Abstract
Previous studies have demonstrated that the galanin system modulates responses to drugs of abuse such as morphine. The current study examined whether genetic deletion of galanin could affect the locomotor and reinforcing effects of cocaine in mice. We examined spontaneous motor activity and cocaine-induced hyperactivity in wild-type (GAL-WT) and knockout mice lacking galanin (GAL-KO) maintained on the 129/OlaHsd background. Our results indicate that cocaine enhanced locomotion (defined as moving more than 5 cm) dose-dependently in GAL-WT and GAL-KO mice. However, general activity (total beam breaks) was increased by cocaine only in GAL-WT mice. An additional experiment indicated that galnon, a non-selective galanin receptor agonist, did not affect cocaine-induced hyperactivity. In a second set of experiments, mice of both genotypes were trained to self-administer cocaine under a fixed ratio schedule and tested with various doses of cocaine under different schedules of reinforcement. This set of experiments showed that cocaine self-administration did not differ markedly between genotypes. However, while GAL-WT mice acquired cocaine self-administration, a median split analysis showed that mice could be divided into large and small drug takers, whereas all GAL-KO mice were small drug takers. Our results indicate that wild-type and galanin knockout mice on a congenic 129/OlaHsd background are responsive to the locomotor effects of cocaine and can acquire i.v. cocaine self-administration. However, the phenotype observed in GAL-KO mice does not support a major role for galanin in cocaine-induced hyperlocomotion and self-administration.
Keywords: Galanin, dopamine, addiction, reward, locomotion, 129/OlaHsd
Introduction
There has been increasing interest in investigating the function of various orexigenic neuropeptides in drug reward. Intrahypothalamic administration of orexin and melanin concentrating hormon (MCH) strongly stimulate feeding behaviors in rodents. Interestingly, both neuropeptides modulate the activity of the mesolimbic dopamine system and can alter the rewarding and hyperlocomotor effects of cocaine (España et al., 2010; Borgland, Taha, Sarti, Fields, & Bonci, 2006; Tyhon et al., 2006; Chung et al., 2009). For example, it has recently been shown that the orexin 1 receptor antagonist, SB-334867, decreased cocaine self-administration when it was administered either peripherally or within the ventral tegmental area (VTA) (España et al., 2010). Moreover, pharmacological blockade of the MCH system using the MCH1R antagonist TPI 1361–17 also reduced cocaine self-administration behaviors (Chung et al., 2009). While orexin and MCH have been suggested to mediate the rewarding effects of cocaine, it has been proposed that galanin, another orexigenic neuropeptide that promotes feeding behaviors when injected into the hypothalamus, exerts an inhibitory function on drug reward, potentially through actions on the mesolimbic dopamine system (Picciotto, Brabant, Einstein, Kamens, & Neugebauer, 2010; Robinson & Brewer, 2008).
In addition to its role in food consumption, galanin affects seizure activity, nociception, depression and anxiety. These behavioral effects have been attributed to the ability of galanin to inhibit neuronal firing and release of various neurotransmitters, including norepinephrine (NE), serotonin (5-HT) and acetylcholine (ACh). Galanin acts through three G protein-coupled receptor subtypes: GalR1, GalR2 and GalR3, all of which can activate Gi and Go proteins (Lang, Gundlach, and Kofler, 2007). GalR1 and GalR3 seem to be similar in their signaling and their activation appears to induce inhibitory responses in the brain. Both receptors can decrease cyclic AMP levels and activate the GIRK family of potassium channels. In contrast, GalR2 receptors are associated with Gq proteins (Wang, Hashemi, Fried, Clemmons, & Hawes, 1998) and can increase phospholipase C and activate PKC (Mahoney et al., 2003; Hawes, Narasimhaiah & Picciotto, 2006).
Many studies have demonstrated functional interactions between galanin and the mesolimbic dopamine system. Galanin reduces stimulation-evoked dopamine release in rat striatal slices through a mechanism involving Gi proteins (Tsuda, Tsuda, Nishio, Masuyama, & Goldstein, 1998). Moreover, intraventricular administration of galanin decreases dopamine synthesis in the striatum, nucleus accumbens and olfactory turbercles and reduces locomotor activity in rats and mice (Ericson & Ahlenius, 1999; Mitsukawa, Lu, Bartfai, 2009; Weiss et al., 2005). Therefore, it has been suggested that the galanin system plays an inhibitory role in drug reward. Consistent with this hypothesis, central administration of galanin decreases the development of conditioned place preference induced by morphine in mice (Zachariou, Parikh, & Picciotto, 1999). Furthermore, knockout mice lacking galanin show increased conditioned place preference for morphine (Hawes et al., 2008) and cocaine (Narasimhaiah, Kamens, & Picciotto, 2009).
The aim of the present study was to examine whether the galanin system attenuates the hyperlocomotor effects and operant responding behaviors maintained by cocaine. To this end, cocaine-induced motor activity and cocaine self-administration behaviors were examined in knockout mice lacking galanin (GAL-KO) maintained on the 129/OlaHsd background. Since the motor effects of cocaine have been reported to be atypical in this mouse strain (Kuzmin, Johansson, Fredholm, & Ogren, 2000), the effects of galnon, a non-selective galanin agonist, were also tested on cocaine-induced hyperactivity in wild-type C57BL/6J mice. This additional experiment allowed us to examine whether changes in cocaine reactivity observed in GAL-KO could have been influenced by the 129/OlaHsd background or by developmental changes resulting from the genetic mutation (Stephens, Mead, & Ripley, 2002) rather than by the loss of galanin alone. Galnon is a low molecular weight, non-peptide galanin agonist that non-selectively activates all three galanin receptors (Saar et al., 2002). This synthetic ligand crosses the blood-brain barrier and affects a number of physiological functions in C57BL6J mice, including seizure activity and feeding behavior. These effects of galnon are blocked by co-administration of the galanin receptor antagonist M35, suggesting that the effects of galnon are mediated through galanin receptors (Saar et al., 2002; Abramov et al., 2004). Since galnon has been shown to induce behavioral changes in C57BL/6J mice through activation of galanin receptors, the effects of galnon on cocaine-induced hyperactivity in this mouse strain can help to determine whether the effects of cocaine in GAL-KO mice are influenced by mouse strain or adaptation to the constitutive mutation.
Methods
Animals
Male mice were used in all experiments. GAL-KO mice were generated on the 129/OlaHsd background according to previously described procedures (Wynick et al., 1998). Exons 1 to 5 of the galanin gene were replaced by a PGK-Neo cassette in reverse orientiation, which removed the signal peptide, coding region and most of the galanin-associated peptide. In homozygous GAL-KO mice, galanin expression was not detected in any of the tissues examined (Wynick et al., 1998). Once generated, chimeras were bred to co-isogenic 129Ola/Hsd wild-type mice to generate mice heterozygous for the galanin knockout allele. These mice became the founders of the new colony. In the current studies, heterozygous mating pairs were bred together to obtain wild-type and knockout mice. To obtain a large number of mice for the behavioral experiments, WT x WT and KO x KO mating pairs were bred together to obtain non-littermate congenic wild-type and GAL-KO mice, representing about 65 % of the tested animals. The remaining 35 % of the tested mice were the direct offspring of heterozygous mating pairs. Littermate and non-littermate mice were represented in each group across the behavioral experiments and did not differ in their behavior in any of the experiments performed. All GAL-WT and GAL-KO mice were between 3 and 7 months of age at the time of the experiments. In every experiment, the two genotypes were age matched and evaluated simultaneously. The genotype of the mice was verified using PCR of tail-tip DNA as has been described (Wynick et al., 1998).
C57BL/6J mice were purchased from Jackson laboratory (Bar Harbor, ME, USA) and were between 2 and 4 months old at the time of the experiments. They were given at least 7 days to habituate to the colony room once they arrived at Yale University before the start of the experiments.
All mice were housed 3 – 5 in standard plastic mouse cages (Allentown Inc, Allentown, NJ, USA) with corncob bedding. Before the start of the behavioral experiments (or just after the catheter implantation in the self-administration experiments), all mice were single housed. Food (standard pellets, Harlan Teklad 2018) and water were available ad libitum unless otherwise noted. The animal room was maintained on a 12:12 h light-dark cycle (lights on at 7:00 a.m.) with an ambient temperature of 19–22°C. All experimental procedures were carried out during the light period of the light-dark cycle, between 9:00 a.m. and 2:00 p.m. All animal studies were performed in strict accordance with the guidelines from the NIH Care and Use of Laboratory Animals Guidelines and were approved by the Yale Animal Care and Use Committee.
Pharmacological treatments
Cocaine hydrochloride (NIDA, Raleigh, NC, USA) was dissolved in an isotonic sterile saline solution (0.9% NaCl). Doses of cocaine HCl always refer to the salt. Galnon (Sigma-Aldrich, Saint Louis, MO, USA) was dissolved in a sterile saline solution (0.9% NaCl) containing 1 % DMSO. In the motor activity experiments, cocaine and galnon were administered intraperitoneally (i.p.) in a volume of 0.01 ml/g body weight. The control treatments for these drugs consisted, respectively, of an equal volume of either saline or 1% DMSO dissolved in saline.
Cocaine-induced hyperactivity
Measurement of locomotor motor activity
Motor activity was measured in eight transparent plastic cages (Allentown Inc, Allentown, NJ, USA; 43 cm × 21 cm × 21cm height) each covered by a removable perforated plastic lid. The mouse was placed in the center of the cage and allowed to move freely for 60 min. During that time, its activity was measured by 16 infrared beam arrays located at a height of 2.5 cm and spaced by 2.5 cm. Motor activity was recorded on a single PC and analyzed by Med-PC IV software (MED Associates, St Albans, VT). General activity (total activity) was defined as all movements detected by the photocells (sum of all beam breaks). Locomotor activity was defined as the consecutive break of beams separated by 5 cm (Trecki & Unterwald, 2009). Generally, a dissociation between these two measures occurs at high doses of cocaine that cause stereotyped behaviors resulting in an increase in general activity. When the animal emits stereotyped behaviors (sniffing, grooming, rearing, biting) while standing, the increase in general activity corresponds to more interruptions of the same photocell and does not result in more consecutive beam breaks separated by a given distance.
