not shown). This result demonstrates that α-MSH plays an important role in inducing Il6 expression in vivo.
The physiological relevance of melanocortin-induced Il6 production in adipose tissue remains unclear. However, pituitary melanocortins appear to have stress-related effects on adipocyte function. Brain-derived α-MSH is released during stress including immobilization stress (Khorram et al. 1985), and the melanocortinergic pathway is rapidly recruited under conditions of emotional stress (Liu et al. 2007). In addition, it has been suggested that in the case of ACTH- or α-MSH-induced lipolysis in adipocytes, stress-induced lipolysis is mediated by pituitary release of melanocortins (Schaffler et al. 2006). Recently, human visceral and subcutaneous adipocytes have also been shown to express the corticotropin-releasing hormone receptor types 1 and 2, which play a major role in coordinating autonomic, cardiovascular, endocrine, and behavioral responses to stress (Seres et al. 2004). These data imply that adipose tissue may be a direct peripheral target organ of stress responses. On the other hand, recent studies have demonstrated that the production of IL6 can be directly stimulated by emotional depression and stressful experiences (Kiecolt-Glaser et al. 2003). In addition, circulating IL6 levels and secretion of IL6 from adipose tissue correlate with insulin resistance (Kristiansen & Mandrup-Poulsen 2005). In this sense, our data suggest that IL6 could be a link between obesity, stress response, and insulin resistance.
To elucidate melanocortin's direct peripheral effects on adipocytes under conditions of stress, in vivo experiments addressing the role of melanocortin in the relationship between stress responses and insulin sensitivity are needed.
In summary, these studies provide direct evidence for peripheral action of melanocortins on Il6 expression and production in adipocytes, and suggest that melanocortins may function as regulators of inflammation by regulating cytokine production. This is the first time that we find melanocortin-mediated Il6 gene expression through adipocyte membrane receptors and signaling pathways through a knockdown experiment using siRNA and in vivo. In addition, our findings provide evidence for the existence of a regulatory loop between the HPA axis and circulating Il6.
Declaration of interest
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
Funding
This work was supported by the National Research Foundation of Korea (NRF) grants (No. 20090063547 and No. 20090081464), Korea Science and Engineering Foundation (KOSEF) grants (No. M10641000077-08N4100-07710), Regional Core Research Program/Anti-aging and Well-being Research Center and Brain Korea 21 program funded by the Korean Ministry of Education, Science and Technology.
References
- Ajuwon KM, Spurlock ME. Palmitate activates the NF-κB transcription factor and induces IL-6 and TNFalpha expression in 3T3-L1 adipocytes. Journal of Nutrition. 2005;135:1841–1846. doi: 10.1093/jn/135.8.1841. [DOI] [PubMed] [Google Scholar]
- An J, Sun Y, Sun R, Rettig MB. Kaposi's sarcoma-associated herpesvirus encoded vFLIP induces cellular IL-6 expression: the role of the NF-κB and JNK/AP1 pathways. Oncogene. 2003;22:3371–3385. doi: 10.1038/sj.onc.1206407. [DOI] [PubMed] [Google Scholar]
- An JJ, Rhee Y, Kim SH, Kim DM, Han DH, Hwang JH, Jin YJ, Cha BS, Baik JH, Lee WT, et al. Peripheral effect of alpha-melanocyte-stimulating hormone on fatty acid oxidation in skeletal muscle. Journal of Biological Chemistry. 2007;282:2862–2870. doi: 10.1074/jbc.M603454200. [DOI] [PubMed] [Google Scholar]
