Abstract
Pancreatic ductal adenocarcinoma (PDAC) is the most common pancreatic malignancy with a dismal prognosis. Developing novel strategies to prevent/delay pancreatic cancer is currently of intense interest. The chemopreventive efficacy of gefitinib, an epidermal growth factor receptor (EGFR) inhibitor, was evaluated on the progression of pancreatic intraepithelial neoplasms (PanINs) to PDAC in conditional LSL-KrasG12D/+ transgenic mice. LSL-KrasG12D/+ and p48Cre/+ mice were bred and off-spring of activated KrasG12D/+ were generated. Six-week old male KrasG12D/+ (20/group) and C57BL/6 wild-type (12/group) mice were fed (AIN-76A) diets containing 0, 100, and 200 ppm gefitinib for 35 weeks. At termination, pancreases were evaluated histopathologically for PanINs and PDAC, and various biomarkers were measured by IHC, immunofluorescence, immunoblotting and/or RT-PCR. Dietary gefitinib at 100 and 200 ppm significantly suppressed PDAC incidence by 77 and 100%, respectively, (p<0.0001) when compared to control diet. Importantly, significant inhibition of carcinoma and a dose-dependent suppression of PanINs (PanIN1 37 – 62%,p<0.002, PanIN2 38–41,p<0.001, and PanIN3 7–34%, p<0.0141) was observed in mice treated with gefitinib. Furthermore, 100 and 200 ppm gefitinib treated mice exhibited 67.6–77.3% of the pancreas to be free from ductal lesions. Also, gefitinib reduced EGFR, PCNA, Cyclin D1, C2GNT, RhoA, β-catenin, p38, pERK, caveolin-1, and mucin, and increased cyclin B1 in the pancreatic lesions/PDAC. In summary, these results demonstrate that gefitinib can prevent pancreatic cancer precursor lesions progression to PDAC in a preclinical model. The present study highlights the promise of chemoprevention and the potential usefulness of EGFR inhibitors in high-risk individuals for pancreatic cancer.
Keywords: EGFR inhibitor, Gefitinib, Chemoprevention, Pancreatic cancer, Kras G12D/+ mice
Introduction
Pancreatic carcinoma is a devastating malignancy with a dismal prognosis, characterized by low responsiveness to conventional chemotherapies, and proving fatal to nearly all who are diagnosed. It is responsible for approximately 260,000 deaths per year worldwide with most of the deaths occurring in developed countries (150,000 annually), (1) and is the fourth leading cause of cancer related deaths in USA. Despite advances in the field of molecular genetics in human pancreatic cancers, the identification of various putative molecular targets associated with the development of targeted therapies has not yet translated to an improved overall patients’ survival (2). The current standard of care for advanced pancreatic ductal adenocarcinoma (PDAC) is infusional gemcitabine, a deoxycytidine analog and inhibitor of nucleic acid synthesis, which prolongs survival by only a few weeks and provides symptomatic improvement in a minority of patients (3). PDAC is generally believed to arise predominantly through progression of pancreatic intraepithelial neoplasia (PanIN), ranging from low-grade PanINs (termed PanIN-1A, -1B) to high-grade PanINs (termed PanIN-2, -3), to ductal adenocarcinoma (4). The preclinical study of PanINs has recently been made possible by the generation of genetically modified animal models, which recapitulate human PanINs on a genetic and histomorphologic level (5). PDAC is characterized by a high frequency of Kras mutations at early stages and the accumulations over time of multiple additional genetic abnormalities (6).
To understand the biology of pancreatic cancer precursor lesions and to discover early detection markers, recent research interest in this field has adopted models and approaches to identify risk factors for the development and inhibition of pancreatic cancers. The conditional KrasG12D/+ model, first described by Hingorani et al. (7), is considered a very valuable tool to study PanIN biology. Mice harboring conditional Kras mutant allele (Kras-LSL.G12D/+) in combination with a pancreas-specific Cre recombinase transgene (p48Cre/+) develop a full range of premalignant lesions in the pancreas, termed pancreatic intraepithelial neoplasia, before succumbing to invasive PDAC and other tumors at late ages (7–10). These mice are an excellent model of PanIN development and are useful for studying tumor progression. Importantly, these mice also serve as a valuable model to evaluate and identify the potential chemopreventive agents which can significantly suppress the progression of PanINs to PADC.
Overexpression of EGF and EGFR has been observed in various malignancies, including carcinomas of the pancreas (11–13), stomach (14) and liver (15), as well as tumors of the brain (16) and is involved in tumor proliferation, survival, metastasis, and induction of angiogenesis. In addition, signaling through EGFR promotes tumor neovascularization and induces resistance to cytotoxic chemotherapy (17). Based on these multiple effects on cancer, the EGFR tyrosine kinase has been recognized as an attractive molecular target for selective treatment of solid tumors with increased EGFR expression levels. Stimulation of EGFR results in activation of multiple intracellular signaling cascades that increase cellular proliferation and prevent programmed cell death (18). The ATP competitive kinase inhibitor gefitinib (Iressa, ZD1839) was the first EGFR-directed small-molecule drug that received approval for the treatment of non – small cell lung cancer (19). Gefitinib is an orally active and selective EGFR-TKI (EGFR-tyrosine kinase inhibitor) that blocks signal transduction pathways responsible for the proliferation and survival of cancer cells, and other host-dependent processes that promote cancer growth. In clinical and preclinical animal models, gefitinib has been shown to be an effective therapeutic agent towards cancers of the lung, breast, colon, prostate, head and neck and other organ sites when administered as a single agent or in combination with other chemotherapeutic agents (20–32). Potential beneficial effects of EGFR inhibitors such as gefitinib on survival of pancreatic cancer patients has been limited (33,34). However, the potential usefulness in the chemoprevention setting has not been established for EGFR inhibitors and/or other molecularly targeted agents. Thus, this study is the first to investigate the chemopreventive effects of gefitinib on PanINs progression to PDAC and on expression of important biomarkers of progression using the conditional LSL-KrasG12D/+ mouse model.
Materials and Methods
Animals, diets and care
All animal experiments were done in accordance with the institutional guidelines of American Council of Animal Care. Breeder pairs of LSL-KrasG12D/+ and p48Cre/+ in the C57BL/6 genetic background were obtained from Dr. Howard Crawford at the University of New York, at Stony Brook, NY. Required quantities of activated KrasG12D/+ mice were generated as described below. Animals were housed in ventilated cages under standardized conditions (21°C, 60% humidity, 12-h light/12-dark cycle, 20 air changes/hour) in the University of Oklahoma Health Sciences Center rodent barrier facility. Semi-purified modified AIN-76A diet ingredients were purchased from the Bioserv, Inc., NJ. The selective EGFR inhibitor gefitinib was procured from the NCI chemoprevention drug repository. Gefitinib (100 and 200 ppm) was premixed with small quantities of casein and then blended in to the diet using a Hobart Mixer. Both control and experimental diets were prepared weekly and stored in the cold room. Agent content in the experimental diets was determined periodically in multiple samples taken from the top, middle, and bottom portions of individual diet preparations to verify uniform distribution. Mice were allowed ad libitum access to the respective diets and to automated tap water purified by reverse osmosis.
