Dectin-2 expression on GM-CSF–cultured bone marrow cells is required for the generation of cysteinyl leukotrienes and Th2 cytokines in response to the house dust mite Dermatophagoides farinae in vivo.
Abstract
The innate signaling pathways for Th2 immunity activated by inhaled antigens are not well defined. We previously identified Dectin-2 as a receptor for glycans in allergen extracts from the house dust mite Dermatophagoides farinae (Df) that mediates cysteinyl leukotriene (cys-LT) generation from pulmonary CD11c+ cells and from GM-CSF–cultured bone marrow cells (BMCsGM-CSF). Using lentiviral knockdown of Dectin-2 in BMCsGM-CSF and adoptive transfer of Df-pulsed BMCsGM-CSF to sensitize naive mice, we now report that Dectin-2 is critical for the development of Df-elicited eosinophilic and neutrophilic pulmonary inflammation and Th2 cytokine generation in the lungs and restimulated lymph nodes. Sensitization with Df-pulsed BMCsGM-CSF from LTC4 synthase (LTC4S)–deficient mice or type 1 cys-LT receptor (CysLT1R)–deficient mice demonstrated that both proteins were required for Df-elicited eosinophilic pulmonary inflammation and Th2 cytokine generation in the lungs and restimulated lymph nodes. Direct sensitization and challenge of Ltc4s−/− and Cysltr1−/− mice confirmed that cys-LTs mediate these parameters of Df-elicited Th2 pulmonary inflammation. Thus, the Dectin-2–cys-LT pathway is critical for the induction of Th2 immunity to a major allergen, in part through CysLT1R. These findings identify a previously unrecognized link between a myeloid C-type lectin receptor and Th2 immunity.
Innate host responses to microbes rely on germline-encoded pattern recognition receptors (PRRs) that recognize conserved groups of molecules, PAMPs (pathogen-associated molecular patterns), that are found in microbes. PRR signaling during antigen presentation plays a critical role in influencing DC activation and cytokine production for tailoring the subsequent adaptive immune response (Iwasaki and Medzhitov, 2004). DCs express four families of PRRs: the toll-like receptors (TLRs), the Nod-like receptors (NLRs), the RIG-I and Mda5 helicases, and the membrane-associated C-type lectin receptors (CLRs). Whereas DC signaling through TLRs in response to some bacterial species is linked to the induction of IL-12 and Th1 immunity (Iwasaki and Medzhitov, 2004), and signaling through CLRs in response to fungi is linked to the generation of IL-6, IL-23, and Th17 immunity (LeibundGut-Landmann et al., 2007; Robinson et al., 2009), only TLR4 on lung stromal cells is clearly linked to the preferential generation of Th2 immunity (Hammad et al., 2009).
Nonmammalian glycans in helminths, fungi, and plants can act as potent Th2 adjuvants. Schistosoma mansoni egg antigen (SEA) elicits Th2 cytokines and antigen-specific IgE in BALB/c mice. This activity is lost with SEA deglycosylation, whereas conjugation of the germane α-fucosylated oligosaccharide, lacto-N-fucopentaose III, to human albumin promotes albumin-specific Th2 cytokine production and albumin-specific IgE (Okano et al., 1999, 2001). Ara h 1, the immunodominant peanut allergen containing xylosylated complex mannose glycans, such as Man3(−4)XylGlcNAc2, confers human monocyte-derived DCs with the capacity to generate IL-4– and IL-13–producing CD4+ T cells from naive CD45RA+ T cells (Shreffler et al., 2006). In mice, the intratracheal administration of soluble β-glucan (β-1,3/1,6-linked) from Candida albicans with OVA augments the levels of elicited pulmonary eosinophilia, pulmonary Th2 cytokine induction, and total and OVA-specific serum IgE produced by the administration of OVA alone (Inoue et al., 2009), and β-1,3/1,6-glucan from baker’s yeast can boost serum levels of OVA-specific IgE and IgG1, but not OVA-specific IgG2a, when added to an OVA footpad injection (Instanes et al., 2004). Thus, N-linked core α-fucosylated and/or β-xylosylated complex mannose glycans found widely in plants, insects, and helminths and β-1,3/1,6-glucans found in fungi appear to be nonmammalian molecular patterns important in triggering Th2 immunity (Altmann, 2007; Wills-Karp et al., 2010). However, although members of the CLR family recognize these motifs—e.g., L-SIGN (liver/lymph node–specific intercellular adhesion molecule 3–grabbing nonintegrin) recognizes α-fucosylated oligosaccharides in SEA (Meyer et al., 2007), DC-SIGN (DC-specific intercellular adhesion molecule 3–grabbing nonintegrin) recognizes xylosylated complex mannose in Ara h 1 (Shreffler et al., 2006), and Dectin-1 recognizes fungal β-glucans (Brown and Gordon, 2001)—a direct role for a myeloid CLR in the generation of Th2 immunity has not been demonstrated.
Dectin-2 is a member of the myeloid CLR family that couples to the FcRγ chain to initiate ITAM (immunoreceptor tyrosine-based activation motif)-dependent signaling and the recruitment and activation of spleen tyrosine kinase (Syk; Ariizumi et al., 2000; Sato et al., 2006). The Dectin-2–Syk pathway activates the CARD9 adaptor protein in DCs to generate NF-κB–dependent IL-2, IL-10, TNF, and IL-23p19 and to promote Th17 immune responses to C. albicans (Robinson et al., 2009). We have previously demonstrated that the Dectin-2–Syk pathway also triggers the generation of cysteinyl leukotrienes (cys-LTs) from pulmonary CD11c+ cells and from GM-CSF–cultured BM cells (BMCsGM-CSF) in response to glycans in allergen extracts from the house dust mite (HDM) species Dermatophagoides farinae (Df) and Dermatophagoides pteronyssinus (Dp; Barrett et al., 2009), the most common aeroallergens to elicit sensitization in humans and potent triggers of Th2- and Th17-dependent bronchial inflammation in mice (Cates et al., 2004; Krishnamoorthy et al., 2008; Hammad et al., 2009; Phipps et al., 2009). Thus, we hypothesized that Dectin-2 is a PRR coupled to the generation of Th2 immune responses via cys-LT signaling.
CD11c+ DCs in the bronchial mucosa sample inhaled antigens and then migrate to the lung-draining lymph nodes while simultaneously activating a maturation program that results in antigen-driven T cell division. This sensitization can be mimicked by the intratracheal or intranasal adoptive transfer of antigen-pulsed myeloid DCs to naive recipient mice and subsequent antigen challenge (Lambrecht et al., 2000a,b,c). We took advantage of this adoptive transfer system and the ability to knock down Dectin-2 in BMCsGM-CSF to examine the role of the Dectin-2–cys-LT pathway in Df-induced Th2 and Th17 pulmonary inflammation. In this paper, we demonstrate that Dectin-2 mediates Df-elicited generation of cys-LTs and cytokines, including TNF, IL-6, IL-10, and IL-23, using lentiviral knockdown of Dectin-2 in BMCsGM-CSF (kdBMCsGM-CSF). Moreover, WT mice sensitized with Df-pulsed kdBMCsGM-CSF and challenged intranasally with Df had significantly reduced pulmonary inflammation and Th2 cytokine generation from lung and restimulated parabronchial lymph nodes as compared with mice sensitized with Df-pulsed BMCsGM-CSF infected with a vector control (cBMCsGM-CSF), thereby identifying Dectin-2 as a critical PRR for Th2 immunity elicited by HDM. Furthermore, we show that the genetic absence of either LTC4 synthase (LTC4S), the critical enzyme in cys-LT generation, or the type 1 cys-LT receptor (CysLT1R) on Df-pulsed BMCsGM-CSF similarly impairs Df-induced Th2 pulmonary inflammation in lung and lymph nodes, thereby demonstrating that cys-LT production and CysLT1R-mediated function promote Th2 sensitization.
RESULTS
Dectin-2 mediates Df-elicited production of IL-6, IL-10, IL-23, and TNF by BMCsGM-CSF
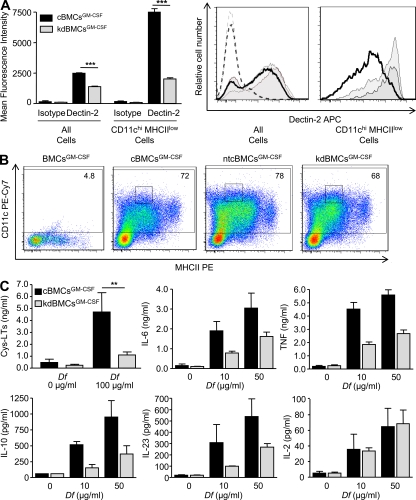
Before assessing the role of Dectin-2 in Df-initiated cytokine responses in vitro and in vivo using GM-CSF–expanded BM cultures, we further characterized the BMCsGM-CSF from WT BALB/c mice. At day 7, BMCsGM-CSF were 74.9 ± 0.7% CD11c+ by flow cytometry. The CD11c+ cell population expressed CD11b (97.7 ± 0.4%) and MHCII (88.5 ± 0.2%; Fig. S1 A and not depicted). Although 89 ± 3.6% of CD11c+ cells expressed Dectin-2, the range of expression was broad with CD11chiMHCIIlow cells expressing the highest levels (Fig. S1 A). 92.4 ± 0.2% of Dectin-2+ cells were CD11c+ (Fig. S1 B). 7.6 ± 0.2% of Dectin-2+ cells were CD11c− and expressed CD11b and the monocyte marker Ly6C (Fig. S1 B). Analysis of stained cytospins revealed that 12.4 ± 0.5% of BMCsGM-CSF were granulocytes (not depicted), all of which were Dectin-2− by immunocytochemistry (Fig. S1 C).
