Abstract
Aims
Hypertrophic cardiomyopathy (HCM) is a frequent cause of sudden cardiac death (SCD) due to exercise-related ventricular arrhythmias (ERVA); however the pathological substrate is uncertain. The aim was to determine the prevalence of ERVA and their relation with fibrosis as determined by cardiac magnetic resonance imaging (CMR) in carriers of an HCM causing mutation.
Methods
We studied the prevalence and origin of ERVA and related these with fibrosis on CMR in a population of 31 HCM mutation carriers.
Results
ERVA occurred in seven patients (23%) who all showed evidence of fibrosis (100% ERVA(+) vs. 58% ERVA(-), p = 0.04). No ventricular tachycardia or ventricular fibrillation occurred. In patients with ERVA, the extent of fibrosis was significantly larger (8 ± 4% vs. 3 ± 4%, p = 0.02). ERVA originated from areas with a high extent of fibrosis or regions directly adjacent to these areas.
Conclusions
ERVA in HCM mutation carriers arose from the area of fibrosis detected by CMR; ERVA seems closely related to cardiac fibrosis. Fibrosis as detected by CMR should be evaluated as an additional risk factor to further delineate risk of SCD in carriers of an HCM causing mutation.
Keywords: Hypertrophic cardiomyopathy, Magnetic resonance imaging, Exercise, Fibrosis, Arrhythmias
Introduction
Hypertrophic cardiomyopathy (HCM) is a common inherited cardiac disorder which is estimated to occur in one out of 500 patients within the general population [1]. An autosomal dominant mutation is found in approximately 50–60% of cases, most frequently in genes that code for sarcomeric proteins [2]. These mutations often lead to thickening of the left ventricular (LV) wall, myocyte disarray, and cardiac fibrosis [3]. Important clinical consequences of HCM are heart failure and potentially life-threatening ventricular arrhythmias. HCM is the most common cause of sudden cardiac death (SCD) under the age of 35 years, especially in young athletes. About one sixth of the cases of SCD occur during or immediately after moderate to severe physical activity [4, 5].
Because of the phenotypic heterogeneity of HCM it is important to find predictors and mechanisms that can explain SCD, particularly in relation to exercise [6, 7]. The precise mechanism of underlying arrhythmias remains unknown and it is currently unsettled whether SCD is triggered by pro-arrhythmic changes in hypertrophied cardiac myocytes themselves or rather by changes in the myocardial structure due to myocardial fibrosis [8–11].
Since SCD in HCM patients is often related to exercise and to fibrosis, we sought to determine the prevalence of ventricular arrhythmias during exercise-ECG testing in a population of proven carriers of an HCM causing mutation and relate this to fibrosis as determined with cardiac magnetic resonance imaging (CMR).
Materials and methods
Study patients
A cohort of 31 genetically proven HCM mutation carriers (probands and family members) were seen between March 2004 and February 2008 in the Maastricht University Medical Center, the Netherlands. We included all carriers, symptomatic and asymptomatic, where both a CMR and exercise test were available for analysis.
CMR
Patients were examined with a clinical 1.5T Gyroscan Intera MRI scanner (Philips Medical Systems, Best, the Netherlands) equipped with a dedicated five-element phased array surface coil and cardiac software. ECG-gated multi-slice multi-phase images were acquired for functional analysis during multiple breath holds using a steady-state free precession sequence (slice thickness 6 mm, slice gap 4 mm, repetition time/echo time 3.8/1.9 ms, flip angle 50°, field of view 350 mm, matrix 256 × 256, 22–25 phases per cardiac cycle) in vertical long-axis (VLA), horizontal long-axis (HLA), LV outflow tract (LVOT) view and contiguous short-axis (SAX) slices covering the entire left ventricle.
Ten minutes after 0.2 mmol/kg intravenous contrast administration (Gadolinium-DTPA, Magnevist, Schering, Berlin, Germany) a Look-Locker (LL) sequence was applied in SAX orientation to determine the inversion time to optimally ‘null’ LV myocardium for the subsequent scan. Immediately after the LL scan, late gadolinium enhancement (LGE) multislice images were obtained using a 3D inversion-recovery gradient-echo sequence, covering the entire LV in the HLA, VLA and SAX orientation.
