Abstract
Pyridinium-based oxime compounds have been utilized worldwide as antidotes following exposure to anticholinesterase agents. In the event of combined chemical and biological incident, it is of vital importance to know the ability of antidotes to provide additional protection against biological threats. This paper reports results of in vitro antimicrobial and antiprotozoal activities of a series of quaternary pyridinium oximes against a number of lower pathogenicity BSL-1 and 2 agents. In general, our compound panel had little to no antimicrobial action except for thiophene- and benzothiophene-substituted monoquaternary pyridinium compounds 21 and 24 that showed moderate antibacterial activity against Staphylococus aureus and methicillin resistant S. aureus with IC50 values ranging from 12.2–17.7 µg/mL. Compounds 21 and 24 also exhibited antileishmanial activity against Leishmania donovani with IC50 values of 19 and 18 µg/mL, respectively. Another monoquaternary pyridinium compound with a bromobutyl side chain 17 showed antimalarial activity against both a chloroquine sensitive and resistant strains of Plasmodium falciparum with IC50 values of 3.7 and 4.0 µg/mL, respectively. None of the bisquaternary pyridinium compounds showed antimicrobial, or antiprotozoal activity. None of the compounds showed cytotoxic effects towards mammalian kidney fibroblasts. Results of this study indicate that the pyridinium compounds, some of which are already in use as antidotes, do not have significant antimicrobial and antiprotozoal activities and cannot be relied upon for additional protection in the event of combined chemical-biological incident.
Keywords: Pyridinium oximes, antimalarial, antimicrobial, antileishmanial, biological threats
Quaternary pyridinium bearing an oxime functional group are well known for their acetylcholinesterase reactivation activities and are used as antidotes in organophosphorus (OP) poisoning (1, 2). Examples of well known AChE reactivators are pralidoxime, HI-6, trimedoxime and obidoxime. In the event of combined chemical-biological incident, it is of prime importance to provide antidotes against both chemical as well as biological threats. Quaternary pyridinium oximes are widely used as antidotes for chemical (nerve agent) poisoning but the ability of these antidotes to provide additional support against biological threats is unknown. Thus, it is important to know whether pyridinium oxime antidote provides additional protection against biological threats besides their AChE reactivation ability. Therefore, herein our aim was to screen series of quaternary pyridinium oximes with known AChE reactivation ability for their ability to provide protection against biological threats.
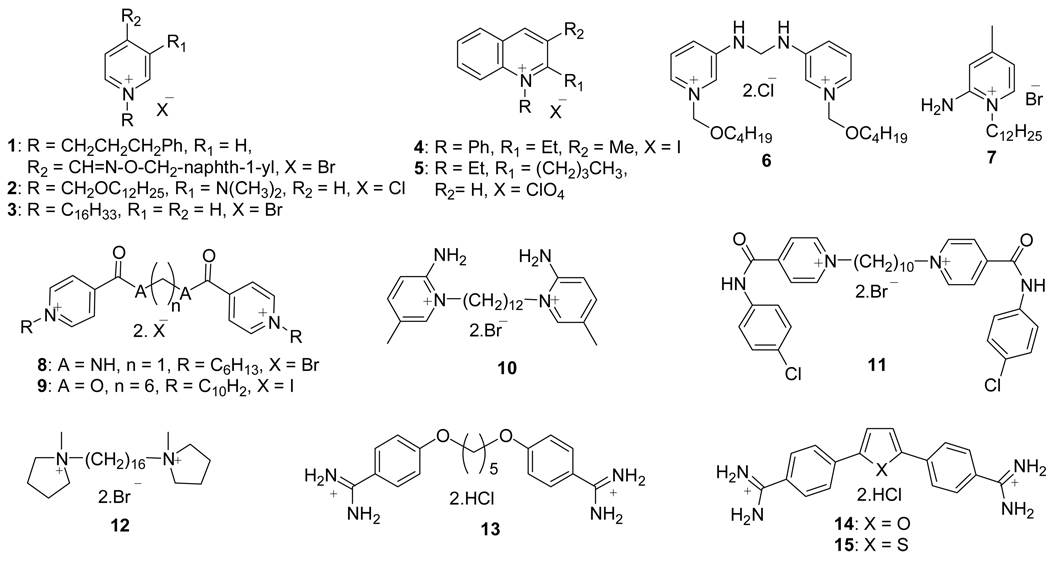
Besides this main objective, literature reports on interesting antimicrobial and antiprotozoal activities of several quaternary pyridinium class of compounds (3) prompted us to evaluate pyridinium class of AChE reactivators for these activities. Quaternary salts exhibit antimicrobial properties by adsorption to the cell wall of microorganisms, and bind to other biochemical components causing disruption of cellular processes which leads to cessation of bacterial growth and thus cell death (3). Chemical structures and IC50/ MIC/ MBC values of representative mono- and bis-quaternary pyridinium compounds with antimicrobial 1–9, (4–11) antimalarial 10–14 (12–16) and antileishmanial 15 (17) activities are shown in Figure 1 and Table 1 respectively.
