Antibody-mediated gp120 shedding and HIV neutralization exhibit similar kinetics and thermodynamic requirements.
Abstract
Interference with virus entry is known to be the principle mechanism of HIV neutralization by antibodies, including 2F5 and 4E10, which bind to the membrane-proximal external region (MPER) of the gp41 envelope protein. However, to date, the precise molecular events underlying neutralization by MPER-specific antibodies remain incompletely understood. In this study, we investigated the capacity of these antibodies to irrevocably sterilize HIV virions. Long-term effects of antibodies on virions can differ, rendering neutralization either reversible or irreversible. MPER-specific antibodies irreversibly neutralize virions, and this capacity is associated with induction of gp120 shedding. Both processes have similar thermodynamic properties and slow kinetics requiring several hours. Antibodies directed to the CD4 binding site, V3 loop, and the MPER can induce gp120 shedding, and shedding activity is detected with high frequency in plasma from patients infected with divergent genetic HIV-1 subtypes. Importantly, as we show in this study, induction of gp120 shedding is closely associated with MPER antibody inhibition, constituting either a primary event leading to virion neutralization or representing an immediate consequence thereof, and thus needs to be factored into the mechanistic processes underlying their activity.
Neutralization of HIV by antibodies is generally attributed to antibody occupancy of the envelope trimers and interference with viral attachment to host cell receptors or entry, but the precise underlying molecular mechanisms leading to neutralization by most neutralizing antibodies identified to date await clarification (Zwick and Burton, 2007). A considerable effort in the development of HIV vaccines has been directed toward eliciting neutralizing antibody responses that mimic activities of the membrane-proximal external region (MPER)–specific mAbs 2F5 and 4E10. Information on the structural composition of the MPER has broadened in recent years, and studies of epitope accessibility have highlighted the possibility that neutralization by MPER-specific antibodies may require the recognition of their epitopes in the context of membrane lipids (Cardoso et al., 2007; Montero et al., 2008; Alam et al., 2009) and may depend on the formation of the prehairpin intermediate state during viral entry (Frey et al., 2008, 2010; Alam et al., 2009). Whether these antibodies can act on free virus in the absence of target cells and receptor engagement remains uncertain, and thus the precise window of action of these antibodies, their modes of epitope recognition, and a detailed mechanism of neutralization still await further definition.
Our understanding of the biochemical activity and biological function of neutralizing antibodies is shaped by studies that have assessed the initial interaction between antibodies and HIV and their capacity to block virus entry in relatively short-term experimental settings but have left the fate of neutralized virions largely unexplored. HIV neutralization is commonly assessed under conditions that do not allow discrimination between actions of the neutralizing antibody on the free virus and actions after receptor engagement, as antibody is usually present throughout all steps, including preincubation and infection. In this study, we specifically investigated the actions of MPER mAbs on virions in the absence of target cell interactions to determine whether and to what extent this type of antibody can contribute to clearance of virus particles in vivo.
Whether neutralization causes an irreversible deactivation of HIV or whether virions can regain activity after antibody dissociation may also significantly impact the in vivo efficacy of a given antibody. In vivo virions can persist for extended periods of time before encountering appropriate target cells or being cleared by phagocytes. The sequestration of HIV by DCs is well documented in this respect. Trapped by DCs, the virus can remain infectious for several days and efficiently be transferred to CD4+ T cells (Turville et al., 2004; Yu et al., 2008). Should neutralization be reversible, trafficking of antibody-opsonized virus to intracellular compartments or anatomical sites with lower antibody concentration could potentially lead to antibody dissociation and reconstitute the virus’s infectivity. Achieving irreversible neutralization is thus clearly desirable for both natural and vaccine-elicited immune responses.
In this study, we aimed to obtain insight into the long-term effect of broadly neutralizing antibodies on cell-free HIV particles and their capacity to irreversibly inactivate the virus. Most notably, the MPER-specific antibodies potently induced gp120 shedding upon prolonged contact with the virus, rendering neutralization irreversible. The kinetic and thermodynamic requirements of the shedding process were virtually identical to those of neutralization, identifying gp120 shedding as a key process associated with HIV neutralization by MPER antibodies.
RESULTS AND DISCUSSION
Kinetics of HIV neutralization
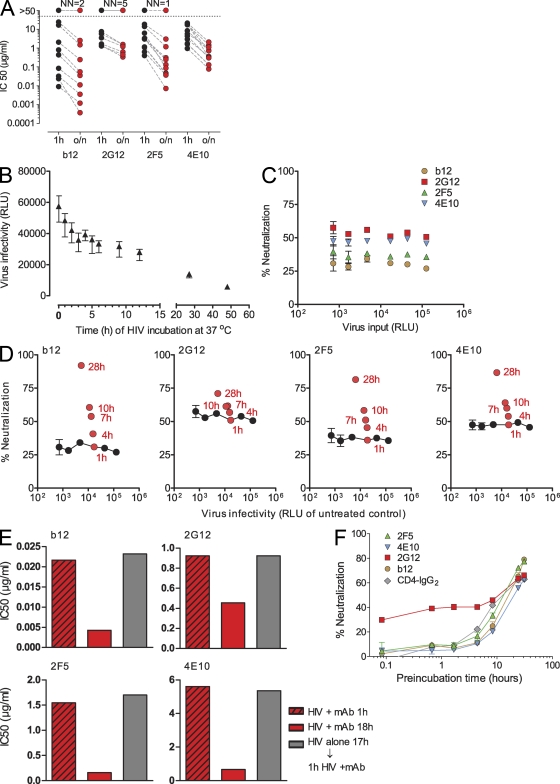
To study the effects of long-term interaction of HIV with neutralizing antibodies, we compared short- (1 h) and long-term (overnight; 16–18 h) inhibitory activity of the neutralizing antibodies b12 (gp120 CD4 binding site [CD4bs] specific; Barbas et al., 1992), 2G12 (gp120 carbohydrate specific; Trkola et al., 1996), and the gp41 MPER–specific antibodies 2F5 (Muster et al., 1993) and 4E10 (Stiegler et al., 2001; Zwick et al., 2001) against a panel of envelope-pseudotyped luciferase reporter viruses (Fig. 1 A and Table S1). Although long-term coincubation of virus with mAb 2G12 showed no or only modest increases in neutralization, prolonged coincubation markedly improved the neutralizing capacity of 2F5, 4E10, and b12. As noted previously (Binley et al., 2004), neutralization activity of MPER mAbs, in particular, increased by more than one order of magnitude upon extended interaction with several of the tested isolates. We ascertained that these increases in inhibitory activity upon prolonged interaction with the virus were a consequence of the antibodies’ action and not a result of spontaneous decay of virions. Although HIV infectivity decreases upon prolonged incubation at 37°C, this loss is moderate (<1 log within 24 h; Fig. 1 B). To study HIV neutralization by mAbs in vitro, antibodies are commonly supplied in stoichiometric excess over the viral target, allowing robust assessment of neutralization activity over a wide range of virus input. This was also true in our assay setup, in which we measured the virtually identical neutralization capacity of b12, 2G12, 2F5, and 4E10 over a >2-log range of virus inputs (Fig. 1 C). Assessment of mAb-independent decrease in virus infectivity and the enhanced mAb neutralization activity during long-term incubation in parallel confirmed that the moderate loss observed in infectivity does not impact the assessment of neutralization activity in our experimental setup (Fig. 1 D). Increases in the neutralization capacity of 2F5, 4E10, and b12 upon prolonged incubation are thus caused by the antibodies’ action. However, under the same conditions, long-term treatment by 2G12 induced no equivalent improvement in neutralization. To formally exclude the influence of virus decay, we performed a series of further control experiments (Fig. 1 E) in which virus was subjected to a 1- (condition 1) and 18-h (condition 2) preincubation with mAbs at 37°C. Condition 3 controlled for viral decay during the 18-h incubation by incubating the virus, in absence of mAbs, for 17 h at 37°C, followed by a 1-h mAb treatment. The latter confirmed that viral decay had no general influence on neutralization activity, as conditions 1 and 3 resulted in identical inhibitory activity.
Figure 1.
Kinetics and reversibility of HIV neutralization. (A) Time dependence of neutralization activity was probed by preincubating serial dilutions of mAbs b12, 2G12, 2F5, and 4E10 with 11 different R5 and X4 pseudoviruses (Tables S1 and S2) for 1 h or overnight (o/n; 16–18 h) at 37°C before infection of TZM-bl target cells. The 50% inhibitory concentration (IC50) for each mAb/treatment condition is depicted. IC50 values were derived from sigmoid dose–response curve fits of pooled data of two to three independent experiments. The number of viruses not neutralized (NN) at the highest mAb concentration probed is indicated on top of each antibody panel. (B) Decay of HIV upon long-term exposure to 37°C. A fixed virus input (JR-FL enveloped-pseudotyped virus; 3,000 TCID50/well in culture medium; no mAbs) was incubated for the indicated time periods at 37°C before infection of TZM-bl target cells, and virus infectivity was monitored by luciferase reporter gene production 72 h after infection. Relative light units (RLU) measured per well are depicted. Values are means of 15 replicas, and error bars indicate SEM. One out of five independent experiments is shown. (C) mAb neutralization is constant over a wide range of viral input. Serial dilutions of JR-FL envelope-pseudotyped virus were pretreated for 1 h at 37°C with a fixed concentration of each mAb at concentrations that typically yield neutralization below 70% (0.02 µg/ml mAb b12, 1 µg/ml 2G12, 1.5 µg/ml 2F5, and 7 µg/ml 4E10). In parallel, for each virus dilution, a mock treatment (incubation without mAb) was performed. The viral infectivity of each virus dilution (relative light units of mock-treated sample, measured 72 h after infection) is plotted on the x axis. The y axis indicates the neutralization each antibody achieved at the respective virus input. Error bars indicate SEM. (D) Enhanced mAb neutralization over prolonged incubation is not caused by spontaneous virus decay. In experiments depicted by red symbols, virus input and mAb concentration were kept constant, and only incubation time was varied. In experiments depicted by black symbols, incubation time (1 h) and mAb concentration were kept constant, and only virus input was varied. mAbs were probed at concentrations that typically yield neutralization below 70% (0.02 µg/ml mAb b12, 1 µg/ml 2G12, 1.5 µg/ml 2F5, and 7 µg/ml 4E10). For the red symbols, JR-FL pseudo virus was pretreated with the respective mAbs for the indicated times at 37°C. In parallel, for each time point, a mock control (incubation in medium without mAb) was performed. The viral infectivity measured for the mock treatment is plotted on the x axis for each time point (relative light units, measured 72 h after TZM-bl cell infection). The y axis indicates the neutralization each antibody achieved at after the respective preincubation time. For the black symbols, JR-FL pseudo virus was titrated and preincubated for 1 h at 37°C with the respective mAbs or a mock control (incubation in medium without mAb). The viral infectivity of the mock-treated samples is plotted on the x axis for the respective virus input. The y axis indicates the neutralization activity each antibody achieved at the respective virus input. Data points are means of 20 replicates. Error bars indicate SEM. One of two independent experiments is shown. (E) Increases in inhibitory activity upon prolonged interaction with the virus are a consequence of the antibodies’ action. Envelope-pseudotyped JR-FL was incubated with serial dilutions of b12, 2G12, 2F5, and 4E10 or left untreated for either 1 or 18 h. Alternatively, virus was left untreated for 17 h before being treated for 1 h with neutralizing antibodies. Pretreated virus was then added to TZM-bl cells, and virus infectivity was measured by luciferase reporter gene production 72 h after infection. The 50% inhibitory concentration (IC50) for each mAb/treatment condition is depicted. IC50 values were derived from sigmoid dose–response curve fits of pooled data of three independent experiments. (F) Time of addition experiments were conducted to probe the kinetics of mAb neutralization. Antibody concentrations were chosen to yield neutralization of ∼70% after 20 h of preincubation to allow for the monitoring of increases in neutralization activity over time. 0.03 µg/ml b12, 12 µg/ml 2G12, 2.5 µg/ml 2F5, 8.5 µg/ml 4E10, and 0.1 µg/ml CD4-IgG2 were incubated with JR-FL pseudovirus for the indicated time periods at 37°C. Percent neutralization of virus infectivity on TZM-bl cells was calculated in reference to the respective mock-treated virus control of each time point. One of six independent experiments is shown. Data points depict means and SEM of duplicate measurements.
