Abstract
In recent years increasing evidence is pointing toward white matter abnormalities in schizophrenia and other psychiatric disorders. The present paper will provide an overview over the role of myelin in cognition and brain function, and its potential involvement in brain disorders. Furthermore, we will examine one particular experimental model for the study of dysmyelination, created by the administration of the toxin cuprizone. Cuprizone, a copper chelator, causes white matter abnormalities in rodents. The administration of cuprizone during specific developmental periods allows for the targeting of specific brain areas for dysmyelination. Thus, cuprizone can be used to study the pathogenesis and pathophysiology of myelin deficiencies in the central nervous system, and its effect on behaviors relevant to psychiatric disorders.
Keywords: schizophrenia, white matter, myelin, oligodendrocytes, cuprizone, copper, prefrontal cortex
2. INTRODUCTION
Although we tend to believe that intellectual aptitude and brain activity are a consequence of neuronal function, this assumption reflects only a part of the narrative. Non-neuronal cells, and in particular glia, are more than just ‘glue’ that keeps the neurons together, as their name would make us believe (glia = Greek for ‘glue’)(1). Here we will focus on one particular type of glia, oligodendrocytes, and their role in schizophrenia and other psychiatric disorders.
The main task of oligodendrocytes is to form myelin, a specialized membrane that is used to insulate axons. Myelinated axons facilitate neuronal performance by increasing the speed of neuronal conductance. As will be outlined below, a strong correlation has been observed between strength of white matter within a brain area and performance on tasks specific for that brain area. Since white matter is composed of myelinated axons, such findings emphasize the role of myelination in cognitive aptitude.
Cognitive deficits are observed in psychiatric disorders such as schizophrenia, which in itself might not be a strong argument for an involvement of myelin. However, certain characteristics of schizophrenia, such as a parallel between age of onset of the disorder and timing of myelination, together with a proposed ‘disconnectivity’, have made myelination a compelling target for study in the disorder. Thus, a number of schizophrenia studies have found increased neuronal density in the context of smaller brain volumes and unchanged total neuron numbers, indicating a reduction in neuropil (i.e. myelinated axons); postmortem studies have shown pathological changes in oligodendrocytes; and diffusion tensor imaging (DTI) studies have shown alterations in fiber bundles. Here we will illustrate findings of myelin abnormalities in schizophrenia and other psychiatric disorders. Moreover, we will take another look at an old hypothesis of schizophrenia, disturbances in copper metabolism, and the potential relationship to myelination. Finally, recent results in rodent studies with the copper chelator cuprizone, and the potential of this model to study the pathophysiology of myelin deficits and their role in schizophrenia-relevant behavioral traits, will be discussed.
3. STRUCTURE AND FUNCTION OF OLIGODENDROCYTES
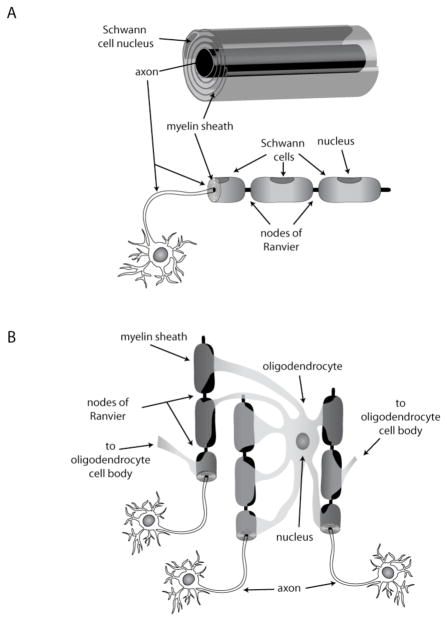
Oligodendrocytes, the cellular units of oligodendroglia, insulate neuronal axons in the central nervous system by wrapping them with a multilamellar membrane. Unlike their counterparts in the peripheral nervous system, the Schwann cells (figure 1A), oligodendrocytes ensheath up to 60 different axons (2), (Figure 1B). The myelin segments along the axons are interspersed with small gaps, the nodes of Ranvier, where sodium channels are gathered in high density and spatially separated from potassium channels at the paranodal region (3–6). Membrane depolarization can only occur at the nodes of Ranvier, leading to saltatory (‘jumping’) conductance (7). This setup enables the axon to propagate electrical signals more rapidly and efficiently, to minimize diffusion and to avoid non-specific cross over of signals to unrelated neuronal circuits.
Figure 1. Myelin-producing cells.
Schwann cells in the peripheral nervous system (PNS) are individually wrapped around axon segments, A. In the CNS, oligodendrocytes provide numerous myelin segments to a number of different axons, B.
4. MYELIN: A MAJOR PLAYER IN COGNITION AND INTELLECTUAL PERFORMANCE
The quality of cognitive processing is dependent upon effective communication between many different brain areas. Sensory stimuli are compared against each other, crosschecked with past experiences and responses determined by cognitive and emotional modalities. Myelination influences processing speed and strength and plays a vital role in signal propagation. Thus it is not surprising that myelination of brain regions during development corresponds to the progression of specific cognitive functions (8, 9). In typically developing adolescents, white matter coherence correlates with visuospatial, psychomotor, and language skills (10). Prefrontal cortex (PFC) development and myelination correlate with the development of cognitive functions such as executive decision-making and impulse control (11). High verbal intellectual abilities are accompanied by heightened white matter development in late childhood in corresponding brain regions (12). In the adult brain, white matter structure has been correlated with IQ, working memory, attention, aptitude in reading, and musical talent (8, 13–20). Training and experience are capable of changing white matter; for example, the number of hours spent practicing the piano correlates with the organization of fiber tracts in regions associated with musical ability (18). Similarly, training of working memory leads to white matter changes and facilitates connectivity in the corpus callosum, the area that connects both cerebral hemispheres, and other brain areas (21). The inverse is also true; severely neglected children have a significantly smaller corpus callosum (22). Taken together, white matter plays an important role in learning, memory and overall cognitive abilities.
5. EVOLUTION AND ONTOGENY OF MYELIN
5.1. Evolution of myelin
Human oligodendrocytes and myelin sheaths have their developmental origin in vertebrates (23). However, a strong evolutionary pressure toward myelination resulted in the independent appearance of myelin several times in evolution (23–25). All myelin, regardless of species, provides multilamellar membrane wrapping and promotes ‘saltatory’ conductance (24, 25).
5.2. Embryonic development of oligodendrocytes and oligodendrocyte regeneration in the adult brain
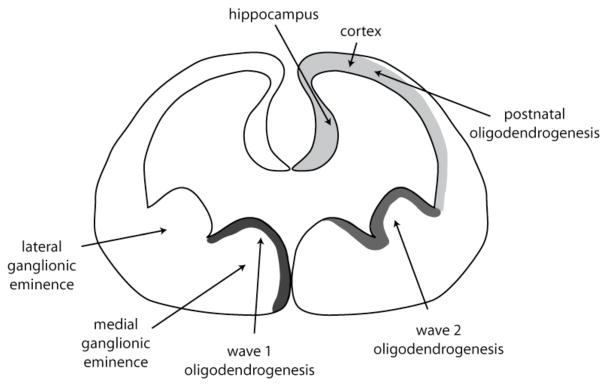
Oligodendrocytes are the last cell type to arise and develop in any brain area, following the generation of neurons and astrocytes. During early rodent brain development precursors of the oligodendrocyte lineage arise predominantly in the ventral ventricular zone of the neural tube, though there is also recent indication for some dorsal sources (26, 27). Oligodendrocytes in the cortex originate from three sources that are sequentially triggered: the medial ganglionic eminence and lateral ganglionic eminence in the embryonic brain, followed by derivation from oligodendrocyte progenitors (OPCs) located directly in the cortex (figure 2). As the forebrain develops, cells of the oligodendrocyte lineage start to appear in the neuroepithelium of the medial ganglionic eminence, from where they migrate into the forebrain (27). A second wave of oligodendrocyte precursors is derived from the lateral ganglionic eminence. Around birth and thereafter, postnatal and adult OPCs located in the cortex start to generate oligodendrocytes. These OPCs can be activated throughout the organism’s lifetime in response to brain injury and pathological conditions (28, 29). In contrast, the corpus callosum, striatum and fimbria fornix receive migrating OPCs from the subventricular zone in adulthood (30).
Figure 2. Developmental origin of oligodendrocytes.
During embryogenesis in the mouse, oligodendrocytes are first generated in the medial ganglionic eminence, followed by generation in the lateral ganglionic eminence. From there, oligodendrocytes migrate into other brain areas. Around birth and thereafter, OPCs in the cortex and the hippocampus generate oligodendrocytes as needed.
The majority of CNS myelination occurs postnatally and continues into adulthood. For example, a recent longitudinal DTI study in human adolescence showed continued microstructural changes in white matter during late adolescence suggesting ongoing refinement of myelination into adulthood (31). In a similar study it was shown that myelination correlates with the phylogenetic age of brain areas, whereby ‘older’ areas and projections such as the ones to the brainstem are fully myelinated during adolescence, whereas interhemispheric connections and prefrontal brain areas involved in executive control are still getting myelinated in adulthood (32). Such findings, which have been previously shown in postmortem brains using histological staining techniques (33) suggest that executive control of behavior is still immature in adolescence, raising challenging legal questions about extent of criminal culpability in teenagers and potential for rehabilitation (34). It seems that myelination is an ongoing process into early aging, as gray matter is thinning and white matter increasing up to the fifth decade of life (35).
6. DISORDERS OF MYELIN
Traditionally, myelin alterations have been investigated for their role in pathologic conditions such as multiple sclerosis (MS), leukodystrophies, and other diseases with known white matter pathology (36, 37). More recently, subtle changes in white matter have been found in numerous conditions not traditionally associated with myelin. For example, moderate to severe white matter disturbances accompany cognitive decline in aging and in dementia (38–44). Even in healthy individuals white matter abnormalities are associated with cognitive impairment (42, 45). Myelin alterations have also been found in mental illnesses and drug and alcohol addictions (46–49). These observations have led to an examination of co-morbidities between the more classic demyelination disorders and mental illnesses including addiction.
6.1. Typical demyelinating disorders: multiple sclerosis and leukodystrophies
Multiple sclerosis is an inflammatory demyelinating disease of the central nervous system affecting 1 in 1,000 individuals, with age of onset generally in the early to mid twenties (15–45 years) (50). Symptoms and severity vary individually depending on the location of the white matter lesion(s), with periods of remission interspersed with relapses. The defining characteristics of MS are typically motor-related symptoms such as fatigue and weakness, visual loss, loss of balance, muscle spasms, loss of- or slurred speech, and bowel and bladder incontinence. In addition, symptoms such as cognitive impairment and psychosis are also prevalent (51–53). Cognitive impairment has been observed in 50–70% of MS patients (54) including during the initial demyelinating episode (55), and is progressive throughout the disease (56). The specific location of white matter lesions determines cognitive impairment and decline, as well as the occurrence of dementia (57). Individuals with MS tend to have a higher incidence of psychiatric issues including depression, anxiety, bipolar disorder, and substance abuse (58), which is again associated with lesion location.
Leukodystrophies are hereditary, i.e. caused by genetic mutations that affect myelination. Many leukodystrophies are lysosomal storage disorders leading to progressive degeneration of the white matter, frequently accompanied by psychiatric problems. For example, metachromatic leukodystrophy presents in early adulthood with psychosis, disorganized thoughts and delusions, and is frequently diagnosed as schizophrenia (59–62). Myelin loss is particularly pronounced in the frontal lobe, which explains the psychiatric symptoms and may cause dementia in the later stages of the disease. Interestingly, metachromatic leukodystrophy has been termed ‘a valuable model for the study of psychosis’ (63).
Cognitive decline with white matter damage is observed not only in demyelinating and genetic disorders of myelin but also in infectious diseases such as HIV, progressive multifocal leukoencephalopathy, traumatic brain injury, and neoplastic white matter tumors (39).
6.2. White matter abnormalities in schizophrenia
Many psychiatric disorders including obsessive-compulsive disorder, ADHD, depression, psychosis, and bipolar disorder are accompanied by myelin deficits (8). Schizophrenia is perhaps the most investigated psychiatric disorder in regard to white matter abnormalities, with the most consistent findings. The disease is characterized by altered perception of reality, most often in forms of disorganized thought processes and speed, auditory, visual, or paranoid hallucinations, and/or social dysfunction. Like MS, schizophrenia has an early peak in age of onset, typically in the late teens to early twenties, and is accompanied by cognitive deficits (64, 65).
Schizophrenia is a systems-wide disorder with genetic and environmental contributions (66, 67). More than one gene is involved, more than one brain area is affected, and communication between brain areas seems disrupted (68, 69). These features of the disorder are highly compatible with disturbances in myelin, though they do neither elucidate the sequence of events (i.e. primary abnormalities in myelination or secondary adjustment to a pathological event), nor do they exclude that diverse pathological events, including but not limited to myelination, can cause a variety of symptoms collectively referred to as ‘schizophrenia’. Following is a summary of experimental approaches and results that highlight white matter abnormalities in schizophrenia.