To assess the role of Dectin-2 in Df-induced cytokine production, we infected day-1 BMCsGM-CSF with lentivirus expressing a short hairpin (sh) RNA for Dectin-2 (kd-BMCsGM-CSF), a nontargeting sequence (nontargeting control BMCGM-CSF [ntcBMCGM-CSF]), or an empty vector alone (cBMCsGM-CSF). After puromycin selection of infected cells, kdBMCsGM-CSF demonstrated a significant 43.9% reduction in Dectin-2 expression by flow cytometry, as compared with cBMCsGM-CSF (Fig. 1 A, left). A CD11chi MHCIIlow subgroup, which was 5 ± 0.8% of total cells, expressed high levels of Dectin-2 in control vector BMCGM-CSF cultures and demonstrated a significant 73.6% reduction in Dectin-2 expression in knockdown BMCGM-CSF cultures (Fig. 1 A, left). Dectin-2 expression on all BMCsGM-CSF and on CD11chi MHCIIlow cells from ntcBMCGM-CSF was comparable to cBMCsGM-CSF and distinct from the reduction observed in kdBMCsGM-CSF (Fig. 1 A, right). Day-7 cBMCsGM-CSF, ntcBMCsGM-CSF, and kdBMCsGM-CSF showed similar expression of CD11c and MHCII (Fig. 1 B). The knockdown was confirmed by bioassay, in which kdBMCsGM-CSF demonstrated a 79.6% reduction in cys-LT generation after stimulation for 1 h with 100 µg/ml Df (Fig. 1 C). In parallel, we stimulated cells at 0, 10, or 50 µg/ml Df for 4 or 30 h. At 4 h, kdBMCsGM-CSF stimulated with Df had significant reductions in the generation of IL-6, IL-10, IL-23, and TNF, as compared with cBMCsGM-CSF (Fig. 1 C). There was no reduction in IL-2. At 30 h, kd-BMCsGM-CSF had significant reductions in IL-6, IL-10, and TNF, as compared with cBMCsGM-CSF, and a lower level of IL-23 that did not reach statistical significance (Fig. S2 A). kdBMCsGM-CSF had no reduction in TNF generation in response to LPS or to curdlan (Fig. S2 B) and no reduction in cys-LT generation in response to curdlan (Fig. S2 B), indicating the functional integrity of TLR4 and Dectin-1.
Figure 1.
Dectin-2 mediates Df-elicited cytokine production by BMCsGM-CSF. BMCsGM-CSF were infected with viral particles containing Dectin-2 shRNA (kdBMCsGM-CSF), a vector control (cBMCsGM-CSF), or a nontargeting vector control (ntcBMCGM-CSF), selected with puromycin, and harvested at day 7. (A) Dectin-2 expression on all BMCsGM-CSF and on CD11chi MHCIIlow cells was assessed by flow cytometry. Left, results are means ± SEM (n = 4–6 mice per group) from three independent experiments. ***, P = 0.0001. Significance was determined with an unpaired Student’s t test. Right, a representative histogram of Dectin-2 expression on all BMCsGM-CSF and on CD11chiMHCIIlow cells from cBMCsGM-CSF (gray), ntcBMCGM-CSF (light gray), and kdBMCsGM-CSF (heavy black line) cultures is compared. Isotype control staining on all BMCsGM-CSF is also shown for cBMCsGM-CSF (gray dotted), ntcBMCsGM-CSF (light gray dotted), and kdBMCsGM-CSF (heavy black line dotted). (B) Representative histograms are shown of CD11c expression and MHCII expression on cBMCsGM-CSF, ntcBMCsGM-CSF, and kdBMCsGM-CSF, with the percentage of CD11c positive cells noted, as gated by the isotype control staining on a mixture of cBMCsGM-CSF, ntcBMCsGM-CSF, and kdBMCsGM-CSF, designated BMCsGM-CSF. MHCII staining was performed with nonsaturating antibody concentrations. Gating on a CD11chiMHCIIlow Dectin-2hi subset is also indicated (small boxes). (C) BMCsGM-CSF were stimulated with Df at the indicated concentrations and the supernatant contents were assessed by ELISA for cys-LTs at 60 min and for cytokines at 4 h. Results for cys-LT generation are means ± SEM (n = 6–8 mice per group) from four independent experiments. **, P = 0.02. Significance was determined with an unpaired Student’s t test. Results for cytokines are means ± SEM (n = 5–6 mice per group) from three independent experiments. P = 0.01 for IL-6, P = 0.04 for IL-10, P = 0.03 for IL-23, and P = 0.0001 for TNF. Significance was determined with two-way ANOVA (p-values refer to the integrated differences in cBMCsGM-CSF and kdBMCsGM-CSF over the varied doses).
BMCGM-CSF Dectin-2 mediates Df-elicited Th2 pulmonary inflammation
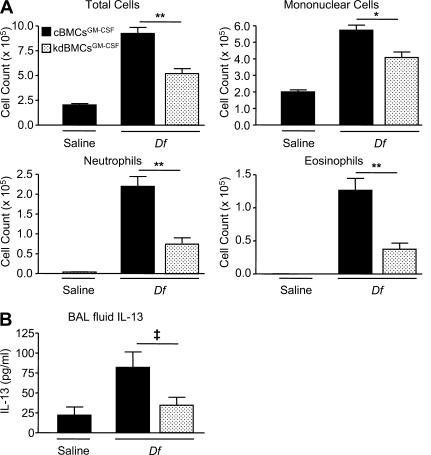
We next assessed whether Dectin-2 influenced the ability of BMCsGM-CSF to initiate a Df-induced immune response in an adoptive transfer model. We stimulated day-7 kdBMCsGM-CSF and cBMCsGM-CSF with 100 µg/ml Df or saline for 24 h, adoptively transferred 104 cells into WT recipients by intranasal injection on day 8, challenged recipients with 2 µg Df intranasally on day 22 and day 23, and euthanized the recipients for assay on day 25. Df-pulsed cBMCsGM-CSF were able to sensitize recipients for Df challenge, inducing a robust inflammatory response with recruitment of mononuclear cells, neutrophils, and eosinophils in bronchoalveolar lavage (BAL) fluid. After Df challenge, recipients sensitized with kd-BMCsGM-CSF had significant reductions in total cell counts of 43.6%, in mononuclear cells of 28.9%, in neutrophils of 65.9%, and in eosinophils of 70.3%, as compared with recipients of cBMCsGM-CSF (Fig. 2 A). To determine whether the reduction in BAL fluid inflammation was associated with a reduction in T cell cytokines, ELISA was performed on the BAL fluid supernatants for IL-13, IL-17A, and IFN-γ (Fig. 2 B). Recipients sensitized with kdBMCsGM-CSF had a significant 59.7% reduction in BAL fluid IL-13, as compared with mice sensitized with cBMCsGM-CSF. The levels of IL-17A and IFN-γ were below the limits of detection.
Figure 2.
BMCGM-CSF Dectin-2 is required for Df-elicited Th2 pulmonary inflammation. WT mice were sensitized by intranasal administration of 104 saline-pulsed or Df-pulsed kdBMCsGM-CSF or cBMCsGM-CSF at day 8 of culture, challenged with 2 µg Df on day 22 and day 23, and analyzed on day 25. (A) Cells from the BAL fluid were counted, cytospin preparations were stained, and 400 cells/slide were counted for specific cell types. Results are means ± SEM (n = 13–14 mice per group) from three independent experiments. **, P = 0.0001; *, P = 0.001. Significance was determined with an unpaired Student’s t test. (B) ELISA was performed on BAL fluid supernatants. Results are means ± SEM (n = 14 mice per group) from three independent experiments. ‡, P = 0.05.
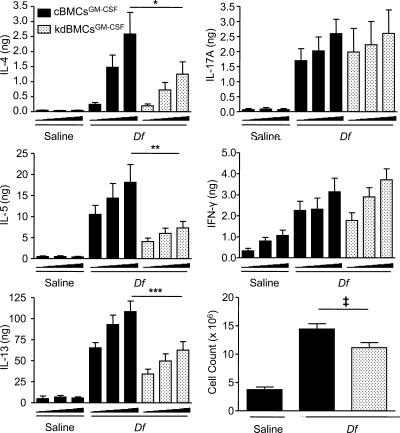
To determine whether the reduction in BAL fluid inflammatory cells and cytokines reflected an impairment in the generation of Th2 and/or Th17 immune responses in the lymph nodes, we assessed cytokine generation from the lung-draining lymph nodes of the three groups of mice. Upon ex vivo restimulation with 0, 1, or 5 µg/ml Df for 72 h, recipients sensitized with Df-pulsed cBMCsGM-CSF generated robust production of T cell cytokines, as compared with recipients of saline-pulsed cBMCsGM-CSF. Lymph node cells from recipients sensitized with Df-pulsed kdBMCsGM-CSF had significant reductions at the 5-µg/ml dose in the total generation of IL-4, IL-5, and IL-13 per mouse of 51.7, 59.6, and 42.2%, respectively, compared with recipients of Df-pulsed cBMCsGM-CSF (Fig. 3). IL-17A and IFN-γ were not different between the Df-pulsed groups. The reduction in IL-4, IL-5, and IL-13 generated by recipients of Df-pulsed kdBMCsGM-CSF was the result of both a reduction in concentration of each cytokine per million cells plated and an 18.9% reduction in the total lymph node cell count (Fig. 3). The reductions in concentration were significant for IL-5 and IL-13, whereas the trend for IL-4 did not reach significance. Thus, the cytokine findings of a reduced Th2 response in the restimulated nodal cells reflected the reduced eosinophil count and the reduced IL-13 level in the BAL fluid. There was no reduction in IL-17A generation to account for the notable neutrophil reduction in the BAL fluid of mice sensitized with kdBMCsGM-CSF.
Figure 3.
