Abstract
Background
Neurotensin receptors (NTS) regulate a variety of the biological functions of neurotensin (NT) in the central nervous system. Although NT and NT receptor type 1 (NTS1) are implicated in some of the behavioral effects of ethanol, the functional roles of NT receptor type 2 (NTS2) in ethanol intoxication and consumption remain unknown. Here we investigated behavioral effects mediated by NTS2 in response to ethanol, which are implicated in ethanol consumption and preference, using NTS2 null mice.
Method
First, we examined ethanol-induced locomotion, ataxia, hypnosis, and hypothermia in NTS2 null mice. Next, we measured ethanol consumption and preference in NTS2 null mice by giving them free choice between ethanol- and tap water-containing bottles. Then using a brain-permeable neurotensin analog, NT69L, we examined the role of NTS2 in locomotor activity and ataxia. Finally, we examined the effect of NT69L on ethanol consumption and preference in NTS2 null mice.
Results
We found that NTS2 null mice appear less sensitive to the acute hypnotic effects of ethanol and consumed more ethanol compared to wild-type littermates in a two-bottle choice experiment, even though ethanol-induced locomotion, ataxia, and hypothermia were similar between genotypes. Interestingly, the administration of NT69L for 4 consecutive days significantly reduced alcohol consumption and preference in wild-type littermates as well as in NTS2 null mice.
Conclusions
Our findings suggest that NTS2 regulates ethanol-induced hypnosis and ethanol consumption.
Keywords: Neurotensin, G-Protein Coupled Receptor, NT69L, Alcohol Preference, Knockout Mice
INTRODUCTION
Alcohol use disorders impose major public health and social problems with substantial worldwide economic loss (Harwood et al., 1998), but limited treatment methods are available mainly due to the complex neurobiological nature of the disease (Koob and Le Moal, 2006; Koob and Volkow, 2010). Investigating the diverse action of neurotransmitters is an important step towards the understanding of molecular bases of alcoholism as well as to develop novel therapeutic methods (Choi et al., 2008).
Neurotensin (NT) is a tridecapeptide neurotransmitter in the brain and is widely expressed not only in the central nervous system, but also in peripheral tissues (Caceda et al., 2006; Carraway and Leeman, 1973; Tyler-McMahon et al., 2000a). NT exerts its effects through three NT receptors: NT receptor types 1, 2 and 3 (NTS1, NTS2 and NTS3) (Vincent et al., 1999). Both NTS1 and NTS2 are G-protein coupled receptors with a 64% amino acid sequence homology in rodents (Chalon et al., 1996; Vincent et al., 1999), and affect downstream signaling of the Phospholipase C β (PLCβ)- Protein Kinase C (PKC) pathway (Perron et al., 2007). However, structurally unrelated NTS3 is a single transmembrane protein (Mazella et al., 1998) that functions in hormone trafficking or NT uptake (Navarro et al., 2001). NTS1 shows a slightly higher affinity for NT than does NTS2, whereas NTS3 has an over 1000-fold lower affinity for NT relative to the other two receptors.
NT receptors are expressed ubiquitously throughout the CNS (Caceda et al., 2006; Chalon et al., 1996; Mazella, 2001; Sarret et al., 2003a). NTS2 is expressed in the substantia nigra, ventral tegmental area, olfactory bulb, bed nucleus of the stria terminalis, preoptic nucleus, amygdaloid complex, anterior thalamic nucleus, cerebellar cortex, periaqueductal grey, dorsal raphe, reticular nucleus, and raphe magnus (Mazella et al., 1996; Sarret et al., 1998; Sarret et al., 2003b; Walker et al., 1998). NTS1 expression overlaps with that of NTS2 in most brain regions, with the exception of the amygdala where NTS1 is not expressed (Caceda et al., 2006; Tanaka et al., 1990; Vita et al., 1993). NTS3 is also broadly expressed in the brain (Caceda et al., 2006; Mazella, 2001; Sarret et al., 2003a).
A large body of evidence supports a role for NT transmission in ethanol-mediated physiological and behavioral changes. NT mimics some of the disruptive effects of a hypnotic dose of ethanol on thermoregulation and locomotor activity (Clineschmidt et al., 1979; Hernandez et al., 1984; Jolicoeur et al., 1981; Nemeroff et al., 1977) and enhances behavioral sensitivity to ethanol-induced hypothermia, locomotor inhibition, and sedation (Erwin et al., 1987; Erwin and Su, 1989; Luttinger et al., 1981; Widdowson, 1987). Significantly lower concentrations of NT have been measured in the prefrontal cortex of alcohol-preferring rats in comparison to non-preferring rats (Ehlers et al., 1999). Furthermore, quantitative trait loci (QTL) mapping studies have shown genetic correlations between alcohol sensitivity and NTS1 in several brain regions (Erwin et al., 2001; Erwin et al., 1997; Radcliffe et al., 2000). Recently, we reported that some of the behavioral effects implicated in ethanol preference and consumption are mediated by NTS1 (Lee et al., 2010).
The synthetic NT analog, NT69L, is a brain-permeable, degradation-resistant peptide that can be administered systematically (Boules et al., 2006). NT69L binds with highest affinity to human NTS1 followed by NTS2 (Kd = 1.55 and 2.10 nM, respectively) (Boules et al., 2006) and shows a prolonged half-life (>130 h) compared to that of NT (Tyler-McMahon et al., 2000b). Our recent work showing that NT69L markedly reduces ethanol preference and consumption in mice provides new evidence that NT signaling regulates ethanol intoxication and intake (Lee et al., 2010).
Although NT and NTS1 contribute to some of the behavioral effects of ethanol, the functional roles of NTS2 in ethanol intoxication, consumption or preference remain unknown. Here we demonstrate some of the behavioral effects in response to ethanol or NT69L mediated by NTS2, which are implicated in ethanol consumption and preference. Our findings provide possible behavioral mechanisms underlying increased alcohol consumption in NTS2 null mice.
MATERIALS AND METHODS
Animals
NT receptor type 2 (NTS2) null mice were generated as described (Liang et al., 2010) and maintained in C57BL/6J background. Mice were crossed with wild-type 129X1/SvJ mice and then crossed between a heterozygous population to generate F2 NTS2 null mice with a ~50% C57BL/6J and ~50% 129X1/SvJ genetic background. The C57BL/6J and 129X1/SvJ mice were purchased from the Jackson laboratory (Bar Harbor, ME). Mice were genotyped by PCR utilizing purified tail DNA (DNeasy Kit, Qiagen) with two NTS2-specific primers (5’GTC CAT TCC CCA CCT CAG AAG 3’, 5’GCA CCC TCC TGG TAT CAC ACT G 3’) and a neo primer (5’CCT TCT ATC GCC TTC TTG ACG AG 3’) which produced 367 bp for wild-type and 563 bp for NTS2 null alleles as described (Liang et al., 2010). Only male mice were used in experiments when they reached 6 to 12 weeks old. All mice used in the experiments were group-housed (4-5 mice per group), with the exception of the group that was individually housed during the two-bottle choice experiment, in standard Plexiglas cages with rodent chow and water available ad libitum. The cages were maintained on a 12-h light/dark cycle with lights on at 6:00 a.m. The same group of mice was used for open-field activity, ataxia, hypothermia, and BEC experiments. We used different groups of mice for LORR, two-bottle choice drinking experiments, and for examining the effect of NT69L. Animal care and handling procedures were approved by Mayo Clinic Institutional Animal Care and Use Committee in accordance with NIH guidelines.
