Abstract
During immunostaining of specific proteins in tissue sections using monoclonal and polyclonal antibodies visualization of general tissue staining/background or major structural features is helpful to pinpoint precise localization of the protein of interest. Often in skeletal muscle research immunostaining with antibodies against connective tissue or plasma membrane proteins (collagen 1, laminin, caveolin 3) are used for this purpose. Although immunostaining for these proteins works well, it is time consuming, costly, limits the number of antibodies against protein of interest that can be used on a single section, and is not applicable to some staining techniques. Lectins were frequently used in earlier publications for skeletal muscle fiber boundaries and connective tissue visualization, but are not common in the current research studies. The present paper investigates co-staining of muscle, bone, ligament and tendon tissue sections with fluorescently tagged wheat germ agglutinin (WGA) lectin as a tool for visualization of connective tissue. The results of the current study show that fluorescent WGA lectin co-staining is a cost-effective, fast and convenient method for connective tissue visualization, especially in the studies where extensive washes reduce staining of the structures that are the primary interest of the investigation.
Keywords: immunostaining, intramyocellular lipid droplets, aggrecan
INTRODUCTION
Lectins are proteins of plant origin that bind different carbohydrate motifs (reviewed in De Hoff et al 2009). They are important reagents used for studies of changes in the carbohydrate composition of glycoproteins and proteoglycans (reviewed in Pilobello and Mahal 2007; Zhao et al 2008). Carbohydrate composition is critically important for functions of the glycoproteins and proteoglycans and altering glycosylation or blocking carbohydrate motifs of these proteins with lectins can modify protein function (Earl and Baum 2008; Kostrominova and Tanzer 1995) and contribute to the development of the impaired/diseased conditions (Saldova et al. 2008; Tajima et al. 2005; O’Connell et al. 2008). For example, impaired sialyl O-glycan formation in alpha-dystroglycan recognized by increased binding of the PNA lectin in distal myopathy with rimmed vacuoles patients can contribute to the pathology of this disease (Tajima et al. 2005). Mammalian cells also can express lectins (Cambi and Figdor 2009). Skeletal muscle expresses trans-membrane protein with carbohydrate recognition domain of C-type lectin in the extracellular portion that might be important for muscle differentiation and function (Weng et al. 2003).
In earlier studies lectins were frequently used for visualization of the connective tissue in different organs (Thoss and Roth 1977; Söderström 1987), including visualization of skeletal muscle fibers (Pena et al. 1981; Capaldi et al. 1985; Di Iorio and Cotrufo 1985). For example Pena and colleagues (1981) investigated use of seven plant lectins, including WGA, for visualization of the human skeletal muscle fiber boundaries in cryostat sections. Di Iorio and Cotrufo (1985) showed that WGA binding sites are abundant and the abundance increases following denervation of skeletal muscle in rats. Denervation significantly increased concanavalin A (ConA) lectin binding to the 75–80 kDa highly charged isomers in the sarcolemma of fast but not slow rat muscle (Iannello and Jeffrey 1990). In recent years very few studies have used this approach (Mozdziak et al. 1996; Tajima et al. 2005; O’Connell et al. 2008) and these studies were focused on the characterization of the changes in glycoprotein composition rather than using lectins as markers for skeletal muscle fiber boundaries.
Lectins were used previously in bone/tendon/ligament research (Schünke et al. 1985; Maffulli et al. 2002; Lyons et al. 2007) although their use as co-staining markers for connective tissue visualization in immunohistochemical studies was very limited.
The current study evaluated the use of lectin staining as a marker for the routine visualization of fiber outlines in skeletal muscle as well as for the connective tissue visualization in bone/tendon/ligament. The data presented here show that sialic acid/N-acetylglucosamine binding fluorescently tagged WGA lectin is a cost-effective, fast and convenient method for connective tissue visualization in many areas of research.