BMCGM-CSF Dectin-2 is required for lymph node Th2 cytokine production elicited by Df. WT mice were sensitized with saline-pulsed or Df-pulsed kdBMCsGM-CSF or cBMCsGM-CSF and challenged with Df as described in the Fig. 2 legend. Parabronchial lymph node cells were isolated, counted, and restimulated for 72 h with 0, 1, or 5 µg/ml Df, and cytokines in the supernatant were measured by ELISA. Total cytokine production per mouse is shown. Results are means ± SEM (n = 10–13 mice per group) from three independent experiments. Triangles under x axes indicate 0, 1, and 5 µg/ml Df, from left to right. *, P = 0.04; **, P = 0.01; ***, P = 0.007; ‡, P = 0.03. Significance was determined with an unpaired Student’s t test.
BMCGM-CSF cys-LT generation mediates Df-elicited Th2 pulmonary inflammation
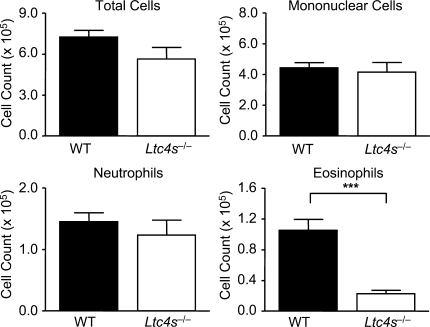
As Dectin-2 can elicit generation of both cys-LTs and proinflammatory cytokines, we next determined the contribution of these lipid mediators to Df-induced pulmonary inflammation in the adoptive transfer model. We generated BMCsGM-CSF from BALB/c WT and LTC4S-deficient (Ltc4s−/−) mice, pulsed them with Df, sensitized WT recipients via intranasal transfer of Df-pulsed BMCsGM-CSF, and challenged recipients intranasally with Df to elicit memory responses. Whereas WT BMCsGM-CSF sensitized recipients for robust pulmonary inflammation with recruitment of mononuclear cells, neutrophils, and eosinophils to the airway, mice sensitized with Df-pulsed Ltc4s−/− BMCsGM-CSF had a selective 78.4% reduction in the recruitment of eosinophils to the BAL fluid with Df challenge (Fig. 4).
Figure 4.
BMCGM-CSF LTC4S is required for Df-elicited eosinophilic pulmonary inflammation. WT mice were sensitized by intranasal administration of 104 Df-pulsed WT or Ltc4s−/− BMCsGM-CSF, challenged with 2 µg Df on day 22 and day 23, and analyzed on day 25. Cells from the BAL fluid were counted, cytospin preparations were stained, and 400 cells/slide were counted for specific cell types. Results are means ± SEM (n = 13–14 mice per group) from four independent experiments. ***, P = 0.0001. Significance was determined with an unpaired Student’s t test.
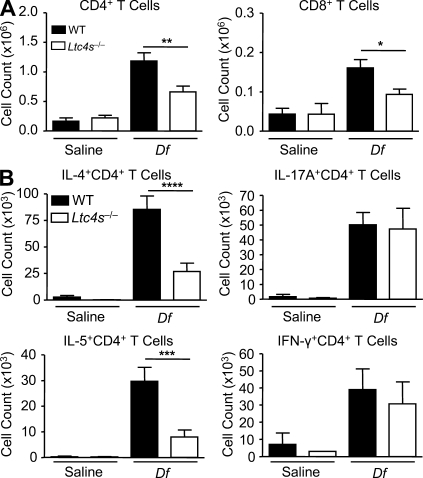
To determine whether the attenuation of eosinophil recruitment in mice sensitized with Ltc4s−/− BMCsGM-CSF reflected a decrement in Th2 cells in the lung, we characterized the CD4+ and CD8+ T cell populations recruited to the lung in another cohort sensitized and challenged in the same manner. Pulmonary mononuclear cells were isolated, stimulated with PMA and ionomycin in the presence of monensin, permeabilized, and stained for CD4, CD8, IL-4, IL-5, IL-17A, and IFN-γ. Fig. 5 A shows that mice sensitized with Df-pulsed WT BMCsGM-CSF and challenged with Df had recruitment of both CD4+ and CD8+ T cells to the lung, as compared with mice sensitized with saline-pulsed WT BMCsGM-CSF. Recruitment of CD4+ and CD8+ T cells was significantly reduced by 44.4 and 41.9%, respectively, in mice sensitized with Df-pulsed Ltc4s−/− BMCsGM-CSF. Recruited CD4+ T cells produced IL-4, IL-5, IL-17A, and IFN-γ (Fig. 5 B and Fig. S3). There was no significant induction of these cytokines from CD8+ T cells. Recipients of Df-pulsed Ltc4s−/− BMCsGM-CSF had a significant 68.6% reduction in the number of IL-4+ CD4+ T cells and a significant 73.0% reduction in the number of IL-5+CD4+ T cells, as compared with mice sensitized with Df-pulsed WT BMCsGM-CSF, whereas the numbers of IL-17A+ and IFN-γ+CD4+ T cells were unchanged. Thus, the selective decline in BAL fluid eosinophils was paralleled by a selective reduction in Th2 cells recruited to lung.
Figure 5.
BMCGM-CSF LTC4S is required for Df-elicited CD4+ Th2 cell recruitment to the lung. WT mice were sensitized with saline-pulsed or Df-pulsed WT or Ltc4s−/− BMCsGM-CSF and challenged with Df as described in the legend to Fig. 4. Pulmonary mononuclear cells were isolated, stimulated with 50 ng/ml PMA and 1 µM ionomycin for 10 h in the presence of 2.5 µM monensin, stained for cell surface expression of CD4 and CD8 (A) and for intracellular expression of IL-4, IL-5, IL-17A, and IFN-γ (B), and analyzed by flow cytometry. Gating on cell size, on CD4 and CD8 expression, the total number of cells recruited to the lung is shown. Results are means ± SEM (n = 11 mice per group) from two experiments. *, P = 0.02; **, P = 0.005; ***, P = 0.002; ****, P = 0.0008. Significance was determined with an unpaired Student’s t test.
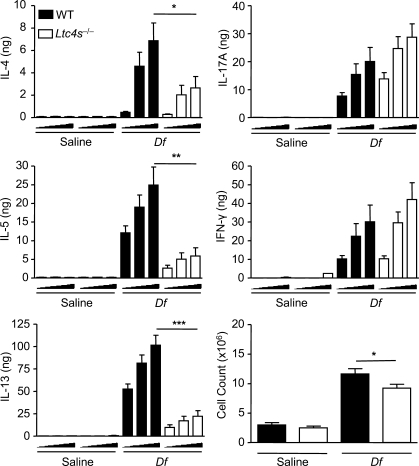
We next assessed the lung-draining lymph nodes to determine whether the profile of cytokine production was similarly impaired. Cells from the lung-draining lymph nodes of sensitized and challenged recipients were restimulated ex vivo with Df at 0, 1, or 5 µg/ml, and cytokines in the supernatant were measured at 72 h. Recipients sensitized with Df-pulsed WT BMCsGM-CSF generated robust production of T cell cytokines, as compared with recipients of saline-pulsed WT BMCsGM-CSF. Mice sensitized with Df-pulsed Ltc4s−/− BMCsGM-CSF had significant reductions in the total generation of IL-4, IL-5, and IL-13 per mouse of 61.5, 76.4, and 78.2%, respectively, as compared with mice sensitized with Df-pulsed WT BMCsGM-CSF (Fig. 6). The reduction in IL-4, IL-5, and IL-13 generation by recipients of Df-pulsed Ltc4s−/− BMCsGM-CSF was the result of both a reduction in concentration of each cytokine per million cells plated and a 20.5% reduction in the total lymph node cell count (Fig. 6). The reductions in concentration were significant for IL-5 and IL-13, whereas the trend for IL-4 did not reach significance. There was no difference in the total generation of IL-17A or IFN-γ per mouse. Thus, the attenuation in pulmonary eosinophil and Th2 cell recruitment to the lung in mice sensitized with Df-pulsed Ltc4s−/− BMCsGM-CSF appeared to reflect a selective defect in Th2 immunity to Df.
Figure 6.
BMCGM-CSF LTC4S is required for lymph node Th2 cytokine production elicited by Df. WT mice were sensitized with saline-pulsed or Df-pulsed WT or Ltc4s−/− BMCsGM-CSF and challenged with Df as described in the legend to Fig. 4. Parabronchial lymph node cells were isolated, counted, and restimulated for 72 h with 0, 1, or 5 µg/ml Df, and cytokines in the supernatant were measured by ELISA. Total cytokine production per mouse is shown. Results are means ± SEM (n = 12–15 mice per group) from three experiments. Triangles under x axes indicate 0, 1, and 5 µg/ml Df, from left to right. *, P = 0.04; **, P = 0.001; ***, P = 0.0001. Significance was determined with an unpaired Student’s t test.
To confirm that MHCII-dependent antigen presentation by BMCsGM-CSF was required to sensitize recipient mice for the generation of eosinophilic pulmonary inflammation and Th2 cytokine induction after Df challenge, we sensitized WT C57BL/6 mice with Df-pulsed WT or MHCII-deficient (MHCIIΔ/Δ) BMCsGM-CSF. After Df challenge, recipients of Df-pulsed MHCIIΔ/Δ BMCsGM-CSF had significant reductions in BAL fluid total cells of 88.3%, in monocytes of 66.9%, in neutrophils of 97%, and in eosinophils of 99%, as compared with recipients of Df-pulsed WT BMCsGM-CSF (Fig. S4 A). Lymph node cells were isolated, stimulated with PMA and ionomycin in the presence of monensin, permeabilized, and stained for CD4, CD8, IL-4, IL-5, IL-17A, and IFN-γ. As shown for a representative plot (Fig. S4 B), IL-4, IL-5, and IL-17A were induced in CD4+ lymphocytes from recipients of WT, but not MHCIIΔ/Δ, BMCsGM-CSF. We did not detect IL-4, IL-5, or IL-17A in any other cell population. Thus, MHCII-mediated antigen presentation from transferred BMCsGM-CSF was necessary to sensitize WT recipients for the appearance of a cellular infiltrate in the BAL fluid and CD4+ Th2 cells in the draining lymph nodes.