- Antunes TT, Gagnon A, Chen B, Pacini F, Smith TJ, Sorisky A. Interleukin-6 release from human abdominal adipose cells is regulated by thyroid-stimulating hormone: effect of adipocyte differentiation and anatomic depot. American Journal of Physiology. Endocrinology and Metabolism. 2006;290:E1140–E1144. doi: 10.1152/ajpendo.00516.2005. [DOI] [PubMed] [Google Scholar]
- Antunes TT, Gagnon A, Langille ML, Sorisky A. Thyroid-stimulating hormone induces interleukin-6 release from human adipocytes through activation of the nuclear factor-κB pathway. Endocrinology. 2008;149:3062–3066. doi: 10.1210/en.2007-1588. [DOI] [PubMed] [Google Scholar]
- Bohm M, Luger TA, Tobin DJ, Garcia-Borron JC. Melanocortin receptor ligands: new horizons for skin biology and clinical dermatology. Journal of Investigative Dermatology. 2006;126:1966–1975. doi: 10.1038/sj.jid.5700421. [DOI] [PubMed] [Google Scholar]
- Boston BA. The role of melanocortins in adipocyte function. Annals of the New York Academy of Sciences. 1999;885:75–84. doi: 10.1111/j.1749-6632.1999.tb08666.x. [DOI] [PubMed] [Google Scholar]
- Brown BL, Albano JD, Ekins RP, Sgherzi AM. A simple and sensitive saturation assay method for the measurement of adenosine 3′:5′-cyclic monophosphate. Biochemical Journal. 1971;121:561–562. doi: 10.1042/bj1210561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler AA, Marks DL, Fan W, Kuhn CM, Bartolome M, Cone RD. Melanocortin-4 receptor is required for acute homeostatic responses to increased dietary fat. Nature Neuroscience. 2001;4:605–611. doi: 10.1038/88423. [DOI] [PubMed] [Google Scholar]
- Chen W, Kelly MA, Opitz-Araya X, Thomas RE, Low MJ, Cone RD. Exocrine gland dysfunction in MC5-R-deficient mice: evidence for coordinated regulation of exocrine gland function by melanocortin peptides. Cell. 1997;91:789–798. doi: 10.1016/s0092-8674(00)80467-5. [DOI] [PubMed] [Google Scholar]
- Chen BC, Liao CC, Hsu MJ, Liao YT, Lin CC, Sheu JR, Lin CH. Peptidoglycan-induced IL-6 production in RAW 264.7 macrophages is mediated by cyclooxygenase-2, PGE2/PGE4 receptors, protein kinase A, I kappa B kinase, and NF-κB. Journal of Immunology. 2006;177:681–693. doi: 10.4049/jimmunol.177.1.681. [DOI] [PubMed] [Google Scholar]
- Cone RD. Studies on the physiological functions of the melanocortin system. Endocrine Reviews. 2006;27:736–749. doi: 10.1210/er.2006-0034. [DOI] [PubMed] [Google Scholar]
- Diya Z, Lili C, Shenglai L, Zhiyuan G, Jie Y. Lipopolysaccharide (LPS) of Porphyromonas gingivalis induces IL-1beta, TNF-alpha and IL-6 production by THP-1 cells in a way different from that of Escherichia coli LPS. Innate Immunity. 2008;14:99–107. doi: 10.1177/1753425907088244. [DOI] [PubMed] [Google Scholar]
- Fang X, Yu S, Bast RC, Liu S, Xu HJ, Hu SX, LaPushin R, Claret FX, Aggarwal BB, Lu Y, et al. Mechanisms for lysophosphatidic acid-induced cytokine production in ovarian cancer cells. Journal of Biological Chemistry. 2004;279:9653–9661. doi: 10.1074/jbc.M306662200. [DOI] [PubMed] [Google Scholar]
- Fasshauer M, Kralisch S, Klier M, Lossner U, Bluher M, Klein J, Paschke R. Adiponectin gene expression and secretion is inhibited by interleukin-6 in 3T3-L1 adipocytes. Biochemical and Biophysical Research Communications. 2003;301:1045–1050. doi: 10.1016/s0006-291x(03)00090-1. [DOI] [PubMed] [Google Scholar]
- Fernandez-Real JM, Ricart W. Insulin resistance and chronic cardiovascular inflammatory syndrome. Endocrine Reviews. 2003;24:278–301. doi: 10.1210/er.2002-0010. [DOI] [PubMed] [Google Scholar]
