Abstract
Arterial remodeling over time is a cornerstone of normal systemic aging. The age-associated arterial structural and functional changes in the intima, the media, and the adventitia are closely linked to angiotensin II (Ang II) signaling. A growing line of evidence indicates that essential elements of Ang II signaling, which encompasses milk fat globule epidermal growth factor-8, calpain-1, transforming growth factor-β1, matrix metalloproteinase-2/9, monocyte chemoattractant protein-1, nicotinamide adenine dinucleotide phosphate-oxidase, and reactive oxygen species, are upregulated within the central arterial wall in rats, nonhuman primates, and humans during aging. In vitro studies show that the elevation of Ang II signaling induces the accumulation of collagen and advanced glycated end-products, the degradation of elastin, and the increased cell cycle disorder, invasion, and hypertrophy of endothelial and vascular smooth muscle cells. Further, in vivo studies demonstrate that increased Ang II signaling accelerates arterial aging. Conversely, attenuating Ang II signaling via an inhibition of angiotensin conversing enzyme or a blockade of AT1 activation retards age-associated arterial remodeling. This review attempts to integrate complex facts of Ang II signaling within the aged central arterial wall and may shed light on new therapeutic targets for arterial aging.
Keywords: aging, proinflammation, arterial remodeling
Introduction
Central arteries are composed of the tunica intima, tunica media, and tunica adventitia. The cellular components and ground materials of each layer constantly remodeled as a result of changes in the intrinsic and extrinsic microenvironment throughout a lifetime [1–3]. Within the aged arterial wall, the luminal-lined endothelial cells (EC) become frail, decreasing in number, capability of attachment, replication, and mobility, with concurrent increases in cellular size and secretion of inflammatory and adhesion molecules [1–3]. The intramurally predominant contractile arterial smooth muscle cells (VSMC) become synthetic, exhibiting enhancement in the capability of migration/invasion, proliferation, and secretion [4–9]. Elastic laminae fatigue, collapse, and fracture while collagen glycosylates, accumulates, and deposits [1].
Age-associated changes in the intramural and extramural signals within arterial walls govern the behavior of vascular cells and their interaction with extracellular matrices [1–3]. These changes orchestrate arterial remodeling with aging, and may result from proinflammatory angiotensin II (Ang II) signaling cascades, including increases in milk fat globule epidermal growth factor-8 (MFG-E8), calpain-1, matrix metalloproteinase type -2/-9 (MMP-2/9), monocyte chemoattractant protein-1 (MCP-1), transforming growth factor-beta 1 (TGF-β1) activation, nicotinamide adenine dinucleotide phosphate-oxidase(NAD[P]H) expression, and reactive oxygen species (ROS) (Table 1) [1–13]. Augmentation of these Ang II biosignals appear to be the foundation of the molecular mechanisms of age-associated arterial structural remodeling, resulting in intima-media thickening, elastin fragmentation, collagen deposition, and functional contraction/relaxation alterations [1–13].
Table 1.
Arterial Remodeling: Impact of Dietary Sodium, Aging, Hypertension, and Atherosclerosis and Ang II Signaling
| Aging | ||||||||
|---|---|---|---|---|---|---|---|---|
| Dietary Salt | Humans (>65 yrs) |
Monkeys (15–20 yrs) |
Rats (24–30 mos) |
Rabbits (3–6 yrs) |
Hypertension | Atherosclerosis | Ang II Signaling | |
| Lumenal dilation | ? | + | + | + | + | ? | ? | ? |
| ↑ Stiffness | + | + | + | + | + | + | + | + |
| Endothelial dysfunction | + | + | + | + | + | + | + | + |
| Diffuse Intimal Thickening | + | + | + | + | + | + | + | + |
| Lipid involvement | ± | − | − | − | − | ± | + | + |
| ↑ VSMC number | + | + | + | + | + | + | + | + |
| Macrophages | ± | + | − | − | + | + | + | + |
| ↑ Matrix | + | + | + | + | + | + | + | + |
| ↑ Local ACE-ANGII- AT1 | + | + | + | + | + | + | + | + |
| MMP/Calpain dysregulation | + | + | + | + | ? | + | + | + |
| ↑ MCP-1/CCR2 | + | + | + | + | + | + | + | + |
| ↑ ICAM-1 | + | ? | ? | + | ? | + | + | + |
| ↑ TGF-B | + | + | + | + | ? | + | + | + |
| ↑ NADPH Oxidase | + | ? | ? | + | ? | + | + | + |
| ↓ Nitric Oxide Bioavailability | + | ? | ? | + | + | + | + | + |
| HYPERTENSION | ± | ± | ± | ± | ? | + | ± | + |
| ATHEROSCLEROSIS | ± | ± | − | − | − | ± | + | + |
?, information unknown ; +, increase; and −, decrease.
In this article, we review the role of molecular cascades that involve Ang II signaling pathways in rats, nonhuman primates, and humans during aging both in vivo and in vitro. We also discuss the progress of interventions for arterial aging, e.g., inhibition of Ang II signaling. This growing body of evidence provides a complex picture of Ang II signaling within the aged central arterial wall, and may shed light on new therapeutic targets for arterial aging.
Arterial aging in humans
Arterial pressure
Arterial pressure is the maximal aortic pressure following cardiac ejection of blood into arteries and is determined by the interplay between the heart and the vasculature. With advancing age, systolic blood pressure rises until the eighth or ninth decade, while diastolic blood pressure increases until the fifth decade, after which it plateaus or decreases [14]. Consequently, pulse pressure continually increases while mean arterial pressure increases and ultimately levels off with advancing age due in large part to increased arterial stiffness [14].
Arterial dilatation
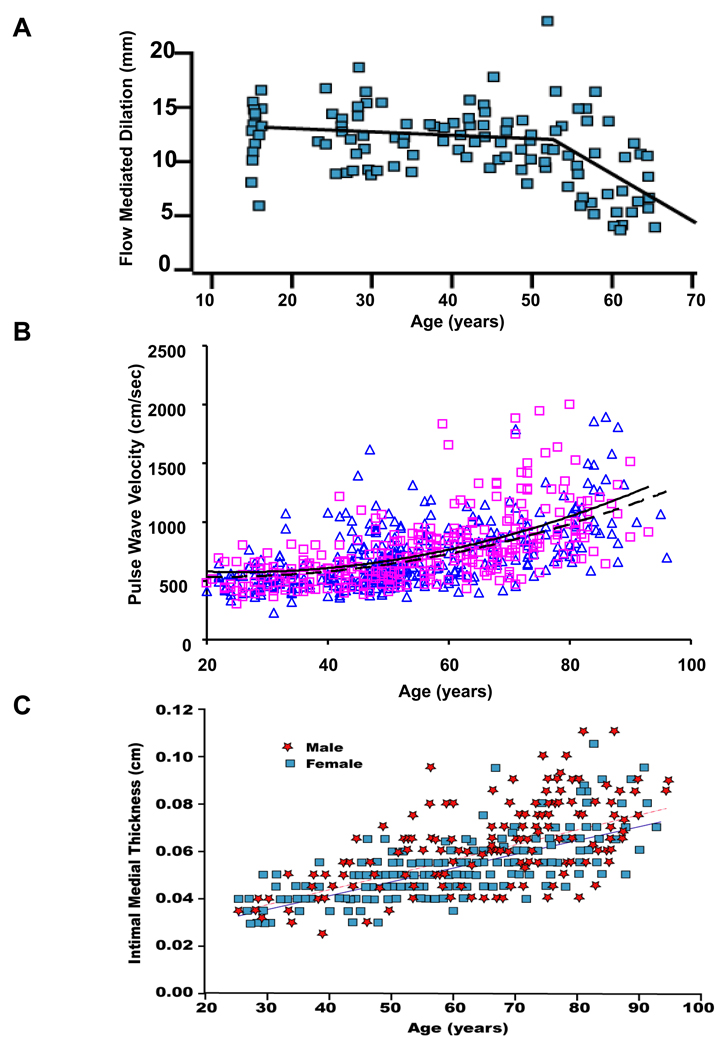
The vascular endothelium is a single layer of cells lining all blood vessels in the body, and it has emerged as a pivotal player in vascular aging. The intact endothelium is an essential element for vasodilatation in response to an increase in blood flow-associated shear stress [15]. Endothelial function of brachial or coronary arteries determined by flow-mediated vasoreactivity has been shown to decline with advancing age and is nitride oxide (NO) signaling-dependent (Figure 1A) [15]. Notably, this age-related decline appears to occur earlier in men than in women [15]. In women, however, a steep decline commences at around the time of the menopause [15].
Figure 1. Age-associated changes in arterial function in humans.
A. Flow mediated induced dilation in the brachial artery of apparently healthy men. From Celermajer et al [15]. Age-associated increase in carotid-femoral pulse wave velocity (B), an index of central arterial stiffness, and intimal medial thickness (C) in healthy men (red) and women (blue). Best-fitting age regression curves are shown for men (solid lines) and women (dashed lines). From Vaitkevicius PV et al [16] and Nagai Y et al [18].
Pulse wave velocity
Arterial stiffness depends on intrinsic stress/strain relationships that are affected by structural properties of the blood vessel wall and smooth muscle tone. Among the various indexes of arterial stiffness, carotid-femoral pulse wave velocity (PWV) has emerged as the “gold-standard” for the non-invasive evaluation of large arterial stiffness. PWV is measured as the distance between the carotid and femoral sampling sites divided by the time delay for the onset of the pressure wave between these two sites. The increase in arterial wall blood pressure and reduction in endothelial function with increasing age are accompanied by an increase in PWV (Figure 1B) [16]. Interestingly, improvement of aerobic capacity by exercise reduces PWV, and may mitigate the stiffening of the artery that accompanies normative aging [16].
Arterial structure
Alterations in blood pressure, endothelial function, and arterial stiffness with advancing age accompany structural remodeling in the central arterial system. Central elastic arteries become dilated with age, which may, depending on the level of intimal thickening, lead to an increase in lumen size [17]. Arterial intimal medial thickness (IMT) also increases with advancing age (Figure 1C) [18]. Post-mortem studies indicate that in humans, this increase is mainly due to intimal rather than medial thickening [19].
Arterial aging in experimental models
Studies of experimental animal models have greatly enhanced our understanding of age-associated alterations in arterial structure and function in humans and the pathogenesis of age-associated arterial disease. The age-associated cellular and molecular alterations of the central arteries of rats, rabbits, and nonhuman primates in the absence of clinical arterial diseases are quite similar to those observed in “grossly normal arterial segments” in humans (Table 1) [2, 4].
Intima
The intima between the luminal surface and the internal elastic lamina of the artery is a “frontline battle field” of human vascular diseases such as hypertension, atherosclerosis, restenosis, and stroke. A series of studies show that age dramatically alters the property of this zone, and its interaction with the medial layer in various species including rats, nonhuman primates, and humans [1–13].
Endothelium
Endothelial structure
The numerous proinflammatory molecular and cellular alterations in arterial heterogeneous EC that occur with aging are likely implicated in age-associated endothelial disorder and age-associated atherosclerosis [13, 20–23]. Electron micrographs show that end-to-end inter-EC conjunctions increase, but stronger and more complicated overlapping or interdigital junctions decrease with advancing age. The immunostaining reveals that the aortic connexins CX37 and CX43 progressively decrease with increasing age. This suggests their involvement with aging-impaired phenomena such as dynamic intercellular material and signaling communication and homeostatic flux involving endothelial cellular inclusions, which are enhanced with aging [22,24]. Furthermore, EC become senescent, which appears flattened, enlarged, and enriched with inflammatory mediators [22, 23]. The decreased EC replicatve capacity that occurs with aging is linked to proinflammation and telomere shortening and increases susceptibility to apoptosis [13, 20, 22, 23].
Endothelial regeneration
The maintenance of an intact arterial endothelium over a lifetime may involve circulating endothelial progenitor cells (EPC), which are recruited to patch damaged regions via differentiation into mature endothelial cells [25–32]. Aging retards arterial reendothelialization after balloon injury, suggesting that aging affects the ability of progenitor cells to repair the damaged endothelium [26, 28]. Increasing age was shown to be associated with a reduction of the number and self renewable capacity of circulating EPC in subjects [29–31]. Additionally, the colony-forming unit and migratory capacity of blood-derived EPC become significantly declined with advancing age; this effect can be prevented by treatment with insulin growth factor-1 (IGF-1) or by aerobic exercise [28, 29]. Furthermore, age may interfere with the survival of circulating stem/progenitor cells since EPC telomere length declines with aging [30–32]. Edelberg et al. reported that only transplantation of young bone marrow-derived cells into aged mice restored myocardial angiogenesis and vascular function, whereas aged mice bone marrow was ineffective [33]. These findings provide the first circumstantial evidence of dysfunctional regulation of EPC in elderly individuals. Interestingly, the capacity of reendothelization by transplanted old EPC at injured sites of young animals was significantly greater than their old counterparts, suggesting that a young niche partially restores reduced function of old EPC via the IP3/Akt signaling pathway [34]. These findings support the idea that the dysfunction of the EPC must be stimulated in vivo and most likely expanded ex vivo to improve regeneration and impaired arterial function with aging [25].
Endothelial dilatation
In old rats, NO- and O2-mediated endothelium-dependent relaxation to acetylcholine was markedly reduced compared with young and middle-aged rats, due to a decrease of NO release and an increase of O2− generation [35, 36].