Breeding and Genotyping analysis
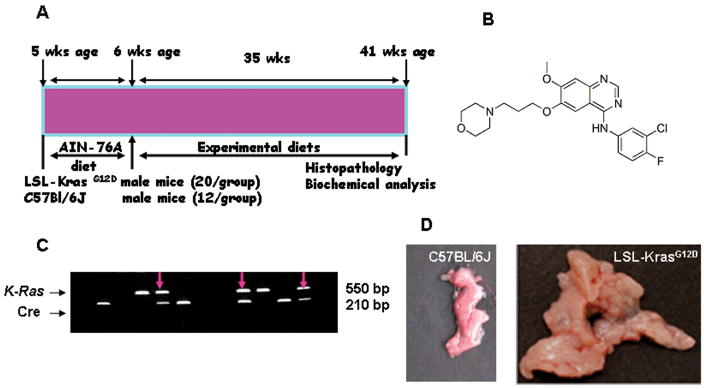
LSL-KrasG12D/+ and p48Cre/+ mice were maintained in a C57BL/6 heterozygous genetic background. LSL-KrasG12D/+ and p48cre/+ mice were bred and the offspring of activated male KrasG12D/+ were generated at required quantities. The genetic background of each pup was confirmed by tail DNA extraction and PCR as described elsewhere (6). Briefly, genomic DNA was extracted from snap-frozen tail tissue samples using the mini-prep kit (Invitrogen). PCR was performed for K-ras and Cre using the following conditions, denaturation at95°C for 5′, followed by 35 cycles at 95°C for 1′, 60°C for 1′, and 72°C for 1′. Oligonucleotide primer sequences used were as follows: K-ras 5′-CCTTTACAAGCGCACGCAGAG-3′ sense, 5′-AGCTAGCCACCATGGCTTGAGTAAGTCTGCA-3′ anti-sense; and Cre 5′-ACCGTCAGTACGTGAGATATCTT-3′ sense and 5′-ACCTGAAGATGTTCGCGATTATCT-3′ antisense. PCR products were separated on a 2% agarose gel. Successful recombination yields are 550 and 210-bp products.
Bioassay: Chemopreventive efficacy of gefitinib
Genotyped male KrasG12D/+ transgenic mice were used in the efficacy study. The experimental protocol is summarized in Fig. 1A. Five week old mice were selected and randomized so that average body weights in each group were equal (n=20/group p48Cre/+/LSL-KrasG12D/+ mice and n=12 C57BL/6 wild-type mice) and were fed with AIN-76A diet for one week. At 6 weeks of age, mice were fed with control or experimental diets containing 0 ppm, 100 ppm or 200 ppm gefitinib in the diet until termination of the study. Mice were routinely checked for signs of weight loss or any signs of toxicity, any abnormalities. Food intake and body weight of each animal were measured once weekly for first 6 weeks and then once a month till termination. After 35 weeks (~9 months) on experimental diets, all mice were euthanized by CO2 asphyxiation and necropsied, pancreases were collected, weighed and snap frozen in liquid nitrogen for further analysis. Pancreases (head to tail) that required histopathologic and IHC evaluation to identify PanIN lesions and PDAC for evaluation of various molecular markers were fixed in 10% neutral-buffered formalin.
Figure 1.
A. Experimental design for chemopreventive efficacy evaluation of gefitinib in male p48cre/+-LSL-KrasG12D/+ mice. At 6-weeks of age groups (20/group activated KrasG12D/+ or 12/group wild-type) of mice were fed experimental diets containing 0, 100 or 200 ppm gefitinib continuously for 35 weeks and each pancreas were evaluated histopathologically and various marker expressions, as described in the text. B. Structure of EGFR inhibitor, gefitinib; C. Genotyping of p48cre/+ and LSL-KrasG12D/+ offspring by PCR; and D. Pancreas of wild-type and activated KrasG12D/+ mice at 41 weeks of age. As shown in the Figure 1D, KrasG12D/+ mice pancreas was increased in size, weight and thickness compared to normal mice pancreas.
Histologic evaluation
Formalin-fixed, paraffin-embedded tissues were sectioned (4 μm) and stained with H&E. Twenty sections of each pancreas were histologically evaluated by a pathologist blinded to the experimental groups. PanIN lesions and carcinoma were classified according to histopathologic criteria as recommended elsewhere (5). To quantify the progression of PanIN lesions, the total number of ductal lesions and their grade were determined. Pancreatic ducts of the entire fixed specimen (head, body, and tail of the pancreatic sections) were analyzed for each animal. The relative proportion of each PanIN lesion to the overall number of analyzed ducts was recorded for each animal. Similarly, pancreatic carcinoma and normal appearing pancreatic tissue were evaluated for all the animals.
Immuno-histochemistry and Immunoflourescence histochemistry
The effects of gefitinib on expression of proliferating cell nuclear antigen (PCNA), β-catenin, EGFR and Cav-1 were evaluated by immunohistochemistry (IHC) and/or immunofluorescence histochemistry (IFHC). Briefly, for IHC of PCNA, β-catenin, EGFR and Cav-1, paraffin sections were deparaffinized in xylene, rehydrated through graded ethanol solutions and washed in PBS. Antigen retrieval was carried out by heating sectionsin 0.01M citrate buffer (pH 6) for 30 minutes in a boiling water bath. Endogenous peroxidase activity was quenched by incubation in 3% H2O2 in PBS for 5 minutes. Nonspecific binding sites were blocked using Protein Block for 20 minutes. Sections were then incubated overnight at 4°C with 1:300 dilutions of monoclonal antibodies against PCNA, β-catenin, EGFR and Cav-1 (AbCam/Santa Cruz Biotechnology, CA). After several washes with PBS, the slides were incubated with appropriate secondary antibody for two hours and then washed and incubated with avidin biotin-complex reagent (Zymed Laboratories). After rinsing with PBS, the slides were incubated with the chromogen 3, 3″-diaminobenzidine (DAB) for three minutes, then rinsed and counterstained with hematoxylin… Substituted non-immune rabbit immunoglobulins for primary antibodies were used as negative controls. For IFHC, after overnight incubation with primary antibody, the slides were rinsed thrice with PBS for 5 min and then were incubated with secondary antibody tagged with FITC/TRITC in dark for one hour. Slides were then washed with PBS for 5 min thrice in dark room and incubated with 0.5μg/mL DAPI for 5 min. Slides were rinsed with PBS and observed for fluorescence under FITC/TRITC filters using Olympus microscope IX701 and digital computer images were recorded by Olympus DP70 camera.
