Abstract
In contrast to fast-twitch skeletal muscle fibers of the chicken, slow-twitch fibers are fatigue-resistant. In fast fibers, the fatigue process has been related to KATP channels. In the present study, we investigated the action of glibenclamide (an anti-diabetic sulphonylurea that acts on KATP channels) on fatigued slow skeletal muscle, studying twitch and tetanus tension after inducing the muscle to fatigue by continuous electrical stimulation. Our results showed that glibenclamide (150 μM) increased post-fatigue twitch tension by about 25% with respect to the fatigued condition (P < 0.05). In addition, glibenclamide (150 μM) increased post-fatigue tetanic tension (83.61 ± 15.7% in peak tension, and 85.0 ± 19.0% in tension-time integral, P = 0.02, and 0.04, respectively; n = 3). Moreover, after exposing the muscle to a condition that inhibits mitochondrial ATP formation in order to activate KATP channels with cyanide (10 mM), tension also diminished, but in the presence of glibenclamide the effect produced by cyanide was abolished. To determine a possible increase in intracellular calcium concentration, the effects of glibenclamide on caffeine-evoked contractures were explored. After muscle pre-incubation with glibenclamide (150 μM), tension of caffeine-evoked contractures increased (6.5 ± 1.5% in maximal tension, and 5.9 ± 3.8% in tension-time integral, P < 0.05). These results suggest a possible role of KATP channels in the fatigue process, since glibenclamide increases twitch and tetanus tension in fatigued slow muscle of the chicken and during metabolic inhibition, possibly by increasing intracellular calcium.
Keywords: Glibenclamide, Fatigue, Skeletal muscle
Introduction
Slow-twitch skeletal muscle, in contrast to fast-twitch muscle is more resistant to fatigue. During muscle contraction, ATP is necessary and muscle demand increases when it is continuously stimulated; so its availability is reduced through fatiguing exercise. There are ionic channels capable of sensing the demands of ATP as ATP-dependent potassium channels (KATP) (Noma 1983; Inagaki and Seino 1998). These KATP channels are inhibited when intracellular ATP concentration increases to mM levels.
During fatigue, intracellular ATP decreases leading to a reduction in ATP hydrolysis in order to protect the cells, thus KATP channels are sensors of ATP and ADP intracellular rate (Inagaki and Seino 1998). The activation of KATP channels succeeds when the cells are compromised in ATP-demanding functions (Yokoshiki et al. 1997) such as fatigue or metabolic inhibition. These channels couple the metabolic state of the cell to its electrical activity and they have been reported in cardiac tissue (Noma 1983) and pancreatic tissue functionality linked to insulin secretion (Ashcroft et al. 1984; Cook and Hales 1984; Rorsman and Trube 1985), in amphibian skeletal muscle (Spruce et al. 1985, 1987), in mammalian skeletal muscle (Burton et al. 1988), in avian fast skeletal muscle (Thomas and Hume 1993; Fosset et al. 1995), in smooth muscle (Davies et al. 1991), and in the central nervous system (Ashford et al. 1988).
Some authors have explored the role of KATP channels in skeletal muscle using agonists of these channels, suggesting that their activation is involved in the diminution of force when muscle is fatigued (Weselcouch et al. 1993; Light and French 1994; Wickenden et al. 1996; Matar et al. 2000, 2001). However, other authors used KATP channel antagonists, such as glibenclamide (a broadly used antidiabetic drug), but did not find effects on fatigue in their results (Light et al. 1994; Van Lunteren et al. 1998; Matar et al. 2000; Gong et al. 2003). Others reported acceleration of fatigue (Comtois et al. 1994), or an increase in tetanic force and in intracellular Ca2+ levels (Duty and Allen 1995).
Thus, in the present paper, the effects of glibenclamide on twitch and tetanus tension of fatigued slow skeletal muscle fibers of the chicken, as well as its action during metabolic inhibition (low ATP), were studied.
Methods
Ethical approval
Chickens were used in accordance with the Institute for Laboratory Animal Research (ILAR) (1996) Guide for the Care and Use of Laboratory Animals and the Ethics Committee of the Centro Universitario de Investigaciones Biomedicas of the Universidad de Colima approved the protocol. To minimize animal pain and distress, as well as for muscle extraction, chickens were previously anesthetized with chloroform, followed by cervical dislocation and decapitation, ensuring a fast and complete separation of the head from the body [American Veterinary Medical Association AVMA (2007) Guidelines on Euthanasia].
Dissection
The anterior latissimus dorsi (ALD) muscle was chosen for this investigation as it is exclusively made up of slow muscle fibers. Muscles were carefully dissected out, together with a piece of the humerus bone and a portion of the vertebral cord from 1 to 2-week-old chickens (arbor acres). The vertebral cord was pinned at the bottom of the experimental chamber and 3.0 surgical silk threads were tied around the humerus bone to attach muscle bundle (1–2 mm thick) distal end to a force transducer (Grass FT03, West Warwick, RI, USA) by means of a lightweight wire. To record force, the transducer was wired to an amplifier (Cyberamp 320, Axon Instruments, Foster City, CA, USA) and connected to an analog-to-digital converter (DMA TL-1, Axon Instruments) with a 5 Hz sampling rate. Data were acquired using the subroutine Clampex of pClamp 8.0 (Axon Instruments) in a desk computer.
Fatigue protocols
To establish a fatigue protocol in the slow latissimus dorsi muscle, different electrical stimulation frequencies were used to produce twitches or tetanus. Twitches were evoked by repetitive supramaximal 300 ms square-wave pulses (Grass S-88 stimulator and SIU-5 stimuli isolation unit) at 0.2 Hz until bundle contractions were approximately 30–40% of the control contraction (about 60 min). Tetanic contractions were evoked every 10 s by trains of 10 ms pulses delivered at 5 (low frequency fatigue) or 50 (high frequency fatigue) Hz for 5 s until force fell to around 30% of initial tension (about 10 min). Pulses were delivered across platinum wire electrodes situated at either side of the muscle. Each protocol consisted of a period with initial stimulation to induce fatigue (about 30% of initial tension), after which glibenclamide was applied to the bath for 5 min, maintaining the stimulation. It was followed by washout with Ginsborg normal saline. After drug washout, electrical stimulation continued to record tension for 15 min for post-fatigue analysis.