Endothelial permeability
Age alters endothelial cell integrity, shape, and population density, sugar coating of glycocalyx, magnitude of negative charge surface characteristics, and endothelial-endothelial communication [13, 20, 22–24, 37]. Those alterations affect the endothelial cell surface’s physical and chemical “barrier” [37], contributing to an increase in endothelial permeability. This may lead to a chain of aberrant fluid and macromolecule albumin interstitial mass transport within the aged arterial wall [22, 38]. These changes in macromolecular transport suggest that the entry of plasma macromolecules in the aged arterial wall might be enhanced, whereas the efflux through the media may be impeded. As a result, these molecules may become trapped in the subendothelium, forming a fertile microenviroment for the pathogenesis of age-associated arterial disease.
Subendothelium
The arterial subendothelial region progressively grows with age in various species, including humans.
In FXBN rats, small disoriented VSMC with enriched nuclei are found within the thickened intima of old rats. The number of arterial intimal VSMC and amount of intimal collagen types I and III and elastin-like materials are markedly increased with aging [6, 7, 12, 13]. Notably, inflammatory cells, i.e. macrophages and lymphocytes, are not detected in the thickened intima [7].
Old monkeys (~20 yrs) have a thicker intima than young (~7 yrs) [11], and electron microscopy of these thickened intimal regions reveals infiltrated cells and matrix connective tissue deposition between an intact endothelium and fragmented internal elastic lamina [13]. Nearly all of these intimal cells stained positively for α-SMA, a marker of VSMC [11]. As in rats, inflammatory cells have not been observed in the old thickened intima in nonhuman primates [11].
Grossly normal aortic intimal thickness and cell infiltration of specimens from older human donors (over 65 yrs) is markedly increased compared to those from younger donors (~20 yrs) with an absence of the aggregated lipid deposition in either age group [8]. The vast majority of cells within the intima also stained positively for α-SMA [8], and SMemb, a marker for the fetal-type VSMC. In addition, aging increased intimal collagen type I, type III, and other ground materials in the human aortic wall [8]. Unlike aged rats and monkeys, sporadic clusters of macrophages (CD-68 positive cells) were more numerous within aortic walls of older versus younger human subjects, and activated mast cells were also occasionally detected in the intima of older rather than younger samples [8].
Taken together, these findings suggest that unique human age–associated subendothelial remodeling may be an underlying molecular and cellular mechanism for the increased prevalence of arterial diseases in the elderly.
Media
The media, nestled between the internal and external elastic lamina of larger arteries, provides the vessel’s second line of defense. It is composed of an arrangement of VSMC, the predominant cell type around the elastic lamina.
VSMC in situ
The aorta of aged rats contains an increased number of intimal VSMC, but medial VSMC appear to be reduced in number. Aged VSMC exhibit greatly heterogeneous features [2]. One subset of old medial cells appear polyploid and larger in size than those in the aorta from young adult rats [5, 39, 40]. Interestingly, the majority of those polyploid cells with enriched NAD(P)H oxidase ultimately reach a cell-cycle arrest status [40]. Another subset of cells, like intimal VSMC, appear tiny in size and may have powerful invasiveness [8, 11].
The principal physical barriers to cell movement in the intact vessel are the elastic laminae and basement membrane (BM). The BM surrounds each VSMC, providing a microenviroment, and is mainly composed of type IV collagen, laminin, and fibronectin. VSMC are surrounded by and embedded in a variety of matrix protein barriers that must be restructured for proliferation and migration to occur. The detailed properties of fresh, enzymatically dissociated single medial VSMC from old rat aortae compared to the cells from young rats are described later on the section of Ang II signaling and arterial cell aging in vitro.
Extracellular matrix
The deposition of collagen within all arterial compartments and elastin fiber fracture is a hallmark of age-associated arterial remodeling [12]. The close association of elastin, collagen, glycoaminoglycans, and VSMC in the mammalian aortic wall causes alterations in viscoelastic characteristics that account for many of its static and dynamic mechanical features [13, 41, 42]. The elastin lamella, and the contents of its adjacent interlamellar zone (which includes VSMC), represent the unit of structure and function of the mammalian aortic wall, and is closely linked to elasticity, which progressively deteriorates with advancing age [12, 42].
Amount of elastin, composed of tropoelastin and mcrofibrils and constituting ~30% of the dry weight of larger arteries, decreases with aging [43]. An imbalance of synthesis and degradation of tropoelastin and mirofibrils leads to a reduction in the mature elastin formation within the aged arterial wall [44, 45]. The steady-state level of aortic tropoelastin mRNA decreases dramatically with increasing age [44]. Furthermore, precursor tropoelastin molecules are secreted from VSMC into the extracellular region and attach to microfibrils, but do not form a meshwork of mature elastin fibers due to insufficient cross-linking resulting from age-declined lysyl oxidase [46].
A change in the solubility and distribution of degenerated and glycated collagen as “matricrine” also occurs with age, and it has been proposed that age-associated changes involve tighter collagen fiber packing, increased stiffness, and increased tensile strength [4, 12, 45–48].
Although the total mucopolysaccharide “ground substance” is unaltered with aging, biglycan, dermatan sulphate, and chondroitin sulfate increase, hyaluronic acid decreases, and chondroitin-4,6-sulphate and heparan sulphate remain constant [4, 48–50]. Chondroitin sulfate B is associated with coarse collagen and elasticity changes, and its increased relative abundance to the A and C mucopolysaccharides lend credence to its role in strengthening the arterial wall with age [50].
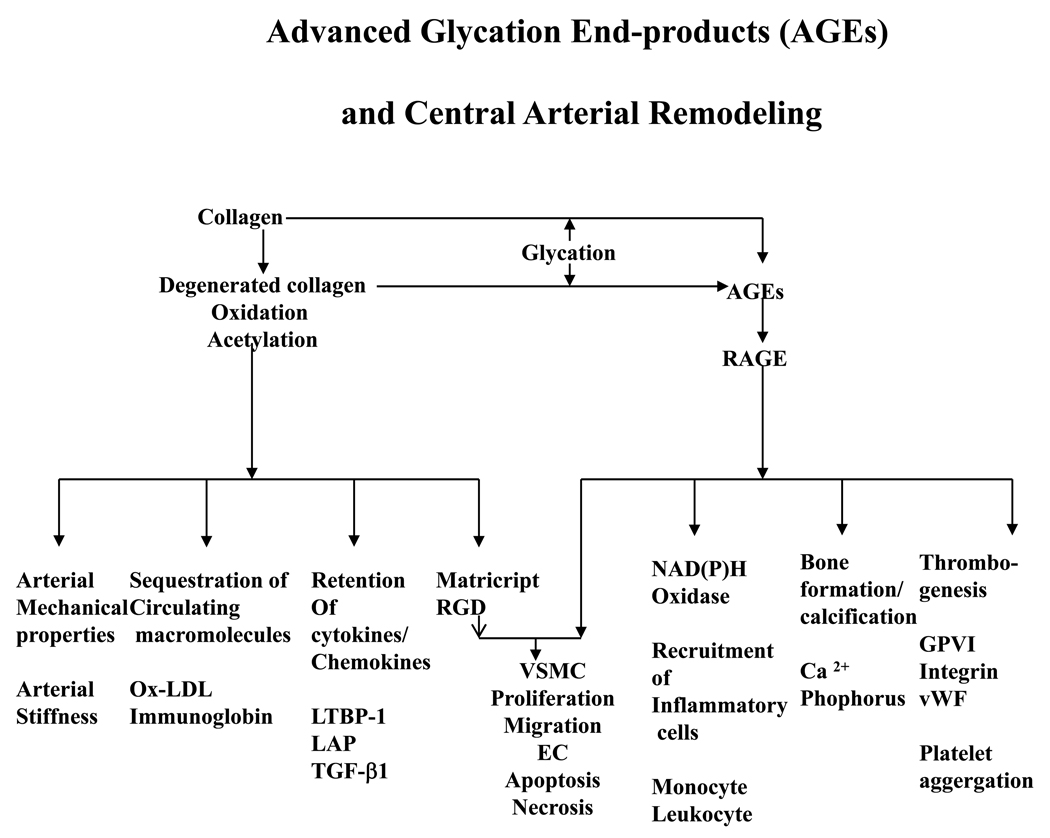
With increasing age, free amino groups on these collagen proteins become more susceptible to nonenzymatic glycation, oxidation, and nitration [47, 51]. As a result, even in euglycemia, they have a propensity to form advanced glycated end-products (AGEs) via the Maillard reaction due to local glucose metabolism disorders [47, 51]. A summary of the role of AGEs in age-associated arterial remodeling is illustrated in Figure 2 [52].
Figure 2.
A schematic depiction of the effects of AGEs and their potential role in arterial remodeling. From Wang M et al [52].
With aging, the glycoprotein component of elastic fibrils decreases and eventually disappears, and concurrently bound Ca2+ content increases [47, 53]. This increased biomineralization accompanies a gradual accretion of collagen-like protein, which is associated with an increase in the content of more polar amino acids [54]. Recent studies also indicate that cross-linked collagen and elastin contribute to the development of vascular calcification, which increases with aging [55, 56]. The expression of the procalcification genes calpain-1, bone morphogenetic protein (BMP)-2, osteopontin, osteonectin, matrix Gla protein, and periostin (osteoblast-specific factor 2, OSF-2) increase while the anticalcification genes fetuin-A and B decrease within the arterial wall with aging [10, 55–57]. These molecules bind Ca2+ and enable both osteogenic and chondrogenic transdifferentiation of VSMC, myo-fibroblasts, and pericytes, thus contributing to arterial ectopic calcification [55, 56].
Adventitia
The adventitia serves as the artery’s final line of defense, and functions as a dynamic compartment for trafficking across the artery wall. It is composed of bundles of thick collagen, disordered elastin fibers, vasa vasorum, nerve bundles, lymphoid organization, sporadic fibroblasts, and smooth muscle progenitor cells [58–63]. The adventitia and adjacent perivascular white adipose tissue, similar to the intima, is a major sources of matrix metalloproteinase, vasoactive factors, including cytokines/chemokines, reactive oxygen species (ROS), and growth factors [58–63]. A growing body of evidence indicates that adventitial remodeling intertwines with VSMC growth, proliferation/invasion, differentiation, and proinflammation [58–63]. Changes occurring in the adventitia could be a signal of impending vascular disorders [58, 59, 61–63]. With increasing age T lymphocytes infiltrate the adventitia and the vasa vasorum continues to develop, resulting in increased vascularity, and possibly facilitating both intima and adventitia inflammation, which is closely associated with atherogenesis [59]. Other studies suggest that in the arterial wall, the adventitia serves as the site of local immune responses during arterial aging and atherogenesis, and is the primary compartment of T cells, which grow in number with age and promote medial VSMC invasion to the intima [63].
Essential components of Ang II signaling cascades within the arterial wall
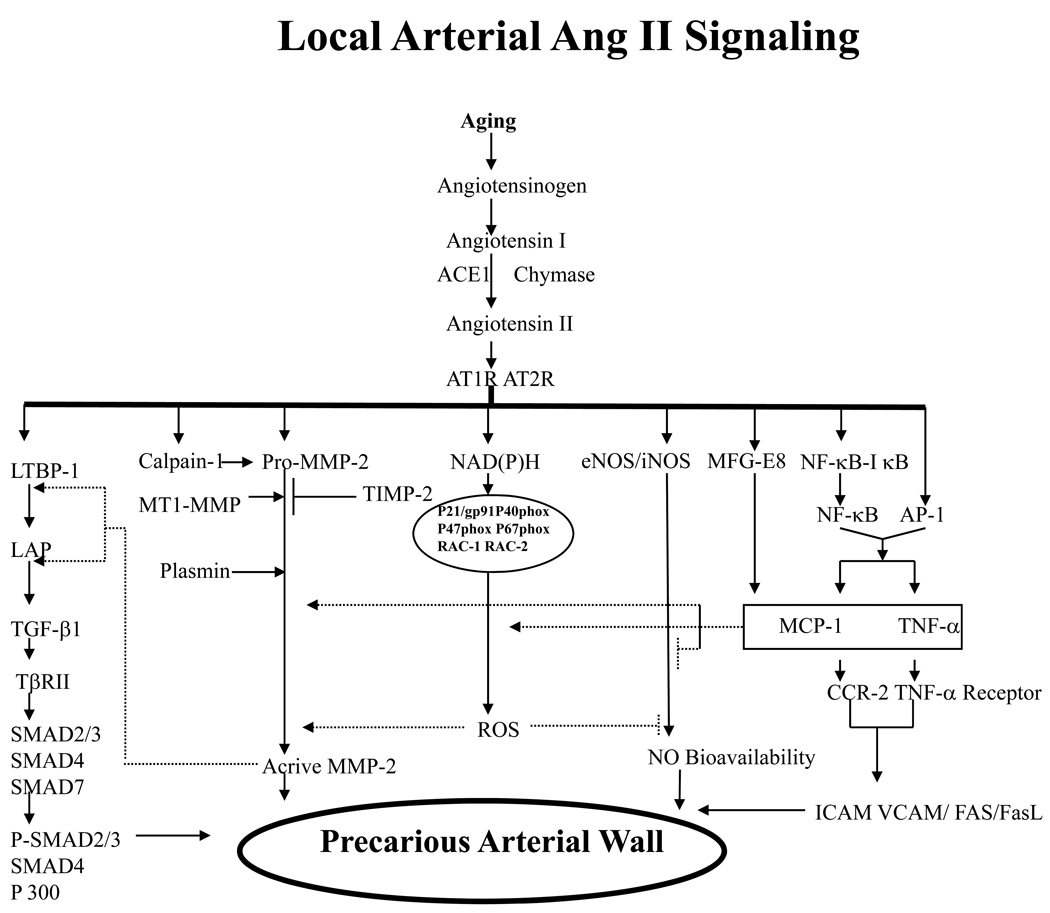
A growing body of evidence demonstrates that the molecules of the Ang II signaling cascade, including MFG-E8, calpain-1, MMP-2/-9, MCP-1, TGF-β1, PDGF-BB, and ROS, are upregulated within the aged arterial wall, and may play causal roles in age-related arterial structural and functional remodeling (Table 1 and Figure 3) [1–3, 52].