We next defined the specific kinetics of mAb binding to virions (Fig. S1, A and B) and neutralization (Fig. 1 F) in time of addition experiments. To monitor increases in neutralization activity over time, fixed antibody doses that typically yield 70% neutralization of JR-FL pseudovirus only after 20-h preincubation were chosen. 2G12 rapidly bound to virions and instantly neutralized, but this activity only moderately improved upon sustained treatment, suggesting that easy access to its well exposed glycan epitope fosters a comparatively fast and efficient binding (Fig. S1 B) and neutralization reaction. In contrast, both binding and neutralization by b12 and the MPER mAbs gradually and substantially increased over time. The latter clarifies a longstanding debate as it confirms that 2F5 and 4E10 can access the MPER on virions before receptor engagement. Yet, in agreement with previous studies, the slow kinetics of binding and neutralization (>7 h; Fig. 1 F and Fig. S1) suggest that access to the MPER epitope is highly restricted (Binley et al., 2003; Frey et al., 2008). Ineffective binding could be a consequence of steric interference of antibody binding to the MPER epitope, which is thought to be partially buried in the viral membrane. In fact, efficient MPER mAb binding may depend on a prior association of the mAbs with the viral membrane (Cardoso et al., 2007; Alam et al., 2009; Buzon et al., 2010; Frey et al., 2010). Alternatively, envelope conformations that expose the MPER may only infrequently be formed.
Is neutralization by antibodies reversible?
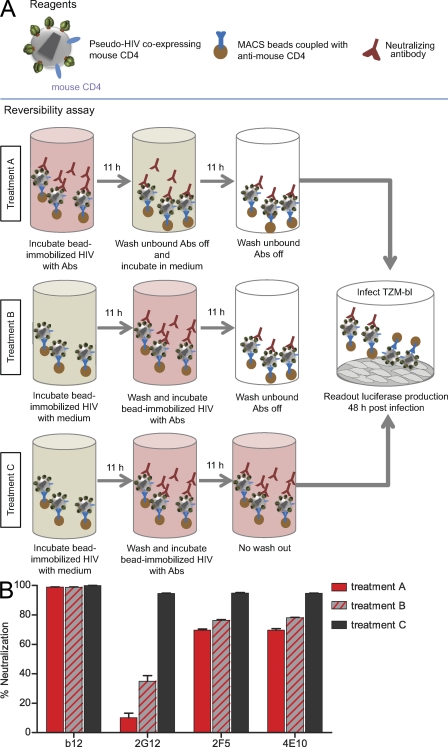
One specific purpose of studying long-term effects of antibody action was to determine whether neutralization of HIV is a reversible event. To probe reversibility, we generated JR-FL pseudovirus that coexpresses mouse CD4, allowing rapid separation of virions and unbound antibodies using magnetic beads (Fig. 2). We subjected virions to neutralizing antibodies, followed by the opportunity for antibody dissociation and removal of unbound antibody (treatment A). Two sets of controls determined the extent of neutralization by antibody-treated virus without dissociation (treatment B) and the maximal inhibitory activity without removal of antibody (treatment C). Most interestingly, neutralization by the MPER mAbs proved to be irreversible. Removal of excess antibody (treatment B) led to only a small reduction in 2F5 and 4E10 neutralization activity (24% and 22%, respectively), likely accounting for ongoing neutralization when mAbs were not removed. Importantly, MPER mAb–opsonized virus remained stably neutralized over the 11-h dissociation period (treatment A). Virtually complete irreversibility of neutralization was observed for mAb b12, as the virus remained neutralized throughout the observation period. In contrast, 2G12 immediately lost the majority of its neutralization activity once excess antibody was removed (treatment B), and the virus was able to regain its infectivity almost completely (treatment A), indicating that neutralization by 2G12 is reversible and steered by a high off rate in binding to the HIV envelope.
Figure 2.
Reversibility of HIV neutralization. (A and B) Reversibility of neutralization was probed as depicted in the flow chart. Antibody concentrations were chosen to yield neutralization activities of ∼90% after 11 h of preincubation to allow for monitoring decreases in neutralization activity after the dissociation step. JR-FL neutralization by 0.1 µg/ml b12, 15 µg/ml 2G12, 11.4 µg/ml 2F5, and 45 µg/ml 4E10 was probed under the indicated treatment conditions: after antibody dissociation (treatment A), neutralization without dissociation (treatment B), and without removal of antibody (treatment C). Means and SEM of triplicates measurements of one of three independent experiments are shown. Abs, antibodies.
Neutralizing antibodies induce gp120 shedding
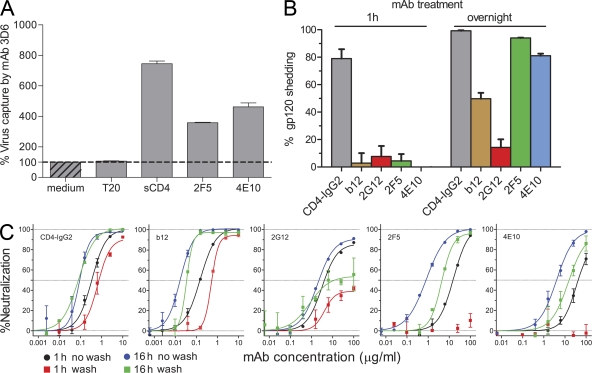
Having established that certain neutralizing antibodies irrevocably inactivate HIV, we investigated plausible mechanisms of this process. To probe whether neutralizing antibodies induce conformational changes in the envelope trimer that irrevocably perturb functionality, we assessed the capacity of opsonized JR-FL pseudovirus to bind to 3D6, an antibody specific for the immunodominant (cluster I) region in gp41 inaccessible on intact envelope trimers (Sattentau et al., 1995; Finnegan et al., 2002). Soluble CD4 (sCD4) treatment is known to induce conformational changes within gp120 (Kwong et al., 1998) and to cause shedding of gp120 (Moore et al., 1990), both of which lead to exposure of otherwise hidden domains in gp41 (Sattentau et al., 1995; Finnegan et al., 2002). In line with this, we observed a strong increase in 3D6 binding (7.5-fold) to virions upon sCD4 treatment (Fig. 3 A). Most strikingly, treatment with mAbs 2F5 or 4E10 also led to a pronounced exposure of cluster I epitopes on virions, whereas the gp41-targeting inhibitor T-20 had no such effect, suggesting that MPER mAbs either induced gp120 shedding or caused an extensive structural reorganization of gp120 and gp41 molecules within the trimer.
Figure 3.
MPER-specific antibodies induce gp120 shedding. (A) To assess exposure of gp41 epitopes upon neutralizing antibody treatment of virions, the capacity of antibody-treated JR-FL pseudovirus to bind to the gp41 cluster I mAb 3D6 was assessed. Virus was pretreated for 18 h with 10 µg/ml sCD4, 100 µg/ml 2F5, 100 µg/ml 4E10, or 10 µg/ml of the fusion inhibitor T-20 or left untreated (medium control). Virus was then captured onto 3D6-coated ELISA plates and quantified by p24 ELISA. Error bars indicate SEM. One of three independent experiments is shown. (B) To assess gp120 shedding, JR-FL virus coexpressing mouse CD4 was pretreated with 10 µg/ml CD4-IgG2, 10 µg/ml b12, 100 µg/ml 2G12, 100 µg/ml 2F5, and 100 µg/ml 4E10 or left untreated for either 1 h or overnight (18–20 h). Virus was immobilized on anti-CD4–coated magnetic beads, and gp120 was quantified by ELISA. Percent gp120 shedding induced by the respective conditions in relation to mock-treated virus was determined. Data were normalized to p24 content (ELISA) to ensure that identical numbers of virions were assessed under each condition. Mean and SEM of three independent experiments are depicted. (C) Neutralization activity against free virus was determined as depicted in the flow chart shown in Fig. S1. JR-FL pseudovirus was pretreated with serial dilutions of CD4-IgG2, b12, 2G12, 2F5, and 4E10 for either 1 or 16 h, with a maximal mAb concentration identical to the shedding assay shown in B. Virus was then bead immobilized, washed, and used to infect TZM-bl cells. In comparison, the entire preincubation mix without washing was transferred onto TZM-bl cells. In the latter case, 1-h preincubation corresponds to the conditions in a conventional neutralization assay, and 16-h preincubation corresponds to the long-term incubation depicted in Fig. 1. Percent neutralization induced by the respective conditions in relation to mock-treated virus was determined. Mean and SEM of two independent experiments are depicted.
To directly probe for gp120 shedding, JR-FL pseudovirus was first treated with neutralizing antibodies. After bead immobilization (via coexpressed mouse CD4), unbound antibody and potentially shed gp120 was removed, and virus-associated gp120 content was quantified (Fig. 3 B). Comparing mock-treated with CD4-IgG2 (tetrameric CD4 Ig)– and neutralizing mAb–treated JR-FL pseudovirus, we observed remarkable potency of the MPER mAbs to induce gp120 shedding upon long-term interaction with the virus. Likewise, antibody b12 induced gp120 shedding, whereas 2G12 had no such effect. Of note, in contrast to CD4-IgG2, which induced rapid shedding, short-term incubation with the probed neutralizing mAbs had only minimal influence on gp120 dissociation (Fig. 3 B). The latter may account for the difficulty of earlier studies to identify induction of gp120 shedding by CD4bs antibodies, including b12 (Moore et al., 1990; McDougal et al., 1996; Poignard et al., 1996). Although sCD4 has been long known to induce dissociation of gp120 (Moore et al., 1990), to what extent shedding contributes to sCD4 inhibitory activity has never been fully unraveled as these processes can differ in concentration and temperature dependence (McDougal et al., 1996; Chertova et al., 2002). Importantly, sCD4 mediates weaker shedding and neutralization of PBMC-derived viruses than T cell line–adapted strains, likely accounting for the failure of recombinant CD4-based therapies (Daar et al., 1990; Moore et al., 1992; Orloff et al., 1993). Previous investigations into shedding as a potential mechanism for antibody neutralization of HIV yielded inconclusive results (Moore et al., 1990; McDougal et al., 1996; Poignard et al., 1996). MPER-specific antibodies had not been investigated, and neutralizing CD4bs mAbs (including b12) were reported to lack shedding activity (McDougal et al., 1996; Poignard et al., 1996). Although several mAbs directed to other domains in gp120 were found to partially dissociate the envelope of T cell line–adapted strains and a direct association between antibody neutralization and gp120 shedding was inferred to, a causal link between these modes of action was not formally experimentally established (Poignard et al., 1996). Thus, the relevance of shedding as a mechanism of HIV neutralization by antibodies has remained to some extent uncertain. The potential in vivo significance of the shedding process was equally unclear as activity against primary viruses was not confirmed (McDougal et al., 1996; Poignard et al., 1996).
