Abstract
In this study, we used surface‐based morphometry to examine whether age‐related changes in gray matter tissue thickness and depth of sulcal regions at high spatial resolution across the cortex differed in children with childhood absence epilepsy (CAE) compared to healthy control subjects. In addition, the possibility of variable brain‐cognition relationships in the CAE compared to the control group was investigated. The main findings of this study are as follows: (1) From the developmental perspective, children with CAE did not demonstrate the normal regional age‐related changes involving a decrease in cortical thickness and increase in sulcal depth. (2) None of the seizure variables, including age of onset, seizure frequency, and AEDs had a significant effect on the association between age and cortical morphometry measures in the CAE population. (3) Even though the CAE group had mean VIQ and PIQ scores in the average range, our findings suggest that they use different brain regions to perform these cognitive functions compared to healthy controls. This first study on brain morphometry and cognition in children with childhood absence seizures has important implications for advancing our understanding of brain development and cognitive comorbidity in CAE, as well as for revisiting the clinical notion that CAE is a benign disorder. Hum Brain Mapp, 2011. © 2010 Wiley‐Liss, Inc.
Keywords: cortical morphometry, childhood absence epilepsy, IQ, cortical thickness, sulcal depth, development, cognition, generalized linear model
INTRODUCTION
Quantitative neuroimaging studies of common localization‐related forms of epilepsy, such as temporal lobe epilepsy, have shown neuroanatomic abnormalities to extend far outside the zone of seizure onset to affect temporal and extratemporal regions of the cortical mantle both contralateral as well as ipsilateral to the side of seizure onset, also affecting a diversity of subcortical regions (e.g., hippocampus, amygdala, thalamus, and caudate), cerebellum, and white matter diffusely [Bernhardt et al., 2008; Keller and Robert, 2008; Lin et al., 2007; McDonald et al., 2008; Mueller et al., 2009]. These abnormalities have been shown to be of clinical consequence through their association with a diversity of cognitive and behavioral abnormalities [Hermann et al., 2004; Keller et al., 2009; Trenerry et al., 1993].
Much less work of this type has been conducted with patients with idiopathic generalized epilepsies in general and childhood absence epilepsy in particular. The literature to date, however, has identified volumetric abnormalities in the thalamus, frontal lobe, and temporal lobe of adults and adolescents with primary generalized epilepsies, some of whom had childhood absence epilepsy (CAE), [Betting et al., 2006a, b; Chan et al., 2006; Pardoe et al., 2008]. Thalamic findings include both atrophy in adolescents and young adults [Chan et al., 2006] and hypertrophy (increased thalamic volume) in adults with CAE [Betting et al., 2006b]. Cortical findings include increased gray matter concentration in the superior mesiofrontal region in adults [Betting et al., 2006b] and larger right temporal and frontal lobe white matter volumes in adolescents and young adults with CAE [Chan et al., 2006]. Furthermore, a recent volumetric study demonstrated reduced gray matter volume in the orbital frontal gyrus and temporal lobe of children with CAE that is unrelated to illness variables [Caplan et al., in press]. The importance of these findings is underscored by involvement of these brain regions in the onset and spread of seizures in CAE [Holmes et al., 2004; Tucker et al., 2007], as well as in the normal development of cognition [Alvarez and Emory, 2006], language [Szaflarski et al., 2006], and behavior [De Bellis et al., 2002; McClure et al., 2007; Sowell et al., 2003].
CAE is among the most common forms of childhood epilepsy [Pavone et al., 2001]; however, the degree to which the structural abnormalities, described above, are specific to CAE remains unclear because these prior imaging studies were conducted in mixed samples of subjects with idiopathic generalized seizures, only some of whom had CAE. Furthermore, since these studies were conducted with adolescents, young adults, and adults, evidence is lacking on whether their findings represent the effects of CAE on brain development during childhood and early adolescence or long‐term effects of uncontrolled idiopathic generalized seizures, and in particular CAE.
These questions are directly addressed in this article. By examining brain development in CAE, this study also sheds light on the traditional notion of CAE as a benign disorder. This concept has been challenged by the evidence that on‐going seizures might underlie or contribute to the cognitive, linguistic, and behavioral comorbidities of CAE [Caplan et al., 2008; Henkin et al., 2003, 2005; Lagae et al., 2001; Mandelbaum and Burack, 1997; Pavone et al., 2001; Williams et al., 1996; Wirrell et al., 1997]. In this investigation, we used surface‐based morphometry to examine whether age‐related changes in gray matter (GM) tissue thickness and depth of sulcal regions at high spatial resolution across the cortex differed in children with CAE compared to healthy control subjects. In addition, the possibility of variable brain‐cognition relationships in the CAE compared to the control group was investigated. Prior volumetric studies in children with recent onset seizures [Hermann et al., 2006], chronic idiopathic epilepsy with complex partial seizures [Daley et al., 2007], and CAE [Caplan et al., in press] demonstrated a dissociation of age, IQ, and brain volumes compared to normal age‐ and gender‐matched control subjects. We, therefore, predicted a similar dissociation in the normal age‐related morphometric development and in the morphometry‐IQ correlation [Shaw et al., 2006a]. Finally, we also investigated the relationship of GM thickness and sulcal depth with seizure control, age of onset, and antiepileptic drug (AED) treatment within the CAE group.
METHODS
Subjects
The study included 24 CAE children, aged 5.5–15.8 years with IQ scores of 70 and above, and 28 age matched normal children (Table I). We determined socioeconomic status using the Hollingshead‐2‐factor index [Hollingshead, 1973], based on parent occupational and educational status. There were no significant differences in the demographic variables of the CAE and normal groups. Three CAE children received no AEDs, 14 were on AED monotherapy, and 7 were on AED polytherapy.
Table I.
