Abstract
A 53-year-old man, who is otherwise healthy and has a 20-year history of occasional heartburn, reports having had worsening heartburn for the past 12 months, with daily symptoms that disturb his sleep. He reports having had no dysphagia, gastrointestinal bleeding, or weight loss and in fact has recently gained 20 lb (9 kg). What would you advise regarding his evaluation and treatment?
THE CLINICAL PROBLEM
Gastroesophageal reflux disease is the most common gastrointestinal diagnosis recorded during visits to outpatient clinics.1 In the United States, it is estimated that 14 to 20% of adults are affected, although such percentages are at best approximations, given that the disease has a nebulous definition and that such estimates are based on the prevalence of self-reported chronic heartburn.2 A current definition of the disorder is “a condition which develops when the reflux of stomach contents causes troublesome symptoms (i.e., at least two heartburn episodes per week) and/or complications.”3 Several extraesophageal manifestations of the disease are well recognized, including laryngitis and cough (Table 1). With respect to the esophagus, the spectrum of injury includes esophagitis (Fig. 1A), stricture (Fig. 1B), the development of columnar metaplasia in place of the normal squamous epithelium (Barrett’s esophagus) (Fig. 1C), and adenocarcinoma (Fig. 1D). Of particular concern is the rising incidence of esophageal adenocarcinoma, an epidemiologic trend strongly linked to the increasing incidence of this condition.4–6 There were about 8000 incident cases of esophageal adenocarcinoma in the United States in 2004,7 which represents an increase by a factor of 2 to 6 in disease burden during the past 20 years.8,9
Table 1.
Symptoms and Conditions Associated with Gastroesophageal Reflux Disease.
| Esophageal syndromes |
| Injury (with or without esophageal symptoms) |
| Reflux esophagitis: necrosis of esophageal epithelium causing erosions or ulcers at or immediately above the gastroesophageal junction |
| Stricture: a persistent luminal narrowing of the esophagus caused by reflux-induced inflammation |
| Barrett’s esophagus: endoscopically suspected and histologically confirmed metaplasia in the distal esophagus, usually with the added stipulation that it be specialized intestinal metaplasia |
| Esophageal adenocarcinoma |
| Symptoms with or without esophageal injury |
| Common symptoms: heartburn, regurgitation, dysphagia, chest pain |
| Less common symptoms: odynophagia (pain with swallowing), water brash (excessive salivation prompted by acid reflux), subxiphoid pain, nausea |
| Extraesophageal syndromes |
| Association with gastroesophageal reflux disease established but good evidence for causation only when accompanied by an esophageal syndrome |
| Chronic cough |
| Laryngitis (hoarseness, throat clearing): reflux usually a cofactor along with excessive use of the voice, environmental irritants, and smoking |
| Asthma (reflux as a cofactor leading to poorly controlled disease) |
| Erosion of dental enamel |
| Proposed association with gastroesophageal reflux disease but neither association nor causation established |
| Pharyngitis |
| Sinusitis |
| Recurrent otitis media |
| Idiopathic pulmonary fibrosis |
Figure 1. Spectrum of Esophageal Injury in Gastroesophageal Reflux Disease.
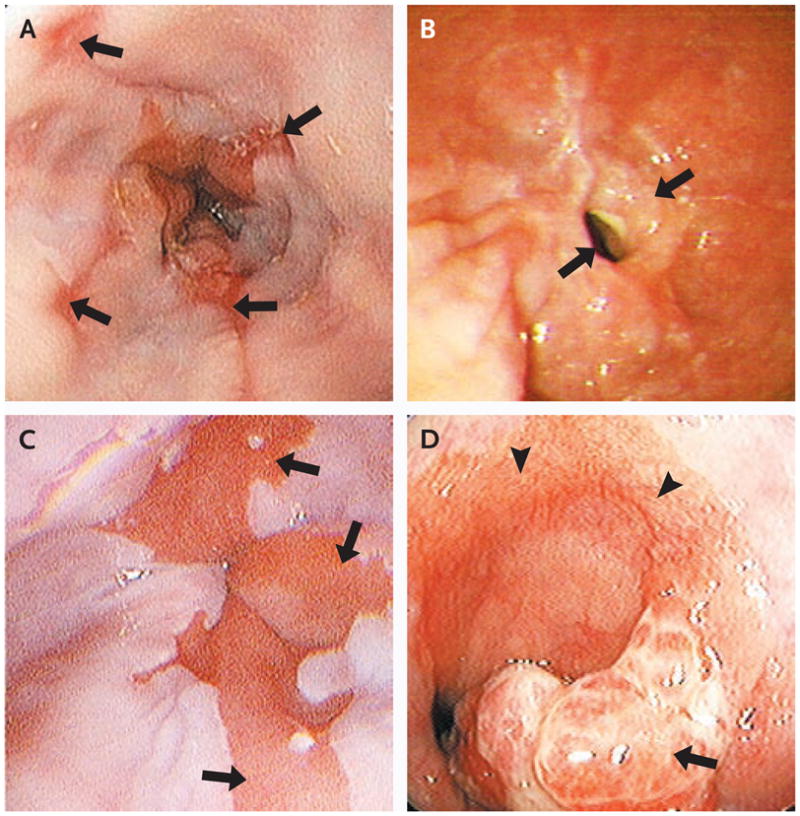
Gastroesophageal reflux is associated with esophagitis caused by erosions of the distal esophageal mucosa (Panel A, arrows), distal esophageal stricture as a consequence of chronic erosive esophagitis (Panel B, arrows), Barrett’s esophagus with columnar metaplasia of the normal squamous epithelium (Panel C, arrows), and esophageal adenocarcinoma (Panel D, arrow), shown here in a patient with Barrett’s esophagus (arrowheads).