6.2.1. Post-mortem and imaging studies show myelin abnormalities in schizophrenia
Reductions in neuropil, first found in post-mortem studies, have long been proposed in schizophrenia (70, 71). These reductions suggest a decrease in dendritic spine density as well as a decrease in myelin (72). Ultrastructural post-mortem studies have demonstrated pathological changes in oligodendrocytes in schizophrenia and bipolar disorder (73). Decreased numbers of oligodendrocytes were found in schizophrenia, bipolar disorder, and in major depression, particularly in layer VI of the PFC (74). Oligodendrocytes were reduced around capillaries in the PFC in schizophrenia, as well as around neurons (75, 76). Immunostaining of oligodendrocytes with 2′,3′-cyclic nucleotide-3′-phosphodiesterase in the superior frontal gyrus yielded similar results (77).
Multiple structural and functional magnetic resonance imaging (fMRI) studies along with DTI studies have demonstrated white matter abnormalities in schizophrenia including reduced density, altered integrity, changes in white matter volume, myelin/axonal disruption and abnormal organization of fiber tracts (8, 78, 79). The alterations in myelin were present among first-episode psychosis patients, under treatment with atypical or typical antipsychotics, and in drug-free patients, indicating that the white matter changes are not solely a result of length of disease or treatment (80–86). However, length of disease does cause a further decline in white matter organization (87).
A vast number of DTI studies have been carried out in the last couple of years in schizophrenic patients. These studies demonstrated an inverse relationship of DTI fractional anisotropy (FA; a measure of white matter health) with positive symptom scores in association fibers (88), as well as a correlation with memory functions (89). A recent study used DTI to differentiate patients with schizophrenia from healthy volunteers with 98% accuracy (90). In monozygotic twins it was shown that anatomical connectivity in medial PFC is heritable (91). In addition, altered medial frontal white matter integrity was found in non-affected relatives of schizophrenic patients. These findings suggest that reduced white matter integrity in medial frontal regions are associated with the genetic liability to schizophrenia, and that myelin abnormalities can be used as an endophenotype (91).
6.2.2. Microarray- and gene expression studies highlight myelin abnormalities in schizophrenia
A microarray study of gene expression levels in the post-mortem dorsolateral PFC of patients with schizophrenia showed that genes expressed in oligodendrocytes were decreased (92). The group of oligodendrocyte-specific genes included myelin associated glycoprotein, myelin basic protein, proteolipid protein, myelin oligodendrocyte glycoprotein, and others. The findings were confirmed in a number of follow-up studies (93–95). A decrease in oligodendrocyte-specific mRNA levels was also observed in other brain areas (96–101) and in mood disorders (93, 102, 103). No difference in the expression levels of oligodendrocyte transcripts was observed between medicated and unmedicated schizophrenic patients, suggesting that these changes are not secondary to treatment with antipsychotic drugs (92, 101).
6.2.3. Factors important for oligodendrocyte development and myelination are known to be affected in schizophrenia
A large number of factors including guidance cues, growth factors and chemokines are necessary to direct OPC migration, proliferation and maturation (2). Small differences in the level or distribution of these factors can presumably have deleterious effects. During development, the number of axons to be myelinated needs to be carefully matched with an appropriate number of oligodendrocytes. After myelination, oligodendrocytes and axons communicate via complex reciprocal interactions. Here we will mention a few of the factors involved in development and communication that might be of particular relevance to schizophrenia. Abnormalities in gene expression patterns will be excluded and are the subject of a chapter below.
6.2.3.1. Electrical activity
Electrical activity stimulates myelination in the CNS, as shown in studies with pharmacological agents that increase or decrease action potential firing (6, 104). Hypoactivity in neuronal circuits, as has been suggested for the PFC in schizophrenia, might thus lead to deficient myelination, creating a vicious cycle of sub-optimal information transfer in response to incomplete myelination (105).
6.2.3.2. Glutamate
Communication between oligodendrocytes and axons is accomplished by a number of neurotransmitters and receptors, with glutamate playing a particularly critical role (106).
Abnormalities in the glutamate system have been indicated in schizophrenia, leading to the ‘glutamate hypothesis’ of schizophrenia (107, 108). These abnormalities, predicting a hypofunction in the glutamate system, will impair the coordination between axons and oligodendrocytes. The careful matching of axon number/axon length and oligodendrocyte number/myelin segments could thus be derailed (106).
6.2.3.3. Neuregulin 1 (NRG1) and ErbB4 (v-erb-a erythroblastic leukemia viral oncogene homolog 4)
NRG belongs to a family of epidermal growth factor-like ligands that interact with ErbB receptor tyrosine kinases. Genetic abnormalities in NRG1 and ErbB4 are among the most consistent findings in schizophrenia (109–112). In the peripheral nervous system (PNS), NRG1 is a critical axonal signal that controls Schwann cell development and myelin sheath thickness (113, 114). Levels of NRG1 type III are a key instructive signal that determines the ensheathment fate of axons (115). Similar functions in the CNS are presumed but not proven to date.
6.2.3.4. Disrupted in schizophrenia (Disc1)
DISC1 was first described as a DNA breakpoint in a large Scottish pedigree with high incidence of schizophrenia, bipolar disorder and other mental illnesses (116, 117). In zebra fish Disc1 is vital for oligodendrocyte development by promoting specification of Olig2-positive cells (118). These defects are comparable to disruption of NRG1 and ErbB signaling. Thus, Disc1 and NRG1 function in common or related pathways to control development of oligodendrocytes. In a rodent model this was further demonstrated that down-regulation of Disc1 results in accelerated differentiation and neuronal integration (28, 119).
6.2.3.5. Reelin (RELN)
A decrease in RELN levels and increased methylation within its promoter region has been associated with schizophrenia in various paradigms (120–123). RELN plays also a role in myelination. It is a secreted extracellular matrix protein which in the PNS regulates Schwann cell-axon interactions (124). In the CNS, RELN is expressed in embryonic and postnatal neurons during periods of neuronal and glial migration (125).
6.2.3.6. Oligodendrocyte lineage transcription factor 2 (OLIG2)
OLIG2 encodes a transcription factor that controls oligodendrocyte development (126). In schizophrenia a significant disease association was found with several markers in the OLIG2 gene (127). Interestingly, an interaction between OLIG2 and ErbB4 was also observed in that sample. A study in Chinese Han schizophrenia patients confirmed an association between schizophrenia and OLIG2 (128), while a study in Japanese patients did not find an association (129).
6.2.3.7. Brain-derived neurotrophic factor (BDNF)
The BDNF gene is a risk factor for schizophrenia, psychosis and mood disorders (130–138), although these findings might not hold up across different populations (139, 140). BDNF knockout mice demonstrated that BDNF plays a role in OPC development in the basal forebrain (141). These findings follow previous studies that demonstrated that BDNF elicits increases in the expression of myelin basic protein in the basal forebrain but not the cortex (142).
6.3. White matter abnormalities in other psychiatric disorders
6.3.1. Mood disorders
Bipolar disorder and major depressive disorder patients have shown myelin abnormalities in postmortem tissue, imaging, and genetic analysis (73, 102, 103, 143, 144). Some of the most consistent findings in bipolar disorder are an increased number of white matter hyperintensities; the underlying mechanism of the hyperintensity is unknown but may be due to demyelination (144). Structural abnormality data have not been consistent with both positive and negative findings, however this most likely reflects subtle changes in white matter structure compared to a global defect in myelin. Most DTI studies in bipolar patients have shown reduced fractional anisotropy and elevated apparent diffusion coefficient in the frontal cortex, corpus callosum, and internal capsule suggesting a loss of connectivity in white matter (143, 144).
6.3.2. Drugs of abuse
Among cocaine addicts, myelin-related genes such as PLP and MOBP are down-regulated in the nucleus accumbens, however genes involved in myelin development such as Olig2 and Sox10 are unaltered (145). Larger white-matter volume and abnormal tract morphology have been observed in chronic methamphetamine users (146–149). The alterations to white matter are present even during the early period of abstinence (150). Cognitive alterations are also present among chronic methamphetamine users including impaired inhibition, information processing, learning and memory, attention and psychomotor speed (151–153). Interestingly, psychiatric symptoms including psychosis, anxiety, suicidal ideation, and hostility are common in methamphetamine-abusing subjects during intoxication and abstinence (154–156).
7. COPPER METABOLISM, THE CUPRIZONE MODEL OF DEMYELINATION, AND THEIR RELEVANCE TO SCHIZOPHRENIA
7.1. Cuprizone and copper
Cuprizone (CPZ; biscyclohexanone oxalyldihydrazone) is a copper (Cu) chelator discovered in the early 1950s (157, 158). CPZ selectively injures oligodendrocytes, but the underlying mechanisms of CPZ-induced demyelination are not well understood. CPZ has been used as a model for MS in mice, but seemed to be without effect in rats. As will be shown below, the latter might not be accurate. Indeed, CPZ can be used to study the effect of oligodendrocyte disruption in the CNS on behaviors relevant for schizophrenia (159).
7.1.1. Role of copper in the CNS
Since CPZ is a Cu chelator, the role of Cu in the CNS deserves a closer look. Copper is an important catalytic and structural cofactor in a wide array of biochemical processes with a narrow range of optimal concentration (160). It has been estimated that 0.2% to 0.5% of the eukaryotic proteome depends on Cu or are involved in Cu management (161). Among the enzymes that use Cu as a co-factor are superoxide dismutase-1 (162), monoamine oxidase (163), dopamine-β-hydroxylase (dopamine beta-monooxygenase, 164), the cytochrome c oxidase family (165), and cytochrome c oxidase assembly protein (166). Excess Cu is highly toxic requiring a close control of the transport, uptake, release and storage of Cu (167, 168).
It is not known if the chelated Cu is inactivated by CPZ or if it is trapped but still reactive. Thus, CPZ toxicity might be caused by an increase in Cu, due to a cellular enrichment of Cu-CPZ, or a decrease in Cu, due to inactivation by CPZ. One hint to the dilemma might be that supplemental administration of Cu failed to reduce CPZ-induced toxicity, pointing to Cu being still active (169). However, a review by a group with a strong commitment to CPZ research claims that Cu levels are reduced after CPZ treatment (170). Because neither data nor a specific reference is provided, it is difficult to further assess this statement.
Taken together, while there is no doubt that CPZ is a Cu chelator that affects oligodendrocyte viability in the mouse (see below), the mechanism of action remains largely unknown.
7.1.2. Genetic disorders of copper metabolism
Genetic disorders that are accompanied by too high or too low Cu levels are well known. Wilson’s disease is characterized by dramatic build-up of intracellular copper with subsequent neurologic abnormalities (171, 172). MRIs of Wilson’s disease patients show abnormalities in extrapyramidal and pyramidal white matter tracts (173). Interestingly, Wilson’s patients have a host of psychiatric manifestations from the affective disorder and schizophrenia spectrum, which often precede the diagnosis of Wilson’s disease (174–176). In a subgroup of these patients the psychiatric symptoms improve with therapies that lower Cu levels, while other patients respond to antipsychotic drugs (174).
Low Cu levels can be equally damaging (177). The X-linked recessive disorder Menkes’ disease is characterized by generalized Cu deficiency (172). Severe neurologic impairment is evident within the first two months after birth and progresses rapidly to decerebration and death (178). Pathological abnormalities include extensive myelin loss (179), which at one point led to the suggestion to group the disorder with leukodystrophies (180). A gene expression microarray study found genes involved in myelination, energy metabolism, and translation to be downregulated (181), and MRI scans showed delayed myelination even in treated patients (182).
7.1.3. Copper levels in schizophrenia
It has long been theorized that excess tissue copper can cause schizophrenia (183). This theory has neither been compellingly demonstrated nor convincingly refuted (184). Most recently, plasma Cu concentrations were shown to be elevated in schizophrenic patients ((185), see also (186) for an earlier reference), and a study of trace metals in scalp hair samples of schizophrenic patients showed an increase in Cu concentrations (187). A caveat of the latter study, pointed out by the investigators, is that most of the patients were poor, middle-aged and divorced. Thus, the alterations might be a consequence nutritional status and socioeconomic factors. On the other hand, a number of studies found no changes in Cu levels. For example, Cu levels in the CSF (188), as well as in post-mortem brains (189) were reported to be normal in schizophrenic patients, and in contrast to the recent study mentioned above (187), a previous study found a reduction in Cu in the hair of schizophrenic patients (190). Treatment with antipsychotic drugs may contribute to the increases in Cu levels (191), although some of the initial studies were carried out prior to the introduction of antipsychotic drugs (183).
At this point, we cannot exclude a genetically predisposing difference in Cu metabolism in schizophrenia, though we are far from a convincing proof. For example, ceruloplasmin, a plasma metalloprotein, carries 90 percent of the plasma Cu (192) and, in the brain, is synthesized and released by glia (predominantly astrocytes, 193, 194). In schizophrenia, ceruloplasmin levels are increased in the CNS (195), and correlated with increased Cu levels (196). One could thus imagine a subset of patients with a genetic predisposition that includes altered ceruloplasmin activity in glia and a higher vulnerability when exposed to Cu. Alternatively, Cu dysregulation, if real, could be an epiphenomenon of nutritional status, disease treatment, or due to secondary pathophysiological mechanisms.
