Abstract
Members of the mitogen-activated protein kinase (MAPK) cascade such as extracellular signal-regulated kinase (ERK), c-Jun N-terminal kinase (JNK) and p38 are implicated as important regulators of cardiomyocyte hypertrophic growth in culture. However, the role that individual MAPK pathways play in vivo has not been extensively evaluated. Here we generated nine transgenic mouse lines with cardiac-restricted expression of an activated MEK1 cDNA in the heart. MEK1 transgenic mice demonstrated concentric hypertrophy without signs of cardiomyopathy or lethality up to 12 months of age. MEK1 transgenic mice showed a dramatic increase in cardiac function, as measured by echocardiography and isolated working heart preparation, without signs of decompensation over time. MEK1 transgenic mice and MEK1 adenovirus-infected neonatal cardiomyocytes each demonstrated ERK1/2, but not p38 or JNK, activation. MEK1 transgenic mice and MEK1 adenovirus-infected cultured cardiomyocytes were also partially resistant to apoptotic stimuli. The results of the present study indicate that the MEK1–ERK1/2 signaling pathway stimulates a physiologic hypertrophy response associated with augmented cardiac function and partial resistance to apoptotsis.
Keywords: cardiac/cardiomyopathy/hypertrophy/MAPK/transgenic
Introduction
While initially a compensatory response, sustained cardiac hypertrophy is associated with decompensation, dilated cardiomyopathy, arrhythmia, fibrotic disease, sudden death or overt heart failure (Levy et al., 1990). In recent years, much investigation has centered around defining the intracellular signaling pathways that are associated with hypertrophy and dilated cardiomyopathy. Members of the mitogen-activated protein kinase (MAPK) signaling cascade are thought to be important regulators of cardiac hypertrophy (reviewed in Sugden and Clerk, 1998).
MAPK signaling pathways consist of a sequence of successively acting kinases that ultimately result in the dual phosphorylation and activation of terminal effector kinases such as p38, c-Jun N-terminal kinases (JNKs) and extracellular signaling-regulated kinases (ERKs) (Widmann et al., 1999). The MAPK signaling cascade is initiated in cardiac myocytes by activation of G-protein coupled receptors, receptor tyrosine kinases, and by stress stimuli (reviewed in Sugden and Clerk, 1998). Once activated, p38, JNKs and ERKs each phosphorylate a wide array of intracellular targets, which includes numerous transcription factors resulting in the reprogramming of cardiac gene expression.
The role that MEK1–ERK1/2 plays in the regulation of cardiac hypertrophy is currently an area of ongoing debate. ERK1/2 has been shown to be activated in cultured neonatal rat cardiomyocytes by agonist stimulation and cell stretching (Bogoyevitch et al., 1993; Yamazaki et al., 1993; Clerk et al., 1994; Post et al., 1996; Zou et al., 1996). Transfection of a constitutively active MEK1 factor augmented atrial natriuretic factor (ANF) promoter activity in cultured cardiomyocytes while a dominant-negative MEK1 construct attenuated promoter activity (Gillespie-Brown et al., 1995). Using antisense oligonucleotides, Glennon et al. (1996) demonstrated that ERK signaling is necessary for phenylephrine-induced cardiomyocyte hypertrophy in culture. Similarly, using the MEK1 inhibitor PD98059, Clerk et al. (1998) reported that ERKs were required for sarcomeric organization induced by hypertrophic agonists. However, a number of additional studies have disputed the importance of MEK1–ERK1/2 in the regulation of cardiac hypertrophy (Thorburn et al., 1994; Post et al., 1996; Ramirez et al., 1997; Zechner et al., 1997; Choukroun et al., 1998), and one study even suggested that ERK activation in response to ANF treatment is associated with prevention of cardiomyocyte hypertrophy (Silberbach et al., 1999).
To evaluate the MEK1–ERK1/2 signaling pathway as a potential regulator of cardiac hypertrophy in vivo, a series of transgenic mice were generated expressing a mutant MEK1 (activated) protein in the heart. Expression of activated MEK1 in the mouse heart promoted specific activation of ERK1/2, but not JNK1/2 or p38, which was associated with a long-standing physiologic hypertrophy response characterized by increased cardiac function and partial resistance to apoptotic stimuli.
Results
Generation of MEK1 transgenic mice
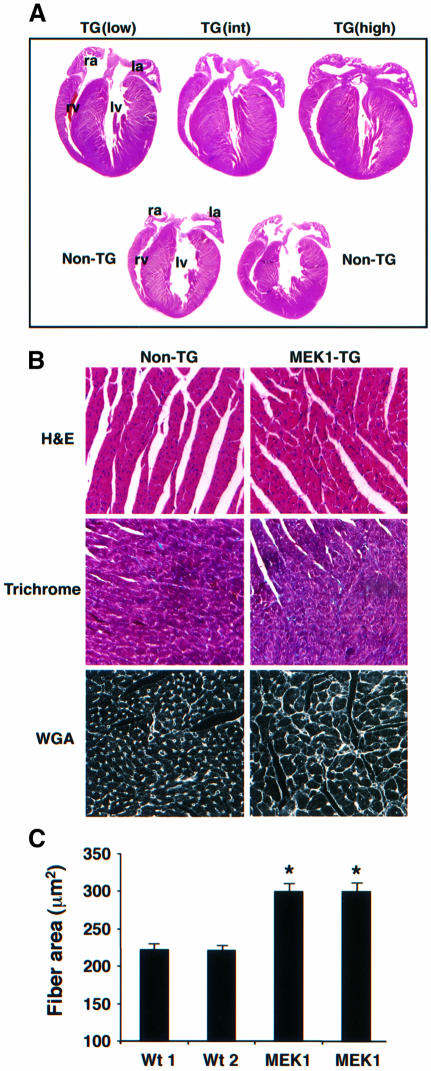
To examine the role that the MEK1–ERK1/2 signaling pathway might play in regulating cardiac hypertrophy in vivo, transgenic mice were generated expressing an activated form of MEK1 (Ser217/221 to Glu) under the control of the cardiac-specific α-myosin heavy chain promoter. Nine independent MEK1 transgenic lines were successfully generated, each demonstrating concentric hypertrophy that was independent of transgene integration number (from two to >40 integrations). None of the transgenic lines was characterized by premature lethality or cardiac dilation (currently up to 12 months of age), suggesting that the hypertrophy response was compensated. Histological analysis performed at 8 weeks of age demonstrated a consistent phenotype of concentric ventricular hypertrophy in low-, intermediate- or high-copy-number transgenic lines compared with non-transgenic littermate mice (Figure 1A). Hematoxylin–eosin- and trichrome-stained histological sections from 8-week-old high-copy-number mice demonstrated overtly normal cytoarchitecture in the heart without interstitial cell fibrosis (Figure 1B). Trichrome staining of histological heart sections also revealed a lack of significant interstitial fibrosis in MEK1 transgenic mice at 6 months of age, despite a persistent hypertrophic phenotype (data not shown). Quantitative assessment of myofibrillar cross-sectional area from wheatgerm agglutinin–tetramethyl rhodamine isothiocyanate (TRITC) (50 µg/ml) stained histological tissue sections demonstrated significant hypertrophy in high or intermediate lines compared with hearts from wild-type littermate mice at 8 weeks of age (Figure 1B and C). Collectively, these data indicate that expression of an activated MEK1 cDNA in the heart promotes cardiac hypertrophy in vivo.
Fig. 1. Cardiac histological analysis of MEK1 transgenic mice. (A) Macroscopic hematoxylin–eosin-stained histological section of hearts from a low, intermediate (int) and high transgene copy number MEK1 line at 8 weeks of age. ra, right atrium; la, left atrium; rv, right ventricle; lv, left ventricle. (B) Microscopic histological analysis revealed no histopathology in hematoxylin–eosin- or trichrome-stained heart sections from high-copy-number MEK1 transgenic mice. Wheat germ agglutinin (WGA)–TRITC-stained histological sections demonstrated noticeably larger myofibrils in high-copy-number MEK1 transgenic hearts. Identical histological observations were made in intermediate- and low-copy-number MEK1 lines (data not shown). (C) Myofibrillar cross-sectional areas were quantified from WGA–TRITC-stained histological sections. At least 150 fibers were measured each in two wild-type (wt) hearts and a high and intermediate MEK1 transgenic heart. *P <0.05 compared with wild-type hearts.