Caffeine contractures
Caffeine-evoked-tension has been reported to be due to calcium release from the sarcoplasmic reticulum (SR) (Caputo 1966; Klein et al. 1990; Huerta and Stefani 1981; Muñiz et al. 1992), but in slow fibers, Huerta and Stefani (1981) and Shabala et al. (2008) suggest that caffeine also opens the L-type Ca2+ channels from the sarcolemma, assuring Ca2+ entry from the external media. Thus, contractures in the ALD muscle were induced by applying 8 mM caffeine. The time course of caffeine contractures was 5 min. Protocol consisted of 5 min exposure to caffeine contracture followed by a resting period of 30 min including 5 min of glibenclamide exposure. After that, 5 min caffeine (6 mM) contracture was recorded in the presence of glibenclamide. After 30 min washout, a final caffeine contracture was obtained.
Solutions
Muscles were immersed in normal Ginsborg saline (Ginsborg 1960; Page 1969) composed of (in mM): 167 NaCl, 5 KCl, 2 MgCl2, 5 CaCl2, 2 imidazole-chloride, and pH was adjusted to 7.4. Normal Ginsborg was supplemented with 2 g/l glucose, but mannitol instead of glucose was used in the experimental fatigue to prevent blocking of KATP channels by glucose (Tsuura et al. 1993). Solutions containing glibenclamide, caffeine, cyanide or verapamil (Sigma Co., St. Louis, MO, USA) were obtained by adding the proper volume of stock solutions (glibenclamide 2 mM in NaOH 0.05 M, NaCN 100 mM in water, caffeine 100 mM in water and verapamil 100 mM) to the mannitol Ginsborg solution. Solutions entered via a three-way tap located at one end of the central channel of the experimental chamber.
Statistical methods
Tension was measured as the maximum tension from the basal line to the peak before, during, and after addition of the experimental drug (peak tension) and tension-time integral was obtained from the area under the twitch profile. Each experimental condition was then compared. Data analysis were carried out using the pClamp 8.0 (Axon Instruments, Foster City, CA, USA) and subroutine and graphs were elaborated using Sigmaplot 8.0 software. Results were expressed as means ± S.E.M. followed by the n value. Mean comparison was applied by Student t test, accepting a significant effect when P was <0.05.
Results
Glibenclamide increases twitch tension of fatigued slow skeletal muscle fibers
There are some reports indicating the presence of KATP channels in the membranes of mammalian and avian fast skeletal muscle fibers and it has been suggested that these kinds of channels may have a functional role in muscular fatigue. However, the physiological role of these K+ channels during the fatigue process and metabolic inhibition is not yet fully established (Davies et al. 1991; Nichols and Lederer 1991; Fosset et al. 1995; Minami et al. 2004; Cifelli et al. 2007).
To establish a fatigue model, we used slow skeletal muscle fibers of the chicken and found that this kind of muscle fiber became fatigued after 60 min of stimulation with a low frequency (0.2 Hz). This represents a 60% reduction in the control. Once the fatigue model was established, we studied the effect of glibenclamide (a KATP channel blocker, Van Lunteren et al. 1998), during low frequency fatigue. Glibenclamide blocks KATP channels at a concentration of 100 μM (Light and French 1994). Figure 1a shows a representative trace of an experiment in which the effect of glibenclamide (150 μM) on slow skeletal muscle fibers of the chicken was examined. The first twitch shown corresponds to the start of the experiment (control). The second one shows the single twitch once the fibers were fatigued according to the fatigue model. The third twitch corresponds to the recording after the addition of glibenclamide (150 μM), in which an increase in tension when compared with the twitch in the fatigued state can be observed. This increase is around 25% higher than the tension in fatigued condition (P < 0.05). Finally, the fourth twitch corresponds to the twitch once the glibenclamide was washed out from the bath.
Fig. 1.
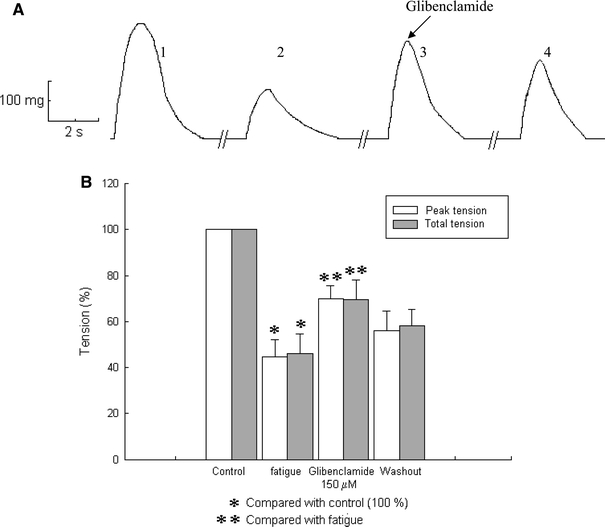
Effects of 150 μM glibenclamide on twitch at 60% of fatigue. a In representative trace 1, the recorded tension at the start of the experiment. In trace 2, the recorded tension approximately 1 h after stimulating at a frequency of 0.2 Hz. In trace 3, 5 min in the presence of glibenclamide increased the tension in the fatigued muscle. Trace 4 shows the recorded tension 10 min after glibenclamide washout. b Graphs showing the effects of glibenclamide on single twitch tension. Glibenclamide produced a significant increase in both total and maximal tension of twitches in fatigued muscle fibers (n = 4). Asterisks indicate P < 0.05
In Fig. 1b, the results obtained using glibenclamide on fatigued muscle fibers are shown in the graph. Mean tension recorded during fatigue was approximately 45% (44.54 ± 7.66% in averaged peak tension and 45.94 ± 8.79% in tension-time integral), with respect to the control (P < 0.05). When glibenclamide was added to the bath, averaged peak tension increased to 69.86 ± 6.09%, and tension-time integral increased to 69.53 ± 8.20%. Both were statistically different with respect to fatigued tension (P < 0.05). After glibenclamide washout, averaged peak tension was 56.13 ± 9.68% and tension-time integral was 57.42 ± 8.17% (n = 4). These results show that glibenclamide, a KATP channels blocker, increases tension of low frequency fatigued muscle fibers.