Alcian blue staining for mucinous PanINs
The effect of gefitinib on mucin secretion in the pancreas was evaluated by alcian blue staining. Briefly, paraffin sections were deparaffinized in xylene, rehydratedthrough graded ethanol solutions as described above and stained with alcian blue for 5 min and then washed with PBS for 5 min. Sections were counterstained with aqueous neutral red, washed with distilled water and mounted with a cover slip. Mucin staining (blue in color) was imaged using an Olympus IX 70 microscope as described above.
RT-PCR for p38, C2GNT, Cyclin D1, Cyclin B1 mRNA expression
Total RNA from pancreas samples was extracted using the Totally RNA™ Kit (Ambion) as per the manufacturer’s instructions. Equal quantities of DNA-free RNA were used in reverse transcription reactions for making cDNA using SuperScript™ reverse transcriptase (Invitrogen). PCR reactions were performed for p38, C2GNT, Cyclin D1 and Cyclin B1 using the following conditions. For p38, denaturation at94°C for 3min, followed by 35 cycles at 94°C for30 seconds, 60°C for 20 seconds, and 72°C for 45 seconds. Oligonucleotide primer sequences used for p38 were: 5′-TCCTCAGGGTTGCCCTTGCCA-3′ sense, 5′-TATGTGCAGCCGCCCTCCCT3′ anti-sense. For C2GNT, denaturation at 94°C for 2 min, followed by 30 cycles at 94°C for30 seconds, 50°C for 30 seconds, and 72°C for 1 min and 15 seconds. Oligonucleotide primer sequences used for C2GNT were as follows: 5′-AGAGGAAGACGCCGCCACCT-3′ sense, 5′-CGCTGGAGGGTGGCCCAAAG-3′ anti-sense. For Cyclin B1, denaturation at 94°C for 2 min, followed by 35 cycles at 94°C for 30 seconds, 58°C for 30 seconds, and 72°C for 45 seconds. Oligonucleotide primer sequences used for cyclin B1 gene were as follows: 5′-ACTTCCTCCGTAGAGCATC-3′ sense, 5′-GCAGAGTTGGTGTCCATTC-3′ anti-sense. For Cyclin D1, denaturation at94°C for 3 min, followed by 35 cycles at 94°C for 30 seconds, 60°C for 20 seconds, and 72°C for 45 seconds. Oligonucleotide primer sequences used for cyclin D1 gene were as follows: 5′-ATGGAACACCAGCTCCTGTG-3′ sense, 5′-ACCTCCAGCATCCAGGTGGC-3′ anti-sense. PCR was done using the Taq polymerase, 10 mM dNTP, and buffers from Invitrogen. The PCR products were visualized and photographed under UV illumination.
Western-blot analysis of protein expression
Pancreases harvested from mice fed with or without gefitinib were homogenized and lysed in ice cold lysis buffer [50 mmol/L Tris (pH 7.4), 150 mmol/L NaCl, 1 mmol/L EDTA, 1% NP40, 50 mmol/L NaF, 1 mmol/L sodium orthovanadate, 1 mmol/L phenylmethylsulfonyl fluoride, 1 mmol/L DTT, and protease inhibitor cocktail]. After a brief vortexing, the lysates were separated by centrifugation at 12,000 × g for 15 minutes at 4°C, and protein concentrations were measured by the Bio-Rad Protein Assay reagent (Hercules, CA). An aliquot (50 μg protein/lane) of the total protein was separated by 10% SDS-PAGE and transferred to nitrocellulosemembranes. After blocking with 5% milk powder, membranes were probed for expression of RhoA, pERK, PCNA and β-catenin in hybridizing solution [1:500, in TBS-Tween 20 solution] using respective primary antibodies (Santa Cruz Biotechnology, Santa Cruz, CA), and then probed with HRP conjugated secondary antibodies. Detection was performed using the SuperSignal® West Pico Chemiluminescence procedure (Pierce, Rockford, IL). The bands were captured on Ewen Parker, Blue sensitive X-ray films.
Statistical analysis
The data are presented as mean ± SE. Differences in body weights were analyzed by ANOVA. Statistical differences between control and treated groups were evaluated using unpaired t-test with Welch’s correction. Differences between groups are considered significant at p<0.05.
Results
General observations
KrasG12D/+ mice fed the control and experimental diets had steady body weight gains. At the end of the experiment, wild-type mice had slightly higher body weight gains (p>0.05) in comparison to the KrasG12D/+ mice. No significant body weight changes were observed within the treatment groups and the control group during the course of the study (Fig 2A). None of the animals fed the experimental diets exhibited any observable toxicity or any gross changes attributable to liver, kidney, or lung toxicity with notable difference in the pancreatic weights as described below.
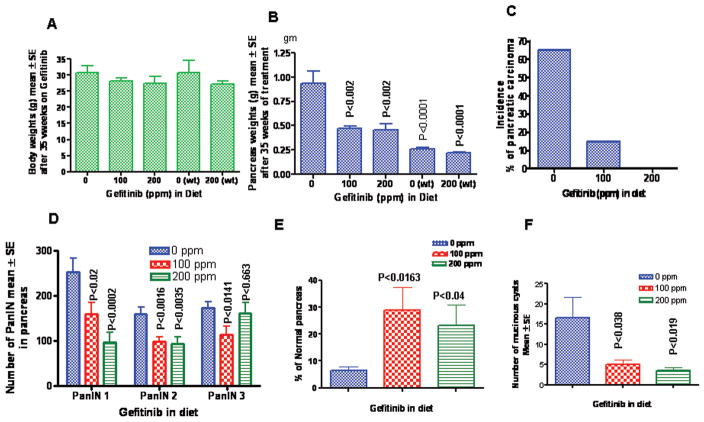
Figure 2.
A. Effect of gefitinib on the body weight gain (Mean±SE, N=20 or 12). No significant differences were observed between the KrasG12D/+ or wild-type mice treated with gefitinib. B. Pancreatic weights (Mean±SE, N=20 or 12) at the termination of the experiment and significant differences between the wild-type and KrasG12D/+ or treatment groups were analyzed by unpaired “t”-test with Welch’s correction C. Effect of gefitinib on the incidence (percentage of mice with carcinomas) of pancreatic ductal adenocarcinoma. Significance in the incidence was analyzed by Fisher’s exact test. Effect of gefitinib on the PanINs multiplicity (Mean±SE) (Fig. D); and percentage of normal pancreas (Fig. E) and number of mucinous cysts (Fig. F). Fig. D–F, significance were analyzed by unpaired ‘t’-test with Welch’s correction, values are considered statistically significant p<0.05.