High-copy-number MEK1 transgenic mice demonstrated a uniform profile of increased heart:body weight ratio of 34, 41 and 23% at 3 weeks, 8 weeks and 6 months of age, respectively (P <0.001) (Table I). This effect was relatively independent of transgene copy number as low, intermediate or high MEK1 transgenic lines each demonstrated increased heart:body weight ratios at 8 weeks (P <0.001) (Table I). To assess cardiac morphology in a functioning heart more accurately, echocardiography was performed on 8-week-old MEK1 transgenic and littermate control mice. MEK1 transgenic mice were characterized by a 54 and 50% increase in septal thickness and left ventricular posterior wall thickness, respectively (P <0.01, P <0.005) (Table II). Echocardiography also demonstrated reduced left ventricular chamber dimension during both systole and diastole in MEK1 transgenic mice, which was associated with increased fractional shortening (Table II). These analyses indicate that MEK1 transgenic mice have concentric cardiac hypertrophy.
Table I. Heart-to-body-weight ratios of MEK1 transgenic mice.
| Non-transgenic | MEK1-TG | % increase | P | |
|---|---|---|---|---|
| 3 weeks (high) | ||||
| number | n = 6 | n = 9 | ||
| height/body wt | 5.52 ± 0.17 | 7.40 ± 0.28 | +34 | <0.001 |
| 8 weeks (low) | ||||
| number | n = 7 | n = 6 | ||
| height/body wt | 4.85 ± 0.09 | 5.39 ± 0.09 | +11 | <0.001 |
| 8 weeks (intermediate) | ||||
| number | n = 7 | n = 6 | ||
| height/body wt | 4.85 ± 0.09 | 6.67 ± 0.31 | +37 | <0.0001 |
| 8 weeks (high) | ||||
| number | n = 7 | n = 28 | ||
| height/body wt | 4.85 ± 0.09 | 6.88 ± 0.20 | +41 | <0.0001 |
| 6 months (high) | ||||
| number | n = 7 | n = 6 | ||
| height/body wt | 5.89 ± 0.20 | 7.22 ± 0.19 | +23 | <0.0001 |
Heart:body-weight ratios were calculated from non-transgenic or MEK1 transgenic mice at the indicated times. At 8 weeks of age, lines of MEK1 mice corresponding to low-, intermediate- or high-copy transgene number were evaluated for heart weights. Values given are ±SEM and P values were calculated by Student’s t-test.
Table II. Echocardiography of MEK1 transgenic mice demonstrates concentric hypertrophy and enhanced fractional shortening.
| Non-transgenic (n = 6) | MEK1-TG (n = 6) | % change | P | |
|---|---|---|---|---|
| 8 weeks | ||||
| septal thickness | 0.65 ± 0.07 | 1.0 ± 0.09 | +54 | 0.01 |
| LV posterior wall thickness | 0.48 ± 0.02 | 0.72 ± 0.06 | +50 | 0.004 |
| LVED | 3.9 ± 0.1 | 3.0 ± 0.2 | –23 | 0.003 |
| LVES | 2.2 ± 0.1 | 1.2 ± 0.2 | –45 | 0.001 |
| FS | 0.44 ± 0.02 | 0.60 ± 0.04 | +36 | 0.005 |
Measurements are given in millimeters, except fractional shortening (FS), which is unitless. Values were quantified from three separate M-mode measurements and averaged between six mice in each group. LV, left ventricle; LVED, left ventricular end diastolic dimension; LVES, left ventricular end systolic dimension.
To evaluate heart function in MEK1 transgenic mice independent of neuro-humoral status, isolated working heart preparations were performed. MEK1 transgenic hearts were characterized by significantly greater left ventricular function (dP/dtmax) at both 2 months (33%) and 6 months (29%) of age compared with wild-type littermate hearts (Table III). In addition, isolated MEK1 transgenic hearts were able to develop higher left ventricular systolic pressures (+16 and +12% at 2 and 6 months, respectively), further demonstrating their hyperfunctional state ex vivo (Table III). This increase in cardiac function was also observed by Langendorff heart preparation at 2 and 6 months of age (Table III). Diastolic function (dP/dtmin) was reduced in MEK1 transgenic hearts in a manner that is consistent with an increase in septal and left ventricular wall thickness (greater stiffness) seen in concentric hypertrophy (Table III).
Table III. Working heart and Langendorff heart preparation at 2 and 6 months of age reveals enhanced function in MEK1 transgenic mice.
| Non-transgenic2 months | MEK1-TG2 months | % change | P | Non-transgenic6 months | MEK1-TG6 months | % change | P | |
|---|---|---|---|---|---|---|---|---|
| Working heart | n = 6 | n = 6 | n = 5 | n = 4 | ||||
| LV dP/dtmax | 6310 ± 168 | 8376 ± 156 | +33 | <0.001 | 5703 ± 201 | 7336 ± 231 | +29 | <0.001 |
| LV dP/dtmin | 4899 ± 99 | 3453 ± 65 | –30 | <0.001 | 4645 ± 141 | 3410 ± 81 | –27 | <0.001 |
| LVSP | 111 ± 2 | 129 ± 2 | +16 | <0.001 | 108 ± 3 | 121 ± 3 | +12 | <0.01 |
| Langendorff | n = 3 | n = 6 | n = 3 | n = 4 | ||||
| LV dP/dtmax | 3035 ± 54 | 4920 ± 191 | +62 | <0.001 | 2720 ± 40 | 4478 ± 66 | +65 | <0.001 |
Maximum and minimum index of contractility was measured by isolated left ventricular ejecting, working heart preparation. Values are given as the first derivative of the slope of the left ventricular pressure (LV) gradient versus time (dP/dt; units: mmHg/ms). Langendorff preparation also demonstrated enhanced functional performance in MEK1 transgenic hearts. Pacing was performed in both preparations (405 ± 2 beats per minute) so that only differences in contractility affected total output. LVSP, left ventricular systolic pressure.
No loss of systolic functional performance occurred between 2 and 6 months of age, indicating that MEK1 transgenic mice have compensated hypertrophy. While MEK1 transgenic mice demonstrated enhanced cardiac function and enhanced left ventricular systolic pressure in an isolated work-performing heart preparation, conscious MEK1 transgenic mice did not have altered blood pressure compared with littermate mice (data not shown).
Molecular characterization of MEK1 transgenic mice
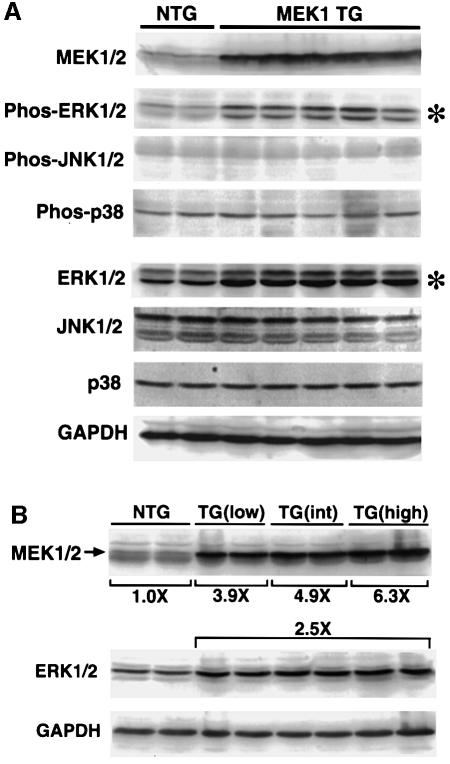
Protein extracts were generated from the hearts of five high-copy-number MEK1 transgenic mice and two littermate controls at 8 weeks of age to verify overexpression of the transgene-encoded MEK1 protein. Western blotting with a MEK1/2 antibody demonstrated robust overexpression of protein in the heart (Figure 2A). Western blotting with phospho-specific ERK1/2 antibody demonstrated a specific activation of ERK1/2 in MEK1 transgenic hearts, but not of JNK1/2 (p46 and p54) or p38 (Figure 2A). Interestingly, total ERK1/2 protein was significantly increased in MEK1 transgenic hearts, suggesting that increased levels of MEK1 protein stabilize or upregulate ERK1/2 protein in the heart (Figure 2A). JNK1/2 and p38 protein levels did not vary between MEK1 transgenic and control hearts (Figure 2A).
Fig. 2. Quantitation of MEK1/2 protein levels and ERK1/2 activation in MEK1 transgenic hearts. (A) Western blotting was performed on protein extracts from two non-transgenic (NTG) hearts and five high-copy MEK1 transgenic hearts at 8 weeks. Quantitative analysis revealed a significant increase in MEK1/2 protein, ERK1/2 protein (*) and phosphorylated ERK1/2 (*) analyzed with a phospho-specific antibody. However, JNK1/2 and p38 phosphorylation and total protein levels were unaffected. (B) Western blotting for MEK1/2 and ERK1/2 protein levels across low-, intermediate (int)- and high-copy-number MEK1 transgenic lines revealed an increase in MEK1/2 protein with increasing copy number (two separate hearts each). However, ERK1/2 protein levels remained elevated and did not increase significantly as copy number increased. GAPDH protein levels were invariant.