Th2 differentiation may be altered by the maturation of DCs, their expression of costimulatory molecules, their production of proinflammatory cytokines, or their ability to migrate to the lung-draining lymph nodes. Flow cytometry revealed comparable cell surface expression of CD80, CD86, CD40, OX40L, CCR7, PD-L1, and ICOSL on CD11c+MHCII+ WT and Ltc4s−/− BMCsGM-CSF after Df loading (Fig. S5). We assessed cytokine production by WT and Ltc4s−/− BMCsGM-CSF stimulated with Df and found no significant difference in the ability to generate TNF, IL-10, IL-6, IL-2, or IL-23 (Fig. S6). IL-12p70 was not detected from WT or Ltc4s−/− BMCsGM-CSF (unpublished data). BMCsGM-CSF can chemotax to LTD4 in a CysLT1R-dependent fashion, and cys-LTs have been reported to enhance DC migration to the DC chemotactic ligand for CCR7, CCL19 (Robbiani et al., 2000). We conjugated Df to Alexa Fluor 647 and assessed the migration of intranasally transferred Alexa Fluor 647–Df-pulsed BMCsGM-CSF to the regional lymph nodes 24 h later. We found that Alexa Fluor 647–Df-pulsed WT and Ltc4s−/− BMCsGM-CSF trafficked to the regional lymph nodes to a similar extent and comprised 7.9 ± 0.75 and 13.5 ± 4.7% of CD11chiMHCIIhi cells, respectively, at 24 h over two experiments (Fig. S7).
CysLT1R function conditions BMCsGM-CSF for Th2 immune responses
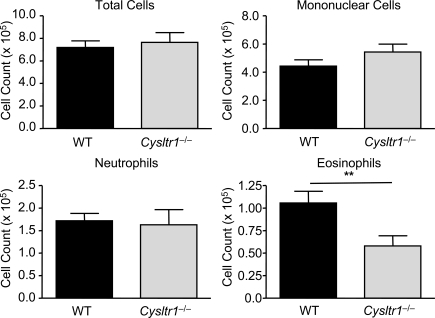
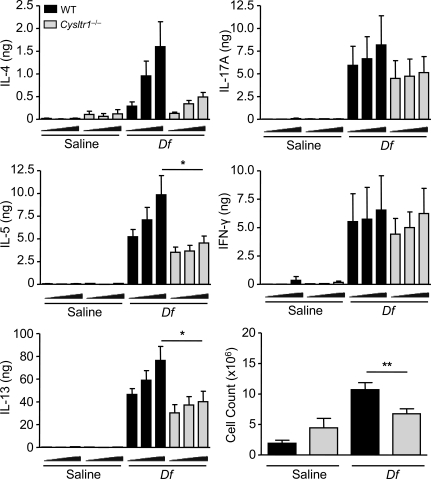
We next sought to determine whether the Th2 priming function of cys-LTs was mediated through CysLT1R. WT recipients were sensitized by intranasal transfer of Df-pulsed WT or CysLT1R-deficient (Cysltr1−/−) BMCsGM-CSF and directly challenged with Df. Although Df-pulsed WT BMCsGM-CSF initiated a characteristic robust pulmonary inflammation with the recruitment of mononuclear cells, neutrophils, and eosinophils to the airway, mice sensitized with Df-pulsed Cysltr1−/− BMCsGM-CSF had a selective 45.1% reduction in eosinophil recruitment to the BAL fluid (Fig. 7). Restimulation of lung-draining lymph node cells demonstrated that recipients sensitized with Df-pulsed WT BMCsGM-CSF generated robust production of T cell cytokines, as compared with recipients of saline-pulsed WT BMCsGM-CSF. At 5 µg/ml Df, mice sensitized with Df-pulsed Cysltr1−/− BMCsGM-CSF had significant reductions in the total generation of IL-5 and IL-13 per mouse of 53.7 and 47.4%, respectively, as compared with recipients of Df-pulsed WT BMCsGM-CSF, and a 69.3% fall in IL-4 that did not reach statistical significance (Fig. 8). The reductions in IL-5 and IL-13 were the result of a significant 36.7% reduction in the total lymph node cell count (Fig. 8). There was no significant difference in the generation of IL-17A or IFN-γ between mice sensitized with WT BMCsGM-CSF or Cysltr1−/− BMCsGM-CSF. Thus, Cysltr1−/− BMCsGM-CSF had a selective impairment in the generation of Th2 immunity that was similar to the findings with Ltc4s−/− BMCsGM-CSF.
Figure 7.
Df-elicited eosinophilic pulmonary inflammation is dependent on BMCGM-CSF CysLT1R. WT mice were sensitized with intranasal administration of 104 Df-pulsed WT or Cysltr1−/− BMCsGM-CSF, challenged with 2 µg Df on day 22 and day 23, and analyzed on day 25. Cells from the BAL fluid were counted, cytospin preparations were stained, and 400 cells/slide were counted for specific cell types. Results are means ± SEM (n = 6–7 mice per group) from two experiments. **, P = 0.02. Significance was determined with an unpaired Student’s t test.
Figure 8.
Df-elicited Th2 cytokine generation is dependent on BMCGM-CSF CysLT1R. WT mice were sensitized with saline-pulsed or Df-pulsed WT or Cysltr1−/− BMCsGM-CSF and challenged with Df as described in the legend to Fig. 7. Parabronchial lymph node cells were isolated, counted, and restimulated for 72 h with 0, 1, or 5 µg/ml Df, and cytokines in the supernatant were measured by ELISA. Total cytokine production per mouse is shown. Results are means ± SEM (n = 7 mice per group) from two experiments. Triangles under x axes indicate 0, 1, and 5 µg/ml Df, from left to right. *, P = 0.03; **, P = 0.02. Significance was determined with an unpaired Student’s t test.
Cys-LTs mediate Df-elicited active sensitization and Th2 pulmonary inflammation
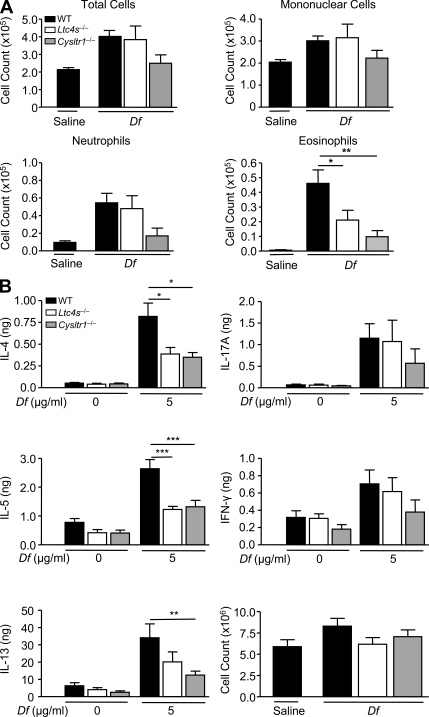
To demonstrate that cys-LT generation and CysLT1R function are each required for Th2 pulmonary inflammation to Df in a direct immunization model, we sensitized and challenged WT, Ltc4s−/−, and Cysltr1−/− mice on a BALB/c background with intranasal Df on days 0, 1, 15, and 16 and assessed pulmonary inflammation in the BAL fluid and Th2 cytokine production from restimulated lung-draining lymph node cells. Although WT mice developed a neutrophilic and eosinophilic inflammatory response in the BAL fluid (Fig. 9 A), Ltc4s−/− and Cysltr1−/− mice had significant reductions in eosinophil recruitment by 48 and 76%, respectively. Cysltr1−/− mice had an additional reduction in neutrophil recruitment to the BAL fluid that did not reach statistical significance. Cytokines from restimulated lung-draining lymph node cells showed significant 53 and 57% reductions in IL-4 and significant 54 and 50% reductions in IL-5 in Ltc4s−/− and Cysltr1−/− mice, respectively, as compared with WT (Fig. 9 B). Cysltr1−/− mice had a significant 63% reduction in IL-13, whereas in Ltc4s−/− mice the reduction did not reach statistical significance. There were additional reductions in IL-17A and in IFN-γ from Cysltr1−/− mice that did not reach statistical significance.
Figure 9.
Df-elicited eosinophilic pulmonary inflammation and Th2 cytokine generation is dependent on LTC4S and CysLT1R. WT (black), Ltc4s−/− (white), or Cysltr1−/− (gray) mice were sensitized with 10 µg Df or saline on days 0 and 1, challenged with 10 µg Df on days 15 and 16, and analyzed on day 18. (A) Cells from the BAL fluid were counted, cytospin preparations were stained, and 400 cells/slide were counted for specific cell types. Results are means ± SEM (n = 7–12 mice per group) from three experiments. *, P = 0.05; **, P = 0.01. (B) Parabronchial lymph node cells were isolated, counted, and restimulated for 72 h with 0 or 5 µg/ml Df, and cytokines in the supernatant were measured by ELISA. Total cytokine production per mouse is shown. Results are means ± SEM (n = 7–12 mice per group) from three experiments. *, P = 0.05; **, P = 0.01; ***, P = 0.001. Significance was determined with a one-way ANOVA.