MATERIALS AND METHODS
Animals
The breeding pair of Sod1−/+ male and Sod1−/+ female mice, were obtained from The Jackson Laboratory (Sod1tm1Leb; stock # 002972) and were bred and genotyped at the Indiana University. At 8–10 months of age, the adult male Sod1+/+ mice were anesthetized with Avertin and the tibialis anterior (TBA) and gastrocnemius (GTN) muscles were dissected from each leg. Following removal of the muscles, the mice were euthanized with an overdose of Avertin and the thorax was opened to ensure the immediate death of the mouse.
10 μg/ml solution of Notexin Np (Accurate Chemicals & Scientific Corporation, Westbury, NY) was injected into TBA muscle of Avertin anesthetized adult male Sod1+/+ mice in the amount of 50 μl per muscle. TBA muscle was dissected seven days after injection, frozen in isopentane and stored at −70°C until sectioning.
Male and pregnant female (for obtaining 3 and 21 day-old neonates) Fischer 344 rats were obtained from Charles River Laboratories, Inc. (Wilmington, MA). Intraperitoneal injection of Evans Blue dye was performed as previously described (Hamer et al. 2002). In short, 4–6 month old male rats were injected with a 1% solution of Evans Blue Dye (1% of body mass). Seventeen hours later rats were anesthetized with sodium pentobarbital and TBA muscles were dissected, frozen in isopentane and stored at −70°C until sectioning. The bovine eyes used in the experiments were obtained from a local slaughterhouse shortly after the cow’s death. Extraocular muscles (EOM) were dissected from the eyes, placed in ice-cold Ringer solution and transported to the laboratory where they were frozen in isopentane and stored at −70°C until sectioning.
All animal care and animal surgeries were in accordance with The Guide for Care and Use of Laboratory Animals (Public Health Service, 1996, NIH Publication No. 85-23); the experimental protocol was approved by the Indiana University Committee for the Use and Care of Animals.
Histochemical and Immunohistochemical Analysis
For histochemical analysis, unfixed samples were placed into TBS medium (Triangle Biological Sciences, Durham, NC), frozen in cold isopentane and stored at −70°C until needed. Samples were sliced with a cryostat at a thickness of approximately 12 μm, adhered to Superfrost Plus microscopy slides and used for staining. Immunofluorescent staining with specific antibodies was performed to detect the presence of blood vessels (CD-31), laminin, cathepsin K, slow myosin and aggrecan. Frozen sections were fixed with ice cold methanol for 10 min and rinsed 3 times with Phosphate Buffered Saline (PBS). For myosin immunostaining methanol fixation was omitted and sections were air dried and boiled in PBS for 5 min as previously described (Mundegar et al., 2008). Sections were blocked for 30 min with PBS-0.05%Tween20 (PBST) containing 20% calf serum (PBST-S) at room temperature. Sections were incubated overnight at 4°C with the primary antibodies in PBST-S. The following primary antibodies were used: mouse anti-rat CD31 antibody (AbD Serotec, Oxford, UK; 1:100 dilution); mouse anti-slow myosin (clone A4.84) antibody (Developmental Studies Hybridoma Bank; 1:5 dilution); rabbit anti-laminin antibody (Chemicon International, Temecula, CA; 1:100 dilution); rabbit anti-cathepsin K and rabbit anti-aggrecan (both from Abcam, Cambridge, MA; 1:100 dilution). One hour room temperature incubation with Cy2 or Cy3-conjugated anti-mouse or anti-rabbit antibody (Jackson ImmunoResearch Lab., West Grove, PA; 1:1000 dilution) was used for visualization. Sections incubated only with Cy3-conjugated anti-mouse or anti-rabbit antibody were used as negative controls.
For lipid staining, tissue sections were incubated in 3% paraformaldehyde for 20 min at room temperature, washed 3 times with PBS and incubated with 1 μg/ml BODIPY 493/503 (Molecular Probes, Eugene, OR) for 20 min at room temperature. For visualization of neuromuscular junctions (NMJ), muscle sections were incubated for 10 min at room temperature in the solution of alpha-bungarotoxin (α-BTX-rhodamine; Sigma, St. Louis, MO; 1μg/ml). Previous publications have shown that avidin selectively binds and can be used as a specific marker for mast cells in tissue sections (Fritz et al. 1984). For visualization of mast cells, muscle sections were incubated for 20 min at room temperature in avidin DCS-fluorescein solution (Vector Laboratories, Burlingame, CA; 1:100 dilution).