To analyze more carefully this increase in cardiac ERK1/2 protein levels identified in MEK1 transgenic hearts, quantitative western blotting was performed from low, intermediate and high transgene containing lines. The data demonstrate a 3.9-, 4.9- and 6.3-fold increase in MEK1/2 protein from a low-, intermediate- and high-copy-number line, respectively (from two hearts each) (Figure 2B). Despite differing levels of MEK1/2 protein expression, a consistent 2.5-fold increase in total ERK1/2 protein was observed, indicating a saturating effect even at lower MEK1 protein levels (Figure 2B) (see Discussion). Taken together, these data indicate that MEK1 overexpression upregulates both ERK1/2 activation and total ERK1/2 protein in the heart, but has no effect on p38 or JNK1/2.
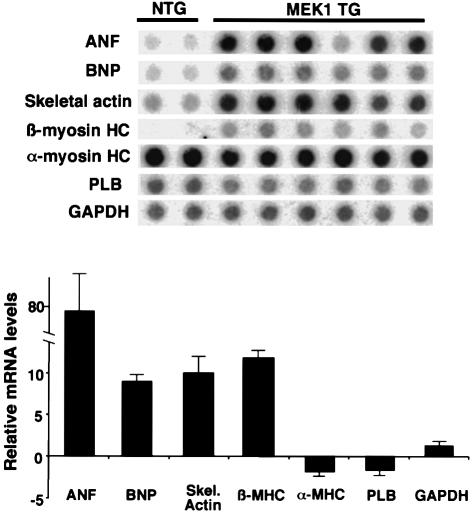
The molecular program of cardiac hypertrophy is often associated with altered expression of contractile genes or fetal encoded genes in the heart. Accordingly, RNA dot blotting was performed to quantify expression of levels of α-myosin heavy chain (α-MHC), β-myosin heavy chain gene (β-MHC), skeletal α-actin, ANF, brain natriuretic peptide (BNP) and phospholamban (PLB). Expression of β-MHC, ANF, BNP and skeletal α-actin was significantly increased in high-copy-number MEK1 transgenic hearts compared with littermate non-transgenic hearts at 8 weeks of age (P <0.05). PLB and α-MHC levels were not significantly different (Figure 3). These changes in gene expression demonstrate activation of the molecular program for cardiac hypertrophy in MEK1 transgenic mice.
Fig. 3. RNA dot blotting of hypertrophy-associated genes in MEK1 hearts. Hearts from high expressing MEK1 transgenic mice were harvested at 8 weeks of age and total ventricular RNA was isolated and subjected to dot-blot hybridization to measure levels of ANF, BNP, skeletal α-actin, α-MHC, β-MHC, PLB and GAPDH as a loading control. The quantitation of these data is shown as a bar graph. Error bars represent the SEM.
MEK1 adenovirus induces cardiac hypertrophy in vitro
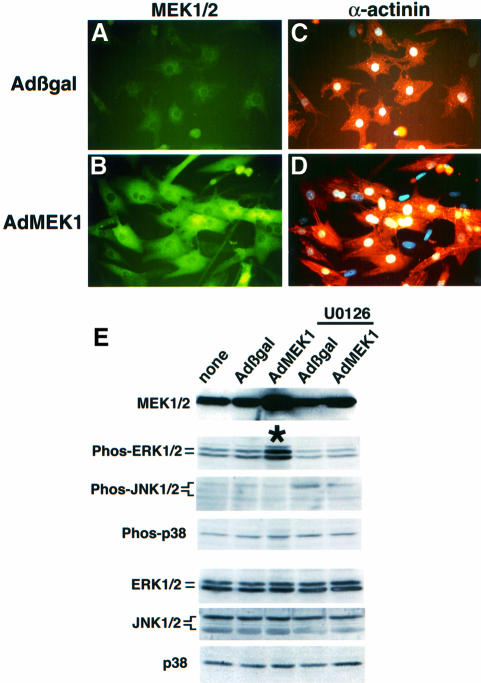
To investigate further the ability of MEK1 to induce a hypertrophic response in vitro, an adenovirus expressing constitutively active MEK1 (Ser217/221 to Glu) was generated to allow infection of neonatal rat ventricular cardiomyocytes. Approximately 99% of cultured primary neonatal cardiomyocytes were infected with the MEK1-encoding adenovirus (AdMEK1) at a multiplicity of infection (m.o.i.) of 50 p.f.u./cell compared with uninfected cultures (Figure 4A and B). AdMEK1 infection resulted in a 3- to 4-fold increase in MEK1/2 protein, resulting in a 2-fold increase in ERK1/2 phosphorylation in cardiomyocytes compared with Adβgal-infected cardiomyocytes (Figure 4E). Expression of activated MEK1 did not result in p38 or JNK1/2 (p54 or p46) phosphorylation in adenovirus-infected cardiomyocytes. In addition, AdMEK1 infection did not alter protein levels of ERK1/2, p38 or JNK1/2 after 24 h of infection in culture (Figure 4E).
Fig. 4. Adenoviral MEK1 infection induces hypertrophy in cultured neonatal cardiomyocytes. (A and B) AdMEK1 infection dramatically augmented MEK1/2 protein levels (MEK1/2 antibody in green) in neonatal cardiomyocytes compared with Adβgal-infected cells. (C and D) Cardiomyocytes were co-immunostained for α-actinin (red/orange) to show both sarcomeric organization and myocyte surface area in response to AdMEK1 or Adβgal infection (after 24 h). (E) Western blot analysis from AdMEK1-infected neonatal cardiomyocytes revealed a significant increase in MEK1/2 protein and ERK1/2 phosphorylation. The MEK1 inhibitor U0126 (20 µM) blocked ERK1/2 phosphorylation. AdMEK1 infection did not result in p38 or JNK1/2 (p46 and p54) phosphorylation, nor were ERK1/2, p38 or JNK1/2 protein levels changed after 24 h of infection. Identical results were obtained in three independent experiments.
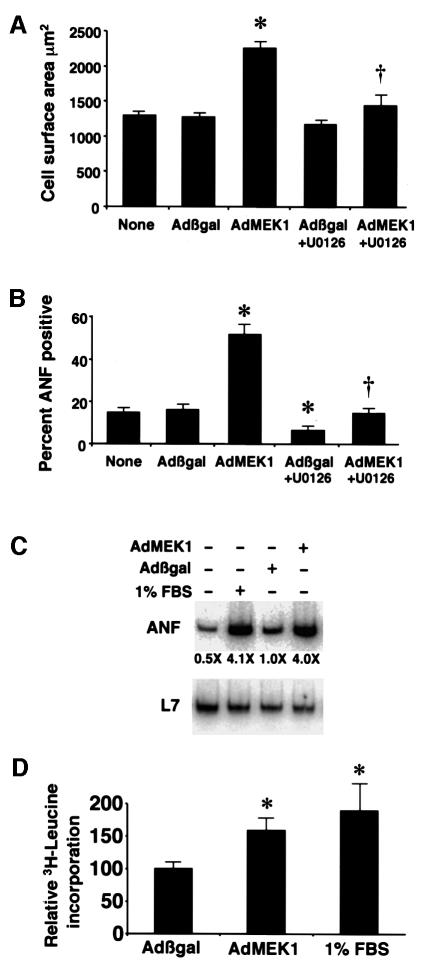
MEK1 adenoviral infection induced a hypertrophic response in cultured neonatal cardiomyocytes characterized by increased cell size and enhanced sarcomeric organization (Figure 4C and D). Control Adβgal infection was associated with an average cell surface area of 1250 ± 46 µm2 compared with 2192 ± 79 µm2 in AdMEK1-infected myocytes (P <0.05) (Figure 5A). U0126, a MEK1 inhibitor (20 µM), attenuated the increase in cell surface area induced by AdMEK1 infection (1435 ± 215 µm2, P <0.05) (Figure 5A). Cardiomyocytes were also immunostained with ANF antibody to quantify the percentage of cells showing perinuclear ANF expression as an indicator of the hypertrophy program, as characterized previously (Taigen et al., 2000). AdMEK1 infection induced a dramatic increase in the percentage of cells expressing ANF (49 ± 4%) compared with Adβgal-infected cells (17 ± 1%) (Figure 5B). U0126 blocked the increase in the percentage of cells expressing ANF due to AdMEK1 infection (14 ± 1%). U0126 also reduced ANF expression in Adβgal-infected cardiomyocytes (6 ± 1%), probably by a reduction in basal ERK1/2 activity. AdMEK1 increased total ANF mRNA levels similarly to 1% fetal bovine serum (FBS) stimulation (Figure 5C). Lastly, AdMEK1 infection (n = 11 individual plates) stimulated a 58% increase in [3H]leucine incorporation after 4 h of incubation in leucine-free media compared with Adβgal-infected cells (n = 11 individual plates) (P <0.05) (Figure 5D). As a control, cultured cardiomyocytes were stimulated with 1% FBS, which also induced a significant increase in protein synthesis rates (n = 7, 88% increase). These results indicate that MEK1 induces cardiomyocyte hypertrophy in vitro through ERK1/2 activation, consistent with the phenotype of MEK1-expressing transgenic mice.
