Abstract
Objectives
Microbial adhesion and biofilms have important implications for human health and disease. Candida albicans is an opportunistic pathogen which forms drug-resistant biofilms that contribute to the recalcitrance of disease. We have developed a high-throughput screen for potentiators of clotrimazole, a common therapy for Candida infections, including vaginitis and thrush. The screen was performed against C. albicans biofilms grown in microtitre plates in order to target the most resilient forms of the pathogen.
Methods
Biofilm growth, in individual wells of 384-well plates, was measured using the metabolic indicator alamarBlue® and found to be very consistent and reproducible. This assay was used to test the effect of more than 120 000 small molecule compounds from the NIH Molecular Libraries Small Molecule Repository, and compounds that enhanced the activity of clotrimazole or acted on the biofilms alone were identified as hits.
Results
Nineteen compounds (0.016% hit rate) were identified and found to cause more than 30% metabolic inhibition of biofilms compared with clotrimazole alone, which had a modest effect on biofilm viability at the concentration tested. Hits were confirmed for activity against biofilms with dose–response measurements. Several compounds had increased activity in combination with clotrimazole, including a 1,3-benzothiazole scaffold that exhibited a >100-fold improvement against biofilms of three separate C. albicans isolates. Cytotoxicity experiments using human fibroblasts confirmed the presence of lead molecules with favourable antifungal activity relative to cytotoxicity.
Conclusions
We have validated a novel approach to identify antifungal potentiators and completed a high-throughput screen to identify small molecules with activity against C. albicans biofilms. These small molecules may specifically target the biofilm and make currently available antifungals more effective.
Keywords: clotrimazole, alamarBlue®, drug resistance
Introduction
Candida albicans is a common member of the human gastrointestinal microbiota and an important opportunistic pathogen. Biofilms of C. albicans often form on indwelling devices, such as blood and urinary catheters and heart valves.1–12 Antifungals are unable to effectively treat biofilm infections and infected prosthetics require device removal to prevent the dissemination of disease. Systemic invasive fungal infections are a cause of high mortality, approaching 40%, despite a variety of antifungal therapeutic options.13–15 Biofilm resistance to antimicrobials may be due to up-regulation of drug efflux pumps,16–18 drug binding to the extracellular matrix,19,20 high cell density within the biofilm21 or the presence of persister cells.22,23
Other important diseases caused by C. albicans are vaginitis and oral thrush. These infections are difficult to treat and recurrent. Recurrence of infection may be due to the presence of biofilms. Most females will develop vaginitis due to C. albicans at some point in their lives.24 Usually cases are treatable with azoles, but 5%–8% resist therapy, producing a chronic disease.25 Weekly prophylactic fluconazole decreased relapse, but did not cure patients.26,27
Oral thrush is a serious disease for immunocompromised patients. Topical clotrimazole and miconazole are standard treatments, but are usually only first-line treatments and temporarily effective.28 Recalcitrance of these infections to antimicrobials is not obvious, since planktonic populations of the same strain can be highly susceptible to a range of antifungals, including azoles, echinocandins and amphotericin B.
Traditionally, antifungals have been identified and validated for efficacy by determining the MIC or the minimal fungicidal concentration against a rapidly growing planktonic population of cells. MIC determination is useful in many regards, for instance, to track the emergence of drug resistance. However, in vivo infection is considerably more complex, and therapeutic failure does not always correlate with increases in MIC for the pathogen. In vivo, pathogens may grow as biofilms, where they are unlikely to experience rapid, exponential growth. This may limit the utility of an in vitro assay to predict the efficacy of a drug in vivo. Indeed, non-growing, slow-growing and biofilm populations are considerably more tolerant to antimicrobials compared with growing cells.29,30 Biofilms may also limit the components of the immune system from the pathogen, further complicating treatment.31,32 Currently, high doses of topically applied clotrimazole cream or troches are used as an alternative to systemic fluconazole treatment for vaginitis and oral thrush. Fluconazole has no microbicidal activity against C. albicans, and resistance is common. Even the most effective cidal antifungals, such as amphotericin B and caspofungin, are not completely active against C. albicans biofilms.22 We reasoned that a molecule that synergizes with a conventional antifungal may overwhelm the biofilm and lead to a more effective therapeutic. Thus, we developed a screen for potentiators of the antifungal clotrimazole, which is based on testing biofilms growing in a microtitre plate with the viability dye alamarBlue®. Hits from the screen were subsequently validated for their ability to inhibit biofilms alone, and in the presence of clotrimazole.