7.2. The cuprizone model of demyelination in the mouse
CPZ has been used in mice to model demyelination and remyelination for MS research (170). The first experiments were performed in the 1960s showing microscopic lesions, edema, astrogliosis, and demyelination along with growth retardation (169). CPZ is typically administered in the chow at concentrations of 0.2 – 0.6% with growth retardation occurring in a dose-dependent manner. Different strains of mice have different degrees of demyelination, which could be an indication that genetic factors influence susceptibility to demyelinating diseases (197, 198), although genetic factors might also influence Cu physiology in this model. The corpus callosum and other major white matter tracts have been predominantly investigated in the CPZ model, but other areas such as the cortex (199), hippocampus (200–202), and cerebellum (203–205) have also shown demyelination. Cuprizone decreases the expression of myelin-specific genes in vivo (206–208) and retards the differentiation of oligodendrocytes in vitro (209). After CPZ treatment in mice, Cu and zinc concentrations increase by over 100% in the brain, with a concomitant decrease in iron (158). During the early stages of exposure to CPZ a decrease in monoamine oxidase and cytochrome c oxidase in the brain and liver of mice is observed (210) along with the development of mega-mitochondria in the liver (211).
Removal of CPZ from the chow allows for remyelination within four to six weeks after onset of exposure, dependent on CPZ dose and the age of the mice. After extended demyelination over 12 weeks, remyelination is either negligible or delayed over weeks of recovery (212, 213). The demyelination-remyelination aspect of the mouse CPZ model is beneficial for the study of the relapsing characteristic of MS.
Not surprisingly, motor deficits are common in mice during CPZ exposure and after withdrawal. These included reduced performance in the rotarod (214) and in wheel running (215, 216). Some open field studies have shown increased exploration in the center together with increased rearing, indicating decreased anxiety (214). However, this has not been repeated in other studies (217).
Of significance to schizophrenia, CPZ impairs spatial working memory in mice, which can be reversed by the antipsychotic drug quetiapine (218). Prepulse inhibition (PPI), a measure of sensory gating known to be disturbed in schizophrenia (219), is also altered in CPZ treated mice (217, 220). Cuprizone-exposed mice display diminished social interaction, another symptom of schizophrenia (221), more time in open arms of the elevated plus maze, and decreased spontaneous alterations in the Y-maze (217).
The timing of CPZ exposure and the age of mice correlate with the severity of the cognitive deficits. Mice exposed to CPZ at an early age (postnatal day 29–56) display working memory deficits immediately after CPZ treatment and after remyelination, whereas mice exposed at a later age (postnatal day 57–84) display working memory deficits only immediately following CPZ exposure (222). This could indicate that an environmental impact affecting oligodendrocyte development might have long-lasting consequences if happening during childhood or early adolescence, whereas the same impact later in life is of lesser significance.
Many of the behavioral changes can be reversed with antipsychotic drugs co-administered with CPZ exposure (217, 220). Mice with co-administered antipsychotic drugs (haloperidol, clozapine, or quetiapine) do not display the PPI deficits, decreased spontaneous alteration in Y maze, or decreased social interaction exhibited by CPZ-only exposed mice. Moreover, the white matter damage induced by CPZ is attenuated in PFC mice given clozapine or haloperidol and in the hippocampus in mice given clozapine or quetiapine. Overall, the CPZ model in mice is a strong indicator that white matter disturbances can cause behavioral deficits similar to the ones observed in schizophrenia, and that these deficits can be reversed with antipsychotic drugs. The caveat in this model might be the motor complications, but these complications can be overcome with careful titration of CPZ.
7.3. The cuprizone model of demyelination in the rat
Although CPZ works well as a demyelinating agent in mice and is used primarily for studies of MS, it does not seem to be useful as an MS model in rats. Whereas one study showed demyelination in young rats at a high concentration of CPZ (223), other studies did not observe demyelination or decreases in oligodendrocyte numbers (170, 224, 225). We were interested in developing a rat model of demyelination in the CNS that leads to oligodendrocyte disturbances in the CNS without triggering motor symptoms. We chose rats since we were interested in the PFC and PFC-mediated behaviors, and rats have an anatomically well-described PFC with established PFC-mediated behaviors (226–228). Given that CPZ was not an obvious MS trigger in rats, we decided to examine it for possible use as a mildly demyelinating agent in the CNS.
Decreased cognitive ability is recognized as a core feature of schizophrenia (229). Cognitive function is a critical determinant for quality of life of schizophrenic patients and long-term outcome of the disease (230–232). Many of the cognitive deficits are mapped to the PFC with schizophrenic patients performing poorly on the Wisconsin Card Sorting Test (WCST), a test dependent on the PFC (233). Since oligodendrocyte pathology is a well-described feature in schizophrenia (see above), we sought to devise an experiment in which to test if oligodendrocyte deficits in the PFC can explain some of the behavioral deficits observed in schizophrenia.
Based on the human data that show that the PFC is fully myelinated only in the late teens to early twenties, we started low-dose (0.2%) CPZ exposure beginning on postnatal day 29, during the juvenile period in the rat. Our hypothesis was that the oligodendrocytes in this final stage of development in the PFC would be more vulnerable than those that had fully matured in other brain regions. Indeed, rats exposed to moderate concentrations of CPZ during adolescents had a decrease in mRNA transcripts and protein levels of oligodendrocyte-specific genes in the PFC (159), similar to what has been observed in schizophrenia and bipolar disorder in humans. Levels of myelin-related genes were not affected in the striatum and hippocampus, two brain areas that should have been completely myelinated before the age of CPZ exposure. The alterations in myelin-related genes were present in the PFC after two and four weeks of CPZ exposure. In addition to the altered myelin genes, glial fibrillary acidic protein (GFAP) was upregulated in the PFC, indicating an activation of astrocytes.
The behavior of CPZ-treated rats was investigated in the attentional set-shifting task (ASST), a modified version of the WCST which reveals impairments in schizophrenia (233, 234). One phase of the ASST, the extra dimensional shift, is impaired by bilateral lesions of the medial PFC and could thus reveal a decline in PFC function (228, 235). Rats treated for two weeks with CPZ demonstrated an increased difficulty to shift attention from one perceptual dimension to another in the extra dimensional shift phase of the ASST (159), while other, arguably more challenging parts of the task, were not affected. The deficit in only the extra dimensional shift phase (shifting strategies) of the task and not during acquisition and reversal-learning indicates specificity for PFC involvement (228). Importantly, CPZ treated rats did not exhibit locomotor problems and had normal weight gain. Thus, the CPZ model in rats can be used to study developmental windows of vulnerability, as well as the pathogenesis and behavioral consequences of dysmyelination.
8. PERSPECTIVES
Overwhelming evidence supports the conclusion that schizophrenia is accompanied by dysmyelination. Little is known about the factors causing myelin deficits in schizophrenia, though it is reasonable to assume that a number of genetic and environmental factors can target different aspects of oligodendrogenesis, axon-oligodendrocyte interaction, and oligodendrocyte viability. The CPZ model of dysmyelination in rodents can help to elucidate windows of vulnerability during brain development and the effects of dysmyelination in particular brain areas on aspects of behavior with relevance to schizophrenia.
Acknowledgments
Supported by Award Number MH74000 from NIMH (CK) and K12GM068543 from NIGMS (NH; PI Dr. Roger Chalkley). The content is solely the responsibility of the authors and does not necessarily represent the official views of the funding institutes or the National Institutes of Health.
Abbreviations
- ASST
attentional set-shifting task
- BDNF
Brain-derived neurotrophic factor
- CPZ
cuprizone
- Cu
copper
- Disc1
Disrupted in schizophrenia
- DTI
diffusion tensor imaging
- ErbB4
v-erb-a erythroblastic leukemia viral oncogene homolog 4
- fMRI
functional magnetic resonance imaging
- GFAP
glial fibrillary acidic protein
- MRI
magnetic resonance imaging
- MS
multiple sclerosis
- NRG1
Neuregulin 1
- OLIG2
Oligodendrocyte lineage transcription factor 2
- OPCs
oligodendrocyte progenitors
- PFC
prefrontal cortex
- PNS
peripheral nervous system
- PPI
prepulse inhibition
- RELN
Reelin
- WCST
Wisconsin Card Sorting Test
References
- 1.Allen NJ, Barres BA. Neuroscience: Glia - more than just brain glue. Nature. 2009;457:675–7. doi: 10.1038/457675a. [DOI] [PubMed] [Google Scholar]
- 2.Miller RH. Regulation of oligodendrocyte development in the vertebrate CNS. Prog Neurobiol. 2002;67:451–67. doi: 10.1016/s0301-0082(02)00058-8. [DOI] [PubMed] [Google Scholar]
- 3.Salzer JL. Nodes of Ranvier come of age. Trends Neurosci. 2002;25:2–5. doi: 10.1016/s0166-2236(00)02006-3. [DOI] [PubMed] [Google Scholar]
- 4.Vabnick I, Shrager P. Ion channel redistribution and function during development of the myelinated axon. J Neurobiol. 1998;37:80–96. [PubMed] [Google Scholar]
- 5.Rasband MN, Trimmer JS. Developmental clustering of ion channels at and near the node of Ranvier. Dev Biol. 2001;236:5–16. doi: 10.1006/dbio.2001.0326. [DOI] [PubMed] [Google Scholar]
- 6.Fields RD, Stevens-Graham B. New insights into neuron-glia communication. Science. 2002;298:556–62. doi: 10.1126/science.298.5593.556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sherman DL, Brophy PJ. Mechanisms of axon ensheathment and myelin growth. Nat Rev Neurosci. 2005;6:683–90. doi: 10.1038/nrn1743. [DOI] [PubMed] [Google Scholar]
- 8.Fields RD. White matter in learning, cognition and psychiatric disorders. Trends Neurosci. 2008;31:361–70. doi: 10.1016/j.tins.2008.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nagy Z, Westerberg H, Klingberg T. Maturation of white matter is associated with the development of cognitive functions during childhood. J Cogn Neurosci. 2004;16:1227–33. doi: 10.1162/0898929041920441. [DOI] [PubMed] [Google Scholar]
- 10.Fryer SL, Frank LR, Spadoni AD, Theilmann RJ, Nagel BJ, Schweinsburg AD, Tapert SF. Microstructural integrity of the corpus callosum linked with neuropsychological performance in adolescents. Brain Cogn. 2008;67:225–33. doi: 10.1016/j.bandc.2008.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liston C, Watts R, Tottenham N, Davidson MC, Niogi S, Ulug AM, Casey BJ. Frontostriatal microstructure modulates efficient recruitment of cognitive control. Cereb Cortex. 2006;16:553–60. doi: 10.1093/cercor/bhj003. [DOI] [PubMed] [Google Scholar]
