Abstract
Background
Omega-3 fatty acid (n-3 FA) blood levels and intakes have been inversely associated with risk for sudden cardiac death, but their relationship with all-cause mortality is unclear. The purpose of this study was to determine the extent to which baseline blood n-3 FA levels are associated with reduced risk for all-cause mortality in patients with stable CHD.
Methods and Results
The Heart and Soul study utilized a prospective cohort design with a median follow-up of 5.9 years. Patients were recruited between 2000 and 2002 from 12 outpatient facilities in the San Francisco Bay Area. Standard cardiovascular risk factors, demographics, socioeconomic status, health behaviors, and inflammatory markers were collected at baseline. Fasting blood levels of eicosapentaenoic and docosahexaenoic acids (EPA+DHA) were measured and expressed as a percent of total blood FAs. Vital status was assessed with annual telephone interviews and confirmed by review of death certificates. There were 237 deaths among 956 patients. Cox proportional hazards models were used to evaluate the extent to which blood EPA+DHA was independently associated with all cause mortality. Compared with patients having baseline EPA+DHA levels below the median (<3.6%), those at or above the median had a 27% decreased risk of death [Hazard Ratio 0.73, 95% Confidence Interval (CI) 0.56 to 0.94]. This association was unaffected by adjustment for age, sex, ethnicity, center, socioeconomic status, traditional cardiovascular risk factors, and inflammatory markers (HR 0.74, 95% CI 0.55 to 1.00, p<0.05).
Conclusions
In these outpatients with stable CHD, blood n-3 FA levels were inversely associated with total mortality independent of standard and emerging risk factors, suggesting that reduced tissue n-3 FA levels may adversely impact metabolism.
Keywords: coronary heart disease, total mortality, whole blood, eicosapentaenoic acid, docosahexaenoic acid, biomarker
Introduction
Multiple lines of evidence (epidemiological, mechanistic, and clinical trial) support the view that the long-chain omega-3 fatty acids (n-3 FAs) eicosapentaenoic and docosahexaenoic acids (EPA and DHA, respectively) reduce the risk for death from coronary heart disease (CHD)1,2. N-3 FAs are polyunsaturated fats that must be obtained from the diet. Lower red blood cell levels of EPA+DHA have been found in patients with acute coronary syndrome3 and primary cardiac arrest4. In prospective studies, n-3 FA levels were also independently associated with risk for CHD events and CHD mortality5,6. Given this prior research, red blood cell levels of EPA+DHA have been proposed as a marker for cardiovascular health and risk of fatal CHD7.
Two randomized controlled trials of n-3 FA supplementation, one in survivors of acute myocardial infarction8 and the other in patients with heart failure,9 found that treatment significantly reduced all-cause mortality. However, observational studies based on estimated fish or n-3 FA intakes have yielded conflicting results regarding overall mortality10–19, but these were not conducted in patients with established CHD where an n-3 FA deficit would be expected to have a significant impact on health. In addition, the use of diet questionnaires instead of biomarkers to assess n-3 FA exposure may partly explain the failure to observe significant associations in some studies.
In this study, we measured blood levels of EPA+DHA and assessed vital status in a prospective cohort of 956 ethnically-diverse adults with CHD. We hypothesized that higher levels of EPA+DHA would be associated with decreased mortality after adjusting for standard cardiovascular risk factors, demographics, and lifestyle factors.
Methods
Participants
As described previously20, participants were recruited from two Department of Veterans Affairs Medical Centers (San Francisco VA Medical Center and the Veterans Affairs Palo Alto Health Care System), one university medical center [University of California, San Francisco (UCSF)], and nine public health clinics in the Community Health Network of San Francisco. Patients were eligible for the study if they met at least one of the following inclusion criteria: (1) history of myocardial infarction; (2) angiographic evidence of >50% stenosis in 1 or more coronary vessels; (3) evidence of exercise-induced ischemia by treadmill or nuclear testing; or (4) history of coronary revascularization. Participants were excluded if they had had an acute coronary syndrome in the prior 6 months, were not able to walk 1 block, or were likely to move out of the area within 3 years. Between September 2000 and December 2002, a total of 1024 individuals enrolled.
The study protocol was approved by the appropriate institutional review boards, and all participants provided written informed consent. Participants underwent a day-long baseline study appointment that included a medical history interview, a physical examination, and a comprehensive health status questionnaire. Prior to the baseline visit, study participants refrained from smoking for five hours, did not take aspirin for one week, and completed an overnight 12-hour fast (except for prescribed medications taken with water). Fasting venous blood samples were drawn, and serum and whole blood were frozen at −70°C. Participants for whom frozen blood was not available (n=37) were excluded and 5 subjects were lost to follow up. For consistency across types of analyses, all subjects missing on one or more confounding variables were eliminated (n=26) resulting in a sample size of 956 participants for this study. Mortality was similar between the 68 excluded patients and the 956 in the analysis (22% and 25%, respectively; p=0.65). The excluded patients were also similar in age, sex, and race (min. p=0.17). Of note, the excluded patients were more likely to be from a Veterans Affairs Medical Center (p=0.002) because we started our recruitment at the VA and did not have the blood draw protocol up and running for the first 35 patients who enrolled.
Predictors: EPA+DHA level
Levels of EPA+DHA in whole blood were measured by capillary gas chromatography as previously described21 and are expressed as a percent of total blood fatty acids. Two erythrocyte control pools were included with each batch to monitor analytical performance. The intra-assay coefficient of variation in the control sample included in each batch was 5.5% for EPA+DHA.
Outcomes: Vital status
Annual telephone interviews were conducted with participants or their proxies to ask about vital status. All reported deaths were confirmed by review of death certificates, which determined event date for analyses. Censoring was defined as time to death or last follow-up.
