Recent work indicates that mitochondrial ROS act via several pathways to elicit proinflammatory cytokines in human and mouse cells.
Abstract
High levels of reactive oxygen species (ROS) are observed in chronic human diseases such as neurodegeneration, Crohn’s disease, and cancer. In addition to the presence of oxidative stress, these diseases are also characterized by deregulated inflammatory responses, including but not limited to proinflammatory cytokine production. New work exploring the mechanisms linking ROS and inflammation find that ROS derived from mitochondria act as signal-transducing molecules that provoke the up-regulation of inflammatory cytokine subsets via distinct molecular pathways.
The pleoitropic effect of inflammatory cytokines demands complex and multifaceted regulation to limit collateral damage to normal tissue. On one level, inflammatory cytokines such as interleukin 1β (IL-1β) and IL-18 exist as inactive zymogens that require proteolytic cleavage mediated by the inflammasome (Martinon et al., 2008). This proteolytic maturation acts in concert with NF-κB–dependent transcriptional activation of genes encoding the zymogens to catalyze a substantial increase in the levels of secreted, bioactive protein. Other inflammatory cytokines such as tumor necrosis factor (TNF) and IL-6 are induced primarily at the transcriptional level and, in the case of TNF, subject to posttranscriptional messenger RNA stabilization by virtue of the dissociation of the mRNA-binding protein tristetraprolin from its adenine- and uridine-rich (ARE) region (Anderson, 2000). Although these are well-established mechanisms of regulating proinflammatory cytokine production, it is becoming apparent that an additional layer of complexity exists; i.e., signals provided by ROS.
The mitochondrion plays a critical role in cell survival, most prominently by generating the vast majority of a cell’s supply of adenosine triphosphate (ATP), but also by influencing apoptosis, cell cycle, and metabolism. Mitochondria generate ATP through aerobic respiration, whereby glucose, pyruvate, and NADH are oxidized, thus generating ROS as a byproduct. In normal circumstances, the deleterious effects caused by the highly reactive nature of ROS are balanced by the presence of antioxidants, including glutathione, carotenoids, and antioxidant enzymes such as catalase and glutathione peroxidase. However, several chronic human diseases associated with inflammation are also characterized by excessive ROS production (Drake et al., 1998; Cominelli, 2004; Reuter et al., 2010). Perhaps not surprisingly, defective mitochondria have also been implicated in human diseases with underlying inflammatory pathologies, such as diabetes mellitus and cardiac dysfunction (DiMauro and Schon, 2003; Nisoli et al., 2007; Patti and Corvera, 2010).
Three recent publications, including Bulua et al. in this issue, indicate that mitochondrial ROS (mtROS) act as signaling molecules to trigger proinflammatory cytokine production (Nakahira et al., 2011; Zhou et al., 2011). These observations provide much needed clarification about the cellular source of ROS that impacts the production of certain inflammatory cytokines. Although all three publications underscore the importance of mtROS-dependent signaling, they differ most notably by describing either inflammasome-dependent or inflammasome-independent roles for ROS.
Using cells from patients with TNFR1-associated periodic syndrome (TRAPS), along with a relevant mouse model, Bulua et al. (2011) demonstrate that mtROS influences the transcription of proinflammatory cytokines such as IL-6. In its most severe form, TRAPS manifests as episodes of fever and severe localized inflammation that is associated with “structural” mutations in TNFR1. These TNFR1 mutations interfere with down-regulation of cell surface TNFR expression, result in retention of the receptor within the endoplasmic reticulum, confer ligand (TNF) independence, and prolong the cytokine response to lipopolysaccharide (LPS; McDermott et al., 1999; Simon et al., 2010; Bulua et al., 2011).
Bulua et al. (2011) found higher baseline levels of mtROS in monocytes and neutrophils from TRAPS patients and in mouse embryonic fibroblasts (MEFs) engineered to express heterozygous TRAPS-associated TNFR1 mutations. The enhancement of ROS levels in the mutant MEFs correlated with an increase in the levels of active JNK and p38, a finding consistent with previous research linking persistent MAPK activation to manifestation of the TRAPS phenotype (Simon et al., 2010). Inhibition of mtROS production inhibited MAPK activation and production of IL-6 and TNF in cells from TRAPS patients (Bulua et al., 2011), a finding consistent with a previous study showing that ROS can inactivate MAPK phosphatases (Kamata et al., 2005). Thus, mitochondria are the cellular source of the excessive ROS in TRAPS, and a delicate equilibrium between MAP kinase and phosphatase activity defines a rheostat regulated primarily by the levels of mtROS, which in turn enables an inflammatory response to proceed in a timely and effective fashion. Notably, LPS-induced IL-6 production was reduced even in healthy cells treated with mtROS inhibitors, indicating that this signaling cascade is relevant to the induction of a normal inflammatory response.