Glibenclamide increases tension of tetanus in fatigued muscle fibers
Another way to induce fatigue in these muscles is by producing tetanic contractions. We used low (5 Hz) and high frequencies (50 Hz) to generate tetanus. Using these protocols tension was diminished 70–80% after 10 min of stimulation, depending on stimulation frequency. Similar results have been reported for fast skeletal muscle fibers showing a diminution of 70%, although this diminution was reached in less time (Duty and Allen 1995).
In Fig. 2a representative traces show the effect of glibenclamide (150 μM) on tetanic tension (5 Hz) of fatigued muscle. In representative trace 1, tetanus recorded at the beginning of the experiment is shown. In trace 2, tetanus recorded after 10 min of stimulation is shown. In trace 3, tetanic tension recorded in the presence of glibenclamide is shown. Under this condition tetanic tension is increased, reversing the fatigue. Trace 4 shows tetanic tension recorded after glibenclamide washout, in which the muscle was further fatigued.
Fig. 2.
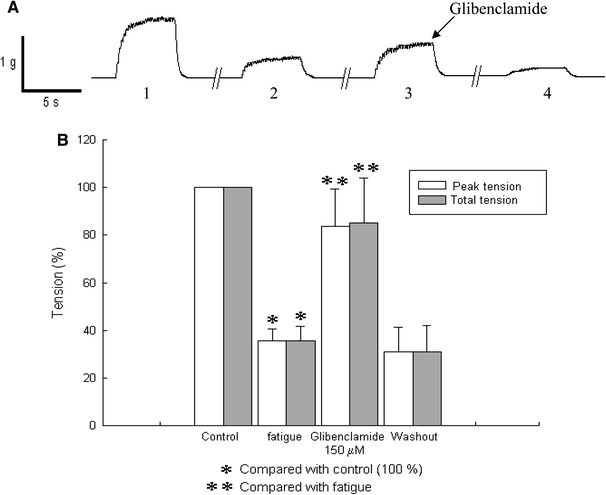
Effects of glibenclamide on tetanic force. a Representative traces showing the effect of glibenclamide (150 μM) on tetanic tension of fatigued muscle fibers. The stimulation frequency used was 5 Hz. 1 Tetanic tension at the start of the experiment (control). 2 Tetanic tension after 10 min of stimulation, muscle fibers were fatigued at this point. 3 When glibenclamide was added to the bath the tetanic tension of the fatigued muscle increased. 4 Tetanic tension after 5 min of glibenclamide washout in which tetanic tension was further decreased is shown. b Graphs showing the effect of glibenclamide on peak tension and tension-time integral of fatigued muscle fibers. Asterisks indicate P < 0.05
Graphs illustrating the effect of glibenclamide (150 μM) on tetanic tension in fatigued muscle fibers are shown in Fig. 2b. Averaged tension was reduced during fatigue by almost 70% (35.64 ± 4.79% in peak tension and 35.81 ± 5.99% in tension-time integral) with respect to the control (P = 0.00001, P = 0.00004, respectively; n = 3). In the presence of glibenclamide tetanic tension increased to 83.61 ± 15.71% in peak tension and to 85.06 ± 19.00% in tension-time integral (P = 0.0267, P = 0.0484 respectively; n = 3). These data correspond to traces 2 and 3 from Fig. 2a. After glibenclamide washout the fatigue process started diminishing tetanic tension again (Figs. 2a, 4).
Fig. 4.
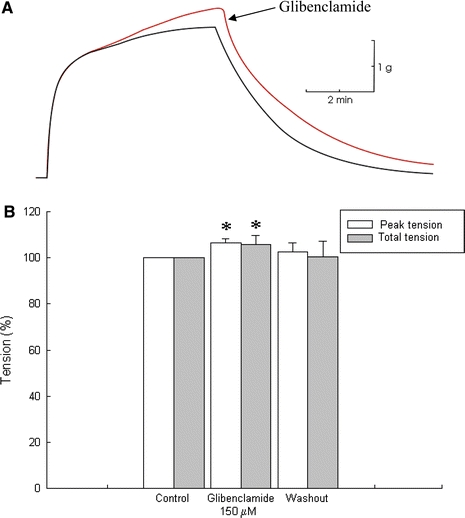
Effects of glibenclamide on caffeine contractures. a Representative traces obtained by inducing contractures with caffeine 8 mM before and after the addition of glibenclamide. Inferior trace corresponds to control caffeine contracture and superior trace corresponds to caffeine contracture in the presence of glibenclamide (150 μM). b Graphs showing the effect of glibenclamide (150 μM) on caffeine contractures. Presence of glibenclamide increases caffeine-induced tension with respect to the control (P < 0.05; n = 3). Asterisks indicate P < 0.05
When a fatigue protocol with high stimulation frequencies was used, we observed a similar effect with glibenclamide (150 μM) after fatigue. The graphs for this effect are shown in Fig. 3. Some groups have used frequencies as high as 100 Hz (e.g., Duty and Allen 1995); but when we used 100 Hz or even 70 Hz the muscle suffered irreversible damage and therefore we chose to use 50 Hz. Under these conditions, tetanic tension during fatigue was reduced to 12.68 ± 4.53% in peak tension and to 10.41 ± 4.98% in tension-time integral with respect to the control. These reductions were statistically significant, having values of P = 0.000001, P = 0.000002, respectively, for peak tension and tension-time integral (n = 3). When glibenclamide (150 μM) was added, peak tension increased to 30.58 ± 5.00% with respect to the fatigued state, and tension-time integral was 28.96 ± 5.64% (n = 3). Student t test showed significance in these results indicating a statistical difference in both parameters (P = 0.03 in peak tension and P = 0.04 in tension-time integral of the tetanus). Thus, our results show that fatigue induced with high frequencies was also reverted in the presence of glibenclamide.