Functional analysis and wall thickness
CMR images were analysed with commercially available software (CAAS MRV 3.0, Pie Medical Imaging BV, Maastricht, the Netherlands). Endocardial and epicardial contours were manually traced in end-diastolic and end-systolic phases on SAX CINE images to determine end-diastolic volume and end-systolic volume, ejection fraction and LV end-diastolic (LVED) mass. Where appropriate LVED was indexed to body surface area. The maximal end-diastolic LV wall thickness (LVEDWT(max)) was taken as the greatest dimension of the LV wall at any site.
Structural abnormalities (Fibrosis)
On the LGE images, the presence and localisation of fibrosis (i.e. hyperenhancement) was assessed by two independent observers who were blinded to the clinical and exercise ECG results. Any discrepancy in analysis between the two readers was then adjudicated by a third observer. The presence of fibrosis was accepted when it was reproducibly observed in all orientations. These areas of fibrosis were subsequently quantified in SAX orientation using a signal intensity (SI) cut-off of at least 5 SD above the SI of remote and adequately ‘nulled’ myocardium [12]. The extent of fibrosis was expressed as percent of LV mass and on a segmental basis according to the American Heart Association (AHA) 17 segment model [12, 13]. Because measurements of the apex were often unreliable, this segment (segment 17) was excluded from further analysis. In addition, the most basal slice having circumferential myocardial muscle at both the end-diastolic and end-systolic phase was selected as the base of the left ventricle to prevent including the LVOT in the measurements. Based on the mean extent of fibrosis per patient, each segment was classified as either having a high (more than or equal to) or low (less than the mean) extent of fibrosis.
Exercise test
All patients underwent a symptom-limited treadmill exercise test according to the Bruce protocol [14]. Standard blood pressure and 12-lead ECG monitoring were performed. Medical therapy at the time of the exercise test was registered. Exercise related ventricular arrhythmias (ERVA) were defined as ventricular premature beats (VPBs), ventricular tachycardia (VT ≥3 beats and frequency ≥100 beats/min) and ventricular fibrillation (VF), occurring during exercise and the post-exercise recovery period. Regarding these VPBs, the number, morphology, bigeminy and time of occurrence during the exercise test were analysed.
Origin of ERVA
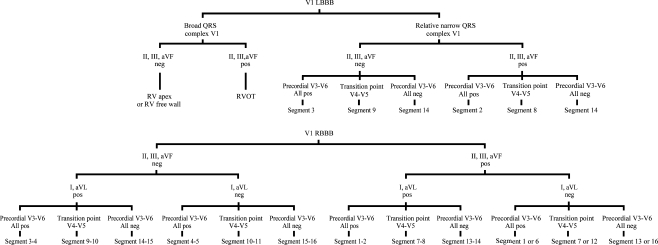
The origin of ERVA was determined according to the diagram in Fig. 1 based on the AHA 17 segment model by an experienced cardiac electrophysiologist blinded to the clinical and CMR data [13]. Patients were categorised into two groups, according to presence or absence of ERVA. Within each patient the origin of ERVA was compared with the site of fibrosis within the 17 segment model (Fig. 2).
Fig. 1.
Analysis method to determine the origin of ventricular arrhythmias. Localisation within the left ventricle: according to the AHA 17 segment model of the left ventricle [13]. LBBB left bundle branch block, etc. Neg negative, Pos positive, RBBB right bundle branch block, RV right ventricle
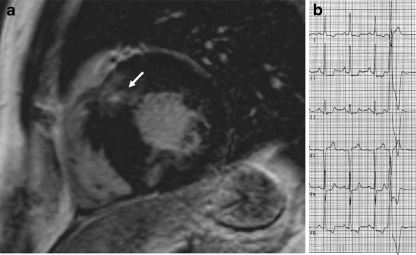
Fig. 2.