Dectin-2 mediates Df-elicited active sensitization and Th2 pulmonary inflammation
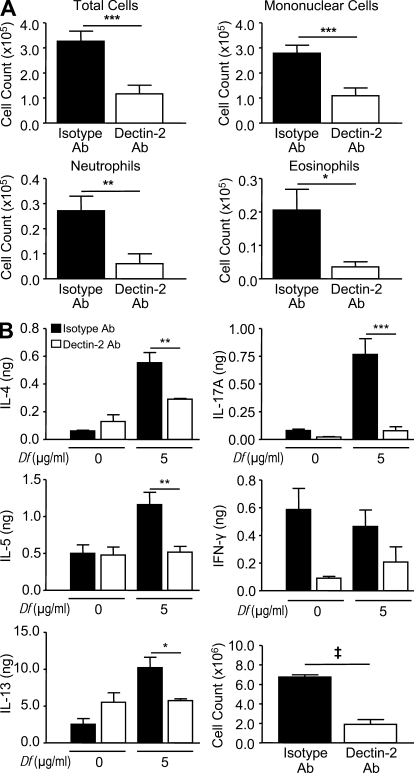
Finally, to assess the role of Dectin-2 in Df-elicited Th2 pulmonary inflammation in the direct immunization model, we sensitized and challenged BALB/c mice with intranasal Df and treated them with a monoclonal antibody to Dectin-2 or the rat IgG2a isotype control on days 0, 2, 15, and 17. Mice treated with the Dectin-2 antibody had significant reductions in BAL fluid total cells (64%), mononuclear cells (61%), neutrophils (78%), and eosinophils (80%), as compared with mice treated with the isotype control (Fig. 10 A). Cytokines from restimulated lung-draining lymph node cells demonstrated that mice treated with the Dectin-2 antibody had significant reductions in the total generation of IL-4, IL-5, IL-13, and IL-17A per mouse of 47, 55, 44, and 90%, respectively, as compared with mice treated with the isotype control (Fig. 10 B). The reduction in IL-4, IL-5, and IL-13 generation by mice treated with the Dectin-2 antibody was the result of a 72% reduction in the total lymph node cell count, whereas the reduction in IL-17A was the result of both a reduction in the concentration of each cytokine per million cells plated and the reduction in the total lymph node cell count (Fig. 10 B).
Figure 10.
Df-elicited pulmonary inflammation and Th2 cytokine generation is dependent on Dectin-2. WT mice were sensitized and challenged as described in the Fig. 9 legend. Mice were injected intraperitoneally with 200 µg Dectin-2 antibody or a rat IgG2a isotype control on days 0, 2, 15, and 17, and analyzed on day 18. (A) Cells from the BAL fluid were counted, cytospin preparations were stained, and 400 cells/slide were counted for specific cell types. (B) Parabronchial lymph node cell counts. Results are means ± SEM (n = 4 mice per group) and are representative of two independent experiments. *, P = 0.04; **, P = 0.02; ***, P = 0.01. (B) Parabronchial lymph node cells were isolated, counted, and restimulated for 72 h with 0 or 5 µg/ml Df, and cytokines in the supernatant were measured by ELISA. Total cytokine production per mouse is shown. Results are means ± SEM (n = 4 mice per group) and are representative of two independent experiments. *, P = 0.04; **, P = 0.03; ***, P = 0.01; ‡, P = 0.0001. Significance was determined with an unpaired Student’s t test.
DISCUSSION
PRR signaling is central to the initiation of Th1 and Th17 adaptive immune responses. We have previously identified Dectin-2 on pulmonary CD11c+ cells and on BMCsGM-CSF as a PRR for clinically relevant allergens including Df, Dp, and Aspergillus fumigatus extract (Barrett et al., 2009). In this study, we demonstrate that Dectin-2 activation elicited by Df in both adoptive transfer and direct sensitization and challenge models triggers Th2 pulmonary inflammation and that this coupling is mediated through the specific actions of cys-LTs. Notably, TLR4 on lung stromal cells is the only PRR previously identified that links HDM recognition to Th2 pulmonary inflammation (Hammad et al., 2009). Arachidonic acid release and cys-LT generation can be elicited by signaling from other myeloid CLRs, such as Dectin-1 and DC-SIGN (Olynych et al., 2006; Suram et al., 2006; Valera et al., 2007), and although these receptors recognize glycans that can serve as adjuvants for a Th2 response (Shreffler et al., 2006; Inoue et al., 2009), no mechanism for this linkage has been suggested. Herein, we observe a link to Th2 immunity not only via Dectin-2–mediated generation of cys-LTs from BMCsGM-CSF but also via CysLT1R function on these cells.
Both human DCs and mouse BMCsGM-CSF express the CysLT1R, and two prior pharmacologic studies with CysLT1R antagonists show that cys-LTs can augment Th2 sensitization in BALB/c mice (Machida et al., 2004; Okunishi et al., 2004). In WT mice sensitized by the adoptive transfer of Df-pulsed BMCsGM-CSF and challenged with Df, the recruitment of eosinophils to lung and the levels of IL-5 in BAL fluid were increased by costimulation with LTD4 and suppressed by the presence of a CysLT1R antagonist during the in vitro Df pulsing (Machida et al., 2004). Systemic administration of a CysLT1R antagonist during OVA sensitization reduced the levels of BAL fluid IL-4, IL-5, and IFN-γ elicited by OVA challenge (Okunishi et al., 2004). However, in the current study, the reductions in Th2 cytokines in restimulated lymph node cells were not accompanied by a reduction in IFN-γ production in WT recipients sensitized with BMCsGM-CSF lacking LTC4S or CysLT1R.
Our findings for a CysLT1R function on BMCsGM-CSF in Th2 sensitization prompt a consideration of possible pathways. We did not find evidence for impaired generation of cytokines such as TNF from Ltc4s−/− BMCsGM-CSF in response to Df (Fig. S6). Nor did we detect any difference in maturation or expression of costimulatory markers between Df-pulsed WT and Ltc4s−/− BMCsGM-CSF (Fig. S5). Cys-LTs can specifically modulate human and mouse DC migration (Robbiani et al., 2000; Thivierge et al., 2006), but we did not see any impairment in the migration of Alexa Fluor 647–Df-pulsed Ltc4s−/− BMCsGM-CSF to the lung-draining lymph nodes, as compared with WT controls (Fig. S7). The CysLT1R ligand, LTD4, can stimulate human neutrophil adhesion to intercellular adhesion molecule 1 in a manner that is dependent on the β2 integrin CD18 and is blocked by the selective CysLT1R antagonist montelukast (Meliton et al., 2010). Moreover, LTD4 can promote α4β1 and α5β1 integrin–dependent adhesion of human CD34+ hematopoietic progenitor cells to endothelial cells, vascular cell adhesion molecule 1, and fibronectin, and such adhesion is blocked by CysLT1R antagonism (Boehmler et al., 2009). As CD18/CD11a and the α4β1 integrin are critical membrane proteins recruited to the DC–T cell immune synapse, CysLT1R facilitation of cell–cell adhesion and the organization of the immune synapse may be a mechanism by which CysLT1R can promote nascent Th2 immune responses.
GM-CSF–expanded BM cultures at day 7 provide a mixed population of cells, including Dectin-2− granulocytes (12.4 ± 0.5%; Fig. S1 C) and Dectin-2+CD11c− cells (7.0 ± 0.5%) that express CD11b and Ly6C, presumably identifying Dectin-2+ monocytes in this culture. The dominant CD11c+ cell population in these cultures expresses CD11b (97.7 ± 0.4%) and MHCII (88.5 ± 2%). Such cells, termed inflammatory DCs, were recently identified as the critical antigen-presenting cells to drive Th2 responses to HDM (Hammad et al., 2010), and our findings suggest that Dectin-2 expression on these cells may be central to this function. As Dectin-2 is highly expressed on immature CD11chiMHCIIlow cells (Fig. 1 A and Fig. S1 A), further studies are needed to determine whether Dectin-2 expression identifies a particular subset of inflammatory DCs in vivo that prime for Th2 immunity.
We found a significant role for Dectin-2–mediated cys-LT production and CysLT1R function in the generation of Th2 immunity to Df in both the adoptive transfer studies with Df-pulsed BMCsGM-CSF and the direct sensitization and challenge of LTC4S- and CysLT1R-deficient strains. However, our studies do not exclude a contribution from the Df-elicited Dectin-2–dependent cytokines. The pharmacologic addition of TNF to OVA/LPS sensitization can restore pulmonary DC activation and migration and the induction of eosinophilic pulmonary inflammation that is impaired in BALB/c mice with defective TLR4 signaling (Eisenbarth et al., 2002). Mice with a targeted deletion of the IL-23α subunit p19 have attenuated Th2 pulmonary inflammation and specific IgE levels after i.p. sensitization with OVA/alum and intranasal challenge with OVA, which are associated with an impairment in T cell GATA-3 induction (Peng et al., 2010). Antigen-presenting cell–derived IL-6 can drive IL-4 production from naive murine CD4+ T cells (Rincón et al., 1997), and neutralization of IL-6 in ex vivo lymph node cell cultures from mice sensitized with HDM-pulsed BMCsGM-CSF diminished the production of IL-13 (Krishnamoorthy et al., 2008). As the generation of TNF, IL-23, and IL-6 was blunted in kdBMCsGM-CSF, these mediators may also contribute to Df-induced Dectin-2–dependent Th2 immunopathology.
We found a robust Th17 immune response in mice receiving Df-pulsed WT BMCsGM-CSF and challenged with Df, as has been noted by others in protocols of intranasal sensitization and challenge with HDM (Krishnamoorthy et al., 2008; Phipps et al., 2009), but we did not find a significant attenuation in lymph node cell production of IL-17A in mice sensitized with Df-pulsed Ltc4s−/− BMCsGM-CSF, Df-pulsed Cysltr1−/− BMCsGM-CSF, or Df-pulsed kdBMCsGM-CSF. The level of recruitment of Th17 cells to the lung in mice sensitized with Df-pulsed Ltc4s−/− BMCsGM-CSF was also comparable to that in mice sensitized with Df-pulsed WT BMCsGM-CSF. Th17 immunity to dust mite may be generated by signaling from other PRRs during sensitization. After intranasal sensitization and challenge with Dp, airway neutrophilia and Dp-specific IL-17A production by restimulated lymph node cells was attenuated in BALB/c mice lacking MyD88 (Phipps et al., 2009). Thus, signaling from another TLR or an IL-1 family member may contribute to the Df-elicited Th17 priming in our adoptive transfer model. In addition, HDM extracts may activate other Syk/CARD9-dependent CLRs to elicit a Th17 response, as the addition of the Dectin-1 agonist curdlan to OVA immunization augments IL-17 production from restimulated lymphocytes in WT but not Dectin-1–null mice (LeibundGut-Landmann et al., 2007). Interestingly, Dectin-2 may have a more significant role in Df-elicited Th17 immunity at the challenge phase, as we did find a reduction in IL-17A production in restimulated lymphocytes from mice treated with the Dectin-2 antibody during both the sensitization and challenge.