Fig. 3.
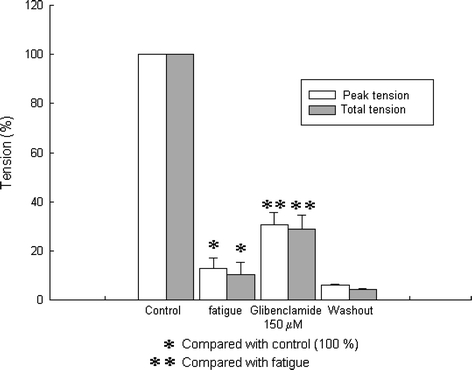
Effects of glibenclamide on tetanic force generated with 50 Hz of stimulation pulses. Glibenclamide increased tetanic tension of fatigued muscle fibers. Asterisks indicate P < 0.05
Effects of glibenclamide on caffeine contractures in slow skeletal muscle fibers
To examine whether or not the effects of glibenclamide were mediated by an increased Ca2+ release from SR, the effect of this drug on contractures induced by caffeine, an alkaloid that mainly induces Ca2+ release from the SR (Caputo 1966; Huerta and Stefani 1981; Muñiz et al. 1992; Shabala et al. 2008) was studied. Moreover, in slow muscle fibers caffeine can open L-type Ca2+ channels in the sarcolemma (Huerta and Stefani 1981; Shabala et al. 2008). In caffeine-induced contractures (8 mM), the addition of glibenclamide (150 μM) caused an increase in amplitude of the contracture with respect to the control. Figure 4a shows a representative trace obtained in this set of experiments. The increase in caffeine contracture observed in the presence of glibenclamide was 6.55 ± 1.57% in maximal tension and 5.93 ± 3.86% in tension-time integral (P < 0.05; n = 3). These effects were reversible after glibenclamide washout, since contracture returned to the initial tension. In Fig. 4b results obtained in these experiments are shown in the graph. They suggest that, at least partly, glibenclamide increased the tension of these slow skeletal muscle fibers by opening Ca2+ channels in the sarcolemma, since the plateau depends on external Ca2+ (Huerta and Stefani 1981).
In order to discard the participation of sarcolemmal L-type Ca2+ channels in the effect observed with caffeine plus glibenclamide, caffeine contractures were induced in the presence of glibenclamide (150 μM) after blocking these channels with verapamil (10 μM). In these experiments, after blocking the L-type Ca2+ channels, a significant increase in both peak tension and tension-time integral (data not shown) was still observed, supporting our hypothesis that the increase in tension caused by glibenclamide is due to an increase in Ca2+ release from the SR.
Glibenclamide blocks the effect of metabolic inhibition on muscle tension
The model for metabolic inhibition induced by cyanide was used according to Gramolini and Renaud (1997) to avoid ATP production and thus to decrease intracellular levels of ATP. Under these conditions the effect of glibenclamide on twitches generated by 0.2 Hz pulses were explored.
Results obtained in this experimental series are shown in Fig. 5. Incubation with cyanide (10 mM) for 5 min produced a rapid diminution in twitch tension as shown in Fig. 5a (second twitch) with respect to the control. This diminution in twitch tension was statistically significant (P < 0.05; Fig. 5b, second bars). After cyanide washout, twitch tension recovered its initial levels. Then, cyanide plus glibenclamide 150 μM was added to the bath for 5 min. In Fig. 5a the third twitch shows tension recorded after 5 min in the presence of cyanide plus glibenclamide and tension similar to that recorded at the start of the experiment (first twitch) can be observed. Thus, the presence of glibenclamide blocked the cyanide-produced effect on force, by avoiding the diminution in tension caused by cyanide alone. The fourth twitch corresponds to the final washout, showing that twitch tension was maintained at values similar to the control condition.
Fig. 5.
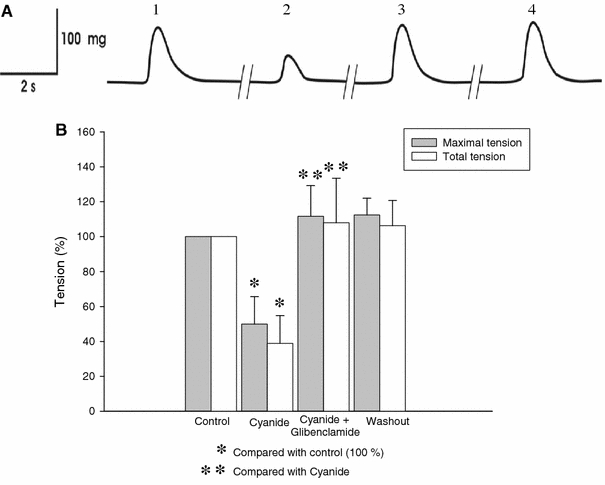
Effects of glibenclamide on twitch tension in skeletal muscle fibers during metabolic inhibition by cyanide. a In trace 1 a twitch at the start of the experiment (control) is shown. Trace 2 shows the twitch after 5 min in the presence of cyanide 10 mM (metabolic inhibition). This effect was totally reversible after cyanide washout. In trace 3, the presence of glibenclamide in the bath avoided the diminution in tension produced by cyanide. In trace 4, the twitch tension at the end of the experiment is similar to that recorded at the start. b Graphs for the effect of glibenclamide on twitch in metabolic inhibition. Twitch tension notably diminished in the presence of cyanide with respect to the control (P < 0.05). Presence of glibenclamide avoided the effect of metabolic inhibition with cyanide. Asterisks indicate P < 0.05
Discussion
KATP channels are involved in the coupling of the metabolic state of the cell to its electric activity. In skeletal muscle, it has been shown that KATP channels have a role in force reduction during some metabolic stress states such as anoxia, ischemia, fatigue, and during metabolic inhibition to preserve ATP and thus prevent muscular damage (Weselcouch et al. 1993; Wickenden et al. 1996; Matar et al. 2000). There are some reports indicating the presence of KATP channels in the membranes of mammalian and avian fast skeletal muscle fibers, and it has been suggested that this kind of channels may have a functional role in muscular fatigue. However, the physiological role of these K+ channels during the fatigue process and metabolic inhibition is not yet fully established (Davies et al. 1991; Nichols and Lederer 1991; Fosset et al. 1995; Minami et al. 2004, Cifelli et al. 2007). In this paper the effect of glibenclamide (a KATP channel blocker, Van Lunteren et al. 1998) during low frequency fatigue was studied.