Procedure
GAL-WT and GAL-KO mice on the 129/OlaHsd background were habituated for 3 days to the testing chambers for 60 min after a saline injection. On the fourth day, mice of each genotype were randomly divided into 4 groups (n =13–20 per group) and received an i.p. injection of saline, 5, 10, or 20 mg/kg cocaine. Each mouse was then immediately placed into the testing chambers for 60 min and activity was recorded.
Four groups of wild-type C57BL/6J mice (9–11 mice per group) were tested using the same procedure as was used for GAL-WT and GAL-KO to determine whether the 129/OlaHsd background contributed to the phenotype observed in GAL-KO mice. This study allowed the establishment of the dose-response curve for cocaine-induced locomotion in C57BL/6J mice. Based on the dose-response study, galnon (0.2–4 mg/kg) was tested in combination with 10 mg/kg cocaine, a dose that induces a moderate elevation of motor activity in C57BL/6J mice. Mice received a saline injection and were habituated to the testing chambers for 60 min. The next day, mice received an i.p. injection of either DMSO (1% DMSO in sterile saline) or galnon (0.2, 1, 2 and 4 mg/kg). Fifteen min later, each mouse received an i.p. injection of either saline or cocaine (10 mg/kg). Mice were then immediately placed into the locomotor boxes for 60 min.
Cocaine self-administration
Surgery
Forty-five male mice (21 GAL-WT and 24 GAL-KO) were anesthetized with a solution of ketamine (130 mg/kg) and xylazine (20 mg/kg) and implanted with an indwelling catheter into the right jugular vein as described previously (Steiner, Hsiung, & Picciotto, 2006). Briefly, the catheter ran subcutaneously to an exit in the mid-scapular region. Catheters were constructed of Silastic tubing (0.3 mm inner diameter, 0.64 mm outer diameter, Dow Corning, Midland, MI, USA) attached to 23-gauge stainless steel tubing (Small Parts, Miami Lakes, FL, USA) surrounded by cranioplastic cement (Plastics One, Roanoke, VA, USA) and secured to Mersilene surgical mesh (Ethicon, Somerville, NJ, USA). After surgery, all mice received daily catheter flushes of 0.05 ml heparinized (100 USP units/ml) bacteriostatic saline containing gentamycin sulfate (0.13 mg/ml). Catheter patency was assessed with daily flushes. Catheters failed in two GAL-KO mice and one GAL-WT mouse before the start of the self-administration experiment. Catheter patency was also lost in one mouse from the GAL-WT saline group on the fifth session of the acquisition phase and one mouse from the GAL-KO cocaine group on the seventh session of the acquisition phase. Data from these mice were excluded from the analysis. All other catheters remained patent throughout the experiment.
Acquisition of cocaine self-administration
Mice were individually housed after surgery and were allowed to recover for 6 – 9 days after surgery. Before the start of the acquisition phase of cocaine self-administration, mice of each genotype were randomly assigned to either the cocaine or the saline group.
The locomotor study indicated that mice on the 129/OlaHsd strain show a ~ 4.5-fold reduction of locomotor activity compared to C57BL/6J mice (Figure 1). Moreover, previous studies have shown that the reinforcing effects of cocaine are diminished in 129 substrains (Thomsen and Caine, 2006) and that 129/OlaHsd mice failed to self-administer cocaine in an acute tail-vein procedure under conditions leading to self-administration of cocaine in C57BL/6J mice (Kuzmin & Johansson, 2000). Therefore, a food shaping procedure was used to promote exploratory behaviors in 129/OlaHsd mice to facilitate cocaine self-administration based on a preliminary study that quickly induced cocaine self-administration in C57BL/6J mice (data not shown). After mice had recovered from surgery, food was restricted until body weight reached 85% of pre-surgery weight. Two days later, mice were introduced into the operant chambers (Med Associates, Georgia, VT, USA) daily for acquisition of cocaine self-administration. A priming infusion lasting 1 s was administered at the start of each session. Each operant chamber had two nose poke holes (referred to as the active and inactive nose poke). During the first 6 sessions of the acquisition phase, a 50 mg piece of a standard food pellet (Harlan Teklad 2018) was introduced into each nose poke aperture to increase exploratory behavior. After each self-administration session, mice received the specific amount of food required to maintain their body weight at 85%.
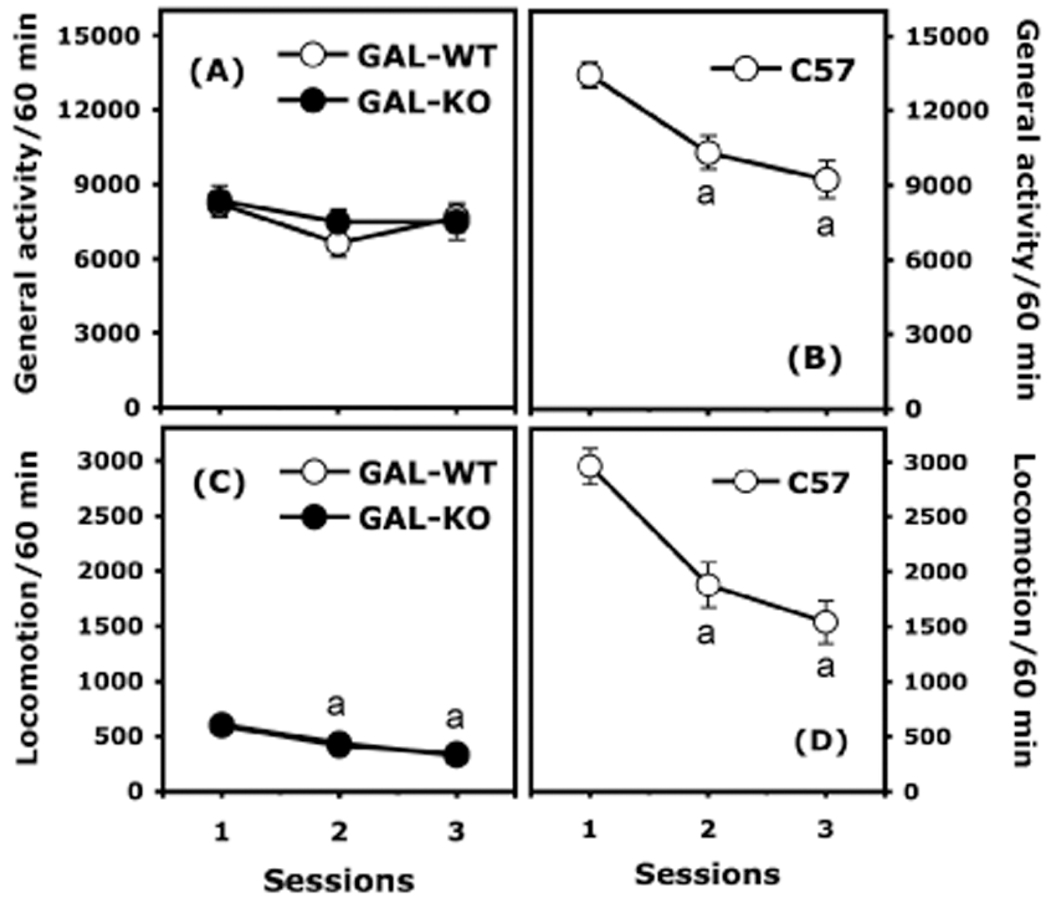
Figure 1.
Spontaneous activity in wild-type (GAL-WT, n = 66) and galanin knockout (GAL-KO, n = 70) mice kept under a 129/OlaHsd background and in wild-type C57BL/6J mice (C57, n = 40). General activity (total beam breaks) and locomotion (breaking 2 beams 5 cm apart) are expressed as mean (±SEM) number of motor counts. Panels A and C represent general activity and locomotor activity in GAL-WT and GAL-KO mice, respectively. Note that the white circles representing general activity of GAL-WT mice are hidden by the black circles representing general activity of the GAL-KO mice. Panels B and D represent general activity and locomotor activity, respectively, in wild-type C57 mice. (a): significantly different from the first session within a specific mouse strain.
During each session of the self-administration experiment, mice were placed in the operant chambers for 60 min. Each chamber was enclosed in a sound- and light-attenuating box and had a cue light above the active nose poke hole and a house light. A syringe pump (Pump model PHM100, Med Associates) was also contained within the outer box and an exhaust fan provided white noise. A response in the active nose poke aperture resulted in the pump turning on for 1 sec to give the infusion, the cue light turning on for 1 sec, and the nose poke light turning off for 10 sec (a time-out period when further active pokes would not result in another infusion). The house light was always illuminated. Mice in the cocaine group received cocaine delivered in 17 µl saline when they visited the active nose poke. Mice in the saline group received 17 µl of saline for visiting the active nose poke. Visits to the inactive nose pokes were recorded but had no consequences in any group.
The acquisition phase lasted 10 days during which cocaine was available on a fixed ratio 1 (FR1) schedule of reinforcement. Each infusion administered 0.84 mg/kg cocaine to mice in the cocaine groups (as in Steiner et al, 2006 where cocaine concentration is expressed as the free base).
Dose-response function of cocaine self-administration
After 10 acquisition sessions, mice that acquired self-administration were tested with various doses of cocaine (0.01, 0.03, 0.11, 0.33, 1.1, 3.3 mg/kg cocaine per infusion) over 6 days according to a Latin square design. Criteria for acquisition of self-administration were the following: (1) at least 3 consecutive sessions in which the number of infusions remained constant within 20% deviation of the mean of these sessions; (2) at least 70% active nose poking over 2 consecutive sessions (Deroche et al., 1997).