Demographic features of study groups
| CAE | Normal | P‐values | |
|---|---|---|---|
| N | 24 | 28 | |
| Age (years) | 9.23 (2.19) | 9.77 (2.20) | 0.38 (t = 0.87, df = 50) |
| Gender (M/F) | 7/17 | 14/14 | 0.01* (χ2 = 7.53, df = 1) |
| Socioeconomic status | |||
| High (i,ii) | 19% | 25% | |
| Low (iii–v) | 81% | 75% | |
| Ethnicity | |||
| Caucasian | 46% | 48% | |
| Non‐Caucasian | 54% | 52% | |
| Age of onset (years) | 6.98 (2.02) | — | |
| Duration (years) | 2.25 (2.19) | — | |
| IQ | |||
| Verbal | 100.12 (20.58) | 112.14 (14.84) | 0.02* (t = 2.44, df = 50) |
| Performance | 102.92 (17.73) | 107.86 (11.60) | 0.23 (t = 1.20, df = 50) |
| Full scale | 101.25 (18.09) | 110.96 (12.73) | 0.03* (t = 2.26, df = 50) |
The primary study inclusion criteria for each CAE subject were a diagnosis of CAE and at least one seizure in the year prior to participation in the study. A pediatric neurologist at each recruitment site diagnosed CAE according to the international classification of epilepsy [Commission, 1989]. All CAE patients had EEG evidence of 3‐Hz spike and wave in addition to absence seizures induced by hyperventilation. We excluded patients with a mixed seizure disorder, previous epilepsy surgery, atypical spike and wave complexes, juvenile myoclonic epilepsy, a neurological illness other than epilepsy, chronic medical illness, imaging evidence for structural brain abnormalities, a metabolic disorder, a hearing disorder, mental retardation based on school/classroom placement, and bilingual speakers of American English who did not attend English speaking schools or speak English at home.
We recruited 32% of the CAE subjects from tertiary centers (i.e., UCLA and USC based clinics) and 68% from community services (i.e., Los Angeles and Anaheim Kaiser Permanente, the Los Angeles and San Diego Chapters of the Epilepsy Foundation of America, private practices). UCLA IRB approved recruitment flyers were available for parents of CAE children at each recruitment site. Interested parents contacted the study coordinator who provided information about the study and used a UCLA IRB approved telephone script to determine if the children met the study's inclusionary but none of its exclusionary criteria. The study coordinator also contacted the child's pediatric neurologist to confirm the child's diagnosis and to rule out exclusionary criteria. One UCLA pediatric neurology investigator (W.D.S.) reviewed the history, EEG records, and diagnosis of each CAE subject from different recruitment sites. If he did not concur with the diagnosis or EEG findings, the child was excluded from the study. The parents and children's medical records provided information on seizure variables (Table I). One CAE subject had slowing on EEG and three had generalized tonic clonic convulsions in addition to absence seizures.
To include control subjects from ethnic and socioeconomic status backgrounds similar to the CAE group, we recruited the normal subjects from four public and two private Los Angeles schools. The study coordinator screened potential participants for neurological, psychiatric, language, and hearing disorders through a telephone conversation with a parent. Given volumetric and morphometric abnormalities in children without epilepsy who have attention deficit hyperactivity disorder [Narr et al., 2009; Sowell et al., 2003], depression [Rosso et al., 2005], and anxiety disorder [De Bellis et al., 2002], we excluded children with these diagnoses in the past or who met criteria for these disorders once enrolled in the study.
MR Acquisition
Structural MR brain image volumes were acquired on a 1.5 Tesla GE Signa magnetic resonance imaging scanner (GE Medical Systems, Milwaukee, WI). The imaging acquisition protocol used to obtain high resolution three‐dimensional (3D) T‐1 weighted spoiled grass (SPGR) sequences included a sagittal plane acquisition with slice thickness of 1.2 mm, repetition time of 24 ms, echo time of 9 ms, flip angle of 22°, acquisition matrix of 256 × 192, FOV 24, and two excitations.
Cognition
The Wechsler Intelligence Scale for Children‐III [WISC‐III; Wechsler, 1991], administered to the children, generated Full Scale (FSIQ), Verbal (VIQ), and Performance IQ scores (PIQ).
Fully Automated Surface‐Based 3D Cortical Morphometry
The skull, scalp, extracranial tissue, cerebellum, and brain stem (at the level of the diencephalon) were removed from each image data using an automated method [Shattuck and Leahy, 2002] followed by quality check. The remaining image volume was then corrected for intensity inhomogeneity using the nonparametric nonuniform intensity normalization (N3) technique [Sled and Pike, 1998]. These processing steps were followed by the reslicing of the remaining image volumes into a standard orientation of the International Consortium for Brain Mapping‐305 (ICBM‐305) average brain by a least‐squares rigid body transformation. The global differences in brain size and shape remained intact during the transformation into the ICBM‐305 average brain space. The resliced image volumes had isotropic voxels each having size of 1 mm × 1 mm × 1 mm. Each individual's cortical surface was extracted using a cortical reconstruction method using implicit surface evolution (CRUISE) technique developed by [Han et al., 2004] and shown to yield an accurate and topologically correct representation that lies at the geometric center of the cortical gray matter tissue [Tosun et al., 2006]. CRUISE is a data‐driven method combining a robust fuzzy segmentation method, an efficient topology correction algorithm, and a geometric deformable surface model. Each resulting cortical surface was represented as a triangle mesh comprising of ∼300,000 mesh nodes. Reconstructed cortical surface for a sample brain is shown in Figure 1.
Figure 1.