Esophagitis occurs when excessive reflux of acid and pepsin results in necrosis of surface layers of esophageal mucosa, causing erosions and ulcers. Impaired clearance of the refluxed gastric juice from the esophagus also contributes to damage in many patients. Whereas some gastroesophageal reflux is normal (and relates to the ability to belch), several factors may predispose patients to pathologic reflux, including hiatus hernia,10,11 lower esophageal sphincter hypotension, loss of esophageal peristaltic function, abdominal obesity,11,12 increased compliance of the hiatal canal,13 gastric hypersecretory states,14 delayed gastric emptying, and overeating. Often multiple risk factors are present.
A consistent paradox in gastroesophageal reflux disease is the imperfect correspondence between symptoms attributed to the condition and endoscopic features of the disease. In a population-based endoscopy study in which 1000 northern Europeans were randomly sampled,15 the prevalence of Barrett’s esophagus was 1.6%, and that of esophagitis was 15.5%. However, only 40% of subjects who were found to have Barrett’s esophagus and one third of those who were found to have esophagitis reported having reflux symptoms. Conversely, two thirds of patients reporting reflux symptoms had no esophagitis. Furthermore, although gastroesophageal reflux is the most common cause of heartburn, other disorders (e.g., achalasia and eosinophilic esophagitis) may also cause or contribute to heartburn.3
STR ATEGIES AND EVIDENCE
DIAGNOSIS
When symptoms of gastroesophageal reflux disease are typical and the patient responds to therapy, no diagnostic tests are necessary to verify the diagnosis.16–18 Rather, the usual reasons prompting diagnostic testing are to avert misdiagnosis, to identify any complications (including stricture, Barrett’s metaplasia, and adenocarcinoma), and to evaluate treatment failures. Important alternative diagnoses to consider include coronary artery disease, gallbladder disease, gastric or esophageal cancer, peptic ulcer disease, esophageal motility disorders, and eosinophilic, infectious, or pill esophagitis.
Endoscopy addresses many of these possibilities with the caveat that evaluation for a potential cardiac cause of the presenting symptoms should always be prioritized. Furthermore, the endoscopist should have a low threshold for obtaining specimens from esophageal or gastric biopsy to detect alternative diagnoses, such as eosinophilic esophagitis and Helicobacter pylori gastritis. Although endoscopy is the primary test in patients whose condition is resistant to empirical therapy, its yield in this setting is low because of the poor correlation between symptoms of gastroesophageal reflux disease and esophagitis, the likelihood that preexisting esophagitis may have resolved with previous therapy, and the poor sensitivity for detecting motility disorders. Physiological testing is not routinely needed but can be helpful in selected patients by identifying subtle motility disorders (esophageal manometry), demonstrating abnormal exposure to esophageal acid in the absence of esophagitis (ambulatory pH monitoring), or most recently, both quantifying exposure to esophageal acid and identifying reflux events regardless of acidic content to assess correlations with symptoms (combined impedance–pH monitoring).19
LIFESTYLE MODIFICATIONS
Many lifestyle modifications are recommended as therapy for gastroesophageal reflux disease (Table 2). These include the avoidance of foods that reduce lower esophageal sphincter pressure and thus predispose to reflux, the limiting of exposure to acidic foods that are inherently irritating, and the adoption of behaviors to minimize reflux or heartburn. Although trials of the clinical efficacy of dietary or behavioral changes are lacking,20 clinical experience suggests that particular patients may benefit from certain measures.17,18 For example, patients with sleep disturbance from nighttime heartburn may benefit from elevation of the head of the bed, but that recommendation is probably superfluous for a patient without nighttime symptoms. Weight reduction should routinely be recommended in overweight patients, given the strong association between an increased body-mass index and the likelihood of symptoms.21
Table 2.
Dietary and Lifestyle Recommendations for the Treatment of Gastroesophageal Reflux Disease.*
| Dietary avoidance |
| Foods that are acidic or otherwise irritative |
| Citrus fruits |
| Tomatoes |
| Onions |
| Carbonated beverages |
| Spicy foods |
| Foods that can cause gastric reflux |
| Fatty or fried foods |
| Coffee, tea, and caffeinated beverages |
| Chocolate |
| Mint |
| Lifestyle |
| Smoking cessation |
| Weight reduction for patients who are overweight (BMI, 25.0–29.9) or obese (BMI, ≥30.0) or whose onset of symptoms was concurrent with weight gain within the normal range (BMI, 18.5–24.9) |
| Reduction in alcohol consumption |
| Nighttime symptoms |
| Avoidance of eating within 3 hr before bedtime |
| Elevation of head of bed |
| Postprandial symptoms |
| Consumption of smaller and more frequent meals |
| Avoidance of lying down after meals |
| Abdominal obesity |
| Avoidance of tight garments |
The rationales for proscribed foods and lifestyle modifications are based on clinical experience or, in some instances, small physiological studies showing a relevant effect, such as the reduction of lower esophageal sphincter pressure. These recommendations should be advocated selectively on the basis of the circumstances of a particular patient. BMI denotes body-mass index, which is calculated as the weight in kilograms divided by the square of the height in meters.