Co-staining of sections with fluorescein conjugated wheat germ agglutinin (WGA-fluorescein; 1μg/ml; Molecular Probes, Eugene, OR) and Alexa Fluor 555 WGA conjugate (WGA-Alexa; 1 μg/ml; Molecular Probes, Eugene, OR) was used for visualization of the connective tissue. Nuclei were stained by 5 min incubation with a DAPI solution (Sigma, St. Louis, MO; 100ng/ml) in PBST. The sections were examined and photographed with a Leica microscope.
RESULTS
Fluorescent WGA lectin co-staining of muscle sections
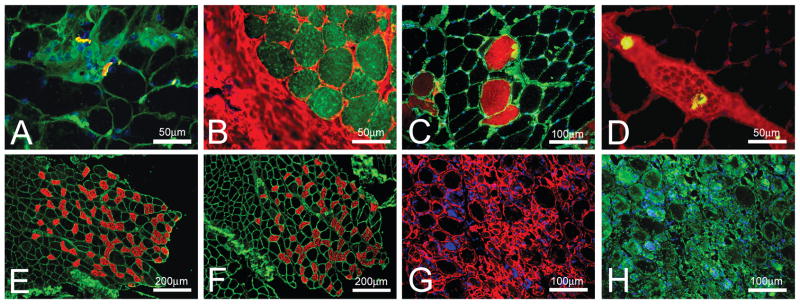
Figure 1 show skeletal muscle sections stained for the visualization of the structures of interest using several different techniques and then co-stained with WGA lectin or anti-laminin antibodies for the muscle fiber outline/connective tissue.α-BTX-rhodamine stained NMJs in bovine EO muscles (red in Figure 1A); but without prominent muscle fiber outlines it was very difficult to localize NMJ in relation to other structures. Co-staining with WGA-fluorescein (green in Figure 1A) clearly showed muscle fiber outlines and localization of the connective tissue allowing NMJ visualization at the surface of the muscle fibers. Similarly in Figure 1B neutral lipid stain allowed visualization of the intramyocellular lipid droplets (green) in bovine EO muscles; but without the staining of the muscle fiber outlines and connective tissue with WGA-Alexa (red) it was difficult to visualize the lipid droplet distribution in the section. Intraperitoneal injection of Evans Blue dye allows visualization of muscle fibers with damaged plasma membrane in rat TBA muscle (red in Figure 1C) even when the fibers appear intact in the Hematoxylin and Eosin stained sections (not shown). WGA-fluorescein (green in Figure 1C) allows visualization of the muscle fiber outlines and distribution of the damaged fibers relative to the intact fibers within the muscle section. Staining with avidin D-fluorescein allows fast and reliable identification of the mast cells in tissue sections (green in Figure 1D). When co-stained with WGA-Alexa (red in Figure 1D) it is possible to localize mast cells to particular structures within the section of plantaris muscle from rats. For example, in Figure 1D mast cells are located within a nerve bundle.
Figure 1.