Figure 3.
Simplified schematic of the pleiotropic roles of increased Ang II signaling on arterial vulnerability. An age-associated increase in Ang II induces TGF-β expression, activates the nuclear factor κB (NF-κB) and MMP systems, promotes reactive oxygen species (ROS) production, and decreases NO bioavailability, contributing to arterial inflammation and fibrosis and resulting in arterial remodeling. ACE indicates angiotensin-converting enzyme; AT1R, Ang II type 1 receptor; LTBP, latent TGF-binding proteins; LAP, latency-associated protein; TβRII, transforming growth factor β receptor type II; VCAM, vascular cell adhesion molecule; FasL, Fas-Fas ligand; SMAD, similar to mother against decapentaplegic; TIMP; tissue inhibitors of metalloproteinases; MT1, membrane type 1; MFG-E8, milk fat globular epiderminal growth factor-8, MFG-E8. Modified from Wang M et al [52].
Ang II
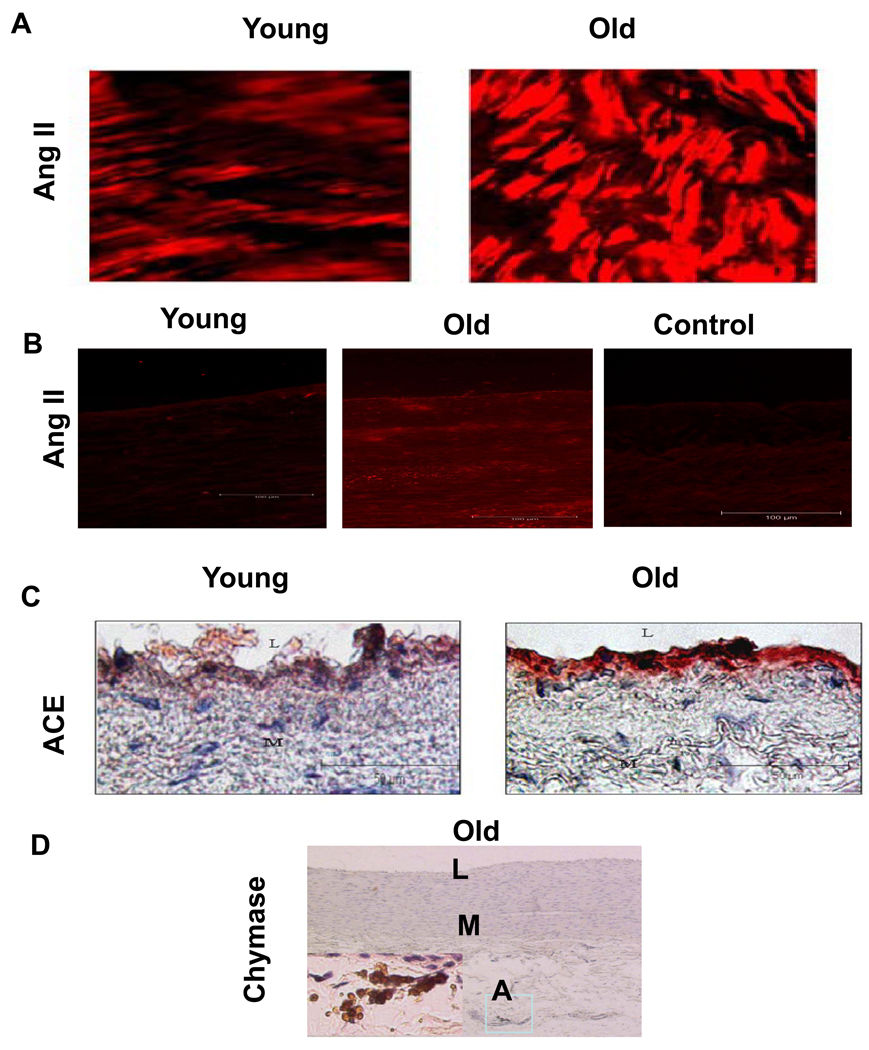
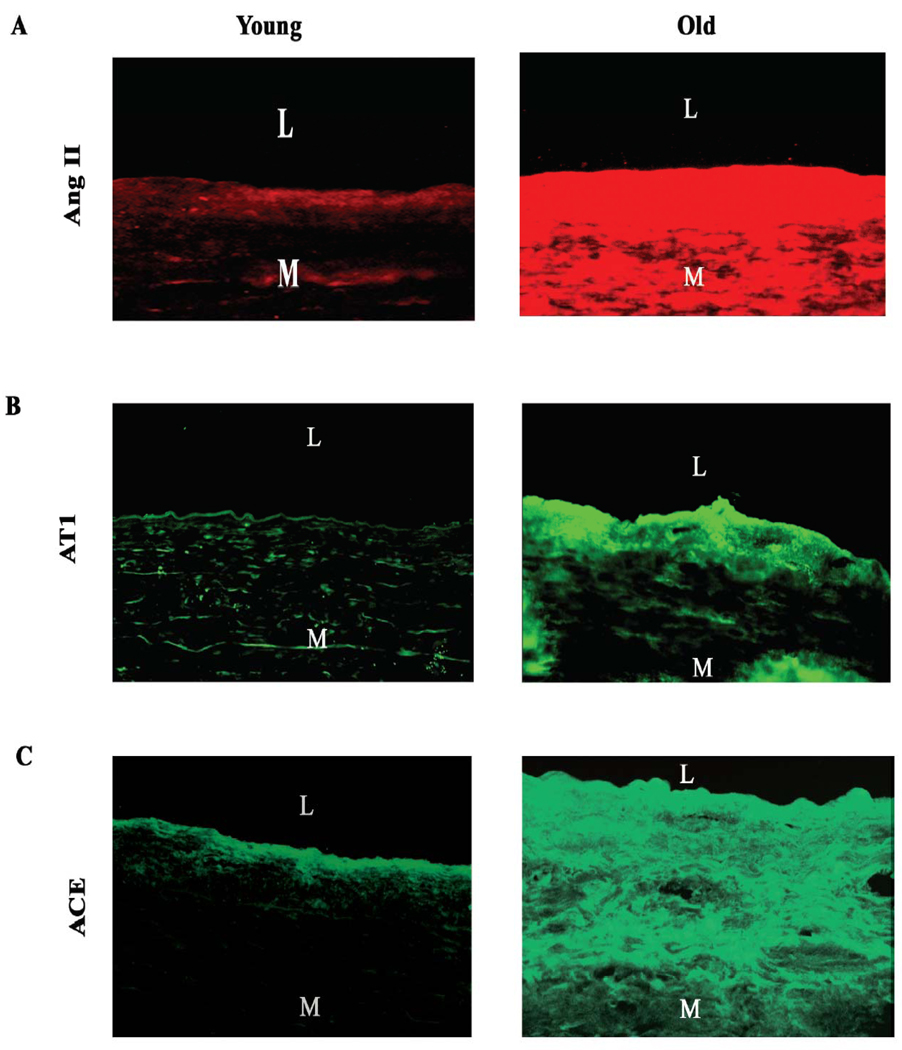
The vast majority of circulating Ang II is the product of Ang I cleavage by angiotensin converting enzyme (ACE). ACE is secreted from the lung epithelial cells, while Ang I is an enzymatic fragment of angiotensinogen (via renin), which is generated from the juxtaglomular apparatus in the kidney. These components have been found to increase within the aged arterial wall in various species [4, 6, 8, 10, 11]. It is known that local Ang II is over 1000-fold more abundant than circulating Ang II, is independently regulated, and plays an important role in vascular pathophysiology with aging [64, 65]. Levels of Ang II protein increase in the aged aortic wall in rats (Figure 4A, red color, right panel) [10]. Further, studies from nonhuman primates also show that Ang II (Figure 4B), ACE (Figure 4C), and adventitial chymase (Figure 4D) staining are increased in the thickened intima of older monkey aortae [11]. In humans, Ang II (Figure 5A), AT1 (Figure 5B), ACE (Figure 5C), and chymase immunofluorescence increase within the grossly normal aortic wall of samples from older donors [8]. Additionally, double staining reveals that intimal Ang II co-expresses with ACE staining in older aortae [8]. Consecutive section staining reveals that most of the chymase was located around or within the granules of mast cells, which also could effectively convert Ang I into Ang II in primates [8, 11, 66].
Figure 4. Ang II and its converting enzymes increase in aged aortic walls from rats and nonhuman primates.
A. Immunolabelled Ang II (red) in the en face medial aortic sections from young (left panel) and old rats (right panels). From Jiang L et al [10]. B. The representative immunofluorescent staining for AngII (red color) from monkeys. C. The representative immunostaining for ACE (red color) with haematoxylin counter stain from monkeys. D. The representative immunostaining for chymase in an old monkey aorta (X 50). Inset, rectangular region under high power (X400). L=lumen, M=media, A=adventitia. L=lumen, M=media. From Wang M et al [11].
Figure 5. Components of renin angiotensin system in human aortic wall.
Immunofluorescence staining for A, Ang II (red color), B, AT1 (green color), and C, ACE (green color). Magnification X200. L=lumen; and M=media. From Wang M et al [8].
MFG-E8
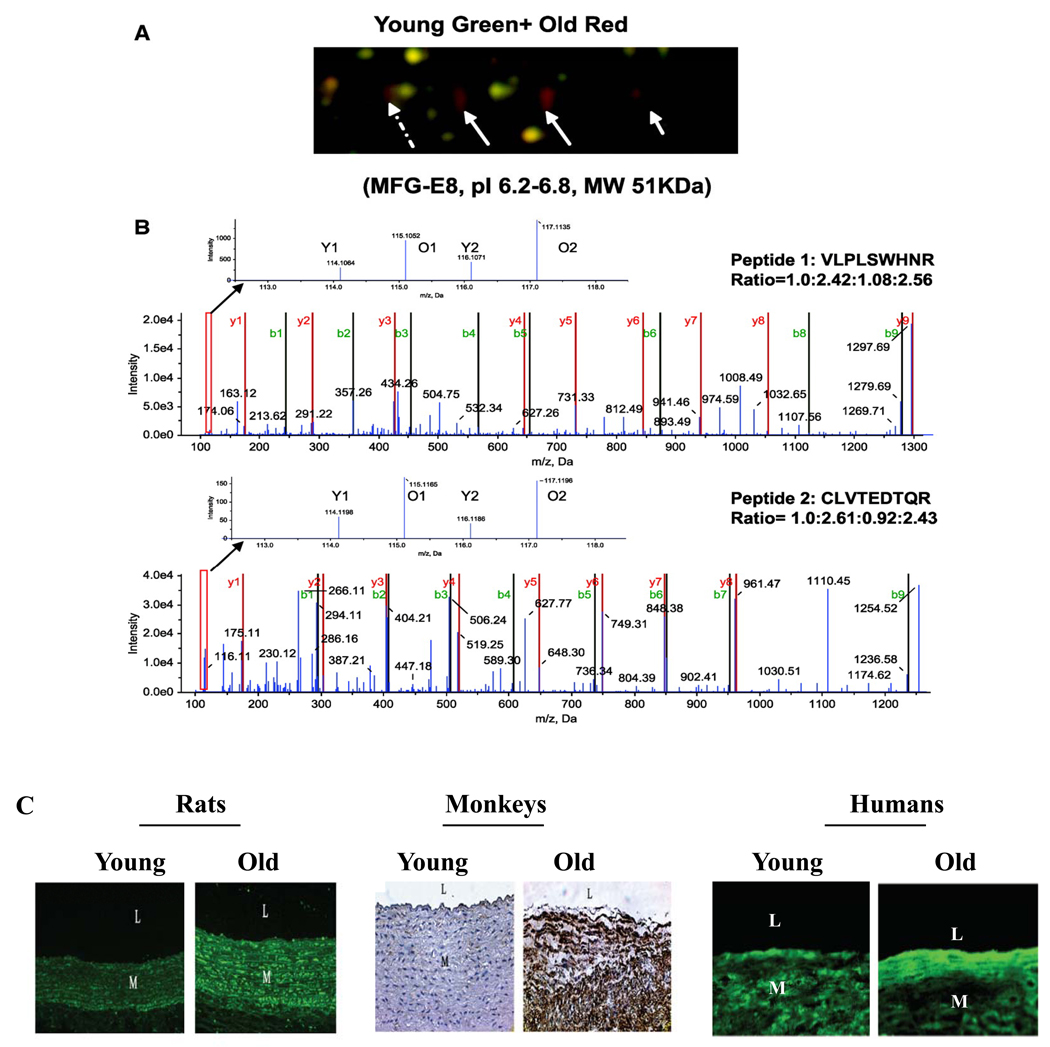
MFG-E8 is a major component of the milk fat globule and may play a diverse role in various developmental homeostatic processes [67]. By supporting cell-cell and cell-lipid interactions, it also facilitates tissue remodeling in various organs and in the vasculature during pathophysiological conditions [4, 67]. Cloned MFG-E8 cDNA encodes 66/53-kDa soluble, heavily glycosylated proteins comprised of an N-terminal signal peptide, two EGF-like repeat domains (EGF1 and EGF2), and two discoidin domains (C1 and C2) found in blood clotting factors V and VIII [67]. MFG-E8 is a secretory adhesion protein with potential acceptor sites, one for αvβ3 and αvβ5, and others for aminolipids such as phosphatidylserine [67]. Importantly, a recent study indicates that MFG-E8 mRNA and protein levels increase with aging in several mammalian species including humans, as determined by 2D DIGE (Figure 6A), iTRAQ (Figure 6B), and immunostaining (Figure 6C) [4]. Dual immunolabeling shows that MFG-E8 colocalizes with both Ang II and MCP-1 within VSMC of the thickened aged aortic wall [4]. The data suggest that arterial MFG-E8 significantly increases with aging and is a potential relay element within the Ang II/MCP-1/VSMC signaling cascade [4].