Open-field activity
Spontaneous locomotor activity was measured in Plexiglas chambers (27 cm × 27 cm; Med Associates, Lafayette, IN) as previously described (Chen et al., 2010; Chen et al., 2007; Lee et al., 2010). The chambers were located in sound-attenuating cubicles and equipped with two sets of 16 pulse-modulated infrared photobeams to record X–Y ambulatory movements at a 50 ms resolution (Med Associates, Lafayette, IN). After a three-day habituation period, mice were weighed, given a 1.5 g/kg ethanol injection [20% ethanol (v/v) in 0.9% NaCl, i.p. injection] and observed in open-field chambers for 1 h. To assess the effect of NT69L, mice were weighed, given 1.0 mg/kg NT69L (0.12 mg/ml in 0.9% NaCl, i.p. injection) and observed in open-field chambers for 1 h. Horizontal distance traveled (cm) was recorded for 3 consecutive 20 min intervals. Distance traveled in 20 min intervals was calculated from the locomotor activity data.
Ataxia
Ethanol- and NT69L-induced ataxia (motor incoordination) was evaluated using a constant velocity rotarod treadmill (UGO Basile, Verese, Italy) (Rustay et al., 2003) at a fixed speed of 20 rpm as described (Chen et al., 2010; Lee et al., 2010). Mice were acclimatized to the rotarod treadmill by placing them on the apparatus 2-3 times. Only mice that were able to remain on the rotarod for 180 sec were used for the ataxia experiments. Five mice were evaluated simultaneously and each mouse was injected (i.p.) with ethanol [1.5 g/kg; 20% (v/v) in 0.9% NaCl] 15 min prior to the actual motor coordination experiment. For the effect of NT69L, mice were given 1.0 mg/kg NT69L (0.12 mg/ml in 0.9% NaCl, i.p. injection) 15 min before putting them on the rotarod. The degree of ataxia was evaluated for each mouse by measuring latency to fall during every sequential 15 min interval for 1 h.
Loss of righting reflex
Mice were given 3.2 g/kg (i.p.) or 3.6 g/kg (i.p.) ethanol [20% (v/v) in 0.9% NaCl] and intermittently placed on their backs to test for a loss of righting reflex. Duration of the loss of righting reflex was determined when the mouse could position itself onto all four paws, three times in a 30 s time period. A one-week interval between the two ethanol doses was used to minimize the effect of repetitive injections.
Hypothermia
The hypothermic effect of ethanol was examined by measuring rectal temperature with the use of a Physiomex (physiological recorder) equipped with a T-type thermocouple (Columbus, OH) at room temperature (22 ± 0.5°C) before and after an intraperitoneal injection of 3.2 g/kg or 3.6 g/kg ethanol [20% (v/v) in 0.9% NaCl]. Rectal temperature was assessed 30 min, 1, 2, and 3 h after the ethanol injection. A one-week interval between ethanol doses was used to minimize the effect of repetitive injections.
Blood ethanol clearance
Blood ethanol clearance was measured by injecting mice with 3.2 g/kg or 3.6 g/kg (i.p.) ethanol [20% (v/v) in 0.9% NaCl] and immediately returning them to their home cages as described (Lee et al., 2010). A one-week interval between ethanol doses was used to minimize the effect of repetitive injections. Approximately 50 μl of blood was collected from each mouse 30 min, 1, 2, and 3 h after the ethanol injection via tail bleeding. Plasma ethanol levels were determined using Analox AM1 (Analox Instrument USA, Lunenburg, MA).
Alcohol self-administration and taste preference
Oral alcohol self-administration and preference were examined using a two-bottle choice experiment (Choi et al., 2004; Lee et al., 2010). Simply, mice were given 24 h access to two bottles, one containing plain tap water and the other containing an ethanol solution. The concentration of ethanol was raised every fourth day, increasing from 3 to 6 to 10% (v/v) ethanol. The positions of the bottles were changed every 2 days to control for position preference. One week after the ethanol self-administration experiment, the same mice were tested for saccharin (sweet) and quinine (bitter) solution intake and preference to detect taste neophobias. Saccharin sodium salt and quinine hemisulphate salt (Sigma, St. Louis, Missouri) were dissolved in tap water. The concentration of saccharin (0.03 and 0.06%) and quinine (30 and 60 μM) were raised every fourth day and the positions of the bottles were changed every 2 days to control for position preference. To examine the effect of NT69L in ethanol consumption and preference, mice were given 1.0 mg/kg NT69L (0.12 mg/ml in 0.9% NaCl, i.p. injection) every 12 h for four additional days while mice steadily consumed 10% (v/v) ethanol in a modified two-bottle drinking experiment as described (Lee et al., 2010). Throughout the experiments, fluid intake and body weight were measured every 2 days using an analytical balance. Average ethanol consumption per day was obtained for each ethanol concentration. Ethanol evaporation was taken into account by having 3-4 control cages with ethanol and water containing bottles but no mouse. These bottles were also measured every 2 days. The total volume of liquid evaporated (water or ethanol) was averaged between the 3-4 control cages and then averaged over the 2-day measurement period to determine per day alcohol or water evaporation. The volume of water or ethanol that evaporated was subtracted from the total consumption of water or ethanol of each mouse. To obtain an accurate measure of ethanol consumption that correlated for individual physical differences in mouse size, grams of ethanol consumed per kilogram of body weight per day were calculated for each mouse. As a measure of relative ethanol preference, ethanol preference ratios were calculated at each ethanol concentration by dividing the total ethanol solution consumption (standardized for evaporation) by the total fluid (ethanol plus water) consumption.
Statistical analyses
All data are presented as mean ± SEM (standard error of the mean). For the rotarod, open field locomotor activity, hypothermia, blood alcohol concentration tests, and the two-bottle choice experiment, statistical analyses were carried out by two-way repeated measures ANOVA followed by Tukey post-hoc test. To examine the effect of NT69L during the two-bottle choice experiment, one-way repeated measures ANOVA followed by Tukey post-hoc test was used. All other data were analyzed using two-tailed student's t-test. Criterion for statistical significance was p < 0.05.