Fluorescent WGA lectin co-staining of muscle sections. (A) a section of bovine EO muscles was stained with α-BTX-rhodamine (red) to visualize neuromuscular junctions and co-stained with WGA-fluorescein (green) to outline muscle fibers and nerve bundles. DAPI (blue) was used to co-stain the nuclei. (B) a section of bovine EO muscles was stained with BODIPY 493/503 (green) to visualize intramyocellular lipid droplets and co-stained with WGA-Alexa to outline muscle fibers and connective tissue. (C) fibers with damaged plasma membrane in rat tibialis anterior muscle were visualized by intra-peritoneal injection of Evans Blue dye (red) and co-stained with WGA-fluorescein (green) to outline all muscle fibers. DAPI (blue) was used to co-stain the nuclei. (D) a section of plantaris muscle from rats was stained with avidin D- fluorescein (green) to visualize mast cells and co-stained with WGA-Alexa to outline muscle fibers and connective tissue. (E) a section of gastrocnemius muscle from mice was stained with antibodies against slow myosin-Cy3 (red) to visualize slow muscle fibers and co-stained with WGA-fluorescein (green) to outline all muscle fibers. (F) a section of gastrocnemius muscle from mice was stained with antibodies against slow myosin-Cy3 (red) to visualize slow muscle fibers and co-stained with antibodies against laminin-Cy2 (green) to outline all muscle fibers. (G) a section of mouse tibialis anterior muscle after 7 day injection of Notoxin was stained with antibodies against laminin-Cy3 (red) to outline muscle fibers. DAPI (blue) was used to co-stain the nuclei. (H) a section of mouse tibialis anterior muscle after 7 day injection of Notoxin (the same view as in G) was stained with WGA-fluorescein (green) to outline muscle fibers. DAPI (blue) was used to co-stain the nuclei.
Fiber typing is often necessary in skeletal muscle research. The majority of the available antibodies against different myosin types are mouse monoclonal antibodies. Using these antibodies on mouse sections is challenging since secondary anti-mouse antibodies produce nonspecific staining when used on mouse skeletal muscle sections. Recently a simple technique for background reduction was introduced (Mundegar et al., 2008) that requires boiling of muscle sections in PBS. Therefore I determined whether boiling the samples would preserve co-staining of muscle fiber outlines with WGA lectin. Figure 1E shows that boiling significantly reduced background staining due to the secondary antibody but did not interfere with primary mouse anti-slow myosin antibody immunostaining (red) and completely preserved WGA lectin staining (green) of gastrocnemius muscle from mice. The WGA lectin staining was practically identical to the immunostaining of the boiled mouse muscle sections with primary mouse anti-slow myosin antibody (red in Figure 1F) and rabbit anti-laminin antibodies (green in Figure 1F).
In my experiments WGA lectin co-staining for visualization of muscle fiber outlines worked very well on muscle samples from young and old, innervated and denervated, mice and rats, as well as on muscle samples from pigs and cows (data not shown). One area where in my opinion using anti-laminin antibody would be more optimal than WGA lectin co-staining are studies of severely damaged muscles (Figure 1G and 1H). In sections with severely damaged muscles fibers, anti-laminin antibody allowed better visualization of muscle fibers outlines in mouse TBA muscle (red in Figure 1G) than staining of the same section with WGA-fluorescein (green in Figure 1H).
Fluorescent WGA lectin co-staining of bone, ligament and tendon sections
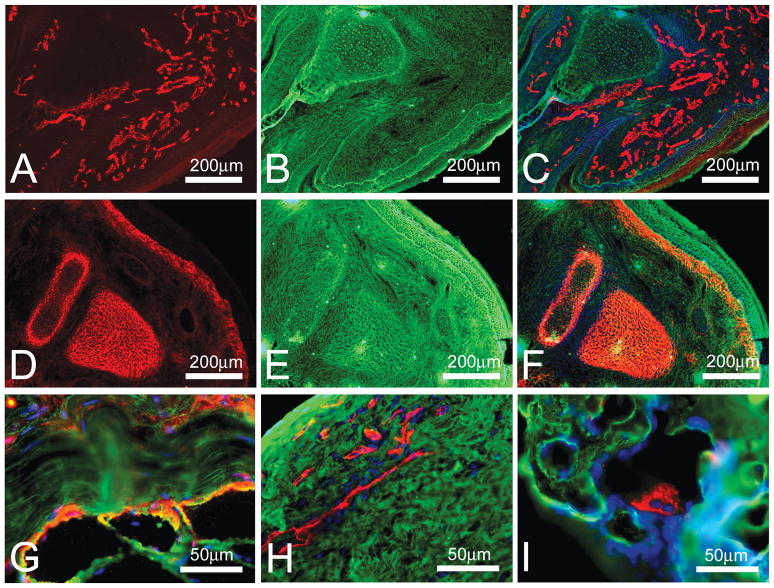