Other Variables
Age, sex, ethnicity, medical history, household income, education level and history of tobacco use were collected by self-report on baseline questionnaires. Household income and education level were included as measures of socioeconomic status, which affects dietary intake of n-3 FAs21. Medical centers were grouped as Veterans Affairs, university medical center, or health clinic. Categories for ethnicity were Hispanic, Asian, White, African American, or Other. Annual household income was grouped as <$10K, $10K to <$20K, $20K to <$40K, and over $40K. Education level was divided into 4 categories: <12th grade, high school graduate or equivalent, some college, or completing a 4-year degree or higher. Body mass index was calculated from height and weight at the baseline visit. During their baseline visit participants were asked how physically active they had been the previous month. Participants selected “not at all”, “a little”, “fairly”, “quite”, “very” or “extremely” active; “fairly and quite” were grouped together as were “very or extremely”. All current medications were collected by inspection of prescriptions brought to the baseline visit. Fasting serum samples were used to measure total cholesterol, high-density lipoprotein cholesterol, triglycerides, high sensitivity C-reactive protein, interleukin-6, and tumor necrosis factor-alpha as previously reported22. All patients underwent complete resting 2-dimensional echocardiography and Doppler examination using an Acuson Sequoia ultrasound system (Siemens Medical Solutions) with a 3.5-MHz transducer. Standard parasternal short-axis and apical 2- and 4-chamber views were obtained and planimetered to determine end-diastolic and end-systolic volumes. The left ventricular ejection fraction (LVEF) was calculated as (end diastolic volume - end systolic volume)/end diastolic volume.
Statistical Analyses
Differences in baseline characteristics were evaluated using Wilcoxon rank-sum test and Chi-square test for continuous and categorical data, respectively. To test the association between blood EPA+DHA levels and all-cause mortality, Kaplan-Meier and Cox proportional hazards regression models were developed. Unadjusted differences in Kaplan-Meier estimated survival functions were tested using the log-rank statistic. Log transformed confidence intervals were calculated for the 75th survival percentile using the method developed by Brookmeyer and Crowley23. The unadjusted model was followed by the sequential addition of potential confounders, including demographics, socioeconomic status, smoking, LVEF, history of hypertension, diabetes, BMI, statin and aspirin use, and physical activity. The final model involved the addition of potential mediators, including lipids and inflammatory markers.
Nonlinear relationships between confounding variables and outcome were examined using Martingale residuals which estimate the excess number of events over time in a Cox model, and can reveal the correct functional form. Linear and logarithmic forms of the continuous covariates were compared24,25. Quadratic relations were inspected by testing the higher order term of the covariates. This resulted in CRP, triglycerides, and total cholesterol being natural log transformed, while a quadratic term was added to age. Standardized score residuals confirmed the proportional hazards assumption. Deviance residuals were used to identify outliers, and their chromatographs were verified for EPA and DHA levels.
Lastly to evaluate possible confounding by other fatty acids the following fatty acids were substituted for EPA+DHA in a univariate Cox model:C18:1 trans, C18:2n6 trans, linoleic acid, arachidonic acid, alpha linolenic acid, docosapenteonoic acid, EPA and DHA individually). If a possible confounding fatty acid had a significant univariate relationship, then it was examined in the sequentially-adjusted models described above. A p-value < 0.05 was used to ascribe statistical significance. Analyses were performed using SAS software (version 9.2; SAS Institute Inc., Cary, NC).
Results
The final cohort of 956 patients was comprised of 396 from VA Medical Centers, 232 from public health clinics and 328 from the UCSF Medical Center. Median follow-up was 5.9 years (inter-quartile range, 4.5–6.0 years) during which 237 (25%) participants died. The whole blood EPA+DHA was greater in those who survived than in those who did not (geometric means 3.8% vs. 3.6%, p=0.05). To evaluate a dose response in EPA+DHA, the unadjusted death rates were examined by quartiles. There was no significant difference between quartile 1 (26.4%) and quartile 2 (29.7%) of EPA+DHA (p=0.42, 2-proportion Z-test), nor between quartile 3 (20.1%) and quartile 4 (22.9%), p=0.45. In other words, death rates exhibited a step function, not a gradient, therefore, the two lower and upper quartiles were each combined (difference p=0.02). All 956 participants were therefore grouped by the median EPA+DHA level (at or above vs. below 3.6%; Table 1). Compared with participants below the median, those at or above the median were older, less likely to be African American or Hispanic, and more likely to be Asian and of higher socioeconomic status. Participants above the median also had a more favorable cardiovascular risk profile (e.g., higher HDL cholesterol and lower total cholesterol, triglyceride, C-reactive protein and IL-6 levels; a lower prevalence of smoking and diabetes; and were more likely to be very or extremely physically active).
Table 1.