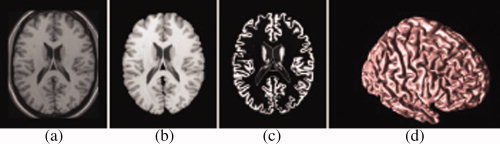
Geometric modeling of cerebral cortex: Axial cross‐section of (a) T1‐weighted MR image, (b) resulting cerebral volume, (c) resulting GM tissue segmentation, and (d) central cortical surface representation. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
Cortical thickness at each point in the cortical GM tissue mantle was defined as the sum of the distances from this point to the GM/WM and GM/CSF tissue boundaries following a flow field which guarantees a one‐to‐one, symmetric, and continuous correspondence between the two tissue boundaries. A flow field with these properties was computed by solving a Laplace's equation with cortical GM tissue mantle as its domain [Tosun et al., 2007; Yezzi and Prince, 2003]. Cortical thickness was estimated in millimeters at 3D image voxels on the GM tissue mantle. Estimated cortical thickness values were mapped onto the corresponding central cortical surface using trilinear interpolation at each mesh vertex. The resulting thickness map for a representative subject is shown in Figure 2.
Figure 2.
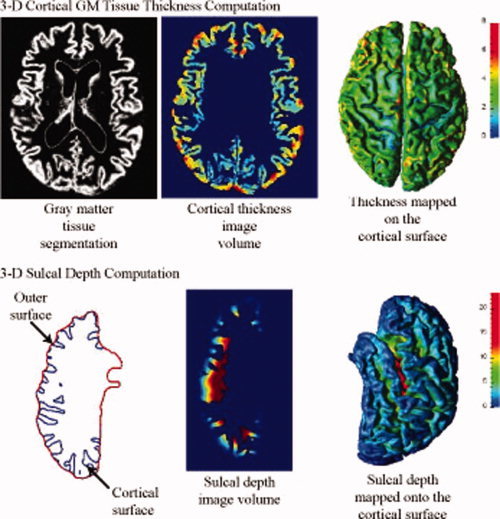
Measures of 3D cortical morphometry: Computation steps for cortical thickness and sulcal depth measures. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
Sulcal regions are defined as the openings surrounded by the buried, inward folded cortex. Sulcal depth measure was defined as a distance from the cortical surface to a reference outer cortical surface that tightly surrounds the cortical surface, outlining the exposed cortex without entering into the sulcal openings. To capture the true depth measure for the sulcal region in medial cortical surface, separate outer surfaces per cortical hemisphere were generated. Focusing on one cortical hemisphere, a geometric deformable surface model expanding the central cortical surface outward at sulcal points was used to construct an outer cortical surface representation [Tosun et al., 2007]. The sulcal depth measure from the central cortical surface to the resulting outer cortical surfaces (one per hemisphere) was estimated using the computation technique described for cortical thickness estimation. This time, the computation domain was defined by the space between the central cortical surface and the outer cortical surface. A cross section from the resulting sulcal depth volume of left cortical hemisphere of a sample subject and the corresponding surface map (i.e., trilinear interpolation of the volumetric data at the triangle mesh nodes of the central cortical surface representation) are shown in Figure 2.
Cortical spatial normalization was used to match anatomically homologous cortical features across subjects. In particular, the central cortical surface model of each subject was spatially normalized with respect to the geometry of a representative reference brain [i.e., the colin27 average brain; Holmes et al., 1998] using an automated surface‐based cortical warping method [Tosun and Prince, 2008]. As a result, individual cortical morphometry measures from homologous surface locations were mapped onto the reference surface, where statistical analyses were carried out.
Surface‐based data smoothing was used to increase the signal‐to‐noise ratio before performing surface‐based statistical analysis. In particular, the estimated value (cortical thickness or sulcal depth measure) at each mesh node was replaced by the convolution of the measure map of interest with a Gaussian kernel centered at this mesh node. The Gaussian kernel domain was defined on each cortical surface over a geodesic neighborhood of radius 10 mm. The size of the smoothing kernel matched the size of the effect we sought while accounting for residual errors in the surface warping. For each subject brain, smoothed measure values at each surface mesh node were transferred onto the anatomically homologous location on the reference brain surface according to the surface correspondence established by the spatial normalization.
Data Analysis
CAE‐related modulation of regional aging effects on cortical morphometry
In this study, we were primarily interested in how CAE might modulate age‐related alterations of cortical morphometry to assess the neurodevelopmental implications of CAE rather than to assess common group differences previously reported. Accordingly, to assess the CAE‐related modulation of regional aging effects on cortical morphometry, we performed the test of equality of slopes as a guide to statistical significance of the differential relationship of age in the two groups of subjects. This is conceptually similar to an interaction between age and diagnostic group. To explore the effects of CAE on regional cortical morphometry independent of participant age, we estimated a generalized linear model (GLM) with diagnosis (i.e., healthy versus CAE) as the classification variable assuming identical age related slopes between groups (main effect). These results are available in Supporting Information figures online. To test whether CAE was associated with different age‐related slopes, we computed another GLM where different age‐related slopes were allowed to emerge (interaction effect). GLMs were followed by a pair‐wise maximum likelihood test to determine if CAE‐related modulation of the interaction effect was significant. In each GLM, sex was included as a covariate, given that each population data set had different male to female ratios (Table I) and evidence for sex‐related differences in the association between IQ and cortical anatomy have been reported in other investigations [Haier et al., 2005; Narr et al., 2007]. The data analysis was repeated for each 3D cortical morphometry measures (i.e., cortical thickness and sulcal depth) separately.
CAE‐related differences in cortical morphometry and IQ associations
GLM analyses were used to examine the CAE‐related relationships between intelligence quotients (VIQ and PIQ) and cortical morphometry measures (cortical thickness and sulcal depth) while removing the variance associated with age and sex. Regional relationships between IQ scores and cortical morphometry measures were examined by testing for the significance of the interaction between diagnosis and slope (IQ versus cortical morphometry measure) from the regression models.
Effects of seizure variables
Subsequently, effects of onset age, seizure frequency, and AEDs on the association between age and cortical morphometry measures in CAE population were also assessed by a generalized linear model analysis, by including these variables as additional predictors in the GLM.