MEDICATION
Abundant data from randomized trials show benefits of inhibiting gastric acid secretion in patients with gastroesophageal reflux disease (Table 3). Reducing the acidity of gastric juice ameliorates reflux symptoms and allows esophagitis to heal. Data from several studies indicate that the likelihood of healing of esophagitis relates directly to the potency of a medication’s antisecretory effect (Table 4).22–29 In a large meta-analysis of 136 randomized, controlled trials involving 35,978 patients with esophagitis, the rate of healing among patients who were treated with proton-pump inhibitors (83%) was greater than that with histamine2-receptor antagonists (H2-blockers) (52%), and both rates were higher than that with placebo (8%).22 In all the trials, antacids were used to treat breakthrough symptoms. There were no major differences in efficacy noted among various proton-pump inhibitors when used in standard doses. The gain achieved in esophagitis healing by using twice the standard dose of a proton-pump inhibitor (as a once-daily initial dose) was modest but significant: an estimated 25 patients would need to be treated with this regimen to benefit 1 patient.22 Data from clinical trials are lacking with respect to the efficacy of double-dose proton-pump inhibitors as a twice-daily regimen for refractory symptoms, as is sometimes used in practice.
Table 3.
Inhibitors of Gastric Acid Secretion Approved for Use by the Food and Drug Administration (FDA).*
| Generic Name | Brand Name | Standard Dose† | Most Common Side Effects‡ |
|---|---|---|---|
| Histamine2-receptor antagonist | Headache, diarrhea, dizziness, fatigue, confusion | ||
| Cimetidine§ | Tagamet¶ | 400 mg twice daily | |
| Famotidine§ | Pepcid¶ | 20 mg twice daily | |
| Nizatidine§ | Axid¶ | 150 mg twice daily | |
| Ranitidine§ | Zantac¶ | 150 mg twice daily | |
| Proton-pump inhibitor | Headache, diarrhea, constipation, abdominal pain | ||
| Omeprazole§ | Prilosec¶ | 20 mg daily | |
| Pantoprazole§ | Protonix | 40 mg daily | |
| Esomeprazole | Nexium | 40 mg daily | |
| Lansoprazole | Prevacid | 30 mg daily | |
| Omeprazole with sodium bicarbonate | Zegerid | 40 mg daily | |
| Rabeprazole | Aciphex | 20 mg daily |
With respect to safety during pregnancy or lactation, omeprazole is a category C drug (no adequate studies or adverse fetal effects in animals). All other drugs are category B drugs (animal studies demonstrate no risk; no human studies). All doses are those commonly prescribed for histamine-2 receptor antagonists or approved by the FDA for proton-pump inhibitors in the treatment of esophagitis.
All doses are those commonly prescribed for histamine-2 receptor antagonists or approved by the FDA for proton-pump inhibitors in the treatment of esophagitis.
The most common side effects (per package inserts and clinical experience) are listed for each therapeutic class, although none of these effects occurred significantly more frequently with drug than with placebo in controlled clinical trials.
This drug is available in a generic form.
This drug is available over the counter without a prescription.
Table 4.
Treatment Data on the Use of Proton-Pump Inhibitors and Histamine2-Receptor Antagonists (H2-Blockers).*
| Healing of esophagitis |
| Proton-pump inhibitor |
| Superior to placebo (83% vs. 18%) at 8 wk; NNTB, 1.722 |
| Superior to H2-blocker (83% vs. 18%)18; relative risk, 0.5122 |
| Superior to H2-blocker (84% vs. 52%)17; relative risk, 0.512 |
| Significant dose–response effect at 4 wk22 |
| Low dose vs. standard dose once daily: NNTB, 10 |
| Standard dose vs. high dose once daily: NNTB, 25 |
| H2-blocker |
| Superior to placebo (41% vs. 20%) at 6 wk; NNTB, 522 |
| No significant dose–response effect (standard dose vs. high dose twice daily)22 |
| Resolution of heartburn† |
| Esophagitis |
| Proton-pump inhibitor superior to placebo (56% vs. 8%) at 4 wk; NNTB, 2 to 323 |
| Proton-pump inhibitor superior to H2-blocker (77% vs. 48%) at 4 to 12 wk24 |
| H2-blocker superior to placebo (56% vs. 45%) at 12 wk25 |
| No significant dose–response effect for proton-pump inhibitor at 4 wk22 |
| Low dose vs. standard dose once daily: 75% vs. 79% |
| Standard dose vs. high dose once daily: 73% vs. 76% |
| Patients without known esophagitis |
| Proton-pump inhibitor superior to placebo (36.7% vs. 9.5%); NNTB, 3 to 423 |
| Proton-pump inhibitor superior to H2-blocker (61% vs. 40%); NNTB, 526 |
| H2-blocker superior to placebo (relative risk, 0.77; 95% CI, 0.60 to 0.99)27 |
| No significant dose–response effect for H2-blocker at 8 wk |
| Standard dose vs. high dose twice daily: 45.8% vs. 44.8%28 |
| Maintenance therapy‡ |
| Remission of esophagitis |
| Proton-pump inhibitor superior to placebo (93% vs. 29%)29 |
| Low dose of proton-pump inhibitor sufficient in 35 to 95% of patients18 |
| Remission of heartburn |
| Acceptable symptom control with low-dose, intermittent therapy with proton-pump inhibitor in 83 to 92% of patients without esophagitis18 |
Relative risk refers to the probability of treatment failure in the active-treatment group. NNTB denotes number of patients needed to treat to benefit one patient.
Resolution of heartburn is generally defined as no symptoms for 7 days.
The duration of maintenance therapy was 6 to 12 months.