Figure 1.
Structures of antimicrobial (1–6, 9), antimalarial (7, 8, 10–14) and antileishmanial (15) pyridinium salts / dications
Table 1.
Antimicrobial and antiprotozoal activities of reported pyridinium quaternary compounds
| Entry | IC50/ EC50/ MIC/ MBC | Reference |
|---|---|---|
| 1 | MIC 9.8 (S. aureus), 39.1 (E. coli), 19.5 (C. albicans), and 9.8 (E. faecalis) µg/mL | (4) |
| 2 | MIC 1.4 (S. aureus), 11 (C. albicans), 6 (B. subtilis) and 22 (E. coli) µM | (5) |
| 3 | MBC 15.6 (S. aureus), 10.4 (E. coli), 16.3 (E. faecalis) µM | (6) |
| 4 – 5 | MBC 62.5 µg/mL (S. aureus, S. albus and MRS) | (7) |
| 6 | MIC 7 (S. aureus), 27 (E. coli), 13.5 (C. albicans) and 14 (B. subtilis) µM | (8) |
| 7 | IC50 1.5 µM (P. falciparum) | (9) |
| 8 | EC50 52 nM (FCR-3 - Chloroquine sensitive strain of P. falciparum) | (10) |
| 9 | MIC 1.1 (S. aureus), 1.4 (MRS), 1.4 (B. subtilis), 5.2 (E. coli) and 13.2 (P. aeruginosa) µM | (11) |
| 10 | IC50 0.5 nM (P. falciparum) | (12) |
| 11 | EC50 8 nM (P. falciparum) | (13) |
| 12 | IC50 0.65 nM (P. falciparum) | (14) |
| 13 | IC50 19 nM (P. falciparum) | (15, 30) |
| 14 | IC50 5.5 nM (P. falciparum) | (16, 30) |
| 15 | IC50 0.82 µM (L. donovani) | (17) |
S. aureus: Staphylococcus aureus; E. coli: Escherichia coli; C. albicans: Candida albicans; E. faecalis: Enterococcus faecalis; M. catarrhalis: Moraxella catarrhalis; B. subtilis: Bacillus subtilis; P. aeruginosa: Pseudomonas aeruginosa; P. falciparum: Plasmodium falciparum; L. donovani: Leishmania donovani; MRS: Methicillin resistant Staphylococcus
Biosafety level 1 and 2 agents have been widely used as model organisms and recently Utrup and Frey (18) used several model organisms as surrogates of biological threat agents. These include Bacillus subtilis for B. anthracis (anthrax), E. coli for Variola major (Smallpox), Pseudomonas fluorescens for Burkholderia pseudomallei (melioidosis) (18). The present paper reports results of an initial screen of a series of quaternary pyridinium oximes (19, 20) against a number of lower pathogenicity BSL-1 and 2 model organisms. Use of model organisms in the first stage screening saves on the time and expense of BSL-3 studies, and allows for a determination of which compounds and BSL-3 organisms are worth further exploration.