All statistical outcomes were corrected for multiple comparisons, using false discovery rate [FDR; Benjamini and Hockberg, 1995] at P < 0.05. All statistical computations were carried out using the statistical package R (http://www.r-project.org/).
RESULTS
Cortical surface P‐value maps (FDR corrected) in Figure 3 depict the regions of brain where age‐cortical morphometry (i.e., cortical thickness and sulcal depth) associations differed between healthy children and children with CAE. Red and green represent areas where healthy children have age‐related cortical thinning (or decrease in sulcal depth) and children with CAE have age‐related thickening (or increase in sulcal depth). This CAE‐related modulation effect on normal age‐related cortical thinning corresponds to significant positive regression coefficient of age‐diagnosis term in GLM. In the same cortical maps, blue and purple indicate age‐related regional cortical thickening (or increase in sulcal depth) in healthy children and age‐related cortical thinning (or decrease in sulcal depth) in children with CAE. Cortical regions with this kind of CAE‐related modulation effect were identified with significant negative regression coefficients of age‐diagnosis terms in GLM. For further clarification, significance maps of effects of aging on cortical thickness and sulcal depth within healthy children and children with CAE populations separately are provided in the Supporting Information figure. In Figure 4, we provided plots of raw data (i.e., cortical thickness and sulcal depth) as functions of age within healthy children and children with CAE groups. Sample points for these raw data plots were selected at cortical regions where significant CAE‐related modulation effects on age‐cortical morphometry associations were observed.
Figure 3.
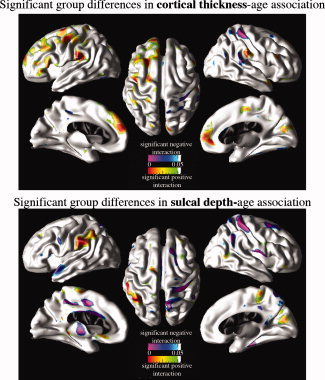
CAE‐related modulation of cortical morphometry‐age associations. Cortical surface P‐value maps show the regional significance of CAE‐related modulation effects on age‐cortical morphometry (i.e., cortical thickness and sulcal depth) associations after removing the partial effects of sex. Hot colors (red and green) imply positive regression coefficients for age‐diagnosis interaction terms in GLM and cold colors (purple and blue) imply negative regression coefficients for age‐diagnosis interaction terms in GLM. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
Figure 4.
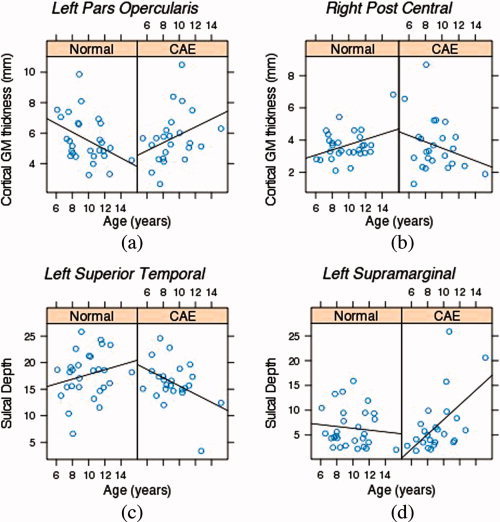
Plots of cortical morphometry (i.e., cortical GM thickness and sulcal depth) as a function of age within healthy children and children with CAE groups separately for sample points in (a) the left pars opercularis with significant positive CAE‐related modulation effect on cortical thickness‐age association, (b) the right post central with significant negative CAE‐related modulation effect on cortical thickness‐age association, (c) the left superior temporal with significant negative CAE‐related modulation effect on sulcal depth‐age association, and (d) the left supramarginal with significant positive CAE‐related modulation effect on CAE‐related modulation effect on sulcal depth‐age association. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
Brain regions with CAE‐related modulation effects on cortical thickness‐age associations include superior (including mesial surface), middle, and inferior frontal cortices, supramarginal gyrus on the left hemisphere, somatosensory region bilaterally; and superior temporal gyrus, lingual gyrus and paracentral gyrus on the right hemisphere.
Sulcal depth‐age associations in CAE children compared to healthy children differs in the left cingulate, right paracentral, and cuneus regions in addition to left inferior parietal lobule, left superior temporal cortex, right post central, and right middle frontal cortices.
Statistically significant group differences in the relationships between IQ measures and cortical morphometry measures after removing the partial effects of sex and age are mapped in Figure 5. The main findings include (1) stronger association (i.e., larger negative slope) of higher VIQ and PIQ scores with more cortical thinning in the left middle frontal, left medial superior frontal gyrus, and right inferior temporal cortices in the healthy group as well as the association of higher VIQ and PIQ scores with thicker cortex (i.e., positive slope) in the left orbito‐frontal region in CAE compared to the negative association in healthy group in the same regions and (2) greater sulcal depth associated with higher VIQ and PIQ scores in the controls compared to the CAE children in the left superior temporal, middle frontal, superior frontal (including mesial surface), parieto‐occipital regions. In Figure 6, plots of raw cortical thickness and sulcal depth measures as a function of IQ (i.e., PIQ and VIQ) for healthy children and children with CAE are shown to elucidate the IQ‐cortical morphometry association differences between groups. These sample cortical points were selected where significant CAE‐related modulation effects on IQ‐cortical morphometry were observed.
Figure 5.