- 12.Tamnes CK, Ostby Y, Walhovd KB, Westlye LT, Due-Tonnessen P, Fjell AM. Intellectual abilities and white matter microstructure in development: A diffusion tensor imaging study. Hum Brain Mapp. doi: 10.1002/hbm.20962. epub 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gold BT, Powell DK, Xuan L, Jiang Y, Hardy PA. Speed of lexical decision correlates with diffusion anisotropy in left parietal and frontal white matter: evidence from diffusion tensor imaging. Neuropsychologia. 2007;45:2439–46. doi: 10.1016/j.neuropsychologia.2007.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schmithorst VJ, Wilke M, Dardzinski BJ, Holland SK. Cognitive functions correlate with white matter architecture in a normal pediatric population: a diffusion tensor MRI study. Hum Brain Mapp. 2005;26:139–47. doi: 10.1002/hbm.20149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Niogi SN, McCandliss BD. Left lateralized white matter microstructure accounts for individual differences in reading ability and disability. Neuropsychologia. 2006;44:2178–88. doi: 10.1016/j.neuropsychologia.2006.01.011. [DOI] [PubMed] [Google Scholar]
- 16.Klingberg T. Development of a superior frontal-intraparietal network for visuo-spatial working memory. Neuropsychologia. 2006;44:2171–7. doi: 10.1016/j.neuropsychologia.2005.11.019. [DOI] [PubMed] [Google Scholar]
- 17.Klingberg T, Hedehus M, Temple E, Salz T, Gabrieli JD, Moseley ME, Poldrack RA. Microstructure of temporo-parietal white matter as a basis for reading ability: evidence from diffusion tensor magnetic resonance imaging. Neuron. 2000;25:493–500. doi: 10.1016/s0896-6273(00)80911-3. [DOI] [PubMed] [Google Scholar]
- 18.Bengtsson SL, Nagy Z, Skare S, Forsman L, Forssberg H, Ullen F. Extensive piano practicing has regionally specific effects on white matter development. Nat Neurosci. 2005;8:1148–50. doi: 10.1038/nn1516. [DOI] [PubMed] [Google Scholar]
- 19.Hyde KL, Zatorre RJ, Griffiths TD, Lerch JP, Peretz I. Morphometry of the amusic brain: a two-site study. Brain. 2006;129:2562–70. doi: 10.1093/brain/awl204. [DOI] [PubMed] [Google Scholar]
- 20.Nestor PG, Kubicki M, Spencer KM, Niznikiewicz M, McCarley RW, Shenton ME. Attentional networks and cingulum bundle in chronic schizophrenia. Schizophr Res. 2007;90:308–15. doi: 10.1016/j.schres.2006.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Takeuchi H, Taki Y, Sassa Y, Hashizume H, Sekiguchi A, Fukushima A, Kawashima R. White matter structures associated with creativity: Evidence from diffusion tensor imaging. Neuroimage. doi: 10.1016/j.neuroimage.2010.02.035. epub 2010. [DOI] [PubMed] [Google Scholar]
- 22.Teicher MH, Dumont NL, Ito Y, Vaituzis C, Giedd JN, Andersen SL. Childhood neglect is associated with reduced corpus callosum area. Biol Psychiatry. 2004;56:80–5. doi: 10.1016/j.biopsych.2004.03.016. [DOI] [PubMed] [Google Scholar]
- 23.Roots BI. The phylogeny of invertebrates and the evolution of myelin. Neuron Glia Biol. 2008;4:101–9. doi: 10.1017/S1740925X0900012X. [DOI] [PubMed] [Google Scholar]
- 24.Hartline DK, Colman DR. Rapid conduction and the evolution of giant axons and myelinated fibers. Curr Biol. 2007;17:R29–35. doi: 10.1016/j.cub.2006.11.042. [DOI] [PubMed] [Google Scholar]
- 25.Schweigreiter R, Roots BI, Bandtlow CE, Gould RM. Understanding myelination through studying its evolution. Int Rev Neurobiol. 2006;73:219–73. doi: 10.1016/S0074-7742(06)73007-0. [DOI] [PubMed] [Google Scholar]
- 26.Rowitch DH. Glial specification in the vertebrate neural tube. Nat Rev Neurosci. 2004;5:409–19. doi: 10.1038/nrn1389. [DOI] [PubMed] [Google Scholar]
- 27.Richardson WD, Kessaris N, Pringle N. Oligodendrocyte wars. Nat Rev Neurosci. 2006;7:11–8. doi: 10.1038/nrn1826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cayre M, Canoll P, Goldman JE. Cell migration in the normal and pathological postnatal mammalian brain. Prog Neurobiol. 2009;88:41–63. doi: 10.1016/j.pneurobio.2009.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McTigue DM, Tripathi RB. The life, death, and replacement of oligodendrocytes in the adult CNS. J Neurochem. 2008;107:1–19. doi: 10.1111/j.1471-4159.2008.05570.x. [DOI] [PubMed] [Google Scholar]
- 30.Menn B, Garcia-Verdugo JM, Yaschine C, Gonzalez-Perez O, Rowitch D, Alvarez-Buylla A. Origin of oligodendrocytes in the subventricular zone of the adult brain. J Neurosci. 2006;26:7907–18. doi: 10.1523/JNEUROSCI.1299-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bava S, Thayer R, Jacobus J, Ward M, Jernigan TL, Tapert SF. Longitudinal Characterization of White Matter Maturation During Adolescence. Brain Res. doi: 10.1016/j.brainres.2010.02.066. epub 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Asato MR, Terwilliger R, Woo J, Luna B. White Matter Development in Adolescence: A DTI Study. Cereb Cortex. doi: 10.1093/cercor/bhp282. epub 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yakovlev PL, Lecours AR. The myelogenetic cycles of regional maturation of the brain. In: Minkowski A, editor. Regional Development of the Brain in Early Life. Blackwell Scientific Publications; Oxford: 1967. [Google Scholar]
- 34.Powell K. Neurodevelopment: how does the teenage brain work? Nature. 2006;442:865–7. doi: 10.1038/442865a. [DOI] [PubMed] [Google Scholar]
- 35.Bartzokis G, Beckson M, Lu PH, Nuechterlein KH, Edwards N, Mintz J. Age-related changes in frontal and temporal lobe volumes in men: a magnetic resonance imaging study. Arch Gen Psychiatry. 2001;58:461–5. doi: 10.1001/archpsyc.58.5.461. [DOI] [PubMed] [Google Scholar]
- 36.O’Riordan JI. Central nervous system white matter diseases other than multiple sclerosis. Curr Opin Neurol. 1997;10:211–4. [PubMed] [Google Scholar]
- 37.Theda C, Moser AB, Powers JM, Moser HW. Phospholipids in X-linked adrenoleukodystrophy white matter: fatty acid abnormalities before the onset of demyelination. J Neurol Sci. 1992;110:195–204. doi: 10.1016/0022-510x(92)90028-j. [DOI] [PubMed] [Google Scholar]
- 38.Pantoni L, Poggesi A, Inzitari D. The relation between white-matter lesions and cognition. Curr Opin Neurol. 2007;20:390–7. doi: 10.1097/WCO.0b013e328172d661. [DOI] [PubMed] [Google Scholar]
- 39.Schmahmann JD, Smith EE, Eichler FS, Filley CM. Cerebral white matter: neuroanatomy, clinical neurology, and neurobehavioral correlates. Ann N Y Acad Sci. 2008;1142:266–309. doi: 10.1196/annals.1444.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hinman JD, Abraham CR. What’s behind the decline? The role of white matter in brain aging. Neurochem Res. 2007;32:2023–31. doi: 10.1007/s11064-007-9341-x. [DOI] [PubMed] [Google Scholar]
- 41.Gootjes L, Teipel SJ, Zebuhr Y, Schwarz R, Leinsinger G, Scheltens P, Moller HJ, Hampel H. Regional distribution of white matter hyperintensities in vascular dementia, Alzheimer’s disease and healthy aging. Dement Geriatr Cogn Disord. 2004;18:180–8. doi: 10.1159/000079199. [DOI] [PubMed] [Google Scholar]
- 42.Frisoni GB, Galluzzi S, Pantoni L, Filippi M. The effect of white matter lesions on cognition in the elderly--small but detectable. Nat Clin Pract Neurol. 2007;3:620–7. doi: 10.1038/ncpneuro0638. [DOI] [PubMed] [Google Scholar]
- 43.Madden DJ, Bennett IJ, Song AW. Cerebral white matter integrity and cognitive aging: contributions from diffusion tensor imaging. Neuropsychol Rev. 2009;19:415–35. doi: 10.1007/s11065-009-9113-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Viana-Baptista M, Bugalho P, Jordao C, Ferreira N, Ferreira A, Forjaz Secca M, Esperanca-Pina JA, Ferro JM. Cognitive function correlates with frontal white matter apparent diffusion coefficients in patients with leukoaraiosis. J Neurol. 2008;255:360–6. doi: 10.1007/s00415-008-0661-9. [DOI] [PubMed] [Google Scholar]
- 45.Schmidt R, Petrovic K, Ropele S, Enzinger C, Fazekas F. Progression of leukoaraiosis and cognition. Stroke. 2007;38:2619–25. doi: 10.1161/STROKEAHA.107.489112. [DOI] [PubMed] [Google Scholar]
- 46.Gallucci M, Amicarelli I, Rossi A, Stratta P, Masciocchi C, Zobel BB, Casacchia M, Passariello R. MR imaging of white matter lesions in uncomplicated chronic alcoholism. J Comput Assist Tomogr. 1989;13:395–8. doi: 10.1097/00004728-198905000-00004. [DOI] [PubMed] [Google Scholar]
- 47.Pfefferbaum A, Sullivan EV, Hedehus M, Adalsteinsson E, Lim KO, Moseley M. In vivo detection and functional correlates of white matter microstructural disruption in chronic alcoholism. Alcohol Clin Exp Res. 2000;24:1214–21. [PubMed] [Google Scholar]
- 48.Romero MJ, Asensio S, Palau C, Sanchez A, Romero FJ. Cocaine addiction: diffusion tensor imaging study of the inferior frontal and anterior cingulate white matter. Psychiatry Res. 2010;181:57–63. doi: 10.1016/j.pscychresns.2009.07.004. [DOI] [PubMed] [Google Scholar]
- 49.Feng Y. Convergence and divergence in the etiology of myelin impairment in psychiatric disorders and drug addiction. Neurochem Res. 2008;33:1940–9. doi: 10.1007/s11064-008-9693-x. [DOI] [PubMed] [Google Scholar]
- 50.Hogancamp WE, Rodriguez M, Weinshenker BG. The epidemiology of multiple sclerosis. Mayo Clin Proc. 1997;72:871–8. doi: 10.4065/72.9.871. [DOI] [PubMed] [Google Scholar]
- 51.Kujala P, Portin R, Ruutiainen J. The progress of cognitive decline in multiple sclerosis. A controlled 3-year follow-up. Brain. 1997;120(Pt 2):289–97. doi: 10.1093/brain/120.2.289. [DOI] [PubMed] [Google Scholar]
- 52.Chiaravalloti ND, DeLuca J. Cognitive impairment in multiple sclerosis. Lancet Neurol. 2008;7:1139–51. doi: 10.1016/S1474-4422(08)70259-X. [DOI] [PubMed] [Google Scholar]
- 53.Genova HM, Sumowski JF, Chiaravalloti N, Voelbel GT, Deluca J. Cognition in multiple sclerosis: a review of neuropsychological and fMRI research. Front Biosci. 2009;14:1730–44. doi: 10.2741/3336. [DOI] [PubMed] [Google Scholar]
- 54.Bobholz JA, Rao SM. Cognitive dysfunction in multiple sclerosis: a review of recent developments. Curr Opin Neurol. 2003;16:283–8. doi: 10.1097/01.wco.0000073928.19076.84. [DOI] [PubMed] [Google Scholar]
- 55.Feinstein A, Kartsounis LD, Miller DH, Youl BD, Ron MA. Clinically isolated lesions of the type seen in multiple sclerosis: a cognitive, psychiatric, and MRI follow up study. J Neurol Neurosurg Psychiatry. 1992;55:869–76. doi: 10.1136/jnnp.55.10.869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hohol MJ, Guttmann CR, Orav J, Mackin GA, Kikinis R, Khoury SJ, Jolesz FA, Weiner HL. Serial neuropsychological assessment and magnetic resonance imaging analysis in multiple sclerosis. Arch Neurol. 1997;54:1018–25. doi: 10.1001/archneur.1997.00550200074013. [DOI] [PubMed] [Google Scholar]
- 57.Comi G, Rovaris M, Falautano M, Santuccio G, Martinelli V, Rocca MA, Possa F, Leocani L, Paulesu E, Filippi M. A multiparametric MRI study of frontal lobe dementia in multiple sclerosis. J Neurol Sci. 1999;171:135–44. doi: 10.1016/s0022-510x(99)00266-x. [DOI] [PubMed] [Google Scholar]
- 58.Chwastiak LA, Ehde DM. Psychiatric issues in multiple sclerosis. Psychiatr Clin North Am. 2007;30:803–17. doi: 10.1016/j.psc.2007.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rauschka H, Colsch B, Baumann N, Wevers R, Schmidbauer M, Krammer M, Turpin JC, Lefevre M, Olivier C, Tardieu S, Krivit W, Moser H, Moser A, Gieselmann V, Zalc B, Cox T, Reuner U, Tylki-Szymanska A, Aboul-Enein F, LeGuern E, Bernheimer H, Berger J. Late-onset metachromatic leukodystrophy: genotype strongly influences phenotype. Neurology. 2006;67:859–63. doi: 10.1212/01.wnl.0000234129.97727.4d. [DOI] [PubMed] [Google Scholar]