Fig. 5. Quantitation of the hypertrophic phenotype of AdMEK1-infected cardiomyocytes. (A) Cell surface area was measured in α-actinin-stained cardiomyocyte cultures using confocal microscopy and digitized imaging (24 h after infection). Cultures were left in serum-free medium with no stimulation or were infected with Adβgal or AdMEK1, or were treated with U0126. (B) Cardiomyocytes were also stained with an ANF-specific antibody to quantify the percentage of cells expressing ANF. The data in (A) and (B) were obtained in two independent experiments. (C) Total ANF mRNA levels were also quantified by RT–PCR, showing similar induction of ANF mRNA between 1% FBS and AdMEK1 infection. (D) Protein synthesis rates were monitored by the incorporation of [3H]leucine in Adβgal-, AdMEK1- and 1% FBS-stimulated cardiomyocytes. *P <0.05 compared with Adβgal; †P <0.05 compared with AdMEK1 infection alone.
AdMEK1 adenoviral infection protects cardiomyocytes from apoptosis
Previous studies have shown that agonist-induced ERK1/2 activation is associated with protection from apoptosis in cultured cardiomyocytes (Parrizas et al., 1997; Sheng et al., 1997; De Windt et al., 2000). To test directly whether MEK1–ERK activation provides protection from apoptotic stimuli in cardiomyocytes, cultures were infected with Adβgal or AdMEK1 and subsequently treated with 2-deoxyglucose in glucose-free media for 12 or 18 h as described previously (Bialik et al., 1999; De Windt et al., 2000). Cardiomyocyte cultures were subsequently fixed and labeled for terminal deoxyribonucleotide transferase (TdT)-mediated dUTP nick-end labeling (TUNEL) reactivity as an indicator of DNA fragmentation (from two independent experiments). AdMEK1 adenoviral infection provided protection from TUNEL compared with Adβgal-infected cultures (Figure 6A). An adenovirus expressing constitutively active form of AKT was also used as an anti-apoptosis control in cultured cardiomyocytes (Sharma et al., 1998; Fujio et al., 2000). As another independent measure of apoptosis, caspase 3 activation was quantified in Adβgal-, AdAKT- and AdMEK1-infected cardiomyocytes in the presence of 2-deoxyglucose. Caspase 3 activity was reduced by ∼50% in both AdAKT- and AdMEK1-infected cardiomyocytes, indicating partial protection (from three independent experiments) (Figure 6B). Collectively, these results demonstrate that AdMEK1 infection partially protects cultured cardiomyocytes from apoptotic stimuli in vitro.
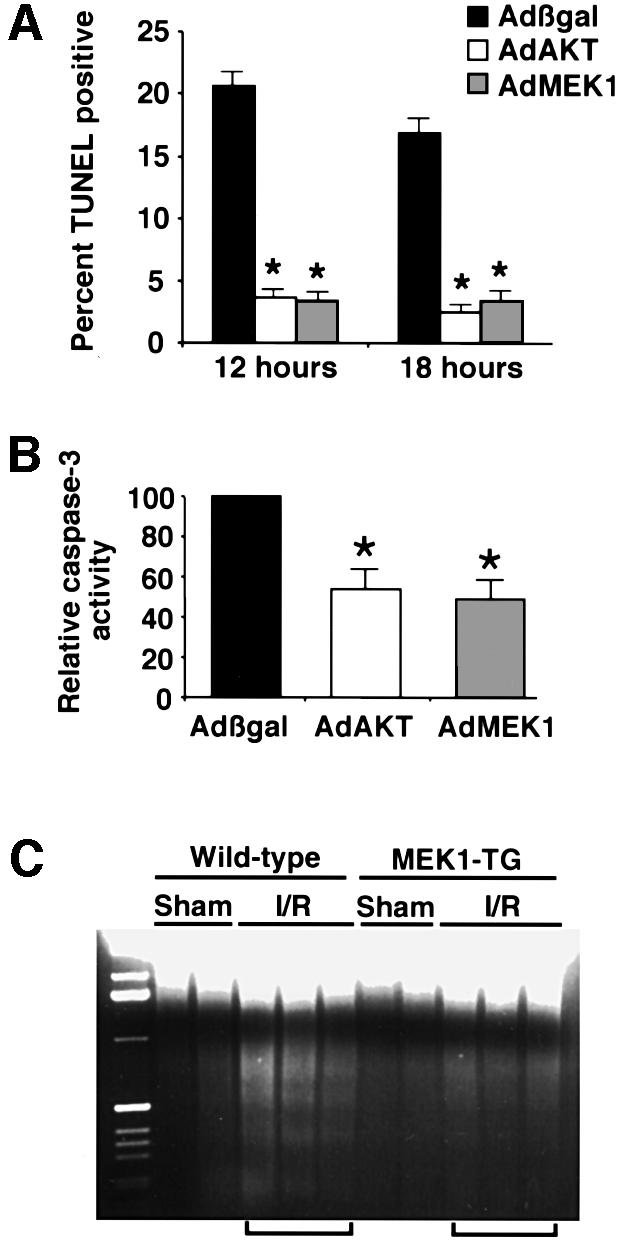
Fig. 6. MEK1 confers protection from TUNEL, caspase 3 activation and DNA laddering. (A) Cultured neonatal cardiomyocytes were infected with Adβgal, AdAkt or AdMEK1 and 24 h later placed in glucose-free and serum-free media in the presence of 2-deoxyglucose to induce apoptosis. The data demonstrate that culturing for 12 or 18 h in the presence of 2-deoxyglucose induced significant TUNEL in Adβgal-infected myocytes, while AdAkt or AdMEK1 infection conferred resistance. (B) AdMEK1 and AdAKT also provided partial protection from caspase 3 activation induced by 2-deoxyglucose. (C) In vivo, MEK1 transgenic and wild-type mice were subjected to 45 min of cardiac ischemia followed by 4 h of reperfusion (I/R). Forty micrograms of genomic DNA were size fractionated on a 1.4% agarose gel containing ethidium bromide to qualitatively analyze DNA laddering in the heart. MEK1 transgenic hearts were partially resistant to DNA laddering following the I/R procedure compared with sham hearts. Wild-type–sham, n = 6; wild-type–I/R, n = 11; MEK1-TG–sham, n = 8; MEK1-TG–I/R, n = 13. *P <0.05 compared with Adβgal.
MEK1 transgenic mice show resistance to ischemia/reperfusion-induced apoptosis
MEK1 transgenic mice or wild-type littermate controls were subjected to 45 min occlusion of the left coronary artery followed by 4 h of reperfusion to induce cardiomyocyte apoptosis as described previously (De Windt et al., 2000). Hearts were collected after the ischemia/reperfusion protocol for an assessment of DNA laddering. Hearts from MEK1 transgenic mice showed a qualitative reduction in the degree of DNA laddering compared with hearts from wild-type littermate mice, suggesting less apoptosis (Figure 6C). The data shown are from three wild-type and three MEK1 transgenic hearts, although a total of 21 MEK1 transgenic mice and 17 wild-type mice were analyzed in two separate experiments. In each case, wild-type hearts demonstrated greater DNA laddering than MEK1 transgenic hearts.
Discussion
To investigate the role of MEK1-ERK1/2 signaling as a potential regulator of cardiac hypertrophy in vivo, we generated transgenic mice expressing an activated form of MEK1 in the heart. Transgenic mice expressing MEK1 were characterized by concentric hypertrophy, hyperdynamic function and resistance to apoptotic stimuli. In sharp contrast, numerous other transgenic mice expressing selected signaling factors in the heart have demonstrated lethal cardiomyopathy.