Dietary administration of gefitinib significantly inhibited PDAC and delayed the progression of -PanIN lesions to PDAC in Kras G12D/+ mice
KrasG12D/+ mice spontaneously develop pancreatic cancer arising through progression of PanINs, ranging from low-grade PanINs (1A and 1B) to high-grade PanINs (PanIN-2, -3). C57BL/6 wild-type mice fed with control diet or experimental diets containing gefitinib showed no evidence of PanIN lesions or carcinoma (data not shown). The efficacy endpoints used in this study were inhibition of PanINs and PDAC. At the termination of the experiment, pancreases were collected and weighed. Pancreases from C57BL/6 wild-type mice fed control or experimental diets weighed about 0.24 (0.21–0.26) gms and did not significantly differ (Fig 2B). However, pancreases of control diet-fed KrasG12D/+ mice weighed 0.95 (0.72–1.4) gms, almost 4.1-fold higher than the wild-type mice pancreas. Whereas a significant decrease in pancreas weights (>50%, p<0.002) was observed in KrasG12D/+ mice fed with gefitinib diet (Fig 2B). Fig 2C summarizes the chemopreventive efficacy of gefitinib on PDAC incidence in KrasG12D/+ mice that were fed control diet with a 65% incidence (percentage of mice with PDAC). Whereas 100 ppm gefitinib-fed mice showed only a 15% incidence (p<0.0001) of PDAC, while 200 ppm gefitinib-fed mice had no evidence of carcinoma by histological analysis. Also, control diet-fed KrasG12D/+ mice developed, on the average, about 253 PanIN1, 159 PanIN2 and 173 PanIN3 lesions, whereas dietary administration of 100 and 200 ppm gefitinib for 35 weeks showed significant inhibition of PanIN 1, 2 and 3 lesions [PanIN1, 37.2–61.6% (p<0.02–0.002); PanIN2 38.4–41.0 (p<0.0016–0.0035); and PanIN3 34 – 7.0% (p<0.014–0.663) respectively (Fig 2D)]. Although a dose-dependent decrease in the incidence of PanIN1 lesions was observed, however, such dose-response effects were not observed for the PanIN2 and 3 lesions (Fig 2D). Furthermore, 100 and 200 ppm gefitinib-fed mice exhibited 67 and 77%, (respectively), normal appearing (free from the PanINs and PDAC) pancreatic tissue in comparison to control group (Fig 2E).
Inhibition of PCNA (Proliferating cell nuclear antigen), β-catenin, EGFR and Cav-1 expression in PanINs and carcinoma of pancreas by gefitinib
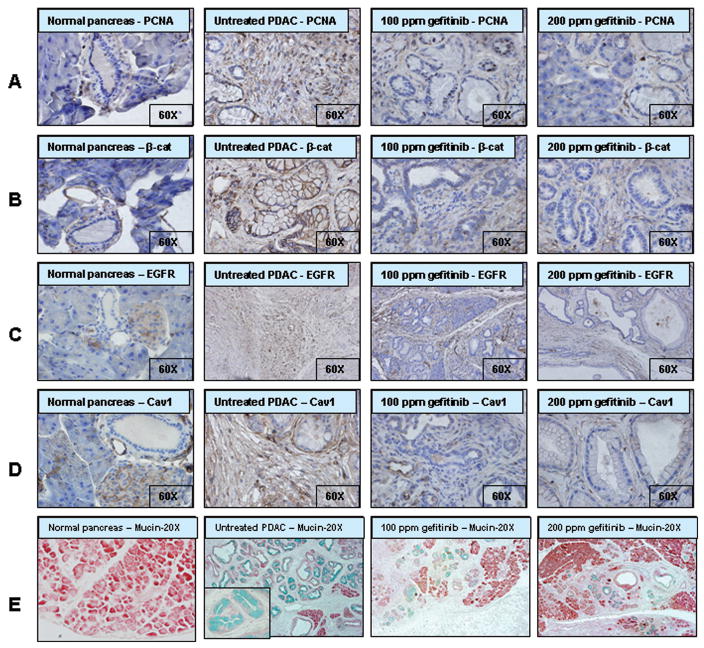
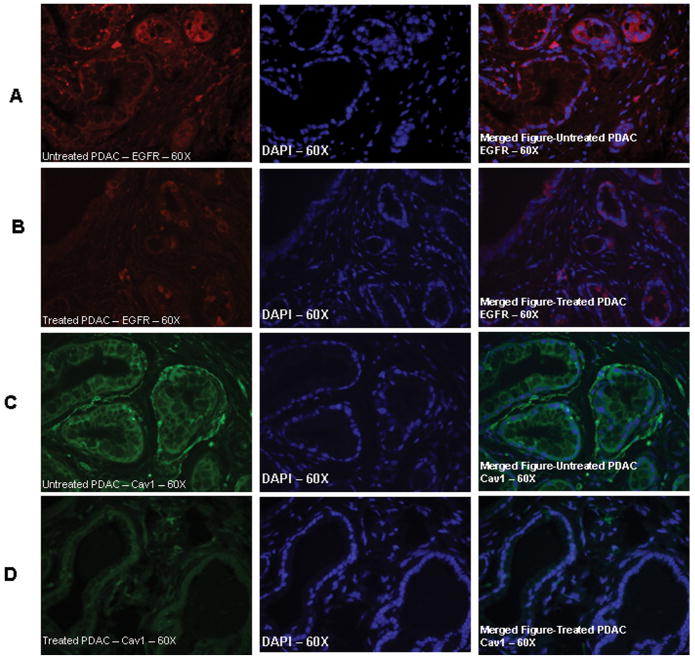
Fig. 3A summarizes the effects of gefitinib on tumor cell proliferation as measured by PCNA overexpression. Qualitative microscopic examination of PCNA-stained sections showed a substantial decrease in PCNA-positive cells in the pancreas of gefitinib treated mice compared with untreated controls. As shown in Fig 3, we observed over-expression of β-catenin, EGFR and Cav-1 in PanIN lesions and PDAC of KrasG12D/+ mice in comparison to the normal pancreases of C57BL/6 wild-type mice. As shown in Fig 2B, minimal to no expression of nuclear accumulation of β-catenin was observed in the pancreatic ductal lesions of KrasG12D/+ mice fed with gefitinib diets when compared to control diet fed KrasG12D/+.mice Similarly, expression levels of EGFR and Cav-1 were significantly suppressed by gefitinib treatment. Also, administration of 100 and 200 ppm gefitinib diet resulted a significant decrease in EGFR and Cav-1 protein expression levels in pancreatic lesions as compared to pancreas of mice fed control diet. Furthermore, IFHC analysis revealed that EGFR and Cav-1 expression was primarily localized to cell membrane (Fig 4A and C). Importantly, membrane expression of EGFR and Cav-1 was significantly reduced in mice fed 200 ppm gefitinib diet as shown in Fig 4B and 4D.
Figure 3.