Characteristics of coronary artery disease patients (N=956) grouped by median blood EPA+DHA values (as % total fatty acids)
| Below Median < 3.6% (n = 478) |
Above Median ≥ 3.6% (n = 478) |
P-value* | |
|---|---|---|---|
| DEMOGRAPHICS | |||
| Age (years) | 65 (57, 73) | 69 (61, 77) | <0.0001 |
| Sex male | 389 (81) | 391 (82) | 0.87 |
| Ethnicity | <0.0001 | ||
| Hispanic | 62 (13) | 21 (4.4) | |
| White | 280 (59) | 298 (62) | |
| African American | 95 (20) | 57 (12) | |
| Asian | 25 (5.2) | 86 (18) | |
| Other | 16 (3.3) | 16 (3.3) | |
| Medical Center | <0.0001 | ||
| Veterans Affairs | 215 (45) | 181 (38) | |
| University | 116 (24) | 212 (44) | |
| Public Health Clinics | 147 (31) | 85 (18) | |
| SOCIOECONOMIC STATUS | |||
| Education level | <0.0001 | ||
| < 12th grade | 82 (17) | 45 (9.4) | |
| H.S. diploma or equiv. | 103 (22) | 68 (14) | |
| Some College | 174 (36) | 152 (32) | |
| B.S. or higher degree | 119 (25) | 213 (45) | |
| Household income | <0.0001 | ||
| Under $10,000 | 147 (31) | 89 (19) | |
| $10,000 to < $20,000 | 122 (26) | 103 (22) | |
| $20,000 to < $40,000 | 106 (22) | 115 (24) | |
| Over $40,000 | 103 (22) | 171 (36) | |
| CARDIOVASCULAR RISK FACTORS | |||
| Smoking Status | <0.0001 | ||
| Never | 113 (24) | 173 (36) | |
| Past | 229 (48) | 253 (53) | |
| Current | 136 (29) | 52 (11) | |
| Left Ventricular Ejection Fraction,% | 63 (57, 67) | 64 (59, 68) | 0.004 |
| Diabetes | 153 (32) | 101 (21) | 0.0001 |
| History of Hypertension | 361 (75.5) | 318 (67) | 0.002 |
| Physically activity | 0.01 | ||
| Not at all | 98 (21) | 86 (18) | |
| A little | 98 (21) | 75 (16) | |
| Fairly or Quite | 148 (31) | 137 (29) | |
| Very or Extremely | 134 (28) | 180 (38) | |
| Body mass index (kg/m2) | 28 (25, 32) | 27 (25, 30) | 0.07 |
| DRUGS | |||
| Statin use | 293 (61) | 325 (68) | 0.03 |
| Aspirin use | 374 (78) | 371 (78) | 0.82 |
| LIPIDS | |||
| EPA+DHA (% of total FA) | 2.8 (2.3, 3.2) | 4.9 (4.1, 6.6) | <0.0001 |
| HDL cholesterol (mmol/L§) | 1.1 (0.9, 1.3) | 1.2 (1.0, 1.4) | 0.0002 |
| Total cholesterol (mmol/L§) | 4.6 (4.0, 5.4) | 4.3 (3.8, 5.0) | 0.0004 |
| Triglyceride (mmol/L§) | 1.4 (0.9, 2.2) | 1.1 (0.8, 1.5) | <0.0001 |
| INFLAMMATORY MARKERS/ASPIRIN | |||
| C-Reactive Protein (mg/L) | 2.8 (1.2, 6.1) | 1.8 (0.7, 3.7) | <0.0001 |
| IL-6 (pg/mL) | 2.9 (1.9, 4.8) | 2.2 (1.4, 3.7) | <0.0001 |
| TNF-α (pg/mL) | 3.8 (2.5, 6.0) | 3.7 (2.6, 5.3) | 0.40 |
Wilcoxon rank-sum test for continuous variables, Chi-square test for categorical variables;
Median (Inter-quartile range);
n (%);
for cholesterol convert from mmol/L to mg/dL by multiplying times 38.6; for triglycerides, by 88.5.
The hazard ratio for those at or above the median level (adjusted for age, sex, ethnicity, and medical center) was reduced by 39% for all causes deaths (p<0.0001, Table 2, Model 1). After further adjustment for potential confounders and mediators the relative risk reduction ranged from 26–31%, and the effect remained significant (p<0.05) throughout all covariate models (Table 2, Models 2–4). In the fully adjusted model and independent of EPA+DHA, other highly significant relations with total mortality were observed for increasing age and IL-6 (directly) and higher LVEF and statin use (inversely; Table 3). Using median blood EPA+DHA categories, Kaplan-Meier estimated survival curves demonstrated a significant difference in time to death (Figure; Logrank p=0.02). Time to 25% mortality was 5.1 years (95% CI: 4.7 to 6.2) and 6.3 years (95% CI: 5.9 to 6.9) in those participants whose blood EPA+DHA were below and at or above the median, respectively.
Table 2.
Hazard ratios of blood EPA+DHA with all cause mortality (N=956)
| Hazard Ratio (95% CI) | |
|---|---|
| Median groups ≥ 3.6% vs. <3.6% | |
| Unadjusted | 0.73 (0.56 to 0.94) |
| Adjusted | |
| Model 1 | 0.61 (0.46 to 0.80) |
| Model 2 | 0.69 (0.52 to 0.93) |
| Model 3 | 0.70 (0.52 to 0.94) |
| Model 4 | 0.74 (0.55 to 1.00) |
Model 1 - age, age2, sex, ethnicity, medical center
Model 2 - model 1 + SES [education, income], smoking, LVEF, DM, physical activity, BMI, History of Hypertension
Model 3 - model 2 + stain use, lipids [HDL, Ln(total cholesterol), Ln(triglyceride)]
Model 4 - model 3 + aspirin use, inflammatory [Ln(CRP), IL6, TNF alpha]
Table 3.
Fully adjusted hazard ratios between baseline variables and all causes mortality (N=956)*
| Variable | Hazard Ratio (95% CI) | P-value |
|---|---|---|
| Blood EPA+DHA above vs. below 3.6% | 0.74 (0.55 to 1.00) | 0.049 |
| Age (mean = 67 years; 5 years older) | 1.31 (1.22 to 1.41) | 0.0003 |
| Diabetes Mellitus | 1.48 (1.09 to 2.03) | 0.01 |
| Education | 0.04 | |
| Less than 12th grade vs. High School (HS) diploma | 0.54 (0.34 to 0.85) | |
| Some college or trade school vs. HS diploma | 0.91 (0.63 to 1.31) | |
| Bachelor or higher college degree vs. HS diploma | 0.77 (0.52 to 1.14) | |
| Physically Active | 0.01 | |
| A little vs. Not at all | 0.77 (0.51 to 1.17) | |
| Fairly or Quite vs. Not at all | 0.68 (0.47 to 0.98) | |
| Very or Extremely vs. Not at all | 0.53 (0.36 to 0.78) | |
| BMI per 1-unit increase (kg/m2) | 0.95 (0.92 to 0.98) | 0.002 |
| Left Ventricular Ejection Fraction per 1% increase | 0.97 (0.96 to 0.98) | <0.0001 |
| Use of Statin Medication | 0.59 (0.44 to 0.80) | 0.0007 |
| Interleukin-6 per 1-unit increase (pg/mL) | 1.11 (1.05 to 1.17) | <0.0001 |
| Tumor necrosis factor-α per 1-unit increase (pg/mL) | 1.02 (1.00 to 1.05) | 0.03 |
| Sex male | 1.47 (0.91 to 2.38) | 0.11 |
| Ethnicity | 0.88 | |
| Hispanic vs. White | 0.79 (0.44 to 1.40) | |
| Asian vs. White | 0.83 (0.51 to 1.35) | |
| African American vs. White | 1.00 (0.66 to 1.52) | |
| Other vs. White | 0.99 (0.49 to 2.03) | |
| Medical Center | 0.19 | |
| Clinics vs. VA Medical Center | 1.41 (0.95 to 2.09) | |
| University vs. VA Medical Center | 1.25 (0.88 to 1.78) | |
| Household Income | 0.50 | |
| $10K to $20K vs. under $10K | 1.06 (0.72 to 1.57) | |
| $20K to $40K vs. under $10K | 0.90 (0.60 to 1.34) | |
| Over $40K vs. under $10K | 0.77 (0.49 to 1.21) | |
| Smoking Status | 0.12 | |
| Past vs. Never | 1.29 (0.93 to 1.80) | |
| Current vs. Never | 1.58 (1.00 to 2.49) | |
| History of Hypertension | 1.03 (0.76 to 1.41) | 0.84 |
| HDL cholesterol (mmol/L) | 1.01 (0.99 to 1.02) | 0.41 |
| Loge(Total cholesterol, mmol/L) | 0.52 (0.23 to 1.14) | 0.10 |
| Loge(Triglyceride, mmol/L) | 1.20 (0.89 to 1.60) | 0.23 |
| Aspirin use | 1.04 (0.74 to 1.45) | 0.82 |
| Loge(C-Reactive Protein, mg/L) | 1.07 (0.94 to 1.22) | 0.29 |
Figure.