In my laboratory, in addition to skeletal muscle, we also study bone, ligament and tendon structure under different physiological conditions. Therefore I determined whether fluorescent WGA lectin co-staining could be beneficial for immunostaining of these tissues. The results showed that fluorescent WGA lectin co-staining aids in visualization of the general tissue background and allows better localization of the specific staining relative to the general section outline (Figure 2). In Figure 2A blood vessels were visualized in the developing rat palm. Although the blood vessels were clearly marked (red in Figure 2A) it was difficult to relate their distribution relative to the other structures in the developing rat palm. Combination of blood vessels (red)/WGA lectin (green)/DAPI (blue) staining allowed visualization of the blood vessels as well as other structures (Figure 2C) and clearly showed that at this stage of development cartilaginous template for the developing bone lacked blood vessels (central area in Figure 2C). WGA lectin co-staining (green in 2E-F) was also beneficial for the visualization of the aggrecan-positive structures (red in 2D and 2F) in the developing rat palm relative to the other structures (Figure 2F). WGA lectin co-staining (green) was also helpful for precise localization of paxillin (red) to the area of the myotendinous junction (MTJ; Figure 2G), localization of the blood vessels (red) to the peripheral layer of the adult rat ligament (Figure 2H), and localization of the osteoclast (red) on the surface of the developing bone (Figure 2I).
Figure 2. Fluorescent WGA lectin co-staining of bone, ligament and tendon sections.
(A) a section of a palm from 3-day old rat was stained with antibodies against CD31-Cy3 (red) to visualize blood vessels. (B) the same section shown in A was co-stained with WGA-fluorescein (green) to outline general tissue structure. (C) combined image of CD31-Cy3 (red) and WGA-fluorescein (green) staining. DAPI (blue) was used to co-stain the nuclei. (D) a section of a palm from 3-day old rat was stained with antibodies against aggrecan-Cy3 (red) to visualize cartilage templates of the developing bones. (E) the same section shown in D was co-stained with WGA-fluorescein (green) to outline general tissue structure. (F) combined image of aggrecan-Cy3 (red) and WGA-fluorescein (green) staining. DAPI (blue) was used to co-stain the nuclei. (G) a section of MTJ from tibialis anterior muscle of adult rat was stained with antibodies against paxillin-Cy3 (red) to visualize MTJ and co-stained with WGA-fluorescein (green) to outline general tissue structure and DAPI (blue) to co-stain the nuclei. (H) a section of a ligament from adult rat was stained with antibodies against CD31-Cy3 (red) to visualize blood vessels and co-stained with WGA-fluorescein (green) to outline general tissue structure and DAPI (blue) to co-stain the nuclei. (I) a section of bone from 21 day-old rat was stained with antibodies against cathepsin K-Cy3 (red) to visualize osteoclasts and co-stained with WGA-fluorescein (green) to outline general tissue structure and DAPI (blue) to co-stain the nuclei.
DISCUSSION
Immunofluorescence is a sensitive, reliable and frequently used method for visualization of the specific cells and structures. Often when visualizing a specific structure with one primary antibody, a co-staining with a different primary antibody is used for outlining general tissue structure. For example, in skeletal muscle histological studies immunostaining with anti-laminin antibodies is often used for skeletal muscle fiber outlining (Sajko et al 2004; Kostrominova et al. 2005; Mundegar et al. 2008). Although this technique works very well there are several drawbacks associated with it. First, there is limit to the number of primary antibodies that can be used on the same section. If mouse or rabbit anti-laminin antibody is used for muscle fiber visualization then primary antibody made in the same host can not be used for the visualization of the specific cells/structures of interest. Second, while staining for specific cells/structures of interest often requires only 20–30 minutes (lipid staining with BODIPY 493/503 or NMJ staining with α-BTX-rhodamine), co-staining with anti-laminin antibody can take several hours. Third, for some staining techniques the extensive washing required for immunofluorescent analysis can reduce staining of the structures that are the primary interest of the investigation (lipid staining with BODIPY 493/503).