Figure 6. Age-associated arterial MFG-E8 expression.
A. Enlarged region of 2D DIGE gel map showing that MFG-E8 is more abundant in aged aortae than in young adult samples. Three solid white arrows point to gel spots identified as MFG-E8. Identifications were performed using light chromatography–MS/MS. B. iTRAQ MS spectra showing that MFG-E8 is more abundant in aged than young adult aorta. C. Immunohistostaining for MFG-E8 within the rat, monkey, and human thoracic aortic wall. L=lumen; M=media. Modified from Fu Z et al [4].
MMP-2/-9
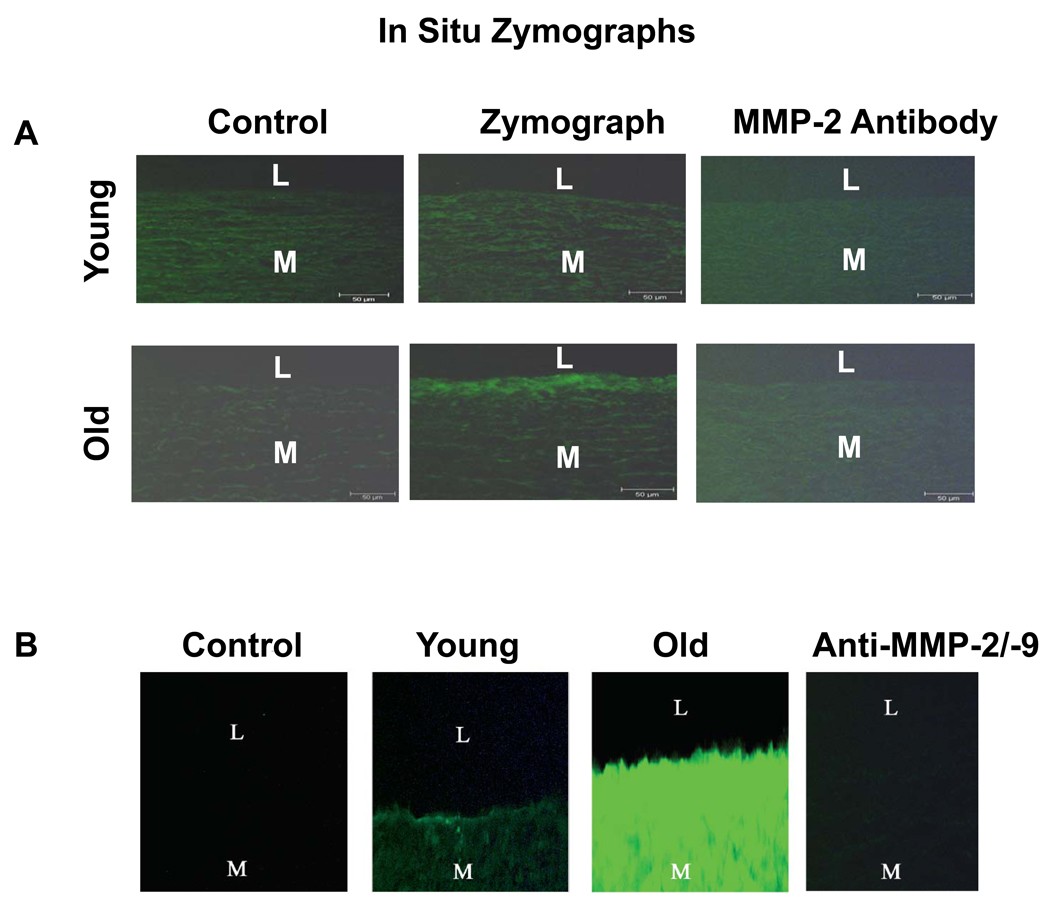
MMP-2/-9 belongs to the zinc-containing endopeptidases, degrades native type IV, V, VII and X collagen, denatured collagen, and elastin, promotes matrix protein degradation in arterial wall, and facilitates VSMC migration. Enhanced MMP-2/-9 expression and activity are linked to increased Ang II signaling, and occur during arterial aging in various species [4, 6, 8, 10]. Aortic MMP-2 activation in situ is progressively elevated with aging, and is mainly localized to the intima, the internal elastic lamina, and elastin fibers in the innermost tunica media. It colocalizes with EC and VSMC [12]. Age-associated MMP-2 activation also occurs in nonhuman primates (Figure 7A) and colocalizes with EC and VSMC [11]. Increased MMP-2 activation is largely confined to the thickened intima of old monkey aortae (Figure 7A, lower middle panel) [11]. In humans, both MMP-2 and MMP-9 activity in situ are enhanced in the grossly normal aortic segments with aging (Figure 7B) [8].
Figure 7. In situ gelatin zymograms.
A. In situ gelatin zymographs of monkey aortae. Controls were from incubation in the absence of gelatin (left panels). Protease activity (green color) is localized mainly in the aortic intima of the old monkey (middle panel). A specific antibody to MMP-2 blocked digestion of the substrate (right panels). From Wang M et al [11]. B. In situ gelatin zymographs of humans. Note the markedly enhanced total gelatin activity in vivo (bright green fluorescence) in old vs. young, and its inhibition by Anti MMP-2/-9 antibodies. L=lumen; I=intimae; M=media, and STD= standard protein. From Wang M et al [8]. L=lumen, M=media.
Modulators of MMP-2 activation
In VSMC, cleavage and activation of MMP-2 can be achieved by a novel membrane-type matrix metalloproteinase (MT1-MMP). MT1-MMP is synthesized as a proform, which can be activated via cleavage by the intracellular protease furin or by extracellular plasmin serving as an activator of MMP-2. TIMP-2, one of the endogenous tissue inhibitors of MMP-2, has a role in the formation of a membrane-bound ternary complex consisting of MT1-MMP, TIMP-2, and latent MMP-2. “Free” MT1-MMP located in proximity to this complex is presumed to cleave proMMP-2 bound to the MT1-MMP/TIMP-2 as “cognitive receptor”. At high concentrations TIMP-2 inhibits MMP-2 activation, presumably by blocking the activity of MT1-MMP [12].
Dysregulation of MMP-2 activation has been observed in arterial walls in rats and nonhuman primates with aging [11, 12]. In rats, intimal and medial MMP-2 increase with aging; intimal MT1-MMP increases while medial MT1-MMP remains constant, and intimal TIMP-2 remains constant while medial TIMP-2 decreases. Thus, ratios of MMP-2/TIMP2 and MT1-MMP/TIMP2 are enhanced, contributing to increased MMP-2 activation within the aging arterial wall [12]. As in rats, the ratios of intimal MMP-2 and MT-1 MMP to TIMP-2 also increase in nonhuman primates with age [11].
The serine protease plasmin can induce a complete conversion of the intermediate MMP-2 form to the mature form, and can also inactivate TIMP-2. Pro-MMP-2 activation is inhibited by plasminogen activator inhibitors-1 (PAI-1) or anti- urokinase plasminogen activator (uPA) antibodies. Tissue plasminogen activator (tPA) and uPA bind to the endogenous uPA receptor (uPAR), resulting in the conversion of plasminogen to plasmin. Thus, a delicate balance among activators and inhibitors of plasmin may control the activation status of MMP-2 and its potential impact on arterial remodeling with aging [12]. Indeed, intimal tPA, uPA, and uPAR progressively increase with aging, but intimal PAI-1 remains constant. Medial tPA and uPA remain constant with aging, but uPAR increases while PAI-1 decreases [7, 12]. Thus, ratios of tPA/PAI-1 and uPA/PAI-1 both increase in the intima and the media, which also contribute to age-associated arterial MMP-2 activation.
TGF-β1
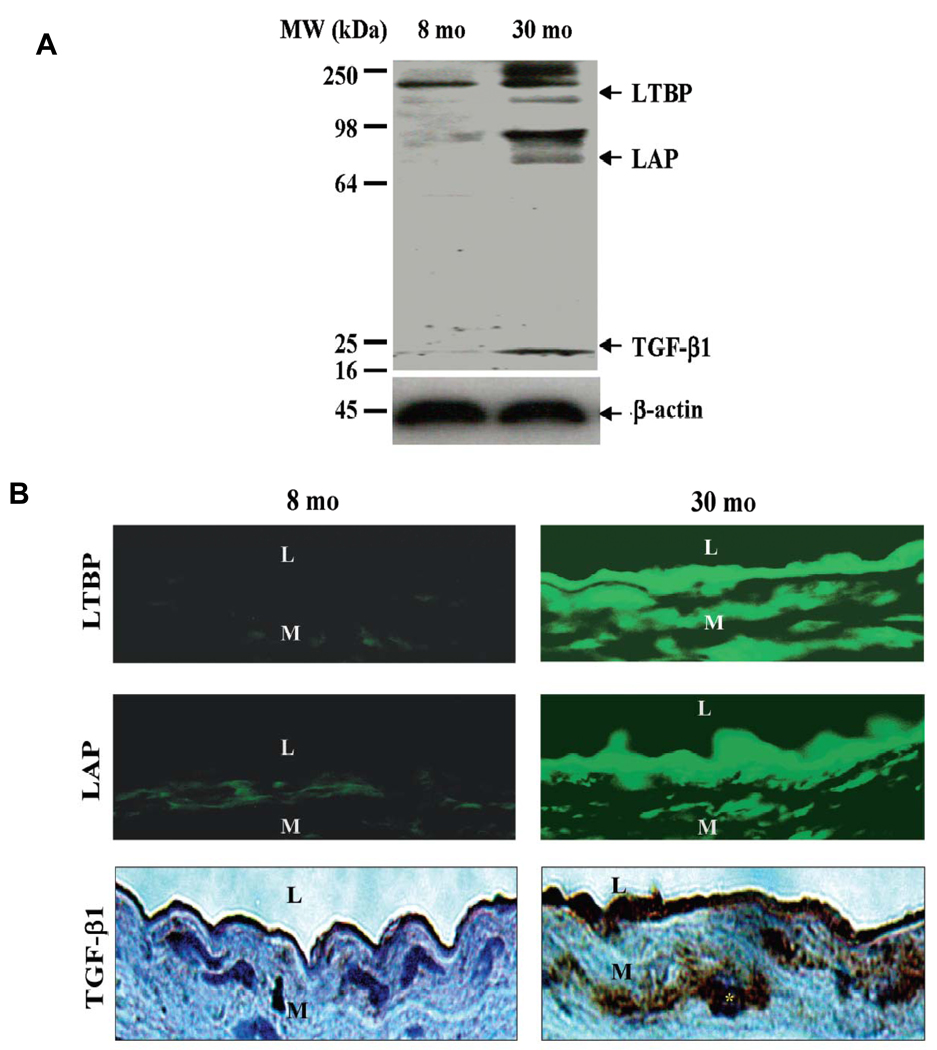
Arterial TGF-β1 is a pluripotent growth factor implicated in various aspects of vascular development and structural remodeling in health and disease via a regulation of collagen and fibronectin expression [6, 7]. TGF-β1 transcription, translation, and activity increase within the aorta of old rats compared to young animals [7]. Three TGF-β1-related components have been found in PAGE gels of rat aortic protein, corresponding to the molecular weights of activated TGF-β1 (~20 kDa), latent associated protein (LAP)-bound TGF-β1 (~75 kDa), and the latent TGF binding protein-1 (LTBP-1)-bound to precursor TGF-β1 (190–250 kDa) (Figure 8A) [7]. Aortic TGF-β1 was mainly present (98%) in the latent form, bound to LTBP and LAP, and all bands, including that of the active form of TGF-β1, increased with aging [7]. The abundance of TGF-β1, LAP, and LTBP-1 proteins increased within the aged aortic wall, particularly within the thickened intima (Figure 8B) [7]. TGF-β1 expression within the aortic walls of aged rats was dramatically increased in both intracellular and extracellular regions. Interestingly, the stronger immunostaining signal for TGF-β1 protein was present within the nuclei and the perinuclear area of vascular cells (Figure 8B, right bottom panel, star), suggesting an increased de novo synthesis of cellular TGF-β1 protein within the aged arterial wall [7].
Figure 8. Rat aortic TGF-β1 protein expression.
A. Western Blots for TGF-β1. B. Immunofluorescence staining for LTBP (upper panels, FITC, green color) and LAP (middle panels), and immunohistochemical staining for TGF-β1 (lower panels, DAB, brown color). L=lumen; M=media. From Wang M et al [7].
Activated TGF-β1 via MMP-2 activation exerts its biological effects by binding its TβRII receptor [7]. Aortic TβRII transcription and translation is also increased with aging [7]. Increases in TβRII are widely distributed within the wall of the aged aorta. The increase in active TGF-β1/TβRII may result in the activation of the SMAD signaling pathways [7]. Indeed, the receptor-regulated phosphorylated p-SMAD2/3, and the common-mediator SMAD4, were increased within the aged aortic wall while the antagonistic or inhibitory SMAD7 protein decreased by 20% with age within the arterial media [7].