In the adoptive transfer model, although the reduction in eosinophil recruitment to the BAL fluid in mice sensitized with Df-pulsed kdBMCsGM-CSF paralleled the reduction in lung and lymph node Th2 responses and was dependent on BMCsGM-CSF cys-LT production and CysLT1R-mediated function, the significant fall in Dectin-2–dependent neutrophil recruitment to the BAL fluid was not accompanied by a reduction in IL-17A and was not dependent on cys-LTs. Dectin-2 activates the FcRγ chain–, Syk-, and CARD9-dependent signaling pathway in DCs. This pathway is shared with macrophage-inducible C-type lectin for the production of neutrophil chemoattractants, such as CXCL2, in mouse macrophages (Yamasaki et al., 2008), suggesting a mechanism by which Dectin-2 signaling may recruit neutrophils independently of IL-17A.
Earlier in vivo studies identified a role for Dectin-2 signaling in the generation of Th17 immunity to C. albicans (Robinson et al., 2009; Saijo et al., 2010) and in regulatory T cell function in a model of UV radiation–induced tolerance (Aragane et al., 2003). Our study not only extends the biology of Dectin-2 to Th2 cells but also provides a link via the cys-LT pathway to pathological Th2 inflammation elicited by HDM, the most common aeroallergen worldwide.
MATERIALS AND METHODS
Reagents and mice.
Lyophilized extracts of Df (Greer Laboratories) were reconstituted in endotoxin-free PBS (Sigma-Aldrich). DNase-1 (Roche), ionomycin (Calbiochem), and collagenase IV (Worthington Biochemical) were obtained as noted. LPS from E. coli O55:B5, curdlan, PMA, monensin, and puromycin were obtained from Sigma-Aldrich. Ltc4s−/− mice on a BALB/c background (N10 and N11), Cysltr1−/− mice on a BALB/c background (N10), and WT littermate controls were generated and maintained in our laboratory (Kanaoka et al., 2001; Maekawa et al., 2002). MHCIIΔ/Δ mice and the C57BL/6 controls were obtained from The Jackson Laboratory (Madsen et al., 1999). All studies were approved by the Institutional Animal Care and Use Committee of the Dana-Farber Cancer Institute.
BMCsGM-CSF generation and Df stimulation.
BMCsGM-CSF were generated with GM-CSF, as previously described (Barrett et al., 2009), according to the protocol for BM-derived DC generation by Lutz et al. (1999). In brief, BM cells were harvested and resuspended at 4 × 105 cells/ml in RPMI 1640 medium supplemented with 10% heat-inactivated FCS, 100 U/ml penicillin, 100 µg/ml streptomycin, 2 mM l-glutamine, 50 µM 2-mercaptoethanol, and 40 ng/ml of recombinant mouse GM-CSF (Peprotech). 10 ml of this suspension was plated in each Petri dish and cultured at 37°C in a 5% CO2 incubator. On day 3 of culture, 10 ml of complete media with GM-CSF was added to each plate. On day 6 of culture, 10 ml of media with GM-CSF was exchanged from each plate. The floating cell population was harvested at day 7, washed, and counted. For cell stimulations, Df was used at the concentrations noted with a final cell concentration of 106 cells/ml.
Cytospin and immunocytochemistry.
To assess the cell composition of day-7 cultures, 1.5 × 105 cells in 200 µl were cytospun, fixed, and stained with Hema 3 Stain Set (Thermo Fisher Scientific). Cells were identified by morphological criteria. 400 cells per slide were counted. To assess Dectin-2 expression by immunocytochemistry, cells were fixed in 4% paraformaldehyde, washed, cytospun, and stained using the rat ABC staining system (Santa Cruz Biotechnology, Inc.), according to the manufacturer’s instructions. In brief, the slides were blocked with 1.5% goat serum, incubated with rat anti–Dectin-2 (AbD Serotec) or a rat IgG2a isotype control Ab (BD) at 1:20 at 25°C for 1 h, washed, and incubated with biotin-conjugated goat anti–rat antibody for 30 min. Detection was accomplished using avidin-biotinylated horseradish peroxidase and diaminobenzidine substrate. Hematoxylin was used for counterstaining. The pictures were taken by a digital camera (DXM 1200; Nikon) with ACT-1 (version 2.70; Nikon) image acquisition software. 500 cells per sample were assessed.
Flow cytometry.
To assess Dectin-2 expression on day 7, BMCsGM-CSF were harvested, washed, blocked in PBS containing 1 mM EDTA and 10% donkey serum (Jackson ImmunoResearch Laboratories, Inc.), and serially stained with rat anti–Dectin-2 (Abcam) or rat IgG2a isotype control Ab (BD) at 1 µg/0.2 ml/106 cells, donkey anti–rat Ig-allophycocyanin (Jackson ImmunoResearch Laboratories, Inc.) at 1:100 dilution, and either rat anti–mouse CD11c-PECy7, MHCII-PE, CD80-PE, CD86-PE, CD40-PE, OX40L-PE, CCR7-PE, PD-L1-PE, and ICOSL-PE (BD) or rat anti–mouse CD11c-Pacific Blue, MHCII-FITC, CD11b-PECy7, and Ly6c-APCCy7 (eBioscience). MHCII-PE staining was performed at nonsaturating antibody concentrations. Analyses were performed on a FACSCanto II flow cytometer (BD), and data were analyzed with FlowJo (7.5; Tree Star, Inc.).
Lentiviral knockdown of Dectin-2 in BMCsGM-CSF.
Infectious viral particles were prepared and titered as previously described (Barrett et al., 2009) using the shRNA construct TRCN0000066785 for mouse Dectin-2 in the pLKO1 lentiviral vector (Open Biosystems), an empty vector control (Open Biosystems), and a nontargeting sequence (Sigma-Aldrich). BMCGM-CSF cultures were begun on day 0, infected on day 1 at a multiplicity of infection of 10, and selected with 2 µg/ml puromycin from days 4–7. BMCGM-CSF cultures infected with lentivirus contained a mean of 76.8 ± 5% CD11c+ cells at day 7, as detected by flow cytometry.
Cys-LT and cytokine measurement.
Cys-LTs in the supernatants of stimulated cells were measured by enzyme immunoassay according to the manufacturer’s protocol (Cayman Chemical). The lower limit of detection was 3.9 pg/ml. IL-2, IL-6, IL-10, IL-23, and TNF were measured by ELISA (eBioscience) with lower limits of detection at 8, 78, 7.8, 7.8, and 156 pg/ml, respectively.
Sensitization and challenge protocols.
For adoptive transfer of Df-pulsed BMCsGM-CSF into WT recipients and subsequent Df challenge, BMCsGM-CSF were grown to day 7 and stimulated with saline or Df at 100 µg/ml for 24 h. On day 8, nonadherent cells were washed twice in PBS, counted, and resuspended in PBS. WT recipient mice were sensitized intranasally with 104 cells/50 µl, challenged with 2 µg Df intranasally on day 22 and day 23, and euthanized 48 h after the last challenge with an overdose of pentobarbital for analysis of BAL fluid, pulmonary mononuclear cell harvest, and lung-draining lymph node restimulation. After adoptive transfer of BMCsGM-CSF from MHCIIΔ/Δ mice or C57BL/6 WT mice, recipient C57BL/6 mice were challenged with 10 µg Df intranasally on days 20, 22, and 24 and euthanized on day 25. For the active sensitization and challenge protocol, Ltc4s−/−, Cysltr1−/−, or BALB/c WT mice were sensitized (days 0 and 1) and challenged (days 15 and 16) with 10 µg Df intranasally and euthanized on day 18. For antibody blocking, 200 µg Dectin-2 antibody (clone D2.11E4; Thermo Fisher Scientific) or a rat IgG2a control (BioLegend) was injected intraperitoneally into BALB/c WT mice on days 0, 2, 15, and 17. On days 0 and 15 the antibody was injected 6 h before Df.
BAL.
Mice were killed, the trachea was exposed and cannulated with a 22-gauge angiocatheter, and the bronchoalveolar space was lavaged three times with 0.75 ml PBS with 1 mM EDTA. The BAL fluid was centrifuged, and the pelleted cells were resuspended in PBS with 1% FCS and counted. Then, 1.5 × 105 cells in 200 µl were cytospun, fixed, and stained with Hema 3 Stain Set (Thermo Fisher Scientific). Cells were identified by morphological criteria. 400 cells per slide were counted. IL-13, IL-17A, and IFN-γ levels in the BAL fluid supernatants were measured by ELISA (eBioscience) with lower limits of detection at 7.8, 7.8, and 31.2 pg/ml, respectively.
Intracellular cytokine staining of pulmonary mononuclear cells and lymph node cells.