Experimental section
General procedure for synthesis of quaternary pyridinium compounds
Hydroxyiminomethylpyridine (1 mmol) and the alkylating agent (1.5 mmol) in solvent (20 mL) were stirred at 60–80 °C for 1–24 h. The mixture was cooled to rt, the precipitate collected, washed with acetone (3 × 20 mL), dried under vacuum, and characterized by 1H NMR, 13C NMR, ESI-MS and IR (19). Spectral data for representative compounds is as follows: Bromothiophen-2-yl)-methyl-2-hydroxyiminomethylpyridinium chloride (21): Brownish black viscous oil; yield: 74%; 1H NMR (400 MHz, CD3OD): δ 8.82 (t, J = 7.2 Hz, 1H), 8.64 (m, 1H), 8.44 (t, J = 12 Hz, 1H), 8.27 (d, J = 8.0 Hz, 1H), 8.09 (m, 1H), 7.19 (d, J = 4.0 Hz, 1H), 7.06 (d, J = 4.0 Hz, 1H), 6.28 (s, 2H); 13C NMR (100 MHz, CD3OD): δ 147.4, 146.2, 142.0, 141.4, 141.0, 131.0, 128.0, 127.5, 126.9, 125.7, 56.4; ESI-MS: m/z 296.95 [M]+ (calcd for [C11H10BrN2OS]+ 297.97); 1-(Benzothiophene-3-yl)-methyl-2-hydroxyiminomethylpyridinium chloride (24): Greenish black viscous oil; yield: 64%; 1H NMR (400 MHz, CD3OD): δ 8.96 (s, 1H), 8.67 (s, 1H), 8.51 (m, 2H), 8.04 (m, 1H), 7.92 (m, 1H), 7.85 (m, 1H), 7.51 (s, 1H), 7.44 (m, 2H), 6.30 (s, 2H); 13C NMR (100 MHz, CD3OD): δ 148.6, 146.1, 145.9, 145.7, 143.1, 141.8, 140.9, 127.9, 127.7, 126.4, 125.5, 125.1, 123.1, 121.2, 56.3; ESI-MS: m/z 269.1 [M]+ calculated for [C15H13N2OS]+.
Biological testing
Antimicrobial, antimalarial, antileishmanial activities and cytotoxicity testing were performed using the protocols as reported by Samoylenko et al (21).
Results and Discussion
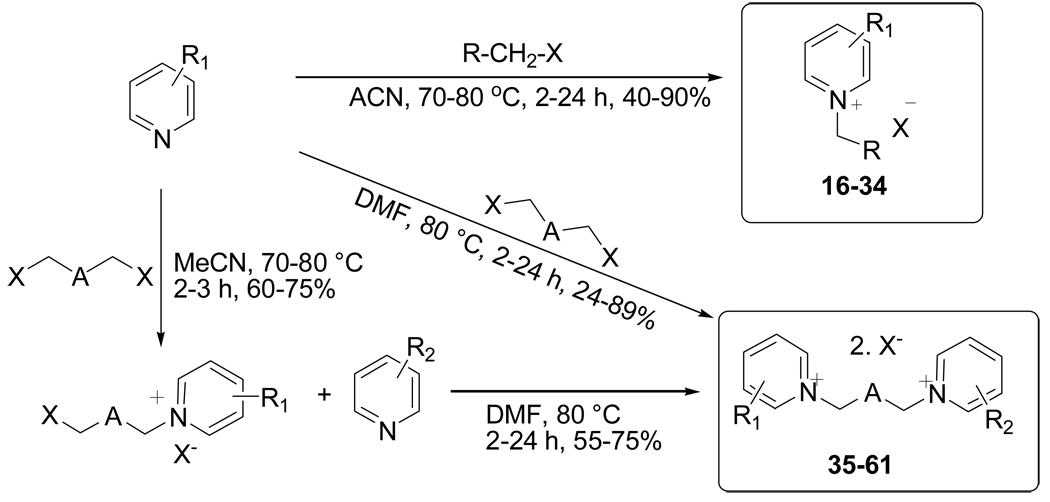
Mono- (16–34) and bisquaternary (35–61) pyridinium compounds used in this study were synthesized using a general synthetic strategy as depicted in Figure 2 and were characterized by 1H NMR, 13C NMR and ESIMS spectral data (19, 22). All synthesized compounds were screened for in vitro antimicrobial, antimalarial, antileishmanial and cytotoxic activities. Susceptibility testing for antifungal and antibacterial activity was done using a modified CLSI (formerly NCCLS) methods (21). Organisms include the fungi Candida albicans, C. glabrata, C. krusei, Aspergillus fumigates, Cryptococcus neoformans and the bacteria Staphylococcus aureus, methicillin resistant S. aureus (MRS), Pseudomonas aerugenosa, Escherichia coli. Ciprofloxacin (0.07 and 0.08 µg/ml for S. aureus and MRS) and Amphotericin B (IC50 0.76 µg/ml for Cryptococcus neoformans) were used as reference standards. Susceptibility of Mycobacterium intracellulare was done using the modified Alamar Blue procedure of Franzblau et al (23). Of all compounds tested, only monoquaternary pyridinium compounds 21 and 24 showed moderate antibacterial activity against