RESULTS
Ethanol-induced locomotor activity, ataxia, hypnosis, and hypothermia in NTS2 null mice
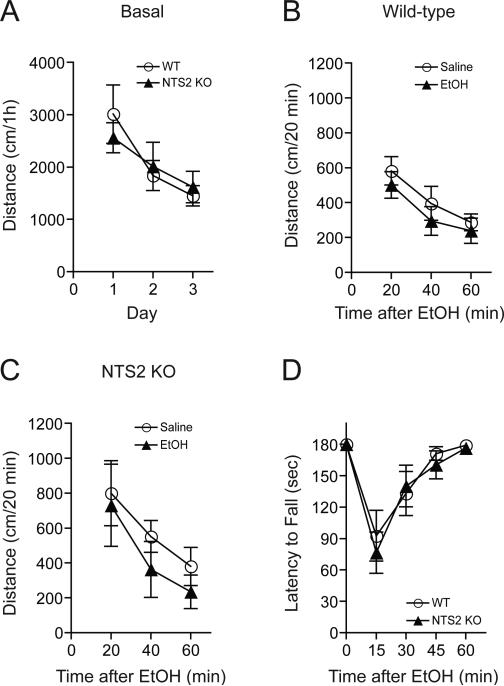
First, we examined ethanol-induced locomotor activity using NTS2 null mice. There were no differences in basal locomotion (Fig. 1A) or locomotion following 1.5 g/kg ethanol treatment (20% ethanol in 0.9% NaCl; i.p. injection) in wild-type mice (Fig. 1B) and NTS2 null mice (Fig. 1C) compared to equivalent volume of saline (i.p.) injection groups. Next, we tested ethanol-induced ataxia with the use of a constant velocity rotarod. NTS2 null mice did not show a significant reduction in ethanol-induced ataxia after a 1.5 g/kg ethanol i.p. injection compared to their wild-type littermates (Fig. 1D).
Fig. 1.
Ethanol-induced locomotor activity and ataxia in NTS2 null and wild-type mice. (A) No significant differences in basal spontaneous locomotor activity were observed in NTS2 null mice compared to their wild-type littermates (n = 13 for each genotype). (B-C) Administration of 1.5 g/kg ethanol (i.p.) did not induce any significant changes in locomotor activity in wild-type mice (B) or NTS2 null mice (C) (n = 8 for each genotype). (D) Similarly, NTS2 null mice showed no significant reduction of ethanol-induced (1.5 g/kg) ataxia in a rotarod test compared to their wild-type littermates (n = 11 for each genotype). All data are expressed as mean ± SEM.
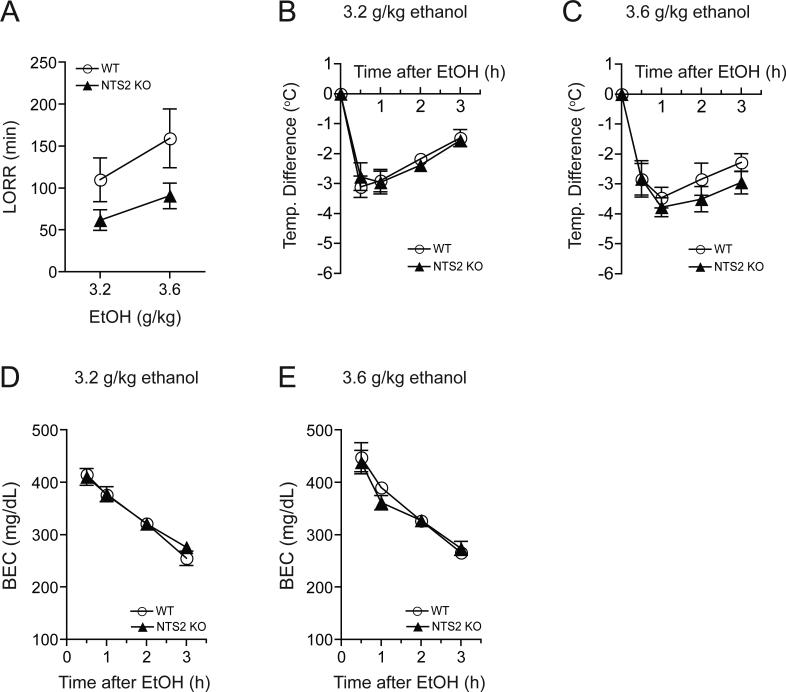
To investigate the role of NTS2 in ethanol-mediated hypnosis, we performed an ethanol-induced loss of righting reflex (LORR) test using 3.2 or 3.6 g/kg ethanol (20% ethanol in 0.9% NaCl; i.p. injection) doses. NTS2 null mice showed a significantly shorter duration of ethanol-induced LORR (Fig. 2A), indicating that NTS2 might contribute to the hypnotic effects of ethanol. Two-way repeated measures ANOVA showed significant effects of genotype [F(1,12) = 5.485, p = 0.034] and ethanol dose [F(1,12) = 5.988, p = 0.031], supporting a difference between genotypes and a longer overall duration of effect after the higher ethanol dose. There was no significant interaction between genotype and ethanol dose.
Fig. 2.
Effect of acute, hypnotic doses of ethanol in NTS2 null and wild-type mice. (A) Reduced hypnotic effect of ethanol measured by the loss of the righting reflex (LORR) in NTS2 null mice, compared to wild-type littermates (n = 8 for each genotype and ethanol dose). There were significant effects of genotype (p = 0.034) and ethanol dose (p = 0.031), without a significant interaction between genotype and ethanol dose. (B-C) However, no significant differences in ethanol-mediated hypothermia after 3.2 g/kg (B) or 3.6 g/kg (C) ethanol administration were observed between NTS2 null (n = 9 for each ethanol dose) and their wild-type littermates (n = 11 for each ethanol dose). (D-E) Similarly, no significant difference in blood ethanol clearances after acute administration of 3.2 g/kg (D) or 3.6 g/kg (E) ethanol were observed in NTS2 null mice compared to their wild-type littermates (n = 10 for each genotype and ethanol dose). All data are expressed as mean ± SEM.
Interestingly, with the administration of either 3.2 g/kg ethanol (Fig. 2B) or 3.6 g/kg ethanol (Fig. 2C), NTS2 null mice displayed similar ethanol-induced hypothermia compared to their wild-type littermates. Also there was no significant difference in basal body temperatures between NTS2 null and their wild-type littermates (data not shown).
Since individual differences in ethanol absorption, distribution, or clearance could contribute to altered acute hypnotic ethanol responses, we measured blood ethanol concentrations 30 min, 1, 2 and 3 h after an injection of 3.2 g/kg (i.p.) ethanol (Fig. 2D) or 3.6 g/kg (i.p.) ethanol (Fig. 2E). Blood ethanol concentrations were similar between NTS2 null mice and wild-type mice at both ethanol concentrations, indicating that there are no alterations in ethanol metabolism between genotypes.