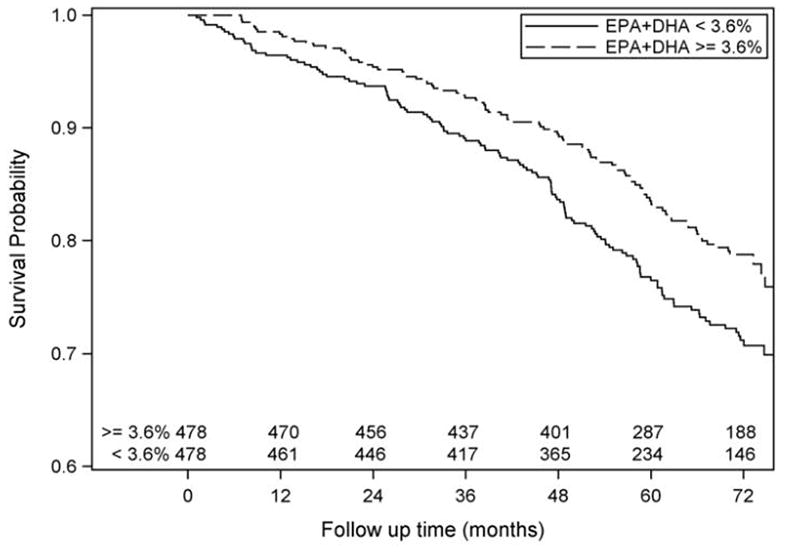
Kaplan-Meier estimated survival functions for at or above vs below the median value (3.6%) of whole blood EPA+DHA. The number of subjects at risk is shown for each category by 12-month intervals. Logrank p=0.02.
To determine if other FAs could be influencing the differences in mortality associated with levels of EPA+DHA, we substituted several other fatty acids for EPA+DHA in a univariate Cox model. EPA and DHA individually had significant inverse associations with all-causes mortality; additionally C18:1 trans and C18:2n6 trans had significant direct associations (Table 4). Each of the individual fatty acids remained significant when adjusted for demographics included in Model 1. Only EPA was significant after further adjustment for LVEF, risk markers and socioeconomic factors found in Model 2. However, the effect of EPA was attenuated when lipids from Model 3 were included. Both trans fats (the only other fatty acids that showed univariate relations with total mortality) and their interaction terms with the median EPA+DHA were included in the final Model 4 to test for moderating relationships. The median level of EPA+DHA (at or above vs. below 3.6%) remained significant (p = 0.04), but the trans fats did not (min p = 0.22).
Table 4.
Univariate Analysis of Individual Fatty Acids (as % of total fatty acids) with All-Causes Mortality. Estimates (95% CI) are per a 1% absolute increase.
| Fatty Acid | (n = 719) Lived Mean (SD) |
(n = 237) Died Mean (SD) |
Unadjusted Cox PH Regression Estimate (95% CI) |
|---|---|---|---|
| C18:1 trans – Elaidic | 1.33(0.60) | 1.51(0.71) | 1.38 (1.15, 1.65) |
| C18:2n6 trans – | 0.69(0.26) | 0.76(0.30) | 1.69 (1.18, 2.43) |
| C18:2n6 – Linoleic (LA) | 22.96(3.61) | 23.22(3.59) | 1.02 (0.98, 1.06) |
| C20:4n6 – Arachidonic (AA) | 11.83(2.28) | 11.57(2.33) | 0.95 (0.90, 1.01) |
| C18:3n3 – alpha Linolenic (ALA) | 0.50(0.22) | 0.49(0.25) | 0.90 (0.51, 1.58) |
| C20:5n3 – Eicosapentaenoic (EPA) | 0.93(0.88) | 0.80(0.76) | 0.81 (0.66, 0.98) |
| C22:5n3 – Docosapenteonoic (DPA) | 1.24(0.29) | 1.22(0.29) | 0.72 (0.46, 1.13) |
| C22:6n3 – Docosahexaenoic (DHA) | 3.30(1.36) | 3.13(1.23) | 0.91 (0.82, 1.00) |
Discussion
In patients with stable CHD, we found that higher baseline blood levels of EPA+DHA were associated with an increase in survival time. These findings were independent of traditional cardiovascular risk factors, serum lipids, and of inflammatory markers, and they harmonize with our recent observation that, in this same cohort, the rate of telomeric shortening (a surrogate for cellular aging) was inversely and independently related to blood EPA+DHA levels26.
