By 2020, chronic obstructive pulmonary disease (COPD) is projected to become the 5th and 3rd leading cause of disability and death worldwide, respectively.1 Given their advanced age and high prevalence of multimorbidity, older persons with COPD are particularly vulnerable to adverse outcomes.2 The Journal has recently published two comprehensive reviews regarding COPD in older persons.3,4 Together, these reviews provide an excellent summary of current practice guidelines regarding the diagnosis, staging, and management of COPD.3,4 These guidelines have largely been informed by the Global Initiative for Obstructive Lung Disease (GOLD) and a combined task force from the American Thoracic and European Respiratory Societies (ATS/ERS).5,6 Although a consensus has emerged regarding principles of COPD management, the applicability of these guidelines to the diagnosis and staging of COPD in older persons is uncertain.7–11 The primary problem is that the diagnostic and staging criteria espoused by GOLD and the ATS/ERS do not adequately address key aging-related changes in pulmonary function, leading to frequent misidentification of COPD and misclassification of COPD severity.7–11 Consequently, current practice guidelines for COPD may lead to inappropriate pharmacotherapy in older persons, as well as delays in the consideration of other diagnoses. In this commentary, we discuss the age-related limitations inherent in existing diagnostic and staging criteria for COPD and, in response, provide alternative strategies.
Because of progressive small airways’ disease and parenchymal destruction, COPD is defined by chronic airflow limitation that is not fully reversible.5,6,12 The airflow limitation is established spirometrically, based solely on a reduced ratio of forced expiratory volume in 1-second (FEV1) to forced vital capacity (FVC), with severity subsequently classified (“staged”) according to the FEV1.5,6 In other words, airflow limitation is characterized by a greater reduction in a timed lung volume—FEV1, than in an untimed lung volume—FVC. Normal aging, however, is also associated with airflow limitation.10,13 Developmentally, after achieving peak pulmonary function at about 20 years of age, airflow limitation progressively worsens across the lifespan, principally due to increasing rigidity of the chest wall and decreasing elastic recoil of the lung.10,13 In addition, between-subject variability in spirometric performance increases progressively with age, so that the coefficient of variation is at least 50% greater in healthy 80-year olds than healthy 40-year olds.10
Because current spirometric criteria do not account for these age-related changes, the diagnosis and staging of COPD become increasingly inaccurate with advancing age, affecting persons 65 years or older most notably, but also persons who are middle-aged.7–11 For example, as shown in Table 1, GOLD sets a diagnostic threshold for a reduced FEV1/FVC below 0.70, yielding a COPD prevalence of about 40% in persons aged 65-to-80 years.7–9,14 This high rate likely includes a large number of false-positive cases, because age-related airflow limitation reduces the FEV1/FVC below 0.70 starting at about 40-to-50 years of age.8,10 Alternatively, the ATS/ERS establishes a diagnostic threshold for the FEV1/FVC based on the lower limit of normal (ATS/ERS-LLN), calculated as the 5th percentile of the distribution of reference values (i.e., the predicted values, as determined in a reference population of healthy never-smokers).6 While yielding a more modest COPD prevalence of about 20% in persons aged 65-to-80 years,8,14 this approach also likely leads to a substantial number of false-positive cases.8 This is because the calculation of the ATS/ERS-LLN does not consider the spread of the reference data (i.e., the coefficient of variation) and is often based on reference equations that incorrectly model the relationship between spirometric lung function and its predictor variables of age, height, sex, and ethnicity.8,10
Table 1.
Current spirometric criteria for establishing and staging COPD
| GOLD | ATS/ERS | ||
|---|---|---|---|
| Establishing COPD FEV1/FVC |
Staging COPD FEV1 (%Pred)* |
Establishing COPD FEV1/FVC |
Staging COPD FEV1 (%Pred)* |
| <0.70 | Mild: ≥80 | <ATS/ERS-LLN | Mild: ≥70 |
| Moderate: 50–79 | Moderate: 60–69 Moderately severe: 50–59 |
||
| Severe: 30–49 | Severe: 35–49 | ||
| Very severe: <30 | Very Severe: <35 | ||
Abbreviations: COPD, Chronic Obstructive Pulmonary Disease; GOLD, Global Initiative for Obstructive Lung Disease; ATS/ERS, American Thoracic and European Respiratory Societies; FEV1/FVC, ratio of forced expiratory volume in 1 second (FEV1) to forced vital capacity (FVC); LLN, lower limit of normal; %Pred, percent predicted.