For pulmonary mononuclear cell assessment, mice were killed, and the lungs were perfused with 10 ml of sterile PBS, homogenized with sterile scalpels, and digested in a shaker for 30 min with 500 U/ml of type IV collagenase and 0.02 mg/ml DNase I at 37°C. The cells were washed, filtered through a 70-µm cell strainer, and separated on a NycoPrep gradient (Axis-Shield) at 600 g for 20 min at 4°C. Harvested mononuclear cells were washed; resuspended in RPMI 1640 medium supplemented with 10% heat-inactivated FCS, 100 U/ml penicillin, 100 µg/ml streptomycin, 0.1 mM nonessential amino acids, 0.043 mM 2-ME, 2 mM l-glutamine, 0.025 M Hepes buffer, and 1 mM sodium pyruvate; plated at 2 × 106 cells/ml in 24-well plate; and stimulated with 50 ng/ml PMA and 1 µM ionomycin for 2 h at 37°C. 2.5 µM monensin was added and the cells were incubated for an additional 8 h. Cells were harvested, treated with DNase I at a concentration of 60 µg/ml for 15 min at 37°C, washed, fixed with fixation buffer (eBioscience) for 15 min, washed again, and blocked in 300 µl permeabilization buffer (eBioscience) with 1% mouse IgG (Sigma-Aldrich) and 1% anti–mouse CD16/CD32 (BD) for 20 min. Cells were then stained with anti–mouse CD4-PE-Cy7 (clone RM4-5; BD), anti–mouse CD8α-allophycocyanin (clone 53–6.7; BD), anti–mouse IL-4–PE (clone 11B11; BD), anti–mouse IL-5-PE (clone TRFK5; BD), anti–mouse IL-17A–PE (clone TC11-18H10; BD), anti–mouse IFN-γ–PE (clone XMG1.2; eBioscience), or rat IgG1-PE isotype control Ab (BD) at a concentration of 0.2 µg/0.1 ml/2 × 106 cells. Analyses were performed on a FACSCanto flow cytometer, and data were analyzed with FlowJo. For lymph node cell assessment, the parabronchial lymph nodes were excised from each mouse. Single cell suspensions were generated, filtered through a 70-µm cell strainer, washed, resuspended in the RPMI medium described in this section, and counted. Cell stimulation, washing, fixation, permeabilization, and staining were as in this section with the exception that PMA, ionomycin, and then monensin treatment was done for a total of 6 h, and cells were stained with anti–mouse CD4-allophycocyanin (clone RM4-5; BD) and anti–mouse CD8α–PE-Cy7 (clone 53–6.7; BD).
Cytokine assays from lung-draining lymph nodes.
Single cell suspensions from the parabronchial lymph nodes were generated. Lymph node cells were cultured at 4 × 106 cells/ml with 0, 1, or 5 µg/ml Df for 72 h at 37°C in RPMI medium with 10% FCS. The concentrations of IL-4, IL-5, IL-13, IL-17A, and IFN-γ in the supernatants were measured with ELISA kits (eBioscience) with lower limits of detection at 39 pg/ml, 117 pg/ml, 390 pg/ml, 39 pg/ml, and 156 pg/ml, respectively. The total concentration of cytokine generated per mouse is reported.
BMCGM-CSF migration.
Df was conjugated to Alexa Fluor 647 with a Protein Labeling kit (Invitrogen) according to the manufacturer’s protocol and was dialyzed with a 10K membrane (Thermo Fisher Scientific) against PBS for 6 h. Day-7 BMCsGM-CSF were stimulated with 100 µg/ml Alexa Fluor 647–Df for 18 h, and 104, 105, or 106 cells were transferred intranasally into mice. 24 and 48 h later, the lymph nodes were harvested, digested with collagenase IV and DNase I for 30 min at 37°C, and filtered. Single cell suspensions were blocked in PBS supplemented with 0.5% heat-inactivated FCS, 0.05% NaN3, 1% mouse IgG, and 1% anti–mouse CD16/CD32. The cells were stained with anti–mouse CD11c-PE-Cy7 (clone HL3; BD) and anti–mouse MHCII-PE (clone M5/114.15.2; BD) at 0.2 µg/100 µl/106 cells.
Statistical analysis.
All experiments were repeated at least three times, except where noted. Results are expressed as means ± SEM. The Student’s t test was used for statistical analysis, except where noted. A value of P < 0.05 was considered significant.
Online supplemental material.
Fig. S1 depicts CD11c, MHCII, Dectin-2, CD11b, and Ly6C expression on BMCsGM-CSF. Fig. S2 shows Df-elicited cytokine production by BMCsGM-CSF at 30 h and the generation of cys-LTs and TNF in response to curdlan and LPS. Fig. S3 shows a representative plot of intracellular cytokine staining in CD4+ lymphocytes from the lung of WT recipients sensitized with Df-pulsed WT and Ltc4s−/− BMCsGM-CSF and challenged with Df. Fig. S4 shows the difference in BAL fluid cell infiltrate and in intracellular cytokine staining in CD4+ lymph node cells from WT recipients sensitized with Df-pulsed WT or MHCIIΔ/Δ BMCsGM-CSF. Fig. S5 shows comparable cell surface expression of costimulatory markers in WT and Ltc4s−/− BMCsGM-CSF. Fig. S6 shows comparable production of Df-elicited cytokines at 4 and 24 h by WT and Ltc4s−/− BMCsGM-CSF. Fig. S7 shows migration of Alexa Fluor 647–Df-pulsed WT and Ltc4s−/− BMCsGM-CSF to the lung-draining lymph nodes in WT mice. Online supplemental material is available at http://www.jem.org/cgi/content/full/jem.20100793/DC1.
Acknowledgments
This work is supported by National Institutes of Health grants P01HL36110, R01HL090630 (to Y. Kanaoka), and K08AI080948 (to. N.A. Barrett). N.A. Barrett is supported by the Joycelyn C. Austen Fund for the Career Development of Women Physician Scientists.
The authors have no conflicting financial interests.
Footnotes
Abbreviations used:
- BAL
- bronchoalveolar lavage
- BMCGM-CSF
- GM-CSF–cultured BM cell
- CLR
- C-type lectin receptor
- cys-LT
- cysteinyl leukotriene
- CysLT1R
- type 1 cys-LT receptor
- Df
- Dermatophagoides farinae
- Dp
- Dermatophagoides pteronyssinus
- HDM
- house dust mite
- LTC4S
- LTC4 synthase
- NLR
- Nod-like receptor
- ntcBMCGM-CSF
- nontargeting control BMCGM-CSF
- PRR
- pattern recognition receptor
- SEA
- Schistosoma mansoni egg antigen
- Syk
- spleen tyrosine kinase
- TLR
- toll-like receptor
References
- Altmann F. 2007. The role of protein glycosylation in allergy. Int. Arch. Allergy Immunol. 142:99–115 10.1159/000096114 [DOI] [PubMed] [Google Scholar]
- Aragane Y., Maeda A., Schwarz A., Tezuka T., Ariizumi K., Schwarz T. 2003. Involvement of dectin-2 in ultraviolet radiation-induced tolerance. J. Immunol. 171:3801–3807 [DOI] [PubMed] [Google Scholar]
- Ariizumi K., Shen G.L., Shikano S., Ritter R., III, Zukas P., Edelbaum D., Morita A., Takashima A. 2000. Cloning of a second dendritic cell-associated C-type lectin (dectin-2) and its alternatively spliced isoforms. J. Biol. Chem. 275:11957–11963 10.1074/jbc.275.16.11957 [DOI] [PubMed] [Google Scholar]
- Barrett N.A., Maekawa A., Rahman O.M., Austen K.F., Kanaoka Y. 2009. Dectin-2 recognition of house dust mite triggers cysteinyl leukotriene generation by dendritic cells. J. Immunol. 182:1119–1128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boehmler A.M., Drost A., Jaggy L., Seitz G., Wiesner T., Denzlinger C., Kanz L., Möhle R. 2009. The CysLT1 ligand leukotriene D4 supports α4β1- and α5β1-mediated adhesion and proliferation of CD34+ hematopoietic progenitor cells. J. Immunol. 182:6789–6798 10.4049/jimmunol.0801525 [DOI] [PubMed] [Google Scholar]
- Brown G.D., Gordon S. 2001. Immune recognition. A new receptor for beta-glucans. Nature. 413:36–37 10.1038/35092620 [DOI] [PubMed] [Google Scholar]
- Cates E.C., Fattouh R., Wattie J., Inman M.D., Goncharova S., Coyle A.J., Gutierrez-Ramos J.C., Jordana M. 2004. Intranasal exposure of mice to house dust mite elicits allergic airway inflammation via a GM-CSF-mediated mechanism. J. Immunol. 173:6384–6392 [DOI] [PubMed] [Google Scholar]
- Eisenbarth S.C., Piggott D.A., Huleatt J.W., Visintin I., Herrick C.A., Bottomly K. 2002. Lipopolysaccharide-enhanced, toll-like receptor 4–dependent T helper cell type 2 responses to inhaled antigen. J. Exp. Med. 196:1645–1651 10.1084/jem.20021340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammad H., Chieppa M., Perros F., Willart M.A., Germain R.N., Lambrecht B.N. 2009. House dust mite allergen induces asthma via Toll-like receptor 4 triggering of airway structural cells. Nat. Med. 15:410–416 10.1038/nm.1946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammad H., Plantinga M., Deswarte K., Pouliot P., Willart M.A., Kool M., Muskens F., Lambrecht B.N. 2010. Inflammatory dendritic cells—not basophils—are necessary and sufficient for induction of Th2 immunity to inhaled house dust mite allergen. J. Exp. Med. 207:2097–2111 10.1084/jem.20101563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue K., Takano H., Koike E., Yanagisawa R., Oda T., Tamura H., Adachi Y., Ishibashi K., Ohno N. 2009. Candida soluble cell wall β-glucan facilitates ovalbumin-induced allergic airway inflammation in mice: possible role of antigen-presenting cells. Respir. Res. 10:68 10.1186/1465-9921-10-68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Instanes C., Ormstad H., Rydjord B., Wiker H.G., Hetland G. 2004. Mould extracts increase the allergic response to ovalbumin in mice. Clin. Exp. Allergy. 34:1634–1641 10.1111/j.1365-2222.2004.02076.x [DOI] [PubMed] [Google Scholar]