MEK1 expression directs ERK1/2 activation in cardiac myocytes
Despite the wide range in MEK1 transgene copy number (2 to >40), very little variation was observed in ERK1/2 phosphorylation or in the degree to which total ERK1/2 protein was increased in the heart. These data suggest that increased ERK1/2 activity is fully saturated even in low expressing MEK1 lines. The mechanism of this saturation may relate to the manner in which MEK1 activates ERK1/2 through the formation of a large multi-subunit complex in the cytoplasm (reviewed in Garrington and Johnson, 1999). Such a model also explains why high levels of activated MEK1 expression did not result in non-specific activation of p38 or JNK1/2. The observation that even modest overexpression of activated MEK1 promoted robust ERK1/2 phosphorylation is also consistent with the enzymic properties (ultrasensitivity) described for this signaling pathway (Huang and Ferrell, 1996). Another consideration is that the activated MEK1 cDNA (Ser217/221 to Glu) is only ∼1% as active compared with Raf-1 activation of MEK1 in vitro (Cowley et al., 1994). However, since the mutant MEK1 protein is resistant to phosphatase-mediated inactivation, its chronic state of low activity affords significant ERK1/2 phosphorylation (Cowley et al., 1994).
MEK1 transgenic mice display concentric and compensated cardiac hypertrophy
A significant finding of the present study is that ERK1/2 activation in vivo results in long-standing concentric hypertrophy that is associated with enhanced cardiac pump function. Over the years, multiple transgenic models of cardiac hypertrophy have been described. However, to the best of our knowledge, almost all other transgenic models described to date demonstrate a cardiomyopathic state. For example, transgenic overexpression of a dominant-negative Kv4.2 potassium channel in the mouse heart induced concentric hypertrophy with augmented maximum rate of contractility by 3 weeks of age, but by 3 months of age, this index was dramatically reduced and histopathology was observed (Wickenden et al., 1999). In contrast, MEK1 transgenic mice demonstrated a sustained hypertrophic phenotype associated with increased cardiac left ventricular function and no signs of histopathology well into adulthood (Table III). However, it is possible that significantly older MEK1 transgenic mice could still transit into a more cardiomyopathic phenotype, since we only evaluated mice up to 12 months of age.
Intracellular signaling pathways and maladaptive hypertrophy
A large number of transgenic overexpression studies have been published that implicate a wide array of intracellular signaling pathways in the hypertrophic response. As discussed above, almost all of these studies have demonstrated a deleterious or myopathic phenotype associated with transgene overexpression. Overexpression of PKCβ1, Gαq, Gαs, angiotensin type-1 receptor, β2-adrenergic (depending on expression level), β1-adrenergic, Ras, Rac1, RhoA, calcineurin, local-form IGF-1 and NFAT3 have each been associated with the induction of a cardiomyopathic phenotype characterized by hypertrophy, dilation or a mix of both states (Hunter et al., 1995; Iwase et al., 1996; Bowman et al., 1997; D’Angelo et al., 1997; Hein et al., 1997; Wakasaki et al., 1997; Molkentin et al., 1998; Delaughter et al., 1999; Dorn et al., 1999; Engelhardt et al., 1999; Sah et al., 1999; Du et al., 2000; Paradis et al., 2000; Sussman et al., 2000). More recently, transgenic mice overexpressing the MAPKKK signaling factor TAK1 were generated that showed significant p38 activation and dilated hypertrophic cardiomyopathy and post-natal lethality (Zhang et al., 2000). Consistent with this report, our attempts to generate stable transgenic mice expressing activated forms of MKK6 (activates p38) or MKK7 (activates JNK) were unsuccessful because of post-natal lethality associated with dilated cardiomyopathy (O.F.Bueno and J.D.Molkentin, unpublished observations). Collectively, these data support the general notion that reactive signaling pathways are desirable targets for inhibition in the treatment of hypertrophy or cardiomyopathy.
In contrast, very few studies have identified intracellular signaling pathways that are of potential benefit to the heart when activated. Mild overexpression of the β2-adrenergic receptor or a general enhancement of endogenous β-adrenergic receptor activity were each shown to provide protection from cardiomyopathy in Gq transgenic or muscle lim protein (MLP) deleted mice (Rockman et al., 1998; Dorn et al., 1999). In addition, basal signaling through the gp130 receptor was shown to provide cardioprotective signals in vivo (Hirota et al., 1999). These studies have implicated gp130 and mild β2-adrenergic signaling as potentially of benefit to the heart. It will be interesting to determine whether the MEK1–ERK1/2 signaling pathway is involved downstream of gp130 and β2-adrenergic signaling as part of the mechanism of cardioprotection.
While the initial presentation of cardiac hypertrophy is associated with increased risk of future heart failure, hypertrophy itself is not necessarily a disease state. Indeed, persistent aerobic conditioning in animals or human athletes induces a compensated hypertrophy that is thought to be of benefit to the heart (Seals et al., 1994; Woodiwiss and Norton, 1995).
MEK1–ERK1/2 act as an anti-apoptotic signaling pathway
Previous studies have demonstrated that MEK1–ERK activation protects cardiac myocytes from apoptotic stimuli in culture. The anti-apoptotic effects of IGF-1, cardiotrophin 1 or phenylephrine were each shown to require ERK1/2 activation (Parrizas et al., 1997; Sheng et al., 1997; De Windt et al., 2000). Furthermore, ERK1/2 signaling was shown to attenuate oxidation- or daunomycin-induced cardiomyocyte apoptosis in vitro (Aikawa et al., 1997; Adderley and Fitzgerald, 1999; Zhu et al., 1999).
While previous reports have shown that stress- or agonist-induced ERK1/2 activation is associated with protection from apoptosis, it was not known whether direct ERK1/2 activation promoted cytoprotection. While the data presented here suggest that ERK1/2 activation is cardioprotective, the downstream mechanism of this protection is unknown. Indeed, of the reports demonstrating a cardioprotective role for MEK1–ERK1/2 signaling in the face of serum deprivation or oxidative damage, only cyclooxygenase-2 was implicated as a downstream mediator of protection (Adderley and Fitzgerald, 1999).
The observation that MEK1–ERK1/2 activation induces compensated hypertrophy, while p38 and JNK probably induce a cardiomyopathy, is consistent with the phenotype of Ras-expressing transgenic mice. Overexpression of constitutively active Ras mutant in the mouse heart was shown to induce cardiac hypertrophy with a myopathic phenotype (Hunter et al., 1995). Ras is a direct upstream activator of Raf-1, which in turn leads directly to MEK1 and ERK1/2 activation in the heart. However, Ras activation also leads to the activation of the other MAPK signaling branches (reviewed in Vojtek and Der, 1998). Indeed, Ras transgenic mice were shown to have significant JNK activation in the heart (Ramirez et al., 1997). The MEK1 transgenic mice have a similar profile of concentric hypertrophy to that demonstrated in Ras-expressing transgenic mice, but unlike the Ras mice, MEK1 mice appear to maintain a compensated state without increased mortality. These observations support the notion that JNK and p38 activation in the heart are associated with cardiomyopathy and might constitute potential targets for pharmacologic intervention, while ERK1/2 activation appears to be involved in a beneficial form of cardiac hypertrophy that could be advantageous to a failing or dilated myocardium.
Materials and methods
Primary cardiomyocyte cultures
Cardiomyocyte cultures were prepared from 1- to 2-day-old rat pups as described previously (Taigen et al., 2000). Dissected ventricles were placed in an isotonic salt solution containing 116 mM NaCl, 5.4 mM KCl, 20 mM HEPES, 0.9 µM Na2HPO4, 5.4 mM MgSO4, 5 mM glucose. The ventricular tissue was then subjected to multiple rounds of enzymic digestion using 84 U/ml collagenase type I (Worthington, Lakewood, NJ) and 0.05% pancreatin (Sigma, St Louis, MO). After isolation, cardiomyocytes were cultured overnight in M199 medium supplemented with 15% FBS, penicillin/streptomycin (100 U/ml) and l-glutamine (2 mM). The following day, medium was removed and cardiomyocytes were cultured in serum-free medium supplemented with Nutridoma (Boehringer Mannheim, Indianapolis, IN). Cardiomyocytes were seeded at a pre-adherent density of 0.5–1.0 and 1.5–2.5 million cells per dish on gelatinized 6- and 10-cm culture dishes, respectively. Dishes were incubated overnight at 37°C in a humidified incubator with 5% CO2.