The response of heartburn to various therapeutic agents is less predictable than that of esophagitis.30 Although, as in the case of esophagitis, trials suggest that proton-pump inhibitors are superior to H2-blockers and that both are superior to placebo for the treatment of heartburn, observed efficacy rates are lower for heartburn than for esophagitis and vary widely among studies. This variation is probably due to the heterogeneity of the study populations and the fact that the outcome measure in most trials of proton-pump inhibitors was a complete resolution of symptoms rather than substantial improvement. The effectiveness of proton-pump inhibitors, as compared with placebo, for healing esophagitis (typically, 90% vs. 15%) is always greater than that for complete resolution of heartburn in the same trials (typically, 40% vs. 15%).
Reflux symptoms tend to be chronic with or without the presence of esophagitis. Data from controlled trials lasting 6 to 12 months showed that continued use of proton-pump inhibitors prevented the recurrence of esophagitis and maintained relief of symptoms (Table 4). An uncontrolled observational study showed continued effectiveness of proton-pump inhibitors in maintaining healing of esophagitis for up to 11 years.31 Thus, a common management strategy is indefinite treatment with proton-pump inhibitors or H2-blockers as necessary to maintain symptom control. Adding a dose of an H2-blocker before bedtime to a twice-daily regimen of proton-pump inhibitors has been advocated on the basis of a pharmacodynamic study suggesting additive inhibition of nocturnal acid secretion.32 However, this practice has not been supported by studies using clinical end points, and other pharmacodynamic data have shown rapid tachyphylaxis of the effect of H2-blockers.19
The most common side effects of proton-pump inhibitors are headache, diarrhea, constipation, and abdominal pain. Although in clinical trials these symptoms were not significantly more common with proton-pump inhibitors than with placebo, they have been confirmed in some patients with a test–retest strategy. Potential risks of long-term use of proton-pump inhibitors include secondary hypergastrinemia, malabsorption, and hypochlorhydria.33 These risks are mainly theoretical, but large, population-based, epidemiologic studies have suggested that long-term use of proton-pump inhibitors was associated with an increased risk of hip fracture by a factor of 1.4 in subjects over the age of 50 years (presumably attributable to calcium malabsorption),34 an increase in the risk of infectious gastroenteritis by a factor of 1.5,35 and a doubling of the risk of Clostridium difficile colitis.36 Available agents are categorized as either category C (omeprazole) or category B (H2-blockers and other proton-pump inhibitors) for use during pregnancy. Data on hundreds of accidental exposures to proton-pump inhibitors during pregnancy, as compared with matched controls, have shown no appreciable increase in the risk of birth defects.37
SURGERY
Surgery, most commonly Nissen fundoplication, in which the proximal stomach is wrapped around the distal esophagus to create an antireflux barrier, is an alternative management approach to chronic gastroesophageal reflux disease. After the adoption of a laparoscopic technique in 1991, the number of fundoplications that were performed annually in adults in the United States nearly tripled by 1999 (to more than 30,000 cases) but has steadily declined since then.38 Poorer-than-anticipated outcomes, including patient dissatisfaction in community practice, may partially explain this trend.39,40
As with therapy with proton-pump inhibitors, evidence supporting the effectiveness of fundoplication is stronger for treating esophagitis than for treating reflux symptoms. At the 7-year follow-up in one study of patients with esophagitis who were randomly assigned to receive either continuous omeprazole therapy (20 to 60 mg per day) or fundoplication, rates of recurrent esophagitis were similar between the two groups (10.3% and 11.8%, respectively).41 In studies in which the assessment of symptoms was restricted to the control of heartburn and acid regurgitation in patients with esophagitis, there was significantly improved control with surgery, as compared with therapy with proton-pump inhibitors.41 However, potential benefits of surgery must be weighed against potential deleterious effects.19,42 These include the inherent risks associated with surgery and the frequent need for revision surgery, the risk of severe dysphagia (about 6% overall),43 increased flatulence,41 an inability to belch,41 and increased bowel symptoms (e.g., diarrhea, bloating, abdominal pain, and constipation).44 Reported rates of reoperation because of disruption or complications are as high as 7% within 1 to 3 years.19 Up to 60% of patients who had undergone such surgery continued to use medication for reflux symptoms when they were assessed 10 to 12 years after surgery.45 Follow-up of patients who have received medical therapy, as compared with surgery, have shown no significant differences in the prevalence of Barrett’s esophagus or in the incidence of adenocarcinoma (estimated at less than 0.01% per year).46–48
AREAS OF UNCERTAINT Y
The optimal criteria are unclear for the diagnosis of gastroesophageal reflux disease and for the assessment of whether extraesophageal symptoms, such as laryngitis and chronic cough, are attributable to reflux. In addition, there is uncertainty regarding the risk–benefit profile of indefinitely continuing medication to suppress acid secretion and the optimal degree of acid inhibition.