Voluntary ethanol consumption and preference in NTS2 null mice
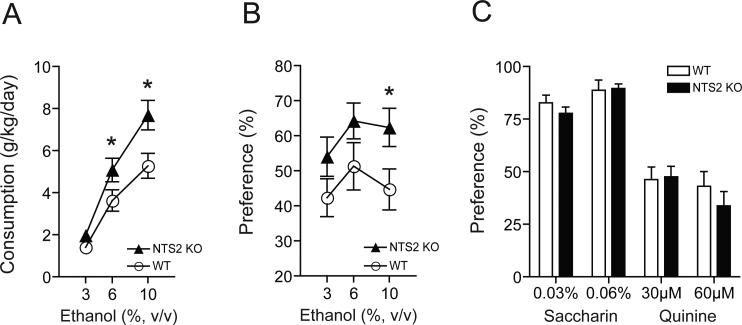
Next, we examined whether absence of NTS2 could alter ethanol consumption or preference using a two-bottle choice drinking experiment (Choi et al., 2004; Lee et al., 2010). NTS2 null mice consumed significantly more ethanol during the 6 and 10% (v/v) ethanol periods compared to their wild-type littermates (Fig. 3A). Two-way repeated measures ANOVA showed significant effects of genotype [F(1,52) = 8.123, p = 0.008] and ethanol concentrations [F(2,52) = 63.012, p < 0.001], but did not show any significant effect of the interaction between genotype and ethanol concentrations. The Tukey post-hoc test showed a significant difference between the ethanol consumption of NTS2 null mice and wild-type mice at 6 and 10% (v/v) ethanol concentrations (p = 0.032 and p = 0.002, respectively). Also, NTS2 null mice showed a significantly higher ethanol preference compared to their wild-type littermates (Fig. 3B). Two-way repeated measures showed a significant effect of genotype [F(1,50) = 5.439, p = 0.028], but did not show any significant effects of ethanol concentrations or interaction between genotype and ethanol concentrations. Also we did not observe any changes in water consumption or mouse body weight between NTS2 null and wild-type mice before or after the two-bottle choice drinking experiment (data not shown).
Fig. 3.
Ethanol consumption and preference of NTS2 null and wild-type mice. (A-B) NTS2 null mice (n = 14) showed increased ethanol consumption (A) and preference (B) in a two-bottle choice experiment. *p < 0.05 compared to the wild-type mice (n = 13) at the same ethanol concentration (Tukey test). (C) No differences in genotype-associated taste preference (saccharin for sweet and quinine for bitter taste) of NTS2 null mice compared to wild-type littermates (n = 10 for each genotype). All data are expressed as mean ± SEM.
Since differential taste reactivity may affect alcohol consumption or preference, we performed a taste preference experiment using saccharin (sweet) and quinine (bitter) solutions (Choi et al., 2002). In this experiment, NTS2 null mice did not show any significant differences in saccharin or quinine preference over water compared to wild-type mice (Fig. 3C). Thus, NTS2 null mice voluntarily consume and prefer more ethanol than their wild-type mice, which is not due to genotype-associated taste preference differences.
NT69L-induced locomotor activity and ataxia in NTS2 null mice
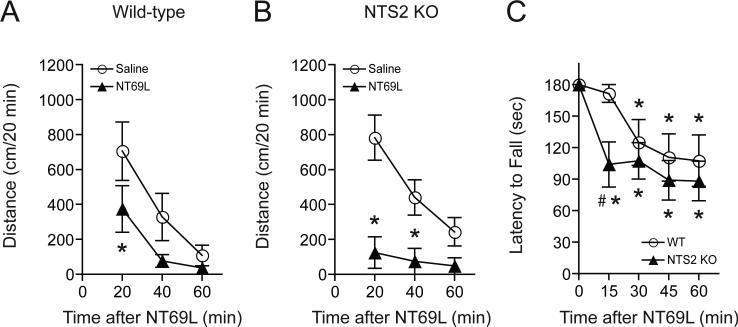
We measured locomotor activity after mice were given either 1.0 mg/kg NT69L (0.12 mg/mL NT69L in 0.9% NaCl; i.p. injection) or an equivalent volume of saline (i.p.). Interestingly, 1.0 mg/kg acute NT69L (i.p.) induced a significant reduction of locomotor activation in both wild-type (Fig. 4A) and NTS2 null mice (Fig. 4B) compared to their respective saline-treated groups. Two-way repeated measures ANOVA for wild-type mice showed significant effects of treatment [F(1,15) = 6.090, p = 0.038] and time [F(2,15) = 22.187, p < 0.001], but did not show any significant effect of the interaction between treatment and time. Two-way repeated measures ANOVA for NTS2 null mice showed significant effects of treatment [F(1,10) = 17.386, p = 0.009], time [F(2,10) = 15.231, p < 0.001], and the interaction between treatment and time [F(2,10) = 14.292, p = 0.001]. The Tukey post-hoc test revealed that wild-type littermates showed reduced locomotion at 20 min after 1.0 mg/kg NT69L treatment, whereas NTS2 null mice showed a more significant locomotion suppression from 20 min to 40 min after the 1.0 mg/kg NT69L treatment, exhibiting a prolonged locomotion suppression effect of NT69L.
Fig. 4.
NT69L-induced locomotor activity and ataxia in NTS2 null and wild-type mice. (A-B) 1.0 mg/kg NT69L (in saline, i.p.) induced a significant reduction in locomotor activation compared to their saline-treated groups both in wild-type mice (A) and NTS2 null mice (B) (n = 9 for wild-type mice and n = 6 for NTS2 null mice; *p < 0.05 by t-test). (C) NTS2 null mice showed an earlier activation of NT69L-mediated ataxia at 15 min after NT69L injection, but a similar NT69L-mediated ataxic compared to their wild-type littermates at 1.0 mg/kg NT69L from 30 min to 60 min after NT69L injection (n = 10 for each genotype). All data are expressed as mean ± SEM. *p < 0.05 compared to their saline-treated groups both in NTS2 null mice and wild-type littermates (Tukey test). #p < 0.05 compared to wild-type littermates (Tukey test).
Next, we examined NT69L-induced ataxia in wild-type and NTS2 null mice. A similar ataxic response was observed between wild-type and NTS2 null mice from 30 min to 60 min when compared to their respective saline-treated groups (Fig. 4C). One-way repeated measures ANOVA showed a significant ataxic effect of NT69L both in wild-type littermates [F(4,36) = 7.115, p < 0.001] and in NTS2 null mice [F(4,36) = 14.69, p < 0.001]. Interestingly, the Tukey post-hoc test revealed that NTS2 null mice showed an ataxic effect from 15 min after the 1.0 mg/kg NT69L treatment, whereas wild-type mice only displayed the effect from 30 min after the treatment. Consistently, two-way repeated measures ANOVA followed by the Tukey post-hoc test to compare between genotypes revealed that NTS2 null mice exhibited significant NT69L-induced ataxia at 15 min compared to wild-type littermates although no effect of genotype was observed. Taken together, these results suggest that NTS2 might counteract the motor-suppressant and ataxic effects of NT.