Pharmacological interventions, such as IL-1β antagonists, which are used to treat cryopyrinopathies caused by perturbed inflammasome activation, have limited efficacy for the treatment of TRAPS. Although it is anticipated that mechanisms of reducing mtROS levels may be of therapeutic utility for TRAPS, it remains to be determined how mtROS levels themselves are modulated. However, some insight is provided by the observation that macrophages lacking NLRP3, caspase-1, or IL-1R produce normal levels of TNF and IL-6 after LPS stimulation (Bulua et al., 2011). These observations confirm that neither mtROS production nor the signals transduced by mtROS depend on activation of the inflammasome. In a critical mechanistic divergence, two recent studies (Nakahira et al., 2011; Zhou et al., 2011) identify an inflammasome-dependent role for mtROS (Fig. 1).
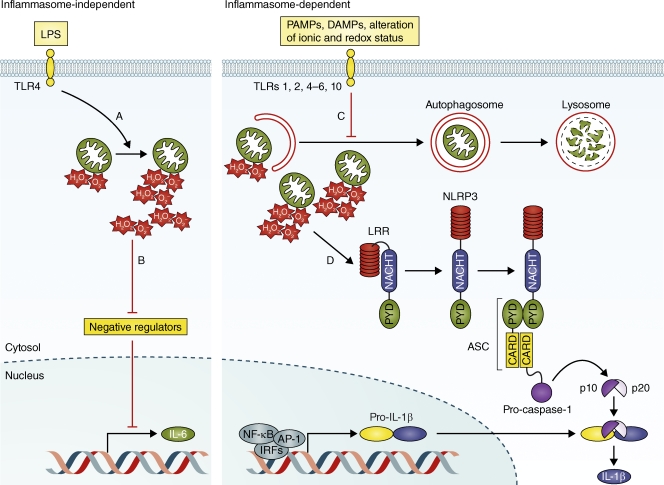
Figure 1.
Putative mechanisms of ROS-dependent proinflammatory cytokine production in normal, healthy cells. During inflammasome-independent proinflammatory cytokine production (left), mtROS modulates the equilibrium between positive and negative regulators, which results in an increase in the rate of transcription of cytokines such as IL-6. Several steps in this pathway require further investigation. Specifically, how does activation of TLR4 by LPS result in an increase in ROS production from an individual mitochondrion (A), and how does mtROS inhibit negative regulators (e.g., MAPK phosphatases) of cytokine gene transcription (B)? Inflammasome-dependent proinflammatory cytokine production (right) requires inhibition of autophagy and mitophagy to increase the net amount of mtROS within the cell. The mechanism of this inhibition is unknown (C). Once mtROS accumulates, it activates the NLRP3 inflammasome, but the mechanism of this activation remains undefined (D). Once activated, the NLRP3 inflammasome undergoes a conformational change and assembles into a homooligomeric complex (one subunit is depicted) that catalyzes the activation of pro–caspase-1. In the case of IL-β, the active p10-p20 caspase-1 heterodimer cleaves pro–IL-1β to produce a mature form that is subsequently exported from the cell. AP-1, activator protein 1; ASC, apoptosis-associated speck-like protein; CARD, caspase recruitment domain; DAMP, damage-associated molecular patterns; IRF, interferon response factor; LRR, leucine-rich repeat; PAMP, pathogen-associated molecular patterns; PYD, pyrin domain.
The inflammasome does not regulate transcription directly, but rather is responsible for the activation of caspase-1, an enzyme responsible for the proteolytic maturation of IL-1β and IL-18. Since the initial identification of inflammasomes, much interest has been given to identifying the initiating events that lead to the assembly of these multiprotein scaffolds. The multiplicity and apparent divergent nature of NLRP3-dependent stimuli have identified this particular inflammasome as a focus of investigation. Models posited for the activation of the NLRP3 inflammasome include direct interaction with cognate ligand, and conformational alteration induced by secondary messengers or alterations in the intracellular milieu resulting from lysosomal rupture (Latz, 2010). Consistent with a secondary messenger model, Zhou et al. (2011) and Nakahira et al. (2011) demonstrate that after an inflammatory stimulus, the accumulation of damaged mitochondria precipitates an increase in mtROS production, which in turn enhances inflammasome activation.