Considering that MPER antibodies even at high concentration (100 µg/ml) did not induce shedding after short-term (1 h) preincubation (Fig. 3 B), whereas after 1-h preincubation, potent inhibition by the same mAbs can be seen in a conventional neutralization assay (Fig. 1), it was prudent to explore whether shedding is a mere artifact of the mAb’s interaction with the virus or is intimately connected with the neutralization process. In conventional neutralization assays, antibody is present during the preincubation period and also during the entire infection process. Thus, for MPER antibodies, for which an action after receptor engagement has been postulated (Cardoso et al., 2007; Frey et al., 2008, 2010; Alam et al., 2009), experimental conditions have to be chosen that allow discrimination between actions on the free virus and actions that occur in the context of virus–host cell interactions. We thus performed neutralization experiments in which virus was only exposed to antibody during the preincubation period and compared these activities with those in a conventional assay format, in which antibody was present throughout (Fig. 3 C and Fig. S1, C and D). The results were strikingly clear; neither 2F5 nor 4E10 were capable of neutralizing JR-FL when only present during the 1-h preincubation period. They only neutralized when either was allowed to interact with the cell free virus for prolonged periods or when present during both preincubation and the entire infection period. These data confirm that neutralization of free virions by MPER antibodies in the absence of receptor interactions requires prolonged interaction of the mAbs with the HIV particle and thus follows the reactivity pattern observed for MPER-induced gp120 shedding.
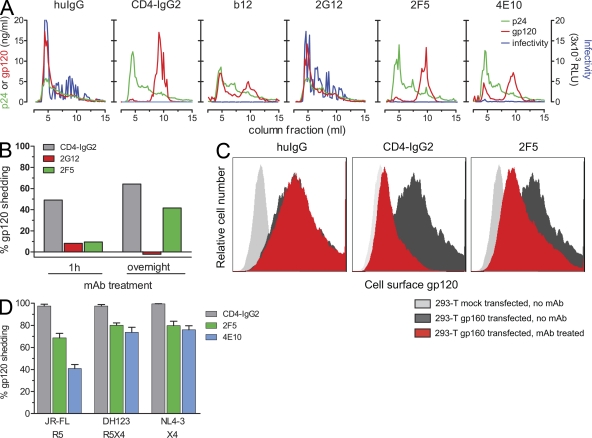
Our finding that MPER mAbs induce gp120 shedding is of obvious interest not only as it provides a long sought for insight into their mechanism of action but also as this process leads to irreversible neutralization of HIV. We confirmed our results by alternative experimental approaches: studying gp120 shedding from virions using size-exclusion chromatography (Fig. 4 A) and shedding from envelope-expressing cells by Western blot and flow cytometry (Fig. 4, B and C; and Fig. S2). The same pattern of reactivity as in the bead-immobilized virion shedding assay was observed in these experiments: MPER mAbs and CD4bs-directed agents induced envelope dissociation, whereas 2G12 did not. To obtain insights into a potential physiological relevance of the shedding process, we verified our initial results (obtained with pseudovirus expressing the primary virus envelope JR-FL) with replication-competent, PBMC-derived viruses (Fig. 4 D). As observed for pseudovirus, mAbs 2F5 and 4E10 induced potent gp120 shedding from the probed R5, R5X4, and X4 isolates, demonstrating that shedding of primary viruses is possible and may thus occur in vivo.
Figure 4.
MPER antibodies induce gp120 shedding from virions and HIV envelope–expressing cells. (A) To analyze gp120 shedding by size-exclusion chromatography, JR-FL pseudovirus was treated with 25 µg/ml of CD4-IgG2, b12, 2G12, 2F5, 4E10, or total human IgG (huIgG) for 20–25 h. Samples were then separated on a Sephacryl S-1000 column, and collected fractions were analyzed for virion content and viral infectivity (relative light units [RLU] luciferase reporter production; relative light units are depicted in units of 3 × 103). One of three independent experiments is shown. (B) Shedding of gp120 from envelope-expressing cells was detected by Western blot analysis. 293-T cells expressing ΔCT JR-FL gp160 were treated with 10 µg/ml CD4-IgG2, 50 µg/ml 2G12, 50 µg/ml 2F5, or 50 µg/ml human IgG as control for the indicated time periods to allow for shedding. After cell lysis, total protein was separated by SDS-PAGE and analyzed by Western blotting for gp120 content. gp120 content was quantified by densitometric analysis, and shedding induced by neutralizing mAbs was expressed in relation to human IgG control. One of three independent experiments is shown. (C) 293-T cells that were mock transfected or transfected with ΔCT JR-FL gp160 were treated with 10 µg/ml CD4-IgG2, 50 µg/ml 2F5, or 50 µg/ml human IgG as control for 20 h to allow for shedding. Cell surface–associated gp120 was detected by flow cytometry upon staining with biotinylated 2G12 and streptavidin-APC. One of three independent experiments is shown. (D) Induction of gp120 shedding from PBMC-derived, replication-competent HIV isolates was analyzed using PBMC-derived viruses JR-FL, DH123, and NL4-3. Coreceptor usage of viruses is indicated. Viruses were incubated with 10 µg/ml CD4-IgG2, 100 µg/ml 2F5, and 100 µg/ml 4E10 or left untreated for 24 h (mock control). The extent of gp120 shedding in mAb-treated samples was analyzed as described in Fig. 3 B. Data are expressed in relation to the mock control. Mean and SEM of three independent experiments are depicted.
Is gp120 shedding a cause or a byproduct of antibody neutralization?
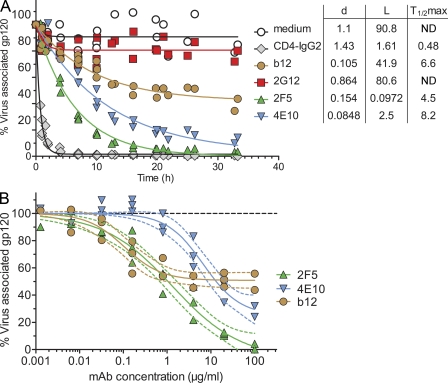
To probe whether gp120 shedding is mechanistically linked with the neutralization process or whether these two activities are independent, separable, or occur consecutively, we first defined the kinetics of the antibody-induced gp120 shedding process in detailed time of addition experiments (Fig. 5 A). The loss of gp120 triggered by the probed mAbs proved to be strikingly different over the 33 h of monitoring. CD4-IgG2 provoked instant and almost complete shedding. 2F5 and 4E10 reacted more rapidly than b12 and caused almost complete gp120 shedding upon long-term treatment, indicating that the antibodies must be able to release gp120 from both functional (trimeric) and nonfunctional envelope species (monomers and dimers) present on viral particles (Moore et al., 2006). Intriguingly, even after long-term incubation, b12 only achieved shedding of 58% of JR-FL gp120. The dose dependency of the b12 shedding process followed a similar pattern. gp120 shedding continuously increased in a dose-dependent manner for 2F5 and 4E10, whereas shedding by b12 plateaued at ∼50% and could not be augmented by increasing doses (Fig. 5 B). Determining precisely which functional properties of b12 shape its shedding activity will require further investigation. For instance, partial gp120 depletion could result if mAb binding prompts shedding of neighboring units in a trimer (or dimer) but not of the unit the mAb is bound to. In general, differences in stoichiometric requirements, steric constraints, and mAb-induced conformational changes (Kwong et al., 2002) could be envisioned to cause the observed variable patterns of shedding activity among antibodies.
Figure 5.
Kinetics of neutralizing antibody-induced gp120 shedding. (A) Shedding kinetics were determined over a 33-h time period. JR-FL pseudovirus was treated with fixed concentrations of CD4-IgG2 (10 µg/ml), b12 (10 µg/ml), 2G12 (100 µg/ml), 2F5 (100 µg/ml), and 4E10 (100 µg/ml) or left untreated (medium control) for the indicated time periods at 37°C, and gp120 shedding was assessed as described in Fig. 3 B. The rate of gp120 loss was fitted according to the formula L + (100 − L) × exp(−d × time). L denotes the maximal level of gp120 loss (lowest level of gp120) inflicted by the respective antibody. The rate of gp120 loss is characterized by a constant d (units per hour). T1/2 max denotes the time until half maximal gp120 loss was reached. An overlay of five independent experiments is depicted. (B) The dose dependency of the shedding process was assessed by treating JR-FL pseudovirus with increasing concentrations of b12, 2F5, and 4E10 or by leaving the pseudovirus untreated (medium control) for 22 h at 37°C. Shedding was analyzed as described in Fig. 3 B. Data are expressed in relation to the medium control. Pooled data from three independent experiments are depicted. Curves depict sigmoid dose–dependent fits with variable slope and constraining upper limits at 100%. The dotted curves indicate the respective 95% confidence intervals.
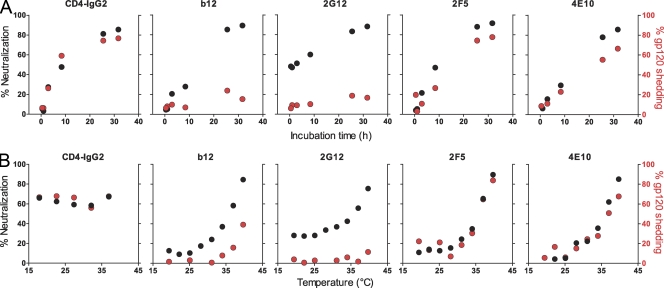
To explore whether the shedding process is an integral component of neutralization by individual antibodies, we monitored the kinetics of neutralization and shedding in parallel (Fig. 6 A). Each mAb was probed at a fixed antibody concentration that typically yields 70% neutralization of JR-FL pseudovirus after 20-h preincubation of mAb and virus at 37°C but has (with the exception of 2G12) no notable effect on virus infectivity upon short incubation (Fig. 1 F). Neutralization activity therefore depended on interaction with the virus during the preincubation period and thus allowed direct assessment of the impact of shedding on the antibodies’ neutralization activity. Strikingly, the timing of JR-FL neutralization and shedding was virtually identical for the two MPER antibodies, indicating that the process of shedding is causally linked to their mechanism of neutralization. Although we cannot formally rule out that MPER binding itself confers neutralization in the absence of shedding, the kinetics of the processes were so closely related that, if these events were indeed functionally separable, shedding must occur very rapidly after JR-FL neutralization by MPER antibodies. In contrast to MPER antibodies, no time-dependent association between shedding and neutralization of JR-FL pseudovirus was detected for b12. Although b12 induced shedding of 50% of JR-FL gp120 at concentrations >0.1 µg/ml (Fig. 5 B), lower concentrations (0.03 µg/ml; Fig. 6 A), which are perfectly capable of inducing neutralization, failed to induce potent gp120 shedding. The latter observation strongly suggests that the primary mechanism of b12 neutralization is independent of shedding. Likewise, as shown above (Figs. 1 F, 3 B, and 5 A), 2G12 neutralized without inducing gp120 shedding.