Although it was not the purpose of this study to formally evaluate the prognostic value of blood n-3 fatty acid levels with respect to total mortality, we were able to gain some insights that may be used in future studies with that goal. Based on a review of the literature, an RBC EPA+DHA level of 4% had originally been proposed7,27, as segregating intermediate from high-risk patients. This value found some support in a subsequent cross-sectional analysis3. In the current study RBCs were not available, only whole blood. Fortunately, with our method there is a very strong correlation (R=0.96, p<0.0001; unpublished findings) between the EPA+DHA content of RBCs and whole blood, and both metrics have been associated with risk of CHD events, including primary cardiac arrest4 and sudden cardiac death6. Converting the median whole blood EPA+DHA value observed in our study of 3.6% to the RBC-based equivalent gave an omega-3 index median value of 4.6%, not far from the originally proposed 4%. Although these results suggest that there may be a threshold above which little additional risk reduction may be expected, a very low risk value (previously proposed as 8%7) could not be evaluated here owing to the very few patients who had levels this high.
The relations between the intake of long-chain n-3 FA and all-cause mortality (as opposed to cardiovascular) have been described in many different study populations, but are inconsistent. Based on estimated fish or n-3 consumption, 4 studies found significant inverse associations with mortality10–13, whereas 8 studies did not10,11,14–19. A 5-year follow-up of 415 Swedish CHD patients28 reported a trend (p=0.06) for reduced total mortality associated with increasing fish intake, but no relations with CHD mortality (p=0.73). Positive findings on total mortality and n-3 FAs from prospective cohort studies from China29, Japan11 and Korea30 (regions where CHD is not the major cause of death), harmonize with our findings. In a recent report from Norway, elderly, hospitalized patients (mean age 82 years) with serum phospholipid EPA levels in the lowest quartile were at twice the risk for death over 3 years compared to those subjects in the 3 higher quartiles31. Higher RBC EPA and DHA levels in centenarians compared to elderly (61–99 years of age) controls32 also supports the beneficial effect of higher omega-3 levels on total mortality. Anderson et al. reported that reduced plasma EPA levels were associated with greater 20-year mortality in a mixed ethnic population in London33. Finally, in a meta-analysis of the effects of dietary and pharmacologic treatments for dyslipidemia, total mortality was reduced by only two agents: omega-3 fatty acids and statins34.
Some of the conflicting findings from other prospective cohort studies may stem from the use of dietary questionnaires, which may not provide accurate estimates of n-3 FA exposure35. Even if intakes could be precisely determined, direct measurement of blood levels of EPA+DHA provides information on inter-individual differences in FA metabolism that diet assessments cannot capture. We recently reported that non-fried fish consumption explained only 37% of the variability in the omega-3 index36, and when combined with information on fish oil supplementation, only 47% of the variation was explained37. In addition to dietary intake, it has been suggested that genetic factors may play a role in establishing blood EPA and DHA content. One single nucleotide polymorphism (rs3834458) in the promoter region of the gene encoding delta-6 desaturase (the enzyme that controls the conversion of alpha-linolenic acid to EPA) is associated with lower plasma and adipose EPA levels38. This polymorphism explains about 5% of the variability in plasma phospholipid EPA levels39. Since only about half of the variability in the blood n-3 FA levels can be explained by known factors, the estimation of n-3 FA exposure is best performed by direct blood measurement.
Increased n-3 FA levels may decrease mortality through a variety of mechanisms. In prior studies, increased intakes of n-3 FAs have been reported to reduce heart rate40, inhibit platelet function41, and at high doses, to reduce serum triglyceride2,42 and inflammatory marker levels43. These factors may contribute to an observed increase in plaque stability44 and to a reduced risk for arrhythmias45 and death from heart failure9. These FAs also modulate a wide variety of fundamental physiological functions. At the cellular level, they affect cell signaling, alter membrane composition46, and modulate the behavior of ion channels. This can lead to changes in several biologic systems, for example, alterations in immune function via interleukin signaling47 and prevention of apoptosis48. N-3 FAs are also converted into a variety of eicosanoids, such as prostaglandins, leukotrienes, prostacyclins, thromboxanes, and neuroprotectins. These substances, in turn, mediate inflammation, vasoconstriction, and platelet aggregation49. In our model 4, adjustment for inflammatory biomarkers attenuated (but did not eliminate) the association between EPA+DHA levels and total mortality, suggesting the beneficial effects of n-3 fatty acids may be partially mediated by a reduction in systemic inflammation50.
Our findings suggest that testing for blood EPA+DHA levels may have clinical utility. Unlike several other risk factors which cannot be easily modified, the level of EPA+DHA in the blood can be raised by increased consumption of these fatty acids in either oily fish (salmon, mackerel, herring, sardines, albacore tuna, etc.) or in fish oil supplements. Both approaches are safe and inexpensive, and importantly, have been shown to reduce total mortality in randomized trials8,9,51.
There are several strengths of this study, including a large and rigorously-defined sample, detailed FA analysis, the inclusion of plasma lipid and inflammatory covariates, a racially diverse sample, and an unambiguous endpoint. There were also several limitations. This was an observational study, and thus unmeasured confounding factors may have contributed to our findings. The cohort was mostly older males, all with known CHD, and thus our results may not be generalizable to younger or female patients, or those without CHD. In addition, we measured blood levels of n-3 FAs at one point in time, and levels may have fluctuated during the follow-up period. Finally, without detailed dietary analyses, we were unable to determine the extent to which low n-3 FA intakes (versus differences in metabolism) were the cause of the low EPA+DHA in those at highest risk for death, although as noted above, only about half of the variability in this marker can be explained by dietary differences.
Conclusion
A reduced blood EPA+DHA level is independently related to risk for death in patients with CHD. These findings suggest that EPA+DHA tissue levels may be playing an optimizing role in normal cellular metabolism which, if not provided, leads to premature death. Further studies are needed in larger and more diverse populations to more clearly define the utility of blood EPA+DHA in risk prediction and to determine risk threshold levels over a wider range of values.
Acknowledgments
Non-authors who have made substantial contributions to this work include Brenda Collison-Schmidt, BA and Andrew Christianson, BS, both from Sanford Research/USD, for conducting the blood fatty acid analysis. They received no additional compensation for this work.