Materials and methods
Biofilm growth conditions
Wild-type C. albicans strain CAF2-1 was streaked onto YPD agar medium (10 g/L yeast extract and 20 g/L Bacto peptone) and incubated at 30°C for 48 h. A single colony from the YPD plate was used to inoculate 100 mL liquid YPD in a 1 L baffled Erlenmeyer flask and the culture was incubated at 30°C for 24 h. Cells were concentrated by centrifugation and resuspended in 15% glycerol in phosphate-buffered saline. Stocks of the suspension in 15% glycerol were divided into single-use aliquots and frozen in liquid nitrogen and stored at −80°C. Twenty-four hour biofilms were formed by standard methods according to Ramage et al.33 Briefly, cells from the frozen stock were resuspended in RPMI 1640 with l-glutamine and 0.165 M MOPS (BioWhittaker). The OD600 of the suspension was adjusted to 0.1, approximately 1 × 106 cfu/mL. Suspensions of 30 μL were aliquoted into wells of flat-bottom 384-well (Greiner 7 81 086) microtitre plates. The plates were incubated for 24 h at 37°C, allowing biofilm formation.
High-throughput screen (HTS)
C. albicans biofilms were grown in 384-well plates. Biofilm growth medium was removed by inverting and discarding. The medium was replaced with 10 μL of fresh RPMI 1640 growth medium containing clotrimazole (145 μM). Compounds from the Molecular Libraries Small Molecule Repository (MLSMR) were initially transferred into a separate intermediate 384-well plate containing RPMI 1640 and clotrimazole (145 μM). At the time of screening, four separate compound pin transfers from a 1536-well compound plate were made into a single 384-well intermediate plate to yield a mixture of four compounds per well. A volume of 114 nL of compound (2.5 mM in DMSO) was transferred into 25 μL of RPMI 1640 medium with clotrimazole to yield a final concentration of 11.4 μM per compound. Next, 20 μL of this mixture was transferred into the biofilm-containing plates using a PerkinElmer Evolution P3 pipetting platform, yielding a final concentration of 7.6 μM per compound. Each HTS plate contained mixtures of compounds in columns 3–22 (experimental), biofilms containing clotrimazole and no compound in columns 2 and 24 (negative control), and biofilms containing a lethal mixture of clotrimazole (145 μM) and chlorhexidine (400 μM) in columns 1 and 23 (positive control). Biofilms were incubated for 48 h at 37°C. After incubation, medium was removed manually without biofilm disruption and replaced with 1% alamarBlue® in PBS. Biofilms were incubated at 37°C for 2 h and alamarBlue® reduction was measured using a Perkin-Elmer Envision microplate fluorimeter with excitation and emission at 535 and 590 nm, respectively.
Data analysis and retesting
Data were analysed in ActivityBase (IDBS, Guildford, UK). Biofilm metabolic inhibition, measured by alamarBlue® reduction, was calculated for each compound using the following equation:
 |
Wells were scored as hits if percentage inhibition was >30%. Individual compounds from the mixtures of four were retested and a compound was scored as a hit if the percentage inhibition was greater than 30% in the retest.
IC50 testing
Biofilms were grown in 384-well plates as described previously. C. albicans strain SC5314 and a fluconazole-resistant strain (29E) isolated from a cancer patient23 were tested in addition to CAF2-1. Hits from the MLSMR were resupplied directly from their commercial vendor and dissolved in DMSO. Compounds were serially diluted (2-fold from 100 mM to 3 μM) using a PerkinElmer Evolution P3 pipetting platform. From this dilution plate, 108 nL of each compound was transferred into a separate intermediate 384-well plate containing 25 μL RPMI 1640 alone (columns 1 and 23) and RPMI 1640 with clotrimazole (145 μM) (columns 2–22 and 24). Biofilm growth medium was removed by inversion and discarded. The medium was replaced with 10 μL of fresh RPMI 1640 growth medium and medium containing 145 μM clotrimazole. Next, 20 μL of medium containing clotrimazole and compound was transferred from the intermediate plate to each well of the biofilm-containing plates. Final concentrations for each compound ranged from 288 μM to 8 nM and each compound was tested for activity against biofilms alone and along with clotrimazole. Biofilms were incubated and assayed for metabolic activity as described previously. The concentration of each compound that inhibited biofilm metabolic activity by 50% was calculated by non-linear regression (four-parameter logistic fit) using XLfit (IDBS, Guildford, UK) and reported as the IC50.