Cocaine self-administration under higher schedules of reinforcement
Following the dose-response sessions, 10 GAL-WT (5 GAL-WT mice that acquired cocaine self-administration and 5 GAL-WT from the saline group) and 11 GAL-KO mice (5 GAL-KO mice that acquired cocaine self-administration and 6 GAL-KO mice from the saline group) were tested for 1 day on an FR1 schedule. From that day on, each infusion administered 0.84 mg/kg cocaine to mice in the cocaine groups and saline to mice in the control groups. The schedule of reinforcement was then increased to FR2 for 2 days. The two following days, an FR4 schedule was used as a transition before progressive ratio (PR) testing. Mice experienced 2 PR sessions during which the nose pokes required for each successive infusion increased by progressive increments according to the following series: 1, 2, 4, 6, 9, 12, 16, 20, 25, 30, 36, 42, 49, 56, 64, 72, 81, 90, 100, 110, and 121 (Colby, Whisler, Steffen, Nestler, & Self, 2003).
Statistical analysis
Spontaneous levels of activity (general activity and locomotion) during the habituation sessions in GAL-WT and GAL-KO mice were analyzed using two-way mixed-model analyses of variance (ANOVA), with genotype (GAL-WT and GAL-KO, 2 levels) defined as a between-subject factor and the habituation sessions as a repeated measure. General activity and locomotion recorded following cocaine administration were treated with mixed-model three-way ANOVAs with the genotype (GAL-WT and GAL-KO, 2 levels) and the cocaine treatment (0, 5, 10 and 20 mg/kg, 4 levels) defined as between-subject factors and the time course (10-min intervals, 6 levels) as a repeated measure. Activity scores in wild-type C57BL/6J mice during the habituation sessions and on the cocaine test were analyzed in the same way as in 129/OlaHsd mice (except that the factor “genotype” was omitted). The data (general activity and locomotion) collected in the experiment testing galnon on cocaine-induced hyperactivity were treated with mixed-model three-way ANOVAs with galnon treatment (vehicle, 1, 2 and 4 mg/kg galnon, 4 levels) and cocaine treatment (0 and 10 mg/kg, 2 levels) defined as between-subject factors and the time course (10-min intervals, 6 levels) as a repeated measure.
The percentage of active nose poking for a given 60-min session was defined as the number of active nose pokes divided by the total number of nose pokes multiplied by 100. The active nose poking percentage of any mouse that did not perform 10 nose pokes over the 60-min session was replaced by 50% (Steiner et al., 2006). The number of infusions and the percentage of active nose poking during the acquisition phase of cocaine self-administration were treated using mixed-model three-way ANOVAs with genotype (GAL-WT and GAL-KO, 2 levels) and cocaine treatment (saline vs cocaine, 2 levels) defined as between-subject factors and the daily conditioning sessions (10 levels) as a repeated measure. The number of infusions and the percentage of active nose poking recorded in the dose-response sessions were treated using mixed-model two-way ANOVAs with genotype (GAL-WT and GAL-KO, 2 levels) defined as a between-subject factor and cocaine doses (0.03, 0.11, 0.33, 1.1, 3.3 mg/kg, 5 levels) considered as a repeated measure. Average infusions obtained on FR2, FR4, and PR sessions were used in the statistical analysis and treated using separate two-way ANOVAs with genotype (GAL-WT and GAL-KO, 2 levels) and cocaine treatment (saline vs cocaine, 2 levels) defined as between-subject factors.
Where necessary, square root transformations normalized raw data prior to ANOVA, more nearly meeting the assumption of homogeneity of variances (following a significant Leven’s test; Zar 1999). For the sake of clarity, means of the raw values are presented in the graphs. Relevant between-mean differences were assessed via the Newman-Keuls post hoc test. Significance was always set at p < 0.05.
Results
Cocaine-induced hyperactivity
Spontaneous activity
When placed in the activity boxes on 3 consecutive days, general activity (total beam breaks) of GAL-WT and GAL-KO mice was similar and did not vary significantly over the sessions (Figure 1A). Two-way ANOVA (Genotype X Session, with the session considered as a repeated measure) did not detect any effect of genotype, of session, nor any interaction between these factors. In contrast, general activity recorded in C57BL/6J mice decreased significantly over the sessions, F(2, 78) = 20.963, p < 0.0001 (Figure 1B). Note that levels of general activity in C57BL/6J mice were higher than in 129/OlaHsd mice, especially in the first habituation session; however, the strain difference became smaller over the sessions and C57BL/6J were only 20% more active than the 129/OlaHsd mice by the third session.
Two-way ANOVA (Genotype X Session, with the session considered as a repeated measure) calculated on the locomotor activity scores (2 beams broken, at least 5 cm apart) in 129/OlaHsd mice yielded a significant effect of session, F(2, 268) = 43.061, p < 0.0001, but no genotype effect and no Session X Genotype interaction. Locomotor activity decreased over the sessions in GAL-WT and GAL-KO mice to a similar extent (Figure 1C), indicating that both genotypes habituated to the open field. In C57BL/6J mice, locomotor activity decreased over the habituation sessions, F(2, 78) = 34.639, p < 0.0001, but remained more than four times higher than in 129/OlaHsd mice (Figure 1D). Together, these results indicate that mice on the 129/OlaHsd strain show low levels of spontaneous locomotor activity (exploratory behaviors) but that basal levels of activity are normal in this genetic background.
Cocaine-induced hyperactivity in galanin knockout and wild-type mice on a 129/OlaHsd background
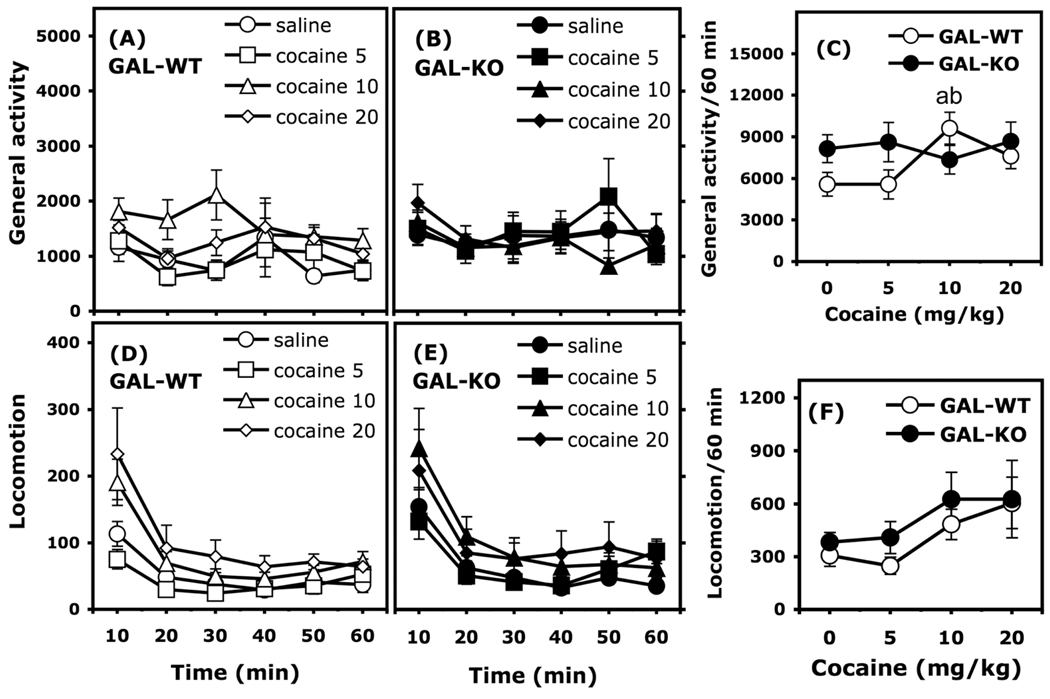
When general activity (total beam breaks) was considered as the dependent variable, cocaine enhanced motor activity only in GAL-WT mice (Fig. 2). Three-way ANOVA (Genotype X Cocaine X Time, with the time across the session considered as a repeated measure) revealed a significant Genotype X Cocaine interaction, F(3, 128) = 2.870, p < 0.05, and a significant effect of the time across the session, F(5, 640) = 5.839, p <0.0001. Post hoc comparisons calculated on the general activity scores recorded over the entire 60-min session indicated that GAL-WT mice treated with 10 mg/kg cocaine were more active than GAL-WT mice treated with saline or 5 mg/kg cocaine (Figure 2C, Newman-Keuls, p <0.05). Note that GAL-KO mice treated with saline tended to be more active than the respective GAL-WT group, however the difference did not reach statistical significance (Newman-Keuls, p = 0.16).
Figure 2.
Dose-response curve of the motor effects of cocaine (5–20 mg/kg, i.p.) in GAL-WT and GAL-KO mice. General activity (total beam breaks) and locomotion (breaking 2 beams 5 cm apart) are expressed as mean (±SEM) number of motor counts. The time course of general activity in 10-min intervals is represented in Panels A and B. The time course of locomotion in 10-min intervals is represented in Panels D and E. The action of cocaine on general activity and locomotor activity recorded during the entire 60-min session are represented in Panels C and F, respectively. Group sizes are as follows: GAL-WT, n = 13–19/group; GAL-KO, n = 15–20/group. (a): significantly different from the group injected with saline within a given genotype. (b): significantly different from the group injected with 5 mg/kg cocaine within a given genotype.
Three-way ANOVA (Genotype X Cocaine X Time, with the time across the session considered as a repeated measure) computed on the locomotor scores (breaking of 2 beams, 5 cm apart) recorded in GAL-WT and GAL-KO mice indicated a significant effect of cocaine, F(3, 128 = 2.887, p <0.05), a significant effect of the time across the session, F(5, 640) = 108.748, p <0.0001, and a significant Time X Cocaine interaction, F(15, 640) = 2.677, p < 0.001. Over the 60-min session, post hoc comparisons did not detect any differences (Newman-Keuls, p >0.070). Post hoc analysis calculated on the Time X Cocaine interaction indicated that 20 mg/kg cocaine significantly increased locomotor activity during the first 10 min of the session in both GAL-WT and GAL-KO mice (Newman-Keuls, p <0.05).