Immunocytochemistry
Cardiomyocytes were processed for immunofluorescence as described previously (De Windt et al., 2000; Taigen et al., 2000). Fixed cells were incubated with anti-α-actinin antibody (Sigma) and anti-ANF polyclonal antiserum (Peninsula Laboratories, San Carlos, CA) in blocking buffer for 1 h at dilutions of 1:800 and 1:400, respectively. Secondary antibodies were combinations of TRITC- and fluorescein isothiocyanate (FITC)-conjugated goat anti-mouse and goat anti-rabbit, respectively, at a dilution of 1:400. Nuclear staining was performed with 0.5 µg/ml bisbenzimide. For quantification of ANF expression, cardiomyocytes showing intense perinuclear staining were counted from 15 randomly selected fields. For quantification of cell surface area, α-actinin-labeled cardiomyocytes were scanned with a confocal microscope and surface area was measured using a Sun system workstation. At least 100 cardiomyocytes in 15–25 fields were examined.
Plasmid construction
The expression vector for the activated form of MEK1 (Ser217/221 to Glu) was kindly provided by Dr C.J.Marshall (Cowley et al., 1994). To generate recombinant adenovirus expressing MEK1, XhoI sites were generated at both ends of the MEK1 cDNA using PCR and the product was subcloned into a SalI site of pACCMVpLpA (Gomez-Foix et al., 1992) or the 5.5 kb murine cardiac α-MHC promoter construct (from Dr Jeffrey Robbins).
Replication-deficient adenovirus production
The construction, characterization and procedures of cardiomyocyte infections with replication-deficient adenovirus were performed as described previously (De Windt et al., 2000). Briefly, the pACCMVpLpA-MEK1 plasmid was co-transfected with pJM17 in HEK293 cells (Gomez-Foix et al., 1992). The adenovirus was plaque purified, expanded and titered by detection of the formation of visible plaques in HEK293 monolayers. Cardiomyocyte cultures were infected at a m.o.i. of 50 p.f.u./cell in 2 ml (6 cm plate) or 5 ml (10 cm plate) of Dulbecco’s modified Eagle’s medium (DMEM) (Gibco-BRL, Rockville, MD) supplemented with 2% FBS for 2 h at 37°C in a humidified incubator with 5% CO2. Subsequently, the cells were cultured in serum-free M199 medium for an additional 24 h prior to treatment.
SDS–PAGE and immunoblot analysis
Protein samples were prepared from cultured cardiomyocytes or heart tissue using extraction buffer (Taigen et al., 2000). Samples containing 50–100 µg were thawed on ice, diluted 1:1 with Laemmli loading buffer, subjected to electrophoresis on SDS–polyacrylamide gels and transferred onto Hybond-P membranes (Amersham Pharmacia Biotech, Buckinghamshire, UK). Membranes were then blocked for 2 h at room temperature with 5% non-fat milk in TBST (0.1% Tween 20, 10 mM Tris pH 7.5, 150 mM NaCl) followed by overnight incubation with primary antiserum at 4°C. Chemiluminescent detection was performed with Vistra ECF (Amersham) and scanned utilizing a Storm 860 (Molecular Dynamics, Sunnyvale, CA). Antibodies included: MEK1/2, phospho-ERK1/2, phospho-p38, p38, phospho-JNK (New England Biolabs, Beverly, MA), JNK (Santa Cruz Biotechnology, Santa Cruz, CA) and ERK1/2 (Transduction Laboratories, Lexington, KY). The phospho-JNK antibody that was used also recognizes phospho-ERK1 (p44), so a phospho-ERK1 peptide was co-incubated to eliminate cross-reactivity when phospho-JNK1/2 blots were performed.
RT–PCR mRNA and leucine incorporation assays
Analysis of mRNA levels for ANF and L7 (ribosomal protein control) from cardiomyocyte cultures was performed by semi-quantitative RT–PCR (Titan one tube RT–PCR; Boehringer Mannheim) in the presence of [γ-32P]dCTP with primers designed to detect rat gene products exactly as described previously (Taigen et al., 2000). The determination of protein synthesis rates in cultured cardiomyocytes by [3H]leucine incorporation has been described previously (Sadoshima et al., 1992). Briefly, cardiomyocytes were infected with adenovirus overnight, pre-incubated with leucine-free RPMI medium for 1 h, followed by incubation with 5 µCi/ml [3H]leucine for 6 h. Plates were washed twice with phosphate-buffered saline, and 10% trichloroacetic acid was added at 4°C and incubated for 60 min to precipitate protein. The precipitate was then washed twice with 95% ethanol and resuspended in 0.5 N NaOH, and incorporated [3H]leucine was measured by scintillation counting.
TUNEL, caspase 3 activation and DNA laddering assay
In situ DNA fragmentation was detected using TUNEL in 7 µm paraffin-embedded tissue sections or in fixed cultured cardiomyocytes using the CardioTACSTM kit from Trevigen (Gaithersburg, MD) according to the manufacturer’s instructions. After the TUNEL procedure, labeled nuclei were visualized by FITC–Extra avidin (Sigma) incubation (1:400 dilution). The total number of nuclei versus the TUNEL-positive nuclei was scored in 15 randomly selected fields. DNA laddering assays were performed from the left ventricles of whole hearts as described previously (Bialik et al., 1997; De Windt et al., 2000). Caspase 3 activation was assayed in cultured cardiomyocytes infected with the indicated adenovirus after 48 h in three individual experiments. The experimental procedures and assay conditions were according to the manufacturer’s instructions (BIOMOL AK-703 kit, BioMol, Plymouth meeting, PA), which uses photometric indication of cleavage of a caspase 3 peptide substrate.
Hypertrophic marker analysis by RNA dot blotting
mRNA levels of hypertrophic molecular markers were quantified by dot blotting as described previously (Jones et al., 1996). Total RNA was extracted from left ventricular tissue using Trizol reagent (Gibco-BRL) according to the manufacturer’s recommendations. The RNA was quantified, denatured and blotted on nitrocellulose filters using a dot-blot filtration manifold (Bio-Rad, Melville, NY). Hybridization signals were quantified using a Storm 860 PhosphorImager and ImageQuant software (Molecular Dynamics). Loading was normalized to glyceraldehyde 3-phosphate dehydrogenase (GAPDH) signal.
Isolated working mouse heart preparation and echocardiography
The isolated ejecting mouse heart preparation and Langendorff preparation used in the present study have been described in detail previously (Gulick et al., 1997). Briefly, hearts were removed from anesthetized mice and a 20 gauge cannula was tied to the aortic stump. Aortic flow was measured with the Transonic Flow Probe Model T206 (Transonic Systems Inc., Ithaca, NY). A silastic fluid-filled catheter was tied into a left pulmonary vein to accommodate regulation of venous return. Temperature was maintained at 37.4°C with water-jacketed catheters and recirculated Krebs–Henseleit solution [118 mM NaCl, 4.7 mM KCl, 2.5 mM CaCl2, 1.2 mM MgSO4, 1.2 mM KH2PO4, 25 mM NaHCO3, 0.5 mM Na-EDTA and 5.5 mM d(+)-glucose] continuously gassed with 95% O2/5% CO2. A Statham pressure transducer was placed in the left ventricle to record pressures. All data were acquired and differentiated using DigiMed Systems Analyzers BPA-2000, HPA-200, HPA-210 and LPA-200 (Micro-Med, Inc., Louisville, KY). A customized software package was subsequently used to calculate the various hemodynamic parameters (Gulick et al., 1997). All animals were received and characterized in a blinded manner.
For echocardiography, MEK1 transgenic mice or littermate wild-type mice at 8 weeks of age were anesthetized with 2% isoflurane and hearts were visualized using a Hewlett Packard Sonos 5500 instrument and a 15 MHz transducer. Cardiac ventricular dimensions were measured on M-mode images at least three times for the number of animals indicated.
Statistical analysis
All results are presented as mean ± standard error of the mean (SEM). All statistical analyses were performed using InStat software (GraphPad Software, San Diego, CA). Differences between experimental groups were analyzed using Student’s t-test. P values <0.05 were considered significant.
Acknowledgments
Acknowledgements
We would like to thank Jon Neumann and the MMD program awarded to the University of Cincinnati for transgenic mouse production and Atsushi Sanbe from Jeffrey Robbin’s laboratory for helping with blood pressure measurements. This work was supported by NIH grants HL69562, HL-62927 and HL52318, and a Pew charitable trust Scholar Award (J.D.M.). L.J.D.W. was supported by a post-doctoral fellowship from the American Heart Association (#9920571V) and O.B was supported by NIH training grant T32 HL07752.