Funding Sources: The Heart and Soul study was funded by the VA Epidemiology (Merit Review) Program; the VA Health Services Research and Development service Career Development Program; the National Heart Lung and Blood Institute (R01 HL079235); the American Federation for Aging Research (Paul Beeson Scholars Program); the Robert Wood Johnson Foundation (Generalist Physician Faculty Scholars Program); the Ischemia Research and Education Foundation; and the Nancy Kirwan Heart Research Fund. The funding organizations had no role in the design or conduct of the study; collection, management, analysis, or interpretation of the data; or preparation, review, or approval of the manuscript.
Footnotes
Conflict of Interest Disclosures: Harris: WSH is a scientific advisor to the following companies with interests in n-3 FAs: Monsanto Company, GlaxoSmithKline, Acasti Pharma, Neptune, and Unilever. He is also the founder and owner of OmegaQuant, LLC, a company that offers blood fatty acid testing.
Reference List
- 1.Wang C, Harris WS, Chung M, Lichtenstein AH, Balk EM, Kupelnick B, Jordan HS, Lau J. n-3 Fatty acids from fish or fish-oil supplements, but not {alpha}-linolenic acid, benefit cardiovascular disease outcomes in primary- and secondary-prevention studies: a systematic review. Am J Clin Nutr. 2006;84:5–17. doi: 10.1093/ajcn/84.1.5. [DOI] [PubMed] [Google Scholar]
- 2.Harris WS, Miller M, Tighe AP, Davidson MH, Schaefer EJ. Omega-3 fatty acids and coronary heart disease risk: clinical and mechanistic perspectives. Atherosclerosis. 2008;197:12–24. doi: 10.1016/j.atherosclerosis.2007.11.008. [DOI] [PubMed] [Google Scholar]
- 3.Block RC, Harris WS, Reid KJ, Sands SA, Spertus JA. EPA and DHA in Blood Cell Membranes from Acute Coronary Syndrome Patients and Controls. Atherosclerosis. 2007;197:821–828. doi: 10.1016/j.atherosclerosis.2007.07.042. [DOI] [PubMed] [Google Scholar]
- 4.Siscovick DS, Raghunathan TE, King I, Weinmann S, Wicklund KG, Albright J, Bovbjerg V, Arbogast P, Smith H, Kushi LH, Cobb LA, Copass MK, Psaty BM, Lemaitre R, Retzlaff B, Childs M, Knopp RH. Dietary intake and cell membrane levels of long-chain n-3 polyunsaturated fatty acids and the risk of primary cardiac arrest. J Am Med Assoc. 1995;274:1363–1367. doi: 10.1001/jama.1995.03530170043030. [DOI] [PubMed] [Google Scholar]
- 5.Harris WS, Poston WC, Haddock CK. Tissue n-3 and n-6 fatty acids and risk for coronary heart disease events. Atherosclerosis. 2007;193:1–10. doi: 10.1016/j.atherosclerosis.2007.03.018. [DOI] [PubMed] [Google Scholar]
- 6.Albert CM, Campos H, Stampfer MJ, Ridker PM, Manson JE, Willett WC, Ma J. Blood levels of long-chain n-3 fatty acids and the risk of sudden death. N Engl J Med. 2002;346:1113–1118. doi: 10.1056/NEJMoa012918. [DOI] [PubMed] [Google Scholar]
- 7.Harris WS, von Schacky C. The Omega-3 Index: a new risk factor for death from coronary heart disease? Prev Med. 2004;39:212–220. doi: 10.1016/j.ypmed.2004.02.030. [DOI] [PubMed] [Google Scholar]
- 8.Marchioli R, Barzi F, Bomba E, Chieffo C, Di Gregorio D, Di Mascio R, Franzosi MG, Geraci E, Levantesi G, Maggioni AP, Mantini L, Marfisi RM, Mastrogiuseppe G, Mininni N, Nicolosi GL, Santini M, Schweiger C, Tavazzi L, Tognoni G, Tucci C, Valagussa F. Early protection against sudden death by n-3 polyunsaturated fatty acids after myocardial infarction: time-course analysis of the results of the Gruppo Italiano per lo Studio della Sopravvivenza nell’Infarto Miocardico (GISSI)-Prevenzione. Circulation. 2002;105:1897–1903. doi: 10.1161/01.cir.0000014682.14181.f2. [DOI] [PubMed] [Google Scholar]
- 9.GISSI-HF investigators. Effect of n-3 polyunsaturated fatty acids in patients with chronic heart failure (the GISSI-HF trial): a randomised, double-blind, placebo-controlled trial. Lancet. 2008;372:1223–1230. doi: 10.1016/S0140-6736(08)61239-8. [DOI] [PubMed] [Google Scholar]
- 10.Folsom AR, Demissie Z. Fish intake, marine omega-3 fatty acids, and mortality in a cohort of postmenopausal women. Am J Epidemiol. 2004;160:1005–1010. doi: 10.1093/aje/kwh307. [DOI] [PubMed] [Google Scholar]
- 11.Nagata C, Takatsuka N, Shimizu H. Soy and fish oil intake and mortality in a Japanese community. Am J Epidemiol. 2002;156:824–831. doi: 10.1093/aje/kwf118. [DOI] [PubMed] [Google Scholar]
- 12.Hu FB, Cho E, Rexrode KM, Albert CM, Manson JE. Fish and long-chain omega-3 fatty acid intake and risk of coronary heart disease and total mortality in diabetic women. Circulation. 2003;107:1852–1857. doi: 10.1161/01.CIR.0000062644.42133.5F. [DOI] [PubMed] [Google Scholar]
- 13.Dolecek TA. Epidemiological Evidence of Relationships between Dietary Polyunsaturated Fatty Acids and Mortality in the Multiple Risk Factor Intervention Trial. Proc Soc Exper Bio Med. 1992;200:177–182. doi: 10.3181/00379727-200-43413. [DOI] [PubMed] [Google Scholar]
- 14.Fraser GE, Shavlik DJ. Risk factors for all-cause and coronary heart disease mortality in the oldest-old. The Adventist Health Study. Arch Intern Med. 1997;157:2249–2258. [PubMed] [Google Scholar]