NTS2 null mice consume less alcohol voluntarily after NT69L treatment
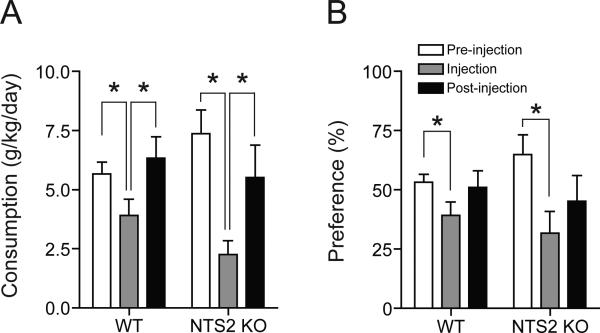
To examine whether NT analog NT69L could regulate ethanol consumption or preference, we evaluated oral ethanol self-administration using a modified two-bottle choice experiment (Lee et al., 2010) with NTS2 null mice. NTS2 null mice consumed significantly less ethanol during the NT69L-injection period as compared to pre- or post-injection periods, similar to the wild-type mice (Fig. 5A). During the post-injection period, the ethanol consumption returned to pre-injection levels. One-way repeated measures ANOVA for wild-type mice showed a significant effect of NT69L treatment [F(2,14) = 22.396, p < 0.001]. Tukey post-hoc test showed a significant difference between pre-injection and NT69L-injection periods (p < 0.001), and between NT69L-injection and post-injection periods (p < 0.001), but showed no difference between pre- and post-injection periods (p > 0.05). Similarly, one-way repeated measures ANOVA for NTS2 null mice showed a significant effect of NT69L treatment [F(2,12) = 17.892, p < 0.001]. Tukey post-hoc test showed a significant difference between pre-injection and NT69L-injection periods (p < 0.001), and between NT69L-injection and post-injection periods (p < 0.001), but showed no difference between pre- and post-injection periods (p > 0.05). For the ethanol preference, NTS2 null mice preferred significantly less ethanol during the NT69L-injection period compared to pre- or post-treatment periods, which is similar to that of wild-type mice (Fig. 5B). Unlike for ethanol consumption, the effect of NT69L on ethanol preference was not fully recovered 4 days after the injection period. One-way repeated measures ANOVA for wild-type mice showed a significant effect of NT69L treatment [F(2,11) = 4.236, p = 0.043]. Tukey post-hoc test showed a significant difference between pre-injection and NT69L-injection periods (p = 0.047), and, but showed no differences between NT69L-injection and post-injection periods and between pre- and post-injection periods. Similarly, one-way repeated measures ANOVA for NTS2 null mice showed a significant effect of NT69L treatment [F(2,11) = 7.331, p = 0.009]. Tukey post-hoc test showed a significant difference between pre-injection and NT69L-injection periods (p = 0.012), and between pre- and post-injection periods (p = 0.039), but showed no difference between NT69L-injection and post-injection periods. Since the ethanol preference ratio can be influenced by water consumption, we measured water consumption and found no difference in NTS2 null mice water consumption throughout the two-bottle choice experiment (data not shown). Taken together, our results indicate that NT69L reduced ethanol consumption and preference significantly compared to baseline values in both NTS2 null and wild-type mice.
Fig. 5.
Effect of NT69L on ethanol consumption and preference in NTS2 null and wild-type mice. (A-B) Ethanol consumption (A) and preference (B) for ethanol are significantly reduced both in wild-type (n = 9) and NTS2 null mice (n = 8) during the NT69L-injection period. Post-injection period indicates 4 days immediately following the NT69L-injection period, whereas pre-injection period refers to 4 days prior to the NT69L-injection period. All injection periods were preformed while mice stably consumed 10% ethanol. All data are expressed as mean ± SEM. *p < 0.05 compared to the pre- or post-injection period as indicated (Tukey test).
DISCUSSION
Our results suggest that NTS2 is implicated in the hypnotic effect of ethanol and in voluntary ethanol drinking behavior. Our data indicate that NTS2 null mice are more resistant to the hypnotic effect of alcohol and drink more ethanol compared to wild-type littermates. However, we found that NTS2 null mice did not display any changes in ethanol-induced locomotor activity or ataxia. Considering a common inverse relationship between ethanol sensitivity and ethanol drinking (Bowers et al., 2000; Choi et al., 2004; Li et al., 1993; Naassila et al., 2002), it is likely that the augmented ethanol consumption of NTS2 null mice is related to insensitivity to ethanol-induced sedation. Surprisingly, however, NTS2 null mice did not display reduced sensitivity to ethanol-induced locomotor activity or ataxia, suggesting that the role of NTS2 in ethanol intake is independent of these effects. This result is in contrast to our recent report that mice lacking NTS1, which show similarly elevated levels of ethanol intake, are resistant to ethanol-induced ataxia on the rotarod (Lee et al., 2010). Thus, with regard to ethanol consumption and sensitivity, it appears that NTS1 is involved in the effect of lower, ataxic doses of ethanol, whereas NTS2 might be responsible for the effect of higher, hypnotic doses of ethanol.
While NTS1 and NTS2 are co-expressed in most brain regions (Boudin et al., 1996; Fassio et al., 2000) and share the same PLCβ-PKC downstream signaling pathway (Perron et al., 2007), studies have shown the genetic deletion of NTS1 or NTS2 in mice leads to different physiological and behavioral changes. NTS2 null mice show reductions in thermal nociception (Maeno et al., 2004), stress-induced analgesia (Lafrance et al., 2010), and fear memory (Yamauchi et al., 2007), while NTS1 null mice are insensitive to NT-induced hypolocomotion (Lee et al., 2010) and inhibition of feeding (Remaury et al., 2002).
It is possible that changes in NTS1 may contribute to some of the ethanol-related measures in the present study. Perron et al. (2007) demonstrated that heterodimerization of NTS1 and NTS2 can occur in COS-7 cells, which could decrease cell surface density of NTS1 without affecting downstream signaling. However, this group found that upon activation, surface NTS1 receptors are more resistant to down-regulation in cells co-expressing NTS1 and NTS2 than in cells expressing NTS1 alone, suggesting that NTS2 stabilizes NTS1 in the plasma membrane. Another recent study showed that NTS1 null and NTS2 null mice exhibit reciprocal up-regulation of NTS1 and NTS2 mRNA expressions compared to wild-type mice in the whole brain (Liang et al., 2010). Thus, up-regulation of NTS1 may explain the normal level of sensitivity to ethanol-induced ataxia and locomotor suppression exhibited by NTS2 null animals. This would further support involvement of the NTS1 receptor in mediating ethanol's ataxic and locomotor effects.
NTS2 may regulate ethanol consumption by altering striatal dopamine signaling involved in reinforcement and reward-seeking behavior. The shell region of the ventral striatum (NAc) receives a dopaminergic projection from the ventral tegmental area (VTA) (Parkinson et al., 1999) and regulates the hedonic properties of rewarding stimuli (Everitt and Robbins, 2005; Everitt and Wolf, 2002). Ethanol (Howard et al., 2009; Robinson et al., 2009; Yim and Gonzales, 2000) and other drugs of abuse increase dopamine levels in this brain region. NT signaling through both NTS1 and NTS2 receptors has been shown to inhibit dopamine receptors in the striatum, with pharmacological effects similar to those of dopamine D2 receptor antagonists (Ferraro et al., 2008; Fuxe et al., 1992). Furthermore, both NTS1 null and NTS2 null mice show elevated basal dopamine levels (Li et al., 2010) and a higher D2/D1 receptor mRNA ratio (Liang et al., 2010). Future investigation clarifying the relationship between NTS2, NTS1, and the dopaminergic system will be essential to understanding the role of NTS2-mediated signaling in ethanol drinking behavior.