Particularly controversial is the appropriate role of endoscopy in screening patients for Barrett’s esophagus and in surveillance of those with known Barrett’s esophagus.49 The risk of esophageal adenocarcinoma in patients with Barrett’s esophagus is 0.50 to 0.75% per year,50 and survival rates for esophageal adenocarcinoma are substantially greater among those whose cancers are detected early (58% for tumors detected in situ, as compared with 10% for tumors with regional spread at 5 years).51 Thus, screening patients for Barrett’s esophagus, followed by surveillance of affected patients for the development of dysplasia and adenocarcinoma, potentially allows for early diagnosis of esophageal carcinoma or even prevention of cancer by ablation of dysplastic lesions. Yet, despite widespread use of endoscopy for screening for Barrett’s esophagus, evidence that this strategy reduces the rate of death from esophageal adenocarcinoma is lacking. For such a strategy to significantly reduce mortality on a population basis, patients with Barrett’s esophagus must constitute a substantial fraction of those at risk for cancer, reflux symptoms should be predictive of finding Barrett’s esophagus on endoscopy, and the detection of Barrett’s esophagus should improve the clinical outcome.7 However, the above-mentioned population-based data indicate that the presence of Barrett’s esophagus was poorly correlated with reflux symptoms.15 Furthermore, in a case–control study, more than 40% of patients with esophageal adenocarcinoma reported having no antecedent reflux symptoms.4 Similarly, in a Kaiser Permanente cohort study, 454 of 589 patients with esophageal adenocarcinoma or adenocarcinoma of the gastric cardia had no identifiable Barrett’s metaplasia evident in pathological specimens, and only 23 of 64 patients who had undergone endoscopy before cancer detection had received the diagnosis of Barrett’s esophagus.52 Consistent with these observations, two large surveillance programs for Barrett’s esophagus concluded that even though a small number of incident esophageal adenocarcinomas were detected, there was no improvement in survival attributable to surveillance.53,54 However, these management trials used esophagectomy as the treatment for high-grade dysplasia or intramucosal cancer within Barrett’s esophagus. Current management for these lesions is shifting rapidly toward less morbid techniques, such as mucosal ablation and endoscopic mucosal resection, potentially improving outcomes.49
GUIDELINES FROM PROFESSIONAL SOCIETIES
Guidelines for the treatment of gastroesophageal reflux disease in adults have been published by the American College of Gastroenterology,16 the Canadian Gastroenterology Association,17 and the American Gastroenterological Association Institute.18 These guidelines agree closely in cases in which the evidence is strongest, most notably in the use of antisecretory medications to treat esophagitis or heartburn, as summarized in Table 4. Similarly, the guidelines agree that dysphagia should be evaluated with endoscopy. The greatest discrepancy among guidelines is in recommendations for or against endoscopy for chronic symptoms of gastroesophageal reflux disease with the goal of detecting Barrett’s esophagus and thus reducing the risk of esophageal adenocarcinoma. The Canadian guideline does not advocate screening endoscopy, noting that the procedure “has not been shown to reduce mortality from esophageal adenocarcinoma.” The position statement of the American Gastroenterological Association Institute concluded that there was insufficient evidence to recommend for or against endoscopy to screen for Barrett’s esophagus or to diminish the risk of esophageal adenocarcinoma. In contrast, the American College of Gastroenterology recommends consideration of endoscopy in patients with symptoms “suggesting complicated disease (dysphagia, odynophagia, bleeding, weight loss, or anemia), those at risk for Barrett’s esophagus, or when the patient and physician feel early endoscopy to be appropriate” — conditions that might encompass the entire population of patients with gastroesophageal reflux disease.
CONCLUSIONS AND RECOMMENDATIONS
The patient in the vignette reports a history of frequent heartburn consistent with gastroesophageal reflux disease. Clinical experience suggests that dietary changes may be beneficial if there are obvious dietary precipitants (coffee, chocolate, or fatty foods) and that lifestyle changes are warranted to reduce obesity, smoking, or excessive alcohol use if present. However, lifestyle modification alone is unlikely to eliminate his symptoms.
I would also recommend therapy with a proton-pump inhibitor and would anticipate the need for maintenance therapy, given the patient’s long history of symptoms. In this case, after 8 to 12 weeks of a standard dose of a proton-pump inhibitor, I would advise the patient to titrate the dose to find the lowest dose that provides satisfactory control of heartburn. A reasonable target is 80% symptom relief; patients often continue to have symptoms triggered by overindulgence. Occasional breakthrough symptoms can be treated with antacids as necessary. Although proton-pump inhibitors are more effective in general than H2-blockers, the latter will suffice for some patients, and some will find as-needed therapy to be sufficient. Other patients will require twice-daily therapy with a proton-pump inhibitor; in such cases, medication should be taken 30 to 60 minutes before breakfast and dinner. There is no evidence that the risk of esophageal adenocarcinoma is reduced by any current medical or surgical therapy.46 Patients whose heartburn has not adequately responded to twice-daily therapy with a proton-pump inhibitor should be referred for specialist evaluation. If a patient has symptoms refractory to proton-pump inhibitors (especially those attributable to regurgitation) or cannot tolerate such therapy, antireflux surgery may be considered; patients should understand that there are associated risks and that medication is often still needed after surgery.
It should be recognized that data are limited to guide the use of endoscopy in patients with gastroesophageal reflux disease. Consistent with current guidelines,15–17 this procedure is routinely recommended for patients with odynophagia, gastrointestinal blood loss, anemia, or dysphagia. A patient’s anxiety and preference to undergo the procedure may also be an indication. The question of whether to screen other patients remains controversial, with various professional societies providing conflicting opinions. I do not routinely recommend endoscopy in patients without these indications, given the low absolute risk of esophageal cancer in patients with gastroesophageal reflux disease and the lack of data to show that endoscopic screening results in better outcomes.
Acknowledgments
Supported by a grant (R01 DC00646) from the Public Health Service.
Footnotes
Dr. Kahrilas reports receiving consulting fees from AstraZeneca, Procter & Gamble, and Xenoport. No other potential conflict of interest relevant to this article was reported.
An audio version of this article is available at www.nejm.org.