Effect of gefitinib on cell proliferation and targeted markers in pancreatic tumors. Immunohistochemical analysis of proliferating cell nuclear antigen (PCNA) (A), β-catanin (B); EGFR (C), and Caveolin-1 (D) expressions in normal pancreatic tissues and ductal adenocarcinomas. Immunoblotting was performed with paraffin embed and micro-sectioned pancreatic tissues as described in the methods section. Fig 3E, Effect of gefitinib on the inhibition of PanINs thereby mucin expression (blue color) in pancreatic tissues by alcin blue staining method.
Figure 4.
Effect of gefitinib on EGFR and caveolin-1 expression in pancreatic ductal adenocarcinoma, localization is shown by immuno-flourescence histochemical method as described in the text. A. EGFR expression in the PDAC of KrasG12D/+ mice fed with control diet. As shown in the merged Figure A (right) EGFR expression is localized to cell membrane and in gefitinib (100 ppm) treated mouse pancreatic lesion showed significantly decreased levels of EGFR levels. Fig 4C, depicts the caveolin-1 expression in PDAC in control diet fed mice and mostly localized to membrane and in gefitinib treated mice PDAC had a limited expression.
Inhibition of PCNA, β-catenin, RhoA, pERK, p38, Cyclin D1 and Cycin B1 expression
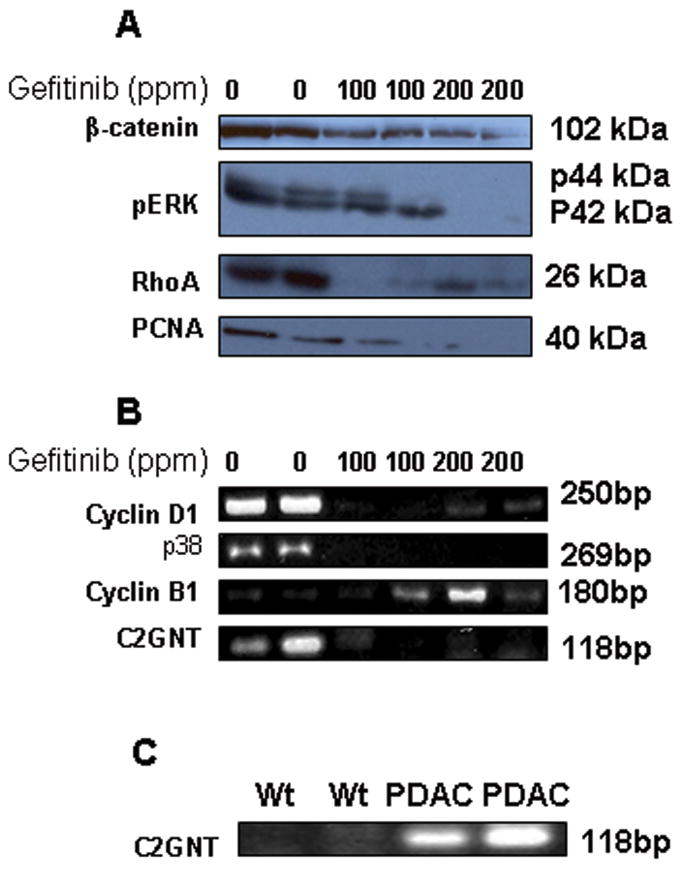
Western blot analysis demonstrated that pancreatic tissues from gefitinib-fed mice exhibited significantly reduced expression of PCNA and β-catenin compared to control diet-fed mice (Fig 5A). These results further confirm the immunohistological observations described above. In addition, Rho A and pERK, which are known to be upregulated due to the Kras mutations in various malignancies, are down-regulated in the pancreases of mice fed gefitinib compared to control diet (Fig 5A). Similarly, significant down-regulation of p38 mRNA expression was observed in the pancreatic tumor tissues of gefitinib-fed mice compared to control diet-fed mice. Also a significant decrease of Cyclin D1 and an increase in Cyclin B1 mRNA were observed in gefitinib fed mice (Fig 5B).
Figure 5.
A. Effect of gefitinib on protein expression levels of β-catenin, p-ERK, Rho A and PCNA. Protein expression was analyzed by Western blot analysis as described in the text. B. Effect of gefitinib in the mRNA expression levels of Cyclin D1, p38, Cyclin B1 and C2GNT by RT-PCR method. C. Expression levels of C2GNT mRNA levels in the normal pancreas of wild-type and PDAC of KrasG12D/+ mice.
Inhibition of mucin production and C2GNT expression in pancreatic lesions
Alcian blue staining demonstrated very high mucin expression levels in PanIN lesions of 10 month old KrasG12D/+ mice, whereas such expression was not detected in normal pancreas (Fig 3E). This was further confirmed by RT-PCR analysis of C2GNT levels (Fig 5C) in pancreatic tissues. Significant inhibition in the number of mucinous cysts/glands was observed in gefitinib treated mice (Fig 2F and 3E, 69–79%, p<0.04–0.02). Similarly, this was consistent with a significant decrease in mucin and C2GNT expression levels with gefitinib treatment in KrasG12D/+ mice pancreas in comparison to the control diet fed mice pancreas (Fig 5B).
DISCUSSION
Substantial efforts in the molecular genetics of human PDAC have delineated a number of common genetic alterations. Among the most common alterations in PDAC are activating mutations in the Kras proto-oncogene, and such mutations are found in almost 95% of human pancreatic cancer cases (35). Although other common alterations involve inactivation of tumor suppressor genes: p16INK4a (90% of cases), p53 (approximately 75% of cases), SMAD4 (55%), and BRCA2 (10%) (36), these alterations accumulate with increasing frequency as PanINs progress to PDAC (37). Results from our efficacy study clearly suggest that an early intervention with an EGFR inhibitor, gefitinib, significantly suppresses the PanINs lesions and their progression to PDAC in this pancreatic cancer model. The results have important implications for the human pancreatic cancer chemoprevention.
The role of EGFR in pancreatic tumor promotion is well established (38,39). In pancreatic cancer patients, EGFR over-expression is detected in approximately 90% of clinical specimens (38,39). Thus, targeting EGFR with small molecule inhibitors has lead to development of clinically successful agents like gefitinib and erlotinib. Studies have been shown that EGFR inhibitors are promising for the treatment of pancreatic cancer when they are combined with gemcitabine (40). Like many chemotherapeutic agents, the effectiveness of EGFR inhibitors on survival in advanced stage PDAC patients has been modest (41). However, there are several lines of evidence that gefitinib inhibits EGFR in various cancers in different mouse models (42–45) Since dysregulation of EGFR-signaling may occur during progression of PanINs, application of EGFR inhibitors may be more effective in suppressing the PDAC, and the present study clearly support this concept. A strong dose-responsive protective effect of gefitinib against Kras driven progression of PanINs to PDAC was observed in the present study. This is the first study to validate pronounced chemopreventive beneficial effects of EGFR-inhibitors in a transgenic mouse model of pancreatic cancer.. To our knowledge there is only one report on chemoprevention of PanIN lesions. This study used a selective COX-2 inhibitor nimesulide to inhibit PanIN lesions in the Kras transgenic mice, however the number of mice used in this study were only six (46).