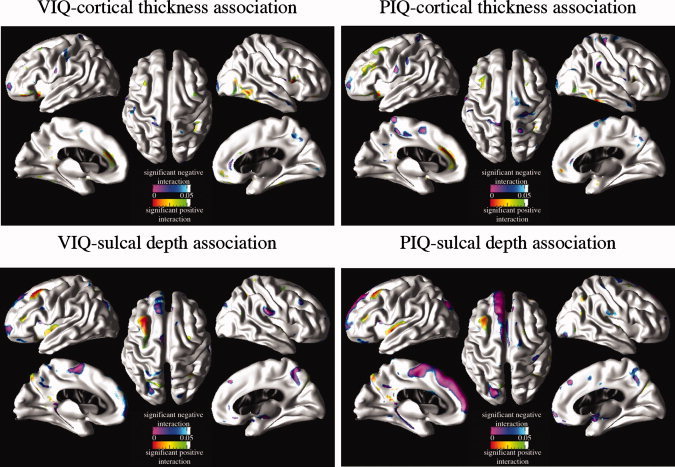
CAE‐related modulation of cortical morphometry‐IQ associations. Cortical surface p‐value maps show the regional significance of CAE‐related modulation effects on IQ (i.e., VIQ and PIQ)‐cortical morphometry (i.e., cortical thickness and sulcal depth) associations after removing the partial effects of sex. Hot colors (red and green) imply positive regression coefficients for IQ‐diagnosis interaction terms in GLM and cold colors (purple and blue) imply negative regression coefficients for IQ‐diagnosis interaction terms in GLM. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
Figure 6.
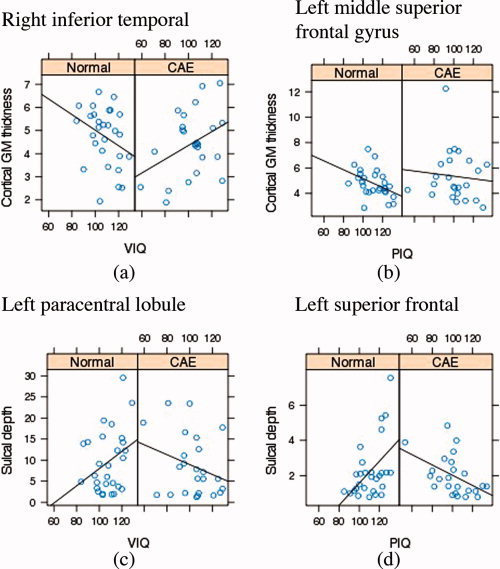
Plots of cortical morphometry (i.e., cortical GM thickness and sulcal depth) as a function of IQ (i.e., VIQ and PIQ) within healthy children and children with CAE groups separately for sample points in (a) right inferior temporal with significant positive CAE‐related modulation effect on cortical thickness‐VIQ association, (b) the left middle frontal with significant positive CAE‐related modulation effect on cortical thickness‐PIQ association, (c) the left parieto‐occipital region with significant negative CAE‐related modulation effect on sulcal depth‐VIQ association, and (d) the left superior temporal with significant negative CAE‐related modulation effect on CAE‐related modulation effect on sulcal depth‐PIQ association. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
After correction for multiple comparisons, none of the seizure variables, including age of onset, seizure frequency, and AEDs had a significant effect on the association between age and cortical morphometry measures in the CAE population; therefore the P‐value maps from these analyses are not included in this article.
DISCUSSION
This first study on brain morphometry and cognition in children with childhood absence seizures has important implications for advancing our understanding of brain development and cognitive comorbidity in CAE, as well as for revisiting the clinical notion that CAE is a benign disorder.
CAE‐Related Modulation of Regional Aging Effects on Cortical Morphometry
From the developmental perspective, children with CAE did not demonstrate the normal age‐related changes involving a decrease in cortical thickness and increase in sulcal depth in the left superior (including mesial surface), middle, and inferior frontal lobe, somatosensory region, and superior temporal gyrus. Unlike the control group, there also appeared to be an age‐related thinning in the right posterior central gyrus and posterior part of the superior temporal gyrus, lingual gyrus, and paracentral gyrus. In addition, the CAE group did not demonstrate the normal rate of increase in sulcal depth with age, particularly in the left superior temporal sulcus, left cingulate, right post central, and right middle frontal; with normal rate of decrease in sulcal depth with age in the right paracentral and cuneus regions in addition to left inferior parietal lobule. Thus, the neurodevelopmental abnormalities in CAE appear to be diverse in nature and extent with sparing of some cortical regions and involvement of others, as noted in [Blumenfeld, 2005].
Predominant involvement of the somatosensory region in the CAE group corroborates evidence in both animal [Bouilleret et al., 2009; Chahboune et al., 2009; Polack et al., 2009] and human studies [Blumenfeld, 2005] for the role of this brain region in this disorder. However, participation mainly of the left hemisphere in the between group morphometric differences underscores the importance of revisiting older concepts of generalized rather than focal effects of this disorder [Holmes et al., 2004; Meeren et al., 2005]. Interestingly, cortical thickness studies of adult temporal lobe epilepsy patients with and without mesial temporal sclerosis also show pronounced thinning in the sensorimotor region [Bernhardt et al., 2008; McDonald et al., 2008; Mueller et al., 2009], so this may not be a CAE specific finding.
Given the cross‐sectional nature of these findings, the increased cortical thickness in the left hemisphere of the CAE group could represent either a developmental delay or irreversible abnormality in the pruning process. The lack of an association of seizure variables with the findings in the CAE group imply that fixed abnormalities of brain structure rather than on‐going effects of seizures underlie the neurodevelopmental abnormalities of this disorder. Similarly, the previously mentioned childhood CAE volumetric study also reported no association of fronto‐temporal volumes with illness variables despite significantly smaller orbital frontal and temporal lobe gray matter volumes in the CAE compared to the normal age and gender matched control group [Caplan et al., in press;. Likewise, Betting et al., 2006b] found no association between seizure variables and abnormal increase in gray matter concentration in the mesial superior frontal region in adults with absence seizures.
Furthermore, involvement of the mesial superior frontal region with increased cortical thickness in the CAE children in our study and greater gray matter concentration in adults with absence seizures [Betting et al., 2006b] suggests that these abnormalities begin in childhood and continue through adulthood. Localization of these morphometric abnormalities in the frontal lobe, particularly superior and medial, also supports the predominance of EEG findings in this brain region in both children and adults with CAE [Holmes et al., 2004].