Cytotoxicity
Cryopreserved adult human fibroblasts were obtained from Lifeline Cell Technology (Walkersville, MD, USA). Fibroblasts were suspended in Eagle's Minimum Essential Medium (ATCC catalogue no. 30-2003) with 10% fetal bovine serum and seeded into a 75 cm2 tissue culture flask according to the manufacturer's recommendations. Cells were incubated at 37°C and 10% CO2 until nearly confluent. Fibroblasts were trypsinized and passaged into clear-bottomed, black 96-well plates (Corning Costar 3603) and grown until 70% confluence. Experimental compounds were diluted into a separate plate, using a 1:2 ratio in fresh medium, from concentrations ranging from 16 to 500 μM. After dilution, the fibroblast medium was aspirated from the fibroblast plate and replaced with fresh medium containing the experimental compounds. The plates were incubated at 37°C and 10% CO2. After 24 h, fibroblasts were washed three times with fresh medium and incubated for an additional 18 h. Next, 10% alamarBlue® was added to each well and the plates were incubated for 2 h. The percentage metabolic activity of fibroblasts in each well was calculated based on measurements from a fluorescent plate reader and negative control wells without experimental compounds. The highest concentration of each compound that caused greater than 50% reduction in metabolic activity was reported as the cytotoxic concentration.
Results
HTS optimization
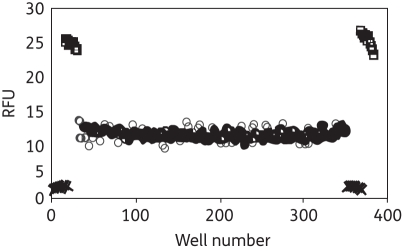
In order to determine whether biofilms could be grown reproducibly in 384-well plates, C. albicans cells were seeded and incubated for 24 h, in a manner similar to biofilm growth in 96-well plates.33 Visual and microscopic inspection of these plates revealed that attached biofilms were present in each well. Biofilm metabolic activity was measured with alamarBlue® (1%) in order to determine biofilm growth and variability. Fluorescent intensity readings, corresponding to biofilm metabolic activity, were measured every 20 min for 4 h. On average, alamarBlue® was found to be almost completely reduced after 2 h of incubation, with only a minimal increase in fluorescence detected thereafter. Next, we calculated the variability of biofilm metabolic activity between individual wells of the microplate. Biofilms were found to grow very consistently and reproducibly in 384-well plates. In fact, the coefficient of variation, defined as standard deviation/mean × 100, was found to be only 3.3%. The consistency of growing biofilms in the 384-well microplate format was quantified by calculation of the Z′-factor, which is a standard measure of the robustness and feasibility of an HTS. The Z′-factor determines the magnitude of the difference between the positive and negative controls relative to the sum of the respective standard deviations:
where SD+ = positive control standard deviation, SD− = negative control standard deviation, Ave+ = positive control average and Ave− = negative control average. In our assay, the positive control consisted of microtitre wells seeded with C. albicans biofilms in the presence of a combination of clotrimazole and chlorhexidine, while negative control wells contained biofilms and clotrimazole alone (Figure 1). Clotrimazole was added to the negative control wells at 145 μM, since this concentration caused a mid-range reduction of alamarBlue® signal compared with positive and negative controls. Chlorhexidine is known to kill biofilms, so it was added to clotrimazole as the positive control. The Z′ was found to be 0.90, well above the Z′ > 0.5 generally regarded as being suitable for an HTS. In addition to Z′, a variety of other standard HTS assay performance parameters, including coefficient of variation (<10) and signal-to-background ratio (>10), were measured to determine suitability for HTS. The parameters determined for the 384-well alamarBlue® assay showed it to be suitable for HTS, comparing favourably with criteria for HTS accepted into the NIH Molecular Libraries Screening Center Network.
Figure 1.
Fluorescent intensity of untreated biofilms (squares), those treated with clotrimazole alone (circles) and those treated with a combination of clotrimazole and chlorhexidine (crosses). Biofilms were grown for 24 h, challenged with antimicrobials for 48 h and assayed with alamarBlue® for 2 h.
HTS
More than 120 000 compounds were screened as mixtures of four compounds per well in 384-well plates. Individual wells that showed activity were parsed and retested to attribute activity to a specific compound. In total, only 19 compounds were identified that inhibited biofilm metabolic activity more than 30%. The overall hit rate from the screen was surprisingly low, approximately 0.016%. The results from the entire screen are included in an Excel file (available as Supplementary data at JAC Online), along with the PubChem identification number of each compound.