Cocaine-induced hyperactivity in wild-type C57BL/6J mice
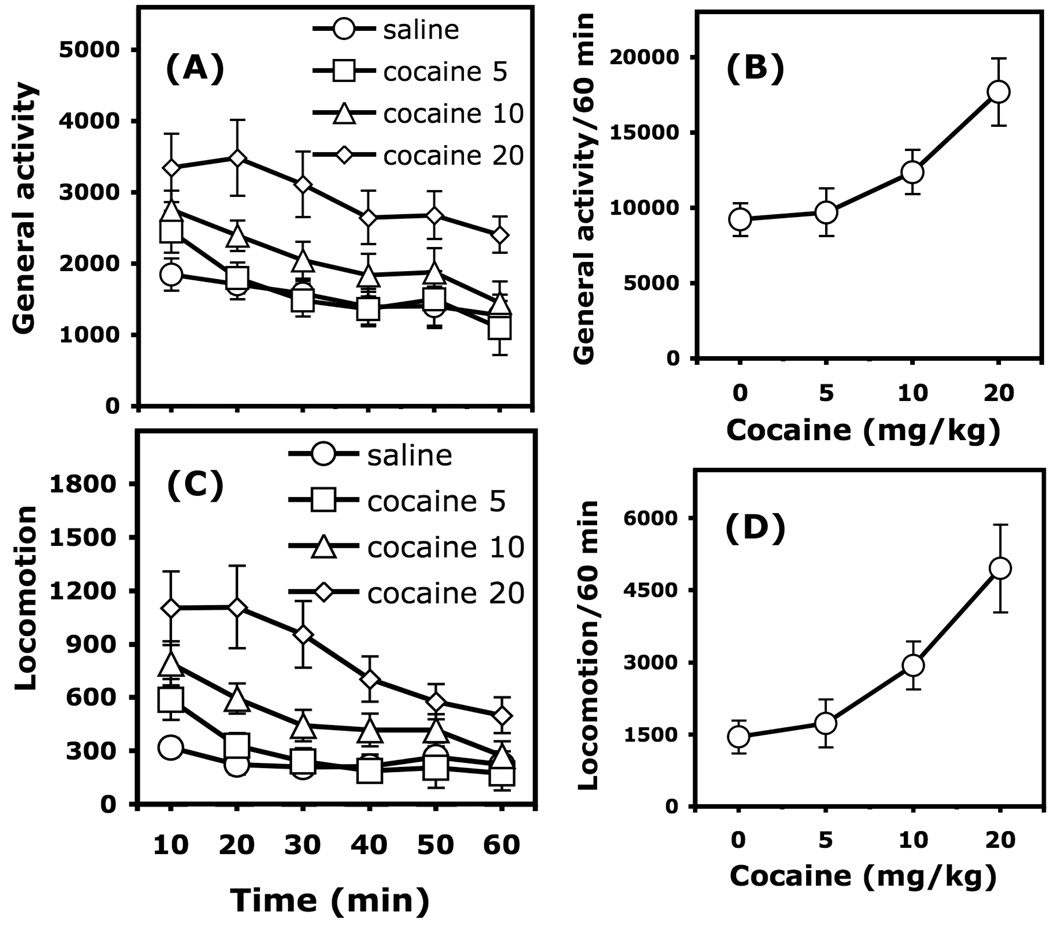
In C57BL/6J mice, cocaine increased general activity and locomotor activity dose-dependently over the entire 60-min session (Figure 3). Two-way ANOVA (Cocaine X Time, with the time across the session considered as a repeated measure) calculated on general activity scores indicated a significant effect of cocaine, F(3, 36) = 4.191, p <0.05, and a significant effect of the time course across the session, F(5, 180) = 15.148, p < 0.0001. Two-way ANOVA calculated on locomotor scores indicated a significant effect of cocaine, F(3, 36) = 5.313, p <0.01, a significant effect of the time course of the session, F(5, 180) = 34.036, p < 0.0001, and a significant interaction between these factors, F(15, 180) = 2.078, p < 0.05. Post hoc comparisons calculated over the entire session indicated that levels of general activity and locomotion in mice treated with 20 mg/kg cocaine were significantly higher than those of saline-treated mice (Newman-Keuls test, p < 0.05).
Figure 3.
Dose-response curve of the motor effects of cocaine (5–20 mg/kg, i.p.) in C57BL/6J mice (n = 9–11/group). General activity (total beam breaks) and locomotion (breaking 2 beams 5 cm apart) are expressed as mean (±SEM) number of motor counts. The time course of general activity in 10-min intervals is represented in Panel A. The time course of locomotion in 10-min intervals is represented in Panel C. The action of cocaine on general activity and locomotor activity recorded during the entire 60-min session are represented in Panels B and D, respectively. (a): significantly different from the group injected with saline.
Action of galnon on cocaine-induced hyperactivity in wild-type C57BL/6J mice
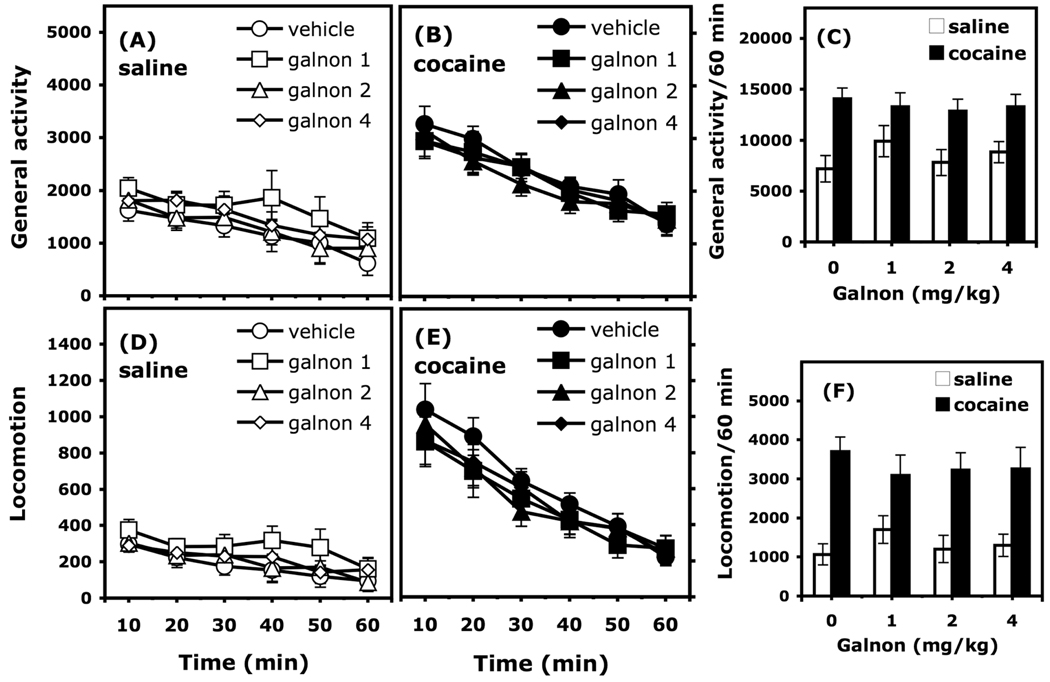
Galnon did not significantly affect general activity or locomotion induced by 10 mg/kg cocaine, a dose that causes a moderate elevation of motor activity (Figure 4). Three way-ANOVAs (Galnon X Cocaine X Time, with the time across the session considered as a repeated measure) calculated on general activity and on locomotor scores both indicated significant effects of cocaine (General activity: F(1, 88) = 31.036, p < 0.0001; Locomotion: F(1, 88) = 52.447, p < 0.0001), of the time course of the session (General activity: F(5, 440) = 53.727, p <0.0001; Locomotion: F(5, 440) = 96.080, p <0.0001) and significant interactions between these factors (General activity: F(5, 440) = 5.423, p < 0.0001; Locomotion: F(5, 440) = 5.574, p < 0.0001). Using the same protocol, an additional experiment (12 mice/group) indicated that 0.2 mg/kg galnon did not significantly affect spontaneous activity or the stimulating effects of cocaine (data not shown).
Figure 4.
Effects of galnon (1–4 mg/kg, i.p.) on spontaneous motor activity and cocaine-induced hyperactivity (10 mg/kg, i.p.) in C57BL/6J mice (n = 12/group). General activity (total beam breaks) and locomotion (breaking 2 beams 5 cm apart) are expressed as mean (±SEM) number of motor counts. The time course of general activity in 10-min intervals is represented in Panels A and B. The time course of locomotion in 10-min intervals is represented in Panels D and E. The action of galnon on general activity and locomotor activity induced by cocaine recorded during the entire 60-min session are represented in Panels C and F, respectively.
Cocaine self-administration
Acquisition of cocaine self-administration
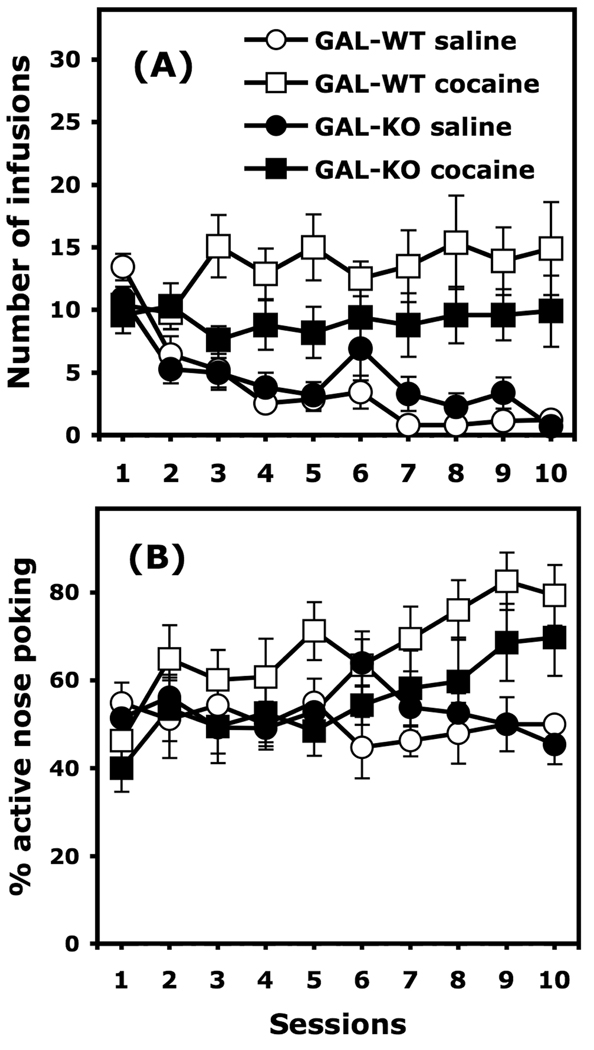
Both GAL-WT and GAL-KO mice on the 129/OlaHsd background can acquire intrajugular self-administration of cocaine (0.84 mg/kg/infusion) under a FR1 schedule of reinforcement (Figure 5A). Three-way ANOVA (Genotype X Cocaine X Session, with the session considered as a repeated measure) calculated on the infusions earned during the acquisition phase yielded a significant effect of cocaine, F(1, 36) = 33.995, p < 0.0001, of the session, F(9, 324) = 10.748, p < 0.0001 and a Session X Cocaine interaction F(9, 324) = 10.257, p <0.0001. The Genotype X Cocaine X Session interaction, F(9, 324) = 2.056, p < 0.05 was significant, indicating that acquisition of cocaine self-administration was somewhat affected by the genetic deletion of galanin. However, Post Hoc comparisons did not detect differences between GAL-KO and GAL-WT mice that self-administered cocaine for any of the sessions of the acquisition phase.