S. aureus and methicillin-resistant S. aureus (MRS): compound 21: IC50 15.3 and 14.2 µg/mL; compound 24: IC50 12.2 and 17.7 µg/mL for S. aureus and MRS, respectively (Table 2). Both compounds 21 and 24 were inactive against all other test organisms. All other compounds showed no in vitro antifungal or antibacterial activity. Similar observations in the activity profile has been reported by Sidorchuk et al for quaternary quinolinium salts (7).
Figure 2.
General Scheme for synthesis of mono- (16–34) and bis- (35–61) quaternary pyridinium compounds (R1 = CHNOH, CONH2; R2 = CHNOH; Position of R1 or R2: 2, 3 or 4; X = Br, Cl, OMs; R = CH2CH2Br, CH2CH2CH2Br, heterocycles – thiophene, furan or isoxazole; A = O, CH2, CH2CH2, heterocycles - thiophene-furan or isoxazole).
Table 2.
Antibacterial and antifungal activities of pyridinium oximes
| IC50 (MIC) µg/mL |
|||||||||
|---|---|---|---|---|---|---|---|---|---|
| Entry |  |
Antibacterial |
Antifungal |
||||||
| S. aureus | MRS |
M. intracellulare |
C. neoformans |
C. glabrata |
A. fumigatus |
||||
| 21 | R = 5-bromothiophen-2-yl, R1 = 2-CHNOH, X = Cl |
15.27 | 14.19 | NA | na | >20 | na | ||
| 24 | R = benzothiophen-3-yl, R1 = 2-CHNOH, X = Cl |
12.23 (20) |
17.72 (20) | >20 | na | na | na | ||
| 25 | R = benzothiophen-3-yl, R1 = 3-CHNOH, X = Cl |
na | na | na | na | na | >20 | ||
| 33 | R = 3-methyl-isoxazol-5-yl, R1 = 3-CHNOH, X = Br |
na | na | na | >20 | na | na | ||
| 45 | R1 = 4-CONH2, R2 = 2- CHNOH, A = CH2CH2, X = Br |
na | na | na | >20 | na | na | ||
| 46 | R1 = 4-CONH2, R2 = 3- CHNOH, A = CH2, X = Br |
na | na | na | >20 | na | na | ||
| 50 | R1 = R2 = 2-CHNOH, A = thiophen-2,5-yl, X = Cl |
na | na | na | >20 | na | na | ||
| 51 | R1 = R2 = 3-CHNOH, A = thiophen-2,5-yl, X = Cl |
na | na | na | >20 | na | na | ||
| 52 | R1 = R2 = 4-CHNOH, A = thiophen-2,5-yl, X = Cl |
na | na | na | >20 | na | na | ||
| 53 | R1 = 4-CONH2, R2 = 4- CHNOH, A = thiophen-2,5- yl, X = Cl |
na | na | na | >20 | na | na | ||
| 58 | R1 = R2 = 4-CONH2, A = furan-2,5-yl, X = Br |
na | na | na | >20 | na | na | ||
| 60 | R1 = R2 = 3-CHNOH, A = isoxazol,3,5-yl, X = Br |
na | na | na | >20 | na | na | ||
| 61 | R1 = R2 = 4-CHNOH, A = isoxazol,3,5-yl, X = Br |
na | na | na | >20 | na | na | ||
| Ciprofloxacin | 0.1 (0.5) | 0.09 (0.25) |
0.39 (0.5) | nt | nt | nt | |||
| Amphotericin B | nt | nt | nt | 1.28 (2.5) | 0.7 (1.25) |
0.98 (2.5) | |||
IC50, the concentration that affords 50% inhibition of bacterial/ fungal growth; na, not active; nt, not tested; MIC (Minimum inhibitory concentration) is the lowest concentration (µg/ml) that allows no detectable growth; *None of the compound was active against C. albicans, C. krusei, E. coli and P. aeruginosa (data not shown).