A review of the scientific literature for fast and reliable methods for skeletal muscle fiber visualization showed several earlier publications describing the use of lectins. Several different lectins were described for skeletal muscle fiber visualization (Pena et al. 1981; Capaldi et al. 1985), but surprisingly these lectins have not found a wide application in recent studies. More recent papers using lectin staining on skeletal muscle were focused on the characterization of the changes in glycoprotein composition (Tajima et al. 2005; O’Connell et al. 2008) rather than using lectins as markers for skeletal muscle fiber boundaries. For example O’Connell and colleagues (2008) using WGA lectin for proteomic profiling of the proteins differentially expressed in skeletal muscles of young and old rats, showed increased N-glycosylation of the pyruvate kinase isoform M1 in aging muscle. Kirkeby and colleagues (1997) investigated preferential accumulation of complex-type oligosaccharides recognized by Datura stramonium agglutinin in type IIa muscle fibers. In another study, comparison of skeletal muscles from old and young rats showed preferential cytoplasmic binding of seven lectins to the angulated aging fibers (Kirkeby 1994).
The present study evaluated the use of lectin staining as a marker for routine visualization of skeletal muscle fiber outlines. In our previous studies we had used laminin immunostaining for this purpose (Kostrominova et al. 2005; Kostrominova et al. 2007). Although it always worked well, anti-laminin antibodies are expensive and immunostaining is a time consuming procedure. In preliminary experiments I evaluated several previously described lectins for visualization of skeletal muscle fiber outlines: ConA, WGA and Ricinus communis agglutinin (RCA; Kostrominova, unpublished data). Even though all three lectins worked well in muscle sections, WGA staining produced better results in all applications in my experiments.
Although fluorescent WGA lectin was suitable in almost all muscle sections tested, in severely damaged muscles laminin staining produced better results. In other studies, lectin staining was evaluated in bupivicaine-induced necrosis and regeneration of the rat skeletal muscle (Helliwell 1988). Compared to the desmin staining that was lost during early stages of necrosis, lectin binding to the periphery of the muscle fibers was preserved (Helliwell 1988). Gulati and Zalewski (1985) also showed that out of four lectins studied only WGA had intense binding in the myogenic zone of regenerating muscle after auto-transplantation.
In addition to successfully using WGA staining on muscle sections we extended the application of this lectin to the visualization of connective tissue in bone/tendon/ligament and it worked extremely well. The data presented in this manuscript illustrate how fluorescent WGA lectin can be routinely used as a co-staining marker for visualization of connective tissue in bone/tendon/ligament research in immunohistochemical experiments.
In conclusion, this paper further characterized a fast, reliable and inexpensive method of fluorescent WGA lectin staining that can be used for skeletal muscle fiber and general connective tissue visualization during immunofluorescent analysis. This method is a much less expensive and time consuming alternative for the currently widely used anti-laminin and anti-collagen type 1 antibody immunostaining. The examples of WGA lectin co-staining, in combination with a variety of staining techniques should encourage other investigators to use this method more often in their research.
Acknowledgments
This study was supported by a subcontract to TK on NIH grant R41AR055604 (Patrick McDonough, PI).
The author is grateful to Drs. Stephen Echtenkamp and Patric Bankston for helpful suggestions during preparation of the manuscript.