Figure 5.
Acquisition of cocaine (0.84 mg/kg/infusion) self-administration under an FR1 schedule of reinforcement in GAL-WT and GAL-KO mice congenic for the 129/OlaHsd background. Panel A represents the number of infusions earned in each daily 60-min session. Panel B represents the ratio of selectivity for the active nose-poke for each of these sessions. Data are expressed as mean (±SEM) number of infusions (Panel A) or mean (±SEM) percentage of active nose poking (Panel B). Group sizes are as follows: GAL-WT, n = 9–10/group; GAL-KO, n = 10–11/group.
129/OlaHsd mice conditioned with cocaine visited the active nose poke significantly more frequently than the inactive nose poke at the end of the acquisition phase (Figure 5B). This assumption is supported by a three-way ANOVA (Genotype X Cocaine X Session, with the session considered as a repeated measure) calculated on the percentage of active nose poking that yielded a significant effect of cocaine, F(1, 36) = 38.89, p < 0.05 and a Session X Cocaine interaction, F(9, 324) = 3.024, p < 0.01.
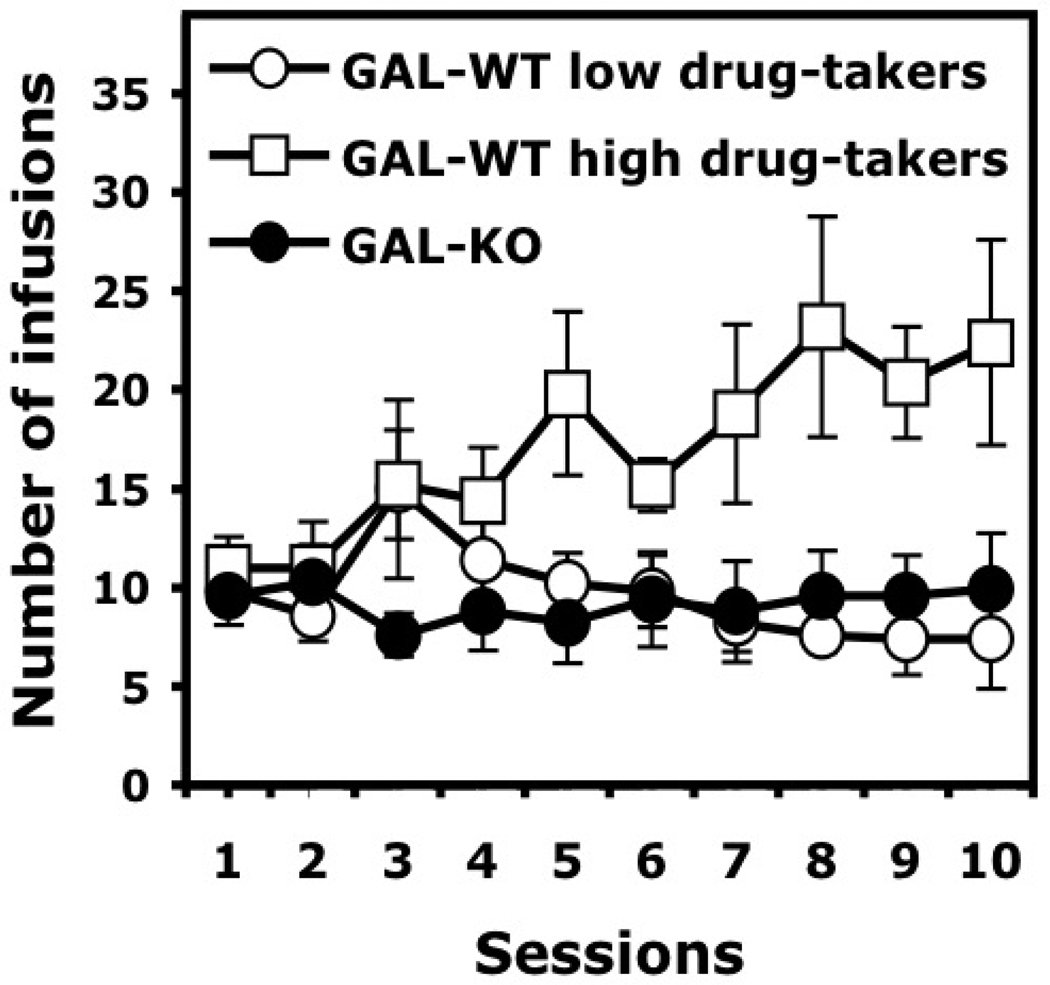
It is important to note that examination of the acquisition data suggested that GAL-WT mice showed large individual differences in their propensity to self-administer cocaine (Figure 5A). This suggests that wild-type 129/OlaHsd mice may fall into two groups, high drug-takers and low drug-takers, as observed in outbred Sprague-Dawley rats. Therefore, the number of infusions received during the last two days of the acquisition phase was averaged, and the mice were separated into two groups using the median split of those means (Puhl, Fang and Grigson, 2009). This analysis allowed the identification of two different sub-populations of GAL-WT mice (below and above the median = 13.5 infusions): low drug-takers (n = 5) and high drug-takers (n = 5). In contrast, the data were more homogeneous in GAL-KO mice (Figure 6). Two-way ANOVA applied on the acquisition data with the groups considered as a between subject factor (three levels: GAL-WT low drug-takers, GAL-WT high drug-takers, GAL-KO) and the sessions considered as a repeated measure revealed a significant group effect, F(2, 17) = 5.720, p < 0.05 and a significant Group X Session interaction, F(18, 154) = 2.149, p < 0.01). In addition, Post Hoc comparisons calculated over the acquisition phase taken entirely indicated that the number of infusions of GAL-KO mice were significantly different than those earned by GAL-WT high drug-takers (Newman-Keuls, p <0.01) but did not differ from GAL-WT low drug-takers (Newman-Keuls, p = 0.89). Taken together, the results indicate that the acquisition of cocaine self-administration in GAL-KO mice is qualitatively similar to that observed in GAL-WT mice identified as a sub-group of low drug-takers.
Figure 6.
Acquisition of cocaine (0.84 mg/kg/infusion) self-administration under an FR1 schedule of reinforcement in GAL-WT and GAL-KO mice congenic for the 129/OlaHsd background. Data are expressed as mean (±SEM) number of infusions earned in each daily 60-min session. GAL-WT mice were separated into two sub-populations: low drug-takers and high drug-takers. GAL-KO mice essentially behaved as low drug-takers in their propensity to self-administer cocaine during the acquisition phase. Group sizes are as follows: GAL-WT low drug-takers, n = 5/group; GAL-WT high drug-takers, n = 5/group; GAL-KO, n = 10/group.
Dose-response function of cocaine self-administration
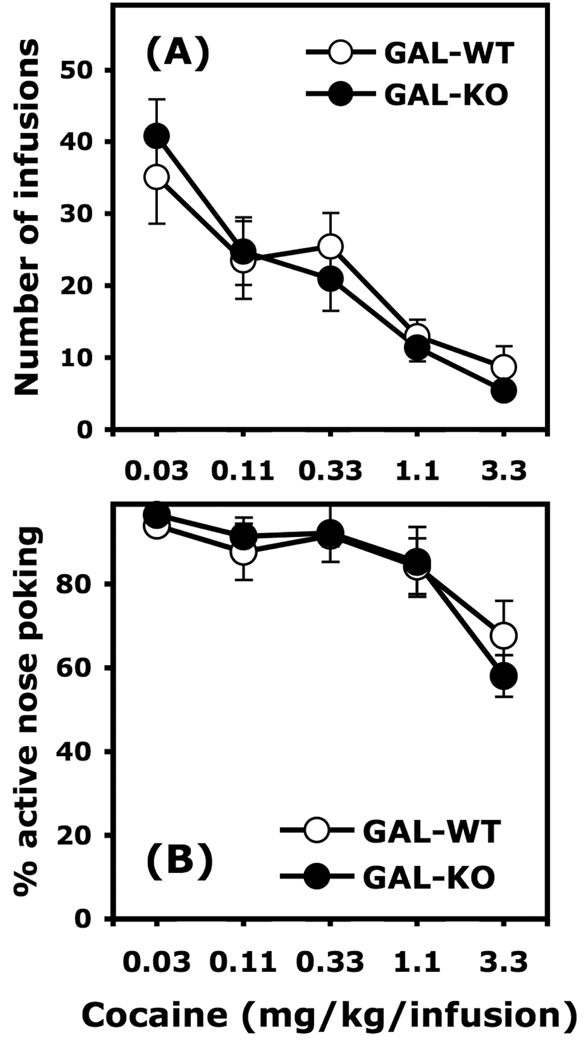
The dose-response curve of cocaine self-administration under an FR1 schedule was similar in GAL-WT and GAL-KO mice (Figure 7). The two-way ANOVA (Genotype X Cocaine Dose, with the cocaine dose considered as a repeated measure) calculated on the number of infusions revealed a significant effect of cocaine dose, F(4, 40) = 25.040, p < 0.0001, but no effect of genotype and no interaction between these factors. Post hoc comparisons indicated that the lowest doses of cocaine (0.03 and 0.11 mg/kg/infusion) maintained the highest rate of infusion in GAL-WT and GAL-KO mice relative to the highest cocaine doses (Newman-Keuls, p < 0.01).