References
- 1.Shaheen NJ, Hansen RA, Morgan DR, et al. The burden of gastrointestinal and liver diseases, 2006. Am J Gastroenterol. 2006;101:2128–38. doi: 10.1111/j.1572-0241.2006.00723.x. [DOI] [PubMed] [Google Scholar]
- 2.Camilleri M, Dubois D, Coulie B, et al. Prevalence and socioeconomic impact of functional gastrointestinal disorders in the United States: results from the US Upper Gastrointestinal Study. Clin Gastroenterol Hepatol. 2005;3:543–52. doi: 10.1016/s1542-3565(05)00153-9. [DOI] [PubMed] [Google Scholar]
- 3.Vakil N, van Zanten SV, Kahrilas P, Dent J, Jones R. The Montreal definition and classification of gastroesophageal reflux disease: a global evidence-based consensus. Am J Gastroenterol. 2006;101:1900–20. doi: 10.1111/j.1572-0241.2006.00630.x. [DOI] [PubMed] [Google Scholar]
- 4.Lagergren J, Bergström R, Lindgren A, Nyrén O. Symptomatic gastroesophageal reflux disease as a risk factor for esophageal adenocarcinoma. N Engl J Med. 1999;340:825–31. doi: 10.1056/NEJM199903183401101. [DOI] [PubMed] [Google Scholar]
- 5.Dent J, El-Serag HB, Wallander MA, Johansson S. Epidemiology of gastrooesophageal reflux disease: a systematic review. Gut. 2005;54:710–7. doi: 10.1136/gut.2004.051821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.el-Serag HB, Sonnenberg A. Opposing time trends of peptic ulcer and reflux disease. Gut. 1998;43:327–33. doi: 10.1136/gut.43.3.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dellon ES, Shaheen NJ. Does screening for Barrett’s esophagus and adenocarcinoma of the esophagus prolong survival? J Clin Oncol. 2005;23:4478–82. doi: 10.1200/JCO.2005.19.059. [DOI] [PubMed] [Google Scholar]
- 8.Pohl H, Welch HG. The role of over-diagnosis and reclassification in the marked increase of esophageal adenocarcinoma incidence. J Natl Cancer Inst. 2005;97:142–6. doi: 10.1093/jnci/dji024. [DOI] [PubMed] [Google Scholar]
- 9.Forman D. Re: the role of overdiagnosis and reclassification in the marked increase of esophageal adenocarcinoma incidence. J Natl Cancer Inst. 2005;97:1013–4. doi: 10.1093/jnci/dji180. [DOI] [PubMed] [Google Scholar]
- 10.Kahrilas PJ, Shi G, Manka M, Joehl RJ. Increased frequency of transient lower esophageal sphincter relaxation induced by gastric distension in reflux patients with hiatal hernia. Gastroenterology. 2000;118:688–95. doi: 10.1016/s0016-5085(00)70138-7. [DOI] [PubMed] [Google Scholar]
- 11.de Vries DR, van Herwaarden MA, Smout AJ, Samsom M. Gastroesophageal pressure gradients in gastroesophageal reflux disease: relations with hiatal hernia, body mass index, and esophageal acid exposure. Am J Gastroenterol. 2008;103:1349–54. doi: 10.1111/j.1572-0241.2008.01909.x. [DOI] [PubMed] [Google Scholar]
- 12.Corley DA, Kubo A, Levin TR, et al. Abdominal obesity and body mass index as risk factors for Barrett’s esophagus. Gastroenterology. 2007;133:34–41. doi: 10.1053/j.gastro.2007.04.046. [DOI] [PubMed] [Google Scholar]
- 13.Pandolfino JE, Shi G, Trueworthy B, Kahrilas PJ. Esophagogastric junction opening during relaxation distinguishes non-hernia reflux patients, hernia patients and normals. Gastroenterology. 2003;125:1018–24. doi: 10.1016/s0016-5085(03)01210-1. [DOI] [PubMed] [Google Scholar]
- 14.Hirschowitz BI, Simmons J, Johnson LF, Mohnen J. Risk factors for esophagitis in extreme acid hypersecretors with and without Zollinger-Ellison syndrome. Clin Gastroenterol Hepatol. 2004;2:220–9. doi: 10.1016/s1542-3565(04)00009-6. [DOI] [PubMed] [Google Scholar]
- 15.Ronkainen J, Aro P, Storskrubb T, et al. Prevalence of Barrett’s esophagus in the general population: an endoscopic study. Gastroenterology. 2005;129:1825–31. doi: 10.1053/j.gastro.2005.08.053. [DOI] [PubMed] [Google Scholar]
- 16.DeVault KR, Castell DO. Updated guidelines for the diagnosis and treatment of gastroesophageal reflux disease. Am J Gastroenterol. 2005;100:190–200. doi: 10.1111/j.1572-0241.2005.41217.x. [DOI] [PubMed] [Google Scholar]
- 17.Armstrong D, Marshall JK, Chiba N, et al. Canadian Consensus Conference on the management of gastroesophageal reflux disease in adults — update 2004. Can J Gastroenterol. 2005;19:15–35. doi: 10.1155/2005/836030. [DOI] [PubMed] [Google Scholar]
- 18.Kahrilas PJ, Shaheen NJ, Vaezi M, et al. AGAI medical position statement: management of gastroesophageal reflux disease. Gastroenterology. 2008;135:1383–91. doi: 10.1053/j.gastro.2008.08.045. [DOI] [PubMed] [Google Scholar]
- 19.Kahrilas PJ, Shaheen NJ, Vaezi M. AGAI technical review: management of gastroesophageal reflux disease. Gastroenterology. 2008;135:1392–1413. doi: 10.1053/j.gastro.2008.08.044. [DOI] [PubMed] [Google Scholar]