The aim of this work was to investigate the effect of glibenclamide on fatigued slow skeletal muscle of the chicken. Previously glibenclamide has been found to have a higher effect on slow-twitch muscle fiber tension than in fast-twitch muscle fibers of the chicken (Sánchez-Pastor et al. 2002). Therefore, a fatigue model for skeletal muscle fibers of the chicken was designed using simple twitch and tetanus to induce fatigue in ALD muscle. ALD muscle is a slow-twitch muscle and fatigue was induced by stimulation pulses at a frequency of 0.2 Hz, reaching a 60–70% fatigue in approximately 60 min of stimulation, and by a stimulation frequency of 5 and 50 Hz, reaching 70–80% fatigue in 10 min. When higher stimulation frequencies (70, 100 and 140) were applied, as reported in other works using different experimental models (Westerblad and Allen 1991; Light and French 1994; Duty and Allen 1995; Matar et al. 2000), muscle fibers suffered irreversible damage. At the age of the animals used, ALD muscle has small physical dimensions, facilitating oxygen diffusion. Muscle and state of contraction was monitored at the beginning and at the end of the fatigue experiments through high-potassium contracture, so it could be analyzed before and after washout. Only those muscles that recovered from fatigue and from the effects of the drugs used were taken into account for analysis.
The participation of KATP channels in the fatigue process has been studied using specific blockers but the role of these kinds of channels is still controversial (Comtois et al. 1994; Matar et al. 2000; Cifelli et al. 2007). The present work shows that glibenclamide improves muscle tension (twitch tension and tetanic tension) in the fatigued state, which coincides with previous work on mammalian fast skeletal muscle (Duty and Allen 1995). However, in the present experiments, when fatigue was induced with tetanic contractions (at high frequencies), further diminution in tension shortly after glibenclamide washout was observed. This could be from fiber damage due to the fact that KATP channels remained closed by glibenclamide, thus avoiding the cellular protection in which KATP channels have been involved. This situation was not observed with fatigue at low frequencies.
Moreover, it has been shown that after a fatiguing period muscle contractile capabilities are diminished (Fitts and Metzger 1988). This effect has been associated with a diminution in intracellular Ca2+ concentration (Allen et al. 2001) affecting the excitation–contraction coupling. Thus, the effects of glibenclamide could be due to an involvement of this drug in one of the steps involved in excitation contraction coupling and in the modulation of intracellular Ca2+ during fatigue in slow skeletal muscle fibers.
The effect of glibenclamide is the improvement of tension in the fatigue state, which is attributed to KATP channel blockade, together with an indirect effect on Ca2+ release from the SR and/or a greater Ca2+ entry (Duty and Allen 1995; Jones 1996). KATP channel blockade by glibenclamide would reduce K+ efflux from the muscle fiber leading to elongation of the action potential (Castle and Haylett 1987; Light and French 1994), which in turn would increase intracellular Ca2+ release (Duty and Allen 1995). Another possibility is that myofilament Ca2+ sensitivity is increased (Duty and Allen 1995). However, more experiments are needed to explain this mechanism.
The present work also showed that glibenclamide increases tension in caffeine contractures even when sarcolemmal Ca2+ channels were blocked, suggesting a greater Ca2+ release from the SR (Fig. 6).
Fig. 6.