- 15.Mann JI, Appleby PN, Key TJ, Thorogood M. Dietary determinants of ischaemic heart disease in health conscious individuals. Heart. 1997;78:450–455. doi: 10.1136/hrt.78.5.450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nakamura Y, Ueshima H, Okamura T, Kadowaki T, Hayakawa T, Kita Y, Tamaki S, Okayama A. Association between fish consumption and all-cause and cause-specific mortality in Japan: NIPPON DATA80, 1980–99. Am J Med. 2005;118:239–245. doi: 10.1016/j.amjmed.2004.12.016. [DOI] [PubMed] [Google Scholar]
- 17.Osler M, Andreasen AH, Hoidrup S. No inverse association between fish consumption and risk of death from all-causes, and incidence of coronary heart disease in middle-aged, Danish adults. J Clin Epidemiol. 2003;56:274–279. doi: 10.1016/s0895-4356(02)00600-5. [DOI] [PubMed] [Google Scholar]
- 18.Daviglus ML, Stamler J, Orencia AJ, Dyer AR, Liu K, Greenland P, Walsh MK, Morris D, Shekelle RB. Fish consumption and the 30-year risk of fatal myocardial infarction. N Eng J Med. 1997;336:1046–1053. doi: 10.1056/NEJM199704103361502. [DOI] [PubMed] [Google Scholar]
- 19.Kromhout D, Bosschieter EB, de Lezenne Coulander C. The inverse relation between fish consumption and 20-year mortality from coronary heart disease. N Engl J Med. 1985;312:1205–1209. doi: 10.1056/NEJM198505093121901. [DOI] [PubMed] [Google Scholar]
- 20.Whooley MA, de Jonge P, Vittinghoff E, Otte C, Moos R, Carney RM, Ali S, Dowray S, Na B, Feldman MD, Schiller NB, Browner WS. Depressive symptoms, health behaviors, and risk of cardiovascular events in patients with coronary heart disease. JAMA. 2008;300:2379–2388. doi: 10.1001/jama.2008.711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cohen BE, Garg SK, Ali S, Harris WS, Whooley MA. Red blood cell docosahexaenoic acid and eicosapentaenoic acid concentrations are positively associated with socioeconomic status in patients with established coronary artery disease: data from the heart and soul study. J Nutr. 2008;138:1135–1140. doi: 10.1093/jn/138.6.1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Marcus GM, Whooley MA, Glidden DV, Pawlikowska L, Zaroff JG, Olgin JE. Interleukin-6 and atrial fibrillation in patients with coronary artery disease: data from the Heart and Soul Study. Am Heart J. 2008;155:303–309. doi: 10.1016/j.ahj.2007.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brookmeyer R, Crowley JP. A Confidence Interval for the Median Survival Time. Biometrics. 1982;38:29–41. [Google Scholar]
- 24.Therneau TM, Grambsch PM, Fleming TR. Martingale-based residuals for survival models. Biometrika. 1990;77:147–160. [Google Scholar]
- 25.Lin DY, Wei LJ, Ying Z. Checking the Cox model with cumulative sums of martingale-based residuals. Biometrika. 1993;80:557–572. [Google Scholar]
- 26.Farzaneh-Far R, Lin J, Epel ES, Harris WS, Blackburn EH, Whooley MA. Association of marine omega-3 fatty acid levels with telomeric aging in patients with coronary heart disease. JAMA. 2010;303:250–257. doi: 10.1001/jama.2009.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Harris WS. The omega-3 index as a risk factor for coronary heart disease. Am J Clin Nutr. 2008;87:1997S–2002S. doi: 10.1093/ajcn/87.6.1997S. [DOI] [PubMed] [Google Scholar]
- 28.Erkkila AT, Lehto S, Pyorala K, Uusitupa MI. n-3 Fatty acids and 5-y risks of death and cardiovascular disease events in patients with coronary artery disease. Am J Clin Nutr. 2003;78:65–71. doi: 10.1093/ajcn/78.1.65. [DOI] [PubMed] [Google Scholar]
- 29.Yuan JM, Ross RK, Gao YT, Yu MC. Fish and shellfish consumption in relation to death from myocardial infarction among men in Shanghai, China. Am J Epidemiol. 2001;154:809–816. doi: 10.1093/aje/154.9.809. [DOI] [PubMed] [Google Scholar]
- 30.Lee SH, Shin MJ, Kim JS, Ko YG, Kang SM, Choi D, Jang Y, Chung N, Shim WH, Cho SY, Manabe I, Ha JW. Blood eicosapentaenoic acid and docosahexaenoic acid as predictors of all-cause mortality in patients with acute myocardial infarction--data from Infarction Prognosis Study (IPS) Registry. Circ J. 2009;73:2250–2257. doi: 10.1253/circj.cj-09-0327. [DOI] [PubMed] [Google Scholar]
- 31.Lindberg M, Saltvedt I, Sletvold O, Bjerve KS. Long-chain n-3 fatty acids and mortality in elderly patients. Am J Clin Nutr. 2008;88:722–729. doi: 10.1093/ajcn/88.3.722. [DOI] [PubMed] [Google Scholar]
- 32.Rabini RA, Moretti N, Staffolani R, Salvolini E, Nanetti L, Franceschi C, Mazzanti L. Reduced susceptibility to peroxidation of erythrocyte plasma membranes from centenarians. Exp Gerontol. 2002;37:657–663. doi: 10.1016/s0531-5565(02)00006-2. [DOI] [PubMed] [Google Scholar]
- 33.Anderson SG, Sanders TAB, Cruickshank JK. Plasma fatty acid composition as a predictor of arterial stiffness and mortality. Hypertension. 2009;53:839–845. doi: 10.1161/HYPERTENSIONAHA.108.123885. [DOI] [PubMed] [Google Scholar]