- Franchimont N, Durant D, Rydziel S, Canalis E. Platelet-derived growth factor induces interleukin-6 transcription in osteoblasts through the activator protein-1 complex and activating transcription factor-2. Journal of Biological Chemistry. 1999;274:6783–6789. doi: 10.1074/jbc.274.10.6783. [DOI] [PubMed] [Google Scholar]
- Fried SK, Bunkin DA, Greenberg AS. Omental and subcutaneous adipose tissues of obese subjects release interleukin-6: depot difference and regulation by glucocorticoid. Journal of Clinical Endocrinology and Metabolism. 1998;83:847–850. doi: 10.1210/jcem.83.3.4660. [DOI] [PubMed] [Google Scholar]
- Gohil BC, Rosenblum LA, Coplan JD, Kral JG. Hypothalamic–pituitary–adrenal axis function and the metabolic syndrome X of obesity. CNS Spectrums. 2001;6:581–586. doi: 10.1017/s1092852900002121. (589) [DOI] [PubMed] [Google Scholar]
- Harmer SC, Pepper DJ, Cooke K, Bennett HP, Bicknell AB. Evidence of a possible role for Lys-gamma3-MSH in the regulation of adipocyte function. Journal of Endocrinology. 2008;196:149–158. doi: 10.1677/JOE-07-0391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoch M, Hirzel E, Lindinger P, Eberle AN, Linscheid P, Martin I, Peters T, Peterli R. Weak functional coupling of the melanocortin-1 receptor expressed in human adipocytes. Journal of Receptors and Signal Transduction. 2008;28:485–504. doi: 10.1080/10799890802442622. [DOI] [PubMed] [Google Scholar]
- Hoggard N, Hunter L, Duncan JS, Rayner DV. Regulation of adipose tissue leptin secretion by alpha-melanocyte-stimulating hormone and agouti-related protein: further evidence of an interaction between leptin and the melanocortin signalling system. Journal of Molecular Endocrinology. 2004;32:145–153. doi: 10.1677/jme.0.0320145. [DOI] [PubMed] [Google Scholar]
- Holz GG, Kang G, Harbeck M, Roe MW, Chepurny OG. Cell physiology of cAMP sensor Epac. Journal of Physiology. 2006;577:5–15. doi: 10.1113/jphysiol.2006.119644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwen KA, Senyaman O, Schwartz A, Drenckhan M, Meier B, Hadaschik D, Klein J. Melanocortin crosstalk with adipose functions: ACTH directly induces insulin resistance, promotes a pro-inflammatory adipokine profile and stimulates UCP-1 in adipocytes. Journal of Endocrinology. 2008;196:465–472. doi: 10.1677/JOE-07-0299. [DOI] [PubMed] [Google Scholar]
- Jun DJ, Lee JH, Choi BH, Koh TK, Ha DC, Jeong MW, Kim KT. Sphingosine-1-phosphate modulates both lipolysis and leptin production in differentiated rat white adipocytes. Endocrinology. 2006;147:5835–5844. doi: 10.1210/en.2006-0579. [DOI] [PubMed] [Google Scholar]
- Kamimura D, Ishihara K, Hirano T. IL-6 signal transduction and its physiological roles: the signal orchestration model. Reviews of Physiology, Biochemistry and Pharmacology. 2003;149:1–38. doi: 10.1007/s10254-003-0012-2. [DOI] [PubMed] [Google Scholar]
- Kaufman D, Banerji MA, Shorman I, Smith EL, Coplan JD, Rosenblum LA, Kral JG. Early-life stress and the development of obesity and insulin resistance in juvenile bonnet macaques. Diabetes. 2007;56:1382–1386. doi: 10.2337/db06-1409. [DOI] [PubMed] [Google Scholar]
- Khorram O, Bedran de Castro JC, McCann SM. Stress-induced secretion of alpha-melanocyte-stimulating hormone and its physiological role in modulating the secretion of prolactin and luteinizing hormone in the female rat. Endocrinology. 1985;117:2483–2489. doi: 10.1210/endo-117-6-2483. [DOI] [PubMed] [Google Scholar]