Figure 7.
Intravenous self-administration of various doses of cocaine (0.03, 0.11, 0.33, 1.1, 3.3 mg/kg/infusion) under a FR1 schedule of reinforcement in GAL-WT (n = 7) and GAL-KO (n = 5) mice kept under a 129/OlaHsd background. Panel A represents the number of infusions earned in each 60-min test session. Panel B represents the ratio of selectivity for the active nose poke for each of these sessions. Data are expressed as mean (±SEM) number of infusions (Panel A) or mean (±SEM) percentage of active nose poking (Panel B).
The two-way ANOVA (Genotype X Cocaine Dose, with cocaine dose considered as a repeated measure) calculated on the percentage of active nose poking revealed a significant effect of cocaine dose, F(4, 40) = 10.265, p < 0.0001. Neither the effect of genotype nor the Genotype X Cocaine Dose interaction were significant. The percentage of active nose poking when mice self-administered 3.3 mg/kg/infusion was lower than when higher doses were self-administered (Newman-Keuls test, p <0.01). This effect probably does not suggest that 3.3 mg/kg/infusion disrupted discrimination between nose pokes, but rather results from an artefact of the method used to calculate selectivity. At the highest dose, most mice did not obtain 10 infusions over the session and were therefore given the 50% default value for selectivity, which artificially lowered the average percentage of active nose poking.
After completion of the dose-response sessions, self-administration of 0.01 mg/kg/infusion was also evaluated in 5 GAL-WT and 5 GAL-KO mice. The mean (± SEM) number of infusions was 25.6 ± 6.89 in GAL-WT and 17.2 ± 2.03 in GAL-KO mice during that session. A bilateral t test indicated that there was no significant difference in the number of infusions obtained between genotypes, t(8) = 1.111, p = 0.298.
Cocaine self-administration under higher schedules of reinforcement
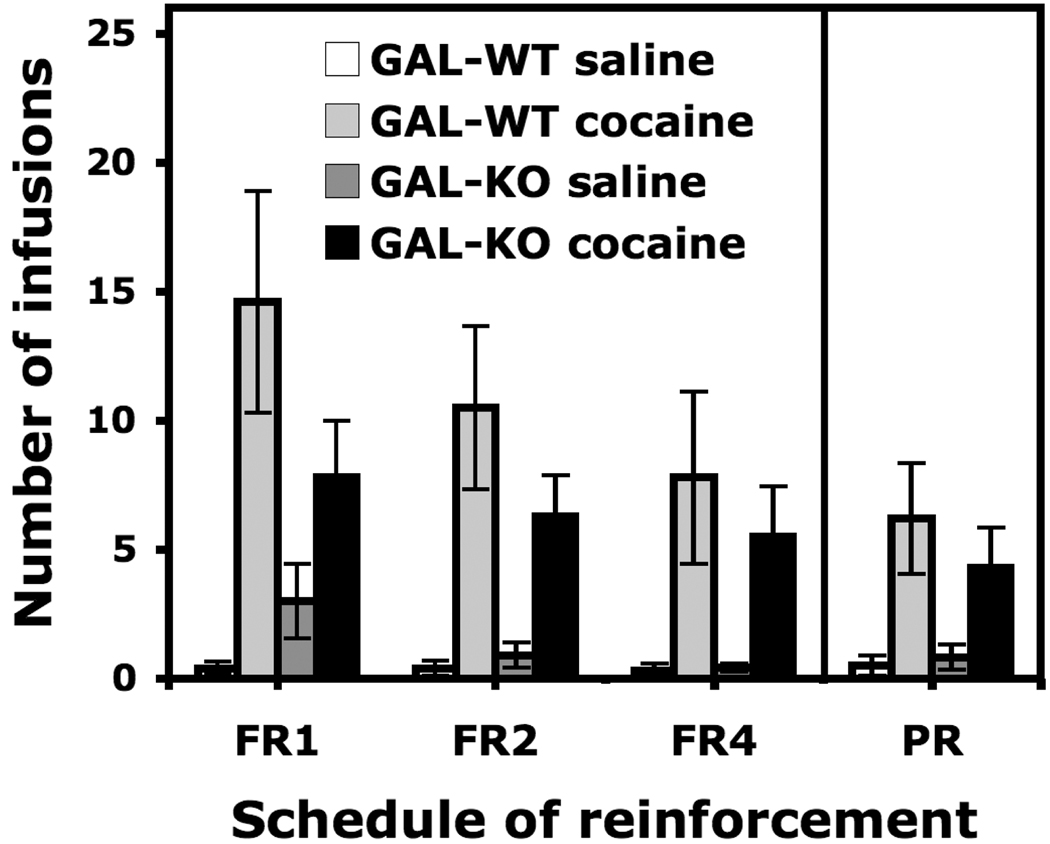
When the schedule of reinforcement was increased from an FR1 to an FR4 schedule, the number of infusions obtained by GAL-WT and GAL-KO mice in the cocaine groups was significantly higher than in the saline groups (Figure 8). A two-way ANOVA (Genotype X Cocaine) calculated on the average number of infusions earned during the two days of the FR2 schedule of reinforcement revealed a significant effect of cocaine, F(1, 17) = 39.505, p < 0.0001. However, neither the effect of genotype nor the genotype by cocaine interaction were significant. A significant effect of cocaine was also found when calculated on the average number of infusions earned during the FR4 sessions, F(1, 17) = 19.674, p < 0.001. Under the PR schedule of reinforcement, the number infusions remained significantly higher in the cocaine groups compared to the saline groups (Figure 8). A two-way ANOVA (Genotype X Cocaine) calculated on the average number of infusions earned during the two PR sessions revealed a significant effect of cocaine, F(1, 17) = 17.930, p < 0.001, but no genotype effect and no genotype by cocaine interaction.
Figure 8.
Intravenous self-administration of cocaine (0.84 mg/kg/infusion) under different schedules of reinforcement (FR1, FR2, FR4 and PR) in GAL-WT and GAL-KO congenic for the 129/OlaHsd background. The average number of infusions during two FR2 sessions, 2 FR4 sessions and 2 sessions of PR are shown. Data are expressed as mean (±SEM) number of infusions. Group sizes are as follows: GAL-WT, n = 5/group; GAL-KO, n = 5–6/group.
Discussion
The current study shows that cocaine can increase general activity (beam breaks) in GAL-WT mice on the 129/OlaHsd background, an effect that was not observed in isogenic GAL-KO mice. Nevertheless, cocaine enhanced locomotion (beam breaks 5 cm apart) dose-dependently in both genotypes to a similar extent. Further, galnon (0.2 – 4 mg/kg, i.p.), a non-selective galanin receptor agonist, did not significantly alter cocaine-induced hyperactivity in C57BL/6J mice. Finally, our results indicate that 129/OlaHsd mice can acquire i.v. cocaine self-administration under various schedules of reinforcement, an effect that was apparent in both GAL-WT and GAL-KO mice. While GAL-WT mice acquired cocaine self-administration, a median split analysis showed that mice could be divided into large and small drug takers, whereas all GAL-KO mice were small drug takers.
The current results show that cocaine increased locomotion in 129/OlaHsd mice to a lesser extent than in C57BL/6J mice, an effect in agreement with a previous report that showed poor cocaine-induced hyperactivity in this 129 substrain (Kuzmin, Johansson, Fredholm, & Ogren, 2000). In addition, there are other strain differences that complicate the interpretation of the changes we observed in knockout mice lacking galanin. In C57BL/6J mice, cocaine elevated locomotor activity and general activity dose-dependently in a similar way, with 20 mg/kg enhancing both measures maximally. In wild-type 129/OlaHsd mice, cocaine also caused a maximal increase in locomotion at 20 mg/kg, an effect that was unaffected by the genetic deletion of galanin. However, general activity of these mice was significantly elevated by cocaine only at 10 mg/kg. Unexpectedly, none of the cocaine doses we have tested altered general activity in GAL-KO mice.
To investigate whether the changes in cocaine-induced hyperactivity observed in GAL-KO mice were likely to be mediated through acute galanin signaling during the task, the effect of the small molecule galanin agonist galnon was tested in C57BL/6J mice treated with cocaine. Galnon (0.2–4 mg/kg, i.p.) did not significantly alter hyperactivity induced by 10 mg/kg cocaine, a dose that causes a moderate increase in motor activity. These data suggest that the changes in cocaine reactivity observed in galanin knockout mice are unlikely related to an acute role of galanin in cocaine-induced hyperactivity. Instead, changes in response to cocaine in GAL-KO mice may result from adaptations to the constitutive mutation, since GAL-KO mice on the 129/OlaHsd background show a selective loss of septo-hippocampal cholinergic cells. This cellular loss has been associated with deficits in acetylcholine transmission in the hippocampus (O’Meara et al., 2000). In agreement with that view, the septo-hippocampal cholinergic system has been suggested to mediate the arousing effects of cocaine that can translate into increases in general activity (Yabase, Carino, & Horita, 1990; Yoshida, Gjerde, Carino, Halpern, & Horita, 1993).