- 60.Freeman SH, Hyman BT, Sims KB, Hedley-Whyte ET, Vossough A, Frosch MP, Schmahmann JD. Adult onset leukodystrophy with neuroaxonal spheroids: clinical, neuroimaging and neuropathologic observations. Brain Pathol. 2009;19:39–47. doi: 10.1111/j.1750-3639.2008.00163.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hyde TM, Ziegler JC, Weinberger DR. Psychiatric disturbances in metachromatic leukodystrophy. Insights into the neurobiology of psychosis. Arch Neurol. 1992;49:401–6. doi: 10.1001/archneur.1992.00530280095028. [DOI] [PubMed] [Google Scholar]
- 62.Baumann N, Turpin JC, Lefevre M, Colsch B. Motor and psycho-cognitive clinical types in adult metachromatic leukodystrophy: genotype/phenotype relationships? J Physiol Paris. 2002;96:301–6. doi: 10.1016/s0928-4257(02)00019-0. [DOI] [PubMed] [Google Scholar]
- 63.Black DN, Taber KH, Hurley RA. Metachromatic leukodystrophy: a model for the study of psychosis. J Neuropsychiatry Clin Neurosci. 2003;15:289–93. doi: 10.1176/jnp.15.3.289. [DOI] [PubMed] [Google Scholar]
- 64.Rajji TK, Ismail Z, Mulsant BH. Age at onset and cognition in schizophrenia: meta-analysis. Br J Psychiatry. 2009;195:286–93. doi: 10.1192/bjp.bp.108.060723. [DOI] [PubMed] [Google Scholar]
- 65.Lewis R. Should cognitive deficit be a diagnostic criterion for schizophrenia? J Psychiatry Neurosci. 2004;29:102–13. [PMC free article] [PubMed] [Google Scholar]
- 66.van Os J, Kapur S. Schizophrenia. Lancet. 2009;374:635–45. doi: 10.1016/S0140-6736(09)60995-8. [DOI] [PubMed] [Google Scholar]
- 67.Mittal VA, Ellman LM, Cannon TD. Gene-environment interaction and covariation in schizophrenia: the role of obstetric complications. Schizophr Bull. 2008;34:1083–94. doi: 10.1093/schbul/sbn080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Minzenberg MJ, Laird AR, Thelen S, Carter CS, Glahn DC. Meta-analysis of 41 functional neuroimaging studies of executive function in schizophrenia. Arch Gen Psychiatry. 2009;66:811–22. doi: 10.1001/archgenpsychiatry.2009.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ford JM, Krystal JH, Mathalon DH. Neural synchrony in schizophrenia: from networks to new treatments. Schizophr Bull. 2007;33:848–52. doi: 10.1093/schbul/sbm062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Selemon LD, Goldman-Rakic PS. The reduced neuropil hypothesis: a circuit based model of schizophrenia. Biol Psychiatry. 1999;45:17–25. doi: 10.1016/s0006-3223(98)00281-9. [DOI] [PubMed] [Google Scholar]
- 71.Heckers S, Heinsen H, Geiger B, Beckmann H. Hippocampal neuron number in schizophrenia. A stereological study. Arch Gen Psychiatry. 1991;48:1002–8. doi: 10.1001/archpsyc.1991.01810350042006. [DOI] [PubMed] [Google Scholar]
- 72.Glantz LA, Lewis DA. Decreased dendritic spine density on prefrontal cortical pyramidal neurons in schizophrenia. Arch Gen Psychiatry. 2000;57:65–73. doi: 10.1001/archpsyc.57.1.65. [DOI] [PubMed] [Google Scholar]
- 73.Uranova N, Orlovskaya D, Vikhreva O, Zimina I, Kolomeets N, Vostrikov V, Rachmanova V. Electron microscopy of oligodendroglia in severe mental illness. Brain Res Bull. 2001;55:597–610. doi: 10.1016/s0361-9230(01)00528-7. [DOI] [PubMed] [Google Scholar]
- 74.Uranova NA, Vostrikov VM, Orlovskaya DD, Rachmanova VI. Oligodendroglial density in the prefrontal cortex in schizophrenia and mood disorders: a study from the Stanley Neuropathology Consortium. Schizophr Res. 2004;67:269–75. doi: 10.1016/S0920-9964(03)00181-6. [DOI] [PubMed] [Google Scholar]
- 75.Vostrikov V, Orlovskaya D, Uranova N. Deficit of pericapillary oligodendrocytes in the prefrontal cortex in schizophrenia. World J Biol Psychiatry. 2008;9:34–42. doi: 10.1080/15622970701210247. [DOI] [PubMed] [Google Scholar]
- 76.Vostrikov VM, Uranova NA, Orlovskaya DD. Deficit of perineuronal oligodendrocytes in the prefrontal cortex in schizophrenia and mood disorders. Schizophr Res. 2007;94:273–80. doi: 10.1016/j.schres.2007.04.014. [DOI] [PubMed] [Google Scholar]
- 77.Hof PR, Haroutunian V, Friedrich VL, Jr, Byne W, Buitron C, Perl DP, Davis KL. Loss and altered spatial distribution of oligodendrocytes in the superior frontal gyrus in schizophrenia. Biol Psychiatry. 2003;53:1075–85. doi: 10.1016/s0006-3223(03)00237-3. [DOI] [PubMed] [Google Scholar]
- 78.Dwork AJ, Mancevski B, Rosoklija G. White matter and cognitive function in schizophrenia. Int J Neuropsychopharmacol. 2007;10:513–36. doi: 10.1017/S1461145707007638. [DOI] [PubMed] [Google Scholar]
- 79.Kubicki M, Park H, Westin CF, Nestor PG, Mulkern RV, Maier SE, Niznikiewicz M, Connor EE, Levitt JJ, Frumin M, Kikinis R, Jolesz FA, McCarley RW, Shenton ME. DTI and MTR abnormalities in schizophrenia: analysis of white matter integrity. Neuroimage. 2005;26:1109–18. doi: 10.1016/j.neuroimage.2005.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Bagary MS, Symms MR, Barker GJ, Mutsatsa SH, Joyce EM, Ron MA. Gray and white matter brain abnormalities in first-episode schizophrenia inferred from magnetization transfer imaging. Arch Gen Psychiatry. 2003;60:779–88. doi: 10.1001/archpsyc.60.8.779. [DOI] [PubMed] [Google Scholar]
- 81.Cheung V, Cheung C, McAlonan GM, Deng Y, Wong JG, Yip L, Tai KS, Khong PL, Sham P, Chua SE. A diffusion tensor imaging study of structural dysconnectivity in never-medicated, first-episode schizophrenia. Psychol Med. 2008;38:877–85. doi: 10.1017/S0033291707001808. [DOI] [PubMed] [Google Scholar]
- 82.Gasparotti R, Valsecchi P, Carletti F, Galluzzo A, Liserre R, Cesana B, Sacchetti E. Reduced fractional anisotropy of corpus callosum in first-contact, antipsychotic drug-naive patients with schizophrenia. Schizophr Res. 2009;108:41–8. doi: 10.1016/j.schres.2008.11.015. [DOI] [PubMed] [Google Scholar]
- 83.Chan WY, Yang GL, Chia MY, Lau IY, Sitoh YY, Nowinski WL, Sim K. White matter abnormalities in first-episode schizophrenia: A combined structural MRI and DTI study. Schizophr Res. doi: 10.1016/j.schres.2009.12.012. epub 2010. [DOI] [PubMed] [Google Scholar]
- 84.Perez-Iglesias R, Tordesillas-Gutierrez D, McGuire PK, Barker GJ, Roiz-Santianez R, Mata I, de Lucas EM, Rodriguez-Sanchez JM, Ayesa-Arriola R, Vazquez-Barquero JL, Crespo-Facorro B. White Matter Integrity and Cognitive Impairment in First-Episode Psychosis. Am J Psychiatry. doi: 10.1176/appi.ajp.2009.09050716. epub 2010. [DOI] [PubMed] [Google Scholar]
- 85.Moriya J, Kakeda S, Abe O, Goto N, Yoshimura R, Hori H, Ohnari N, Sato T, Aoki S, Ohtomo K, Nakamura J, Korogi Y. Gray and white matter volumetric and diffusion tensor imaging (DTI) analyses in the early stage of first-episode schizophrenia. Schizophr Res. 2010;116:196–203. doi: 10.1016/j.schres.2009.10.002. [DOI] [PubMed] [Google Scholar]
- 86.Kyriakopoulos M, Vyas NS, Barker GJ, Chitnis XA, Frangou S. A diffusion tensor imaging study of white matter in early-onset schizophrenia. Biol Psychiatry. 2008;63:519–23. doi: 10.1016/j.biopsych.2007.05.021. [DOI] [PubMed] [Google Scholar]
- 87.Friedman JI, Tang C, Carpenter D, Buchsbaum M, Schmeidler J, Flanagan L, Golembo S, Kanellopoulou I, Ng J, Hof PR, Harvey PD, Tsopelas ND, Stewart D, Davis KL. Diffusion tensor imaging findings in first-episode and chronic schizophrenia patients. Am J Psychiatry. 2008;165:1024–32. doi: 10.1176/appi.ajp.2008.07101640. [DOI] [PubMed] [Google Scholar]
- 88.Skelly LR, Calhoun V, Meda SA, Kim J, Mathalon DH, Pearlson GD. Diffusion tensor imaging in schizophrenia: relationship to symptoms. Schizophr Res. 2008;98:157–62. doi: 10.1016/j.schres.2007.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Rametti G, Junque C, Falcon C, Bargallo N, Catalan R, Penades R, Garzon B, Bernardo M. A voxel-based diffusion tensor imaging study of temporal white matter in patients with schizophrenia. Psychiatry Res. 2009;171:166–76. doi: 10.1016/j.pscychresns.2008.05.003. [DOI] [PubMed] [Google Scholar]
- 90.Ardekani BA, Tabesh A, Sevy S, Robinson DG, Bilder RM, Szeszko PR. Diffusion tensor imaging reliably differentiates patients with schizophrenia from healthy volunteers. Hum Brain Mapp. doi: 10.1002/hbm.20995. epub 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Camchong J, Lim KO, Sponheim SR, Macdonald AW. Frontal white matter integrity as an endophenotype for schizophrenia: diffusion tensor imaging in monozygotic twins and patients’ nonpsychotic relatives. Front Hum Neurosci. 2009;3:35. doi: 10.3389/neuro.09.035.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Hakak Y, Walker JR, Li C, Wong WH, Davis KL, Buxbaum JD, Haroutunian V, Fienberg AA. Genome-wide expression analysis reveals dysregulation of myelination-related genes in chronic schizophrenia. Proc Natl Acad Sci U S A. 2001;98:4746–51. doi: 10.1073/pnas.081071198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Tkachev D, Mimmack ML, Ryan MM, Wayland M, Freeman T, Jones PB, Starkey M, Webster MJ, Yolken RH, Bahn S. Oligodendrocyte dysfunction in schizophrenia and bipolar disorder. Lancet. 2003;362:798–805. doi: 10.1016/S0140-6736(03)14289-4. [DOI] [PubMed] [Google Scholar]
- 94.Peirce TR, Bray NJ, Williams NM, Norton N, Moskvina V, Preece A, Haroutunian V, Buxbaum JD, Owen MJ, O’Donovan MC. Convergent evidence for 2′,3′-cyclic nucleotide 3′-phosphodiesterase as a possible susceptibility gene for schizophrenia. Arch Gen Psychiatry. 2006;63:18–24. doi: 10.1001/archpsyc.63.1.18. [DOI] [PubMed] [Google Scholar]
- 95.Sugai T, Kawamura M, Iritani S, Araki K, Makifuchi T, Imai C, Nakamura R, Kakita A, Takahashi H, Nawa H. Prefrontal abnormality of schizophrenia revealed by DNA microarray: impact on glial and neurotrophic gene expression. Ann N Y Acad Sci. 2004;1025:84–91. doi: 10.1196/annals.1316.011. [DOI] [PubMed] [Google Scholar]
- 96.Dracheva S, Davis KL, Chin B, Woo DA, Schmeidler J, Haroutunian V. Myelin-associated mRNA and protein expression deficits in the anterior cingulate cortex and hippocampus in elderly schizophrenia patients. Neurobiol Dis. 2006;21:531–40. doi: 10.1016/j.nbd.2005.08.012. [DOI] [PubMed] [Google Scholar]
- 97.Aston C, Jiang L, Sokolov BP. Microarray analysis of postmortem temporal cortex from patients with schizophrenia. J Neurosci Res. 2004;77:858–66. doi: 10.1002/jnr.20208. [DOI] [PubMed] [Google Scholar]
- 98.Davis KL, Stewart DG, Friedman JI, Buchsbaum M, Harvey PD, Hof PR, Buxbaum J, Haroutunian V. White matter changes in schizophrenia: evidence for myelin-related dysfunction. Arch Gen Psychiatry. 2003;60:443–56. doi: 10.1001/archpsyc.60.5.443. [DOI] [PubMed] [Google Scholar]
- 99.Haroutunian V, Katsel P, Dracheva S, Stewart DG, Davis KL. Variations in oligodendrocyte-related gene expression across multiple cortical regions: implications for the pathophysiology of schizophrenia. Int J Neuropsychopharmacol. 2007;10:565–73. doi: 10.1017/S1461145706007310. [DOI] [PubMed] [Google Scholar]
- 100.Katsel P, Davis KL, Haroutunian V. Variations in myelin and oligodendrocyte-related gene expression across multiple brain regions in schizophrenia: a gene ontology study. Schizophr Res. 2005;79:157–73. doi: 10.1016/j.schres.2005.06.007. [DOI] [PubMed] [Google Scholar]
- 101.McCullumsmith RE, Gupta D, Beneyto M, Kreger E, Haroutunian V, Davis KL, Meador-Woodruff JH. Expression of transcripts for myelination-related genes in the anterior cingulate cortex in schizophrenia. Schizophr Res. 2007;90:15–27. doi: 10.1016/j.schres.2006.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Sokolov BP. Oligodendroglial abnormalities in schizophrenia, mood disorders and substance abuse. Comorbidity, shared traits, or molecular phenocopies? Int J Neuropsychopharmacol. 2007;10:547–55. doi: 10.1017/S1461145706007322. [DOI] [PubMed] [Google Scholar]