- Kiecolt-Glaser JK, Preacher KJ, MacCallum RC, Atkinson C, Malarkey WB, Glaser R. Chronic stress and age-related increases in the proinflammatory cytokine IL-6. PNAS. 2003;100:9090–9095. doi: 10.1073/pnas.1531903100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kristiansen OP, Mandrup-Poulsen T. Interleukin-6 and diabetes: the good, the bad, or the indifferent? Diabetes. 2005;54:S114–S124. doi: 10.2337/diabetes.54.suppl_2.s114. [DOI] [PubMed] [Google Scholar]
- Lagathu C, Bastard JP, Auclair M, Maachi M, Capeau J, Caron M. Chronic interleukin-6 (IL-6) treatment increased IL-6 secretion and induced insulin resistance in adipocyte: prevention by rosiglitazone. Biochemical and Biophysical Research Communications. 2003;311:372–379. doi: 10.1016/j.bbrc.2003.10.013. [DOI] [PubMed] [Google Scholar]
- Legrand-Poels S, Schoonbroodt S, Piette J. Regulation of interleukin-6 gene expression by pro-inflammatory cytokines in a colon cancer cell line. Biochemical Journal. 2000;349:765–773. doi: 10.1042/bj3490765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Libermann TA, Baltimore D. Activation of interleukin-6 gene expression through the NF-κB transcription factor. Molecular and Cellular Biology. 1990;10:2327–2334. doi: 10.1128/mcb.10.5.2327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu XH, Kirschenbaum A, Yao S, Levine AC. Cross-talk between the interleukin-6 and prostaglandin E(2) signaling systems results in enhancement of osteoclastogenesis through effects on the osteoprotegerin/receptor activator of nuclear factor-κB (RANK) ligand/RANK system. Endocrinology. 2005;146:1991–1998. doi: 10.1210/en.2004-1167. [DOI] [PubMed] [Google Scholar]
- Liu J, Garza JC, Truong HV, Henschel J, Zhang W, Lu XY. The melanocortinergic pathway is rapidly recruited by emotional stress and contributes to stress-induced anorexia and anxiety-like behavior. Endocrinology. 2007;148:5531–5540. doi: 10.1210/en.2007-0745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Mohamed-Ali V, Goodrick S, Rawesh A, Katz DR, Miles JM, Yudkin JS, Klein S, Coppack SW. Subcutaneous adipose tissue releases interleukin-6, but not tumor necrosis factor-alpha, in vivo. Journal of Clinical Endocrinology and Metabolism. 1997;82:4196–4200. doi: 10.1210/jcem.82.12.4450. [DOI] [PubMed] [Google Scholar]
- Mohamed-Ali V, Flower L, Sethi J, Hotamisligil G, Gray R, Humphries SE, York DA, Pinkney J. β-Adrenergic regulation of IL-6 release from adipose tissue: in vivo and in vitro studies. Journal of Clinical Endocrinology and Metabolism. 2001;86:5864–5869. doi: 10.1210/jcem.86.12.8104. [DOI] [PubMed] [Google Scholar]
- Niederberger E, Geisslinger G. The IKK-NF-κB pathway: a source for novel molecular drug targets in pain therapy? FASEB Journal. 2008;22:3432–3442. doi: 10.1096/fj.08-109355. [DOI] [PubMed] [Google Scholar]
- Norman D, Isidori AM, Frajese V, Caprio M, Chew SL, Grossman AB, Clark AJ, MichaelBesser G, Fabbri A. ACTH and alpha-MSH inhibit leptin expression and secretion in 3T3-L1 adipocytes: model for a central-peripheral melanocortin-leptin pathway. Molecular and Cellular Endocrinology. 2003;200:99–109. doi: 10.1016/s0303-7207(02)00410-0. [DOI] [PubMed] [Google Scholar]
- Persson E, Voznesensky OS, Huang YF, Lerner UH. Increased expression of interleukin-6 by vasoactive intestinal peptide is associated with regulation of CREB, AP-1 and C/EBP, but not NF-κB, in mouse calvarial osteoblasts. Bone. 2005;37:513–529. doi: 10.1016/j.bone.2005.04.043. [DOI] [PubMed] [Google Scholar]