Autophagy is the process of “self-digestion” that occurs by delivery of self-components to the lysosomal machinery. Mitophagy is a specialized form of autophagy that refers to the specific catabolism of mitochondria. Pharmacological inhibition of autophagy by treatment of macrophages with 3-methyladenine resulted in the accumulation of damaged mitochondria and an increase in the net amount of mtROS (Zhou et al., 2011). IL-1β secretion induced by mtROS elevation was abolished in Nlrp3−/− and Asc−/− cells, thus confirming that the NLRP3 inflammasome is activated in a ROS-dependent manner. In bone marrow-derived macrophages (BMDMs), genetic deletion of the autophagy-related protein LC3B, which prevents the formation and maturation of autophagosomes, also enhanced caspase-1 activation and production of IL-1β and IL-18 in response to the inflammasome-dependent stimuli LPS and ATP (Nakahira et al., 2011). Similar results were observed after deletion of the critical upstream regulator of autophagy, Beclin 1. In addition, perturbation of basal levels of autophagy had a profound impact on the integrity of the mitochondria, as Beclin or LC3-deficient macrophages generated more mitochondrial superoxide anion radical (O2−) in the absence of stimulation. Thus, autophagy regulates baseline mtROS production from individual mitochondria by a yet to be identified mechanism.
The release of mitochondrial DNA (mtDNA) in to the cytosol after treatment of BMDMs with LPS and ATP potentiated caspase-1 activation (Nakahira et al., 2011). Thus, mtDNA is an additional secondary messenger that acts as a surrogate marker of mitochondrial dysfunction and also activates caspase-1, presumably to amplify the inflammatory response. This amplification loop depends on NLRP3, as NLRP3 deficiency suppressed the release of mtDNA after LPS and ATP stimulation.
Collectively, these two investigations clearly established that in normal healthy cells the process of mitophagy is a critical negative regulator of inflammasome activation. This implies that active inhibition of autophagy is required for proinflammatory signaling to proceed, for damaged mitochondria to accumulate, for intracellular mtROS to increase, for mtDNA release, and for the NLRP3 inflammasome to be activated. Consequently, a pressing question is, how do NLRP3-dependent stimuli inhibit autophagy?
The intimate relationship between mtROS production and proinflammatory cytokine activation is now well established. The fact that different cytokines are subjected to distinct regulatory events, simplistically defined as either transcriptional or posttranslational, suggests that the generality of any given mechanism is likely to be limited. The distinct temporal regulation of IL-1β (early response) and TNF and IL-6 (late response) also suggests that different cytokines will be influenced in distinct ways by the production of mtROS. For example, are MAPK phosphatases only inhibited in the presence of higher levels of mtROS that presumably accumulate at later stages of an inflammatory response? The cellular source of physiologically relevant ROS is also stimulus dependent, as inflammatory signaling downstream of TLR ligation is dependent on mtROS, whereas crystalline particles such as asbestos and silica require both NADPH oxidase and mtROS (Dostert et al., 2008; Nakahira et al., 2011; Zhou et al., 2011). This underscores the importance of defining the physiologically relevant amount and source of ROS for each class of stimuli.
Further experiments will illuminate whether mtROS impacts other proinflammatory transcriptional networks, even though it does not modulate IRF3-dependent induction of IFN-γ (Bulua et al., 2011). Consequently, elucidating how ROS signaling acquires specificity for any given transcriptional network is of critical importance. Because of the highly reactive nature of ROS, its signal transduction properties are likely to include direct chemical modification of its targets and/or catalysis of enzymatic reactions (e.g., H2O2). Indeed, the redox regulator superoxide (SOD-1) modifies the redox-sensitive cysteines of caspase-1 to modulate its activation (Meissner et al., 2008). NLRP3 and ASC relocalize to the mitochondria after inflammasome activation (Zhou et al., 2011), but whether this relocalization facilitates such modifications has yet to be demonstrated and is much anticipated. Another important issue is whether mtROS, or ROS in general, influences the activation of other inflammasomes such as IPAF/NLRC4 or AIM2 inflammasomes. The observation that macrophages lacking the IPAF/NLRC4 inflammasome respond normally to artificial induction of ROS and that mtROS production is dispensable for activation of IPAF and AIM2 inflammasomes (Zhou et al., 2011) argues against this and raises the question of how specificity for the NLRP3-inflammasome is acquired. Answers to such challenging questions will provide further refinement of the model of ROS-dependent proinflammatory cytokine production, as well as information necessary for the development of novel therapeutic interventions for ROS-driven human diseases.