Our cortical morphometry findings involving more cortical thinning in the right cuneus and precuneus of the normal group versus CAE are interesting in light of involvement of these brain regions in basic visual processing and visuo‐spatial imagery [Cabeza and Nyberg, 1997; Cabeza and Nyberg, 2000]. Despite lack of involvement of this brain region in animal studies and minimal involvement in human EEG studies, they support prior findings of impaired visual memory in CAE [Pavone et al., 2001] and are congruent with previous fMRI [Archer, 2003] and voxel‐based morphometry studies [Pardoe et al., 2006] reporting significant BOLD signal and GM volume decreases in these cortical regions in idiopathic generalized epilepsy patients with mean age 14.6 years.
However, the relative lack of involvement of the temporal lobe, other than the superior temporal gyrus in our CAE group, is surprising since Holmes et al. [ 2004] and Tucker et al. [ 2007] have shown spread of absence seizures from orbital frontal and dorsolateral prefrontal regions to the temporal lobe in adults with idiopathic generalized seizures, some of whom had childhood onset of CAE. Since the reported age‐related normal decrease in cortical thickness of the temporal lobe occurs after that of the frontal lobe during adolescence [Lenroot and Giedd, 2006], our finding might reflect the younger age of the children in our study.
In terms of comorbidities, localization of increased cortical thickness in the superior temporal lobe, mainly the posterior part, is particularly interesting given the role of this brain region in language [Friederici et al., 2009] and evidence for both basic [Caplan et al., in press] and higher level linguistic deficits [Caplan et al., 2006] in children with CAE despite average IQ scores. As for cognition, the between group differences in the association of VIQ with more cortical thinning in the orbital frontal and anterior superior temporal region in the normal group in contrast to the CAE group might indirectly reflect the gray matter volumetric abnormalities in these brain regions in children with CAE [Caplan et al., in press]. Both these brain regions play an important role in linguistic competence [Demonet et al., 2005; Duffau et al., 2005; Friederici et al., 2009]. The orbital frontal gyrus is also integrally involved in the onset of absence seizures [Tucker et al., 2007] and inhibits thalamocortical circuitry via the nucleus reticularis of the thalamus [see review in Holmes et al., 2004].
CAE‐Related Differences in Cortical Morphometry and IQ Associations
Even though the CAE group had mean VIQ and PIQ scores in the average range, our findings suggest that they use different brain regions to perform these cognitive functions compared to healthy controls. Thus, greater sulcal depth was related to higher VIQ and PIQ scores in the controls in the left superior temporal gyrus, middle frontal, and superior frontal gyrus. However, in CAE the relationship of VIQ with sulcal depth in the left inferior frontal and sulcal region between the paracentral lobule and superior frontal region highlights involvement of traditional non‐language‐related brain regions in the verbal skills of these children and raises the possibility of plasticity and reorganization in the absence of a structural lesion.
The association of PIQ with shallow sulci in the left mesial and superior frontal regions in the CAE group also highlights involvement of different brain regions in the skills tapped by PIQ and VIQ in the CAE group. The similar distribution in the relationship of morphometric measures with both VIQ and PIQ in the normal group provides additional evidence for perturbed brain development despite average functioning in CAE.
Taken together, the study's findings imply that the average intellectual functioning of these children reflects marked plasticity and reorganization of brain development due to the distributed neuropathology underlying CAE. In support of this explanation, age, IQ, and fronto‐temporal volumes were also associated with different brain region in children with CAE and typically developing children [Caplan et al., in press].
Limitations
Study limitations involve the sample (i.e., size, gender, seizure control, comorbid psychiatric diagnoses in CAE group), between group differences in IQ, and cross‐sectional study design. In terms of sample related limitations, the study was conducted on a relatively small sample, albeit within a relatively narrow age range. Although the sample included more girls than boys as characteristic of the illness [Loiseau and Hirsch, 2002], all analyses controlled for gender effects. Regarding seizure control, study inclusionary criteria stipulated that all CAE subjects had at least one seizure in the year prior to their participation in the study. Since the CAE morphometric findings were unrelated to seizure variables, the findings do not appear to reflect inclusion of a skewed sample. Given evidence for cortical thinning in children with attention deficit hyperactivity disorder [ADHD; Luders et al., 2009] in medial and superior prefrontal and precentral regions [Shaw et al., 2006b] and the presence of ADHD without hyperactivity in 36% of the sample, our findings might not be generalizable to CAE subjects without ADHD. Information on the presence of lip and eyelid myoclonia, features reflecting increased severity of illness and poor outcome, was not available.
Although the mean FSIQ of the normal group was one standard deviation higher than the CAE group, whose mean IQ was the same as the general population, the IQ‐cortical morphometry findings in the normal group were similar to those described in other typically developing children [Shaw et al., 2006a]. Finally, a prospective study rather than the cross‐sectional design of the current study is needed to confirm that brain development in CAE is unrelated to effects of on‐going seizures.
In conclusion, identification of morphometric abnormalities in children with CAE highlights the importance of revisiting the traditional concept that CAE is a benign disorder. However, these findings also suggest that reorganization of brain structure/functional relationships is associated with average intellectual functioning in children with CAE.
Supporting information
Additional Supporting Information may be found in the online version of this article.