NT69L, the brain permeable and longer acting analog of NT, was used to examine the effect of NT in NTS2 and wild-type mice. Previously, we found that mice lacking NTS1 are insensitive to the motor suppressing and ataxic effects of NT69L (Lee et al., 2010). In contrast, NTS2 null mice showed more severe locomotor suppressing and ataxic effects of NT69L compared to their wild-type littermates. The hypersensitivity to these activity measures during NT69L treatment was accompanied by a greater reduction in ethanol consumption in NTS2 null mice versus wild-type controls. As NT69L-induced deficits in locomotor activity mimic those mediated by ethanol (Clineschmidt et al., 1979; Hernandez et al., 1984; Jolicoeur et al., 1981; Nemeroff et al., 1977), it is unclear why NTS2 null mice showed a similar response to ethanol-induced motor deficits while they were more sensitive to those elicited by NT69L. These results may reflect the up-regulation of NTS1 receptors secondary to NTS2 deletion (Liang et al., 2010). This idea is supported by the finding that NT69L preferentially binds to NTS1 over NTS2 (Boules et al., 2006). Taken together, these lines of evidence indicate that NTS1 mediates the motor suppressing and ataxic effects of NT, while NTS2 acts to oppose these effects. However, more research will be necessary to test this hypothesis, and to characterize the mechanisms behind the observed differences between ethanol- and NT69L-induced behavioral changes.
In summary, the present study supplies further evidence for the involvement of NT signaling in regulating sensitivity to ethanol-induced motor deficits and ethanol intake in mice. Moreover, the NT analog NT69L appears to be effective in reducing ethanol intake, particularly in NTS2 null mice. While additional experimentation is needed to clarify the pharmacodynamic or other changes that may influence ethanol-related measures in NTS2 null animals, these data provide a strong case for NT signaling as a promising pharmacological target in the treatment of alcohol use disorders.
ACKNOWLEDGEMENTS
We thank S. Choi for mouse husbandry. We thank M. Boules for providing NT69L compounds. We thank D. Frederixon and C. Ruby for editing the manuscript. This project was funded by the Samuel Johnson Foundation for Genomics of Addiction Program at Mayo Clinic to D.-S. C and in part by grants from the National Institutes of Health (NIH) to D.-S. C (AA015164, AA018779, AA017830-Project 1).
REFERENCES
- Boudin H, Pelaprat D, Rostene W, Beaudet A. Cellular distribution of neurotensin receptors in rat brain: immunohistochemical study using an antipeptide antibody against the cloned high affinity receptor. J Comp Neurol. 1996;373:76–89. doi: 10.1002/(SICI)1096-9861(19960909)373:1<76::AID-CNE7>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- Boules M, Fredrickson P, Richelson E. Bioactive analogs of neurotensin: focus on CNS effects. Peptides. 2006;27:2523–2533. doi: 10.1016/j.peptides.2005.12.018. [DOI] [PubMed] [Google Scholar]
- Bowers BJ, Collins AC, Tritto T, Wehner JM. Mice lacking PKC gamma exhibit decreased anxiety. Behav Genet. 2000;30:111–121. doi: 10.1023/a:1001951104208. [DOI] [PubMed] [Google Scholar]
- Caceda R, Kinkead B, Nemeroff CB. Neurotensin: role in psychiatric and neurological diseases. Peptides. 2006;27:2385–2404. doi: 10.1016/j.peptides.2006.04.024. [DOI] [PubMed] [Google Scholar]
- Carraway R, Leeman SE. The isolation of a new hypotensive peptide, neurotensin, from bovine hypothalami. J Biol Chem. 1973;248:6854–6861. [PubMed] [Google Scholar]
- Chalon P, Vita N, Kaghad M, Guillemot M, Bonnin J, Delpech B, Le Fur G, Ferrara P, Caput D. Molecular cloning of a levocabastine-sensitive neurotensin binding site. FEBS Lett. 1996;386:91–94. doi: 10.1016/0014-5793(96)00397-3. [DOI] [PubMed] [Google Scholar]
- Chen J, Nam HW, Lee MR, Hinton DJ, Choi S, Kim T, Kawamura T, Janak PH, Choi D-S. Altered glutamatergic neurotransmission in the striatum regulates ethanol sensitivity and intake in mice lacking ENT1. Behav Brain Res. 2010;208:636–642. doi: 10.1016/j.bbr.2010.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Rinaldo L, Lim SJ, Young H, Messing RO, Choi DS. The type 1 equilibrative nucleoside transporter regulates anxiety-like behavior in mice. Genes Brain Behav. 2007;6:776–783. doi: 10.1111/j.1601-183X.2007.00311.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi DS, Karpyak VM, Frye MA, Hal-Flavin DK, Mrazek DA. Drug Addiction. In: Waldman SA, Terzic A, editors. Pharmacology and Therapeutics: Principles to Practice. Bermedica Production, Ltd.; 2008. pp. 817–835. [Google Scholar]
- Choi DS, Cascini MG, Mailliard W, Young H, Paredes P, McMahon T, Diamond I, Bonci A, Messing RO. The type 1 equilibrative nucleoside transporter regulates ethanol intoxication and preference. Nat Neurosci. 2004;7:855–861. doi: 10.1038/nn1288. [DOI] [PubMed] [Google Scholar]
- Choi DS, Wang D, Dadgar J, Chang WS, Messing RO. Conditional rescue of protein kinase C epsilon regulates ethanol preference and hypnotic sensitivity in adult mice. J Neurosci. 2002;22:9905–9911. doi: 10.1523/JNEUROSCI.22-22-09905.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clineschmidt BV, McGuffin JC, Bunting PB. Neurotensin: antinocisponsive action in rodents. Eur J Pharmacol. 1979;54:129–139. doi: 10.1016/0014-2999(79)90415-1. [DOI] [PubMed] [Google Scholar]
- Ehlers CL, Somes C, Li TK, Lumeng L, Kinkead B, Owens MJ, Nemeroff CB. Neurontensin studies in alcohol naive, preferring and non-preferring rats. Neuroscience. 1999;93:227–236. doi: 10.1016/s0306-4522(99)00113-x. [DOI] [PubMed] [Google Scholar]
- Erwin VG, Gehle VM, Davidson K, Radcliffe RA. Confirmation of correlations and common quantitative trait loci between neurotensin receptor density and hypnotic sensitivity to ethanol. Alcohol Clin Exp Res. 2001;25:1699–1707. [PubMed] [Google Scholar]