Development of pancreatic lesions and their progression to PDAC is complex. It is well known that the endogenous mouse models show very few and less extensive acinar-ductal metaplasia (ADM) compared to the acinar promoter models which are characterized by prominent and extensive acinar-ductal metaplasia (5,47) In the present study we have utilized endogenous Kras mouse model and have evaluated the pancreases after over 10 months age where we did not find statistically significant number of ADM. However, there were very few infrequent ADMs (acinar to ductal metaplasia) and MMLs (mucinous metaplastic lesions) seen in untreated p48cre/+-LSL-KrasG12D/+ mice pancreas. Moreover, our histopathological observations suggest that mucinous ducts were rare. suggesting, there was no clear evidence of acini becoming metaplastic ducts. Almost all the ducts present seemed to come from the periacinar ducts that normally exist.
To understand the possible mechanism(s) of PanINs progression to PDAC and the inhibitory effects of gefitinib, we analyzed a number of key molecular markers associated with EGFR pathway and pancreatic tumor growth (48–52). Higher expression levels of EGFR were associated with Cav-1 expression and positively correlated with histological grade and advanced tumor stage. Clinical studies have shown that Cav-1 expression is associated with pancreatic tumor progression and poor prognosis for patient survival (51). In the present study, over-expression of Cav-1 was corroborated with proliferation markers and several of the down-stream signal molecules, such as RhoA, pERK and the MAPK signaling molecule p38. The high expression levels of proliferation markers PCNA, β-catenin and cyclin D1 observed in KrasG12D/+ mice pancreatic lesions were significantly inhibited by dietary gefitinib in correlation with EGFR and Cav-1 expressions. EGFR and Cav-1 co-expression levels are known to regulate a number of signaling pathways including PI3K-AKt and mitogen-activated pathways (MAPK) that contribute to the progression and development of PDAC (52). Our results support previous observations that oncogenic Kras enhances progression of pancreatic ductal cells to a malignant phenotype through the activation of MAPK and RhoA. Also, a dose dependent decrease in cyclin D1 and increase in cyclin B1 was observed in the pancreas of gefitinib fed Kras G12D/+ mice. These results suggest that the cells are arrested in G2/M phase, which is similar to pancreatic cancer cell growth inhibition by FTI inhibitor, L-744,832 (53).
There is a strong relationship between the risk of pancreatic carcinoma with the presence of combinations of K-ras gene mutation, papillary growth, and expression of mucins (54). In the present study, gefitinib treatment not only decreased the number of PanIN lesions, but also significantly decreased the expression of mucin and a mucin producing enzyme in these lesions.
In summary, this study demonstrated for the first time that blockade of the EGFR signalling pathway in the pancreatic cancer mouse model by dietary gefitinib, exerts significant chemopreventive efficacy in the inhibition of PanIN formation and their progression to PDAC. Inhibition of PanINs and PDAC by gefitinib is associated with significant suppression of tumor cell proliferation, mucin biosynthesis and multiple signaling pathways, such as AKT, Rho A, MAPK, and Wnt, tumor cell proliferation and mucin biosynthesis. The above results and similarity of this pancreatic mouse model to human premalignant and malignant pancreatic lesions highlight the potential application of EGFR pathway inhibitors in preventing and delaying the progression of pancreatic cancer in clinical setting.
Acknowledgments
We thank the University of Oklahoma Health Sciences Center Rodent Barrier Facility staff. We thank Dr. Doris Mangiaracina Benbrook for editing this manuscript. This work was in part supported by National Cancer Institute CN-N01-53300.
References
- 1.American Cancer Society. Cancer Facts and Figures 2007. Atlanta (GA): 2007. [Google Scholar]
- 2.Eckel F, Schneider G, Schmid RM. Pancreatic cancer: a review of recent advances. Expert Opin Investig Drugs. 2006;15:1395–410. doi: 10.1517/13543784.15.11.1395. [DOI] [PubMed] [Google Scholar]
- 3.Burris HA, Moore MJ, Andersen J, et al. Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: a randomized trial. J Clin Oncol. 1997;15:2403–13. doi: 10.1200/JCO.1997.15.6.2403. [DOI] [PubMed] [Google Scholar]
- 4.Hruban RH, Adsay NV, Albores-Saavedra J, et al. Pathology of genetically engineered mouse models of pancreatic exocrine cancer: consensus report and recommendations. Cancer Res. 2005;66:95–106. doi: 10.1158/0008-5472.CAN-05-2168. [DOI] [PubMed] [Google Scholar]
- 5.Hruban RH, Adsay NV, bores-Saavedra J, et al. Pathology of genetically engineered mouse models of pancreatic exocrine cancer: consensus report and recommendations. Cancer Res. 2006;66:95–106. doi: 10.1158/0008-5472.CAN-05-2168. [DOI] [PubMed] [Google Scholar]
- 6.Tuveson DA, Hingorani SR. Ductal pancreatic cancer in humans and mice. Cold Spring Harb Symp Quant Biol. 2005;70:65–72. doi: 10.1101/sqb.2005.70.040. [DOI] [PubMed] [Google Scholar]
- 7.Hingorani SR, Petricoin EF, Maitra A, et al. Preinvasive and invasive ductal pancreatic cancer and its early detection in the mouse. Cancer Cell. 2003;4:437–450. doi: 10.1016/s1535-6108(03)00309-x. [DOI] [PubMed] [Google Scholar]
- 8.Kawaguchi B, Cooper M, Gannon M, et al. The role of the transcriptional regulator Ptf1a in converting intestinal to pancreatic progenitors. Nat Genet. 2002;32:128–134. doi: 10.1038/ng959. [DOI] [PubMed] [Google Scholar]