MCP-1
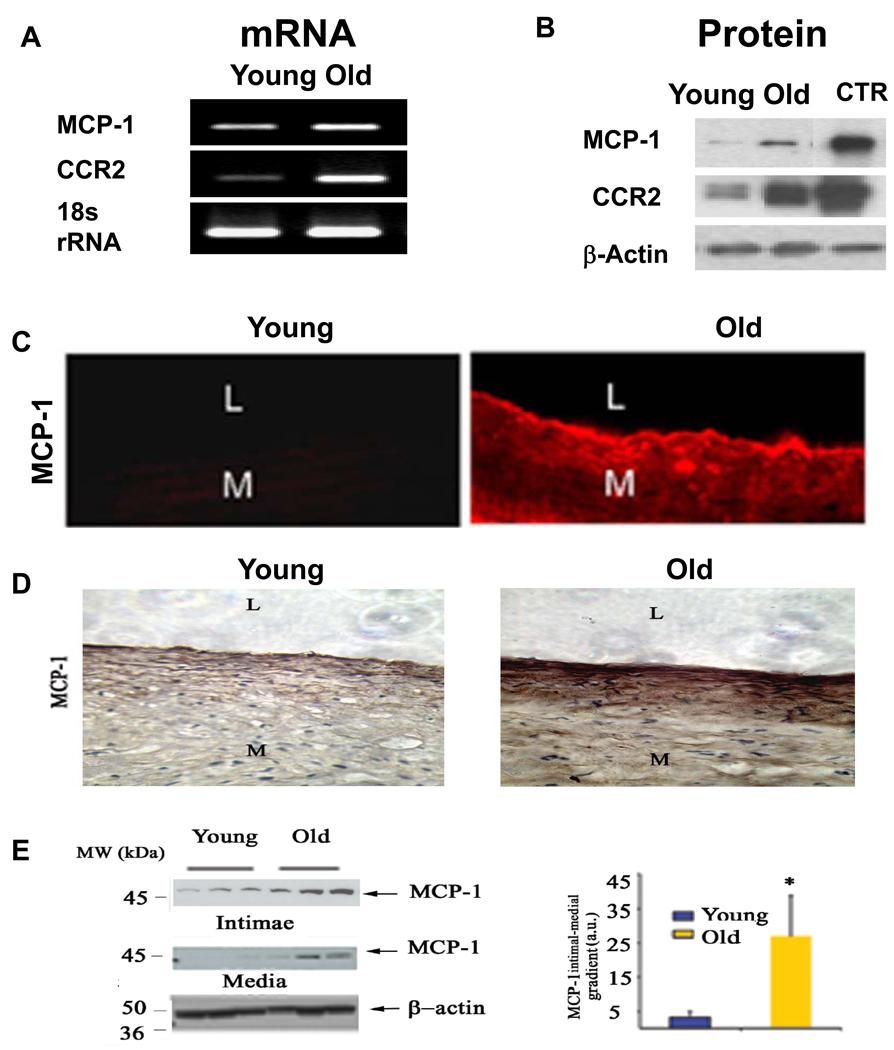
Monocyte chemoattractant protein-1 (MCP-1/CCL2) can induce migration of VSMC through the activation of CCR2 in the aortic wall. Aortic MCP-1 and CCR2 transcriptome, and their protein abundance, increase in rats with aging (Figure 9 A & B) [9]. Increased MCP-1 and CCR2 protein expression within the old rat aorta was localized mainly in the intimal region (Figure 9C) [9]. In humans, the increased MCP-1 within the old aorta resides predominantly within the intima, resulting in a markedly increased, age-associated intimal-medial gradient of the chemoattractant MCP-1 (Figure 9D and Figure 9E) [8].
Figure 9. MCP-1 transcription and translation increase in the aortic wall in rats and humans with aging.
A. A representative agarose gel showing the amplified rat aortic cDNA fragments by Real Time PCR analysis. B Representative Western Blot analysis on protein extracts from rat aortae. C. Immunofluorescence labeled MCP-1 (red) increases in the rat aorta with age, and localizes mainly in the intima. From Spinetti G et al [9]. D. Immunohistochemical staining for MCP-1, detected with DAB in human aortic sections (brown color) (X200). E. Representative Western blots for MCP-1 (left panels), and average data of MCP-1 gradient from intima to media of human aortae. *p <0.05, young versus old. L=lumen; and M=media. From Wang M et al [8].
PDGF-BB
Ang II increases platelet-derived growth factor-BB (PDGF-BB) expression within the arterial wall [6]. PDGF is a chemokine, as well as a potent mitogen for VSMC, and is increased in the old thickened intima containing infiltrated VSMC in rats [6]. Further, increased PDGF-BB, like the increased MCP-1, within the old aorta resides predominantly within the intima. This results in a markedly increased, age-associated intimal-medial gradient of the chemoattractant PDGF-BB, which can be substantially reduced by chronic calorie restriction [4, 6, 8, 68].
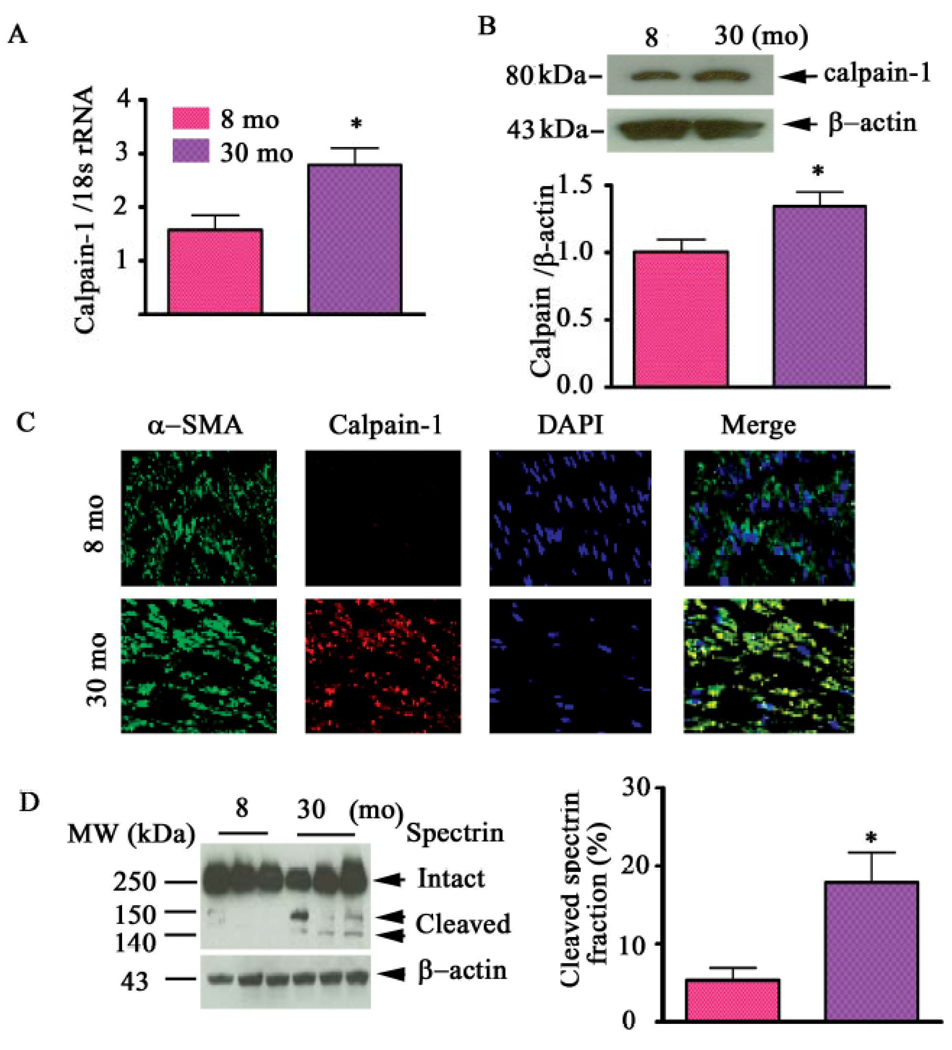
Calpain-1
Calpain-1, a ubiquitous, cytosolic Ca2+ activated neutral protease, is a heterodimeric molecule consisting of a large 80 kDa catalytic and a small 30 kDa regulatory subunit. Calpain-1 activation is linked to the cellular Ca2+ loading status in a dose–dependent manner within living cells and increases in the aged arterial wall [10]. The levels of calpain-1 transcripts (Figure 10A) and protein abundance (Figure 10B) are increased with age, and this increased calpain-1 protein within old rats co-localizes with VSMC (Figure 10C) [10]. Furthermore, calpain-1 activity increases within the aged aorta (Figure 10D) [10].
Figure 10. Calpain-1 transcriptome, protein abundance, and activity increase in the aged aortic wall.
A. Average data of calpain-1 transcriptome. B. Western blots for calpain-1 protein of rat intact aortae (upper panel); average data (lower panel). C. Dual en face fluorescence staining for α-SMA (green) and calpain-1 (red) in the medial aortic sections from young (upper panels) and old rats (lower panels). Nuclei were counterstained with DAPI (blue). Merged image is depicted in right panel (yellow-blue). Magnification: X400. D. Western blot for calpain substrate α-II spectrin from aortic lysates (left panels). Average cleaved spectrin fraction (right panel). *p<0.05, old vs. young. From Jiang L et al [10].
NO bioavailability and ROS
As in humans, NO dependent mechanical and agonist-mediated endothelial vasodilation is attenuated in older rats [13, 16, 35, 36, 69–73]. In rats, arterial arginase activity increases with age and may deplete local substrates for NOS, particularly L-arginine, a precursor for NO [72]. The impairment of endothelial-mediated vasodilitation with aging in humans can, in part, be prevented by L-arginine administration. These findings suggest that NO production becomes reduced with aging [73]. The activity of the endothelial nitric oxide synthase (eNOS) isoform has been shown to be markedly reduced with aging [71]. In other studies in rats, a large age-associated reduction in NO bioavailability occurs in the context of an increase in eNOS expression in the aged aorta [70]. This has been interpreted to reflect enhanced mitochondrial superoxide production coupled to enhanced peroxynitrite formation, as evidenced by increased levels of nitrosylated proteins [70].
ROS are important mediators of NO bioavailability [74]. The NAD(P)H oxidase is a major source of ROS in vascular cells, and is composed of 6 subunits: Rho guanosine triphosphatase (a GTPase, usually Rac1 or Rac20), and five "phox" units of p90, p22, p40, p47, and p67. Among NAD(P)H subunits, p22 phox is higher in the aortic endothelium of old rats compared to that in the young [74]. Within the arterial wall, NADH-driven O 2 − generation increases with aging. The effect of aging on the arterial wall activities of Cu/Zn SOD (SOD1), Mg SOD (SOD2), extracellular matrix superoxide dismutase (ECM-SOD/SOD3), catalase, and glutathione peroxidase-1 remains controversial [75–77]. While superoxide levels increase with age, the abundance and activity of these antioxidant enzymes likely rely on their metabolic environment, and their delicate balance with proxidant factors [75–77].
Ang II signaling and arterial cell aging in vitro
Endothelial cells
An increase in the number of circulating endothelial cells have been observed in older rats, possibly reflecting an enhancement of endothelial cell turnover with age [20, 23]. Endothelial cells have a finite cell lifespan and eventually enter an irreversible growth arrest, also termed cellular senescence [22, 23]. Both ACE and Ang II have been found in arterial endothelial cells and are increased with aging [8, 11]. The survival capability of endothelial cells isolated from polymorphic ACE II subjects with lower secreted Ang II is 20-fold higher than in cells from polymorphic ACE DD subjects with higher secreted Ang II [78]. The survival capability of ACE DD cells mimics that of ACE II cells after the ACE inhibition by Captopril [78]. Further, a recent study shows that Ang II treatment affects endothelial cell viability in a concentration-dependent manner [79]. Noticeably, Ang II-induced endothelial cells appear flattened, enlarged, and stain positive for senescence associated β-galactosidase (SA β–gal) [79]. The vast majority of these cells remain in the G0–G1 phase, while a small portion stay at the S and G2/M phases [79], suggesting entry into a growth-arrested status. Electronic micrographs show that chromatin is condensed at the periphery of the nuclei, the nuclear membrane is invaginated, and the cytoplasm is vacuolized [79]. These findings suggest that increased Ang II signaling may contribute to endothelial senescence with aging.
VSMC morphology and cytoskeleton remodeling
The diameter of fresh VSMC isolated from old rat aortae was 52.4% larger than that of cells from young animals [5]. Old cells also exhibit decreases in the intensities of smooth muscle myosin, α-SMA, and intact vimentin, and increases in desmin and tubulin compared to their young counterparts.