- 103.Aston C, Jiang L, Sokolov BP. Transcriptional profiling reveals evidence for signaling and oligodendroglial abnormalities in the temporal cortex from patients with major depressive disorder. Mol Psychiatry. 2005;10:309–22. doi: 10.1038/sj.mp.4001565. [DOI] [PubMed] [Google Scholar]
- 104.Demerens C, Stankoff B, Logak M, Anglade P, Allinquant B, Couraud F, Zalc B, Lubetzki C. Induction of myelination in the central nervous system by electrical activity. Proc Natl Acad Sci U S A. 1996;93:9887–92. doi: 10.1073/pnas.93.18.9887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Snitz BE, MacDonald A, 3rd, Cohen JD, Cho RY, Becker T, Carter CS. Lateral and medial hypofrontality in first-episode schizophrenia: functional activity in a medication-naive state and effects of short-term atypical antipsychotic treatment. Am J Psychiatry. 2005;162:2322–9. doi: 10.1176/appi.ajp.162.12.2322. [DOI] [PubMed] [Google Scholar]
- 106.Benarroch EE. Oligodendrocytes: Susceptibility to injury and involvement in neurologic disease. Neurology. 2009;72:1779–85. doi: 10.1212/WNL.0b013e3181a6b123. [DOI] [PubMed] [Google Scholar]
- 107.Coyle JT. Glutamate and schizophrenia: beyond the dopamine hypothesis. Cell Mol Neurobiol. 2006;26:365–84. doi: 10.1007/s10571-006-9062-8. [DOI] [PubMed] [Google Scholar]
- 108.Konradi C, Heckers S. Molecular aspects of glutamate dysregulation: implications for schizophrenia and its treatment. Pharmacol Ther. 2003;97:153–79. doi: 10.1016/s0163-7258(02)00328-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Hahn CG, Wang HY, Cho DS, Talbot K, Gur RE, Berrettini WH, Bakshi K, Kamins J, Borgmann-Winter KE, Siegel SJ, Gallop RJ, Arnold SE. Altered neuregulin 1-erbB4 signaling contributes to NMDA receptor hypofunction in schizophrenia. Nat Med. 2006;12:824–8. doi: 10.1038/nm1418. [DOI] [PubMed] [Google Scholar]
- 110.Li T, Stefansson H, Gudfinnsson E, Cai G, Liu X, Murray RM, Steinthorsdottir V, Januel D, Gudnadottir VG, Petursson H, Ingason A, Gulcher JR, Stefansson K, Collier DA. Identification of a novel neuregulin 1 at-risk haplotype in Han schizophrenia Chinese patients, but no association with the Icelandic/Scottish risk haplotype. Mol Psychiatry. 2004;9:698–704. doi: 10.1038/sj.mp.4001485. [DOI] [PubMed] [Google Scholar]
- 111.Jaaro-Peled H, Hayashi-Takagi A, Seshadri S, Kamiya A, Brandon NJ, Sawa A. Neurodevelopmental mechanisms of schizophrenia: understanding disturbed postnatal brain maturation through neuregulin-1-ErbB4 and DISC1. Trends Neurosci. 2009;32:485–95. doi: 10.1016/j.tins.2009.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Konrad A, Vucurevic G, Musso F, Stoeter P, Dahmen N, Winterer G. ErbB4 genotype predicts left frontotemporal structural connectivity in human brain. Neuropsychopharmacology. 2009;34:641–50. doi: 10.1038/npp.2008.112. [DOI] [PubMed] [Google Scholar]
- 113.Barres BA. The mystery and magic of glia: a perspective on their roles in health and disease. Neuron. 2008;60:430–40. doi: 10.1016/j.neuron.2008.10.013. [DOI] [PubMed] [Google Scholar]
- 114.Nave KA, Salzer JL. Axonal regulation of myelination by neuregulin 1. Curr Opin Neurobiol. 2006;16:492–500. doi: 10.1016/j.conb.2006.08.008. [DOI] [PubMed] [Google Scholar]
- 115.Taveggia C, Zanazzi G, Petrylak A, Yano H, Rosenbluth J, Einheber S, Xu X, Esper RM, Loeb JA, Shrager P, Chao MV, Falls DL, Role L, Salzer JL. Neuregulin-1 type III determines the ensheathment fate of axons. Neuron. 2005;47:681–94. doi: 10.1016/j.neuron.2005.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.St Clair D, Blackwood D, Muir W, Carothers A, Walker M, Spowart G, Gosden C, Evans HJ. Association within a family of a balanced autosomal translocation with major mental illness. Lancet. 1990;336:13–6. doi: 10.1016/0140-6736(90)91520-k. [DOI] [PubMed] [Google Scholar]
- 117.Millar JK, Wilson-Annan JC, Anderson S, Christie S, Taylor MS, Semple CA, Devon RS, St Clair DM, Muir WJ, Blackwood DH, Porteous DJ. Disruption of two novel genes by a translocation co-segregating with schizophrenia. Hum Mol Genet. 2000;9:1415–23. doi: 10.1093/hmg/9.9.1415. [DOI] [PubMed] [Google Scholar]
- 118.Wood JD, Bonath F, Kumar S, Ross CA, Cunliffe VT. Disrupted-in-schizophrenia 1 and neuregulin 1 are required for the specification of oligodendrocytes and neurones in the zebrafish brain. Hum Mol Genet. 2009;18:391–404. doi: 10.1093/hmg/ddn361. [DOI] [PubMed] [Google Scholar]
- 119.Duan X, Chang JH, Ge S, Faulkner RL, Kim JY, Kitabatake Y, Liu XB, Yang CH, Jordan JD, Ma DK, Liu CY, Ganesan S, Cheng HJ, Ming GL, Lu B, Song H. Disrupted-In-Schizophrenia 1 regulates integration of newly generated neurons in the adult brain. Cell. 2007;130:1146–58. doi: 10.1016/j.cell.2007.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Kim S, Webster MJ. The stanley neuropathology consortium integrative database: a novel, web-based tool for exploring neuropathological markers in psychiatric disorders and the biological processes associated with abnormalities of those markers. Neuropsychopharmacology. 2010;35:473–82. doi: 10.1038/npp.2009.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Impagnatiello F, Guidotti AR, Pesold C, Dwivedi Y, Caruncho H, Pisu MG, Uzunov DP, Smalheiser NR, Davis JM, Pandey GN, Pappas GD, Tueting P, Sharma RP, Costa E. A decrease of reelin expression as a putative vulnerability factor in schizophrenia. Proc Natl Acad Sci U S A. 1998;95:15718–23. doi: 10.1073/pnas.95.26.15718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Fatemi SH, Earle JA, McMenomy T. Reduction in Reelin immunoreactivity in hippocampus of subjects with schizophrenia, bipolar disorder and major depression. Mol Psychiatry. 2000;5:654–63. 571. doi: 10.1038/sj.mp.4000783. [DOI] [PubMed] [Google Scholar]
- 123.Grayson DR, Jia X, Chen Y, Sharma RP, Mitchell CP, Guidotti A, Costa E. Reelin promoter hypermethylation in schizophrenia. Proc Natl Acad Sci U S A. 2005;102:9341–6. doi: 10.1073/pnas.0503736102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Panteri R, Mey J, Zhelyaznik N, D’Altocolle A, Del Fa A, Gangitano C, Marino R, Lorenzetto E, Buffelli M, Keller F. Reelin is transiently expressed in the peripheral nerve during development and is upregulated following nerve crush. Mol Cell Neurosci. 2006;32:133–42. doi: 10.1016/j.mcn.2006.03.004. [DOI] [PubMed] [Google Scholar]
- 125.D’Arcangelo G, Miao GG, Chen SC, Soares HD, Morgan JI, Curran T. A protein related to extracellular matrix proteins deleted in the mouse mutant reeler. Nature. 1995;374:719–23. doi: 10.1038/374719a0. [DOI] [PubMed] [Google Scholar]
- 126.Ono K, Takebayashi H, Ikenaka K. Olig2 transcription factor in the developing and injured forebrain; cell lineage and glial development. Mol Cells. 2009;27:397–401. doi: 10.1007/s10059-009-0067-2. [DOI] [PubMed] [Google Scholar]
- 127.Georgieva L, Moskvina V, Peirce T, Norton N, Bray NJ, Jones L, Holmans P, Macgregor S, Zammit S, Wilkinson J, Williams H, Nikolov I, Williams N, Ivanov D, Davis KL, Haroutunian V, Buxbaum JD, Craddock N, Kirov G, Owen MJ, O’Donovan MC. Convergent evidence that oligodendrocyte lineage transcription factor 2 (OLIG2) and interacting genes influence susceptibility to schizophrenia. Proc Natl Acad Sci U S A. 2006;103:12469–74. doi: 10.1073/pnas.0603029103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Huang K, Tang W, Tang R, Xu Z, He Z, Li Z, Xu Y, Li X, He G, Feng G, He L, Shi Y. Positive association between OLIG2 and schizophrenia in the Chinese Han population. Hum Genet. 2008;122:659–60. doi: 10.1007/s00439-007-0434-z. [DOI] [PubMed] [Google Scholar]
- 129.Usui H, Takahashi N, Saito S, Ishihara R, Aoyama N, Ikeda M, Suzuki T, Kitajima T, Yamanouchi Y, Kinoshita Y, Yoshida K, Iwata N, Inada T, Ozaki N. The 2′,3′-cyclic nucleotide 3′-phosphodiesterase and oligodendrocyte lineage transcription factor 2 genes do not appear to be associated with schizophrenia in the Japanese population. Schizophr Res. 2006;88:245–50. doi: 10.1016/j.schres.2006.07.019. [DOI] [PubMed] [Google Scholar]
- 130.Neves-Pereira M, Cheung JK, Pasdar A, Zhang F, Breen G, Yates P, Sinclair M, Crombie C, Walker N, St Clair DM. BDNF gene is a risk factor for schizophrenia in a Scottish population. Mol Psychiatry. 2005;10:208–12. doi: 10.1038/sj.mp.4001575. [DOI] [PubMed] [Google Scholar]
- 131.Jindal RD, Pillai AK, Mahadik SP, Eklund K, Montrose DM, Keshavan MS. Decreased BDNF in patients with antipsychotic naive first episode schizophrenia. Schizophr Res. doi: 10.1016/j.schres.2009.12.035. epub 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Rybakowski JK. BDNF gene: functional Val66Met polymorphism in mood disorders and schizophrenia. Pharmacogenomics. 2008;9:1589–93. doi: 10.2217/14622416.9.11.1589. [DOI] [PubMed] [Google Scholar]
- 133.Rybakowski JK, Borkowska A, Skibinska M, Hauser J. Illness-specific association of val66met BDNF polymorphism with performance on Wisconsin Card Sorting Test in bipolar mood disorder. Mol Psychiatry. 2006;11:122–4. doi: 10.1038/sj.mp.4001765. [DOI] [PubMed] [Google Scholar]
- 134.Xu J, Liu Y, Wang P, Li S, Wang Y, Li J, Zhou D, Chen Z, Zhao T, Wang T, Xu H, Yang Y, Feng G, He L, Yu L. Positive association between the brain-derived neurotrophic factor (BDNF) gene and bipolar disorder in the Han Chinese population. Am J Med Genet B Neuropsychiatr Genet. 153B:275–9. doi: 10.1002/ajmg.b.30953. epub 2010. [DOI] [PubMed] [Google Scholar]
- 135.Palomino A, Vallejo-Illarramendi A, Gonzalez-Pinto A, Aldama A, Gonzalez-Gomez C, Mosquera F, Gonzalez-Garcia G, Matute C. Decreased levels of plasma BDNF in first-episode schizophrenia and bipolar disorder patients. Schizophr Res. 2006;86:321–2. doi: 10.1016/j.schres.2006.05.028. [DOI] [PubMed] [Google Scholar]
- 136.Lohoff FW, Sander T, Ferraro TN, Dahl JP, Gallinat J, Berrettini WH. Confirmation of association between the Val66Met polymorphism in the brain-derived neurotrophic factor (BDNF) gene and bipolar I disorder. Am J Med Genet B Neuropsychiatr Genet. 2005;139B:51–3. doi: 10.1002/ajmg.b.30215. [DOI] [PubMed] [Google Scholar]
- 137.Okada T, Hashimoto R, Numakawa T, Iijima Y, Kosuga A, Tatsumi M, Kamijima K, Kato T, Kunugi H. A complex polymorphic region in the brain-derived neurotrophic factor (BDNF) gene confers susceptibility to bipolar disorder and affects transcriptional activity. Mol Psychiatry. 2006;11:695–703. doi: 10.1038/sj.mp.4001822. [DOI] [PubMed] [Google Scholar]
- 138.Rosa A, Cuesta MJ, Fatjo-Vilas M, Peralta V, Zarzuela A, Fananas L. The Val66Met polymorphism of the brain-derived neurotrophic factor gene is associated with risk for psychosis: evidence from a family-based association study. Am J Med Genet B Neuropsychiatr Genet. 2006;141B:135–8. doi: 10.1002/ajmg.b.30266. [DOI] [PubMed] [Google Scholar]
- 139.Squassina A, Piccardi P, Del Zompo M, Rossi A, Vita A, Pini S, Mucci A, Galderisi S. NRG1 and BDNF genes in schizophrenia: An association study in an Italian case-control sample. Psychiatry Res. 176:82–84. doi: 10.1016/j.psychres.2009.03.017. epub 2010. [DOI] [PubMed] [Google Scholar]
- 140.Kawashima K, Ikeda M, Kishi T, Kitajima T, Yamanouchi Y, Kinoshita Y, Okochi T, Aleksic B, Tomita M, Okada T, Kunugi H, Inada T, Ozaki N, Iwata N. BDNF is not associated with schizophrenia: data from a Japanese population study and meta-analysis. Schizophr Res. 2009;112:72–9. doi: 10.1016/j.schres.2009.03.040. [DOI] [PubMed] [Google Scholar]