- Erwin VG, Korte A, Marty M. Neurotensin selectively alters ethanol-induced anesthesia in LS/Ibg and SS/Ibg lines of mice. Brain Res. 1987;400:80–90. doi: 10.1016/0006-8993(87)90655-x. [DOI] [PubMed] [Google Scholar]
- Erwin VG, Radcliffe RA, Gehle VM, Jones BC. Common quantitative trait loci for alcohol-related behaviors and central nervous system neurotensin measures: locomotor activation. J Pharmacol Exp Ther. 1997;280:919–926. [PubMed] [Google Scholar]
- Erwin VG, Su NC. Neurotensin and ethanol interactions on hypothermia and locomotor activity in LS and SS mice. Alcohol Clin Exp Res. 1989;13:91–94. doi: 10.1111/j.1530-0277.1989.tb00289.x. [DOI] [PubMed] [Google Scholar]
- Everitt BJ, Robbins TW. Neural systems of reinforcement for drug addiction: from actions to habits to compulsion. Nat Neurosci. 2005;8:1481–1489. doi: 10.1038/nn1579. [DOI] [PubMed] [Google Scholar]
- Everitt BJ, Wolf ME. Psychomotor stimulant addiction: a neural systems perspective. J. Neurosci. 2002;22:3312–3320. doi: 10.1523/JNEUROSCI.22-09-03312.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fassio A, Evans G, Grisshammer R, Bolam JP, Mimmack M, Emson PC. Distribution of the neurotensin receptor NTS1 in the rat CNS studied using an amino-terminal directed antibody. Neuropharmacology. 2000;39:1430–1442. doi: 10.1016/s0028-3908(00)00060-5. [DOI] [PubMed] [Google Scholar]
- Ferraro L, Tomasini MC, Mazza R, Fuxe K, Fournier J, Tanganelli S, Antonelli T. Neurotensin receptors as modulators of glutamatergic transmission. Brain Res Rev. 2008;58:365–373. doi: 10.1016/j.brainresrev.2007.11.001. [DOI] [PubMed] [Google Scholar]
- Fuxe K, O'Connor WT, Antonelli T, Osborne PG, Tanganelli S, Agnati LF, Ungerstedt U. Evidence for a substrate of neuronal plasticity based on pre- and postsynaptic neurotensin-dopamine receptor interactions in the neostriatum. Proc Natl Acad Sci U S A. 1992;89:5591–5595. doi: 10.1073/pnas.89.12.5591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harwood HJ, Fountain D, Livermore G. Economic costs of alcohol abuse and alcoholism. Recent Dev Alcohol. 1998;14:307–330. doi: 10.1007/0-306-47148-5_14. [DOI] [PubMed] [Google Scholar]
- Hernandez DE, Nemeroff CB, Valderrama MH, Prange AJ., Jr. Neurotensin-induced antinociception and hypothermia in mice: antagonism by TRH and structural analogs of TRH. Regul Pept. 1984;8:41–49. doi: 10.1016/0167-0115(84)90027-2. [DOI] [PubMed] [Google Scholar]
- Howard EC, Schier CJ, Wetzel JS, Gonzales RA. The dopamine response in the nucleus accumbens core-shell border differs from that in the core and shell during operant ethanol self-administration. Alcohol Clin Exp Res. 2009;33:1355–1365. doi: 10.1111/j.1530-0277.2009.00965.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jolicoeur FB, Barbeau A, Rioux F, Quirion R, St-Pierre S. Differential neurobehavioral effects of neurotensin and structural analogues. Peptides. 1981;2:171–175. doi: 10.1016/s0196-9781(81)80031-9. [DOI] [PubMed] [Google Scholar]
- Koob GF, Le Moal M. Neurobiology of Addiction. Elsevier; London: 2006. [Google Scholar]
- Koob GF, Volkow ND. Neurocircuitry of addiction. Neuropsychopharmacology. 2010;35:217–238. doi: 10.1038/npp.2009.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lafrance M, Roussy G, Belleville K, Maeno H, Beaudet N, Wada K, Sarret P. Involvement of NTS2 receptors in stress-induced analgesia. Neuroscience. 2010;166:639–652. doi: 10.1016/j.neuroscience.2009.12.042. [DOI] [PubMed] [Google Scholar]
- Lee MR, Hinton DJ, Song JY, Lee KW, Choo C, Johng H, Unal SS, Richelson E, Choi D-S. Neurotensin receptor type 1 regulates ethanol intoxication and consumption in mice. Pharm Biochem Behav. 2010;208:235–241. doi: 10.1016/j.pbb.2010.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li TK, Lumeng L, Doolittle DP. Selective breeding for alcohol preference and associated responses. Behavioral Genetics. 1993;23:163–170. doi: 10.1007/BF01067421. [DOI] [PubMed] [Google Scholar]
- Li Z, Liang Y, Boules M, Gordillo A, Richelson E. Effect of amphetamine on extracellular concentrations of amino acids in striatum in neurotensin subtype 1 and 2 receptor null mice: a possible interaction between neurotensin receptors and amino acid systems for study of schizophrenia. Neuropharmacology. 2010;58:1174–1178. doi: 10.1016/j.neuropharm.2010.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang Y, Boules M, Li Z, Williams K, Miura T, Oliveros A, Richelson E. Hyperactivity of the dopaminergic system in NTS1 and NTS2 null mice. Neuropharmacology. 2010;58:1199–1205. doi: 10.1016/j.neuropharm.2010.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luttinger D, Nemeroff CB, Mason GA, Frye GD, Breese GR, Prange AJ., Jr. Enhancement of ethanol-induced sedation and hypothermia by centrally administered neurotensin, beta-endorphin and bombesin. Neuropharmacology. 1981;20:305–309. doi: 10.1016/0028-3908(81)90139-8. [DOI] [PubMed] [Google Scholar]
- Maeno H, Yamada K, Santo-Yamada Y, Aoki K, Sun YJ, Sato E, Fukushima T, Ogura H, Araki T, Kamichi S, Kimura I, Yamano M, Maeno-Hikichi Y, Watase K, Aoki S, Kiyama H, Wada E, Wada K. Comparison of mice deficient in the high- or low-affinity neurotensin receptors, Ntsr1 or Ntsr2, reveals a novel function for Ntsr2 in thermal nociception. Brain Res. 2004;998:122–129. doi: 10.1016/j.brainres.2003.11.039. [DOI] [PubMed] [Google Scholar]
- Mazella J. Sortilin/neurotensin receptor-3: a new tool to investigate neurotensin signaling and cellular trafficking? Cell Signal. 2001;13:1–6. doi: 10.1016/s0898-6568(00)00130-3. [DOI] [PubMed] [Google Scholar]