Supporting Figure 1
Supporting Information
REFERENCES
- Alvarez JA, Emory E ( 2006): Executive function and the frontal lobes: A meta‐analytic review. Neuropsychol Rev 16: 17–42. [DOI] [PubMed] [Google Scholar]
- Archer JS ( 2003): fMRI deactivation of the posterior cingulate during generalized spike and wave. NeuroImage 20: 1915–1922. [DOI] [PubMed] [Google Scholar]
- Benjamini Y, Hockberg Y ( 1995): Controlling the false discovery rate: A practical and powerful approach to mltiple testing. J R Stat Soc 57: 289–300. [Google Scholar]
- Bernhardt B, Worsley KJ, Besson P, Concha L, Lerch JP, Evans ACNB ( 2008): Mapping limbic network organization in temporal lobe epilepsy using morphometric correlations: Insights on the relation between mesiotemporal connectivity and cortical atrophy Neuroimage 15: 515– 524. [DOI] [PubMed] [Google Scholar]
- Betting LE, Mory SB, Li LM, Lopes‐Cendes I, Guerreiro MM, Guerreiro CA, Cendes F ( 2006a) Voxel‐based morphometry in patients with idiopathic generalized epilepsies. Neuroimage 32: 498–502. [DOI] [PubMed] [Google Scholar]
- Betting LE, Mory SB, Lopes‐Cendes I, Li LM, Guerreiro MM, Guerreiro CA, Cendes F ( 2006b) MRI volumetry shows increased anterior thalamic volumes in patients with absence seizures. Epilepsy Behav 8: 575–580. [DOI] [PubMed] [Google Scholar]
- Blumenfeld H ( 2005): Cellular and network mechanisms of spike‐wave seizures. Epilepsia 46: 21–33. [DOI] [PubMed] [Google Scholar]
- Bouilleret V, Hogan RE, Velakoulis D, Salzberg MR, Wang L, Egan GF, O'Brien TJ, Jones NC ( 2009): Morphometric abnormalities and hyperanxiety in genetically epileptic rats: A model of psychiatric comorbidity? Neuroimage 45: 267–274. [DOI] [PubMed] [Google Scholar]
- Cabeza R, Nyberg L ( 1997): Imaging cognition: an empirical review of PET studies with normal subjects. J Cogn Neurosci 9: 1–26. [DOI] [PubMed] [Google Scholar]
- Cabeza R, Nyberg L ( 2000): Imaging cognition II: An empirical review of 275 PET and fMRI studies. J Cogn Neurosci 12: 1–47. [DOI] [PubMed] [Google Scholar]
- Caplan R, Siddarth P, Gurbani S, Lanphier E, Koh S, Sankar R ( 2006): Thought disorder: A developmental disability in pediatric epilepsy. Epilepsy Behav 8: 726–735. [DOI] [PubMed] [Google Scholar]
- Caplan R, Siddarth P, Stahl L, Lanphier E, Vona P, Gurbani S, Koh S, Sankar R, Shields WD ( 2008): Childhood absence epilepsy: Behavioral, cognitive, and linguistic comorbidities. Epilepsia 49: 1838–1846. [DOI] [PubMed] [Google Scholar]
- Caplan R, Levitt J, Siddarth P, Wu KN, Gurbani S, Sankar R, Shields WD: ( 2009) Frontal and temporal volumes in childhood absence epilepsy. Epilepsia 50: 2466–2472. [DOI] [PubMed] [Google Scholar]
- Caplan R, Siddarth P, Vona P, Stahl L, Bailey CE, Gurbani S, Sankar R, Donald WD: ( 2009) Language in pediatric epilepsy. Epilepsia 50: 2397–2407. [DOI] [PubMed] [Google Scholar]
- Chahboune H, Mishra AM, DeSalvo MN, Staib LH, Purcaro M, Scheinost D, Papademetris X, Fyson SJ, Lorincz ML, Crunelli V, et al. ( 2009): DTI abnormalities in anterior corpus callosum of rats with spike‐wave epilepsy. Neuroimage 47: 459–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan CH, Briellmann RS, Pell GS, Scheffer IE, Abbott DF, Jackson GD ( 2006): Thalamic atrophy in childhood absence epilepsy. Epilepsia 47: 399–405. [DOI] [PubMed] [Google Scholar]
- Commission ( 1989): Commission on classification and terminology of the international league against epilepsy. Proposal for revised clinical and electroencephalographic classification of epileptic seizures. Epilepsia 30: 389–399. [DOI] [PubMed] [Google Scholar]
- Daley M, Levitt J, Siddart P, Mormino E, Hojatkashani C, Gurbani S, Shields WD, Sankar R, Toga A, Caplan R ( 2007): Frontal and temporal volumes in children with complex partial seizures. Epilepsy Behav 10: 470–476. [DOI] [PubMed] [Google Scholar]
- De Bellis MD, Keshavan MS, Shifflett H, Iyengar S, Dahl RE, Axelson DA, Birmaher B, Hall J, Moritz G, Ryan ND ( 2002): Superior temporal gyrus volumes in pediatric generalized anxiety disorder. Biol Psychiatry 51: 553–562. [DOI] [PubMed] [Google Scholar]
- Demonet JF, Pernet C, Kouider S, Musso M ( 2005): The dynamics of language‐related brain images. Neurocase 11: 148–150. [DOI] [PubMed] [Google Scholar]
- Duffau H, Gatignol P, Mandonnet E, Peruzzi P, Tzourio‐Mazoyer N, Capelle L ( 2005): New insights into the anatomo‐functional connectivity of the semantic system: A study using cortico‐subcortical electrostimulations. Brain 128: 797–810. [DOI] [PubMed] [Google Scholar]
- Friederici AD, Makuuchi M, Bahlmann J ( 2009): The role of the posterior superior temporal cortex in sentence comprehension. Neuroreport 20: 563–568. [DOI] [PubMed] [Google Scholar]
- Haier RJ, Jung RE, Yeo RA, Head K, Alkire MT ( 2005): The neuroanatomy of general intelligence: Sex matters. NeuroImage 25: 320–327. [DOI] [PubMed] [Google Scholar]
- Han X, Pham DL, Tosun D, Rettmann ME, Xu C, Prince JL ( 2004): CRUISE: cortical reconstruction using implicit surface evolution. NeuroImage 23: 997–1012. [DOI] [PubMed] [Google Scholar]