Example of exercise related arrhythmias and fibrosis in one patient. Late gadolinium enhanced (LGE) short axis image (a) showing fibrosis in the basal anteroseptal segment (arrow). In this patient, a ventricular premature beat originated from the corresponding segment or nearby this segment in the right ventricular outflow tract (b)
Statistical analysis
Data are expressed as mean ± SD. Clinical and demographic characteristics of the ERVA and non-ERVA groups were compared using the Mann-Whitney U test for continuous variables, and the χ2 test for categorised variables expressed as proportions (or Fisher’s exact test for subgroups containing ≤5 observations). The SPSS software version 12.0 (SPSS Inc., Chicago, Illinois) was used for all analyses. A p value of ≤0.05 was considered statistically significant.
Results
Baseline characteristics are shown in Table 1. Thirty-one patients were studied with a mean age of 38 ± 13 years (range 16 to 65 years) and of whom 20 were male (65%). These patients carried five different mutated genes, one patient carried a double-heterozygous mutation. Mean LVED mass and LVEF were 76 ± 23 g/m2 and 67 ± 5% respectively. LVEDWT(max) was 20 ± 7 mm (range 9 to 32 mm). Twenty-three (74%) patients had an LVEDWT(max) of ≥15 mm. Fibrosis was present in 21 (68%) patients. In these patients, the extent of fibrosis was 4.9 ± 4.3% of LV mass. ERVA (VPBs but not VT or VF) occurred in 7 (23%) patients. One patient had a bigeminy and couplet during the recovery phase.
Table 1.
Study patients and subgroups according to exercise related ventricular arrhythmias
| Characteristics | All patients (n = 31) | ERVA status | p valuea | |
|---|---|---|---|---|
| ERVA (+) (n = 7) | ERVA (−) (n = 24) | |||
| Age (years) | 38 ± 13 | 37 ± 6 | 38 ± 15 | NS |
| Male gender, n (%) | 20 (65) | 5 (71) | 15 (63) | NS |
| More than 1 SCD risk factor, n (%) | 21 (68) | 7 (100) | 14 (28) | 0.04 |
| Mutations, n (%) | NS | |||
| MYH7 | 12 (37) b | 2 (29) | 10 (40) b | |
| MYBPC3 | 7 (23) | 4 (57) | 3 (13) | |
| TPM1 | 9 (29) | 1 (14) | 8 (33) | |
| MYL2 | 2 (7) | 0 (0) | 2 (8) | |
| CSRP3 | 2 (5) b | 0 (0) | 2 (6) b | |
| NT-proBNP, pmol/l | 70 ± 56 | 87 ± 53 | 62 ± 57 | NS |
| CMR | ||||
| LVEF, % | 67 ± 5 | 69 ± 3 | 67 ± 6 | NS |
| LVMi, g/m2 | 76 ± 23 | 95 ± 33 | 71 ± 17 | NS |
| LVEDWT(max), mm | 20 ± 7 | 23 ± 7 | 19 ± 7 | NS |
| Fibrosis, n (%) | 21 (68) | 7 (100) | 14 (58) | 0.04 |
| Extent of fibrosis, % | 5 ± 4 | 8 ± 4 | 3.4 ± 3.6 | 0.02 |
| Exercise test, n (%) | ||||
| VT/VF | 0 (0) | 0 | 0 | |
| VPBs | 7 (27) | 7 | 0 | |
| Bigeminy and couplet | 1 (3) | 1 | 0 | |
| β-blocker therapy | 9 (29) | 3 (43) | 6 (25) | NS |
CMR cardiac magnetic resonance imaging, CSRP3 cysteine and glycine-rich protein 3, ERVA exercise related ventricular arrhythmias, LV left ventricle, LVEDWT(max) maximal end-diastolic left ventricle wall thickness, LVEF left ventricle ejection fraction, LVMi left ventricle mass index, MYBPC3 myosin binding protein C, MYH7 myosin heavy chain 7, MYL2 myosin light chain 2, NS not significant, SCD sudden cardiac death, TPM1 alpha tropomyosin 1, VF ventricular fibrillation, VPB ventricular premature beat, VT ventricular tachycardia
aThe p values reflect the comparison of patients with ERVA vs. those without ERVA
bOne patient carried double heterozygous gene mutations
All patients with ERVA had myocardial fibrosis on CMR, whereas fibrosis was only observed in 58% of the patients without ERVA (100% vs. 58%, p = 0.04). In patients with ERVA, the extent of fibrosis was significantly larger than in patients without ERVA (8 ± 4% vs. 3 ± 3%, p = 0.02).