Validation of HTS hits
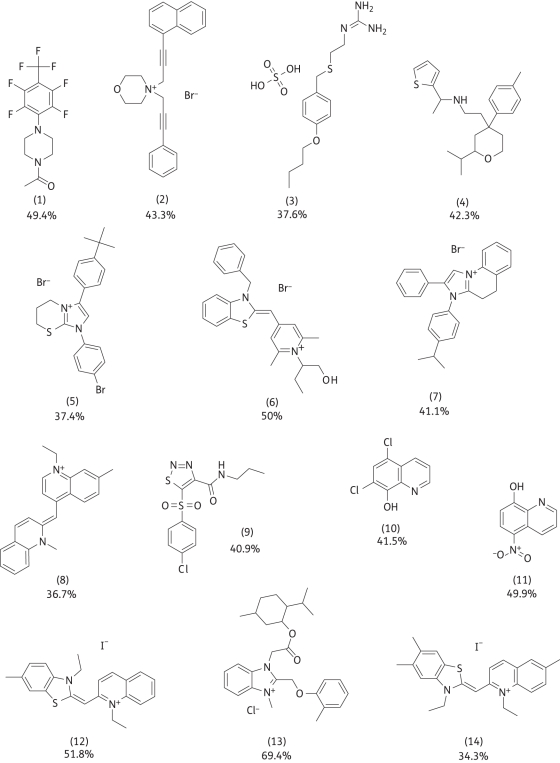
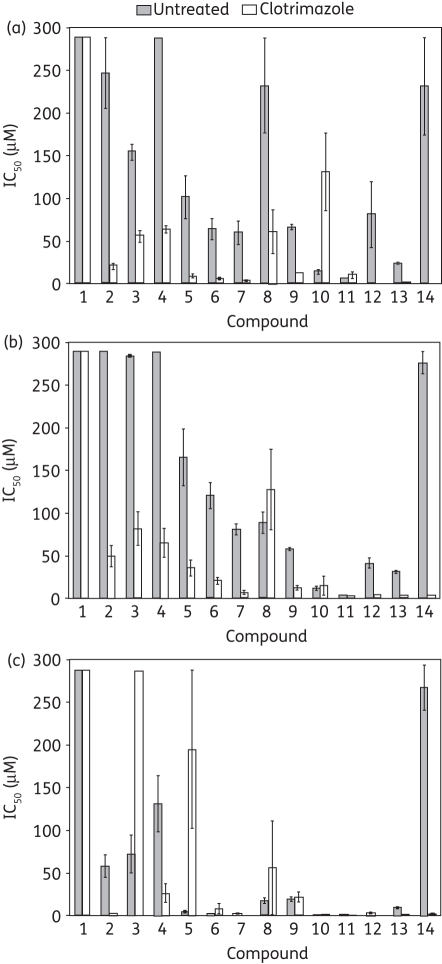
Of the 19 hits, 5 were unavailable for immediate resupply and were excluded from further analysis. The structures of the other hits are detailed, along with the average percentage metabolic inhibition scores from the primary screen and retest validations (Figure 2). It should be noted that the HTS did not discriminate between compounds with direct activity on the biofilm and potentiator compounds that had no effect alone, since each small molecule was tested in combination with clotrimazole. To distinguish between these two possibilities, hits from the HTS were tested in a dose–response experiment for efficacy against biofilms alone and in the presence of 145 μM clotrimazole. Dose–response curves were generated against biofilms of three strains of C. albicans, including a fluconazole-resistant strain isolated from a cancer patient23 (strain 29E). The concentration of each compound required to inhibit biofilm metabolic activity by 50%, the IC50, was calculated from each dose–response using non-linear regression (Table S1, available as Supplementary data at JAC Online). Several compounds had greatly improved activity when tested in combination with clotrimazole compared with the compound alone based on the IC50 (Figure 3). In particular, compounds 2, 7 and 12–14 demonstrated a greater than 8-fold increase in activity together with clotrimazole, across the three strains. As expected, some compounds caused decreases in biofilm metabolism alone. Most compounds had similar relative effects on biofilms produced by three separate strains of Candida (Figure 3a–c). However, differences in susceptibilities between strains were noted for individual compounds. Surprisingly, a fluconazole-resistant strain appeared to have greater overall susceptibilities (Figure 3c) compared with two wild-type strains. Subsequent microscopic examination of biofilms produced by strain 29E revealed sparse biomass and reduced hyphae compared with the other two strains.
Figure 2.
Compounds that inhibited the metabolic activity of C. albicans biofilms, tested in the presence of clotrimazole. For each compound, the average percentage metabolic inhibition from the primary screen and retest confirmation experiments is given.
Figure 3.
Effects of each hit on biofilm metabolism. Dose–response curves were generated from biofilms exposed to a range of concentrations from 288 μM to 8 nM of each compound. Biofilms of C. albicans strains CAF2-1 (a) and SC5314 (b) and fluconazole-resistant clinical isolate 29E (c) were tested with or without clotrimazole for 48 h and the IC50 for each compound was determined.