- 34.Studer M, Briel M, Leimenstoll B, Glass TR, Bucher HC. Effect of different antilipidemic agents and diets on mortality: a systematic review. Arch Intern Med. 2005;165:725–730. doi: 10.1001/archinte.165.7.725. [DOI] [PubMed] [Google Scholar]
- 35.Briefel RR, Sempos CT, McDowell MA, Chien S, Alaimo K. Dietary methods research in the third National Health and Nutrition Examination Survey: underreporting of energy intake. Am J Clin Nutr. 1997;65:1203S–1209S. doi: 10.1093/ajcn/65.4.1203S. [DOI] [PubMed] [Google Scholar]
- 36.Sands SA, Reid KJ, Windsor SL, Harris WS. The impact of age, body mass index, and fish intake on the EPA and DHA content of human erythrocytes. Lipids. 2005;40:343–347. doi: 10.1007/s11745-006-1392-2. [DOI] [PubMed] [Google Scholar]
- 37.Block RC, Harris WS, Pottala JV. Determinants of Blood Cell Omega-3 Fatty Acid Content. Open Biomarkers J. 2008;1:1–6. doi: 10.2174/1875318300801010001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Baylin A, Ruiz-Narvaez E, Kraft P, Campos H. alpha-Linolenic acid, Delta6-desaturase gene polymorphism, and the risk of nonfatal myocardial infarction. Am J Clin Nutr. 2007;85:554–560. doi: 10.1093/ajcn/85.2.554. [DOI] [PubMed] [Google Scholar]
- 39.Schaeffer L, Gohlke H, Muller M, Heid IM, Palmer LJ, Kompauer I, Demmelmair H, Illig T, Koletzko B, Heinrich J. Common genetic variants of the FADS1 FADS2 gene cluster and their reconstructed haplotypes are associated with the fatty acid composition in phospholipids. Hum Mol Genet. 2006;15:1745–1756. doi: 10.1093/hmg/ddl117. [DOI] [PubMed] [Google Scholar]
- 40.Mozaffarian D, Geelen A, Brouwer IA, Geleijnse JM, Zock PL, Katan MB. Effect of fish oil on heart rate in humans: a meta-analysis of randomized controlled trials. Circulation. 2005;112:1945–1952. doi: 10.1161/CIRCULATIONAHA.105.556886. [DOI] [PubMed] [Google Scholar]
- 41.Larson MK, Ashmore JH, Harris KA, Vogelaar JL, Pottala JV, Sprehe M, Harris WS. Effects of omega-3 acid ethyl esters and aspirin, alone and in combination, on platelet function in healthy subjects. Thromb Haemost. 2008;100:634–641. [PubMed] [Google Scholar]
- 42.Harris WS. n-3 Fatty acids and serum lipoproteins: human studies. Am J Clin Nutr. 1997;65 (suppl):1645S–1654S. doi: 10.1093/ajcn/65.5.1645S. [DOI] [PubMed] [Google Scholar]
- 43.Ferrucci L, Cherubini A, Bandinelli S, Bartali B, Corsi A, Lauretani F, Martin A, Andres-Lacueva C, Senin U, Guralnik JM. Relationship of plasma polyunsaturated fatty acids to circulating inflammatory markers. J Clin Endocrinol Metab. 2006;91:439–446. doi: 10.1210/jc.2005-1303. [DOI] [PubMed] [Google Scholar]
- 44.Thies F, Garry JM, Yaqoob P, Rerkasem K, Williams J, Shearman CP, Gallagher PJ, Calder PC, Grimble RF. Association of n-3 polyunsaturated fatty acids with stability of atherosclerotic plaques: a randomised controlled trial. Lancet. 2003;361:477–485. doi: 10.1016/S0140-6736(03)12468-3. [DOI] [PubMed] [Google Scholar]
- 45.London B, Albert C, Anderson ME, Giles WR, Van Wagoner DR, Balk E, Billman GE, Chung M, Lands W, Leaf A, McAnulty J, Martens JR, Costello RB, Lathrop DA. Omega-3 fatty acids and cardiac arrhythmias: prior studies and recommendations for future research: a report from the National Heart, Lung, and Blood Institute and Office Of Dietary Supplements Omega-3 Fatty Acids and their Role in Cardiac Arrhythmogenesis Workshop. Circulation. 2007;116:e320–e335. doi: 10.1161/CIRCULATIONAHA.107.712984. [DOI] [PubMed] [Google Scholar]
- 46.Ma DW, Seo J, Switzer KC, Fan YY, McMurray DN, Lupton JR, Chapkin RS. n-3 PUFA and membrane microdomains: a new frontier in bioactive lipid research. J Nutr Biochem. 2004;15:700–706. doi: 10.1016/j.jnutbio.2004.08.002. [DOI] [PubMed] [Google Scholar]
- 47.Li Q, Wang M, Tan L, Wang C, Ma J, Li N, Li Y, Xu G, Li J. Docosahexaenoic acid changes lipid composition and interleukin-2 receptor signaling in membrane rafts. J Lipid Res. 2005;46:1904–1913. doi: 10.1194/jlr.M500033-JLR200. [DOI] [PubMed] [Google Scholar]
- 48.Akbar M, Calderon F, Wen Z, Kim HY. Docosahexaenoic acid: a positive modulator of Akt signaling in neuronal survival. Proc Natl Acad Sci U S A. 2005;102:10858–10863. doi: 10.1073/pnas.0502903102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ott RL. An Introduction to Statistical Methods and Data Analysis. 4. Belmont, CA: Duxbury Press; 1993. [Google Scholar]
- 50.Massaro M, Habib A, Lubrano L, Del Turco S, Lazzerini G, Bourcier T, Weksler BB, De Caterina R. The omega-3 fatty acid docosahexaenoate attenuates endothelial cyclooxygenase-2 induction through both NADP(H) oxidase and PKC epsilon inhibition. Proc Natl Acad Sci U S A. 2006;103:15184–15189. doi: 10.1073/pnas.0510086103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Burr ML, Fehily AM, Gilbert JF, Rogers S, Holliday RM, Sweetnam PM, Elwood PC, Deadman NM. Effects of changes in fat, fish, and fibre intakes on death and myocardial reinfarction: diet and reinfarction trial (DART) Lancet. 1989;2:757–761. doi: 10.1016/s0140-6736(89)90828-3. [DOI] [PubMed] [Google Scholar]