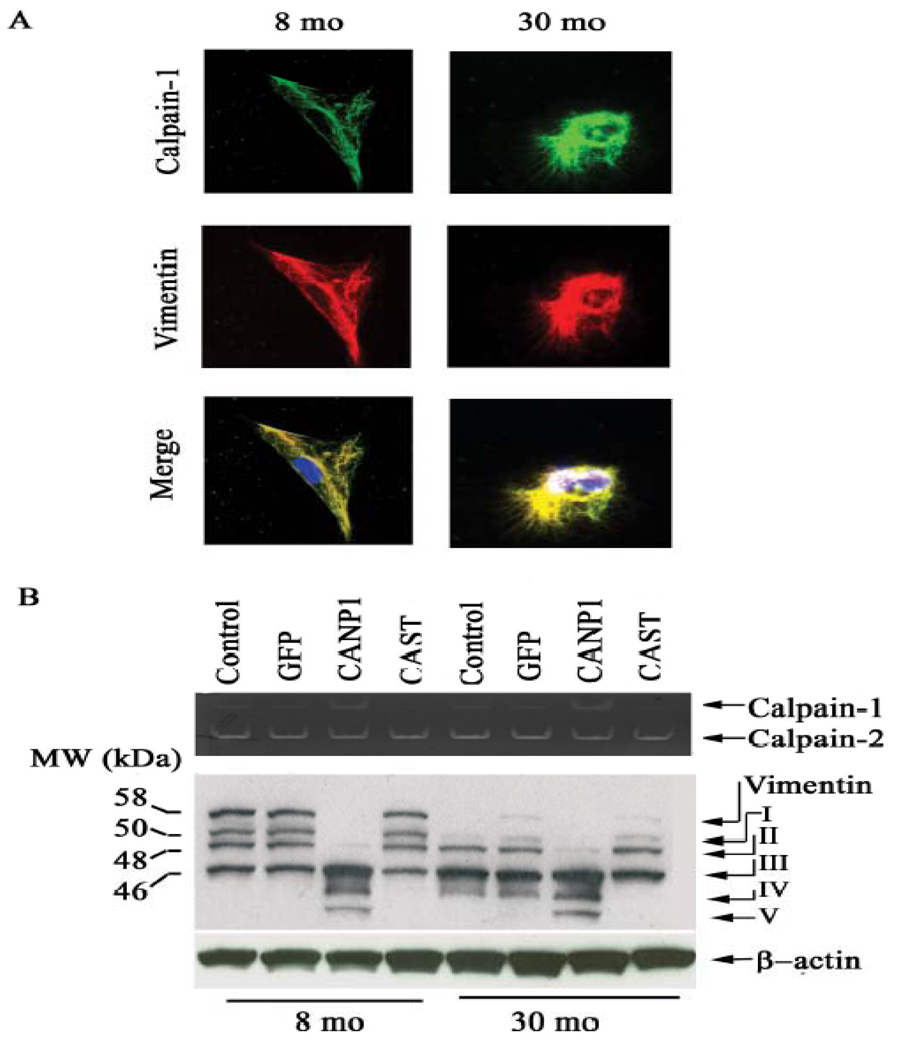
Cytoskeleton remodeling within VSMC likely results from increasing activity of intracellular proteases, i.e., calpain-1, with advancing age. Fig 11A demonstrates that in old VSMC, increased calpain-1 colocalizes with vimentin (lower right panel, yellow color) and α-SMA. Casein zymography (upper panel) confirms that calpain-1 but not calpain-2 activity in VSMC increases with age. Over-expression of calpastatin decreases calpain-1 activity in old cells [10]. In addition, an intact vimentin protein is only observed in early passage young VSMC while old cells solely exhibit small vimentin fragments (Fig. 11B) [10].
Figure 11. Calpain-1 and vimentin interactions within VSMC.
A. Photomicrographs of dual labeling for calpain-1 (green) and vimentin (red) within VSMC from young (left panels) and old rats (right panels); merged image (yellow bottom panels). B. Casein zymogram of VSMC from young and old VSMC infected with a CANP1 or CAST virus (upper panel). Western blots of vimentin from young and old VSMC infected with CANP1 or CAST virus (lower panel). Proteolytic fragments are indicated by arrows and designated I–V. From Jiang L et al [10].
This characteristic age-associated shift of vimentin fragments suggests that arterial wall calpain-1 may cleave the intact vimentin, generating multiple proteolytic fragments. Indeed, infection of adenoviruses harboring calpain-1 (CANP1) into young VSMC dramatically increases calpain-1 activity (Fig. 11B, upper panel), and generates a multiple proteolytic fragment pattern (Fig. 11B, lower panel, 8 mo), similar to old uninfected or CANP-1 infected young cells (Fig. 11B, lower panel, 30 mo) [10]. Remarkably, infection of old VSMC with a CAST adenovirus does indeed inhibit calpain-1 activity (Fig. 11B, upper panel) and generates a pattern of proteolytic products of vimentin resembling that of untreated young cells (Fig.11B, lower panel, 30 mo) [10].
VSMC proinflammatary responses
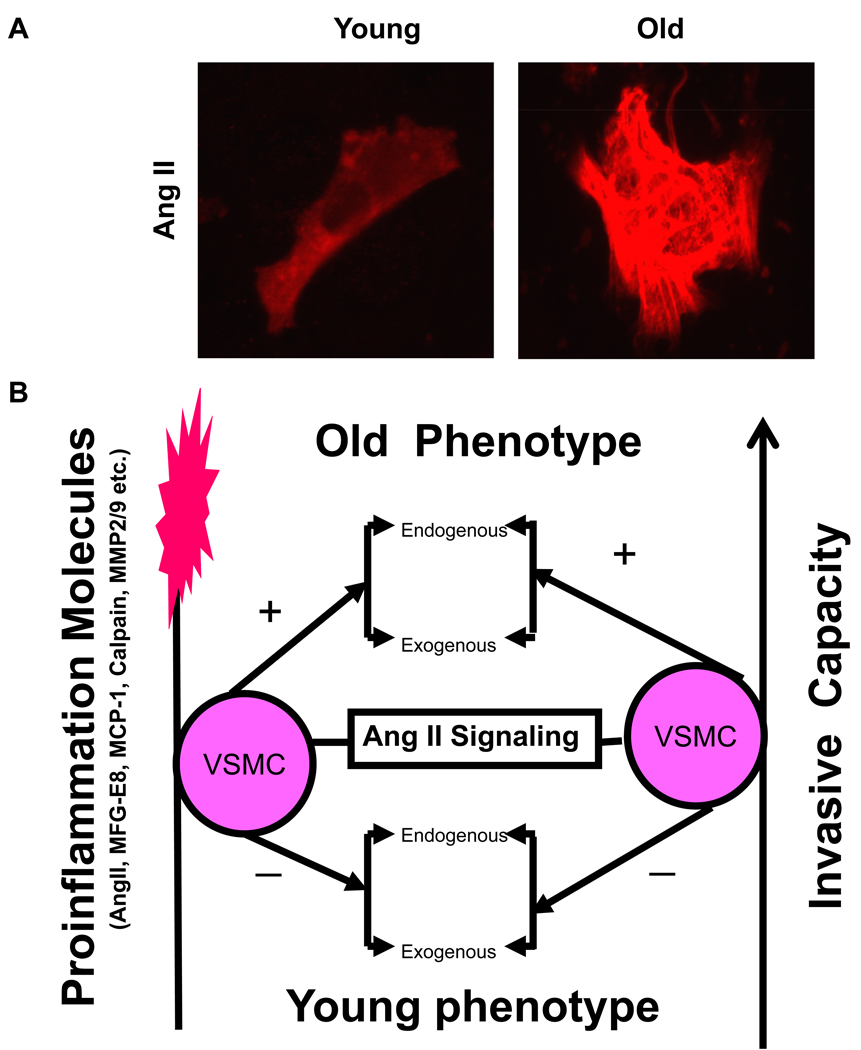
Ang II protein expression increases in aged VSMC (Figure 12A), and its signaling function results in a cellular inflammatory cascade, which is a central molecular event of VSMC aging (Figure 12B) [1–3, 6]. Ang II treatment of VSMC from young rats increases MFG-E8, MMP2, calpain-1 activity, and MCP-1 expression up to the level of untreated VSMC from old rats [6, 8, 10]. These Ang II- and age-associated effects were reduced by Losartan, an AT1 blocker [4, 6, 8, 10]. Interestingly, MFG-E8 treatment increases MCP-1 activity in both young and old VSMC [4]; the increased MMP2 activity induced by Ang II or aging itself is also abolished by Ci 1, a Calpain inhibitor [10]. Transcriptome and protein abundance of MCP-1 and CCR2, key inflammatory molecules which relay Ang II signaling, also increased in VSMC with aging [9].
Figure 12. VSMC phenotypic shift during aging.
A. Representative photomicrographs of VSMC with anti-Ang II antibody staining (red). From Wang M et al [6]. B. Simplified schematic of the Ang II signaling and age-associated phenotypic shift of VSMC. +, increase; and −, decrease.
VSMC Proliferation
Intimal cell proliferation is a "hallmark" of arterial aging. The myointimal hyperplasia that results from vascular transplantation injury and endothelial denudation is age-dependent [80, 81]. Myointimal thickening is more severe in older rats, and aortic segments from old rats transplanted into young syngeneic hosts have a greater proliferative response to injury than in their original environment [80, 81]. This suggests that old VSMC may have higher proliferative capability. The number of cultured VSMC from young and old rats were counted at day 3, 7, and 14 and a growth curve was constructed (Figure 13A) [5]. As early as 3 days, cultured VSMC from old rats displays faster growth rates. This trend continued at day 7 and up to day 14 [5]. Compared to young VSMC, old VSMC have a greater percentage of their population in the S phase and a lower percentage in the G0/G1 phase. These differences may be indicative of the cellular kinetic changes in VSMC via ERK1/2 signaling observed during aging [81, 82].
Figure 13. VSMC during aging.
A. Growth curves of VSMC cultured from young and old rat aortae. The number of VSMC obtained from old rats was significantly higher at days 3, 7 and 14. From Li Z et al [5]. B. Chemotatic response to a PDGF-BB gradient is increased in early passage VSMC from the aortic media of old rats compared with those from younger rats VSMC within the older aorta are "primed" to respond to the growth factor. From Pauly RR et al [84].
VSMC invasion
Intimal VSMC infiltration is a key cellular event in arterial aging, assumed to be the result of the migration/invasion of VSMC from the tunica media to the intima in various species, including humans [8]. Electronic microscopy demonstrates that medial VSMC are able to invade the intima [83]. Early passage aortic VSMC of old rats exhibit an exaggerated chemotactic PDGF-BB response, whereas cells from young aortae require several additional passages in culture to generate an equivalent response (Figure 13B) [84]. Furthermore, exposure of young aortic medial VSMC to the proinflammatory molecules Ang II, MFG-E8, MCP-1, or calpain-1 induces an increase in invasion ability in a dose-dependent manner, up to the level of untreated, old medial VSMC [4, 6, 9, 10]. VSMC isolated from old aortae exhibit increased invasion relative to young VSMC, chemoattracted by a gradient of PDGF-BB or MCP-1. This age difference is abolished or substantially reduced by Losartan, an AT1 atagonist, vCCI, an inhibitor of CCR2 signaling, GM 6001, an MMP inhibitor, Ci 1, a Calpain inhibitor, and by silencing MFG-E8 [4, 6, 9, 10].
Ang II signaling and arterial aging in vivo
Emerging evidence shows that increased Ang II signaling plays a causal role in arterial aging and lifespan in normotensive or hypertensive rodents. Disruption of the Agtr 1a gene that encodes AT1A markedly increases life span in mice [85]. Agtr1a−/− mice develop less oxidative arterial injury [85]. Lifelong treatment with the AT1 blocker fonsartan in young, stroke-prone, spontaneously hypertensive rats (SHR-sp) from the age of one month onward doubles the lifespan to 30 months, which was comparable to the lifespan of normotensive Wistar-Kyoto rats [86]. These effects were correlated with increased eNOS expression in the carotid artery and with markedly decreased tissue ACE expression/activities [86]. Furthermore, lifespan extension and cardiovascular protection by long-term AT1 blockade with fonsartan leads to similar beneficial effects, as observed with long-term ACE inhibition [87, 88]. Further, recent studies show that in vivo chronic infusion of Ang II at concentrations sufficient to elicit an increase in arterial pressure in FXBN rats similar to that associated with age, increases MMP2, calpain-1, and TGF-β1 activity [6, 10]. This infusion also imparts to the central arteries of young rat structural and molecular characteristics of arteries of old, untreated rats [6, 10]. A subpressor infusion of Ang II also increased MMP2 expression and activity, and increased collagen production within the arterial wall [6]. Interestingly, the administration of Phenylephrene, an α-adrenergic agonist to young rats increases arterial Ang II production, and reproduces Ang II effects [6]. These results demonstrate that Ang II signaling can indeed mediate structural, biochemical, and functional features of aging within the arterial wall of young rats. In addition, prior studies that have demonstrated that chronic ACE inhibition, AT1 receptor blockades, and treatment with resveratrol, a blocker of Ang II signaling and a Sirtuin 1 agonist, at an early age markedly delays the progression of age-associated arterial remodeling [87–91]. Studies show that AT1a deficiency effectively protects apoE−/−mice from the intiation and progression of atherogenesis, which is closely associated with the inhibition of age-associated increases in the expression or activation of collagen, 22phox, and MMP-2/9 [92]. Thus, these results support the idea that Ang II signaling is a central pathway which mediates cellular and molecular mechanisms that underlie arterial aging and age-associated arterial diseases.
Concluding remarks
The metabolic, enzymatic, cellular, and molecular alterations that have been implicated in age-associated structural remodeling (Table 1 and Figure 3) are linked to Ang II signaling. These effects are increasingly recognized as major role players in the genesis or promotion of inflammatory arterial diseases such as hypertension and atherosclerosis. Many of the same factors that underlie the age-associated structural and functional alterations of the arterial wall are also implicated in the pathogenesis of clinical arterial disease. These and other previously well defined factors appear to be the “culprits” that accumulate and underlie the deleterious aspects of arterial aging, which are linked to an increased incidence of the quintessential cardiovascular diseases such as hypertension, atherosclerosis, and stroke in the elderly. Therefore, targeting Ang II signaling within the aged central arterial wall is a novel and promising approach to interfere with arterial aging and age-associated arterial disease.