- 141.Vondran MW, Clinton-Luke P, Honeywell JZ, Dreyfus CF. BDNF+/− mice exhibit deficits in oligodendrocyte lineage cells of the basal forebrain. Glia. doi: 10.1002/glia.20969. epub 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Du Y, Fischer TZ, Lee LN, Lercher LD, Dreyfus CF. Regionally specific effects of BDNF on oligodendrocytes. Dev Neurosci. 2003;25:116–26. doi: 10.1159/000072261. [DOI] [PubMed] [Google Scholar]
- 143.Heng S, Song AW, Sim K. White matter abnormalities in bipolar disorder: insights from diffusion tensor imaging studies. J Neural Transm. doi: 10.1007/s00702-010-0368-9. epub 2010. [DOI] [PubMed] [Google Scholar]
- 144.Mahon K, Burdick KE, Szeszko PR. A role for white matter abnormalities in the pathophysiology of bipolar disorder. Neurosci Biobehav Rev. 2010;34:533–54. doi: 10.1016/j.neubiorev.2009.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Albertson DN, Pruetz B, Schmidt CJ, Kuhn DM, Kapatos G, Bannon MJ. Gene expression profile of the nucleus accumbens of human cocaine abusers: evidence for dysregulation of myelin. J Neurochem. 2004;88:1211–9. doi: 10.1046/j.1471-4159.2003.02247.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Oh JS, Lyoo IK, Sung YH, Hwang J, Kim J, Chung A, Park KS, Kim SJ, Renshaw PF, Song IC. Shape changes of the corpus callosum in abstinent methamphetamine users. Neurosci Lett. 2005;384:76–81. doi: 10.1016/j.neulet.2005.04.082. [DOI] [PubMed] [Google Scholar]
- 147.Bartzokis G, Beckson M, Lu PH, Edwards N, Rapoport R, Wiseman E, Bridge P. Age-related brain volume reductions in amphetamine and cocaine addicts and normal controls: implications for addiction research. Psychiatry Res. 2000;98:93–102. doi: 10.1016/s0925-4927(99)00052-9. [DOI] [PubMed] [Google Scholar]
- 148.Chang L, Cloak C, Patterson K, Grob C, Miller EN, Ernst T. Enlarged striatum in abstinent methamphetamine abusers: a possible compensatory response. Biol Psychiatry. 2005;57:967–74. doi: 10.1016/j.biopsych.2005.01.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Thompson PM, Hayashi KM, Simon SL, Geaga JA, Hong MS, Sui Y, Lee JY, Toga AW, Ling W, London ED. Structural abnormalities in the brains of human subjects who use methamphetamine. J Neurosci. 2004;24:6028–36. doi: 10.1523/JNEUROSCI.0713-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Tobias MC, O’Neill J, Hudkins M, Bartzokis G, Dean AC, London ED. White-matter abnormalities in brain during early abstinence from methamphetamine abuse. Psychopharmacology (Berl) 2010;209:13–24. doi: 10.1007/s00213-009-1761-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Baicy K, London ED. Corticolimbic dysregulation and chronic methamphetamine abuse. Addiction. 2007;102(Suppl 1):5–15. doi: 10.1111/j.1360-0443.2006.01777.x. [DOI] [PubMed] [Google Scholar]
- 152.Barr AM, Panenka WJ, MacEwan GW, Thornton AE, Lang DJ, Honer WG, Lecomte T. The need for speed: an update on methamphetamine addiction. J Psychiatry Neurosci. 2006;31:301–13. [PMC free article] [PubMed] [Google Scholar]
- 153.Meredith CW, Jaffe C, Ang-Lee K, Saxon AJ. Implications of chronic methamphetamine use: a literature review. Harv Rev Psychiatry. 2005;13:141–54. doi: 10.1080/10673220591003605. [DOI] [PubMed] [Google Scholar]
- 154.Darke S, Kaye S, McKetin R, Duflou J. Major physical and psychological harms of methamphetamine use. Drug Alcohol Rev. 2008;27:253–62. doi: 10.1080/09595230801923702. [DOI] [PubMed] [Google Scholar]
- 155.Kalechstein AD, Newton TF, Longshore D, Anglin MD, van Gorp WG, Gawin FH. Psychiatric comorbidity of methamphetamine dependence in a forensic sample. J Neuropsychiatry Clin Neurosci. 2000;12:480–4. doi: 10.1176/jnp.12.4.480. [DOI] [PubMed] [Google Scholar]
- 156.Leventhal AM, Kahler CW, Ray LA, Stone K, Young D, Chelminski I, Zimmerman M. Anhedonia and amotivation in psychiatric outpatients with fully remitted stimulant use disorder. Am J Addict. 2008;17:218–23. doi: 10.1080/10550490802019774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Messori L, Casini A, Gabbiani C, Sorace L, Muniz-Miranda M, Zatta P. Unravelling the chemical nature of copper cuprizone. Dalton Trans. 2007:2112–4. doi: 10.1039/b701896g. [DOI] [PubMed] [Google Scholar]
- 158.Zatta P, Raso M, Zambenedetti P, Wittkowski W, Messori L, Piccioli F, Mauri PL, Beltramini M. Copper and zinc dismetabolism in the mouse brain upon chronic cuprizone treatment. Cell Mol Life Sci. 2005;62:1502–13. doi: 10.1007/s00018-005-5073-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Gregg JR, Herring NR, Naydenov A, Hanlin RP, Konradi C. Downregulation of oligodendrocyte transcripts is associated with impaired prefrontal cortex function in rats. Schizophr Res. 2009 doi: 10.1016/j.schres.2009.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Kim BE, Nevitt T, Thiele DJ. Mechanisms for copper acquisition, distribution and regulation. Nat Chem Biol. 2008;4:176–85. doi: 10.1038/nchembio.72. [DOI] [PubMed] [Google Scholar]
- 161.Andreini C, Banci L, Bertini I, Rosato A. Occurrence of copper proteins through the three domains of life: a bioinformatic approach. J Proteome Res. 2008;7:209–16. doi: 10.1021/pr070480u. [DOI] [PubMed] [Google Scholar]
- 162.Fridovich I. Superoxide dismutases. Annu Rev Biochem. 1975;44:147–59. doi: 10.1146/annurev.bi.44.070175.001051. [DOI] [PubMed] [Google Scholar]
- 163.Zhang X, McIntire WS. Cloning and sequencing of a copper-containing, topa quinone-containing monoamine oxidase from human placenta. Gene. 1996;179:279–86. doi: 10.1016/s0378-1119(96)00387-3. [DOI] [PubMed] [Google Scholar]
- 164.Blackburn NJ, Mason HS, Knowles PF. Dopamine-beta-hydroxylase: evidence for binuclear copper sites. Biochem Biophys Res Commun. 1980;95:1275–81. doi: 10.1016/0006-291x(80)91611-3. [DOI] [PubMed] [Google Scholar]
- 165.Horn D, Barrientos A. Mitochondrial copper metabolism and delivery to cytochrome c oxidase. IUBMB Life. 2008;60:421–9. doi: 10.1002/iub.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Takahashi Y, Kako K, Kashiwabara S, Takehara A, Inada Y, Arai H, Nakada K, Kodama H, Hayashi J, Baba T, Munekata E. Mammalian copper chaperone Cox17p has an essential role in activation of cytochrome C oxidase and embryonic development. Mol Cell Biol. 2002;22:7614–21. doi: 10.1128/MCB.22.21.7614-7621.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Harrison MD, Jones CE, Solioz M, Dameron CT. Intracellular copper routing: the role of copper chaperones. Trends Biochem Sci. 2000;25:29–32. doi: 10.1016/s0968-0004(99)01492-9. [DOI] [PubMed] [Google Scholar]
- 168.Puig S, Thiele DJ. Molecular mechanisms of copper uptake and distribution. Curr Opin Chem Biol. 2002;6:171–80. doi: 10.1016/s1367-5931(02)00298-3. [DOI] [PubMed] [Google Scholar]
- 169.Carlton WW. Studies on the induction of hydrocephalus and spongy degeneration by cuprizone feeding and attempts to antidote the toxicity. Life Sci. 1967;6:11–9. doi: 10.1016/0024-3205(67)90356-6. [DOI] [PubMed] [Google Scholar]
- 170.Matsushima GK, Morell P. The neurotoxicant, cuprizone, as a model to study demyelination and remyelination in the central nervous system. Brain Pathol. 2001;11:107–16. doi: 10.1111/j.1750-3639.2001.tb00385.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Wilson SAK. Progressive lenticular degeneration: a familial nervous disease associated with cirrhosis of the liver. Brain. 1912;34:295–507. doi: 10.1093/brain/awp193. [DOI] [PubMed] [Google Scholar]
- 172.de Bie P, Muller P, Wijmenga C, Klomp LW. Molecular pathogenesis of Wilson and Menkes disease: correlation of mutations with molecular defects and disease phenotypes. J Med Genet. 2007;44:673–88. doi: 10.1136/jmg.2007.052746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.van Wassenaer-van Hall HN, van den Heuvel AG, Jansen GH, Hoogenraad TU, Mali WP. Cranial MR in Wilson disease: abnormal white matter in extrapyramidal and pyramidal tracts. AJNR Am J Neuroradiol. 1995;16:2021–7. [PMC free article] [PubMed] [Google Scholar]
- 174.Srinivas K, Sinha S, Taly AB, Prashanth LK, Arunodaya GR, Janardhana Reddy YC, Khanna S. Dominant psychiatric manifestations in Wilson’s disease: a diagnostic and therapeutic challenge! J Neurol Sci. 2008;266:104–8. doi: 10.1016/j.jns.2007.09.009. [DOI] [PubMed] [Google Scholar]
- 175.Wichowicz HM, Cubala WJ, Slawek J. Wilson’s disease associated with delusional disorder. Psychiatry Clin Neurosci. 2006;60:758–60. doi: 10.1111/j.1440-1819.2006.01592.x. [DOI] [PubMed] [Google Scholar]
- 176.Dening TR. Psychiatric aspects of Wilson’s disease. Br J Psychiatry. 1985;147:677–82. doi: 10.1192/bjp.147.6.677. [DOI] [PubMed] [Google Scholar]
- 177.Prohaska JR, Smith TL. Effect of dietary or genetic copper deficiency on brain catecholamines, trace metals and enzymes in mice and rats. J Nutr. 1982;112:1706–17. doi: 10.1093/jn/112.9.1706. [DOI] [PubMed] [Google Scholar]
- 178.Menkes JH, Alter M, Steigleder GK, Weakley DR, Sung JH. A sex-linked recessive disorder with retardation of growth, peculiar hair, and focal cerebral and cerebellar degeneration. Pediatrics. 1962;29:764–79. [PubMed] [Google Scholar]
- 179.Barnard RO, Best PV, Erdohazi M. Neuropathology of Menkes’ disease. Dev Med Child Neurol. 1978;20:586–97. doi: 10.1111/j.1469-8749.1978.tb15277.x. [DOI] [PubMed] [Google Scholar]
- 180.Vagn-Hansen L, Reske-Nielsen E, Lou HC. Menkes’ disease--a new leucodystrophy (?). A clinical and neuropathological review together with a new case. Acta Neuropathol. 1973;25:103–19. doi: 10.1007/BF00687555. [DOI] [PubMed] [Google Scholar]
- 181.Liu PC, Chen YW, Centeno JA, Quezado M, Lem K, Kaler SG. Downregulation of myelination, energy, and translational genes in Menkes disease brain. Mol Genet Metab. 2005;85:291–300. doi: 10.1016/j.ymgme.2005.04.007. [DOI] [PubMed] [Google Scholar]
- 182.Kaler SG, Holmes CS, Goldstein DS, Tang J, Godwin SC, Donsante A, Liew CJ, Sato S, Patronas N. Neonatal diagnosis and treatment of Menkes disease. N Engl J Med. 2008;358:605–14. doi: 10.1056/NEJMoa070613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Heilmeyer L, Keiderling W, Struve C. Kupfer und Eisen als koerpereigene Wirkstoffe und ihre Bedeutung beim Krankheitsgeschehen. Fischer; Jena: 1941. [Google Scholar]
- 184.Bowman MB, Lewis MS. The copper hypothesis of schizophrenia: a review. Neurosci Biobehav Rev. 1982;6:321–8. doi: 10.1016/0149-7634(82)90044-6. [DOI] [PubMed] [Google Scholar]
- 185.Yanik M, Kocyigit A, Tutkun H, Vural H, Herken H. Plasma manganese, selenium, zinc, copper, and iron concentrations in patients with schizophrenia. Biol Trace Elem Res. 2004;98:109–17. doi: 10.1385/BTER:98:2:109. [DOI] [PubMed] [Google Scholar]
- 186.Olatunbosun DA, Akindele MO, Adadevoh BK, Asuni T. Serum copper in schizophrenia in Nigerians. Br J Psychiatry. 1975;127:119–21. doi: 10.1192/bjp.127.2.119. [DOI] [PubMed] [Google Scholar]
- 187.Rahman A, Azad MA, Hossain I, Qusar MM, Bari W, Begum F, Huq SM, Hasnat A. Zinc, manganese, calcium, copper, and cadmium level in scalp hair samples of schizophrenic patients. Biol Trace Elem Res. 2009;127:102–8. doi: 10.1007/s12011-008-8230-8. [DOI] [PubMed] [Google Scholar]
- 188.Shore D, Potkin SG, Weinberger DR, Torrey EF, Henkin RI, Agarwal RP, Gillin JC, Wyatt RJ. CSF copper concentrations in chronic schizophrenia. Am J Psychiatry. 1983;140:754–7. doi: 10.1176/ajp.140.6.754. [DOI] [PubMed] [Google Scholar]
- 189.Kornhuber J, Lange KW, Kruzik P, Rausch WD, Gabriel E, Jellinger K, Riederer P. Iron, copper, zinc, magnesium, and calcium in postmortem brain tissue from schizophrenic patients. Biol Psychiatry. 1994;36:31–4. doi: 10.1016/0006-3223(94)90059-0. [DOI] [PubMed] [Google Scholar]