Figure 6.
Shedding and neutralization activity of MPER mAbs follow identical kinetics and thermodynamic requirements. (A) To compare the kinetics of shedding and neutralization, neutralization on TZM-bl cells and shedding activity (as described in Fig. 3 B) of JR-FL pseudovirus upon treatment with mAbs b12 (0.03 µg/ml), 2G12 (12 µg/ml), 2F5 (2.5 µg/ml), and 4E10 (8.5 µg/ml) was assessed at the indicated times at 37°C. Chosen concentrations correspond to ∼70% neutralization after 20-h incubation at 37°C. One of two independent experiments is depicted. (B) To compare thermodynamic requirements of shedding and neutralization, neutralization on TZM-bl cells and shedding activity (as described in Fig. 3 B) of JR-FL pseudovirus upon treatment with mAbs b12 (0.03 µg/ml), 2G12 (12 µg/ml), 2F5 (2.5 µg/ml), and 4E10 (8.5 µg/ml) for 20 h at the indicated temperature were assessed. One of three independent experiments is depicted. (A and B) Chosen concentrations correspond to ∼70% neutralization after 20-h incubation at 37°C.
We next assessed the thermodynamic requirements of neutralization and shedding activity (Fig. 6 B) using mAb concentrations that yield 70% neutralization after 20-h preincubation at 37°C. CD4-IgG2 potently neutralized virions and induced gp120 shedding even at low temperatures. 2G12 displayed modest neutralization activity at 19.4°C, which increased with temperature. Confirming our previous observations (Figs. 3 B and 4, A and B; and Figs. 5 A and 6 A), no notable shedding of JR-FL gp120 was induced by 2G12 at any of the probed temperatures. Antibodies b12, 2F5, and 4E10 all required temperatures >28°C to neutralize. Strikingly, MPER mAb neutralization and gp120 shedding activity followed an identical temperature profile. Together with the observed close time-dependent synchronization of their activities, these data provide strong evidence that with the majority of isolates, MPER neutralization and shedding are indeed mechanistically linked. The thermodynamic processes observed for b12 were more complex: although the chosen dose (0.03 µg/ml) induced potent neutralization at 37°C, which decreased in a temperature-dependent manner, the same conditions induced only comparatively little shedding, particularly at lower temperatures. Although the pattern was similar (more shedding with higher temperature), neutralization by b12 was not obligatorily linked to shedding. Likewise, the high potency of b12 to irreversibly neutralize HIV appears not to depend entirely on the mAb’s shedding capacity, as complete irreversibility is achieved at antibody doses which only induce partial shedding (Figs. 2 and 5 B). The latter suggests that the degree of conformational fixation of gp120 inflicted upon b12 binding (Zhou et al., 2007) alone mediates irreversible inactivation.
Neutralizing antibodies induce gp120 shedding of genetically diverse viruses
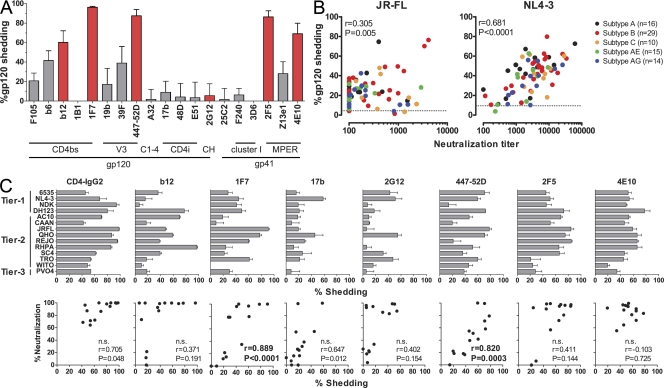
Held together by labile, noncovalent intersubunit interactions between gp41 and gp120 (Finzi et al., 2010; Pancera et al., 2010), the HIV envelope trimer is characterized by a high conformational diversity particularly within gp120, which is central to the envelope’s function in virus entry and immune evasion (Kwong et al., 2002; Chen et al., 2009). Notably, neutralization-sensitive HIV strains have been postulated to present envelope conformations, which, although optimized for receptor interaction, allow easier access for neutralizing antibodies and potentially destabilize the trimer (Park et al., 2000; Pugach et al., 2004). These structural characteristics are also likely to steer the sensitivity of a given virus envelope to antibody-induced shedding. To address this, we first screened a panel of gp120- and gp41-specific antibodies for their shedding activity against JR-FL pseudovirus (Fig. 7 A and Tables S2–S4). With the exception of 2G12, all mAbs that neutralized the virus also induced potent gp120 shedding. Particularly notable were the CD4bs-specific antibody 1F7 (Buchacher et al., 1994; Kunert et al., 1998) and the V3 loop antibody 447-52D (Gorny et al., 1992), which achieved 96.3% and 87.6% shedding, respectively. Interestingly, the CD4bs antibody b6, which is known to bind predominantly nonfunctional envelope forms (Poignard et al., 2003) and lacks neutralization activity against JR-FL, induced partial shedding (41.7%), demonstrating that both neutralizing and nonneutralizing antibodies can participate in this process, presumably by acting against envelope monomers or dimers on the virion surface.
Figure 7.
Antibodies with gp120 shedding activity are commonly elicited in HIV infection. (A) Assessment of gp120 shedding activity induced by HIV envelope–specific mAbs. 20 µg/ml of mAbs directed to diverse epitopes in gp120 and gp41 (Table S3) were assessed for shedding activity (as described in Fig. 3 B) after 20-h treatment of JR-FL pseudovirus at 37°C. Red bars denote mAbs that neutralize JR-FL, and gray bars denote nonneutralizing mAbs. Mean and SEM of three independent experiments are depicted. (B) Shedding activity elicited in HIV infection. 84 plasma samples derived from patients with chronic infection with the indicated HIV subtypes (Table S5) were probed for shedding (as described in Fig. 3 B), and neutralization activity was measured using TZM-bl cells against JR-FL (left) and NL4-3 pseudovirus (right) under identical preincubation conditions (20-h preincubation at 37°C). Correlation coefficient r (Pearson) and p-values are depicted. Data are means of two independent experiments. Dotted lines correspond to spontaneous gp120 shedding induced by healthy donor plasma (mean of three donors). (C) Shedding induced by a wide spectrum of mAbs with divergent specificities. Shedding (as described in Fig. 3 B) and neutralizing activity measured using TZM-bl cells of the indicated mAbs against 14 divergent viruses from tier 1–3 (Tables S2–S4) were determined under identical preincubation conditions (10 µg/ml mAb and 20-h preincubation at 37°C). Correlation coefficient r (Pearson) and p-values are depicted. Significance was assessed after correcting for multiple testing (Bonferroni test). Data are means of two to four independent experiments. Error bars indicate SEM. n.s., not significant.
To explore how ubiquitously neutralizing antibodies cause shedding across divergent strains, we assessed the shedding and neutralizing capacity of seven mAbs against a panel of 14 pseudoviruses encompassing relatively easy to neutralize tier 1 and more resistant tier 2 and 3 isolates (Fig. 7 C and Tables S2–S4). The results confirmed our previous observations with the virus strain JR-FL (Fig. 7 A). mAbs directed to the CD4bs, V3 loop, and the MPER possessed potent shedding activity against the majority of isolates. 2G12, which was ineffective in inducing shedding from JR-FL envelope–bearing virions, induced 30–60% shedding with 5 of the 14 probed viruses. Although clearly not a potent shedding inducer, 2G12 can thus not be considered incapable of inducing shedding. mAb b12 promoted shedding of >30% in 8 of 14 viruses but had only marginal effects against the other 6 isolates, 3 of which were not neutralized by b12. The CD4i (CD4 induced)-specific mAb 17b was largely ineffective in both, inducing neutralization and shedding. 2F5 induced potent shedding in 11 of the 14 probed viruses. A comparatively low level of shedding (<30%) occurred with three viruses, WITO, TRO, and PVO.4, of which the latter two were not neutralized by 2F5. The pattern for 4E10 was similar: neutralization was detected against all 14 viruses, against 13 of which shedding activity was high. The only comparatively shedding-insensitive virus (<30% shedding by 4E10), WITO, was nevertheless neutralized by 4E10. It is to be expected that both shedding efficacy and dependency of the MPER neutralization process on shedding induction vary among divergent virus strains, as the induction of gp120 shedding by sCD4 also varies among virus strains (Moore et al., 1992; McDougal et al., 1996; Chertova et al., 2002). Besides epitope accessibility and antibody affinity, the conformational flexibility of gp120 and the stability of the noncovalent gp120/gp41 association of a specific virus strain will influence shedding efficacy (Moore et al., 1992; Finzi et al., 2010; Pancera et al., 2010). Thus, envelope trimers that can undergo the required conformational changes and tolerate neutralizing mAb binding without jeopardizing subunit association may be less prone to shed gp120 upon interaction with neutralizing antibodies. However, overall, we found that shedding activity is prominently associated with neutralization activity. Of the probed neutralizing antibodies, MPER mAbs yielded the highest and broadest shedding activity overall. Particularly notable again were mAbs 447-52D and 1F7, which both induced potent shedding that correlated with their neutralization activity.
To obtain information on the frequency at which shedding-inducing antibodies are elicited in HIV infection, we analyzed shedding and long-term neutralization activity in 84 plasma samples derived from patients with chronic infection of subtypes A, B, C, CRF01_AE, and CFR02_AG (Fig. 7 B and Table S5). Cross-neutralizing activity against JR-FL was low in most plasma samples (reciprocal neutralization titer <100 in 38 of 84 samples) but was frequently associated with induction of shedding against the same virus. The low plasma neutralization titers against JR-FL did not allow firm conclusions on a mechanistic link between JR-FL directed neutralization and shedding activity to be drawn. In contrast, NL4-3 was neutralized by a high proportion of isolates at relatively high titers (reciprocal neutralization titer >1,000 in 60 of 84 samples). Shedding was induced by plasma of chronic patients across all subtypes and, in accordance with neutralization activity, was higher against NL4-3 than JR-FL. Notably, the neutralization and shedding activity directed toward NL4-3 were tightly correlated, further underlining a potential mechanistic association of these activities.