Cytotoxicity
HTS libraries often contain toxic, promiscuous or generally reactive molecules which do not make good drug candidates. We reasoned that molecules that act in synergy with conventional antifungals and do not have activity alone are not likely to be toxic. In order to confirm this hypothesis, we tested the HTS hits for cytotoxicity against human cell cultures. Fibroblasts were chosen due to their ubiquitous nature and widespread use in cytotoxicity testing. Fibroblasts were grown in 96-well plates and exposed to increasing doses (2-fold increments) of each hit compound for 24 h. After exposure, fibroblast metabolic activity was measured and used as an indicator of cell viability. The concentration of each compound that caused a 50% reduction of fibroblast metabolic activity was reported as the cytotoxic concentration (Table 1). Compounds 12–14, which exhibited dramatically improved potency along with clotrimazole, were also cytotoxic at concentrations at or below 31 μM. The most promising molecules to emerge from the HTS were compounds 2, 7, 10 and 11. These molecules had cytotoxic concentrations at or above 125 μM and had biofilm inhibitory activities less than 10 μM for at least one strain of C. albicans.
Table 1.
Cytotoxic concentration of each hit tested against human fibroblasts
| Compound | Cytotoxic concentration (µM) |
|---|---|
| 1 | 500 |
| 2 | 125 |
| 3 | 62 |
| 4 | 250 |
| 5 | 62 |
| 6 | 31 |
| 7 | 125 |
| 8 | 62 |
| 9 | 16 |
| 10 | 500 |
| 11 | 125 |
| 12 | 16 |
| 13 | 16 |
| 14 | 31 |
Discussion
Fungal infections are often recurrent and difficult to treat; thus there is an urgent need for novel antifungal therapeutics and treatment strategies. Currently available antifungals may be growth inhibitory or cidal to exponentially growing cells, but are largely ineffective against biofilms. We have utilized the intrinsic drug-resistant nature of the biofilm growth state to establish acceptable conditions to screen for antifungal potentiators. The primary screen did not discriminate between compounds that acted directly on the biofilm or those that potentiated clotrimazole, and the overall low hit rate of the screen confirms the notion that biofilms are extremely difficult to eradicate.
Clotrimazole troches (10 mg) and creams (1%) are used to treat oral and vaginal candidiasis. The clotrimazole potentiators identified in this screen have the potential to improve dosing regimens, decrease the acting concentration of drug for these indications, and combat resistance. Also, since the hits identified in our screen have activity against biofilms, they may be especially useful in treating biofilm-related fungal infections. Recent evidence suggests C. albicans forms biofilms on the oral and vaginal mucosa during infection.34,35 Compounds that are particularly effective against biofilm pathologies may prove useful in clearing infections or preventing relapse.
Clotrimazole, like other azole antifungals, is known to target α-14 lanosterol demethylase, causing ergosterol depletion and growth arrest. However, a fluconazole-resistant strain, 29E, was found to produce poor biofilms and this strain had increased susceptibilities to certain molecules (compounds 2, 4, 7 and 10–13) compared with wild-type strains that formed more robust biofilms. Thus, a high level of antifungal drug resistance was conferred by the extent to which a strain was able to form a biofilm. Furthermore, if a susceptible strain with a low MIC is able to form a robust biofilm, the biofilm may provide even greater protection compared with a strain with a higher MIC that cannot form a robust biofilm. Strain 29E did exhibit increased resistance to the combination of compound 3 or 5 and clotrimazole. These compounds may be particularly good substrates of fungal multidrug efflux transporters, which are known to be up-regulated in the presence of azoles36 and in drug-resistant strains.37
Several of the most potent clotrimazole potentiators that were identified in the screen contain a 1,3-benzothiazole scaffold (compounds 6, 12 and 14). This motif is responsible for the biological activity of luciferin,38 found in fireflies, and the pharmaceutical riluzole,39 used to treat amyotrophic lateral sclerosis. Antifungal activities of benzothiazole compounds have been reported40 and 6-amino-2-n-pentylthiobenzothiazole was found to inhibit Candida filamentation,41 which is required for biofilm formation. We are planning to test whether the 1,3-benzothiazole compounds identified in this screen also inhibit filamentation. The apparent cytotoxic liabilities of the 1,3-benzothiazole hits may be irrelevant for topical applications or it may be possible to optimize this scaffold for reduced cytotoxicity by synthesizing and testing chemical analogues of this moiety. Other azole potentiators identified in the literature are known to function by diverse mechanisms, including inhibiting calcineurin,42 HSP9043 or drug efflux pumps.44 The targets and mechanisms of action of the potentiator compounds identified in the screen are currently under investigation in order to determine whether they function by known or novel mechanisms.