The LVEDWT(max) and NT-proBNP levels did not significantly differ between patients with and without ERVA (23 ± 7 mm vs. 19 ± 7 mm, p = 0.2 and 87 ± 53 pmol/l vs. 62 ± 57 pmol/l, p = 0.3, respectively).
No significant differences in concomitant β-blocker therapy, gender, age or mutated gene were found between patients with and without ERVA. All patients with ERVA had one or more risk factors for SCD (100% vs. 58%, p = 0.04) (Table 2) [15]. There was no significant difference in the presence of fibrosis between the carriers of the different gene mutations (p = 0.7).
Table 2.
Relation between the number of risk factors for sudden cardiac death and the presence of exercise related ventricular arrhythmias
| Number of risk factors | ERVA (+) (n = 7) | ERVA (−) (n = 24) |
|---|---|---|
| 0, n (%) | 0 (0) | 10 (42) |
| 1, n (%) | 5 (71) | 11 (46) |
| 2, n (%) | 1 (14) | 2 (8) |
| 3, n (%) | 0 (0) | 1 (4) |
| 4, n (%) | 1 (14) | 0 (0) |
ERVA exercise related ventricular arrhythmias
Origin of ERVA
In four patients ERVA originated from the same segment, as the segment with a high extent of fibrosis (Table 3). In the remaining three patients the ERVA were derived from segments immediately adjacent to segments with a high extent of fibrosis.
Table 3.
Relation between the origin of exercise related ventricular arrhythmias and the localisation of left ventricular fibrosis
| Patient | Origin of the VAa | Segment with a high extent of fibrosisb | Relation between origin of the VA and the segments of fibrosis |
|---|---|---|---|
| 1 | 2 | 2, 4, 8, 10 | Same segment |
| 2 | 15/16 | 2, 8, 10 | Adjacent segment |
| 3 | 8 | 2, 3, 9, 10 | Adjacent segment |
| 4 | 8 | 1, 2, 4, 8, | Same segment |
| 5 | 10/11 | 1, 2, 8, 10 | Same segment |
| 6 | 14 | 1, 2, 7, 8, 10, 12 | Adjacent segment |
| 7 | 10/11 | 3, 4, 5, 10, 11, 12,14 | Same segment |
There was no significant difference between the extent of fibrosis of patients with only ERVA during exercise and patients with ERVA during recovery (10 ± 3% vs. 7 ± 7%, p = 0.7) and there was no significant difference between the extent of fibrosis of patients with one ERVA and patients with more than one ERVA (8 ± 7% vs. 10 ± 3%, p = 0.9).
Discussion
Widespread genetic testing identifies large numbers of carriers of mutations that cause HCM; these carriers often have no or few symptoms. Exercise is considered an important risk factor of SCD in these subjects, but the precise risk of exercise in mutation carriers is not fully defined. The purpose of our study was to investigate the relation between fibrosis and ERVA in a population of HCM mutation carriers. ERVA were found in about one fifth of the patients. Interestingly, we found that ERVA exclusively occurred in patients who demonstrated fibrosis on CMR, and that these arrhythmias originated from areas of fibrosis or regions directly adjacent to these areas. Patients with ERVA had significantly more fibrosis on CMR as compared with patients without ERVA. In addition, all patients with ERVA also had one or more risk factors for SCD. ERVA were not related to age, gender, ejection fraction, NT-proBNP levels, LVEDWT(max) or treatment with β-blockers.