Although compounds 33, 45, 46, 50–53, 58, 60 and 61 showed some antifungal activity against Cryptococcus neoformans with IC50 > 20 µg/mL, none of the compound showed significant antifungal activity against any of the organisms. In vitro antimalarial activity was evaluated against chloroquine sensitive (D6) and chloroquine resistant (W2) clones of P. falciparum. Determination of in vitro antimalarial activity was based on the determination of plasmodial LDH activity (24). Compounds were tested at three concentrations 4.76, 1.59 and 5.29 µg/mL. Chloroquine and artemisinin were used as reference standards. Monoquaternary pyridinium compound 17 showed antimalarial activities against both the clones of Plasmodium falciparum with the IC50 of 3.7 (D6 clone) and 4.0 (W2 clone) µg/mL with selectivity index of more than 1.3 (Table 3). None of the other compounds were active.
Table 3.
Antimalarial and antileishmanial activities of pyridinium oximes
| Entry |  |
Antimalarial | Antileishmanial | Cytotoxicity | ||
|---|---|---|---|---|---|---|
|
P. falciparum |
L. donovani | Vero cells | ||||
| D6 Clone | W2 Clone | |||||
| IC50 µg/mL | IC50 µg/mL | IC90 µg/mL | IC50 ng/mL | |||
| 17 | R = CH2CH2CH2Br, R1 = 4-CONH2, X = Br |
3.7 (S.I. > 1.3) |
4.0 (S.I. > 1.2) |
na | na | nc |
| 21 | R = 5-bromothiophen-2-yl, R1 = 2-CHNOH, X = Cl |
na | na | 19 | 40 | nc |
| 24 | R = benzothiophen-3-yl, R1 = 2-CHNOH, X = Cl |
na | na | 18 | 40 | nc |
| Chloroquine | 0.015 | 0.12 | nt | nt | nt | |
| Artemisinin | 0.010 | 0.0065 | nt | nt | nt | |
| Pentamidine | nt | nt | 1.2 | 5 | nt | |
| Amphotericin B | nt | nt | 0.2 | 0.4 | nt | |
IC50, the concentration that affords 50% inhibition of plasmodial / leishmanial growth; IC90, the concentration that affords 90% inhibition of leishmanial growth; na, not active; nt, not tested; nc, Not cytotoxic; S.I., selectivity index = IC50 vero cells/IC50 P. falciparum.
Antileishmanial activity against L. donovani promastigotes was determined by the Alamar Blue assay (25, 26). Monoquaternary pyridinium compounds bearing thiophene functionality 21 and 24 showed antileishmanial activity against L. donovani with IC50 of 19 and 18 µg/mL, respectively (Table 3). None of the compounds were found to have any cytotoxic effects towards mammalian kidney fibroblasts (vero cells) (27).
Although a series of pyridinium compounds tested in the present study varied in structural features to allow for a critical study of structure-activity relationships, but this class of compound was almost devoid of any significant antimicrobial, antimalarial or antileishmanial properties. None of the compound demonstrated any activities comparable to standards used in the study. Mainly, bisquaternary pyridinium oximes displayed a complete lack of activity. Therefore, a detailed, meaningful analysis of structure-activity relationships was not possible. The only active compounds were two monoquaternary compounds 21 and 24 possessing a thiophene and banzothiophene linker chains with antibacterial and antileishmanial activities and another monoquaternary compound 17 with antimalarial activity. It is noteworthy to mention that compounds 21 and 24 which were already reported to possess AChE reactivation ability (19) also exhibited moderate antimicrobial and antileishmanial activities. In contrast, commercially used bisquaternary pyridinium antidotes trimedoxime (42) and obidoxime (37) with potent AChE reactivation abilities (19) were completely devoid of any antimicrobial or antiprotozoal activities. The major difference between reported antimicrobial / antimalarial quaternary pyridinium salts and compounds studied in this paper is that most of the compounds have long aliphatic chains (C4–C20) (Figure 1). This long aliphatic chain appears important for antimicrobial or antiprotozoal activity of quaternary salts. The poor or complete lack of activity of our compounds may be due to this difference. Thus, this work suggests that the aliphatic chain attached to pyridinium skeleton may be important for retaining antimicrobial/ antiprotozoal activity. Merely, the presence of pyridinium skeleton does not suffice requirements to inhibit microbes or parasites.