We have demonstrated the feasibility of performing an HTS against C. albicans biofilms. As proof of principle, we identified clotrimazole potentiators, which may prove useful for topical applications such as vaginitis or oral thrush. It is likely that screens for potentiators of systemic antifungals could be conducted in a similar manner. For example, the identification of potentiators of caspofungin, which targets the fungal cell wall, may shed light on Candida cell wall biology and lead to improved treatment of systemic fungal infections. These probes may also reveal the extent to which biofilms are responsible for untreatable or recurrent fungal infection in vivo. The small molecule approach to specifically target biofilms that we have described may have important therapeutic and industrial applications.
Funding
This work was supported by NIH grants GM061162 to K. L. and HG003915 to S. L. D. The funder had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript.
Transparency declarations
None to declare.
Disclaimer
The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Supplementary data
References
- 1.Ramage G, Wickes BL, Lopez-Ribot JL. Biofilms of Candida albicans and their associated resistance to antifungal agents. Am Clin Lab. 2001;20:42–4. [PubMed] [Google Scholar]
- 2.Baillie GS, Douglas LJ. Matrix polymers of Candida biofilms and their possible role in biofilm resistance to antifungal agents. J Antimicrob Chemother. 2000;46:397–403. doi: 10.1093/jac/46.3.397. doi:10.1093/jac/46.3.397. [DOI] [PubMed] [Google Scholar]
- 3.Kumamoto CA. Candida biofilms. Curr Opin Microbiol. 2002;5:608–11. doi: 10.1016/s1369-5274(02)00371-5. doi:10.1016/S1369-5274(02)00371-5. [DOI] [PubMed] [Google Scholar]
- 4.Douglas LJ. Candida biofilms and their role in infection. Trends Microbiol. 2003;11:30–6. doi: 10.1016/s0966-842x(02)00002-1. doi:10.1016/S0966-842X(02)00002-1. [DOI] [PubMed] [Google Scholar]
- 5.Kuhn DM, Ghannoum MA. Candida biofilms: antifungal resistance and emerging therapeutic options. Curr Opin Investig Drugs. 2004;5:186–97. [PubMed] [Google Scholar]
- 6.Jabra-Rizk MA, Falkler WA, Meiller TF. Fungal biofilms and drug resistance. Emerg Infect Dis. 2004;10:14–9. doi: 10.3201/eid1001.030119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kojic EM, Darouiche RO. Candida infections of medical devices. Clin Microbiol Rev. 2004;17:255–67. doi: 10.1128/CMR.17.2.255-267.2004. doi:10.1128/CMR.17.2.255-267.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mukherjee PK, Chandra J. Candida biofilm resistance. Drug Resist Updat. 2004;7:301–9. doi: 10.1016/j.drup.2004.09.002. doi:10.1016/j.drup.2004.09.002. [DOI] [PubMed] [Google Scholar]
- 9.Ramage G, Saville SP, Thomas DP, et al. Candida biofilms: an update. Eukaryot Cell. 2005;4:633–8. doi: 10.1128/EC.4.4.633-638.2005. doi:10.1128/EC.4.4.633-638.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lopez-Ribot JL. Candida albicans biofilms: more than filamentation. Curr Biol. 2005;15:R453–5. doi: 10.1016/j.cub.2005.06.020. doi:10.1016/j.cub.2005.06.020. [DOI] [PubMed] [Google Scholar]
- 11.Mukherjee PK, Zhou G, Munyon R, et al. Candida biofilm: a well-designed protected environment. Med Mycol. 2005;43:191–208. doi: 10.1080/13693780500107554. doi:10.1080/13693780500107554. [DOI] [PubMed] [Google Scholar]
- 12.Kumamoto CA, Vinces MD. Alternative Candida albicans lifestyles: growth on surfaces. Annu Rev Microbiol. 2005;59:113–33. doi: 10.1146/annurev.micro.59.030804.121034. doi:10.1146/annurev.micro.59.030804.121034. [DOI] [PubMed] [Google Scholar]
- 13.Zaoutis TE, Heydon K, Localio R, et al. Outcomes attributable to neonatal candidiasis. Clin Infect Dis. 2007;44:1187–93. doi: 10.1086/513196. doi:10.1086/513196. [DOI] [PubMed] [Google Scholar]
- 14.Leleu G, Aegerter P, Guidet B. Systemic candidiasis in intensive care units: a multicenter, matched-cohort study. J Crit Care. 2002;17:168–75. doi: 10.1053/jcrc.2002.35815. doi:10.1053/jcrc.2002.35815. [DOI] [PubMed] [Google Scholar]