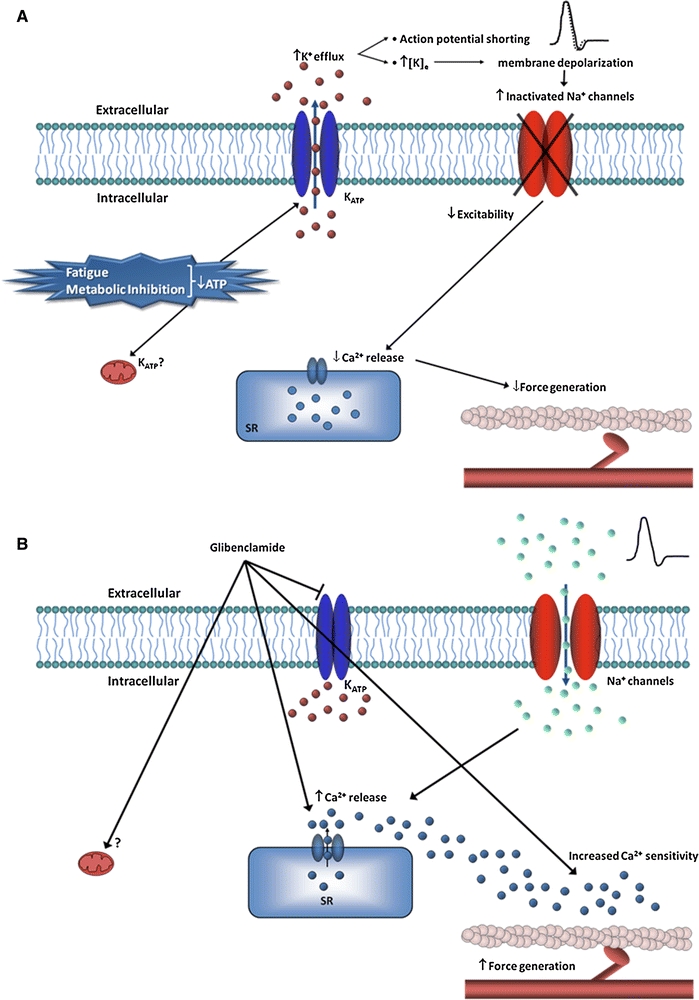
Possible role of KATP channels in skeletal muscle. a During fatigue or metabolic inhibition a reduction in ATP is produced leading to the opening of KATP channels. This causes an increase in K+ efflux in turn causing a shortening in the action potential. This excess in K+ efflux also increases the external K+ concentration, depolarizing the membrane. Both of these processes cause Na+ channel inactivation, reducing excitability. The reduction in excitability is reflected in a smaller Ca2+ release from the sarcoplasmic reticulum, causing less force generation. b In the presence of glibenclamide, there is a blocking of KATP channels at the cell membrane and possibly at the mitochondria. This blocking at the cell surface avoids the shortening of the action potential and enough Na+ channels can be activated causing normal Ca2+ release from the sarcoplasmic reticulum. These Ca2+ ions reach their binding sites at Troponin C, allowing for the formation of cross bridges generating muscle contraction. Moreover, glibenclamide may increase Ca2+ release from the sarcoplasmic reticulum and increase Ca2+ sensitivity of Troponin C, increasing force generation
On the other hand, it is known that ATP production is also diminished during metabolic inhibition (Adler et al. 1999); this diminution would be sensed by KATP channels at the sarcolemma, which in turn would be activated causing a reduction in membrane excitability. To obtain further evidence of KATP channel presence in this muscle and their possible role during metabolic inhibition, the muscles were stimulated repeatedly in the presence of cyanide. Tension recorded under these conditions was diminished. The effect of glibenclamide was then tested by incubating the muscles in cyanide plus glibenclamide. Glibenclamide was found to avoid the diminution in tension caused by cyanide suggesting that KATP channel activation is involved in the action mechanism causing muscle tension reduction during metabolic inhibition. Zhang et al. (2006) reported a marked decrease in tetanic intracellular Ca2+i during fatigue of single soleus fibers exposed to cyanide, and suggested that the opening of KATP was involved in these effects.
Additionally, we cannot rule out the possibility that, as in other tissues such as those of the heart and liver (Inoue et al. 1991; Paucek et al. 1992), mitochondrial KATP channels are present in these skeletal muscle fibers and that the effects of glibenclamide on the fatigued muscle fibers were due to an additional action of this drug on mitochondrial KATP channels (Paucek et al. 1992; García et al. 2009). However, further experiments using a selective inhibitor for these mitochondrial channels such as 5-Hydroxydecanoate are necessary.
The use of skeletal muscle fibers of the chicken provides a useful model for the study of fatigue processes, since ALD muscle is conformed exclusively of slow muscle fibers. Moreover, this type of muscle fiber shares mechanical and electrophysiological properties with mammalian and amphibian slow muscle fibers (Trujillo et al. 2002).
Thus, indirect evidence of possible KATP channel involvement or the stopping of excitation–contraction coupling in the reduction of tension in slow skeletal muscle fibers of the chicken produced by fatigue or metabolic inhibition processes is presented in this paper.
Acknowledgments
This work was partially supported, by a grant from the Ramón Álvarez-Buylla de Aldana Fund (FRABA, to MH and XT). FA and ESP had received CONACYT fellowships. Some portions of this work formed part of the PhD Thesis of FA, at the University of Colima, Colima, Mexico. The authors wish to thank Mr. Ezequiel Viera for his technical assistance. We dedicate this paper to the memory of Dr. J.L. Marín.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
References
- Adler M, Lebeda FJ, Kauffman FC, Deshpande SS. Mechanism of action of sodium cyanide on rat diaphragm muscle. J Appl Toxicol. 1999;19:411–419. doi: 10.1002/(SICI)1099-1263(199911/12)19:6<411::AID-JAT597>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- Allen DG, Lee JA, Westerblad H (2001) Intracellular calcium and tension during fatigue in isolated single muscle fibres from Xenopus laevis. J Physiol 415:433–458 [DOI] [PMC free article] [PubMed]
- American Veterinary Medical Association AVMA (2007) Guidelines on Euthanasia Formerly Report of the AVMA Panel on Euthanasia. American Veterinary Medical Association http://www.avma.org/issues/animal_welfare/euthanasia.pdf accessed 26 Jan 2010
- Ashcroft FM, Harrison DE, Ashcroft SJ. Glucose induces closure of single potassium channels in isolated rat pancreatic beta-cells. Nature. 1984;312:446–448. doi: 10.1038/312446a0. [DOI] [PubMed] [Google Scholar]
- Ashford ML, Sturgess NC, Trout NJ, Gardner NJ, Hales CN. Adenosine 5′-triphosphate-sensitive ion channels in neonatal rat cultured central neurones. Pflugers Arch. 1988;412:297–304. doi: 10.1007/BF00582512. [DOI] [PubMed] [Google Scholar]