Our study suggests that ERVA is a marker of arrhythmogenic cardiac fibrosis as represented by their spatial relationship as well as the higher load of late enhancement on gadolinium-enhanced CMR in arrhythmogenic areas.
Earlier studies also suggested a relation between ventricular arrhythmias and myocardial fibrosis in patients with HCM [10, 16, 17]. In these studies the prevalence of late enhancement was between 41% [17] and 79% [10], which is similar to our study. In contrast to these studies, we considered ERVA rather than spontaneous arrhythmias or arrhythmias on ambulatory monitors. Besides that we used a population of proven carriers of pathogenic HCM mutations. In 2003, Moon et al. [10] described that HCM patients with ≥2 risk factors for SCD including nonsustained ventricular tachycardia on Holter monitoring showed a greater extent of fibrosis. Adabag et al. [17] showed that VPBs on Holter monitoring are related to the presence of fibrosis, but not to the extent of fibrosis. Recently Kwon et al. [15] showed that patients with VT had a significantly higher extent of fibrosis, which is in line with our present findings.
Two other Dutch studies have already described the importance of cardiological evaluation for asymptomatic carriers of HCM causing mutations. Christiaans at al. described a population of 235 asymptomatic mutation carriers with a mutation in myosin binding protein C (MYBPC3); after first cardiological evaluation almost one-quarter were diagnosed with HCM and more than one-tenth of the carriers were at risk for SCD [18]. Michels et al. found an even higher number of asymptomatic mutation carriers who received the diagnosis HCM [19]. They also described the presence of SCD risk factors in carriers with and without HCM. In the same line, Ho et al. showed an early profibrotic response of mutation carriers but without left ventricular hypertrophy [20].
The current risk stratification used for HCM identifies a number of high-risk patients for SCD, but unfortunately still a substantial number of HCM patients die suddenly [21]. Fibrosis as detected by CMR is increasingly recognised as a risk factor for SCD in HCM, but routine screening is not yet advised [9, 15, 16, 21, 22]. Our data lend further support to the idea that myocardial fibrosis in HCM is related to ERVA. However, this study cannot answer whether these ERVA are related to more life-threatening ventricular arrhythmias.
In population-based cohorts with coronary heart disease, VPBs do predict an increased risk of death, but VPBs that were found during the recovery phase appeared to be an even better predictor of an increased risk of death compared with VPBs during exercise [23–25].
There are important limitations to this retrospective study that need to be taken into account. Most importantly, the fact that the registry was undertaken by a referral centre may have resulted in a referral bias towards more severely affected patients. Moreover, it cannot be excluded that there was a selection bias towards the selection of patients with both a CMR and exercise test. This study is limited by its size, and lack of follow-up. As a result, it cannot answer the question whether ERVA should also be considered as a marker of high risk of SCD in the HCM population, as it is in patients with coronary artery disease. However, it may provide the necessary initial observation to fuel a larger study into the predictive role of ERVA and to validate our findings.
The ERVA that were observed originated either from a segment with a high extent of fibrosis or from a segment directly adjacent to this. This imprecision may be explained by the fact that the analysis of the origins of the VPBs is an approximation provided by 12-channel ECG readings, but also by the fact that the exit point is at the edge of the fibrosis area and not necessarily in the area itself. In addition, electrocardiographic and anatomical orientation by CMR are not fully congruent and may lead to imprecision of the origin of the VPB and the localisation of fibrosis.
Conclusion
A substantial proportion of carriers of an HCM causing mutation may show ERVA which—in turn—is associated with a significantly higher extent of ventricular fibrosis. In addition, there was a significant spatial relationship between ERVA and fibrosis supporting the notion that increased fibrosis is arrhythmogenic and possibly related to increased mortality. Future studies are needed to address whether increased late enhancement on CMR represents an increased risk of SCD in HCM mutation carriers.