Conclusions
Although the ability of neutralizing antibodies to protect against HIV infection has been demonstrated in vivo (Mascola et al., 2000; Trkola et al., 2005; Hessell et al., 2009a), whether one or more actions, direct virus neutralization, induction of phagocytosis, inhibition of transfer (e.g., via DCs) to target cells, or killing of infected cells via antibody-dependent cellular cytotoxicity, are key to block transmission is currently not known (Hessell et al., 2007; Huber and Trkola, 2007). Both gp120 shedding and antibody-induced conformational changes can lead to irreversible inactivation of HIV, which may positively influence in vivo efficacy of antibody neutralization (McDougal et al., 1996; Poignard et al., 1996). The impact of antibodies mediating reversible neutralization may depend on rapid clearance of neutralized virions by phagocytes as influences of the milieu (e.g., migration to anatomical sites with lower antibody concentration; engulfment by DCs) may lead to antibody dissociation and reconstitute the virus’s infectivity. Neutralizing antibodies that irrevocably sterilize virions before target cells are encountered would in these scenarios be of clear benefit. However, failure to irreversibly neutralize does not necessarily render an antibody ineffective in vivo. Although reversible, 2G12 neutralization occurs, as we show in this study, more rapidly than neutralization with b12, 2F5, and 4E10, and its immediate action may indeed account for its high in vivo efficacy, particularly in established infection (Trkola et al., 2005; Hessell et al., 2009b).
In HIV vaccine development, a considerable effort has been directed toward eliciting neutralizing antibody responses that match the activities of the MPER-specific antibodies 2F5 and 4E10, yet the molecular basis of their neutralization remained unresolved. Recently, protection by MPER-directed antibodies was suggested not to involve antibody-dependent cellular cytotoxicity, emphasizing the fact that neutralization rather than effector functions dominate the in vivo activity of these mAbs (Hessell et al., 2010). Our current findings underline the importance of the neutralization process to the MPER mAb antiviral activity. Here, we provide compelling evidence that neutralization by MPER mAbs involves induction of gp120 shedding, rendering virus inhibition irreversible. In our study, we explored the interaction of the antibodies with HIV before receptor engagement to obtain information explicitly on the virion-directed activity of the mAbs. Notably, as we show here, virion neutralization by MPER mAbs is a comparatively slow process that requires several hours. In the light of our findings, it will be interesting to verify the postulated action of MPER mAbs after receptor engagement and investigate the contribution of shedding in this process (Binley et al., 2003). MPER antibodies have been shown to preferentially bind to the prehairpin intermediate that forms upon receptor engagement (Cardoso et al., 2007; Frey et al., 2008, 2010; Alam et al., 2009). Whether these confirmations are also adopted spontaneously in the absence of receptor engagement or upon initial, low affinity interaction of MPER mAbs with the viral envelope remains to be determined. Be it upon receptor engagement or mAb interaction, gp120 shedding can be envisioned to be closely associated with the formation of prehairpin intermediate.
Notably, our data only allow us to affirm a causal link between JR-FL virus neutralization and gp120 shedding by MPER antibodies within the temporal resolution of our assay systems. From this we can conclude that these actions are highly synchronized and follow identical kinetic, thermodynamic, and stoichiometric requirements. Yet, this does not exclude that MPER mAb binding alone would suffice to neutralize the virus by blocking the refolding process of gp41 and that shedding occurs as an immediate consequence of mAb binding. Whether a primary event leading to neutralization or a consequence of neutralization, our data clearly indicate that induction of gp120 shedding is closely associated with MPER antibody inhibition of the majority of HIV isolates and needs to be factored into the mechanistic processes underlying HIV neutralization by MPER mAbs.
How can antibody binding principally lead to gp120 dissociation from the envelope trimer? The HIV trimer association is relatively labile as it must support substantial conformational changes and dissociation of the gp120–gp41 heterodimers during interaction with cellular receptors and fusion. In analogy to the entry process, conformational changes resulting from antibody binding or conformational fixation by neutralizing antibodies can be envisioned to similarly disrupt the fragile inter- and intrasubunit associations. It can be speculated that the trimer association may depend on a certain degree of flexibility of all three subunits to allow synchronized movements. Once one unit’s conformation is arrested, for example by mAb binding to the MPER domain and the viral membrane, trimer association may be jeopardized. Alternatively, envelope conformations that expose the MPER and enable 2F5 and 4E10 binding may induce instability of the trimer. The strength of the subunit interaction as well as the degree by which conformational changes are inflicted will likely determine whether and to what extent shedding is associated with the neutralization process of a given virus antibody combination. Related observations have been made in other virus infections in which envelope or capsid conformation changes need to occur before or during neutralization (Witz and Brown, 2001; Selinka et al., 2003; Lok et al., 2008).
A principle question remains: is induction of shedding by neutralizing antibodies of physiological relevance? Clearly it is no prerequisite of neutralization as 2G12 and b12, which both can neutralize without inducing shedding, have proven in vivo efficacy (Trkola et al., 2005; Hessell et al., 2007, 2009b; Mehandru et al., 2007) Likewise, the in vitro and in vivo action of sCD4 has been suggested to be dominated by receptor binding site occupancy rather than by induction of gp120 shedding (Moore et al., 1990, 1992; McKeating et al., 1991; Orloff et al., 1993; McDougal et al., 1996; Haim et al., 2009). Yet, we would argue that the capacity of neutralizing antibodies to induce shedding or otherwise irreversibly neutralize HIV could be expected to substantially impact their in vivo activity. Virus transmission across mucosal surfaces is an inefficient process, and only few founder viruses establish the new infection (Salazar-Gonzalez et al., 2009). Mucus and epithelia trap virus, physically restricting transmission, and the limited number of transmitted virions depend on locating appropriate target cells (Haase, 2010). These processes require time during which virions remain vulnerable to antibody attack. During established infection, the bulk of HIV particles in the periphery are rapidly turned over with an estimated half-life of plasma virus of 28–110 min (Ramratnam et al., 1999). If this turnover is to be influenced by neutralizing antibodies, it requires a more rapid action than MPER antibodies appear capable of. Yet, not all virions are in the periphery and cleared. HIV is known to be trapped in various tissue compartments (e.g., by immature and mature DCs, follicular DCs, and B cells) where virions can be retained in an infectious state for prolonged time periods (Moir et al., 2000; Turville et al., 2004; Bánki et al., 2005; Ho et al., 2007; Yu et al., 2008). Although encompassing only a small fraction of the circulating virions, these trapped HIV particles are thought to contribute substantially toward dissemination and the formation of a viral reservoir in the infected individual. In this setting, antibodies that irreversibly neutralize the virus could indeed be of clear benefit, and antibody activities that require prolonged interaction with the virus may come in play.
Should vaccine-elicited antibody responses be specifically tailored to induce irreversible neutralization? As we show in this study, a variety of neutralizing antibodies induce shedding and thus irreversibly neutralize HIV. Thus, elicitation of antibodies that irreversibly neutralize should be within reach, and it will certainly be of interest to assess the recently identified potently neutralizing antibodies PG9, PG16, HJ16, and VRC01 for their capacity to irreversibly neutralize HIV (Walker et al., 2009; Corti et al., 2010; Pietzsch et al., 2010; Wu et al., 2010). A combination of antibodies that act rapidly and neutralize irreversibly may prove to be advantageous. Intriguingly, 2G12, which lacks the capacity to irreversibly neutralize but instead rapidly binds to and neutralizes HIV, effectively blocks the virus in vivo, as passive immunization trials with combinations of 2G12, 2F5, and 4E10 confirmed (Trkola et al., 2005; Mehandru et al., 2007). Although only 2G12 was assumed to be effective in these trials based on escape virus formation, a matter for speculation remains whether in vivo efficacy may have in part resulted from a rapid but reversible inhibition by 2G12 followed by sustained, irreversible inhibition by the MPER antibodies.
Although commonly low titered and often subneutralizing in vitro, neutralizing antibody concentrations in mucosal fluids nevertheless protected against SHIV transmission in challenge studies (Mascola et al., 2000; Hessell et al., 2009b). Our data provide a possible mechanistic basis for these observations as we demonstrate that upon long-term contact, efficient (and in most cases irreversible) inactivation of HIV occurs at comparatively low antibody concentrations. Although the precise delineation of the molecular processes that induce gp120 shedding upon MPER mAb binding will require further investigation, our current observations reveal important aspects of these antibodies’ mode of action and lay a foundation for the design of vaccines and compounds that induce gp120 shedding and irreversible inactivation in a similar manner.
MATERIALS AND METHODS
Reagents and clinical specimens.
Antibodies used in this study were made available by D. Burton (The Scripps Research Institute, La Jolla, CA), D. Katinger and H. Katinger (Polymun Scientific, Vienna, Austria), J. Robinson (Tulane University Medical Center, New Orleans, LA), S. Zolla-Pazner (New York University School of Medicine, New York, NY), and the AIDS Research and Reference Reagent Program (Division of AIDS, National Institute of Allergy and Infectious Diseases, National Institutes of Health). The precise origin and epitope specificities of monoclonal antibodies used in this study are listed in Table S3. Tetrameric CD4-Ig (CD4-IgG2; Allaway et al., 1995) and sCD4 (Deen et al., 1988) were provided by W. Olson (Progenics Pharmaceuticals, Tarrytown, NY); T-20 (Kilby et al., 1998) was provided by Roche. Human IgG was purchased from Sigma-Aldrich. Plasma samples were obtained from individuals with chronic HIV-1 infection (>6 mo infected) enrolled in (a) the Swiss HIV Cohort Study (Schoeni-Affolter et al., 2010), (b) the Zurich Primary HIV infection study (Huber et al., 2006), and (c) the Swiss Spanish Treatment Interruption trial (Trkola et al., 2004). All experiments were approved by the local ethics committee of the University Hospital Zurich, and written informed consent was obtained from all individuals. Detailed patient demographics are listed in Table S5.
Cells.
293-T and TZM-bl cells were obtained from the American Type Culture Collection and the National Institutes of Health AIDS repository, respectively, and cultured as described previously (Rusert et al., 2009).
Virus preparation.
Envelope-pseudotyped virus was prepared as previously described (Rusert et al., 2009). For generation of envelope-pseudotyped virus with mouse CD4 on the viral surface, 30 µg of mouse CD4 plasmid (mCD4-pCMV-SPORT6, IRAVp968B1245D; RZPD Deutsches Ressourcenzentrum für Genomforschung GmbH) was cotransfected with 7.5 µg of envelope expression plasmid, 22.5 µg of the envelope-deficient HIV-1 backbone vector pNL-luc-AM, and 120 µg polyethylenimine. Stocks of replication-competent virus were prepared as described previously (Rusert et al., 2005).
Neutralization assay using envelope-pseudotyped virus.
The neutralization activity of mAbs and patient plasma was evaluated on TZM-bl cells essentially as described previously (Rusert et al., 2009). Virus input was chosen to yield comparable reporter gene production (10,000–20,000 relative light units) in untreated control samples across divergent viruses. The antibody concentrations causing 50% reduction in viral infectivity (IC50) were calculated in Prism (GraphPad Software, Inc.) by sigmoid dose–response curve fit through the pooled data of two to five independent assays. If the appropriate degree of inhibition was not achieved at the highest or lowest drug concentration, a greater than or less than value was recorded.
gp120 and p24 ELISA.
gp120 and p24 antigen were quantified by ELISA as described previously (Trkola et al., 2004; Rusert et al., 2009).
Probing reversibility of neutralization.