- Burton F, Dorstelmann U, Hutter OF. Single-channel activity in sarcolemmal vesicles from human and other mammalian muscles. Muscle Nerve. 1988;11:1029–1038. doi: 10.1002/mus.880111004. [DOI] [PubMed] [Google Scholar]
- Caputo C. Caffeine- and potassium-induced contractures of frog striated muscle fibers in hypertonic solutions. J Gen Physiol. 1966;50:129–139. doi: 10.1085/jgp.50.1.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castle NA, Haylett DG. Effect of channel blockers on potassium efflux from metabolically exhausted frog skeletal muscle. J Physiol. 1987;383:31–43. doi: 10.1113/jphysiol.1987.sp016394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cifelli C, Bourassa F, Gariépy L, Banas K, Benkhalti M, Renaud JM. KATP channel deficiency in mouse flexor digitorum brevis causes fibre damage and impairs Ca2+ release and force development during fatigue in vitro. J Physiol. 2007;582:843–857. doi: 10.1113/jphysiol.2007.130955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comtois A, Sinderby C, Comtois N, Grassino A, Renaud JM. An ATP-sensitive potassium channel blocker decreased diaphragmatic circulation in anesthetized dogs. J Appl Physiol. 1994;77:127–134. doi: 10.1152/jappl.1994.77.1.127. [DOI] [PubMed] [Google Scholar]
- Cook DL, Hales CN. Intracellular ATP directly blocks K+ channels in pancreatic β-cells. Nature. 1984;311:271–273. doi: 10.1038/311271a0. [DOI] [PubMed] [Google Scholar]
- Davies NW, Standen NB, Stanfield PR. ATP-dependent potassium channels of muscle: their properties, regulation and possible functions. J Bioenerg Biomembr. 1991;23:509–535. doi: 10.1007/BF00785809. [DOI] [PubMed] [Google Scholar]
- Duty S, Allen DG. The effects of glibenclamide on tetanic force and intracellular calcium in normal and fatigued mouse skeletal muscle. Exp Physiol. 1995;80:529–541. doi: 10.1113/expphysiol.1995.sp003865. [DOI] [PubMed] [Google Scholar]
- Fitts RH, Metzger JM (1988) Mechanisms of muscular fatigue. In: Poortmans (ed) Principles of exercise biochemistry. vol 27. S Karger AG, Medical and Scientific Publishers, pp 212–229
- Fosset M, Allard B, Lazdunski M. Coexistence of two classes of glibenclamide-inhibitable ATP-regulated K+ channels in avian skeletal muscle. Pflugers Arch. 1995;431:117–124. doi: 10.1007/BF00374384. [DOI] [PubMed] [Google Scholar]
- García MC, Hernández A, Sánchez JA. Role of mitochondrial ATP-sensitive potassium channels on fatigue in mouse muscle fibers. Biochem Biophys Res Commun. 2009;385:28–32. doi: 10.1016/j.bbrc.2009.05.019. [DOI] [PubMed] [Google Scholar]
- Ginsborg BL. Some properties of avian skeletal muscle fibres with multiple neuromuscular junctions. J Physiol. 1960;154:581–598. doi: 10.1113/jphysiol.1960.sp006597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong B, Legault D, Miki T, Seino S, Renaud JM. KATP channels depress force by reducing action potential amplitude in mouse EDL and soleus muscle. Am J Physiol Cell Physiol. 2003;285:1464–1474. doi: 10.1152/ajpcell.00278.2003. [DOI] [PubMed] [Google Scholar]
- Gramolini A, Renaud JM. Blocking ATP-sensitive K+ channel during metabolic inhibition impairs muscle contractility. Am J Physiol. 1997;272:1936–1946. doi: 10.1152/ajpcell.1997.272.6.C1936. [DOI] [PubMed] [Google Scholar]
- Huerta M, Stefani E. Potassium and caffeine contractures in fast and slow muscles of the chicken. J Physiol. 1981;318:181–189. doi: 10.1113/jphysiol.1981.sp013857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inagaki N, Seino S. ATP-sensitive potassium channels: structures, functions, and pathophysiology. Jpn J Physiol. 1998;48:397–412. doi: 10.2170/jjphysiol.48.397. [DOI] [PubMed] [Google Scholar]
- Inoue I, Nagase H, Kishi K, Higuti T. ATP-sensitive K+ channel in the mitochondrial inner membrane. Nature. 1991;352:244–247. doi: 10.1038/352244a0. [DOI] [PubMed] [Google Scholar]
- Institute for Laboratory Animal Research . Guide for the Care and Use of Laboratory Animals. Washington, DC: National Research Council. National Academic Press; 1996. [Google Scholar]
- Jones DA. High-and low-frequency fatigue revisited. Acta Physiol Scand. 1996;156:265–270. doi: 10.1046/j.1365-201X.1996.192000.x. [DOI] [PubMed] [Google Scholar]
- Klein MG, Simon BJ, Schneider MF. Effects of caffeine on calcium release from the sarcoplasmic reticulum in frog skeletal muscle fibres. J Physiol. 1990;425:599–626. doi: 10.1113/jphysiol.1990.sp018120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Light PE, French RJ. Glibenclamide selectively blocks ATP-sensitive K+ channels reconstituted from skeletal muscle. Eur J Pharmacol. 1994;259:219–222. doi: 10.1016/0014-2999(94)90647-5. [DOI] [PubMed] [Google Scholar]
- Light PE, Comtois AS, Renaud JM. The effect of glibenclamide on frog skeletal muscle: evidence for KATP channel activation during fatigue. J Physiol. 1994;475:495–507. doi: 10.1113/jphysiol.1994.sp020088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matar W, Nosek TM, Wong D, Renaud JM. Pinacidil suppresses contractility and preserves energy but glibenclamide has no effect during muscle fatigue. Am J Physiol Cell Physiol. 2000;278:C404–C416. doi: 10.1152/ajpcell.2000.278.2.C404. [DOI] [PubMed] [Google Scholar]