Trimedoxime, obidoxime, HI-6 and several other oximes are known to be tolerated in high doses in humans and have been used as warfare agents for years (28, 29). It was anticipated that the ability for safely achieving high serum concentrations of these compounds would allow for a significant antimicrobial effect against biological threats and might provide advantage in the event of a combined nerve agent/ biological-agent incident. Unfortunately, based on initial in-vitro studies in model organisms, the compounds appear to be tolerated as well by bacteria and protozoa as seen for mammals. Therefore, the utility of this class of compound does not extend to biological agents.
In conclusion, pyridinium oxime compounds which are widely used as antidotes for nerve gas poisoning, do not have ability to provide protection against microbial and protozoal infections. Results from this work provided important information about antidotes of OP poisoning that they cannot be relied upon for additional protection against biological threats in the event of combined chemical-biological incident.
Supplementary Material
Acknowledgements
The synthesis of compounds was supported by NIH UO1-ES016102 (CMT) and SBIR grants to ATERIS Technologies LLC (R43 ES016392 U44 NS058229). Support from the Core Laboratory for Neuromolecular Production (NIH P30-NS055022) and the Center for Structural and Functional Neuroscience (NIH P20-RR015583) is appreciated. The authors wish to thank Melissa R. Jacob, Shabana I. Khan, Ikhlas A. Khan, Troy J. Smillie, Babu L. Tekwani, and Marsha A. Wright for antimicrobial testing. The antimicrobial/ antiprotozoal testing was supported by the NIH, NAID, Division of AIDS, Grant No. AI 27094 and the USDA Agricultural Research Service Specific Cooperative Agreement No. 58-6408-2-0009.
Footnotes
Chemical structures of all mono- (16–34) and bis- (35–61) quaternary pyridinium oximes.
References
- 1.Bajgar J. Organophosphates/ nerve agent poisoning: mechanism of action, diagnosis, prophylaxis, and treatment. Adv. Clin. Chem. 2004;38:151–216. doi: 10.1016/s0065-2423(04)38006-6. [DOI] [PubMed] [Google Scholar]
- 2.Kassa J. Review of oximes in the antidotal treatment of poisoning by organophosphorus nerve agents. Clin. Toxicol. 2002;40:803–816. doi: 10.1081/clt-120015840. [DOI] [PubMed] [Google Scholar]
- 3.Madaan P, Tyagi VK. Quaternary pyridinium salts: A review. J. Oleo Sci. 2008;57:197–215. doi: 10.5650/jos.57.197. [DOI] [PubMed] [Google Scholar]
- 4.Alptuzuun V, Tasli H, Erciyas E. Synthesis and antimicrobial activities of some pyridinium salts. Ankara Ecz. Fak. Derg. 2006;35:177–188. [Google Scholar]
- 5.Pernak J, Branicka M. The properties of 1-alkoxymethyl-3-hydroxypyridinium and 1-alkoxymethyl-3-dimethylaminopyridinium chlorides. J. Surfactants Deterg. 2003;6:119–123. [Google Scholar]
- 6.Haldar J, Kondaiah P, Bhattacharya S. Synthesis and antibacterial properties of novel hydrolyzable cationic amphiphiles. Incorporation of multiple head groups leads to impressive antibacterial activity. J. Med. Chem. 2005;48:3823–3831. doi: 10.1021/jm049106l. [DOI] [PubMed] [Google Scholar]
- 7.Sidorchuk II, Stadniichuk RF, Tishchenko EI, Bordyakovskya LT. Antimicrobial activity of quaternary quinolinium salts. Pharm. Chem. J. 1978;12:893–895. [Google Scholar]