- 15.Ruhnke M. Epidemiology of Candida albicans infections and role of non-Candida-albicans yeasts. Curr Drug Targets. 2006;7:495–504. doi: 10.2174/138945006776359421. doi:10.2174/138945006776359421. [DOI] [PubMed] [Google Scholar]
- 16.Ramage G, Bachmann S, Patterson TF, et al. Investigation of multidrug efflux pumps in relation to fluconazole resistance in Candida albicans biofilms. J Antimicrob Chemother. 2002;49:973–80. doi: 10.1093/jac/dkf049. doi:10.1093/jac/dkf049. [DOI] [PubMed] [Google Scholar]
- 17.Mukherjee PK, Chandra J, Kuhn DM, et al. Mechanism of fluconazole resistance in Candida albicans biofilms: phase-specific role of efflux pumps and membrane sterols. Infect Immun. 2003;71:4333–40. doi: 10.1128/IAI.71.8.4333-4340.2003. doi:10.1128/IAI.71.8.4333-4340.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Niimi M, Firth NA, Cannon RD. Antifungal drug resistance of oral fungi. Odontology. 2010;98:15–25. doi: 10.1007/s10266-009-0118-3. doi:10.1007/s10266-009-0118-3. [DOI] [PubMed] [Google Scholar]
- 19.Nett J, Lincoln L, Marchillo K, et al. Putative role of β-1,3 glucans in Candida albicans biofilm resistance. Antimicrob Agents Chemother. 2007;51:510–20. doi: 10.1128/AAC.01056-06. doi:10.1128/AAC.01056-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vediyappan G, Rossignol T, d'Enfert C. Interaction of Candida albicans biofilms with antifungals: transcriptional response and binding of antifungals to β-glucans. Antimicrob Agents Chemother. 2010;54:2096–111. doi: 10.1128/AAC.01638-09. doi:10.1128/AAC.01638-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Perumal P, Mekala S, Chaffin WL. Role for cell density in antifungal drug resistance in Candida albicans biofilms. Antimicrob Agents Chemother. 2007;51:2454–63. doi: 10.1128/AAC.01237-06. doi:10.1128/AAC.01237-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.LaFleur MD, Kumamoto CA, Lewis K. Candida albicans biofilms produce antifungal-tolerant persister cells. Antimicrob Agents Chemother. 2006;50:3839–46. doi: 10.1128/AAC.00684-06. doi:10.1128/AAC.00684-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.LaFleur MD, Qi Q, Lewis K. Patients with long-term oral carriage harbor high-persister mutants of Candida albicans. Antimicrob Agents Chemother. 2010;54:39–44. doi: 10.1128/AAC.00860-09. doi:10.1128/AAC.00860-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Workowski KA, Berman SM. Sexually transmitted diseases treatment guidelines. MMWR Recomm Rep. 2006;55:1–94. [PubMed] [Google Scholar]
- 25.Skiest DJ, Vazquez JA, Anstead GM, et al. Posaconazole for the treatment of azole-refractory oropharyngeal and esophageal candidiasis in subjects with HIV infection. Clin Infect Dis. 2007;44:607–14. doi: 10.1086/511039. doi:10.1086/511039. [DOI] [PubMed] [Google Scholar]
- 26.Sobel JD, Wiesenfeld HC, Martens M, et al. Maintenance fluconazole therapy for recurrent vulvovaginal candidiasis. N Engl J Med. 2004;351:876–83. doi: 10.1056/NEJMoa033114. doi:10.1056/NEJMoa033114. [DOI] [PubMed] [Google Scholar]
- 27.Sobel JD, Ferris D, Schwebke J, et al. Suppressive antibacterial therapy with 0.75% metronidazole vaginal gel to prevent recurrent bacterial vaginosis. Am J Obstet Gynecol. 2006;194:1283–9. doi: 10.1016/j.ajog.2005.11.041. doi:10.1016/j.ajog.2005.11.041. [DOI] [PubMed] [Google Scholar]
- 28.Vazquez JA. Therapeutic options for the management of oropharyngeal and esophageal candidiasis in HIV/AIDS patients. HIV Clin Trials. 2000;1:47–59. doi: 10.1310/T7A7-1E63-2KA0-JKWD. doi:10.1310/T7A7-1E63-2KA0-JKWD. [DOI] [PubMed] [Google Scholar]
- 29.Coates AR, Hu Y. New strategies for antibacterial drug design: targeting non-multiplying latent bacteria. Drugs R D. 2006;7:133–51. doi: 10.2165/00126839-200607030-00001. doi:10.2165/00126839-200607030-00001. [DOI] [PubMed] [Google Scholar]
- 30.Lewis K. Persister cells, dormancy and infectious disease. Nat Rev Microbiol. 2007;5:48–56. doi: 10.1038/nrmicro1557. doi:10.1038/nrmicro1557. [DOI] [PubMed] [Google Scholar]