See Fig. 2 for flow chart. Anti–mouse CD4 magnetic beads were incubated with envelope-pseudotyped virus expressing mouse CD4 on the viral surface for 30 min at 4°C. For treatment A, the bead–virus complexes were bound to µMACS columns (Miltenyi Biotec), washed with DME/10% FCS, and subsequently eluted according to the manufacturer’s protocol. Virus–bead complexes were then treated for 11 h at 37°C with neutralizing antibodies and unbound mAbs and afterward removed via column separation as described for the previous step. Antibody-opsonized virus was then incubated in medium for 11 h at 37°C to allow for antibody dissociation. After a final wash step to remove all unbound antibodies and shed gp120, viral infectivity was assessed on TZM-bl cells. To normalize for viral input, p24 content in the virus preparations was quantified by ELISA. Controls to account for neutralization without dissociation were conducted for each antibody (treatment B). For these, virus was treated identically as described for treatment A, with the exception that antibody treatment was performed in the second 11-h incubation step. Time periods of virus incubation and antibody exposure were thus identical in treatment A and B, only the order differed. The maximal inhibitory activity without removal of antibody was probed in treatment C, in which incubation was performed as described for treatment B but without the final removal of the unbound antibody. Percent inhibition was calculated in reference to a mock-treated control (100% virus infectivity).
Assessing neutralization activity against free virus.
See Fig. S1 for flow chart. JR-FL–pseudotyped virus was incubated with neutralizing mAbs for the indicated time period. To separate virions and unbound mAbs, anti-CD44 (BioLegend)–coupled magnetic beads (Dynabeads MyOne streptavidin; Invitrogen) were added to the preincubation mix and allowed to react with virions for 30 min at 4°C. Virus–bead complexes were then separated, washed, and transferred to TZM-bl cells to assess viral infectivity. Percent inhibition was calculated in reference to a mock-treated control (100% virus infectivity).
Virion binding assay.
ELISA plates (Costar) were coated with 2 µg/ml goat anti–human IgG (SouthernBiotech) in NaHCO3, pH 8.5, and blocked with TBS containing 2% BSA. mAb 3D6 was diluted to 10 µg/ml in TBS/2% BSA and captured on coated plates. Subsequently, antibody-covered plates were blocked with human IgG diluted to 10 µg/ml in TBS/2% BSA. JR-FL–pseudotyped virus was pretreated for 18 h at 37°C with 10 µg/ml sCD4, 100 µg/ml 2F5, 100 µg/ml 4E10, or 10 µg/ml of the fusion inhibitor T-20 or left untreated (medium control). Virus was then added to the antibody-covered plates and incubated for 12 h at 37°C. Plates were washed with TBS/2% BSA to remove unbound virus, and bound virus was lysed in TBS containing 1% Empigen. Finally, p24 content was determined by ELISA.
Detection of gp120 shedding from bead immobilized virus by ELISA.
(a) Pseudovirus expressing mouse CD4 on the viral surface was incubated at the indicated time/temperature conditions in the presence or absence of neutralizing antibodies. Virus was then immobilized onto magnetic beads coated with rat anti–mouse CD4 antibodies (Invitrogen) and washed with TBS/2% BSA using a magnetic 96-well plate (OZ Biosciences) to separate virus from unbound antibodies and shed gp120. Finally, the magnetic pellets containing washed virus were lysed in TBS containing 1% Empigen and gp120, and the p24 content of the lysate was quantified by ELISA. The percentage of gp120 shedding induced by the respective conditions is dependent on the experiment, expressed either in relation to the original virus stock (decay experiments; Figs. 5 A and 6 A) or in relation to mock-treated virus controls (all other results). Mock-treated virus controls correspond to 0% shedding and 100% gp120 content. (b) Analysis of gp120 from PBMC-derived, replication-competent virus was essentially identical. The only difference in this set up was that virus was immobilized on magnetic anti-CD44 beads (Miltenyi Biotec) for 1 h at 4°C and washed on µMACS columns. Purified virus was eluted in TBS/1% Empigen and gp120, and the p24 content of the lysate was quantified by ELISA.
Detection of gp120 shedding by gel filtration.
Size-exclusion chromatography was performed as described previously (Moore et al., 1990). In brief, JR-FL–envelope-pseudotyped virions were concentrated by ultracentrifugation over a 32% sucrose cushion in a centrifuge (Centrikon Ultra, Rotor TST 28.38; Kontron Instruments) at 28,000 rpm for 2 h. Aliquots of the concentrated virus (100 ng p24) were treated with 25 µg/ml CD4-IgG2, 25 µg/ml b12, 25 µg/ml 2G12, 25 µg/ml 2F5, and 25 µg/ml 4E10 or 25 µg/ml human IgG for 20–25 h. Samples were then subjected to size-exclusion chromatography using a (10 ml, 16/50) Sephacryl S-1000 column (GE Healthcare) on a purifier system (AEKTA; GE Healthcare). 200-µl fractions were collected and analyzed for p24 and gp120 content by ELISA, and infectivity was probed on TZM-bl reporter cells.
Detection of gp120 shedding by Western blot analysis.
293T cells were transfected in 24-well plates with ΔCT JR-FL gp160, a cytosolic tail truncated gp160 construct known to yield higher envelope trimer expression (Binley et al., 2003), and pCMV-rev (Lewis et al., 1990) using jetPEI (Polyplus Transfection) according to the manufacturer’s instruction. As mock control, empty pcDNA3.1 vector was cotransfected together with pCMV-rev under identical conditions. 20 or 1 h before harvest, the cells were treated with 50 µg/ml total human IgG, 10 µg/ml CD4-IgG2, 50 µg/ml 2G12, or 50 µg/ml 2F5. Cells were then washed to remove shed gp120 in PBS/10 mM EDTA and lysed with 100 µl of 50 mM Tris, 150 mM NaCl, SDS 0.1% (wt/vol), 0.5% (wt/vol) Na deoxycholate, and 1% Triton X-100, pH 7.4 (RIPA buffer). The cell lysates were analyzed on a NuPAGE 4–12% gradient gel (Invitrogen) and subsequently blotted onto a Hybond C membrane (GE Healthcare) and analyzed for gp120 content using goat anti-gp120 antibody (D7324; Aalto) and horseradish peroxidase–conjugated rabbit anti–goat IgG and detection with ECL (GE Healthcare). Imaging analysis was performed using the LAS4000 imaging system (Fujifilm), and densitometry was performed using Multi Gauge version 3.0 software (Fujifilm). All data were normalized to uncleaved (intracellular) gp160 of the respective sample to correct for potential differences in transfection efficiency.
Detection of gp120 shedding by FACS.
293-T cells expressing ΔCT JR-FL gp160 and controls were prepared as described for the analysis of gp120 shedding by Western blot. 20 h before harvest, the culture medium was exchanged for fresh medium containing protein transport inhibitor Brefeldin A (BioLegend) at 1:1,000 to limit incorporation of new trimer molecules in the cell membrane during the following shedding analysis. Cells were then incubated for 1 or 20 h with total human 50 µg/ml IgG, 10 µg/ml CD4-IgG2, or 50 µg/ml 2F5, after which cells were detached from plates with PBS/10 mM EDTA (Invitrogen) and washed twice with PBS, 1% FCS, and 2 mM EDTA. The level of residual surface gp120 was determined by flow cytometry upon staining with biotinylated 2G12 and detection by streptavidin-APC (BioLegend). Viability stain propidium iodide (Invitrogen) was added to all samples 10 min before acquisition, and dead cells were excluded from analysis. Samples were acquired on a CyAn ADP analyzer (Beckman Counter) and analyzed using FlowJo software (Tree Star, Inc.).
Data analysis.
Statistical analysis and fitting were performed using Prism version 5.0 with the exception of data depicted in Fig. 5 A, where fitting was performed using the R language of statistical computing (R Development Core Team, 2008).
Online supplemental material.
Fig. S1 shows virus–antibody interaction kinetics. Fig. S2 depicts gp120 staining on transfected cells after 1-h treatment with CD4-IgG2 and 2F5, demonstrating that short-term treatment with 2F5 does not release gp120. Table S1 provides IC50 concentrations for all virus mAb combinations probed in Fig. 1 A. Table S2 lists characteristics of pseudovirions used. Table S3 lists the characteristics and origin of antibodies used. Table S4, included as an Excel file, provides shedding and neutralization data depicted in Fig. 7 C. Table S5, included as an Excel file, provides shedding and neutralization data depicted in Fig. 7 B. Online supplemental material is available at http://www.jem.org/cgi/content/full/jem.20101907/DC1.
Acknowledgments
We thank W.A. Paxton, L. Hangartner, and J. Pavlovic for helpful discussion and D. Katinger and H. Katinger for providing mAbs. We thank our patients for participating in the clinical studies from which samples for the current analysis were derived. We thank the Swiss HIV Cohort Study (SHCS) for access to samples and J. Böni for the use of the SHCS drug resistance database.
Support was provided by grants from the Swiss National Science Foundation (310000-120739 to A. Trkola, 323530-123719 to I.A. Abela, 324730-130865 to H.F. Günthard, and 315200-114148 to R.R. Regoes). A. Trkola is an Elizabeth Glaser Scientist supported by the Elizabeth Glaser Pediatric AIDS Foundation.
The authors declare no competing financial interests.