(measured / predicted) × 100
The extent of the misidentification of COPD by current spirometric criteria has been evaluated relative to an alternative approach, termed Lambda-Mu-Sigma (LMS).10 This approach, which is widely used to construct growth charts for children, expresses spirometric values as Z-scores, calculated as follows, [(measured ÷ Mu)Lambda minus 1] ÷ (Lambda × Sigma).10 In this equation, “Mu” is the predicted median, representing how a spirometric measure changes based on its predictor variables; “Sigma” is the coefficient of variation, modeling the spread of the reference data and adjusting for non-uniform dispersion; and “Lambda” is a measure of skewness, namely the departure from normality.10 Using the LMS approach, we have shown that, in a representative sample of persons aged 65-to-80 years, a lower limit of normal for the FEV1/FVC set at the 5th percentile of the distribution of Z-scores (LMS-LLN) is associated with an increased risk of death and likelihood of having respiratory symptoms, and yields a COPD prevalence of 13.2%.8 In addition, we have shown that, relative to the LMS-LLN threshold, spirometric criteria based on GOLD and the ATS/ERS yield high false-positive rates for COPD of 63% and 31%, respectively.8 Similar results have been found in middle-aged persons, although the differences were less pronounced.8 Unfortunately, the LMS-LLN approach is currently limited to whites, since Z-scores have not yet been calculated for non-whites.10 Once they are available more broadly, LMS-derived Z-scores will offer the most valid method for establishing the diagnosis of COPD across the adult lifespan.
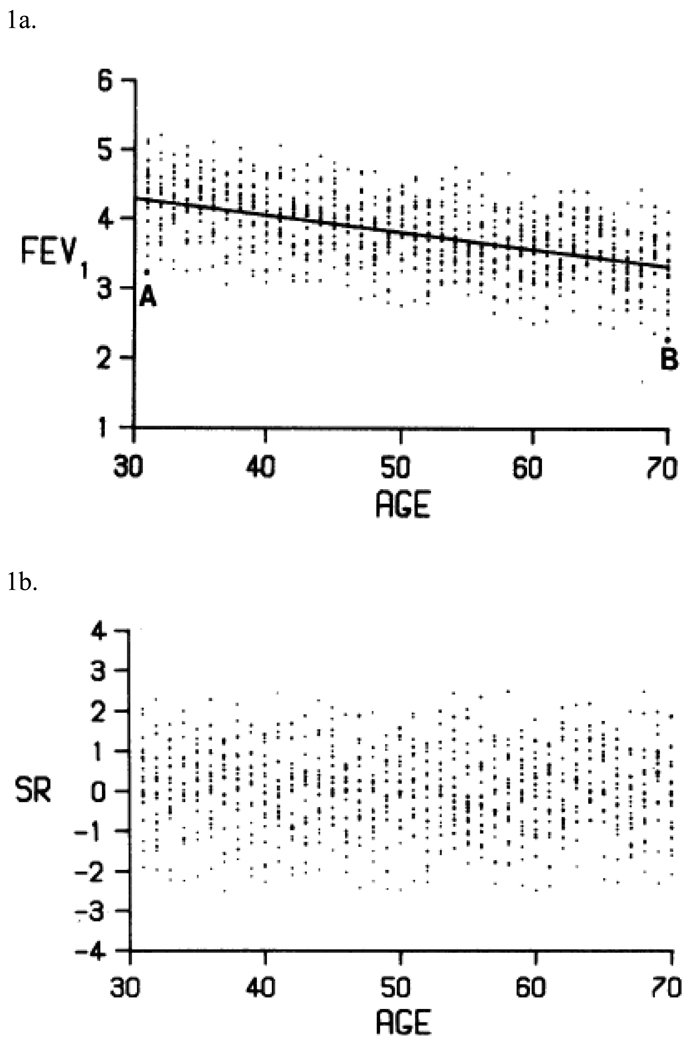
Once a diagnosis has been established, the severity of COPD should next be determined, since it ultimately guides prognosis and therapy.5,6 As shown in Table 1, both GOLD and the ATS/ERS stage COPD severity based on the FEV1, expressed as percent predicted (%Pred), with only modest differences in the recommended cut-points.5,6 The %Pred method, however, is seriously flawed in older persons.15,16 Specifically, GOLD and the ATS/ERS incorrectly assume that a given %Pred cut-point is equivalent for all persons regardless of age, height, sex and ethnicity.15,16 As shown in Figure 1a, a white male of average height has a value for the FEV1 at the lower 95% confidence limit that corresponds to 74%Pred at age 30-years, but only 63%Pred at age 70-years.15 This difference occurs because %Pred assumes that a measured value is proportionate to a predicted value when, in fact, it is proportionate to the spread of the reference data.15,16 More than 20 years ago, Miller and Pincock argued that the use of %Pred for reporting the FEV1 has little to no scientific merit.15 A similar position was adopted by the European Respiratory Society in 1993.16
Figure 1.