- 31.Hoyle BD, Jass J, Costerton JW. The biofilm glycocalyx as a resistance factor. J Antimicrob Chemother. 1990;26:1–5. doi: 10.1093/jac/26.1.1. doi:10.1093/jac/26.1.1. [DOI] [PubMed] [Google Scholar]
- 32.von Eiff C, Heilmann C, Peters G. New aspects in the molecular basis of polymer-associated infections due to staphylococci. Eur J Clin Microbiol Infect Dis. 1999;18:843–6. doi: 10.1007/s100960050417. doi:10.1007/s100960050417. [DOI] [PubMed] [Google Scholar]
- 33.Ramage G, Vande Walle K, Wickes BL, et al. Standardized method for in vitro antifungal susceptibility testing of Candida albicans biofilms. Antimicrob Agents Chemother. 2001;45:2475–9. doi: 10.1128/AAC.45.9.2475-2479.2001. doi:10.1128/AAC.45.9.2475-2479.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Harriott MM, Lilly EA, Rodriguez TE, et al. Candida albicans forms biofilms on the vaginal mucosa. Microbiology. 2010;156:3635–44. doi: 10.1099/mic.0.039354-0. doi:10.1099/mic.0.039354-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dongari-Bagtzoglou A, Kashleva H, Dwivedi P, et al. Characterization of mucosal Candida albicans biofilms. PLoS One. 2009;4:e7967. doi: 10.1371/journal.pone.0007967. doi:10.1371/journal.pone.0007967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hernaez ML, Gil C, Pla J, et al. Induced expression of the Candida albicans multidrug resistance gene CDR1 in response to fluconazole and other antifungals. Yeast. 1998;14:517–26. doi: 10.1002/(SICI)1097-0061(19980430)14:6<517::AID-YEA250>3.0.CO;2-D. doi:10.1002/(SICI)1097-0061(19980430)14:6<517::AID-YEA250>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 37.Holmes AR, Lin YH, Niimi K, et al. ABC transporter Cdr1p contributes more than Cdr2p does to fluconazole efflux in fluconazole-resistant Candida albicans clinical isolates. Antimicrob Agents Chemother. 2008;52:3851–62. doi: 10.1128/AAC.00463-08. doi:10.1128/AAC.00463-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Marques SM, Esteves da Silva JC. Firefly bioluminescence: a mechanistic approach of luciferase catalyzed reactions. IUBMB Life. 2009;61:6–17. doi: 10.1002/iub.134. doi:10.1002/iub.134. [DOI] [PubMed] [Google Scholar]
- 39.Bryson HM, Fulton B, Benfield P. Riluzole. A review of its pharmacodynamic and pharmacokinetic properties and therapeutic potential in amyotrophic lateral sclerosis. Drugs. 1996;52:549–63. doi: 10.2165/00003495-199652040-00010. doi:10.2165/00003495-199652040-00010. [DOI] [PubMed] [Google Scholar]
- 40.Bujdakova H, Kuchta T, Sidoova E, et al. Anti-Candida activity of four antifungal benzothiazoles. FEMS Microbiol Lett. 1993;112:329–33. doi: 10.1111/j.1574-6968.1993.tb06471.x. doi:10.1111/j.1574-6968.1993.tb06471.x. [DOI] [PubMed] [Google Scholar]
- 41.Fabry S, Gaborova S, Bujdakova H, et al. Inhibition of germ tube formation, filamentation and ergosterol biosynthesis in Candida albicans treated with 6-amino-2-n-pentylthiobenzothiazole. Folia Microbiol (Praha) 1999;44:523–6. doi: 10.1007/BF02816254. doi:10.1007/BF02816254. [DOI] [PubMed] [Google Scholar]
- 42.Sanglard D, Ischer F, Marchetti O, et al. Calcineurin A of Candida albicans: involvement in antifungal tolerance, cell morphogenesis and virulence. Mol Microbiol. 2003;48:959–76. doi: 10.1046/j.1365-2958.2003.03495.x. doi:10.1046/j.1365-2958.2003.03495.x. [DOI] [PubMed] [Google Scholar]
- 43.Cowen LE. Hsp90 orchestrates stress response signaling governing fungal drug resistance. PLoS Pathog. 2009;5:e1000471. doi: 10.1371/journal.ppat.1000471. doi:10.1371/journal.ppat.1000471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lamping E, Monk BC, Niimi K, et al. Characterization of three classes of membrane proteins involved in fungal azole resistance by functional hyperexpression in Saccharomyces cerevisiae. Eukaryot Cell. 2007;6:1150–65. doi: 10.1128/EC.00091-07. doi:10.1128/EC.00091-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.