- 190.Tada K, Nogami Y, Nagashima M, Nagase T, Ishiwata H, Motegi Y, Ikeda M. Trace elements in the hair of schizophrenics. Biol Psychiatry. 1986;21:325–8. doi: 10.1016/0006-3223(86)90056-9. [DOI] [PubMed] [Google Scholar]
- 191.Herran A, Garcia-Unzueta MT, Fernandez-Gonzalez MD, Vazquez-Barquero JL, Alvarez C, Amado JA. Higher levels of serum copper in schizophrenic patients treated with depot neuroleptics. Psychiatry Res. 2000;94:51–8. doi: 10.1016/s0165-1781(00)00126-8. [DOI] [PubMed] [Google Scholar]
- 192.Gitlin D, Janeway CA. Turnover of the copper and protein moieties of ceruloplasmin. Nature. 1960;185:693. doi: 10.1038/185693a0. [DOI] [PubMed] [Google Scholar]
- 193.Zahs KR, Bigornia V, Deschepper CF. Characterization of “plasma proteins” secreted by cultured rat macroglial cells. Glia. 1993;7:121–33. doi: 10.1002/glia.440070202. [DOI] [PubMed] [Google Scholar]
- 194.Klomp LW, Gitlin JD. Expression of the ceruloplasmin gene in the human retina and brain: implications for a pathogenic model in aceruloplasminemia. Hum Mol Genet. 1996;5:1989–96. doi: 10.1093/hmg/5.12.1989. [DOI] [PubMed] [Google Scholar]
- 195.Alias AG, Vijayan N, Nair DS, Sukumaran M. Serum ceruloplasmin in schizophrenia: significant increase in acute cases especially in catatonia. Biol Psychiatry. 1972;4:231–8. [PubMed] [Google Scholar]
- 196.Wolf TL, Kotun J, Meador-Woodruff JH. Plasma copper, iron, ceruloplasmin and ferroxidase activity in schizophrenia. Schizophr Res. 2006;86:167–71. doi: 10.1016/j.schres.2006.05.027. [DOI] [PubMed] [Google Scholar]
- 197.Taylor LC, Gilmore W, Matsushima GK. SJL mice exposed to cuprizone intoxication reveal strain and gender pattern differences in demyelination. Brain Pathol. 2009;19:467–79. doi: 10.1111/j.1750-3639.2008.00230.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Skripuletz T, Lindner M, Kotsiari A, Garde N, Fokuhl J, Linsmeier F, Trebst C, Stangel M. Cortical demyelination is prominent in the murine cuprizone model and is strain-dependent. Am J Pathol. 2008;172:1053–61. doi: 10.2353/ajpath.2008.070850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Gudi V, Moharregh-Khiabani D, Skripuletz T, Koutsoudaki PN, Kotsiari A, Skuljec J, Trebst C, Stangel M. Regional differences between grey and white matter in cuprizone induced demyelination. Brain Res. 2009;1283:127–38. doi: 10.1016/j.brainres.2009.06.005. [DOI] [PubMed] [Google Scholar]
- 200.Hoffmann K, Lindner M, Groticke I, Stangel M, Loscher W. Epileptic seizures and hippocampal damage after cuprizone-induced demyelination in C57BL/6 mice. Exp Neurol. 2008;210:308–21. doi: 10.1016/j.expneurol.2007.11.005. [DOI] [PubMed] [Google Scholar]
- 201.Koutsoudaki PN, Skripuletz T, Gudi V, Moharregh-Khiabani D, Hildebrandt H, Trebst C, Stangel M. Demyelination of the hippocampus is prominent in the cuprizone model. Neurosci Lett. 2009;451:83–8. doi: 10.1016/j.neulet.2008.11.058. [DOI] [PubMed] [Google Scholar]
- 202.Norkute A, Hieble A, Braun A, Johann S, Clarner T, Baumgartner W, Beyer C, Kipp M. Cuprizone treatment induces demyelination and astrocytosis in the mouse hippocampus. J Neurosci Res. 2009;87:1343–55. doi: 10.1002/jnr.21946. [DOI] [PubMed] [Google Scholar]
- 203.Groebe A, Clarner T, Baumgartner W, Dang J, Beyer C, Kipp M. Cuprizone treatment induces distinct demyelination, astrocytosis, and microglia cell invasion or proliferation in the mouse cerebellum. Cerebellum. 2009;8:163–74. doi: 10.1007/s12311-009-0099-3. [DOI] [PubMed] [Google Scholar]
- 204.Ludwin SK. Central nervous system demyelination and remyelination in the mouse: an ultrastructural study of cuprizone toxicity. Lab Invest. 1978;39:597–612. [PubMed] [Google Scholar]
- 205.Skripuletz T, Bussmann JH, Gudi V, Koutsoudaki PN, Pul R, Moharregh-Khiabani D, Lindner M, Stangel M. Cerebellar Cortical Demyelination in the Murine Cuprizone Model. Brain Pathol. 2009 doi: 10.1111/j.1750-3639.2009.00271.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Morell P, Barrett CV, Mason JL, Toews AD, Hostettler JD, Knapp GW, Matsushima GK. Gene expression in brain during cuprizone-induced demyelination and remyelination. Mol Cell Neurosci. 1998;12:220–7. doi: 10.1006/mcne.1998.0715. [DOI] [PubMed] [Google Scholar]
- 207.Pasquini LA, Calatayud CA, Bertone Una AL, Millet V, Pasquini JM, Soto EF. The neurotoxic effect of cuprizone on oligodendrocytes depends on the presence of pro-inflammatory cytokines secreted by microglia. Neurochem Res. 2007;32:279–92. doi: 10.1007/s11064-006-9165-0. [DOI] [PubMed] [Google Scholar]
- 208.Seiwa C, Yamamoto M, Tanaka K, Fukutake M, Ueki T, Takeda S, Sakai R, Ishige A, Watanabe K, Akita M, Yagi T, Asou H. Restoration of FcRgamma/Fyn signaling repairs central nervous system demyelination. J Neurosci Res. 2007;85:954–66. doi: 10.1002/jnr.21196. [DOI] [PubMed] [Google Scholar]
- 209.Cammer W. The neurotoxicant, cuprizone, retards the differentiation of oligodendrocytes in vitro. J Neurol Sci. 1999;168:116–20. doi: 10.1016/s0022-510x(99)00181-1. [DOI] [PubMed] [Google Scholar]
- 210.Venturini G. Enzymic activities and sodium, potassium and copper concentrations in mouse brain and liver after cuprizone treatment in vivo. J Neurochem. 1973;21:1147–51. doi: 10.1111/j.1471-4159.1973.tb07569.x. [DOI] [PubMed] [Google Scholar]
- 211.Hoppel CL, Tandler B. Biochemical effects of cuprizone on mouse liver and heart mitochondria. Biochem Pharmacol. 1973;22:2311–8. doi: 10.1016/0006-2952(73)90012-9. [DOI] [PubMed] [Google Scholar]
- 212.Armstrong RC, Le TQ, Flint NC, Vana AC, Zhou YX. Endogenous cell repair of chronic demyelination. J Neuropathol Exp Neurol. 2006;65:245–56. doi: 10.1097/01.jnen.0000205142.08716.7e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Lindner M, Fokuhl J, Linsmeier F, Trebst C, Stangel M. Chronic toxic demyelination in the central nervous system leads to axonal damage despite remyelination. Neurosci Lett. 2009;453:120–5. doi: 10.1016/j.neulet.2009.02.004. [DOI] [PubMed] [Google Scholar]
- 214.Franco-Pons N, Torrente M, Colomina MT, Vilella E. Behavioral deficits in the cuprizone-induced murine model of demyelination/remyelination. Toxicol Lett. 2007;169:205–13. doi: 10.1016/j.toxlet.2007.01.010. [DOI] [PubMed] [Google Scholar]
- 215.Hibbits N, Pannu R, John Wu T, Armstrong RC. Cuprizone demyelination of the corpus callosum in mice correlates with altered social interaction and impaired bilateral sensorimotor coordination. ASN Neuro. 2009;1 doi: 10.1042/AN20090032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Liebetanz D, Merkler D. Effects of commissural de- and remyelination on motor skill behaviour in the cuprizone mouse model of multiple sclerosis. Exp Neurol. 2006;202:217–24. doi: 10.1016/j.expneurol.2006.05.032. [DOI] [PubMed] [Google Scholar]
- 217.Xu H, Yang HJ, Zhang Y, Clough R, Browning R, Li XM. Behavioral and neurobiological changes in C57BL/6 mice exposed to cuprizone. Behav Neurosci. 2009;123:418–29. doi: 10.1037/a0014477. [DOI] [PubMed] [Google Scholar]
- 218.Xiao L, Xu H, Zhang Y, Wei Z, He J, Jiang W, Li X, Dyck LE, Devon RM, Deng Y, Li XM. Quetiapine facilitates oligodendrocyte development and prevents mice from myelin breakdown and behavioral changes. Mol Psychiatry. 2008;13:697–708. doi: 10.1038/sj.mp.4002064. [DOI] [PubMed] [Google Scholar]
- 219.Turetsky BI, Calkins ME, Light GA, Olincy A, Radant AD, Swerdlow NR. Neurophysiological endophenotypes of schizophrenia: the viability of selected candidate measures. Schizophr Bull. 2007;33:69–94. doi: 10.1093/schbul/sbl060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Xu H, Yang H, McConomy B, Browning R, Li X-M. Behavioral and neurobiological changes in C57BL/6 mouse exposed to cuprizone: effects of antipsychotics. Frontiers in Behavioral Neuroscience. 4 doi: 10.3389/fnbeh.2010.00008. epub 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Brunet-Gouet E, Decety J. Social brain dysfunctions in schizophrenia: a review of neuroimaging studies. Psychiatry Res. 2006;148:75–92. doi: 10.1016/j.pscychresns.2006.05.001. [DOI] [PubMed] [Google Scholar]
- 222.Makinodan M, Yamauchi T, Tatsumi K, Okuda H, Takeda T, Kiuchi K, Sadamatsu M, Wanaka A, Kishimoto T. Demyelination in the juvenile period, but not in adulthood, leads to long-lasting cognitive impairment and deficient social interaction in mice. Prog Neuropsychopharmacol Biol Psychiatry. 2009;33:978–85. doi: 10.1016/j.pnpbp.2009.05.006. [DOI] [PubMed] [Google Scholar]
- 223.Adamo AM, Paez PM, Escobar Cabrera OE, Wolfson M, Franco PG, Pasquini JM, Soto EF. Remyelination after cuprizone-induced demyelination in the rat is stimulated by apotransferrin. Exp Neurol. 2006;198:519–29. doi: 10.1016/j.expneurol.2005.12.027. [DOI] [PubMed] [Google Scholar]
- 224.Love S. Cuprizone neurotoxicity in the rat: morphologic observations. J Neurol Sci. 1988;84:223–37. doi: 10.1016/0022-510x(88)90127-x. [DOI] [PubMed] [Google Scholar]
- 225.Purves DC, Garrod IJ, Dayan AD. A comparison of spongiosis induced in the brain by hexachlorophene, cuprizone and triethyl tin in the Sprague-Dawley rat. Hum Exp Toxicol. 1991;10:439–44. doi: 10.1177/096032719101000613. [DOI] [PubMed] [Google Scholar]
- 226.Uylings HB, Groenewegen HJ, Kolb B. Do rats have a prefrontal cortex? Behav Brain Res. 2003;146:3–17. doi: 10.1016/j.bbr.2003.09.028. [DOI] [PubMed] [Google Scholar]
- 227.Uylings HB, van Eden CG. Qualitative and quantitative comparison of the prefrontal cortex in rat and in primates, including humans. Prog Brain Res. 1990;85:31–62. doi: 10.1016/s0079-6123(08)62675-8. [DOI] [PubMed] [Google Scholar]
- 228.Birrell JM, Brown VJ. Medial frontal cortex mediates perceptual attentional set shifting in the rat. J Neurosci. 2000;20:4320–4. doi: 10.1523/JNEUROSCI.20-11-04320.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Harvey PD, Green MF, Keefe RS, Velligan DI. Cognitive functioning in schizophrenia: a consensus statement on its role in the definition and evaluation of effective treatments for the illness. J Clin Psychiatry. 2004;65:361–72. [PubMed] [Google Scholar]
- 230.Green MF, Kern RS, Braff DL, Mintz J. Neurocognitive deficits and functional outcome in schizophrenia: are we measuring the “right stuff”? Schizophr Bull. 2000;26:119–36. doi: 10.1093/oxfordjournals.schbul.a033430. [DOI] [PubMed] [Google Scholar]
- 231.Emsley R, Chiliza B, Schoeman R. Predictors of long-term outcome in schizophrenia. Curr Opin Psychiatry. 2008;21:173–7. doi: 10.1097/YCO.0b013e3282f33f76. [DOI] [PubMed] [Google Scholar]
- 232.Tomida K, Takahashi N, Saito S, Maeno N, Iwamoto K, Yoshida K, Kimura H, Iidaka T, Ozaki N. Relationship of psychopathological symptoms and cognitive function to subjective quality of life in patients with chronic schizophrenia. Psychiatry Clin Neurosci. 2009 doi: 10.1111/j.1440-1819.2009.02033.x. [DOI] [PubMed] [Google Scholar]
- 233.Franke P, Maier W, Hain C, Klingler T. Wisconsin Card Sorting Test: an indicator of vulnerability to schizophrenia? Schizophr Res. 1992;6:243–9. doi: 10.1016/0920-9964(92)90007-r. [DOI] [PubMed] [Google Scholar]
- 234.Haut MW, Cahill J, Cutlip WD, Stevenson JM, Makela EH, Bloomfield SM. On the nature of Wisconsin Card Sorting Test performance in schizophrenia. Psychiatry Res. 1996;65:15–22. doi: 10.1016/0165-1781(96)02940-x. [DOI] [PubMed] [Google Scholar]
- 235.McAlonan K, Brown VJ. Orbital prefrontal cortex mediates reversal learning and not attentional set shifting in the rat. Behav Brain Res. 2003;146:97–103. doi: 10.1016/j.bbr.2003.09.019. [DOI] [PubMed] [Google Scholar]