Acknowledgement
The authors also would like to thank Robert E. Monticone for his assistance in preparing this document.
Sources of Funding
This research was supported by the Intramural Research Program of the National Institute on Aging, National Institutes of Health.
Footnotes
Conflicts of Interest
None
References
- 1.Lakatta EG, Wang M, Najjar SS. Arterial aging and subclinical arterial disease are fundamentally intertwined at macroscopic and molecular levels. Med Clin North Am. 2009;93:583–604. doi: 10.1016/j.mcna.2009.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang M, Monticone RE, Lakatta EG. Arterial aging: a journey into subclinical arterial disease. Curr Opin Nephrol Hypertens. 2010;19:201–207. doi: 10.1097/MNH.0b013e3283361c0b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang M, Lakatta EG. The Salted Artery and Angiotensin II Signaling: A deadly duo in arterial disease. J Hypertens. 2009;27:19–21. doi: 10.1097/HJH.0b013e32831d1fed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fu Z, Wang M, Gucek M, et al. Milk fat globule protein epidermal growth factor-8: a pivotal relay element within the angiotensin II and monocyte chemoattractant protein-1 signaling cascade mediating vascular smooth muscle cells invasion. Circ Res. 2009;104:1337–1346. doi: 10.1161/CIRCRESAHA.108.187088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li Z, Cheng H, Lederer WJ, et al. Enhanced proliferation and migration and altered cytoskeletal proteins in early passage smooth muscle cells from young and old rat aortic explants. Exp Mol Pathol. 1997;64:1–11. doi: 10.1006/exmp.1997.2204. [DOI] [PubMed] [Google Scholar]
- 6.Wang M, Zhang J, Spinetti G, et al. Angiotensin II activates matrix metalloproteinase type II and mimics age-associated carotid arterial remodeling in young rats. Am J Patho. 2005;167:1429–1442. doi: 10.1016/S0002-9440(10)61229-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang M, Zhao D, Spinetti G, et al. Matrix metalloproteinase 2 activation of transforming growth factor-beta1 (TGF-beta1) and TGF-beta1-type II receptor signaling within the aged arterial wall. Arterioscler Thromb Vasc Biol. 2006;26:1503–1509. doi: 10.1161/01.ATV.0000225777.58488.f2. [DOI] [PubMed] [Google Scholar]
- 8.Wang M, Zhang J, Jiang LQ, et al. Proinflammatory profile within the grossly normal aged human aortic wall. Hypertension. 2007;50:219–227. doi: 10.1161/HYPERTENSIONAHA.107.089409. [DOI] [PubMed] [Google Scholar]
- 9.Spinetti G, Wang M, Monticone R, et al. Rat aortic MCP-1 and its receptor CCR2 increase with age and alter vascular smooth muscle cell function. Arterioscler Thromb Vasc Biol. 2004;24:1397–1402. doi: 10.1161/01.ATV.0000134529.65173.08. [DOI] [PubMed] [Google Scholar]
- 10.Jiang L, Wang M, Zhang J, et al. Increased aortic calpain-1 activity mediates age-associated angiotensin II signaling of vascular smooth muscle cells. PLoS ONE. 2008;3:e2231. doi: 10.1371/journal.pone.0002231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang M, Takagi G, Asai K, et al. Aging increases aortic MMP-2 activity and angiotensin II in nonhuman primates. Hypertension. 2003;41:1308–1316. doi: 10.1161/01.HYP.0000073843.56046.45. [DOI] [PubMed] [Google Scholar]
- 12.Wang M, Lakatta EG. Altered regulation of matrix metalloproteinase-2 in aortic remodeling during aging. Hypertension. 2002;39:865–873. doi: 10.1161/01.hyp.0000014506.13322.66. [DOI] [PubMed] [Google Scholar]
- 13.Asai K, Kudej RK, Shen YT, et al. Peripheral vascular endothelial dysfunction and apoptosis in old monkeys. Arterioscler Thromb Vasc Biol. 2000;20:1493–1499. doi: 10.1161/01.atv.20.6.1493. [DOI] [PubMed] [Google Scholar]
- 14.Franklin SS, Gustin WG, W4th, Wong ND, et al. Hemodynamic patterns of age-related changes in blood pressure: the Framingham Heart Study. Circulation. 1997;96:308–315. doi: 10.1161/01.cir.96.1.308. [DOI] [PubMed] [Google Scholar]
- 15.Celermajer DS, Sorensen KE, Spiegelhalter DJ, et al. Aging is associated with endothelial dysfunction in healthy men years before the age-related decline in women. J Am Coll Cardiol. 1994;24:471–476. doi: 10.1016/0735-1097(94)90305-0. [DOI] [PubMed] [Google Scholar]
- 16.Vaitkevicius PV, Fleg JL, Engel JH, et al. Effects of age and aerobic capacity on arterial stiffness in healthy adults. Circulation. 1993;88:1456–1462. doi: 10.1161/01.cir.88.4.1456. [DOI] [PubMed] [Google Scholar]
- 17.Gerstenblith G, Frederiksen J, Yin FC, et al. Echocardiographic assessment of a normal adult aging population. Circulation. 1977;56:273–278. doi: 10.1161/01.cir.56.2.273. [DOI] [PubMed] [Google Scholar]
- 18.Nagai Y, Metter EJ, Earley CJ, et al. Increased carotid artery intimal-medial thickness in asymptomatic older subjects with exercise-induced myocardial ischemia. Circulation. 1998;98:1504–1509. doi: 10.1161/01.cir.98.15.1504. [DOI] [PubMed] [Google Scholar]
- 19.Virmani R, Avolio AP, Mergner WJ, et al. Effect of aging on aortic morphology in populations with high and low prevalence of hypertension and atherosclerosis. Comparison between occidental and Chinese communities. Am J Pathol. 1991;139:1119–1129. [PMC free article] [PubMed] [Google Scholar]
- 20.Schwartz SM, Benditt EP. Aortic endothelial cell replication, I: effects of age and hypertension in the rat. Circ Res. 1977;41:248–255. doi: 10.1161/01.res.41.2.248. [DOI] [PubMed] [Google Scholar]
- 21.Tashiro K, Shimokama T, Haraoka S, et al. Endothelial cell heterogeneity in experimentally-induced rabbit atherosclerosis: demonstration of multinucleated giant endothelial cells by scanning electron microscopy and cell culture. Virchows Archiv. 1994;425:521–529. doi: 10.1007/BF00197556. [DOI] [PubMed] [Google Scholar]
- 22.Kao CH, Chen JK, Yang VC. Ultrastructure and permeability of endothelial cells in branched regions of rat arteries. Atherosclerosis. 1994;105:97–114. doi: 10.1016/0021-9150(94)90012-4. [DOI] [PubMed] [Google Scholar]
- 23.Cavallaro U, Castelli V, Del Monte U, et al. Phenotypic alterations in senescent large-vessel and microvascular endothelial cells. Mol Cell Biol Res Commun. 2000;4:117–121. doi: 10.1006/mcbr.2000.0263. [DOI] [PubMed] [Google Scholar]
- 24.Yeh HI, Dupont E, Coppen S, et al. Gap junction localization and connexin expression in cytochemically identified endothelial cells of arterial tissue. J Histochem Cytochem. 1997;45:539–550. doi: 10.1177/002215549704500406. [DOI] [PubMed] [Google Scholar]
- 25.Dimmeler S, Vasa-Nicotera M. Aging of progenitor cells: limitation for regenerative capacity? J Am Coll Cardiol. 2003;42:2081–2082. doi: 10.1016/j.jacc.2003.09.016. [DOI] [PubMed] [Google Scholar]
- 26.Torella D, Leosco D, Indolfi C, et al. Aging exacerbates negative remodeling and impairs endothelial regeneration after balloon injury. Am J Physiol Heart Circ Physiol. 2004;287:H2850–H2860. doi: 10.1152/ajpheart.01119.2003. [DOI] [PubMed] [Google Scholar]
- 27.Schmidt-Lucke C, Rossig L, Fichtlscherer S, et al. Reduced number of circulating endothelial progenitor cells predicts future cardiovascular events: proof of concept for the clinical importance of endogenous vascular repair. Circulation. 2005;111:2981–2987. doi: 10.1161/CIRCULATIONAHA.104.504340. [DOI] [PubMed] [Google Scholar]
- 28.Thum T, Hoeber S, Froese S, et al. Age-dependent impairment of endothelial progenitor cells is corrected by growth-hormone-mediated increase of insulin-like growth-factor-1. Circ Res. 2007;100:434–443. doi: 10.1161/01.RES.0000257912.78915.af. [DOI] [PubMed] [Google Scholar]
- 29.Hoetzer GL, Van Guilder GP, Irmiger H, et al. Aging, exercise and endothelial progenitor cell clonogenic and migratory capacity in men. J Appl Physiol. 2007;102:847–852. doi: 10.1152/japplphysiol.01183.2006. [DOI] [PubMed] [Google Scholar]
- 30.Kushner EJ, Van Guilder GP, MacEneaney OJ, et al. Aging and endothelial progenitor cell telomere length in healthy men. Clinical Chemistry and Laboratory Medicine. 2009;47:47–50. doi: 10.1515/CCLM.2009.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Minamino T, Komuro I. Vascular aging: insights from studies on cellular senescence stem cell aging, and progeroid syndromes. Nat Clin Pract Cardiovasc Med. 2008;5:637–648. doi: 10.1038/ncpcardio1324. [DOI] [PubMed] [Google Scholar]
- 32.Dimmeler S, Zeiher AM. Vascular repair by circulating endothelial progenitor cells: the missing link in atherosclerosis? J Mol Med. 2004;83:671–677. doi: 10.1007/s00109-004-0580-x. [DOI] [PubMed] [Google Scholar]
- 33.Edelberg JM, Tang L, Hattori K, et al. Young adult bone marrow-derived endothelial precursor cells restore aging-impaired cardiac angiogenic function. Circ Res. 2002;90:e89–e93. doi: 10.1161/01.res.0000020861.20064.7e. [DOI] [PubMed] [Google Scholar]
- 34.Zhu G, Song M, Wang H, et al. Young environment reverses the declined activity of aged rat-derived endothelial progenitor cells: involvement of the phosphatidylinositol 3-kinase/Akt signaling pathway. Ann Vasc Surg. 2009;23:519–534. doi: 10.1016/j.avsg.2008.11.013. [DOI] [PubMed] [Google Scholar]
- 35.van der Loo B, Labugger R, Skepper JN, et al. Enhanced peroxynitrite formation is associated with vascular aging. J Exp Med. 2000;192:1731–1744. doi: 10.1084/jem.192.12.1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tschudi MR, Barton M, Bersinger NA, et al. Effect of age on kinetics of nitric oxide release in rat aorta and pulmonary artery. J Clin Invest. 1996;98:899–905. doi: 10.1172/JCI118872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Danon D, Laver-Rudich Z, Skutelsky E. Surface charge and flow properties of endothelial membranes in aging rats. Mech Ageing Dev. 1980;14:145–153. doi: 10.1016/0047-6374(80)90113-x. [DOI] [PubMed] [Google Scholar]
- 38.Belmin J, Corman B, Merval R, et al. Age-related changes in endothelial permeability and distribution volume of albumin in rat aorta. Am J Physiol. 1993;264:H679–H685. doi: 10.1152/ajpheart.1993.264.3.H679. [DOI] [PubMed] [Google Scholar]
- 39.Nagata Y, Jones MR, Nguyen HG, et al. Vascular smooth muscle cell polyploidization involves changes in chromosome passenger proteins and an endomitotic cell cycle. Exp. Cell Res. 2005;305:277–291. doi: 10.1016/j.yexcr.2004.12.028. [DOI] [PubMed] [Google Scholar]
- 40.Yang D, McCrann DJ, Nguyen H, et al. Increased polyploidy in aortic vascular smooth muscle cells during aging is marked by cellular senescence. Aging Cell. 2007;6:257–260. doi: 10.1111/j.1474-9726.2007.00274.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Arribas SM, Briones AM, Bellingham C, et al. Heightened aberrant deposition of hard-wearing elastin in conduit arteries of prehypertensive SHR is associated with increased stiffness and inward remodeling. Am J Physiol Heart Circ Physiol. 2008;295:H2299–H2307. doi: 10.1152/ajpheart.00155.2008. [DOI] [PubMed] [Google Scholar]
- 42.Silver FH, Horvath I, Foran DJ. Viscoelasticity of the vessel wall: the role of collagen and elastic fibers. Crit Rev Biomed Eng. 2001;29:279–301. doi: 10.1615/critrevbiomedeng.v29.i3.10. [DOI] [PubMed] [Google Scholar]
- 43.Krettek A, Sukhova GK, Libby P. Elastogenesis in human arterial disease: a role for macrophages in disordered elastin synthesis. Arterioscler. Thromb. Vasc. Biol. 2003;23:582–587. doi: 10.1161/01.ATV.0000064372.78561.A5. [DOI] [PubMed] [Google Scholar]
- 44.Sauvage M, Hinglais N, Mandet C, et al. Localization of elastin mRNA and TGF-β1 in rat aorta and caudal artery as a function of age. Cell Tissue Res. 1998;291:305–314. doi: 10.1007/s004410051000. [DOI] [PubMed] [Google Scholar]