- 20.Kaltenbach T, Crockett S, Gerson LB. Are lifestyle measures effective in patients with gastroesophageal reflux disease? An evidence-based approach. Arch Intern Med. 2006;166:965–71. doi: 10.1001/archinte.166.9.965. [DOI] [PubMed] [Google Scholar]
- 21.Jacobson BC, Somers SC, Fuchs CS, Kelly CP, Camargo CA., Jr Body-mass index and symptoms of gastroesophageal reflux in women. N Engl J Med. 2006;354:2340–8. doi: 10.1056/NEJMoa054391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Khan M, Santana J, Donnellan C, Preston C, Moayyedi P. Medical treatments in the short term management of reflux esophagitis. Cochrane Database Syst Rev. 2007;2:CD003244. doi: 10.1002/14651858.CD003244.pub2. [DOI] [PubMed] [Google Scholar]
- 23.Dean BB, Cano AD, Jr, Knight K, Ofman JJ, Fass R. Effectiveness of proton pump inhibitors in nonerosive reflux disease. Clin Gastroenterol Hepatol. 2004;2:656–64. doi: 10.1016/s1542-3565(04)00288-5. [DOI] [PubMed] [Google Scholar]
- 24.Chiba N, De Gara CJ, Wilkinson JM, Hunt RH. Speed of healing and symptom relief in grade II to IV gastroesophageal reflux disease: a meta-analysis. Gastroenterology. 1997;112:1798–810. doi: 10.1053/gast.1997.v112.pm9178669. [DOI] [PubMed] [Google Scholar]
- 25.Sabesin SM, Berlin RG, Humphries TJ, Bradstreet DC, Walton-Bowen KL, Zaidi S. Famotidine relieves symptoms of gastroesophageal reflux disease and heals erosions and ulcerations: results of a multi-center, placebo-controlled, dose-ranging study. Arch Intern Med. 1991;151:2394–400. [PubMed] [Google Scholar]
- 26.Venables TL, Newland RD, Patel AC, Hole J, Wilcock C, Turbitt ML. Omeprazole 10 milligrams once daily, omeprazole 20 milligrams twice daily, or raniditine 150 milligrams twice daily, evaluated as initial therapy for the relief of symptoms of gastrooesophageal reflux disease in general practice. Scand J Gastroenterol. 1997;32:965–73. doi: 10.3109/00365529709011211. [DOI] [PubMed] [Google Scholar]
- 27.van Pinxteren B, Numans ME, Bonis PA, Lau J. Short-term treatment with proton pump inhibitors, H2-receptor antagonists and prokinetics for gastro-oesophageal reflux disease-like symptoms and endoscopy negative reflux disease. Cochrane Database Syst Rev. 2006;3:CD002095. doi: 10.1002/14651858.CD002095.pub3. [DOI] [PubMed] [Google Scholar]
- 28.Kahrilas PJ, Fennerty MB, Joelsson B. High- versus standard-dose raniditine for control of heartburn in poorly responsive gastroesophageal reflux disease: a prospective, controlled trial. Am J Gastroenterol. 1999;94:92–7. doi: 10.1111/j.1572-0241.1999.00777.x. [DOI] [PubMed] [Google Scholar]
- 29.Johnson DA, Benjamin SB, Vakil NB, et al. Esomeprazole once daily for 6 months is effective therapy for maintaining healed erosive esophagitis and for controlling gastroesophageal reflux disease symptoms: a randomized, double-blind, placebo-controlled study of efficacy and safety. Am J Gastroenterol. 2001;96:27–34. doi: 10.1111/j.1572-0241.2001.03443.x. [DOI] [PubMed] [Google Scholar]
- 30.Dent J. Review article: from 1906 to 2006 — a century of major evolution of understanding of gastro-oesophageal reflux disease. Aliment Pharmacol Ther. 2006;24:1269–81. doi: 10.1111/j.1365-2036.2006.03122.x. [DOI] [PubMed] [Google Scholar]
- 31.Klinkenberg-Knol EC, Nelis F, Dent J, et al. Long-term omeprazole treatment in resistant gastroesophageal reflux disease: efficacy, safety, and influence on gastric mucosa. Gastroenterology. 2000;118:661–79. doi: 10.1016/s0016-5085(00)70135-1. [DOI] [PubMed] [Google Scholar]
- 32.Peghini PL, Katz PO, Castell DO. Ranitidine controls nocturnal gastric acid breakthrough on omeprazole: a controlled study in normal subjects. Gastroenterology. 1998;115:1335–9. doi: 10.1016/s0016-5085(98)70010-1. [DOI] [PubMed] [Google Scholar]
- 33.Laine L, Ahnen D, McClain C, Solcia E, Walsh JH. Review article: potential gastrointestinal effects of long-term acid suppression with proton pump inhibitors. Aliment Pharmacol Ther. 2000;14:651–68. doi: 10.1046/j.1365-2036.2000.00768.x. [DOI] [PubMed] [Google Scholar]
- 34.Yang YX, Lewis JD, Epstein S, Metz DC. Long-term proton pump inhibitor therapy and risk of hip fracture. JAMA. 2006;296:2947–53. doi: 10.1001/jama.296.24.2947. [DOI] [PubMed] [Google Scholar]
- 35.Garcia Rodríguez LA, Ruigómez A. Gastric acid, acid-suppressing drugs, and bacterial gastroenteritis: how much of a risk? Epidemiology. 1997;8:571–4. doi: 10.1097/00001648-199709000-00016. [DOI] [PubMed] [Google Scholar]