- Iwasaki A., Medzhitov R. 2004. Toll-like receptor control of the adaptive immune responses. Nat. Immunol. 5:987–995 10.1038/ni1112 [DOI] [PubMed] [Google Scholar]
- Kanaoka Y., Maekawa A., Penrose J.F., Austen K.F., Lam B.K. 2001. Attenuated zymosan-induced peritoneal vascular permeability and IgE-dependent passive cutaneous anaphylaxis in mice lacking leukotriene C4 synthase. J. Biol. Chem. 276:22608–22613 10.1074/jbc.M103562200 [DOI] [PubMed] [Google Scholar]
- Krishnamoorthy N., Oriss T.B., Paglia M., Fei M., Yarlagadda M., Vanhaesebroeck B., Ray A., Ray P. 2008. Activation of c-Kit in dendritic cells regulates T helper cell differentiation and allergic asthma. Nat. Med. 14:565–573 10.1038/nm1766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambrecht B.N., De Veerman M., Coyle A.J., Gutierrez-Ramos J.C., Thielemans K., Pauwels R.A. 2000a. Myeloid dendritic cells induce Th2 responses to inhaled antigen, leading to eosinophilic airway inflammation. J. Clin. Invest. 106:551–559 10.1172/JCI8107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambrecht B.N., Pauwels R.A., Fazekas De St Groth B. 2000b. Induction of rapid T cell activation, division, and recirculation by intratracheal injection of dendritic cells in a TCR transgenic model. J. Immunol. 164:2937–2946 [DOI] [PubMed] [Google Scholar]
- Lambrecht B.N., Peleman R.A., Bullock G.R., Pauwels R.A. 2000c. Sensitization to inhaled antigen by intratracheal instillation of dendritic cells. Clin. Exp. Allergy. 30:214–224 10.1046/j.1365-2222.2000.00818.x [DOI] [PubMed] [Google Scholar]
- LeibundGut-Landmann S., Gross O., Robinson M.J., Osorio F., Slack E.C., Tsoni S.V., Schweighoffer E., Tybulewicz V., Brown G.D., Ruland J., Reis e Sousa C. 2007. Syk- and CARD9-dependent coupling of innate immunity to the induction of T helper cells that produce interleukin 17. Nat. Immunol. 8:630–638 10.1038/ni1460 [DOI] [PubMed] [Google Scholar]
- Lutz M.B., Kukutsch N., Ogilvie A.L., Rössner S., Koch F., Romani N., Schuler G. 1999. An advanced culture method for generating large quantities of highly pure dendritic cells from mouse bone marrow. J. Immunol. Methods. 223:77–92 10.1016/S0022-1759(98)00204-X [DOI] [PubMed] [Google Scholar]
- Machida I., Matsuse H., Kondo Y., Kawano T., Saeki S., Tomari S., Obase Y., Fukushima C., Kohno S. 2004. Cysteinyl leukotrienes regulate dendritic cell functions in a murine model of asthma. J. Immunol. 172:1833–1838 [DOI] [PubMed] [Google Scholar]
- Madsen L., Labrecque N., Engberg J., Dierich A., Svejgaard A., Benoist C., Mathis D., Fugger L. 1999. Mice lacking all conventional MHC class II genes. Proc. Natl. Acad. Sci. USA. 96:10338–10343 10.1073/pnas.96.18.10338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maekawa A., Austen K.F., Kanaoka Y. 2002. Targeted gene disruption reveals the role of cysteinyl leukotriene 1 receptor in the enhanced vascular permeability of mice undergoing acute inflammatory responses. J. Biol. Chem. 277:20820–20824 10.1074/jbc.M203163200 [DOI] [PubMed] [Google Scholar]
- Meliton A.Y., Muñoz N.M., Osan C.M., Meliton L.N., Leff A.R. 2010. Leukotriene D4 activates β2-integrin adhesion in human polymorphonuclear leukocytes. Eur. Respir. J. 35:402–409 10.1183/09031936.00009309 [DOI] [PubMed] [Google Scholar]
- Meyer S., Tefsen B., Imberty A., Geyer R., van Die I. 2007. The C-type lectin L-SIGN differentially recognizes glycan antigens on egg glycosphingolipids and soluble egg glycoproteins from Schistosoma mansoni. Glycobiology. 17:1104–1119 10.1093/glycob/cwm073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okano M., Satoskar A.R., Nishizaki K., Abe M., Harn D.A., Jr 1999. Induction of Th2 responses and IgE is largely due to carbohydrates functioning as adjuvants on Schistosoma mansoni egg antigens. J. Immunol. 163:6712–6717 [PubMed] [Google Scholar]
- Okano M., Satoskar A.R., Nishizaki K., Harn D.A., Jr 2001. Lacto-N-fucopentaose III found on Schistosoma mansoni egg antigens functions as adjuvant for proteins by inducing Th2-type response. J. Immunol. 167:442–450 [DOI] [PubMed] [Google Scholar]
- Okunishi K., Dohi M., Nakagome K., Tanaka R., Yamamoto K. 2004. A novel role of cysteinyl leukotrienes to promote dendritic cell activation in the antigen-induced immune responses in the lung. J. Immunol. 173:6393–6402 [DOI] [PubMed] [Google Scholar]
- Olynych T.J., Jakeman D.L., Marshall J.S. 2006. Fungal zymosan induces leukotriene production by human mast cells through a dectin-1-dependent mechanism. J. Allergy Clin. Immunol. 118:837–843 10.1016/j.jaci.2006.06.008 [DOI] [PubMed] [Google Scholar]
- Peng J., Yang X.O., Chang S.H., Yang J., Dong C. 2010. IL-23 signaling enhances Th2 polarization and regulates allergic airway inflammation. Cell Res. 20:62–71 10.1038/cr.2009.128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phipps S., Lam C.E., Kaiko G.E., Foo S.Y., Collison A., Mattes J., Barry J., Davidson S., Oreo K., Smith L., et al. 2009. Toll/IL-1 signaling is critical for house dust mite-specific helper T cell type 2 and type 17 [corrected] responses. Am. J. Respir. Crit. Care Med. 179:883–893 10.1164/rccm.200806-974OC [DOI] [PubMed] [Google Scholar]
- Rincón M., Anguita J., Nakamura T., Fikrig E., Flavell R.A. 1997. Interleukin (IL)-6 directs the differentiation of IL-4-producing CD4+ T cells. J. Exp. Med. 185:461–469 10.1084/jem.185.3.461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbiani D.F., Finch R.A., Jäger D., Muller W.A., Sartorelli A.C., Randolph G.J. 2000. The leukotriene C(4) transporter MRP1 regulates CCL19 (MIP-3β, ELC)-dependent mobilization of dendritic cells to lymph nodes. Cell. 103:757–768 10.1016/S0092-8674(00)00179-3 [DOI] [PubMed] [Google Scholar]
- Robinson M.J., Osorio F., Rosas M., Freitas R.P., Schweighoffer E., Gross O., Verbeek J.S., Ruland J., Tybulewicz V., Brown G.D., et al. 2009. Dectin-2 is a Syk-coupled pattern recognition receptor crucial for Th17 responses to fungal infection. J. Exp. Med. 206:2037–2051 10.1084/jem.20082818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saijo S., Ikeda S., Yamabe K., Kakuta S., Ishigame H., Akitsu A., Fujikado N., Kusaka T., Kubo S., Chung S.H., et al. 2010. Dectin-2 recognition of alpha-mannans and induction of Th17 cell differentiation is essential for host defense against Candida albicans. Immunity. 32:681–691 10.1016/j.immuni.2010.05.001 [DOI] [PubMed] [Google Scholar]
- Sato K., Yang X.L., Yudate T., Chung J.S., Wu J., Luby-Phelps K., Kimberly R.P., Underhill D., Cruz P.D., Jr, Ariizumi K. 2006. Dectin-2 is a pattern recognition receptor for fungi that couples with the Fc receptor γ chain to induce innate immune responses. J. Biol. Chem. 281:38854–38866 10.1074/jbc.M606542200 [DOI] [PubMed] [Google Scholar]
- Shreffler W.G., Castro R.R., Kucuk Z.Y., Charlop-Powers Z., Grishina G., Yoo S., Burks A.W., Sampson H.A. 2006. The major glycoprotein allergen from Arachis hypogaea, Ara h 1, is a ligand of dendritic cell-specific ICAM-grabbing nonintegrin and acts as a Th2 adjuvant in vitro. J. Immunol. 177:3677–3685 [DOI] [PubMed] [Google Scholar]
- Suram S., Brown G.D., Ghosh M., Gordon S., Loper R., Taylor P.R., Akira S., Uematsu S., Williams D.L., Leslie C.C. 2006. Regulation of cytosolic phospholipase A2 activation and cyclooxygenase 2 expression in macrophages by the β-glucan receptor. J. Biol. Chem. 281:5506–5514 10.1074/jbc.M509824200 [DOI] [PubMed] [Google Scholar]
- Thivierge M., Stankova J., Rola-Pleszczynski M. 2006. Toll-like receptor agonists differentially regulate cysteinyl-leukotriene receptor 1 expression and function in human dendritic cells. J. Allergy Clin. Immunol. 117:1155–1162 10.1016/j.jaci.2005.12.1342 [DOI] [PubMed] [Google Scholar]
- Valera I., Vigo A.G., Alonso S., Barbolla L., Crespo M.S., Fernández N. 2007. Peptidoglycan and mannose-based molecular patterns trigger the arachidonic acid cascade in human polymorphonuclear leukocytes. J. Leukoc. Biol. 81:925–933 10.1189/jlb.0706451 [DOI] [PubMed] [Google Scholar]
- Wills-Karp M., Nathan A., Page K., Karp C.L. 2010. New insights into innate immune mechanisms underlying allergenicity. Mucosal Immunol. 3:104–110 10.1038/mi.2009.138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamasaki S., Ishikawa E., Sakuma M., Hara H., Ogata K., Saito T. 2008. Mincle is an ITAM-coupled activating receptor that senses damaged cells. Nat. Immunol. 9:1179–1188 10.1038/ni.1651 [DOI] [PubMed] [Google Scholar]