Acknowledgments
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
References
- 1.Maron BJ, Gardin JM, Flack JM, et al. Prevalence of hypertrophic cardiomyopathy in a general population of young adults. Echocardiographic analysis of 4111 subjects in the CARDIA Study. Coronary Artery Risk Development in (Young) Adults. Circulation. 1995;92:785–789. doi: 10.1161/01.cir.92.4.785. [DOI] [PubMed] [Google Scholar]
- 2.Elliott P, McKenna WJ. Hypertrophic cardiomyopathy. Lancet. 2004;363:1881–1891. doi: 10.1016/S0140-6736(04)16358-7. [DOI] [PubMed] [Google Scholar]
- 3.Hughes SE, McKenna WJ. New insights into the pathology of inherited cardiomyopathy. Heart. 2005;91:257–264. doi: 10.1136/hrt.2004.040337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Maron BJ, Epstein SE, Roberts WC. Hypertrophic cardiomyopathy: a common cause of sudden death in the young competitive athlete. Eur Heart J. 1983;4(Suppl F):135–144. doi: 10.1093/eurheartj/4.suppl_f.135. [DOI] [PubMed] [Google Scholar]
- 5.Maron BJ, Olivotto I, Spirito P, et al. Epidemiology of hypertrophic cardiomyopathy-related death: revisited in a large non-referral-based patient population. Circulation. 2000;102:858–864. doi: 10.1161/01.cir.102.8.858. [DOI] [PubMed] [Google Scholar]
- 6.Ciro E, Nichols PF, 3rd, Maron BJ. Heterogeneous morphologic expression of genetically transmitted hypertrophic cardiomyopathy. Two-dimensional echocardiographic analysis. Circulation. 1983;67:1227–1233. doi: 10.1161/01.CIR.67.6.1227. [DOI] [PubMed] [Google Scholar]
- 7.Maron BJ. Hypertrophic cardiomyopathy. Lancet. 1997;350:127–133. doi: 10.1016/S0140-6736(97)01282-8. [DOI] [PubMed] [Google Scholar]
- 8.Spirito P, Bellone P, Harris KM, et al. Magnitude of left ventricular hypertrophy and risk of sudden death in hypertrophic cardiomyopathy. N Engl J Med. 2000;342:1778–1785. doi: 10.1056/NEJM200006153422403. [DOI] [PubMed] [Google Scholar]
- 9.Shirani J, Pick R, Roberts WC, et al. Morphology and significance of the left ventricular collagen network in young patients with hypertrophic cardiomyopathy and sudden cardiac death. J Am Coll Cardiol. 2000;35:36–44. doi: 10.1016/S0735-1097(99)00492-1. [DOI] [PubMed] [Google Scholar]
- 10.Moon JC, McKenna WJ, McCrohon JA, et al. Toward clinical risk assessment in hypertrophic cardiomyopathy with gadolinium cardiovascular magnetic resonance. J Am Coll Cardiol. 2003;41:1561–1567. doi: 10.1016/S0735-1097(03)00189-X. [DOI] [PubMed] [Google Scholar]
- 11.Elliott PM, Gimeno Blanes JR, Mahon NG, et al. Relation between severity of left-ventricular hypertrophy and prognosis in patients with hypertrophic cardiomyopathy. Lancet. 2001;357:420–424. doi: 10.1016/S0140-6736(00)04005-8. [DOI] [PubMed] [Google Scholar]
- 12.Bondarenko O, Beek AM, Hofman MB, et al. Standardizing the definition of hyperenhancement in the quantitative assessment of infarct size and myocardial viability using delayed contrast-enhanced CMR. J Cardiovasc Magn Reson. 2005;7:481–485. doi: 10.1081/JCMR-200053623. [DOI] [PubMed] [Google Scholar]
- 13.Cerqueira MD, Weissman NJ, Dilsizian V, et al. Standardized myocardial segmentation and nomenclature for tomographic imaging of the heart: a statement for healthcare professionals from the Cardiac Imaging Committee of the Council on Clinical Cardiology of the American Heart Association. Circulation. 2002;105:539–542. doi: 10.1161/hc0402.102975. [DOI] [PubMed] [Google Scholar]