- Mazella J, Botto JM, Guillemare E, Coppola T, Sarret P, Vincent JP. Structure, functional expression, and cerebral localization of the levocabastine-sensitive neurotensin/neuromedin N receptor from mouse brain. J Neurosci. 1996;16:5613–5620. doi: 10.1523/JNEUROSCI.16-18-05613.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazella J, Zsurger N, Navarro V, Chabry J, Kaghad M, Caput D, Ferrara P, Vita N, Gully D, Maffrand JP, Vincent JP. The 100-kDa neurotensin receptor is gp95/sortilin, a non-G-protein-coupled receptor. J Biol Chem. 1998;273:26273–26276. doi: 10.1074/jbc.273.41.26273. [DOI] [PubMed] [Google Scholar]
- Naassila M, Ledent C, Daoust M. Low ethanol sensitivity and increased ethanol consumption in mice lacking adenosine A2A receptors. J Neurosci. 2002;22:10487–10493. doi: 10.1523/JNEUROSCI.22-23-10487.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarro V, Martin S, Sarret P, Nielsen MS, Petersen CM, Vincent J, Mazella J. Pharmacological properties of the mouse neurotensin receptor 3. Maintenance of cell surface receptor during internalization of neurotensin. FEBS Lett. 2001;495:100–105. doi: 10.1016/s0014-5793(01)02367-5. [DOI] [PubMed] [Google Scholar]
- Nemeroff CB, Bissette G, Prange AJ, Jr., Loosen PT, Barlow TS, Lipton MA. Neurotensin: central nervous system effects of a hypothalamic peptide. Brain Res. 1977;128:485–496. doi: 10.1016/0006-8993(77)90173-1. [DOI] [PubMed] [Google Scholar]
- Parkinson JA, Olmstead MC, Burns LH, Robbins TW, Everitt BJ. Dissociation in effects of lesions of the nucleus accumbens core and shell on appetitive pavlovian approach behavior and the potentiation of conditioned reinforcement and locomotor activity by D-amphetamine. J Neurosci. 1999;19:2401–2411. doi: 10.1523/JNEUROSCI.19-06-02401.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perron A, Sharif N, Sarret P, Stroh T, Beaudet A. NTS2 modulates the intracellular distribution and trafficking of NTS1 via heterodimerization. Biochem Biophys Res Commun. 2007;353:582–590. doi: 10.1016/j.bbrc.2006.12.062. [DOI] [PubMed] [Google Scholar]
- Radcliffe RA, Bohl ML, Lowe MV, Cycowski CS, Wehner JM. Mapping of quantitative trait loci for hypnotic sensitivity to ethanol in crosses derived from the C57BL/6 and DBA/2 mouse strains. A Alcohol Clin Exp Res. 2000;24:1335–1342. [PubMed] [Google Scholar]
- Remaury A, Vita N, Gendreau S, Jung M, Arnone M, Poncelet M, Culouscou JM, Le Fur G, Soubrie P, Caput D, Shire D, Kopf M, Ferrara P. Targeted inactivation of the neurotensin type 1 receptor reveals its role in body temperature control and feeding behavior but not in analgesia. Brain Res. 2002;953:63–72. doi: 10.1016/s0006-8993(02)03271-7. [DOI] [PubMed] [Google Scholar]
- Robinson DL, Howard EC, McConnell S, Gonzales RA, Wightman RM. Disparity between tonic and phasic ethanol-induced dopamine increases in the nucleus accumbens of rats. Alcohol Clin Exp Res. 2009;33:1187–1196. doi: 10.1111/j.1530-0277.2009.00942.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rustay NR, Wahlsten D, Crabbe JC. Assessment of genetic susceptibility to ethanol intoxication in mice. Proc Natl Acad Sci U S A. 2003;100:2917–2922. doi: 10.1073/pnas.0437273100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarret P, Beaudet A, Vincent JP, Mazella J. Regional and cellular distribution of low affinity neurotensin receptor mRNA in adult and developing mouse brain. J Comp Neurol. 1998;394:344–356. [PubMed] [Google Scholar]
- Sarret P, Krzywkowski P, Segal L, Nielsen MS, Petersen CM, Mazella J, Stroh T, Beaudet A. Distribution of NTS3 receptor/sortilin mRNA and protein in the rat central nervous system. J Comp Neurol. 2003a;461:483–505. doi: 10.1002/cne.10708. [DOI] [PubMed] [Google Scholar]
- Sarret P, Perron A, Stroh T, Beaudet A. Immunohistochemical distribution of NTS2 neurotensin receptors in the rat central nervous system. J Comp Neurol. 2003b;461:520–538. doi: 10.1002/cne.10718. [DOI] [PubMed] [Google Scholar]
- Tanaka K, Masu M, Nakanishi S. Structure and functional expression of the cloned rat neurotensin receptor. Neuron. 1990;4:847–854. doi: 10.1016/0896-6273(90)90137-5. [DOI] [PubMed] [Google Scholar]
- Tyler-McMahon BM, Boules M, Richelson E. Neurotensin: peptide for the next millennium. Regul Pept. 2000a;93:125–136. doi: 10.1016/s0167-0115(00)00183-x. [DOI] [PubMed] [Google Scholar]
- Tyler-McMahon BM, Stewart JA, Farinas F, McCormick DJ, Richelson E. Highly potent neurotensin analog that causes hypothermia and antinociception. Eur J Pharmacol. 2000b;390:107–111. doi: 10.1016/s0014-2999(99)00877-8. [DOI] [PubMed] [Google Scholar]
- Vincent JP, Mazella J, Kitabgi P. Neurotensin and neurotensin receptors. Trends Pharmacol Sci. 1999;20:302–309. doi: 10.1016/s0165-6147(99)01357-7. [DOI] [PubMed] [Google Scholar]
- Vita N, Laurent P, Lefort S, Chalon P, Dumont X, Kaghad M, Gully D, Le Fur G, Ferrara P, Caput D. Cloning and expression of a complementary DNA encoding a high affinity human neurotensin receptor. FEBS Lett. 1993;317:139–142. doi: 10.1016/0014-5793(93)81509-x. [DOI] [PubMed] [Google Scholar]
- Walker N, Lepee-Lorgeoux I, Fournier J, Betancur C, Rostene W, Ferrara P, Caput D. Tissue distribution and cellular localization of the levocabastine-sensitive neurotensin receptor mRNA in adult rat brain. Brain Res Mol Brain Res. 1998;57:193–200. doi: 10.1016/s0169-328x(98)00074-6. [DOI] [PubMed] [Google Scholar]
- Widdowson PS. The effect of neurotensin, TRH and the delta-opioid receptor antagonist ICI 174864 on alcohol-induced narcosis in rats. Brain Res. 1987;424:281–289. doi: 10.1016/0006-8993(87)91472-7. [DOI] [PubMed] [Google Scholar]
- Yamauchi R, Wada E, Kamichi S, Yamada D, Maeno H, Delawary M, Nakazawa T, Yamamoto T, Wada K. Neurotensin type 2 receptor is involved in fear memory in mice. J Neurochem. 2007;102:1669–1676. doi: 10.1111/j.1471-4159.2007.04805.x. [DOI] [PubMed] [Google Scholar]
- Yim HJ, Gonzales RA. Ethanol-induced increases in dopamine extracellular concentration in rat nucleus accumbens are accounted for by increased release and not uptake inhibition. Alcohol. 2000;22:107–115. doi: 10.1016/s0741-8329(00)00121-x. [DOI] [PubMed] [Google Scholar]