- 8.Pernak J, Rogoza J, Mirska I. Synthesis and antimicrobial activities of new pyridinium and benzimidazolium chlorides. Eur. J. Med. Chem. 2001;36:313–320. doi: 10.1016/s0223-5234(01)01226-0. [DOI] [PubMed] [Google Scholar]
- 9.Salom-Roig XJ, Hamze A, Calas M, Vial HJ. Dual molecules as new antimalarials. Comb. Chem. High throughput Screening. 2005;8:49–62. doi: 10.2174/1386207053328219. [DOI] [PubMed] [Google Scholar]
- 10.Fujimoto K, Morisaki D, Yoshida M, Namba T, Hye-Sook K, Wataya Y, Kourai H, Kakuta H, Sasaki K. Antimalarial effect of bis-pyridinium salts, N,N'-hexamethylenebis(4-carbamoyl-1-alkylpyridinium bromide) Bioorg. Med. Chem. Lett. 2006;16:2758–2760. doi: 10.1016/j.bmcl.2006.02.030. [DOI] [PubMed] [Google Scholar]
- 11.Maeda T, Manabe Y, Yamamoto M, Yoshida M, Okazaki K, Nagamune H, Kourai H. Synthesis and antimicrobial characteristices of novel biocides, 4,4'-(1,6-hexamethylenedioxydicarbonyl)bis(1-alkylpyridinium iodide)s. Chem. Pharm. Bull. 1999;47:1020–1023. doi: 10.1248/cpb.47.1020. [DOI] [PubMed] [Google Scholar]
- 12.Calas M, Ouattara M, Piquet G, Ziora Z, Bordat Y, Ancelin ML, Escale R, Vial H. Potent antimalarial activity of 2-aminopyridinium salts, amidines, and guanidines. J. Med. Chem. 2007;50:6307–6315. doi: 10.1021/jm0704752. [DOI] [PubMed] [Google Scholar]
- 13.Yoshikawa M, Motoshima K, Fujimoto K, Tai A, Kakuta H, Sasaki K. Pyridinium cationic-dimer antimalarials, unlike chloroquine, act selectively between the schizont stage and the ring stage of Plasmodium falciparum. Bioorg. Med. Chem. 2008;16:6027–6033. doi: 10.1016/j.bmc.2008.04.051. [DOI] [PubMed] [Google Scholar]
- 14.Calas Ml, Cordina Gr, Bompart J, Bari MB, Jei Tb, Ancelin ML, Vial H. Antimalarial activity of molecules interfering with Plasmodium falciparum phospholipid metabolism. Structure-activity relationship analysis. J. Med. Chem. 1997;40:3557–3566. doi: 10.1021/jm9701886. [DOI] [PubMed] [Google Scholar]
- 15.Tidwell RR, Boykin DW. Dicationic DNA minor groove binders as antimicrobial agents. In: Demeunynck M, Bailly C, Wilson WD, editors. Small molecule DNA and RNA binders: from synthesis to nucleic acid complexes. Volume 2. New York: Wiley-VCH; 2003. pp. 414–460. [Google Scholar]
- 16.Ismail MA, Brun R, Wenzler T, Tanious FA, Wilson WD, Boykin DW. Novel dicationic imidazo[1,2-a]pyridines and 5,6,7,8-tetrahydro-imidazo[1,2-a]pyridines as antiprotozoal agents. J. Med. Chem. 2004;47:3658–3664. doi: 10.1021/jm0400092. [DOI] [PubMed] [Google Scholar]
- 17.Brendle JJ, Outlaw A, Kumar A, Boykin DW, Patrick DA, Tidwell RR, Werbovetz KA. Antileishmanial activities of several classes of aromatic dications Antimicrob. Agents Chemother. 2002;46:797–807. doi: 10.1128/AAC.46.3.797-807.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Utrup LJ, Frey AH. Fate of bioterrorism-relevant viruses and bacteria, including spores, aerosolized into an indoor air environment. Exp. Biol. Med. 2004;229:345–350. doi: 10.1177/153537020422900409. [DOI] [PubMed] [Google Scholar]