The current study shows that wild-type 129/OlaHsd mice can acquire intrajugular cocaine self-administration under conditions that have been shown to support cocaine self-administration in C57BL/6J mice (Steiner et al., 2006). These results contrast with a previous report showing that 129/OlaHsd mice failed to self-administer cocaine in an acute tail-vein model (Kuzmin and Johansson, 2000). The discrepancy between these studies suggests that several procedural factors used in the present work are critical for the demonstration of cocaine self-administration in 129/OlaHsd mice. First, mice were food restricted in our study but had unlimited access to food in Kuzmin and Johansson’s research. Food restriction has been shown to facilitate the acquisition of i.v. cocaine self-administration (Campbell and Carroll, 2001). Moreover, the present study used a food shaping procedure in which food pellets were placed in the nose-poke apertures for the first 6 sessions of the acquisition phase of cocaine self-administration. The presence of food strongly motivated the mice to explore both nose pokes. The protocol we used does not rule out the possibility that the emergence of cocaine self-administration in 129/OlaHsd mice over the first six sessions could have been influenced by the concomitant intake of food pellets, which might potentiate the reinforcing effects of cocaine. However, when food was removed from the nose-poke apertures, mice continued to visit the active nose poke to self-administer on average 15 cocaine infusions on the 10th session of the acquisition phase. During that same session, the percentage of active nose poking was on average 80%. In contrast, visits to the nose pokes virtually disappeared at the end of conditioning phase in mice receiving saline infusions. Our results therefore indicate that this procedure can reliably induce cocaine self-administration in 129/OlaHsd mice and suggests its utility in facilitating the development of self-administration behaviors in 129 mouse substrains that poorly react to the behavioral effects of cocaine.
Contrary to our initial hypothesis, acquisition of cocaine self-administration was not facilitated in GAL-KO mice compared to GAL-WT controls. It is noteworthy that wild-type 129/OlaHsd mice showed large individual differences in their propensity to self-administer cocaine. A median split analysis applied on the acquisition data indicated that wild-type 129/OlaHsd mice can be divided into sub-populations: low and high drug-takers, a phenomenon similar to what is observed in outbred Sprague–Dawley rats (Puhl et al., 2009). Acquisition of cocaine self-administration was more homogenous in GAL-KO mice and they essentially behaved as small drug-takers during the acquisition phase. However, the current study indicates that the dose-response curve for cocaine self-administration did not differ between GAL-WT and GAL-KO mice that acquired self-administration. Finally, operant responding for cocaine tended to be decreased rather than increased in GAL-KO mice when cocaine self-administration was examined under higher FR schedules (FR2, FR4) or a PR schedule of reinforcement. In contrast to these results, conditioned place preference induced by cocaine has been shown to be increased in GAL-KO mice (Narasimhaiah et al., 2009). One explanation for the discrepancy between the present results and the place conditioning study is that conditioned place preference and self-administration are not redundant measures that would refer to a unique neuronal system. Many studies have found that the neurobiological mechanisms that underlie these behavioral models of addiction can be dissociated (see Bardo & Bevins, 2000 for a review). For example, knockout mice lacking dopamine D1 receptors do not self-administer cocaine but exhibit normal conditioned place preference for this psychostimulant (Caine et al., 2007; Karasinska, George, Cheng, & O'Dowd, 2005). Another possibility is that the increased cocaine conditioned place preference observed in GAL-KO is related to the loss of medial septal cells these mice present. Lesions of the medial septum have been shown to result in conditioned place preference for a low inactive cocaine dose in rats, an effect that has been associated to the septo-hippocampal system (Gong, Neill, & Justice, 1995).
Overall, the current results suggest that galanin does not play a major role in the locomotor and reinforcing effects of cocaine. Based on previous studies on morphine place preference and withdrawal (Zachariou et al., 2003; Hawes et al., 2008), the galanin system may preferentially modulate opioid dependence but not cocaine addiction. For example, the locomotor and rewarding effects of morphine are enhanced in knockout mice lacking galanin from the same background than those used in the present study (Hawes et al., 2008). The VTA has been suggested to be the site through which galanin inhibits dopamine mesolimbic activity (Ericson and Ahlenius, 1998). In agreement with that view, GalR1 receptors in the midbrain have been shown to inhibit tyrosine hydroxylase (TH) activity (Counts et al., 2002). It is therefore possible that galanin modulates responses to drugs whose primary reinforcing effects are triggered by molecular targets in the VTA, such as morphine that binds to mu opioid receptors located in that brain area (Johnson and North, 1992). In contrast, the reinforcing effects of cocaine result from its ability to increase extracellular dopamine levels through the blockade of the dopamine transporter located in the ventral striatum (Chen et al., 2006). It is important to note that galanin does not appear to exert a simple overall negative effect on dopaminergic activity and drug reward. For example, microinjection of galanin into the paraventricular nucleus of the hypothalamus stimulates the release of dopamine in the nucleus accumbens and enhances alcohol consumption (Rada, Mark, & Hoebel, 1998; Rada, Avena, Leibowitz, & Hoebel, 2004; Schneider, Rada, Darby, Leibowitz, & Hoebel, 2007).
In conclusion, the current study found that the locomotor and reinforcing effects of cocaine are not markedly changed in knockout mice lacking the galanin peptide constitutively. Unexpectedly, the ability of cocaine to increase general activity was altered in GAL-KO mice, an effect that may be unrelated to the loss of galanin signaling based on pharmacological experiments in wild-type C57BL/6J mice. Finally, GAL-KO mice have a similar dose-response function for cocaine self-administration as compared to GAL-WT mice, but during acquisition GAL-KO mice behave like the subset of GAL-WT mice that take smaller amouts of cocaine. Taken together, these results suggest that galanin does not play a significant role in the locomotor and reinforcing effects of cocaine, but may play a more minor modulatory role in acquisition of cocaine self-administration.
Acknowledgements
The authors would like to thank Drs. Rebecca Steiner and Yann Mineur for their help with this study. This work was supported by grants DA15425 and DA00436 from the National Institutes of Health. Dr. Christian Brabant is a research associate under contract with the National Funds for Scientific Research (FNRS, Belgium).
Footnotes
Publisher's Disclaimer: The following manuscript is the final accepted manuscript. It has not been subjected to the final copyediting, fact-checking, and proofreading required for formal publication. It is not the definitive, publisher-authenticated version. The American Psychological Association and its Council of Editors disclaim any responsibility or liabilities for errors or omissions of this manuscript version, any version derived from this manuscript by NIH, or other third parties. The published version is available at www.apa.org/pubs/journals/bne
References
- Abramov U, Florén A, Echevarria DJ, Brewer A, Manuzon H, Robinson JK, Bartfai T, Vasar E, Langel U. Regulation of feeding by galnon. Neuropeptides. 2004;38:55–61. doi: 10.1016/j.npep.2004.01.001. [DOI] [PubMed] [Google Scholar]
- Bardo MT, Bevins RA. Conditioned place preference: what does it add to our preclinical understanding of drug reward? Psychopharmacology. 2003;153:31–43. doi: 10.1007/s002130000569. [DOI] [PubMed] [Google Scholar]
- Borgland SL, Taha SA, Sarti F, 1 Fields HL, Bonci A. Orexin A in the VTA is critical for the induction of synaptic plasticity and behavioral sensitization to cocaine. Neuron. 2006;49:589–601. doi: 10.1016/j.neuron.2006.01.016. [DOI] [PubMed] [Google Scholar]
- Caine SB, Thomsen M, Gabriel KI, Berkowitz JS, Gold LH, Koob GF, Tonegawa S, Zhang J, Xu M. Lack of self-administration of cocaine in dopamine D1 receptor knock-out mice. Journal of Neuroscience. 2007;27:13140–13150. doi: 10.1523/JNEUROSCI.2284-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell UC, Carroll ME. Effects of ketoconazole on the acquisition of intravenous cocaine self-administration under different feeding conditions in rats. Psychopharmacology. 2001;154:311–318. doi: 10.1007/s002130000627. [DOI] [PubMed] [Google Scholar]
- Chen R, Tilley MR, Wei H, Zhou F, Zhou FM, Ching S, Quan N, Stephens RL, Hill ER, Nottoli T, Han DD, Gu HH. Abolished cocaine reward in mice with a cocaine-insensitive dopamine transporter. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:9333–9338. doi: 10.1073/pnas.0600905103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung S, Hopf FW, Nagasaki H, Li C-Y, Belluzzia JD, Bonci A, Civelli O. The melanin-concentrating hormone system modulates cocaine reward. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:6772–6777. doi: 10.1073/pnas.0811331106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colby CR, Whisler K, Steffen C, Nestler EJ, Self DW. Striatal cell type-specific overexpression of DeltaFosB enhances incentive for cocaine. Journal of Neuroscience. 2003;23:2488–2493. doi: 10.1523/JNEUROSCI.23-06-02488.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Counts SE, McGuire SO, Sortwell CE, Crawley JN, Collier TJ, Mufson EJ. Galanin inhibits tyrosine hydroxylase expression in midbrain dopaminergic neurons. Journal of Neurochemistry. 2002;83:442–451. doi: 10.1046/j.1471-4159.2002.01148.x. [DOI] [PubMed] [Google Scholar]