References
- Anderson P. 2000. Post-transcriptional regulation of tumour necrosis factor alpha production. Ann. Rheum. Dis. 59:i3–i5 10.1136/ard.59.suppl_1.i3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bulua A.C., Simon A., Maddipati R., Pelletier M., Park H., Kim K.-Y., Sack M.N., Kastner D.L., Siegel R.M. 2011. Mitochondrial reactive oxygen species promote production of proinflammatory cytokines and are elevated in TNFR1-associated periodic syndrome (TRAPS). J. Exp. Med. 208:519–533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cominelli F. 2004. Cytokine-based therapies for Crohn’s disease—new paradigms. N. Engl. J. Med. 351:2045–2048 10.1056/NEJMp048253 [DOI] [PubMed] [Google Scholar]
- DiMauro S., Schon E.A. 2003. Mitochondrial respiratory-chain diseases. N. Engl. J. Med. 348:2656–2668 10.1056/NEJMra022567 [DOI] [PubMed] [Google Scholar]
- Dostert C., Pétrilli V., Van Bruggen R., Steele C., Mossman B.T., Tschopp J. 2008. Innate immune activation through Nalp3 inflammasome sensing of asbestos and silica. Science. 320:674–677 10.1126/science.1156995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drake I.M., Mapstone N.P., Schorah C.J., White K.L.M., Chalmers D.M., Dixon M.F., Axon A.T.R. 1998. Reactive oxygen species activity and lipid peroxidation in Helicobacter pylori associated gastritis: relation to gastric mucosal ascorbic acid concentrations and effect of H pylori eradication. Gut. 42:768–771 10.1136/gut.42.6.768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamata H., Honda S., Maeda S., Chang L., Hirata H., Karin M. 2005. Reactive oxygen species promote TNFalpha-induced death and sustained JNK activation by inhibiting MAP kinase phosphatases. Cell. 120:649–661 10.1016/j.cell.2004.12.041 [DOI] [PubMed] [Google Scholar]
- Latz E. 2010. The inflammasomes: mechanisms of activation and function. Curr. Opin. Immunol. 22:28–33 10.1016/j.coi.2009.12.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinon F., Major A., Tschopp J. 2008. The inflammasomes: guardians of the body. Annu. Rev. Immunol. 27:229–265 10.1146/annurev.immunol.021908.132715 [DOI] [PubMed] [Google Scholar]
- McDermott M.F., Aksentijevich I., Galon J., McDermott E.M., Ogunkolade B.W., Centola M., Mansfield E., Gadina M., Karenko L., Pettersson T., et al. 1999. Germline mutations in the extracellular domains of the 55 kDa TNF receptor, TNFR1, define a family of dominantly inherited autoinflammatory syndromes. Cell. 97:133–144 10.1016/S0092-8674(00)80721-7 [DOI] [PubMed] [Google Scholar]
- Meissner F., Molawi K., Zychlinsky A. 2008. Superoxide dismutase 1 regulates caspase-1 and endotoxic shock. Nat. Immunol. 9:866–872 10.1038/ni.1633 [DOI] [PubMed] [Google Scholar]
- Nakahira K., Haspel J.A., Rathinam V.A.K., Lee S.-J., Dolinay T., Lam H.C., Englert J.A., Rabinovitch M., Cernadas M., Kim H.P., Fitzgerald K.A., Ryter S.W., Choi A.M. 2011. Autophagy proteins regulate innate immune responses by inhibiting the release of mitochondrial DNA mediated by the NALP3 inflammasome. Nat. Immunol. 12:222–230 10.1038/ni.1980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nisoli E., Clementi E., Carruba M.O., Moncada S. 2007. Defective mitochondrial biogenesis: a hallmark of the high cardiovascular risk in the metabolic syndrome? Circ. Res. 100:795–806 10.1161/01.RES.0000259591.97107.6c [DOI] [PubMed] [Google Scholar]
- Patti M.-E., Corvera S. 2010. The role of mitochondria in the pathogenesis of type 2 diabetes. Endocr. Rev. 31:364–395 10.1210/er.2009-0027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuter S., Gupta S.C., Chaturvedi M.M., Aggarwal B.B. 2010. Oxidative stress, inflammation, and cancer: how are they linked? Free Radic. Biol. Med. 49:1603–1616 10.1016/j.freeradbiomed.2010.09.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon A., Park H., Maddipati R., Lobito A.A., Bulua A.C., Jackson A.J., Chae J.J., Ettinger R., de Koning H.D., Cruz A.C., Kastner D.L., Komarow H., Siegel R.M. 2010. Concerted action of wild-type and mutant TNF receptors enhances inflammation in TNF receptor 1-associated periodic fever syndrome. Proc. Natl. Acad. Sci. USA. 107:9801–9806 10.1073/pnas.0914118107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou R., Yazdi A.S., Menu P., Tschopp J. 2011. A role for mitochondria in NLRP3 inflammasome activation. Nature. 469:221–225 10.1038/nature09663 [DOI] [PubMed] [Google Scholar]