LITERATURE CITED
- Cambi A, Figdor C. Necrosis: C-type lectins sense cell death. Curr Biol. 2009;19:R375–R378. doi: 10.1016/j.cub.2009.03.032. [DOI] [PubMed] [Google Scholar]
- Capaldi MJ, Dunn MJ, Sewry CA, Dubowitz V. Lectin binding in human skeletal muscle: a comparison of 15 different lectins. Histochem J. 1985;17:81–92. doi: 10.1007/BF01003405. [DOI] [PubMed] [Google Scholar]
- De Hoff PL, Brill LM, Hirsch AM. Plant lectins: the ties that bind in root symbiosis and plant defense. Mol Genet Genomics. 2009;282:1–15. doi: 10.1007/s00438-009-0460-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Iorio G, Cotrufo R. ConA and WGA receptors in rat skeletal muscles. Changes in density, distribution and composition following denervation. J Neurol Sci. 1985;67:105–114. doi: 10.1016/0022-510x(85)90026-7. [DOI] [PubMed] [Google Scholar]
- Earl LA, Baum LG. CD45 glycosylation controls T-cell life and death. Immunol Cell Biol. 2008;86:608–615. doi: 10.1038/icb.2008.46. [DOI] [PubMed] [Google Scholar]
- Fritz P, Reiser H, Saal JG, Hadam M, Muller J, Wegner G. Analysis of mast cells in rheumatoid arthritis and osteoarthritis by an avidin-peroxidase staining. Virchows Arch B Cell Pathol Incl Mol Pathol. 1984;47:35–45. doi: 10.1007/BF02890187. [DOI] [PubMed] [Google Scholar]
- Gulati AK, Zalewski AA. An immunofluorescent analysis of lectin binding to normal and regenerating skeletal muscle of rat. Anat Rec. 1985;212:113–117. doi: 10.1002/ar.1092120202. [DOI] [PubMed] [Google Scholar]
- Hamer PW, McGeachie JM, Davies MJ, Grounds MD. Evans Blue Dye as an in vivo marker of myofibre damage: optimising parameters for detecting initial myofibre membrane permeability. J Anat. 2002;200:69–79. doi: 10.1046/j.0021-8782.2001.00008.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helliwell TR. Lectin binding and desmin staining during bupivicaine-induced necrosis and regeneration in rat skeletal muscle. J Pathol. 1988;155:317–326. doi: 10.1002/path.1711550407. [DOI] [PubMed] [Google Scholar]
- Iannello RC, Jeffrey PL. Glycoproteins of rat skeletal muscle sarcolemma: characterization by two-dimensional gel electrophoresis and effect of denervation. Exp Neurol. 1990;108:156–161. doi: 10.1016/0014-4886(90)90023-l. [DOI] [PubMed] [Google Scholar]
- Kirkeby S. Glycosylation pattern and enzyme activities in atrophic, angulated skeletal muscle fibres from ageing rats. Virchows Arch. 1994;424:279–285. doi: 10.1007/BF00194612. [DOI] [PubMed] [Google Scholar]
- Kirkeby S, Animashaun T, Hughes RC. The complex-type oligosaccharide binding lectin Datura stramonium agglutinin detects type II A muscle fibres in the branchial biceps from man and cat. J Muscle Res Cell Motil. 1997;18:31–41. doi: 10.1023/a:1018624715326. [DOI] [PubMed] [Google Scholar]
- Kostrominova TY, Tanzer ML. Rodent myoblast interactions with laminin require cell surface glycoconjugates but not laminin glycosyl groups. J Cell Biochem. 1995;57:163–172. doi: 10.1002/jcb.240570116. [DOI] [PubMed] [Google Scholar]
- Kostrominova TY, Dow DE, Dennis RG, Miller RA, Faulkner JA. Comparison of gene expression of 2-mo denervated, 2-mo stimulated-denervated, and control rat skeletal muscles. Physiol Genomics. 2005;22:227–43. doi: 10.1152/physiolgenomics.00210.2004. [DOI] [PubMed] [Google Scholar]
- Kostrominova TY, Pasyk KA, Van Remmen H, Richardson AG, Faulkner JA. Adaptive changes in structure of skeletal muscles from adult Sod1 homozygous knockout mice. Cell Tissue Res. 2007;327:595–605. doi: 10.1007/s00441-006-0297-y. [DOI] [PubMed] [Google Scholar]