- 9.Jackson EL, Willis N, Mercer K, et al. Analysis of lung tumor initiation and progression using conditional expression of oncogenic K-ras. Genes Dev. 2001;15:3243–3248. doi: 10.1101/gad.943001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Olive KP, Tuveson DA. The Use of Targeted Mouse Models for Preclinical Testing of Novel Cancer Therapeutics. Clin Cancer Res. 2006;15:5277–5287. doi: 10.1158/1078-0432.CCR-06-0436. [DOI] [PubMed] [Google Scholar]
- 11.Pryczynicz A, Guzin K, Ska-ustymowicz, Kemona A, Ewska JC. Expression of EGF and EGFR Strongly Correlates with Metastasis of Pancreatic Ductal Carcinoma. Anticancer Res. 2004;28:1399–1404. [PubMed] [Google Scholar]
- 12.Durkin AJ, Bloomston PM, Rosemurgy AS, et al. Defining the role of the epidermal growth factor receptor in pancreatic cancer grown in vitro. Am J Surg. 2003;186:431–36. doi: 10.1016/j.amjsurg.2003.07.008. [DOI] [PubMed] [Google Scholar]
- 13.Korc M, Chandrasekar B, Yamanaka Y, Friess H, Buchier M, Beger HG. Overexpression of the epidermal growth factor receptor in human pancreatic cancer is associated with concomitant increases in the levels of epidermal growth factor and transforming growth factor alpha. J Clin Invest. 1992;90:1352–1360. doi: 10.1172/JCI116001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Takemura S, Yashiro M, Sunami T, Tendo M, Hirakawa K. Novel models for human scirrhous gastric carcinoma in vivo. Cancer Sci. 2004;95:893–900. doi: 10.1111/j.1349-7006.2004.tb02199.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Borlak J, Meier T, Halter R, Spanel R, Spanel-Borowski L. Epidermal growth factor-induced hepatocellular carcinoma: gene expression profiles in precursor lesions, early stage and solitary tumours. Oncogene. 2005;24:1809–19. doi: 10.1038/sj.onc.1208196. [DOI] [PubMed] [Google Scholar]
- 16.Held-Feindt J, Lutjohann B, Ungefroren H, Mehdorn HM, Mentlein R. Interaction of transforming growth factor-beta (TGF-beta) an epidermal growth factor (EGF) in human glioma cells. J Neurooncol. 2003;63:117–27. doi: 10.1023/a:1023943405292. [DOI] [PubMed] [Google Scholar]
- 17.Li D, Xie K, Wolff R, Abbruzzese JL. Pancreatic cancer. Lancet. 2004;363:1049–57. doi: 10.1016/S0140-6736(04)15841-8. [DOI] [PubMed] [Google Scholar]
- 18.Bruns CJ, Solorzano CC, Habrison MT, et al. Blockade of epidermal growth factor receptor signaling by a novel tyrosine kinase inhibitor leads to apoptosis of endothelial cells and therapy of human pancreatic carcinoma. Cancer Res. 2000;60:2926–35. [PubMed] [Google Scholar]
- 19.Brehmer D, Greff Z, Godl K, et al. Cellular Targets of Gefitinib. Cancer Res. 2005;65(2):379–82. [PubMed] [Google Scholar]
- 20.Cohen EEW, Rosen F, Stadler WM, et al. Phase II trial of ZD1839 in recurrent or metastatic squamous cell carcinoma of the head and neck. J Clin Oncol. 2003;21:1980–87. doi: 10.1200/JCO.2003.10.051. [DOI] [PubMed] [Google Scholar]
- 21.Wirth LJ, Haddad RI, Wieczorek TJ, et al. Phase I study of gefitinib plus celecoxib in patients with metastatic and/or locally recurrent squamous cell carcinoma of the head and neck (SCCHN) Proc Am Soc Clin Oncol. 2004;23:496. doi: 10.1200/JCO.2005.02.4182. [DOI] [PubMed] [Google Scholar]
- 22.Albain K, Elledge R, Gradishar WJ, et al. Open-label, phase II, multicenter trial of ZD1839 (‘Iressa’) in patients with advanced breast cancer. Breast Cancer Res Treat. 2002;76:33. [Google Scholar]
- 23.Baselga J, Albanell J, Ruiz A, et al. Phase II and tumor pharmacodynamic study of gefitinib (ZD1839) in patients with advanced breast cancer. Proc Am Soc Clin Oncol. 2003;22:7. doi: 10.1200/JCO.2005.08.326. [DOI] [PubMed] [Google Scholar]
- 24.Gee JMW, Harper ME, Hutcheson IR, et al. The antiepidermal growth factor receptor agent gefitinib (ZD1839/Iressa) improves antihormone response and prevents development of resistance in breast cancer in vitro. Endocrinology. 2003;144:5105–17. doi: 10.1210/en.2003-0705. [DOI] [PubMed] [Google Scholar]
- 25.Okubo S, Kurebayashi J, Otsuki T, Yamamoto Y, Tanaka K, Sonoo H. Additive antitumour effect of the epidermal growth factor receptor tyrosine kinase inhibitor gefitinib (Iressa, ZD1839) and the antioestrogen fulvestrant (Faslodex, ICI 182,780) in breast cancer cells. Br J Cancer. 2004;90:236–44. doi: 10.1038/sj.bjc.6601504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shou J, Massarweh S, Osborne CK, et al. Mechanisms of tamoxifen resistance: increased estrogen receptor-HER2/neu cross-talk in ER/HER2-positive breast cancer. J Natl Cancer Inst. 2004;96:926–35. doi: 10.1093/jnci/djh166. [DOI] [PubMed] [Google Scholar]
- 27.McKillop D, Raab G, Eidtmann H, et al. Intratumoral and plasma concentrations of gefitinib (‘Iressa’) in breast cancer patients: preliminary results from a presurgical investigatory study (BCIRG 103) J Clin Oncol. 2004;22(Suppl 14S):581. [Google Scholar]
- 28.Fisher GA, Kuo T, Cho CD, et al. A phase II study of gefitinib in combination with FOLFOX-4 (IFOX) in patients with metastatic colorectal cancer. Proc Am Soc Clin Oncol. 2004;23:249. [Google Scholar]
- 29.Doi T, Koizumi W, Siena S, et al. Efficacy, tolerability, and pharmacokinetics of gefitinib (‘Iressa’, ZD1839) in pretreated patients with metastatic gastric cancer. Proc Am Soc Clin Oncol. 2003;22:258. [Google Scholar]
- 30.Barker AJ, Gibson KH, Grundy W, et al. Studies leading to the identification of ZD1839 (Iressa ): an orally active, selective epidermal growth factor receptor tyrosine kinase inhibitor targeted to the treatment of cancer. Bioorg Med Chem Lett. 2001;11:1911–14. doi: 10.1016/s0960-894x(01)00344-4. [DOI] [PubMed] [Google Scholar]
- 31.Feng FY, Lopez CA, Normolle DP, et al. Effect of epidermal growth factor receptor inhibitor class in the treatment of head and neck cancer with concurrent radiochemotherapy in vivo. Clin Cancer Res. 2007;15:2512–8. doi: 10.1158/1078-0432.CCR-06-2582. [DOI] [PubMed] [Google Scholar]