Expressing the forced expiratory volume in 1-second (FEV1) as percent predicted (%Pred)* or standardized residual (SR)†
1a. Reference values for FEV1, in men of height 1.7 meters (5 feet 7 inches). Subjects A (a 30-year old) and B (a 70-year old) are on the lower 95% confidence limit, corresponding to 74%Pred and 63%Pred, respectively.‡
1b. Plot of the data from Figure 1a, expressed as SR. As can be seen, SR values remain constant, regardless of age.‡
* (measured / predicted) × 100
† (measured − predicted) / (standard deviation of the residuals); see text.
‡ Reproduced with permission from reference 15.
To address limitations in the %Pred method, the European Respiratory Society and others have proposed that the FEV1 should be expressed as a Z-score, calculated as a standardized residual (SR): [(measured − predicted) / (standard deviation of the residuals)].7,9,15–18 In this equation, the numerator is termed the “residual,” while the denominator is a constant that quantifies the spread of the reference data (i.e., derived from regression equations).7,9,15,16 Based on the SR, a percentile is then computed (SR-tile), with an easy-to-interpret scale of 0–100 (e.g., an SR of −1.64 corresponds to the 5th SR-tile).7,9,15,16 Importantly, in contrast to %Pred, a given SR-tile value is equivalent for all persons, regardless of age, height, sex and ethnicity (as shown in Figure 1b).7,9,15,16
Using the SR approach in a large cohort of elderly whites and non-whites, with clinically validated cut-points for the FEV1 set at the 5th and 10th SR-tiles,7 we have shown that, among participants who had GOLD-diagnosed COPD, more than half were subsequently staged at an FEV1 ≥80%Pred, a level of severity that neither increased the risk of death nor the likelihood of having respiratory symptoms.9 Moreover, among those who had GOLD-diagnosed COPD and were staged at an FEV1 of 50–79%Pred, the risk of death and likelihood of having respiratory symptoms were overestimated in two thirds and one quarter of participants, respectively.9 These results, together with data presented in Figure 1, challenge the clinical validity not only of GOLD-staged COPD in older persons, but also of the ATS/ERS staging strategy, since the latter relies on similar %Pred cut-points (as shown in Table 1). We are currently evaluating whether the validity of the Z-score approach could be improved by using the LMS method rather than standardized residuals.
In light of the inherent, age-related limitations in the spirometric criteria published by GOLD and the ATS/ERS, a strong rationale exists to redefine COPD. Only by establishing and staging disease based on age-appropriate methods and clinically-meaningful thresholds can practice guidelines be effectively implemented. Therefore, in view of its strong mathematical and clinical rationale, we propose that a Z-score methodology be used as a basis for defining COPD, with diagnostic and staging thresholds for the FEV1/FVC and FEV1 linked to important clinical outcomes, including respiratory symptoms, health-care utilization, and mortality. To move this approach forward, LMS-derived Z-scores will need to be calculated for non-whites, and diagnostic and staging thresholds for the FEV1/FVC and FEV1 will require further validation in other cohorts of middle-age and older persons. A similar Z-score approach has been successfully incorporated into clinical practice for the diagnosis of osteopenia and osteoporis.19
In conclusion, among older persons, current spirometric criteria frequently misidentify COPD and misclassify its severity.7–11 This has important implications for practice guidelines, as many older persons may be inappropriately prescribed COPD-specific pharmacotherapies that could result in medication-related adverse events,20 as well as delays in establishing alternative diagnoses. In contrast, we and others have shown that a Z-score methodology provides a more age-appropriate and clinically valid basis for diagnosing and staging COPD.7–10,15–18
Acknowledgments
Funding support: Dr. Fragoso is currently a recipient of a Career Development Award from the Department of Veterans Affairs and the Yale Claude D. Pepper Older Americans Independence Center (P30AG21342) and an R03 award from the National Institute on Aging (R03AG037051). Dr. Gill is the recipient of an NIA Midcareer Investigator Award in Patient-Oriented Research (K24AG021507).
Footnotes
Conflict of Interest: The editor in chief has reviewed the conflict of interest checklist provided by the authors and has determined that the authors have no financial or any other kind of personal conflicts with this paper.
Author Contributions: The editorial reflects the contributions of both authors.
Sponsor’s Role: The authors report no conflicts of interest.