- 19.Bharate SB, Guo L, Reeves TE, Cerasoli DM, Thompson CM. New series of monoquaternary pyridinium oximes: synthesis and reactivation potency for paraoxon-inhibited electric eel and recombinant human acetylcholinesterase. Bioorg. Med. Chem. Lett. 2009;19:5101–5104. doi: 10.1016/j.bmcl.2009.07.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bharate SB, Guo L, Reeves TE, Cerasoli DM, Thompson CM. Bisquaternary pyridinium oximes: Comparison of in vitro reactivation potency of compounds bearing aliphatic linkers and heteroaromatic linkers for paraoxon-inhibited electric eel and recombinant human acetylcholinesterase. Bioorg. Med. Chem. 2010;18:787–794. doi: 10.1016/j.bmc.2009.11.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Samoylenko V, Ashfaq MK, Jacob MR, Tekwani BL, Khan SI, Manly SP, Joshi VC, Walker LA, Muhammad I. Indolizidine, antiinfective and antiparasitic compounds from Prosopis glandulosa var. glandulosa. J. Nat. Prod. 2009;72:92–98. doi: 10.1021/np800653z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kuca K, Bielavsky J, Cabal J, Bielavska M. Synthesis of a potential reactivator of acetylcholinesterase-1-(4-hydroxyiminomethylpyridinium)-3-(carbamoylpyridinium)-propane dibromide. Tetrahedron Lett. 2003;44:3123–3125. [Google Scholar]
- 23.Franzblau SG, Witzig RS, McLaughlin JC, Torres P, Madico G, Hernandez A, Degnan MT, Cook MB, Quenzer VK, Ferguson RM, Gilman RH. Rapid, low-technology MIC determination with clinical M. tuberculosis isolates by using the microplate Alamar Blue assay. J. Clin. Microbiol. 1998;36:362–366. doi: 10.1128/jcm.36.2.362-366.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Makler MT, Hinrichs DJ. Measurement of the lactate dehydrogenase activity of Plasmodium falciparum as an measurement of parasitemia. Am. J. Trop. Med. Hyg. 1993;48:205–210. doi: 10.4269/ajtmh.1993.48.205. [DOI] [PubMed] [Google Scholar]
- 25.Ma G, Khan SI, Jacob MR, Tekwani BL, Li Z, Pasco DS, Walker LA, Khan IA. Antimicrobial and antileishmanial activities of hypocrellins A and B. Antimicrob. Agents Chemother. 2004;48:4450–4452. doi: 10.1128/AAC.48.11.4450-4452.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mikus J, Steverding D. A simple colorimetric method to screen drug cytotoxicity against Leishmania using the dye Alamar Blue. Parasitol. Int. 2000;48:265–269. doi: 10.1016/s1383-5769(99)00020-3. [DOI] [PubMed] [Google Scholar]
- 27.Mustafa J, Khan SI, Ma G, Walker LA, Khan IA. Synthesis and anticancer activities of fatty acid analogs of podophyllotoxin. Lipids. 2004;39:167–172. doi: 10.1007/s11745-004-1215-5. [DOI] [PubMed] [Google Scholar]
- 28.Kusic R, Boskovic B, Vojvodic V, Jovanovic D. HI-6 in man: blood levels, urinary excretion, and tolerance after intramuscular administration of the oxime to healthy volunteers. Fund. Appl. Toxicol. 1985;5:S89–S97. doi: 10.1093/toxsci/5.6part2.89. [DOI] [PubMed] [Google Scholar]
- 29.Jovanovic D, Maksimovic M, Joksovic V. Oral forms of the oxime HI-6: a study of the pharmacokinetics and tolerance after administration to healthy volunteers. Vet. Hum. Toxicol. 1990;32:419–421. [PubMed] [Google Scholar]
- 30.Wilson WD, Tanious FA, Mathis A, Tevis D, Hall JE, Boykin DW. Antiparasitic compounds that target DNA. Biochimie. 2008;90:999–1014. doi: 10.1016/j.biochi.2008.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.