References
- Adderley S.R. and Fitzgerald,D.J. (1999) Oxidative damage of cardiomyocytes is limited by extracellular regulated kinases 1/2-mediated induction of cyclooxygenase-2. J. Biol. Chem., 274, 5038–5046. [DOI] [PubMed] [Google Scholar]
- Aikawa R., Komuro,I., Yamazaki,T., Zou,Y., Kudoh,S., Tanaka,M., Shiojima,I., Hiroi,Y. and Yazaki,Y. (1997) Oxidative stress activates extracellular signal-regulated kinases through Src and Ras in cultured cardiac myocytes of neonatal rats. J. Clin. Invest., 100, 1813–1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bialik S., Geenen,D.L., Sasson,I.E., Cheng,R., Horner,J.W., Evans,S.M., Lord,E.M., Koch,C.J. and Kitsis,R.N. (1997) Myocyte apoptosis during acute myocardial infarction in the mouse localizes to hypoxic regions but occurs independently of p53. J. Clin. Invest., 100, 1363–1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bialik S., Cryns,V.L., Drincic,A., Miyata,S., Wollowick,A.L., Srinivasan,A. and Kitsis,R.N. (1999) The mitochondrial apoptotic pathway is activated by serum and glucose deprivation in cardiac myocytes. Circ. Res., 85, 403–414. [DOI] [PubMed] [Google Scholar]
- Bogoyevitch M.A., Glennon,P.E. and Sugden,P.H. (1993) Endothelin-1, phorbol esters and phenylephrine stimulate MAP kinase activities in ventricular cardiomyocytes. FEBS Lett., 317, 271–275. [DOI] [PubMed] [Google Scholar]
- Bowman J.C., Steinberg,S.F., Jiang,T., Geenen,D.L., Fishman,G.I. and Buttrick,P.M. (1997) Expression of protein kinase C β in the heart causes hypertrophy in adult mice and sudden death in neonates. J. Clin. Invest., 100, 2189–2195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choukroun G., Hajjar,R., Kyriakis,J.M., Bonventre,J.V., Rosenzweig,A. and Force,T. (1998) Role of the stress-activated protein kinases in endothelin-induced cardiomyocyte hypertrophy. J. Clin. Invest., 102, 1311–1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clerk A., Bogoyevitch,M.A., Anderson,M.B. and Sugden,P.H. (1994) Differential activation of protein kinase C isoforms by endothelin-1 and phenylephrine and subsequent stimulation of p42 and p44 mitogen-activated protein kinases in ventricular myocytes cultured from neonatal rat hearts. J. Biol. Chem., 269, 32848–32857. [PubMed] [Google Scholar]
- Clerk A., Michael,A. and Sugden,P.H. (1998) Stimulation of the p38 mitogen-activated protein kinase pathway in neonatal rat ventricular myocytes by the G protein-coupled receptor agonists, endothelin-1 and phenylephrine: a role in cardiac myocyte hypertrophy? J. Cell Biol., 142, 523–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowley S., Paterson,H., Kemp,P. and Marshall,C.J. (1994) Activation of MAP kinase kinase is necessary and sufficient for PC12 differentiation and for transformation of NIH 3T3 cells. Cell, 77, 841–852. [DOI] [PubMed] [Google Scholar]
- D’Angelo D.D., Sakata,Y., Lorenz,J.N., Boivin,G.P., Walsh,R.A., Liggett,S.B. and Dorn,G.W.,II (1997) Transgenic Gαq overexpression induces cardiac contractile failure in mice. Proc. Natl Acad. Sci. USA, 94, 8121–8126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delaughter M.C., Taffet,G.E., Fiorotto,M.L., Entman,M.L. and Schwartz,R.J. (1999) Local insulin-like growth factor I expression induces physiologic, then pathologic, cardiac hypertrophy in transgenic mice. FASEB J., 13, 1923–1929. [DOI] [PubMed] [Google Scholar]
- De Windt L.J., Lim,H.W., Taigen,T., Wencker,D., Condorelli,G., Dorn,G.W.,II, Kitsis,R.N. and Molkentin,J.D. (2000) Calcineurin-mediated hypertrophy protects cardiomyocytes from apoptosis in vitro and in vivo: an apoptosis-independent model of dilated heart failure. Circ. Res., 86, 255–263. [DOI] [PubMed] [Google Scholar]
- Dorn G.W.,II, Tepe,N.M., Lorenz,J.N., Koch,W.J. and Liggett,S.B. (1999) Low- and high-level transgenic expression of β2-adrenergic receptors differentially affect cardiac hypertrophy and function in Gαq-overexpressing mice. Proc. Natl Acad. Sci. USA, 96, 6400–6405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du X.J., Autelitano,D.J., Dilley,R.J., Wang,B., Dart,A.M. and Woodcock,E.A. (2000) β2-adrenergic receptor overexpression exacerbates development of heart failure after aortic stenosis. Circulation, 101, 71–77. [DOI] [PubMed] [Google Scholar]
- Fujio Y., Nguyen,T., Wencker,D., Kitsis,R.N. and Walsh,K. (2000) Akt promotes survival of cardiomyocytes in vitro and protects against ischemia–reperfusion injury in mouse heart. Circulation, 101, 660–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelhardt S., Hein,L., Wiesmann,F. and Lohse,M.J. (1999) Progressive hypertrophy and heart failure in β1-adrenergic receptor transgenic mice. Proc. Natl Acad. Sci. USA, 96, 7059–7064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrington T.P. and Johnson,G.L. (1999) Organization and regulation of mitogen-activated protein kinase signaling pathways. Curr. Opin. Cell Biol., 11, 211–218. [DOI] [PubMed] [Google Scholar]
- Gillespie-Brown J., Fuller,S.J., Bogoyevitch,M.A., Cowley,S. and Sugden,P.H. (1995) The mitogen-activated protein kinase kinase MEK1 stimulates a pattern of gene expression typical of the hypertrophic phenotype in rat ventricular cardiomyocytes. J. Biol. Chem., 270, 28092–28096. [DOI] [PubMed] [Google Scholar]
- Glennon P.E., Kaddoura,S., Sale,E.M., Sale,G.J., Fuller,S.J. and Sugden,P.H. (1996) Depletion of mitogen-activated protein kinase using an antisense oligodeoxynucleotide approach downregulates the phenylephrine-induced hypertrophic response in rat cardiac myocytes. Circ. Res., 78, 954–961. [DOI] [PubMed] [Google Scholar]
- Gomez-Foix A.M., Coats,W.S., Baque,S., Alam,T., Gerard,R.D. and Newgard,C.B. (1992) Adenovirus-mediated transfer of the muscle glycogen phosphorylase gene into hepatocytes confers altered regulation of glycogen metabolism. J. Biol. Chem., 267, 25129–25134. [PubMed] [Google Scholar]
- Gulick J., Hewett,T.E., Klevitsky,R., Buck,S.H., Moss,R.L. and Robbins,J. (1997) Transgenic remodeling of the regulatory myosin light chains in the mammalian heart. Circ. Res., 80, 655–664. [DOI] [PubMed] [Google Scholar]
- Hein L., Stevens,M.E., Barsh,G.S., Pratt,R.E., Kobilka,B.K. and Dzau,V.J. (1997) Overexpression of angiotensin AT1 receptor transgene in the mouse myocardium produces a lethal phenotype associated with myocyte hyperplasia and heart block. Proc. Natl Acad. Sci. USA, 94, 6391–6396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirota H., Chen,J., Betz,U.A., Rajewsky,K., Gu,Y., Ross,J.,Jr, Muller,W. and Chien,K.R. (1999) Loss of a gp130 cardiac muscle cell survival pathway is a critical event in the onset of heart failure during biomechanical stress. Cell, 97, 189–198. [DOI] [PubMed] [Google Scholar]
- Huang C.Y. and Ferrell,J.E.,Jr (1996) Ultrasensitivity in the mitogen-activated protein kinase cascade. Proc. Natl Acad. Sci. USA, 93, 10078–10083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter J.J., Tanaka,N., Rockman,H.A., Ross,J.,Jr and Chien,K.R. (1995) Ventricular expression of a MLC-2v-ras fusion gene induces cardiac hypertrophy and selective diastolic dysfunction in transgenic mice. J. Biol. Chem., 270, 23173–23178. [DOI] [PubMed] [Google Scholar]
- Iwase M. et al. (1996) Adverse effects of chronic endogenous sympathetic drive induced by cardiac GS α overexpression. Circ. Res., 78, 517–524. [DOI] [PubMed] [Google Scholar]