Footnotes
Abbreviations used:
- CD4bs
- CD4 binding site
- MPER
- membrane-proximal external region
- sCD4
- soluble CD4
References
- Alam S.M., Morelli M., Dennison S.M., Liao H.X., Zhang R., Xia S.M., Rits-Volloch S., Sun L., Harrison S.C., Haynes B.F., Chen B. 2009. Role of HIV membrane in neutralization by two broadly neutralizing antibodies. Proc. Natl. Acad. Sci. USA. 106:20234–20239 10.1073/pnas.0908713106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allaway G.P., Davis-Bruno K.L., Beaudry G.A., Garcia E.B., Wong E.L., Ryder A.M., Hasel K.W., Gauduin M.C., Koup R.A., McDougal J.S., et al. 1995. Expression and characterization of CD4-IgG2, a novel heterotetramer that neutralizes primary HIV type 1 isolates. AIDS Res. Hum. Retroviruses. 11:533–539 10.1089/aid.1995.11.533 [DOI] [PubMed] [Google Scholar]
- Bánki Z., Kacani L., Rusert P., Pruenster M., Wilflingseder D., Falkensammer B., Stellbrink H.J., van Lunzen J., Trkola A., Dierich M.P., Stoiber H. 2005. Complement dependent trapping of infectious HIV in human lymphoid tissues. AIDS. 19:481–486 10.1097/01.aids.0000162336.20439.8d [DOI] [PubMed] [Google Scholar]
- Barbas C.F., III, Björling E., Chiodi F., Dunlop N., Cababa D., Jones T.M., Zebedee S.L., Persson M.A., Nara P.L., Norrby E., et al. 1992. Recombinant human Fab fragments neutralize human type 1 immunodeficiency virus in vitro. Proc. Natl. Acad. Sci. USA. 89:9339–9343 10.1073/pnas.89.19.9339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binley J.M., Cayanan C.S., Wiley C., Schülke N., Olson W.C., Burton D.R. 2003. Redox-triggered infection by disulfide-shackled human immunodeficiency virus type 1 pseudovirions. J. Virol. 77:5678–5684 10.1128/JVI.77.10.5678-5684.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binley J.M., Wrin T., Korber B., Zwick M.B., Wang M., Chappey C., Stiegler G., Kunert R., Zolla-Pazner S., Katinger H., et al. 2004. Comprehensive cross-clade neutralization analysis of a panel of anti-human immunodeficiency virus type 1 monoclonal antibodies. J. Virol. 78:13232–13252 10.1128/JVI.78.23.13232-13252.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchacher A., Predl R., Strutzenberger K., Steinfellner W., Trkola A., Purtscher M., Gruber G., Tauer C., Steindl F., Jungbauer A., et al. 1994. Generation of human monoclonal antibodies against HIV-1 proteins; electrofusion and Epstein-Barr virus transformation for peripheral blood lymphocyte immortalization. AIDS Res. Hum. Retroviruses. 10:359–369 10.1089/aid.1994.10.359 [DOI] [PubMed] [Google Scholar]
- Buzon V., Natrajan G., Schibli D., Campelo F., Kozlov M.M., Weissenhorn W. 2010. Crystal structure of HIV-1 gp41 including both fusion peptide and membrane proximal external regions. PLoS Pathog. 6:e1000880 10.1371/journal.ppat.1000880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardoso R.M., Brunel F.M., Ferguson S., Zwick M., Burton D.R., Dawson P.E., Wilson I.A. 2007. Structural basis of enhanced binding of extended and helically constrained peptide epitopes of the broadly neutralizing HIV-1 antibody 4E10. J. Mol. Biol. 365:1533–1544 10.1016/j.jmb.2006.10.088 [DOI] [PubMed] [Google Scholar]
- Chen L., Kwon Y.D., Zhou T., Wu X., O’Dell S., Cavacini L., Hessell A.J., Pancera M., Tang M., Xu L., et al. 2009. Structural basis of immune evasion at the site of CD4 attachment on HIV-1 gp120. Science. 326:1123–1127 10.1126/science.1175868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chertova E., Bess J.W., Jr, Crise B.J., Sowder R.C., II, Schaden T.M., Hilburn J.M., Hoxie J.A., Benveniste R.E., Lifson J.D., Henderson L.E., Arthur L.O. 2002. Envelope glycoprotein incorporation, not shedding of surface envelope glycoprotein (gp120/SU), is the primary determinant of SU content of purified human immunodeficiency virus type 1 and simian immunodeficiency virus. J. Virol. 76:5315–5325 10.1128/JVI.76.11.5315-5325.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corti D., Langedijk J.P., Hinz A., Seaman M.S., Vanzetta F., Fernandez-Rodriguez B.M., Silacci C., Pinna D., Jarrossay D., Balla-Jhagjhoorsingh S., et al. 2010. Analysis of memory B cell responses and isolation of novel monoclonal antibodies with neutralizing breadth from HIV-1-infected individuals. PLoS ONE. 5:e8805 10.1371/journal.pone.0008805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daar E.S., Li X.L., Moudgil T., Ho D.D.C. 1990. High concentrations of recombinant soluble CD4 are required to neutralize primary human immunodeficiency virus type 1 isolates. Proc. Natl. Acad. Sci. USA. 87:6574–6578 10.1073/pnas.87.17.6574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deen K.C., McDougal J.S., Inacker R., Folena-Wasserman G., Arthos J., Rosenberg J., Maddon P.J., Axel R., Sweet R.W. 1988. A soluble form of CD4 (T4) protein inhibits AIDS virus infection. Nature. 331:82–84 10.1038/331082a0 [DOI] [PubMed] [Google Scholar]
- Finnegan C.M., Berg W., Lewis G.K., DeVico A.L. 2002. Antigenic properties of the human immunodeficiency virus transmembrane glycoprotein during cell-cell fusion. J. Virol. 76:12123–12134 10.1128/JVI.76.23.12123-12134.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finzi A., Xiang S.H., Pacheco B., Wang L., Haight J., Kassa A., Danek B., Pancera M., Kwong P.D., Sodroski J.C. 2010. Topological layers in the HIV-1 gp120 inner domain regulate gp41 interaction and CD4-triggered conformational transitions. Mol. Cell. 37:656–667 10.1016/j.molcel.2010.02.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frey G., Peng H., Rits-Volloch S., Morelli M., Cheng Y., Chen B.C. 2008. A fusion-intermediate state of HIV-1 gp41 targeted by broadly neutralizing antibodies. Proc. Natl. Acad. Sci. USA. 105:3739–3744 10.1073/pnas.0800255105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frey G., Chen J., Rits-Volloch S., Freeman M.M., Zolla-Pazner S., Chen B. 2010. Distinct conformational states of HIV-1 gp41 are recognized by neutralizing and non-neutralizing antibodies. Nat. Struct. Mol. Biol. 17:1486–1491 10.1038/nsmb.1950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorny M.K., Conley A.J., Karwowska S., Buchbinder A., Xu J.Y., Emini E.A., Koenig S., Zolla-Pazner S. 1992. Neutralization of diverse human immunodeficiency virus type 1 variants by an anti-V3 human monoclonal antibody. J. Virol. 66:7538–7542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haase A.T. 2010. Targeting early infection to prevent HIV-1 mucosal transmission. Nature. 464:217–223 10.1038/nature08757 [DOI] [PubMed] [Google Scholar]
- Haim H., Si Z., Madani N., Wang L., Courter J.R., Princiotto A., Kassa A., DeGrace M., McGee-Estrada K., Mefford M., et al. 2009. Soluble CD4 and CD4-mimetic compounds inhibit HIV-1 infection by induction of a short-lived activated state. PLoS Pathog. 5:e1000360 10.1371/journal.ppat.1000360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hessell A.J., Hangartner L., Hunter M., Havenith C.E., Beurskens F.J., Bakker J.M., Lanigan C.M., Landucci G., Forthal D.N., Parren P.W., et al. 2007. Fc receptor but not complement binding is important in antibody protection against HIV. Nature. 449:101–104 10.1038/nature06106 [DOI] [PubMed] [Google Scholar]
- Hessell A.J., Poignard P., Hunter M., Hangartner L., Tehrani D.M., Bleeker W.K., Parren P.W., Marx P.A., Burton D.R. 2009a. Effective, low-titer antibody protection against low-dose repeated mucosal SHIV challenge in macaques. Nat. Med. 15:951–954 10.1038/nm.1974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hessell A.J., Rakasz E.G., Poignard P., Hangartner L., Landucci G., Forthal D.N., Koff W.C., Watkins D.I., Burton D.R.C. 2009b. Broadly neutralizing human anti-HIV antibody 2G12 is effective in protection against mucosal SHIV challenge even at low serum neutralizing titers. PLoS Pathog. 5:e1000433 10.1371/journal.ppat.1000433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hessell A.J., Rakasz E.G., Tehrani D.M., Huber M., Weisgrau K.L., Landucci G., Forthal D.N., Koff W.C., Poignard P., Watkins D.I., Burton D.R.C. 2010. Broadly neutralizing monoclonal antibodies 2F5 and 4E10 directed against the human immunodeficiency virus type 1 gp41 membrane-proximal external region protect against mucosal challenge by simian-human immunodeficiency virus SHIVBa-L. J. Virol. 84:1302–1313 10.1128/JVI.01272-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho J., Moir S., Kulik L., Malaspina A., Donoghue E.T., Miller N.J., Wang W., Chun T.W., Fauci A.S., Holers V.M. 2007. Role for CD21 in the establishment of an extracellular HIV reservoir in lymphoid tissues. J. Immunol. 178:6968–6974 [DOI] [PubMed] [Google Scholar]
- Huber M., Trkola A. 2007. Humoral immunity to HIV-1: neutralization and beyond. J. Intern. Med. 262:5–25 10.1111/j.1365-2796.2007.01819.x [DOI] [PubMed] [Google Scholar]
- Huber M., Fischer M., Misselwitz B., Manrique A., Kuster H., Niederöst B., Weber R., von Wyl V., Günthard H.F., Trkola A.C. 2006. Complement lysis activity in autologous plasma is associated with lower viral loads during the acute phase of HIV-1 infection. PLoS Med. 3:e441 10.1371/journal.pmed.0030441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilby J.M., Hopkins S., Venetta T.M., DiMassimo B., Cloud G.A., Lee J.Y., Alldredge L., Hunter E., Lambert D., Bolognesi D., et al. 1998. Potent suppression of HIV-1 replication in humans by T-20, a peptide inhibitor of gp41-mediated virus entry. Nat. Med. 4:1302–1307 10.1038/3293 [DOI] [PubMed] [Google Scholar]
- Kunert R., Rüker F., Katinger H. 1998. Molecular characterization of five neutralizing anti-HIV type 1 antibodies: identification of nonconventional D segments in the human monoclonal antibodies 2G12 and 2F5. AIDS Res. Hum. Retroviruses. 14:1115–1128 10.1089/aid.1998.14.1115 [DOI] [PubMed] [Google Scholar]
- Kwong P.D., Wyatt R., Robinson J., Sweet R.W., Sodroski J., Hendrickson W.A. 1998. Structure of an HIV gp120 envelope glycoprotein in complex with the CD4 receptor and a neutralizing human antibody. Nature. 393:648–659 10.1038/31405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwong P.D., Doyle M.L., Casper D.J., Cicala C., Leavitt S.A., Majeed S., Steenbeke T.D., Venturi M., Chaiken I., Fung M., et al. 2002. HIV-1 evades antibody-mediated neutralization through conformational masking of receptor-binding sites. Nature. 420:678–682 10.1038/nature01188 [DOI] [PubMed] [Google Scholar]
- Lewis N., Williams J., Rekosh D., Hammarskjöld M.L.C. 1990. Identification of a cis-acting element in human immunodeficiency virus type 2 (HIV-2) that is responsive to the HIV-1 rev and human T-cell leukemia virus types I and II rex proteins. J. Virol. 64:1690–1697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lok S.M., Kostyuchenko V., Nybakken G.E., Holdaway H.A., Battisti A.J., Sukupolvi-Petty S., Sedlak D., Fremont D.H., Chipman P.R., Roehrig J.T., et al. 2008. Binding of a neutralizing antibody to dengue virus alters the arrangement of surface glycoproteins. Nat. Struct. Mol. Biol. 15:312–317 10.1038/nsmb.1382 [DOI] [PubMed] [Google Scholar]
- Mascola J.R., Stiegler G., VanCott T.C., Katinger H., Carpenter C.B., Hanson C.E., Beary H., Hayes D., Frankel S.S., Birx D.L., Lewis M.G. 2000. Protection of macaques against vaginal transmission of a pathogenic HIV-1/SIV chimeric virus by passive infusion of neutralizing antibodies. Nat. Med. 6:207–210 10.1038/72318 [DOI] [PubMed] [Google Scholar]