- 36.Dial S, Alrasadi K, Manoukian C, Huang A, Menzies D. Risk of Clostridium difficile diarrhea among hospital inpatients prescribed proton pump inhibitors: cohort and case-control studies. CMAJ. 2004;171:33–8. doi: 10.1503/cmaj.1040876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Diav-Citrin O, Arnon J, Schechtman S, et al. The safety of proton pump inhibitors in pregnancy: a multicentre prospective controlled study. Aliment Pharmacol Ther. 2005;21:269–75. doi: 10.1111/j.1365-2036.2005.02306.x. [DOI] [PubMed] [Google Scholar]
- 38.Finks JF, Wei Y, Birkmeyer JD. The rise and fall of antireflux surgery in the United States. Surg Endosc. 2006;20:1698–701. doi: 10.1007/s00464-006-0042-3. [DOI] [PubMed] [Google Scholar]
- 39.Vakil N, Shaw M, Kirby R. Clinical effectiveness of laparoscopic fundoplication in a U.S. community. Am J Med. 2003;114:1–5. doi: 10.1016/s0002-9343(02)01390-6. [DOI] [PubMed] [Google Scholar]
- 40.Rantanen TK, Halme TV, Luostarinen ME, Karhumäki LM, Könönen EO, Isolauri JO. The long term results of open antireflux surgery in a community-based health care center. Am J Gastroenterol. 1999;94:1777–81. doi: 10.1111/j.1572-0241.1999.01205.x. [DOI] [PubMed] [Google Scholar]
- 41.Lundell L, Miettinen P, Myrvold HE, et al. Seven-year follow-up of a randomized clinical trial comparing proton-pump inhibition with surgical therapy for reflux oesophagitis. Br J Surg. 2007;94:198–203. doi: 10.1002/bjs.5492. [DOI] [PubMed] [Google Scholar]
- 42.Dominitz JA, Dire CA, Billingsley KG, Todd-Stenberg JA. Complications and antireflux medication use after antireflux surgery. Clin Gastroenterol Hepatol. 2006;4:299–305. doi: 10.1016/j.cgh.2005.12.019. [DOI] [PubMed] [Google Scholar]
- 43.Bais JE, Bartelsman JF, Bonjer HJ, et al. Laparoscopic or conventional Nissen fundoplication for gastro-oesophageal reflux disease: randomised clinical trial. Lancet. 2000;355:170–4. doi: 10.1016/s0140-6736(99)03097-4. [DOI] [PubMed] [Google Scholar]
- 44.Klaus A, Hinder RA, DeVault KR, Achem SR. Bowel dysfunction after laparoscopic antireflux surgery: incidence, severity, and clinical course. Am J Med. 2003;114:6–9. doi: 10.1016/s0002-9343(02)01301-3. [DOI] [PubMed] [Google Scholar]
- 45.Spechler SJ, Lee E, Ahnen D, et al. Long-term outcome of medical and surgical therapies for gastroesophageal reflux disease: follow-up of a randomized controlled trial. JAMA. 2001;285:2331–8. doi: 10.1001/jama.285.18.2331. [DOI] [PubMed] [Google Scholar]
- 46.Tran T, Spechler SJ, Richardson P, El-Serag HB. Fundoplication and the risk of esophageal cancer in gastroesophageal reflux disease: a Veterans Affairs cohort study. Am J Gastroenterol. 2005;100:1002–8. doi: 10.1111/j.1572-0241.2005.41007.x. [DOI] [PubMed] [Google Scholar]
- 47.Ye W, Chow WH, Lagergren J, Yin L, Nyrén O. Risk of adenocarcinomas of the esophagus and gastric cardia in patients with gastroesophageal reflux diseases and after antireflux surgery. Gastroenterology. 2001;121:1286–93. doi: 10.1053/gast.2001.29569. [DOI] [PubMed] [Google Scholar]
- 48.Shaheen N, Ransohoff DF. Gastroesophageal reflux, Barrett esophagus, and esophageal cancer: scientific review. JAMA. 2002;287:1972–81. doi: 10.1001/jama.287.15.1972. [DOI] [PubMed] [Google Scholar]
- 49.Sharma P, McQuaid K, Dent J, et al. A critical review of the diagnosis and management of Barrett’s esophagus: the AGA Chicago Workshop. Gastroenterology. 2004;127:310–30. doi: 10.1053/j.gastro.2004.04.010. [DOI] [PubMed] [Google Scholar]
- 50.Shaheen NJ, Crosby MA, Bozymski EM, Sandler RS. Is there publication bias in the reporting of cancer risk in Barrett’s esophagus? Gastroenterology. 2000;119:333–8. doi: 10.1053/gast.2000.9302. [DOI] [PubMed] [Google Scholar]
- 51.Eloubeidi MA, Mason AC, Desmond RA, El-Serag HB. Temporal trends (1973–1997) in survival of patients with esophageal adenocarcinoma in the United States: a glimmer of hope? Am J Gastroenterol. 2003;98:1627–33. doi: 10.1111/j.1572-0241.2003.07454.x. [DOI] [PubMed] [Google Scholar]
- 52.Corley DA, Levin TR, Habel LA, Weiss NS, Buffler PA. Surveillance and survival in Barrett’s adenocarcinomas: a population-based study. Gastroenterology. 2002;122:633–40. doi: 10.1053/gast.2002.31879. [DOI] [PubMed] [Google Scholar]
- 53.Conio M, Bianchi S, Lapertosa G, et al. Long-term endoscopic surveillance of patients with Barrett’s esophagus: incidence of dysplasia and adenocarcinoma: a prospective study. Am J Gastroenterol. 2003;98:1931–9. doi: 10.1111/j.1572-0241.2003.07666.x. [DOI] [PubMed] [Google Scholar]
- 54.van der Burgh A, Dees J, Hop WC, van Blankenstein M. Oesophageal cancer is an uncommon cause of death in patients with Barrett’s oesophagus. Gut. 1996;39:5–8. doi: 10.1136/gut.39.1.5. [DOI] [PMC free article] [PubMed] [Google Scholar]