REFERENCES
- 1.Raherison C, Girodet PO. Epidemiology of COPD. Eur Resp Rev. 2009;18:213–221. doi: 10.1183/09059180.00003609. [DOI] [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention. Deaths from chronic obstructive pulmonary disease – United States, 2000–2005. MMWR. 2008;57:1229–1232. [PubMed] [Google Scholar]
- 3.Gooneratne NS, Patel NP, Corcoran A. Chronic obstructive pulmonary disease diagnosis and management in older adults. J Am Geriatr Soc. 2010;58:1153–1162. doi: 10.1111/j.1532-5415.2010.02875.x. [DOI] [PubMed] [Google Scholar]
- 4.Albertson TE, Louie S, Chan AL. The diagnosis and treatment of elderly patients with acute exacerbation of chronic obstructive pulmonary disease and chronic bronchitis. J Am Geriatr Soc. 2010;58:570–579. doi: 10.1111/j.1532-5415.2010.02741.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.GOLD executive summary. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2007;176:532–555. doi: 10.1164/rccm.200703-456SO. [DOI] [PubMed] [Google Scholar]
- 6.Pellegrino R, Viegi G, Brusasco V, et al. Interpretative strategies for lung function tests. Eur Respir J. 2005;26:948–968. doi: 10.1183/09031936.05.00035205. [DOI] [PubMed] [Google Scholar]
- 7.Fragoso CAV, Concato J, McAvay G, et al. Defining chronic obstructive pulmonary disease in older persons. Resp Med. 2009;103:1468–1476. doi: 10.1016/j.rmed.2009.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fragoso CAV, Concato J, McAvay G, et al. The ratio of the forced expiratory volume in 1-second to forced vital capacity in establishing chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2010;181:446–451. doi: 10.1164/rccm.200909-1366OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fragoso CAV, Concato J, McAvay G, et al. Chronic obstructive pulmonary disease in older persons: A comparison of two spirometric definitions. Resp Med. 2010;104:1189–1196. doi: 10.1016/j.rmed.2009.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stanojevic S, Wade A, Stocks J, et al. Reference ranges for spirometry across all ages. Am J Respir Crit Care Med. 2008;177:253–260. doi: 10.1164/rccm.200708-1248OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hansen JE, Sun X-G, Wasserman K. Spirometric criteria for airway obstruction. Chest. 2007;131:349–355. doi: 10.1378/chest.06-1349. [DOI] [PubMed] [Google Scholar]
- 12.Hogg JC. Pathophysiology of airflow limitation in chronic obstructive pulmonary disease. Lancet. 2004;364:709–721. doi: 10.1016/S0140-6736(04)16900-6. [DOI] [PubMed] [Google Scholar]
- 13.Meyer KC. Aging. Proc Am Thorac Soc. 2005;2:433–439. doi: 10.1513/pats.200508-081JS. [DOI] [PubMed] [Google Scholar]
- 14.Celli BR, Halbert RJ, Isonaka S, et al. Population impact of different definitions of airway obstruction. Eur Respir J. 2003;22:268–273. doi: 10.1183/09031936.03.00075102. [DOI] [PubMed] [Google Scholar]
- 15.Miller MR, Pincock AC. Predicted values: how should we use them? Thorax. 1988;43:265–267. doi: 10.1136/thx.43.4.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Official statement of the European Respiratory Society. Lung volumes and forced ventilatory flows. Report working party standardization of lung function tests European community for steel and coal. Eur Respir J. 1993;6(S16):5–40. [PubMed] [Google Scholar]
- 17.Fragoso CAV, Gahbauer E, Van Ness P, et al. Reporting peak expiratory flow in older persons. J Gerontol Med Sci. 2007;62A:1147–1151. doi: 10.1093/gerona/62.10.1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fragoso CAV, Gahbauer E, Van Ness P, Concato J, Gill T. Peak expiratory flow predicts subsequent disability and death in older persons. J Am Geriatr Soc. 2008;56:1014–1020. doi: 10.1111/j.1532-5415.2008.01687.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cummings SR, Bates D, Black DM. Clinical use of bone densitometry: Scientific review. JAMA. 2002;288:1889–1897. doi: 10.1001/jama.288.15.1889. [DOI] [PubMed] [Google Scholar]
- 20.Singh S, Loke YK, Furberg CD. Inhaled anticholinergics and risk of major adverse cardiovascular events in patients with chronic obstructive pulmonary disease: A systematic review and meta-analysis. JAMA. 2008;300:1439–1450. doi: 10.1001/jama.300.12.1439. [DOI] [PubMed] [Google Scholar]