- McDougal J.S., Kennedy M.S., Orloff S.L., Nicholson J.K., Spira T.J. 1996. Mechanisms of human immunodeficiency virus Type 1 (HIV-1) neutralization: irreversible inactivation of infectivity by anti-HIV-1 antibody. J. Virol. 70:5236–5245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKeating J.A., McKnight A., Moore J.P. 1991. Differential loss of envelope glycoprotein gp120 from virions of human immunodeficiency virus type 1 isolates: effects on infectivity and neutralization. J. Virol. 65:852–860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehandru S., Vcelar B., Wrin T., Stiegler G., Joos B., Mohri H., Boden D., Galovich J., Tenner-Racz K., Racz P., et al. 2007. Adjunctive passive immunotherapy in human immunodeficiency virus type 1-infected individuals treated with antiviral therapy during acute and early infection. J. Virol. 81:11016–11031 10.1128/JVI.01340-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moir S., Malaspina A., Li Y., Chun T.W., Lowe T., Adelsberger J., Baseler M., Ehler L.A., Liu S., Davey R.T., Jr, et al. 2000. B cells of HIV-1–infected patients bind virions through CD21–complement interactions and transmit infectious virus to activated T cells. J. Exp. Med. 192:637–646 10.1084/jem.192.5.637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montero M., van Houten N.E., Wang X., Scott J.K.C. 2008. The membrane-proximal external region of the human immunodeficiency virus type 1 envelope: dominant site of antibody neutralization and target for vaccine design. Microbiol. Mol. Biol. Rev. 72:54–84 10.1128/MMBR.00020-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore J.P., McKeating J.A., Weiss R.A., Sattentau Q.J. 1990. Dissociation of gp120 from HIV-1 virions induced by soluble CD4. Science. 250:1139–1142 10.1126/science.2251501 [DOI] [PubMed] [Google Scholar]
- Moore J.P., McKeating J.A., Huang Y.X., Ashkenazi A., Ho D.D. 1992. Virions of primary human immunodeficiency virus type 1 isolates resistant to soluble CD4 (sCD4) neutralization differ in sCD4 binding and glycoprotein gp120 retention from sCD4-sensitive isolates. J. Virol. 66:235–243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore P.L., Crooks E.T., Porter L., Zhu P., Cayanan C.S., Grise H., Corcoran P., Zwick M.B., Franti M., Morris L., et al. 2006. Nature of nonfunctional envelope proteins on the surface of human immunodeficiency virus type 1. J. Virol. 80:2515–2528 10.1128/JVI.80.5.2515-2528.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muster T., Steindl F., Purtscher M., Trkola A., Klima A., Himmler G., Rüker F., Katinger H. 1993. A conserved neutralizing epitope on gp41 of human immunodeficiency virus type 1. J. Virol. 67:6642–6647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orloff S.L., Kennedy M.S., Belperron A.A., Maddon P.J., McDougal J.S. 1993. Two mechanisms of soluble CD4 (sCD4)-mediated inhibition of human immunodeficiency virus type 1 (HIV-1) infectivity and their relation to primary HIV-1 isolates with reduced sensitivity to sCD4. J. Virol. 67:1461–1471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pancera M., Majeed S., Ban Y.E., Chen L., Huang C.C., Kong L., Kwon Y.D., Stuckey J., Zhou T., Robinson J.E., et al. 2010. Structure of HIV-1 gp120 with gp41-interactive region reveals layered envelope architecture and basis of conformational mobility. Proc. Natl. Acad. Sci. USA. 107:1166–1171 10.1073/pnas.0911004107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park E.J., Gorny M.K., Zolla-Pazner S., Quinnan G.V.J.C., Jr 2000. A global neutralization resistance phenotype of human immunodeficiency virus type 1 is determined by distinct mechanisms mediating enhanced infectivity and conformational change of the envelope complex. J. Virol. 74:4183–4191 10.1128/JVI.74.9.4183-4191.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pietzsch J., Scheid J.F., Mouquet H., Klein F., Seaman M.S., Jankovic M., Corti D., Lanzavecchia A., Nussenzweig M.C.C. 2010. Human anti–HIV-neutralizing antibodies frequently target a conserved epitope essential for viral fitness. J. Exp. Med. 207:1995–2002 10.1084/jem.20101176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poignard P., Fouts T., Naniche D., Moore J.P., Sattentau Q.J.C. 1996. Neutralizing antibodies to human immunodeficiency virus type-1 gp120 induce envelope glycoprotein subunit dissociation. J. Exp. Med. 183:473–484 10.1084/jem.183.2.473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poignard P., Moulard M., Golez E., Vivona V., Franti M., Venturini S., Wang M., Parren P.W., Burton D.R.C. 2003. Heterogeneity of envelope molecules expressed on primary human immunodeficiency virus type 1 particles as probed by the binding of neutralizing and nonneutralizing antibodies. J. Virol. 77:353–365 10.1128/JVI.77.1.353-365.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pugach P., Kuhmann S.E., Taylor J., Marozsan A.J., Snyder A., Ketas T., Wolinsky S.M., Korber B.T., Moore J.P. 2004. The prolonged culture of human immunodeficiency virus type 1 in primary lymphocytes increases its sensitivity to neutralization by soluble CD4. Virology. 321:8–22 10.1016/j.virol.2003.12.012 [DOI] [PubMed] [Google Scholar]
- R Development Core Team 2008. R: A language and environment for statistical computing. Vienna Austria R Foundation for Statistical Computing. Vol. 1 (09/18/2009). Available at http://www.r-project.org
- Ramratnam B., Bonhoeffer S., Binley J., Hurley A., Zhang L., Mittler J.E., Markowitz M., Moore J.P., Perelson A.S., Ho D.D. 1999. Rapid production and clearance of HIV-1 and hepatitis C virus assessed by large volume plasma apheresis. Lancet. 354:1782–1785 10.1016/S0140-6736(99)02035-8 [DOI] [PubMed] [Google Scholar]
- Rusert P., Kuster H., Joos B., Misselwitz B., Gujer C., Leemann C., Fischer M., Stiegler G., Katinger H., Olson W.C., et al. 2005. Virus isolates during acute and chronic human immunodeficiency virus type 1 infection show distinct patterns of sensitivity to entry inhibitors. J. Virol. 79:8454–8469 10.1128/JVI.79.13.8454-8469.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rusert P., Mann A., Huber M., von Wyl V., Gunthard H.F., Trkola A. 2009. Divergent effects of cell environment on HIV entry inhibitor activity. AIDS. 23:1319–1327 10.1097/QAD.0b013e32832d92c2 [DOI] [PubMed] [Google Scholar]
- Salazar-Gonzalez J.F., Salazar M.G., Keele B.F., Learn G.H., Giorgi E.E., Li H., Decker J.M., Wang S., Baalwa J., Kraus M.H., et al. 2009. Genetic identity, biological phenotype, and evolutionary pathways of transmitted/founder viruses in acute and early HIV-1 infection. J. Exp. Med. 206:1273–1289 10.1084/jem.20090378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sattentau Q.J., Zolla-Pazner S., Poignard P. 1995. Epitope exposure on functional, oligomeric HIV-1 gp41 molecules. Virology. 206:713–717 10.1016/S0042-6822(95)80094-8 [DOI] [PubMed] [Google Scholar]
- Schoeni-Affolter F., Ledergerber B., Rickenbach M., Rudin C., Günthard H.F., Telenti A., Furrer H., Yerly S., Francioli P.; Swiss HIV Cohort Study 2010. Cohort profile: the Swiss HIV Cohort study. Int. J. Epidemiol. 39:1179–1189 10.1093/ije/dyp321 [DOI] [PubMed] [Google Scholar]
- Selinka H.C., Giroglou T., Nowak T., Christensen N.D., Sapp M. 2003. Further evidence that papillomavirus capsids exist in two distinct conformations. J. Virol. 77:12961–12967 10.1128/JVI.77.24.12961-12967.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stiegler G., Kunert R., Purtscher M., Wolbank S., Voglauer R., Steindl F., Katinger H. 2001. A potent cross-clade neutralizing human monoclonal antibody against a novel epitope on gp41 of human immunodeficiency virus type 1. AIDS Res. Hum. Retroviruses. 17:1757–1765 10.1089/08892220152741450 [DOI] [PubMed] [Google Scholar]
- Trkola A., Purtscher M., Muster T., Ballaun C., Buchacher A., Sullivan N., Srinivasan K., Sodroski J., Moore J.P., Katinger H. 1996. Human monoclonal antibody 2G12 defines a distinctive neutralization epitope on the gp120 glycoprotein of human immunodeficiency virus type 1. J. Virol. 70:1100–1108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trkola A., Kuster H., Leemann C., Oxenius A., Fagard C., Furrer H., Battegay M., Vernazza P., Bernasconi E., Weber R., et al. ; Swiss HIV Cohort Study 2004. Humoral immunity to HIV-1: kinetics of antibody responses in chronic infection reflects capacity of immune system to improve viral set point. Blood. 104:1784–1792 10.1182/blood-2004-01-0251 [DOI] [PubMed] [Google Scholar]
- Trkola A., Kuster H., Rusert P., Joos B., Fischer M., Leemann C., Manrique A., Huber M., Rehr M., Oxenius A., et al. 2005. Delay of HIV-1 rebound after cessation of antiretroviral therapy through passive transfer of human neutralizing antibodies. Nat. Med. 11:615–622 10.1038/nm1244 [DOI] [PubMed] [Google Scholar]
- Turville S.G., Santos J.J., Frank I., Cameron P.U., Wilkinson J., Miranda-Saksena M., Dable J., Stössel H., Romani N., Piatak M., Jr, et al. 2004. Immunodeficiency virus uptake, turnover, and 2-phase transfer in human dendritic cells. Blood. 103:2170–2179 10.1182/blood-2003-09-3129 [DOI] [PubMed] [Google Scholar]
- Walker L.M., Phogat S.K., Chan-Hui P.Y., Wagner D., Phung P., Goss J.L., Wrin T., Simek M.D., Fling S., Mitcham J.L., et al. ; Protocol G Principal Investigators 2009. Broad and potent neutralizing antibodies from an African donor reveal a new HIV-1 vaccine target. Science. 326:285–289 10.1126/science.1178746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witz J., Brown F. 2001. Structural dynamics, an intrinsic property of viral capsids. Arch. Virol. 146:2263–2274 10.1007/s007050170001 [DOI] [PubMed] [Google Scholar]
- Wu X., Yang Z.Y., Li Y., Hogerkorp C.M., Schief W.R., Seaman M.S., Zhou T., Schmidt S.D., Wu L., Xu L., et al. 2010. Rational design of envelope identifies broadly neutralizing human monoclonal antibodies to HIV-1. Science. 329:856–861 10.1126/science.1187659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu H.J., Reuter M.A., McDonald D.C. 2008. HIV traffics through a specialized, surface-accessible intracellular compartment during trans-infection of T cells by mature dendritic cells. PLoS Pathog. 4:e1000134 10.1371/journal.ppat.1000134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou T., Xu L., Dey B., Hessell A.J., Van Ryk D., Xiang S.H., Yang X., Zhang M.Y., Zwick M.B., Arthos J., et al. 2007. Structural definition of a conserved neutralization epitope on HIV-1 gp120. Nature. 445:732–737 10.1038/nature05580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zwick M.B., Burton D.R. 2007. HIV-1 neutralization: mechanisms and relevance to vaccine design. Curr. HIV Res. 5:608–624 10.2174/157016207782418443 [DOI] [PubMed] [Google Scholar]
- Zwick M.B., Labrijn A.F., Wang M., Spenlehauer C., Saphire E.O., Binley J.M., Moore J.P., Stiegler G., Katinger H., Burton D.R., Parren P.W. 2001. Broadly neutralizing antibodies targeted to the membrane-proximal external region of human immunodeficiency virus type 1 glycoprotein gp41. J. Virol. 75:10892–10905 10.1128/JVI.75.22.10892-10905.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]