- Rotter V, Nagaev I, Smith U. Interleukin-6 (IL-6) induces insulin resistance in 3T3-L1 adipocytes and is, like IL-8 and tumor necrosis factor-alpha, overexpressed in human fat cells from insulin-resistant subjects. Journal of Biological Chemistry. 2003;278:45777–45784. doi: 10.1074/jbc.M301977200. [DOI] [PubMed] [Google Scholar]
- Saha RN, Jana M, Pahan K. MAPK p38 regulates transcriptional activity of NF-κB in primary human astrocytes via acetylation of p65. Journal of Immunology. 2007;179:7101–7109. doi: 10.4049/jimmunol.179.10.7101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaffler A, Scholmerich J, Buechler C. The role of ‘adipotropins’ and the clinical importance of a potential hypothalamic–pituitary–adipose axis. Nature Clinical Practice. Endocrinology and Metabolism. 2006;2:374–383. doi: 10.1038/ncpendmet0197. [DOI] [PubMed] [Google Scholar]
- Seres J, Bornstein SR, Seres P, Willenberg HS, Schulte KM, Scherbaum WA, Ehrhart-Bornstein M. Corticotropin-releasing hormone system in human adipose tissue. Journal of Clinical Endocrinology and Metabolism. 2004;89:965–970. doi: 10.1210/jc.2003-031299. [DOI] [PubMed] [Google Scholar]
- Shi H, Tzameli I, Bjorbaek C, Flier JS. Suppressor of cytokine signaling 3 is a physiological regulator of adipocyte insulin signaling. Journal of Biological Chemistry. 2004;279:34733–34740. doi: 10.1074/jbc.M403886200. [DOI] [PubMed] [Google Scholar]
- Tan KS, Nackley AG, Satterfield K, Maixner W, Diatchenko L, Flood PM. Beta2 adrenergic receptor activation stimulates pro-inflammatory cytokine production in macrophages via PKA- and NF-κB-independent mechanisms. Cellular Signalling. 2007;19:251–260. doi: 10.1016/j.cellsig.2006.06.007. [DOI] [PubMed] [Google Scholar]
- Tang CH, Chiu YC, Tan TW, Yang RS, Fu WM. Adiponectin enhances IL-6 production in human synovial fibroblast via an AdipoR1 receptor, AMPK, p38, and NF-κB pathway. Journal of Immunology. 2007;179:5483–5492. doi: 10.4049/jimmunol.179.8.5483. [DOI] [PubMed] [Google Scholar]
- Tuyt LM, Dokter WH, Birkenkamp K, Koopmans SB, Lummen C, Kruijer W, Vellenga E. Extracellular-regulated kinase 1/2, Jun N-terminal kinase, and c-Jun are involved in NF-κB-dependent IL-6 expression in human monocytes. Journal of Immunology. 1999;162:4893–4902. [PubMed] [Google Scholar]
- Verhasselt V, Buelens C, Willems F, De Groote D, Haeffner-Cavaillon N, Goldman M. Bacterial lipopolysaccharide stimulates the production of cytokines and the expression of costimulatory molecules by human peripheral blood dendritic cells: evidence for a soluble CD14-dependent pathway. Journal of Immunology. 1997;158:2919–2925. [PubMed] [Google Scholar]
- Wikberg JE, Mutulis F. Targeting melanocortin receptors: an approach to treat weight disorders and sexual dysfunction. Nature Reviews. Drug Discovery. 2008;7:307–323. doi: 10.1038/nrd2331. [DOI] [PubMed] [Google Scholar]
- Yang YK, Harmon CM. Recent developments in our understanding of melanocortin system in the regulation of food intake. Obesity Reviews. 2003;4:239–248. doi: 10.1046/j.1467-789x.2003.00104.x. [DOI] [PubMed] [Google Scholar]
- Yoon SW, Goh SH, Chun JS, Cho EW, Lee MK, Kim KL, Kim JJ, Kim CJ, Poo H. α-Melanocyte-stimulating hormone inhibits lipopolysaccharide-induced tumor necrosis factor-alpha production in leukocytes by modulating protein kinase A, p38 kinase, and nuclear factor κB signaling pathways. Journal of Biological Chemistry. 2003;278:32914–32920. doi: 10.1074/jbc.M302444200. [DOI] [PubMed] [Google Scholar]
- Zhao W, Liu M, Kirkwood KL. p38alpha stabilizes interleukin-6 mRNA via multiple AU-rich elements. Journal of Biological Chemistry. 2008;283:1778–1785. doi: 10.1074/jbc.M707573200. [DOI] [PMC free article] [PubMed] [Google Scholar]