- 14.Bruce RA, Kusumi F, Hosmer D. Maximal oxygen intake and nomographic assessment of functional aerobic impairment in cardiovascular disease. Am Heart J. 1973;85:546–562. doi: 10.1016/0002-8703(73)90502-4. [DOI] [PubMed] [Google Scholar]
- 15.Elliott PM, Poloniecki J, Dickie S, et al. Sudden death in hypertrophic cardiomyopathy: identification of high risk patients. J Am Coll Cardiol. 2000;36:2212–2218. doi: 10.1016/S0735-1097(00)01003-2. [DOI] [PubMed] [Google Scholar]
- 16.Kwon DH, Setser RM, Popovic ZB, et al. Association of myocardial fibrosis, electrocardiography and ventricular tachyarrhythmia in hypertrophic cardiomyopathy: a delayed contrast enhanced MRI study. Int J Cardiovasc Imaging. 2008;24:617–625. doi: 10.1007/s10554-008-9292-6. [DOI] [PubMed] [Google Scholar]
- 17.Adabag AS, Maron BJ, Appelbaum E, et al. Occurrence and frequency of arrhythmias in hypertrophic cardiomyopathy in relation to delayed enhancement on cardiovascular magnetic resonance. J Am Coll Cardiol. 2008;51:1369–1374. doi: 10.1016/j.jacc.2007.11.071. [DOI] [PubMed] [Google Scholar]
- 18.Christiaans I, Birnie E, van Langen IM, et al. The yield of risk stratification for sudden cardiac death in hypertrophic cardiomyopathy myosin-binding protein C gene mutation carriers: focus on predictive screening. Eur Heart J. 2010;31:842–848. doi: 10.1093/eurheartj/ehp539. [DOI] [PubMed] [Google Scholar]
- 19.Michels M, Soliman OI, Phefferkorn J, et al. Disease penetrance and risk stratification for sudden cardiac death in asymptomatic hypertrophic cardiomyopathy mutation carriers. Eur Heart J. 2009;30:2593–2598. doi: 10.1093/eurheartj/ehp306. [DOI] [PubMed] [Google Scholar]
- 20.Ho CY, Lopez B, Coelho-Filho OR, et al. Myocardial fibrosis as an early manifestation of hypertrophic cardiomyopathy. N Engl J Med. 2010;363:552–563. doi: 10.1056/NEJMoa1002659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Maron BJ, Maron MS, Lesser JR, et al. Sudden cardiac arrest in hypertrophic cardiomyopathy in the absence of conventional criteria for high risk status. Am J Cardiol. 2008;101:544–547. doi: 10.1016/j.amjcard.2007.09.101. [DOI] [PubMed] [Google Scholar]
- 22.Nazarian S, Lima JA. Cardiovascular magnetic resonance for risk stratification of arrhythmia in hypertrophic cardiomyopathy. J Am Coll Cardiol. 2008;51:1375–1376. doi: 10.1016/j.jacc.2008.01.010. [DOI] [PubMed] [Google Scholar]
- 23.Frolkis JP, Pothier CE, Blackstone EH, et al. Frequent ventricular ectopy after exercise as a predictor of death. N Engl J Med. 2003;348:781–790. doi: 10.1056/NEJMoa022353. [DOI] [PubMed] [Google Scholar]
- 24.Calkins H. Premature ventricular depolarizations during exercise. N Engl J Med. 2000;343:879–880. doi: 10.1056/NEJM200009213431209. [DOI] [PubMed] [Google Scholar]
- 25.Califf RM, McKinnis RA, McNeer JF, et al. Prognostic value of ventricular arrhythmias associated with treadmill exercise testing in patients studied with cardiac catheterization for suspected ischemic heart disease. J Am Coll Cardiol. 1983;2:1060–1067. doi: 10.1016/S0735-1097(83)80330-1. [DOI] [PubMed] [Google Scholar]