- Jones W.K. et al. (1996) Ablation of the murine myosin heavy chain gene leads to dosage effects and functional deficits in the heart. J. Clin. Invest., 98, 1906–1917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy D., Garrison,R.J., Savage,D.D., Kannel,W.B. and Castelli,W.P. (1990) Prognostic implications of echocardiographically determined left ventricular mass in the Framingham heart study. N. Engl. J. Med., 322, 1561–1566. [DOI] [PubMed] [Google Scholar]
- Molkentin J.D., Lu,J.R., Antos,C.L., Markham,B., Richardson,J., Robbins,J., Grant,S.R. and Olson,E.N. (1998) A calcineurin-dependent transcriptional pathway for cardiac hypertrophy. Cell, 93, 215–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paradis P., Dali-Youcef,N., Paradis,F.W., Thibault,G. and Nemer,M. (2000) Overexpression of angiotensin II type I receptor in cardiomyocytes induces cardiac hypertrophy and remodeling. Proc. Natl Acad. Sci. USA, 97, 931–936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parrizas M., Saltiel,A.R. and LeRoith,D. (1997) Insulin-like growth factor 1 inhibits apoptosis using the phosphatidylinositol 3-kinase and mitogen-activated protein kinase pathways. J. Biol. Chem., 272, 154–161. [DOI] [PubMed] [Google Scholar]
- Post G.R., Goldstein,D., Thuerauf,D.J., Glembotski,C.C. and Brown,J.H. (1996) Dissociation of p44 and p42 mitogen-activated protein kinase activation from receptor-induced hypertrophy in neonatal rat ventricular myocytes. J. Biol. Chem., 271, 8452–8457. [DOI] [PubMed] [Google Scholar]
- Ramirez M.T., Sah,V.P., Zhao,X.L., Hunter,J.J., Chien,K.R. and Brown,J.H. (1997) The MEKK–JNK pathway is stimulated by α1-adrenergic receptor and ras activation and is associated with in vitro and in vivo cardiac hypertrophy. J. Biol. Chem., 272, 14057–14061. [DOI] [PubMed] [Google Scholar]
- Rockman H.A., Chien,K.R., Choi,D.J., Iaccarino,G., Hunter,J.J., Ross,J.,Jr, Lefkowitz,R.J. and Koch,W.J. (1998) Expression of a β-adrenergic receptor kinase 1 inhibitor prevents the development of myocardial failure in gene-targeted mice. Proc. Natl Acad. Sci. USA, 95, 7000–7005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadoshima J.-I., Jahn,L., Takahashi,T., Kulik,T.J. and Izumo S. (1992) Molecular characterization of the stretch-induced adaptation of cultured cardiac cells. J. Biol. Chem., 267, 10551–10560. [PubMed] [Google Scholar]
- Sah V.P., Minamisawa,S., Tam,S.P., Wu,T.H., Dorn,G.W.,II, Ross,J.,Jr, Chien,K.R. and Brown,J.H. (1999) Cardiac-specific overexpression of RhoA results in sinus and atrioventricular nodal dysfunction and contractile failure. J. Clin. Invest., 103, 1627–1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seals D.R., Hagberg,J.M., Spina,R.J., Rogers,M.A., Schechtman,K.B. and Ehsani,A.A. (1994) Enhanced left ventricular performance in endurance trained older men. Circulation, 89, 198–205. [DOI] [PubMed] [Google Scholar]
- Sharma P.M., Egawa,K., Huang,Y., Martin,J.L., Huvar,I., Boss,G.R. and Olefsky,J.M. (1998) Inhibition of phosphatidylinositol 3-kinase activity by adenovirus-mediated gene transfer and its effect on insulin action. J. Biol. Chem., 273, 18528–18537. [DOI] [PubMed] [Google Scholar]
- Sheng Z., Knowlton,K., Chen,J., Hoshijima,M., Brown,J.H. and Chien,K.R. (1997) Cardiotrophin 1 (CT-1) inhibition of cardiac myocyte apoptosis via a mitogen-activated protein kinase-dependent pathway. Divergence from downstream CT-1 signals for myocardial cell hypertrophy. J. Biol. Chem., 272, 5783–5791. [DOI] [PubMed] [Google Scholar]
- Silberbach M., Gorenc,T., Hershberger,R.E., Stork,P.J., Steyger,P.S. and Roberts,C.T.,Jr (1999) Extracellular signal-regulated protein kinase activation is required for the anti-hypertrophic effect of atrial natriuretic factor in neonatal rat ventricular myocytes. J. Biol. Chem., 274, 24858–24864. [DOI] [PubMed] [Google Scholar]
- Sugden P.H. and Clerk,A. (1998) ‘Stress-responsive’ mitogen-activated protein kinases (c-Jun N-terminal kinases and p38 mitogen-activated protein kinases) in the myocardium. Circ. Res., 24, 345–352. [DOI] [PubMed] [Google Scholar]
- Sussman M.A., Welch,S., Walker,A., Klevitsky,R., Hewett,T.E., Price,R.L., Schaefer,E. and Yager,K. (2000) Altered focal adhesion regulation correlates with cardiomyopathy in mice expressing constitutively active rac1. J. Clin. Invest., 105, 875–886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taigen T., De Windt,L.J., Lim,H.W. and Molkentin,J.D. (2000) Targeted inhibition of calcineurin prevents agonist-induced cardiomyocyte hypertrophy. Proc. Natl Acad. Sci. USA, 97, 1196–1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorburn J., Frost,J.A. and Thorburn,A. (1994) Mitogen-activated protein kinases mediate changes in gene expression, but not cytoskeletal organization associated with cardiac muscle cell hypertrophy. J. Cell Biol., 126, 1565–1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vojtek A.B. and Der,C.J. (1998) Increasing complexity of the Ras signaling pathway. J. Biol. Chem., 273, 19925–19928. [DOI] [PubMed] [Google Scholar]
- Wakasaki H., Koya,D., Schoen,F.J., Jirousek,M.R., Ways,D.K., Hoit,B.D., Walsh,R.A. and King,G.L. (1997) Targeted overexpression of protein kinase C β2 isoform in myocardium causes cardiomyopathy. Proc. Natl Acad. Sci. USA, 94, 9320–9325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickenden A.D., Lee,P., Sah,R., Huang,Q., Fishman,G.I. and Backx,P.H. (1999) Targeted expression of a dominant-negative Kv4.2 K+ channel subunit in the mouse heart. Circ. Res., 85, 1067–1076. [DOI] [PubMed] [Google Scholar]
- Widmann C., Gibson,S., Jarpe,M.B. and Johnson,G.L. (1999) Mitogen-activated protein kinase: conservation of a three-kinase module from yeast to human. Physiol. Rev., 79, 143–180. [DOI] [PubMed] [Google Scholar]
- Woodiwiss A.J. and Norton,G.R. (1995) Exercise-induced cardiac hypertrophy is associated with an increased myocardial compliance. J. Appl. Physiol., 78, 1303–1311. [DOI] [PubMed] [Google Scholar]
- Yamazaki T. et al. (1993) Mechanical loading activates mitogen-activated protein kinase and S6 peptide kinase in cultured rat cardiac myocytes. J. Biol. Chem., 268, 12069–12076. [PubMed] [Google Scholar]
- Zechner D., Thuerauf,D.J., Hanford,D.S., McDonough,P.M. and Glembotski,C.C. (1997) A role for the p38 mitogen-activated protein kinase pathway in myocardial cell growth, sarcomeric organization and cardiac-specific gene expression. J. Cell Biol., 139, 115–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang D., Gaussin,V., Taffet,G.E., Belaguli,N.S., Yamada,M., Schwartz,R.J., Michael,L.H., Overbeek,P.A. and Schneider,M.D. (2000) TAK1 is activated in the myocardium after pressure overload and is sufficient to provoke heart failure in transgenic mice. Nature Med., 6, 556–563. [DOI] [PubMed] [Google Scholar]
- Zhu W. et al. (1999) MAPK superfamily plays an important role in daunomycin-induced apoptosis of cardiac myocytes. Circulation, 100, 2100–2107. [DOI] [PubMed] [Google Scholar]
- Zou Y., Komuro,I., Yamazaki,T., Aikawa,R., Kudoh,S., Shiojima,I., Hiroi,Y., Mizuno,T. and Yazaki,Y. (1996) Protein kinase C, but not tyrosine kinases or Ras, plays a critical role in angiotensin II-induced activation of Raf-1 kinase and extracellular signal-regulated protein kinases in cardiac myocytes. J. Biol. Chem., 271, 33592–33597. [DOI] [PubMed] [Google Scholar]