- Deroche V, Caine SB, Heyser CJ, Polis I, Koob GF, Gold LH. Differences in the liability to self-administer intravenous cocaine between C57BL/6 x SJL and BALB/cByJ mice. Pharmacology Biochemistry and Behavior. 1997;57:429–440. doi: 10.1016/s0091-3057(96)00439-x. [DOI] [PubMed] [Google Scholar]
- Ericson E, Ahlenius S. Suggestive evidence for inhibitory effects of galanin on mesolimbic dopaminergic neurotransmission. Brain Research. 1999;822:200–209. doi: 10.1016/s0006-8993(99)01144-0. [DOI] [PubMed] [Google Scholar]
- España RA, Oleson EB, Locke JL, Brookshirem BR, Roberts DCS, Jones SR. The hypocretin-orexin system regulates cocaine self-administration via actions on the mesolimbic dopamine system. European Journal of Neuroscience. 2009;31:336–348. doi: 10.1111/j.1460-9568.2009.07065.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong W, Neill DB, Justice JB. Increased sensitivity to cocaine place-preference conditioning by septal lesions in rats. Brain Research. 1995;683:221–227. doi: 10.1016/0006-8993(95)00376-2. [DOI] [PubMed] [Google Scholar]
- Hawes JJ, Brunzell DH, Narasimhaiah R, Langel U, Wynick D, Picciotto MR. Galanin protects against behavioral and neurochemical correlates of opiate reward. Neuropsychopharmacology. 2008;33:1864–1873. doi: 10.1038/sj.npp.1301579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawes JJ, Narasimhaiah R, Picciotto MR. Galanin and galanin-related peptide modulate neurite outgrowth via PKC-mediated activation of ERK. European Journal of Neuroscience. 2006;23:2937–2946. doi: 10.1111/j.1460-9568.2006.04828.x. [DOI] [PubMed] [Google Scholar]
- Johnson S, North R. Opioids excite dopamine neurons by hyperpolarization of local interneurons. Journal of Neuroscience. 1992;12:483–488. doi: 10.1523/JNEUROSCI.12-02-00483.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karasinska JM, George SR, Cheng R, O'Dowd BF. Deletion of dopamine D1 and D3 receptors differentially affects spontaneous behaviour and cocaine-induced locomotor activity, reward and CREB phosphorylation. European Journal of Neuroscience. 2005;22:1741–1750. doi: 10.1111/j.1460-9568.2005.04353.x. [DOI] [PubMed] [Google Scholar]
- Kuzmin A, Johansson B. Reinforcing and neurochemical effects of cocaine: differences among C57, DBA, and 129 mice. Pharmacology Biochemistry and Behavior. 2000;65:399–406. doi: 10.1016/s0091-3057(99)00211-7. [DOI] [PubMed] [Google Scholar]
- Kuzmin A, Johansson B, Fredholm BB, Ogren SO. Genetic evidence that cocaine and caffeine stimulate locomotion in mice via different mechanisms. Life Science. 2000;66:PL113–PL118. doi: 10.1016/s0024-3205(99)00647-5. [DOI] [PubMed] [Google Scholar]
- Lang R, Gundlach AL, Kofler B. The galanin peptide family: receptor pharmacology, pleiotropic biological actions, and implications in health and disease. Pharmacology & Therapeutics. 2007;115:177–207. doi: 10.1016/j.pharmthera.2007.05.009. [DOI] [PubMed] [Google Scholar]
- Mahoney S-A, Hosking R, Farrant S, Holmes FE, Jacoby AS, Shine J, Iismaa TP, Scott MK, Schmidt R, Wynick D. The second galanin receptor GalR2 plays a key role in neurite outgrowth from adult sensory neurons. Journal of Neuroscience. 2003;23:416–421. doi: 10.1523/JNEUROSCI.23-02-00416.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitsukawa K, Lu X, Bartfai T. Bidirectional regulation of stress responses by galanin in mice: Involvement of galanin receptor subtype 1. Neuroscience. 2009;160:837–846. doi: 10.1016/j.neuroscience.2009.02.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narasimhaiah R, Kamens H, Picciotto M. Effects of galanin on cocaine-mediated conditioned place preference and ERK signaling in mice. Psychopharmacology. 2009;204:95–102. doi: 10.1007/s00213-008-1438-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Meara G, Coumis U, Ma SY, Kehr J, Mahoney S, Bacon A, Allen SJ, Holmes F, Kahl U, Wang FH, Kearns IR, Ove-Ogren S, Dawbarn D, Mufson EJ, Davies C, Dawson G, Wynick D. Galanin regulates the postnatal survival of a subset of basal forebrain cholinergic neurons. Proceedings of the National Academy of Sciences of the United States of America. 2000;97:11569–11574. doi: 10.1073/pnas.210254597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picciotto MR, Brabant C, Einstein EB, Kamens HM, Neugebauer NM. Effects of galanin on monoaminergic systems and HPA axis: Potential mechanisms underlying the effects of galanin on addiction- and stress-related behaviors. Brain Research. 2010;1314:206–218. doi: 10.1016/j.brainres.2009.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puhl MD, Fang J, Grigson PS. Acute sleep deprivation increases the rate and efficiency of cocaine self-administration, but not the perceived value of cocaine reward in rats. Pharmacology Biochemistry and Behavior. 2009;94:262–270. doi: 10.1016/j.pbb.2009.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rada P, Avena NM, Leibowitz SF, Hoebel BG. Ethanol intake is increased by injection of galanin in the paraventricular nucleus and reduced by a galanin antagonist. Alcohol. 2004;33:91–97. doi: 10.1016/j.alcohol.2004.05.002. [DOI] [PubMed] [Google Scholar]
- Rada P, Mark GP, Hoebel BG. Galanin in the hypothalamus raises dopamine and lowers acetylcholine release in the nucleus accumbens: a possible mechanism for hypothalamic initiation of feeding behavior. Brain Research. 1998;798:1–6. doi: 10.1016/s0006-8993(98)00315-1. [DOI] [PubMed] [Google Scholar]
- Robinson J, Brewer A. Galanin: A potential role in mesolimbic dopamine-mediated instrumental behavior. Neuroscience of Biobehavioral Reviews. 2008;32:1485–1493. doi: 10.1016/j.neubiorev.2008.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saar K, Mazarati AM, Mahlapuu R, Hallnemo G, Soomets U, Kilk K, Hellberg S, Pooga M, Tolf BR, Shi TS, Hökfelt T, Wasterlain C, Bartfai T, Langel U. Anticonvulsant activity of a nonpeptide galanin receptor agonist. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:7136–7141. doi: 10.1073/pnas.102163499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider ER, Rada P, Darby RD, Leibowitz SF, Hoebel BG. Orexigenic peptides and alcohol intake: differential effects of orexin, galanin, and ghrelin. Alcoholism: Clinical and Experimental Research. 2007;31:1858–1865. doi: 10.1111/j.1530-0277.2007.00510.x. [DOI] [PubMed] [Google Scholar]
- Steiner RC, Hsiung HM, Picciotto MR. Cocaine self-administration and locomotor sensitization are not altered in CART knockout mice. Behavioural and Brain Research. 2006;171:56–62. doi: 10.1016/j.bbr.2006.03.022. [DOI] [PubMed] [Google Scholar]
- Stephens DN, Mead AN, Ripley TL. Studying the neurobiology of stimulant and alcohol abuse and dependence in genetically manipulated mice. Behavioural Pharmacology. 2002;13:327–345. doi: 10.1097/00008877-200209000-00004. [DOI] [PubMed] [Google Scholar]
- Thomsen M, Caine SB. Cocaine self-administration under fixed and progressive ratio schedules of reinforcement: comparison of C57BL/6J, 129×1/SvJ, and 129S6/SvEvTac inbred mice. Psychopharmacology. 2006;184:145–154. doi: 10.1007/s00213-005-0207-0. [DOI] [PubMed] [Google Scholar]
- Trecki J, Unterwald EM. Modulation of cocaine-induced activity by intracerebral administration of CXCL12. Neuroscience. 2009;161:13–22. doi: 10.1016/j.neuroscience.2009.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuda K, Tsuda S, Nishio I, Masuyama Y, Goldstein M. Effects of galanin on dopamine release in the central nervous system of normotensive and spontaneously hypertensive rats. American Journal of Hypertension. 1998;11:1475–1479. doi: 10.1016/s0895-7061(98)00168-x. [DOI] [PubMed] [Google Scholar]
- Tyhon A, Adamantidis A, Foidart A, Grisar T, Lakaye B, Tirelli E. Mice lacking the melanin-concentrating hormone receptor-1 exhibit an atypical psychomotor susceptibility to cocaine and no conditioned cocaine response. Behavioural and Brain Research. 2006;173:94–103. doi: 10.1016/j.bbr.2006.06.007. [DOI] [PubMed] [Google Scholar]
- Wang S, Hashemi T, Fried S, Clemmons AL, Hawes BE. Differential intracellular signaling of the GalR1 and GalR2 galanin receptor subtypes. Biochemistry. 1998;37:6711–6717. doi: 10.1021/bi9728405. [DOI] [PubMed] [Google Scholar]
- Weiss J, Boss-Williams K, Moore J, Demetrikopoulos M, Ritchie J, West C. Testing the hypothesis that locus coeruleus hyperactivity produces depression-related changes via galanin. Neuropeptides. 2005;39:279–285. doi: 10.1016/j.npep.2004.12.028. [DOI] [PubMed] [Google Scholar]
- Wu WP, Hao JX, Lundström L, Wiesenfeld-Hallin Z, Langel U, Bartfai T, Xu XJ. Systemic galnon, a low-molecular weight galanin receptor agonist, reduces heat hyperalgesia in rats with nerve injury. European Journal of Pharmacology. 2003;482:133–137. doi: 10.1016/j.ejphar.2003.09.018. [DOI] [PubMed] [Google Scholar]
- Wynick D, Small CJ, Bacon A, Holmes FE, Norman M, Ormandy CJ, Kilic E, Kerr NC, Ghatei M, Talamantes F, Bloom SR, Pachnis V. Galanin regulates prolactin release and lactotroph proliferation. Proceedings of the National Academy of Sciences of the United States of America. 1998;95:12671–12676. doi: 10.1073/pnas.95.21.12671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yabase M, Carino MA, Horita A. Cocaine produces cholinergically mediated analeptic and EEG arousal effects in rabbits and rats. Pharmacology Biochemistry and Behavior. 1990;37:375–377. doi: 10.1016/0091-3057(90)90351-h. [DOI] [PubMed] [Google Scholar]
- Yoshida K, Gjerde DK, Carino MA, Halpern LM, Horita A. The effects of SCH 23390 and raclopride on cocaine-induced analepsis and EEG arousal in rabbits. Neuropharmacology. 1993;32:487–492. doi: 10.1016/0028-3908(93)90174-2. [DOI] [PubMed] [Google Scholar]
- Zachariou V, Brunzell DH, Hawes J, Stedman DR, Bartfai T, Steiner RA, Wynick D, Langel U, Picciotto MR. The neuropeptide galanin modulates behavioral and neurochemical signs of opiate withdrawal. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:9028–9033. doi: 10.1073/pnas.1533224100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zachariou V, Parikh K, Picciotto MR. Centrally administered galanin blocks morphine place preference in the mouse. Brain Research. 1999;831:33–42. doi: 10.1016/s0006-8993(99)01476-6. [DOI] [PubMed] [Google Scholar]
- Zar JH. Biostatistical analysis. 4th edition. New Jersey: Prentice Hall; 1999. [Google Scholar]