- 32.Matar P, Rojo F, Cassia R, et al. Combined Epidermal Growth Factor Receptor Targeting with the Tyrosine Kinase Inhibitor Gefitinib (ZD1839) and the Monoclonal Antibody Cetuximab (IMC-C225). Superiority Over Single-Agent Receptor Targeting. Clinical Cancer Res. 2004;10:6487–501. doi: 10.1158/1078-0432.CCR-04-0870. [DOI] [PubMed] [Google Scholar]
- 33.Czito BG, Willett CG, Bendell JC, et al. Increased toxicity with gefitinib, capecitabine, and radiation therapy in pancreatic and rectal cancer: phase I trial results. J Clin Oncol. 2006;24:656–62. doi: 10.1200/JCO.2005.04.1749. [DOI] [PubMed] [Google Scholar]
- 34.Maurel J, Martin-Richard M, Conill C, et al. Phase I trial of gefitinib with concurrent radiotherapy and fixed 2-h gemcitabine infusion, in locally advanced pancreatic cancer. Int J Radiat Oncol Biol Phys. 2006;66:1391–8. doi: 10.1016/j.ijrobp.2006.07.008. [DOI] [PubMed] [Google Scholar]
- 35.Smit VT, Boot AI, Smits AM, et al. KRAS codon mutations occur very frequently in pancreatic adenocarcinomas. Nucleic Acids Res. 1998;16:7773–82. doi: 10.1093/nar/16.16.7773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hezel AF, Kimmelman AC, Stanger BZ, et al. Genetics and biology of pancreatic ductal adenocarcinoma. Genes & Dev. 2006;20:1218–49. doi: 10.1101/gad.1415606. [DOI] [PubMed] [Google Scholar]
- 37.Hruban RH, Wilentz RE, Kern SE. Genetic progression in the pancreatic ducts. Am J Pathol. 2000;156(6):1821–5. doi: 10.1016/S0002-9440(10)65054-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wagner M, Greten FR, Weber CK, et al. Genes Dev. 2001;15:286–293. doi: 10.1101/gad.184701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wagner M, Weber CK, Bressau F, et al. Transgenic overexpression of amphiregulin induces a mitogenic response selectively in pancreatic duct cells. Gastroenterology. 2002;122:1898–12. doi: 10.1053/gast.2002.33594. [DOI] [PubMed] [Google Scholar]
- 40.Steven DL. Mouse models of pancreatic cancer:The fur is finally flying! Cancer Cell. 2004;5:7–11. doi: 10.1016/s1535-6108(03)00337-4. [DOI] [PubMed] [Google Scholar]
- 41.Miksad RA, Schnipper L, Goldstein M. Does a statistically significant survival benefit of erlotinib plus gemcitabine for advanced pancreatic cancer translate into clinical significance and value? J Clin Oncol. 2007;25:4506–07. doi: 10.1200/JCO.2007.13.0401. [DOI] [PubMed] [Google Scholar]
- 42.Ishii Y, Fujimoto S, Fukuda T. Gefitinib Prevents Bleomycin-induced Lung Fibrosis in Mice. Am J Res Critical Care Med. 2006;174:550–556. doi: 10.1164/rccm.200509-1534OC. [DOI] [PubMed] [Google Scholar]
- 43.Hattori K, Iida K, Joraku A, Tsukamoto S, Akaza H, Oyasu R. Chemopreventive effects of cyclooxygenase-2 inhibitor and epidermal growth factor-receptor kinase inhibitor on rat urinary bladder carcinogenesis. B J U Int. 2006;97:640–3. doi: 10.1111/j.1464-410X.2006.06053.x. [DOI] [PubMed] [Google Scholar]
- 44.Ohashi K, Takigawa N, Osawa M, et al. Chemopreventive Effects of Gefitinib on Nonsmoking-Related Lung Tumorigenesis in Activating Epidermal Growth Factor Receptor Transgenic Mice. Cancer Res. 2009;69:7088–95. doi: 10.1158/0008-5472.CAN-08-4205. [DOI] [PubMed] [Google Scholar]
- 45.Schiffer E, Housset C, Cacheux W, et al. Gefitinib, an EGFR inhibitor prevents hepatocellular carcinoma development in the rat liver with cirrhosis. Hepatology. 2005;41:307–14. doi: 10.1002/hep.20538. [DOI] [PubMed] [Google Scholar]
- 46.Funahashi H, Satake M, Dawson D, et al. Delayed Progression of Pancreatic Intraepithelial Neoplasia in a Conditional KrasG12D Mouse Model by a Selective Cyclooxygenase-2 Inhibitor. Cancer Res. 2007;67(15):7068–71. doi: 10.1158/0008-5472.CAN-07-0970. [DOI] [PubMed] [Google Scholar]
- 47.Zhu L, Shi G, Schmidt CM, Hruban RH, Konieczny SF. Acinar Cells Contribute to the Molecular Heterogeneity of Pancreatic Intraepithelial Neoplasia. Am J Pathol. 2007;171:263–73. doi: 10.2353/ajpath.2007.061176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tanase CP, Dima S, Mihai M, et al. Caveolin-1 overexpression correlates with tumour progression markers in pancreatic ductal adenocarcinoma. J Mol Hist. 2009;40:23–29. doi: 10.1007/s10735-008-9209-7. [DOI] [PubMed] [Google Scholar]
- 49.Witkiewicz AK, Nguyen K, Dasgupta A, et al. Co-expression of fatty acid synthase and caveolin-1 in pancreatic ductal adenocarcinoma: Implications for tumor progression and clinical outcome. Cell Cycle. 2008;7(19):3021–25. doi: 10.4161/cc.7.19.6719. [DOI] [PubMed] [Google Scholar]
- 50.Muslimov GF. Role of Epidermal Growth Factor Gene in the Development of Pancreatic Cancer and Efficiency of Inhibitors of This Gene in the Treatment of Pancreatic Carcinoma. Bulletin of Exp Biol and Med. 2008;145:4. doi: 10.1007/s10517-008-0135-1. [DOI] [PubMed] [Google Scholar]
- 51.Suzuoki M, Miyamoto M, Kato K, et al. Impact of caveolin-1 expression on prognosis of pancreatic ductal adenocarcinoma. British J Cancer. 2002;87:1140–44. doi: 10.1038/sj.bjc.6600619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Agnieszka K, Witkiewicz, Nguyen KH, et al. Co-expression of fatty acid synthase and caveolin-1 in pancreatic ductaladenocarcinoma. Cell Cycle. 2008;7(19):3021–25. doi: 10.4161/cc.7.19.6719. [DOI] [PubMed] [Google Scholar]
- 53.Song SY, Meszoely IM, Coffey RJ, Pietenpol JA, Leach SD. K-Ras-independent effects of the farnesyl transferase inhibitor L-744,832 on cyclin B1/Cdc2 kinase activity, G2/M cell cycle progression and apoptosis in human pancreatic ductal adenocarcinoma cells. Neoplasia. 2000;2(3):261–72. doi: 10.1038/sj.neo.7900088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Matsubayashi H, Watanabe H, Nishikura K, Ajioka Y, Kijima H, Saito T. Determination of pancreatic ductal carcinoma histogenesis by analysis of mucous quality and K-ras mutation. Cancer. 1998;82(4):651–60. doi: 10.1002/(sici)1097-0142(19980215)82:4<651::aid-cncr6>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]