- Lyons TJ, Stoddart RW, McClure SF, McClure J. Lectin and other histochemical studies of the articular cartilage and the chondro-osseous junction of the normal human knee joint. J Mol Histol. 2007;38:13–23. doi: 10.1007/s10735-006-9071-4. [DOI] [PubMed] [Google Scholar]
- Maffulli N, Waterston SW, Ewen SW. Ruptured Achilles tendons show increased lectin stainability. Med Sci Sports Exerc. 2002;34:1057–1064. doi: 10.1097/00005768-200207000-00001. [DOI] [PubMed] [Google Scholar]
- Mozdziak PE, Fassel TA, Schultz E, Greaser ML, Cassens RG. A double fluorescence staining protocol to determine the cross-sectional area of myofibers using image analysis. Biotech Histochem. 1996;71:102–107. doi: 10.3109/10520299609117143. [DOI] [PubMed] [Google Scholar]
- Mundegar RR, Franke E, Schafer R, Zweyer M, Wernig A. Reduction of high background staining by heating unfixed mouse skeletal muscle tissue sections allows for detection of thermostable antigens with murine monoclonal antibodies. J Histochem Cytochem. 2008;56:969–975. doi: 10.1369/jhc.2008.950105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connell K, Doran P, Gannon J, Ohlendieck K. Lectin-based proteomic profiling of aged skeletal muscle: decreased pyruvate kinase isozyme M1 exhibits drastically increased levels of N-glycosylation. Eur J Cell Biol. 2008;87:793–805. doi: 10.1016/j.ejcb.2008.04.003. [DOI] [PubMed] [Google Scholar]
- Pena SD, Gordon BB, Karpati G, Carpenter S. Lectin histochemistry of human skeletal muscle. J Histochem Cytochem. 1981;29:542–546. doi: 10.1177/29.4.6166659. [DOI] [PubMed] [Google Scholar]
- Pilobello KT, Mahal LK. Lectin microarrays for glycoprotein analysis. Methods Mol Biol. 2007;385:193–203. doi: 10.1007/978-1-59745-426-1_14. [DOI] [PubMed] [Google Scholar]
- Sajko S, Kubinova L, Cvetko E, Kreft M, Wernig A, Erzen I. Frequency of M-cadherin-stained satellite cells declines in human muscles during aging. J Histochem Cytochem. 2004;52:179–185. doi: 10.1177/002215540405200205. [DOI] [PubMed] [Google Scholar]
- Saldova R, Wormald MR, Dwek RA, Rudd PM. Glycosylation changes on serum glycoproteins in ovarian cancer may contribute to disease pathogenesis. Dis Markers. 2008;25:219–232. doi: 10.1155/2008/601583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schunke M, Schumacher U, Tillmann B. Lectin-binding in normal and fibrillated articular cartilage of human patellae. Virchows Arch A Pathol Anat Histopathol. 1985;407:221–231. doi: 10.1007/BF00737079. [DOI] [PubMed] [Google Scholar]
- Söderström KO. Lectin binding to collagen strands in histologic tissue sections. Histochemistry. 1987;87:557–60. doi: 10.1007/BF00492470. [DOI] [PubMed] [Google Scholar]
- Tajima Y, Uyama E, Go S, Sato C, Tao N, Kotani M, Hino H, Suzuki A, Sanai Y, Kitajima K, Sakuraba H. Distal myopathy with rimmed vacuoles: impaired O-glycan formation in muscular glycoproteins. Am J Pathol. 2005;166:1121–1130. doi: 10.1016/S0002-9440(10)62332-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thoss K, Roth J. The use of fluorescein isothiocyanate labelled lectins for immuno-histological demonstration of saccharides. III. Studies by use of Ricinus communis lectin and wheat germ agglutinin. Exp Pathol (Jena) 1977;14:215–219. doi: 10.1016/s0014-4908(77)80069-0. [DOI] [PubMed] [Google Scholar]
- Weng L, Hubner R, Claessens A, Smits P, Wauters J, Tylzanowski P, Van ME, Merregaert J. Isolation and characterization of chondrolectin (Chodl), a novel C-type lectin predominantly expressed in muscle cells. Gene. 2003;308:21–29. doi: 10.1016/s0378-1119(03)00425-6. [DOI] [PubMed] [Google Scholar]
- Zhao J, Patwa TH, Lubman DM, Simeone DM. Protein biomarkers in cancer: natural glycoprotein microarray approaches. Curr Opin Mol Ther. 2008;10:602–610. [PMC free article] [PubMed] [Google Scholar]