- Matar W, Lunde JA, Jasmin BJ, Renaud JM. Denervation enhances the physiological effects of the K(ATP) channel during fatigue in EDL and soleus muscle. Am J Physiol Regul Integr Comp Physiol. 2001;281:R56–R65. doi: 10.1152/ajpregu.2001.281.1.R56. [DOI] [PubMed] [Google Scholar]
- Minami K, Miki T, Kadowaki T, Seino S. Roles of ATP-Sensitive K+ Channels as Metabolic Sensors. Studies of Kir6.x Null Mice. Diabetes. 2004;53:S176–S180. doi: 10.2337/diabetes.53.suppl_3.S176. [DOI] [PubMed] [Google Scholar]
- Muñiz J, Huerta M, Dueñas J, Trujillo X, Elizalde A. Caffeine and theophylline contractures in tonic skeletal muscle fibres of the frog. Jpn J Physiol. 1992;42:711–720. doi: 10.2170/jjphysiol.42.711. [DOI] [PubMed] [Google Scholar]
- Nichols CG, Lederer WJ. Adenosine triphosphate-sensitive potassium channels in the cardiovascular system. Am J Physiol. 1991;261:H1675–H1686. doi: 10.1152/ajpheart.1991.261.6.H1675. [DOI] [PubMed] [Google Scholar]
- Noma A. ATP-regulated K+ channels in cardiac muscle. Nature. 1983;305:147–148. doi: 10.1038/305147a0. [DOI] [PubMed] [Google Scholar]
- Page SG. Structure and some contractile properties of fast and slow muscles of the chicken. J Physiol. 1969;205:131–145. doi: 10.1113/jphysiol.1969.sp008956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paucek P, Mironova G, Mahdi F, Beavis AD, Woldogiorgis G, Garlid KD. Reconstitution and partial purification of the glibenclamide-sensitive, ATP-dependent K+ channel from rat liver and beef heart mitochondria. J Biol Chem. 1992;267:26062–26069. [PubMed] [Google Scholar]
- Rorsman P, Trube G. Glucose dependent K+ channels in pancreatic b-cells are regulated by intracellular ATP. Pflugers Arch. 1985;405:305–309. doi: 10.1007/BF00595682. [DOI] [PubMed] [Google Scholar]
- Sánchez-Pastor E, Trujillo X, Huerta M, Andrade F (2002) Effects of glibenclamide on twitch and tetanic tension in chicken skeletal muscle fibres. Annual Meeting, Society for Neuroscience, A127463 Orlando, FL, USA
- Shabala L, Sánchez-Pastor E, Trujillo X, Shabala S, Muñiz J, Huerta M. Effects of verapamil and gadolinium on caffeine-induced contractures and calcium fluxes in frog slow skeletal muscle fibers. J Membr Biol. 2008;221:7–13. doi: 10.1007/s00232-007-9079-z. [DOI] [PubMed] [Google Scholar]
- Spruce AE, Standen NB, Stanfield PR. Voltage-dependent ATP-sensitive potassium channels of skeletal muscle membrane. Nature. 1985;316:736–738. doi: 10.1038/316736a0. [DOI] [PubMed] [Google Scholar]
- Spruce AE, Standen NB, Stanfield PR. Studies of the unitary properties of adenosine 5′-triphosphate-regulated potassium channels of frog skeletal muscle. J Physiol. 1987;382:213–236. doi: 10.1113/jphysiol.1987.sp016364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas SA, Hume R. Single potassium channel currents activated by extracellular ATP in developing chick skeletal muscle: a role for second messengers. J Neurophysiol. 1993;69:1556–1566. doi: 10.1152/jn.1993.69.5.1556. [DOI] [PubMed] [Google Scholar]
- Trujillo X, Huerta M, Vasquez C, Andrade F. Adrenaline diminishes K+ contractures and Ba2+-current in chicken slow skeletal muscle fibres. J Muscle Res Cell Motil. 2002;23:157–165. doi: 10.1023/A:1020295702288. [DOI] [PubMed] [Google Scholar]
- Tsuura Y, Ishida H, Okamoto Y, Kato S, Sakamoto K, Horie H, Ikeda H, Okada Y, Seino Y. Glucose sensitivity of ATP-sensitive K+ channels is impaired in β-cells of the GK rat. A new genetic model of NIDDM. Diabetes. 1993;42:1446–1453. doi: 10.2337/diabetes.42.10.1446. [DOI] [PubMed] [Google Scholar]
- Van Lunteren E, Moyer M, Torres A. ATP-sensitive K+ channel blocker glibenclamide and diaphragm fatigue during normoxia and hypoxia. J Appl Physiol. 1998;85:601–608. doi: 10.1152/jappl.1998.85.2.601. [DOI] [PubMed] [Google Scholar]
- Weselcouch EO, Sargent C, Wilde MW, Smith MA. ATP-sensitive potassium channels and skeletal muscle function in vitro. J Pharmacol Exp Ther. 1993;267:410–416. [PubMed] [Google Scholar]
- Westerblad H, Allen DG. Changes of myoplasmic calcium concentration during fatigue in single mouse muscle fibers. J Gen Physiol. 1991;98:615–635. doi: 10.1085/jgp.98.3.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickenden AD, Prior H, Kelly E, Russell K, Poucher SM, Kumar P. The effects of pharmacological modulation of KATP on the guinea pig isolated diaphragm. Eur J Pharmacol. 1996;302:79–88. doi: 10.1016/0014-2999(95)00872-1. [DOI] [PubMed] [Google Scholar]
- Yokoshiki H, Katsube Y, Sunugawa M, Seki T, Sperelakis N. Disruption of actin cytoskeleton attenuates sulfonylurea inhibition of cardiac ATP-sensitive K+ channels. Pflugers Arch. 1997;434:203–205. doi: 10.1007/s004240050384. [DOI] [PubMed] [Google Scholar]
- Zhang SJ, Bruton JD, Katz A, Westerblad H. Limited oxygen diffusion accelerates fatigue development in mouse skeletal muscle. J Physiol. 2006;572:551–559. doi: 10.1113/jphysiol.2005.104521. [DOI] [PMC free article] [PubMed] [Google Scholar]