- 45.Jacob MP. Extracellular matrix remodeling and matrix metalloproteinases in the vascular wall during aging and in pathological conditions. Biomed Pharmacother. 2003;57:195–202. doi: 10.1016/s0753-3322(03)00065-9. [DOI] [PubMed] [Google Scholar]
- 46.Behmoaras J, Slove S, Seve S, et al. Differential expression of lysyl Oxidases LOXL1 and LOX during growth and aging suggests specific roles in elastin and collagen fiber remodeling in rat aorta. Rejuvenation Res. 2008;11:883–889. doi: 10.1089/rej.2008.0760. [DOI] [PubMed] [Google Scholar]
- 47.Bailey AJ, Paul RG, Knott L. Mechanisms of maturation and ageing of collagen. Mech Ageing Develop. 1998;106:1–56. doi: 10.1016/s0047-6374(98)00119-5. [DOI] [PubMed] [Google Scholar]
- 48.Helin P, Garbarsch C, Helin G, et al. Vascular injury compared to ageing of normal rabbit aorta: Biochemical and histochemical studies on time-dependent alterations of vascular connective tissue. Blood Vessel. 1985;22:94–104. [PubMed] [Google Scholar]
- 49.Tovar AM, Cesar DC, Leta GC, et al. Age-related changes in populations of aortic glycosaminoglycans: species with low affinity for plasma low-density lipoproteins, and not species with high affinity, are preferentially affected. Arterioscler Thromb Vasc Biol. 1998;18:604–614. doi: 10.1161/01.atv.18.4.604. [DOI] [PubMed] [Google Scholar]
- 50.Zugibe FT. The demonstration of the individual acid mucopolysaccharides in human aortas, coronary arteries and cerebral arteries. II. Identification and significance with aging. J. Histochem. Cytochem. 1962;10:448–461. [Google Scholar]
- 51.Dyer D, Dunn J, Thorpe S, et al. Accumulation of Maillard products in skin collagen in diabetes and aging. J Clin Invest. 1993;91:2463–2469. doi: 10.1172/JCI116481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wang M, Lakatta EG. Chapter 9. Central Arterial Aging: Humans to Molecules Hypertension in the elderly. In: MichelE Safar, MichelE O’Rourke., editors. Handbook of Hypertension. Vol. 23. Elsevier; 2006. pp. 137–160. [Google Scholar]
- 53.Pasquali-Ronchetti I, Baccarani-Contri M. Elastic fiber during development and aging. Microsc. Res. Tech. 1997;38:428–435. doi: 10.1002/(SICI)1097-0029(19970815)38:4<428::AID-JEMT10>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 54.Giachelli CM, Bae N, Almeida M, et al. Osteopontin is elevated during neointima formation in rat arteries and is a novel component of human atherosclerotic plaques. J Clin Invest. 1993;92:1686–1696. doi: 10.1172/JCI116755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wallin R, Wajih N, Greenwood GT, et al. Arterial calcification: a review of mechanisms, animal models, and the prospects for therapy. Med Res Rev. 2001;21:274–301. doi: 10.1002/med.1010. [DOI] [PubMed] [Google Scholar]
- 56.Atkinson J. Age-related medial elastocalcinosis in arteries: mechanisms, animal models, and physiological consequences. J Appl Physiol. 2008;105:1643–1651. doi: 10.1152/japplphysiol.90476.2008. [DOI] [PubMed] [Google Scholar]
- 57.Westenfeld R, Schäfer C, Krüger T, et al. Fetuin-A protects against atherosclerotic calcification in CKD. J Am Soc Nephrol. 2009;20:1264–1274. doi: 10.1681/ASN.2008060572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Michel JB, Thaunat O, Houard X, et al. Topological determinants and consequences of adventitial responses to arterial wall injury. Arterioscler Thromb Vasc Biol. 2007;27:1259–1268. doi: 10.1161/ATVBAHA.106.137851. [DOI] [PubMed] [Google Scholar]
- 59.Gräbner R, Lötzer K, Döpping S, et al. Lymphotoxin beta receptor signaling promotes tertiary lymphoid organogenesis in the aorta adventitia of aged ApoE−/− mice. J Exp Med. 2009;206:233–248. doi: 10.1084/jem.20080752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Passman JN, Dong XR, Wu SP, et al. A sonic hedgehog signaling domain in the arterial adventitia supports resident Sca1+ smooth muscle progenitor cells. Proc Natl Acad Sci U S A. 2008;105:9349–9354. doi: 10.1073/pnas.0711382105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sierevogel MJ, Velema E, Meer FJ, et al. Matrix metalloproteinase inhibition reduces adventitial thickening and collagen accumulation following balloon dilation. Cardiovascular Research. 2002;55:864–869. doi: 10.1016/s0008-6363(02)00467-4. [DOI] [PubMed] [Google Scholar]
- 62.Aubry M, Riehle DL, Edwards WD, et al. B-Lymphocytes in plaque and adventitia of coronary arteries in two patients with rheumatoid arthritis and coronary atherosclerosis: Preliminary observations. Cardiovascular Pathology. 2004;13:233–236. doi: 10.1016/j.carpath.2004.02.005. [DOI] [PubMed] [Google Scholar]
- 63.Moos M, John N, Gräbner R, et al. The lamina adventitia is the major site of immune cell accumulation in standard chow-fed apolipoprotein E-deficient mice. Arteriosclerosis, Thrombosis, and Vascular Biology. 2005;25:2386–2391. doi: 10.1161/01.ATV.0000187470.31662.fe. [DOI] [PubMed] [Google Scholar]
- 64.Navar LG, Harrison-Bernard LM, Wang CT, et al. Concentrations and actions of intraluminal angiotensin II. J Am Soc Nephrol. 1999;10 Suppl 11:S189–S195. [PubMed] [Google Scholar]
- 65.Diz D, Lewis K. Dahl memorial lecture: the renin-angiotensin system and aging. Hypertension. 2008;52:37–43. [Google Scholar]
- 66.Koka V, Wang W, Huang XR, et al. Advanced glycation end products activate a chymase-dependent angiotensin II-generating pathway in diabetic complications. Circulation. 2006;113:1353–1360. doi: 10.1161/CIRCULATIONAHA.105.575589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Raymond A, Ensslin MA, Shur BD. SED1/MFG-E8: a bi-motif protein that orchestrates diverse cellular interactions. J Cell Biochem. 2009;106:957–966. doi: 10.1002/jcb.22076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wang M, Zhang J, de Cabo R, et al. Calorie restriction reduces MMP-2 actvity and retards age-associated aortic restructuring in rats. Circulation (Supll II) 2006;114:II-335. [Google Scholar]
- 69.Yang YM, Huang A, Kaley G, et al. eNOS uncoupling and endothelial dysfunction in aged vessels. Am J Physiol Heart Circ Physiol. 2009;297:H1829–H1836. doi: 10.1152/ajpheart.00230.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Soucy KG, Ryoo S, Benjo A, et al. Impaired shear stress-induced nitric oxide production through decreased NOS phosphorylation contributes to age-related vascular stiffness. J Appl Physiol. 2006;101:1751–1759. doi: 10.1152/japplphysiol.00138.2006. [DOI] [PubMed] [Google Scholar]
- 71.Cernadas MR, Sánchez de Miguel L, García-Durán M, et al. Expression of constitutive and inducible nitric oxide synthases in the vascular wall of young and aging rats. Circ Res. 1998;83:279–286. doi: 10.1161/01.res.83.3.279. [DOI] [PubMed] [Google Scholar]
- 72.White AR, Ryoo S, Li D, et al. Knockdown of arginase I restores NO signaling in the vasculature of old rats. Hypertension. 2006;47:245–251. doi: 10.1161/01.HYP.0000198543.34502.d7. [DOI] [PubMed] [Google Scholar]
- 73.Chauhan A, More RS, Mullins PA, et al. Aging-associated endothelial dysfunction in humans is reversed by L-arginine. J Am Coll Cardiol. 1996;28:1796–1804. doi: 10.1016/s0735-1097(96)00394-4. [DOI] [PubMed] [Google Scholar]
- 74.Hamilton CA, Brosnan MJ, McIntyre M, et al. Superoxide excess in hypertension and aging: a common cause of endothelial dysfunction. Hypertension. 2001;37:529–534. doi: 10.1161/01.hyp.37.2.529. [DOI] [PubMed] [Google Scholar]
- 75.Csiszar A, Wang M, Lakatta EG, et al. Inflammation and endothelial dysfunction during aging: role of NF-{kappa} B. J Appl Physiol. 2008;105:1333–1341. doi: 10.1152/japplphysiol.90470.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Guo ZM, Yang H, Hamilton ML, et al. Effects of age and food restriction on oxidative DNA damage and antioxidant enzyme activities in mouse aorta. Mech Ageing Dev. 2001;122:1771–1786. doi: 10.1016/s0047-6374(01)00298-6. [DOI] [PubMed] [Google Scholar]
- 77.Demaree SR, Lawler JM, Linehan J, et al. Ageing alters aortic antioxidant enzyme activities in Fischer-344 rats. Acta Physiol Scand. 1999;166:203–208. doi: 10.1046/j.1365-201x.1999.00552.x. [DOI] [PubMed] [Google Scholar]
- 78.Hamdi HK, Castellon RA. Genetic variant of ACE increases cell survival: a new paradigm for biology and disease. Biochem Biophys Res Commun. 2004;318:187–191. doi: 10.1016/j.bbrc.2004.04.004. [DOI] [PubMed] [Google Scholar]
- 79.Shan HY, Bai XJ, Chen XM. Apoptosis is involved in the senescence of endothelial cells induced by angiotensin II. Cell Biol Int. 2008;32:264–270. doi: 10.1016/j.cellbi.2007.09.003. [DOI] [PubMed] [Google Scholar]
- 80.Hariri RJ, Alonso DR, Hajjar DP, et al. Aging and arteriosclerosis: I: development of myointimal hyperplasia after endothelial injury. J Exp Med. 1986;164:1171–1178. doi: 10.1084/jem.164.4.1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hariri RJ, Hajjar DP, Coletti D, et al. Aging and arteriosclerosis. Cell cycle kinetics of young and old arterial smooth muscle cells. Am J Pathol. 1988;131:132–136. [PMC free article] [PubMed] [Google Scholar]
- 82.Gennaro G, Ménard C, Giasson E, et al. Role of p44/p42 MAP kinase in the age-dependent increase in vascular smooth muscle cell proliferation and neointimal formation. Arterioscler Thromb Vasc Biol. 2003;23:204–210. doi: 10.1161/01.atv.0000053182.58636.be. [DOI] [PubMed] [Google Scholar]
- 83.Yoshida Y, Mitsumata M, Ling G, et al. Migration of medial smooth muscle cells to the intima after balloon injury. Ann N Y Acad Sci. 1997;811:459–470. doi: 10.1111/j.1749-6632.1997.tb52027.x. [DOI] [PubMed] [Google Scholar]
- 84.Pauly RR, Passaniti A, Crow M, et al. Experimental models that mimic the differentiation and dedifferentiation of vascular cells. Circulation. 1992;86:III68–III73. [PubMed] [Google Scholar]
- 85.Benigni A, Corna D, Zoja C, et al. Disruption of the Ang II type 1 receptor promotes longevity in mice. J Clin Invest. 2009;119:524–530. doi: 10.1172/JCI36703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Linz W, Heitsch H, Schölkens BA, et al. Long-term angiotensin II type 1 receptor blockade with fonsartan doubles lifespan of hypertensive rats. Hypertension. 2000;35:908–913. doi: 10.1161/01.hyp.35.4.908. [DOI] [PubMed] [Google Scholar]
- 87.Michel JB, Heudes D, Michel O, et al. Effect of chronic ANGI-converting enzyme inhibition on aging processes: II: Large arteries. Am J Physiol. 1994;267:R124–R135. doi: 10.1152/ajpregu.1994.267.1.R124. [DOI] [PubMed] [Google Scholar]
- 88.Huang W, Alhenc Gelas F, Osborne-Pellegrin MJ. Protection of the arterial internal elastic lamina by inhibition of the renin-angiotensin system in the rat. Circ Res. 1998;82:879–890. doi: 10.1161/01.res.82.8.879. [DOI] [PubMed] [Google Scholar]
- 89.Baur JA, Pearson KJ, Price NL, et al. Resveratrol improves health and survival of mice on a high-calorie diet. Nature. 2006;444:337–342. doi: 10.1038/nature05354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Miyazaki R, Ichiki T, Hashimoto T, et al. SIRT1, a longevity gene, downregulates angiotensin II type 1 receptor expression in vascular smooth muscle cells. Arterioscler Thromb Vasc Biol. 2008;28:1263–1269. doi: 10.1161/ATVBAHA.108.166991. [DOI] [PubMed] [Google Scholar]
- 91.Chao HH, Juan SH, Liu JC, et al. Resveratrol inhibits angiotensin II-induced endothelin-1 gene expression and subsequent proliferation in rat aortic smooth muscle cells. Eur J Pharmacol. 2005;515:1–9. doi: 10.1016/j.ejphar.2005.03.035. [DOI] [PubMed] [Google Scholar]
- 92.Eto H, Miyata M, Shirasawa T, et al. The long-term effect of angiotensin II type 1a receptor deficiency on hypercholesterolemia-induced atherosclerosis. Hypertens Res. 2008;31:1631–1642. doi: 10.1291/hypres.31.1631. [DOI] [PubMed] [Google Scholar]