- Henkin Y, Kishon‐Rabin L, Pratt H, Kivity S, Sadeh M, Gadoth N ( 2003): Linguistic processing in idiopathic generalized epilepsy: An auditory event‐related potential study. Epilepsia 44: 1207–1217. [DOI] [PubMed] [Google Scholar]
- Henkin Y, Sadeh M, Kivity S, Shabtai E, Kishon‐Rabin L, Gadoth N ( 2005): Cognitive function in idiopathic generalized epilepsy of childhood. Dev Med Child Neurol 47: 126–132. [DOI] [PubMed] [Google Scholar]
- Hermann B, Seidenberg M, Sears L, Hansen R, Bayless K, Rutecki P, Dow C ( 2004): Cerebellar atrophy in temporal lobe epilepsy affects procedural memory. Neurology 63: 2129–2131. [DOI] [PubMed] [Google Scholar]
- Hermann B, Jones J, Sheth R, Dow C, Koehn M, Seidenberg M ( 2006): Children with new‐onset epilepsy: Neuropsychological status and brain structure. Brain 129: 2609–2619. [DOI] [PubMed] [Google Scholar]
- Hollingshead A ( 1973): Medical sociology: A brief review. Milbank Mem Fund Q Health Soc 51: 531–542. [PubMed] [Google Scholar]
- Holmes CJ, Hoge R, Collins L, Woods R, Toga AW, Evans AC ( 1998): Enhancement of MR images using registration for signal averaging. J Comput Assist Tomogr 22: 324–333. [DOI] [PubMed] [Google Scholar]
- Holmes M, Brown M, Tucker DM ( 2004): Are “generalized” seizures truly generalized? Evidence of localized mesial frontal and frontopolar discharges in absence. Epilepsia 45: 1568–1579. [DOI] [PubMed] [Google Scholar]
- Keller SS, Robert N ( 2008): Voxel‐based morphometry of temporal lobe epilepsy: An introduction nand review of the literature. Epilepsia 49: 741–757. [DOI] [PubMed] [Google Scholar]
- Keller SS, Baker G, Downes JJ, Roberts N ( 2009): Quantitative MRI of the prefrontal cortex and executive function in patients with temporal lobe epilepsy. Epilepsy Behav 15: 186–195. [DOI] [PubMed] [Google Scholar]
- Lagae L, Pauwels J, Monte CP, Verhelle B, Vervisch I ( 2001): Frontal absences in children. Eur J Paediatr Neurol 5: 243–251. [DOI] [PubMed] [Google Scholar]
- Lenroot R, Giedd J ( 2006): Brain development in children and adolescents: Insights from anatomical magnetic resonance imaging. Neurosci Biobehav Rev 30: 718. [DOI] [PubMed] [Google Scholar]
- Lin JJ, Salamon N, Lee AD, Dutton RA, Geaga JA, Hayashi KM, Luders E, Toga AW, Engel JJ, Thompson PM ( 2007): Reduced neocortical thickness and complexity mapped in mesial temporal lobe epilepsy with hippocampal sclerosis. Cereb Cortex 17: 2007–2018. [DOI] [PubMed] [Google Scholar]
- Loiseau P, Panayiotopoulos CP, Hirsch E ( 2002): In: Roger JBM, Dravet C, editors. Childhood absence epilepsy and related syndromes London: John Libbey; pp 285–303. [Google Scholar]
- Luders E, Narr KL, Hamilton LS, Phillips OR, Thompson PM, Valle JS, Del'Homme M, Strickland T, McCracken JT, Toga AW, et al. ( 2009): Decreased callosal thickness in attention‐deficit/hyperactivity disorder. Biol Psychiatry 65: 84–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandelbaum D, Burack G ( 1997): The effect of seizure type and medication on cognitive and behavioral functioning in children with idiopathic epilepsy. Dev Med Child Neurol 39: 731–735. [DOI] [PubMed] [Google Scholar]
- McClure E, Monk CS, Nelson EE, Adler A, Blair RJ, Fromm S, Charney DS, Leibenluft E, Ernst M, Pine DS ( 2007): Abnormal attention modulation of fear circuit function in pediatric generalized anxiety disorder. Arch Gen Psychiatry 64: 97–106. [DOI] [PubMed] [Google Scholar]
- McDonald CR, Hagler DJJ, Ahmadi ME, Tecoma E, Iragui V, Gharapetian L, Dale AM, Halgren E ( 2008): Regional neocortical thinning in mesial temporal lobe epilepsy. Epilepsia 49: 794–803. [DOI] [PubMed] [Google Scholar]
- Meeren H, van Luijtelaar G, Lopes da Silva F, Coenen A ( 2005): Evolving concepts on the pathophysiology of absence seizures: The cortical focus theory. Arch Neurol 62: 371–376. [DOI] [PubMed] [Google Scholar]
- Mueller SG, Laxer KD, Barakos J, Cheong I, Garcia P, Weiner MW ( 2009): Widespread neocortical abnormalities in temporal lobe epilepsy with and without mesial sclerosis. Neuroimage 46: 353–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narr KL, Woods RP, Thompson PM, Szeszko P, Robinson D, Dimtcheva T, Gurbani M, Toga AW, Bilder RM ( 2007): Relationships between IQ and regional cortical gray matter thickness in healthy adults. Cereb Cortex 17: 2163–2171. [DOI] [PubMed] [Google Scholar]
- Narr K, Woods R, Lin J, Kim J, Phillips O, Del'Homme M, Caplan R, Toga A, McCracken J, Levitt J ( 2009): Widespread cortical thinning is a robust anatomical marker for attention deficit/hyperactivity disorder. JACAAP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardoe H, Pell GS, Berg AT, Abbott DF, Jackson GD ( 2006): In proceedings of ISMRM: Voxel‐based morphometry analysis of structural gray matter volume variations across idiopathic and cryptogenic epilepsy syndromes. p 1509. [Google Scholar]
- Pardoe H, Pell GS, Abbott DF, Berg AT, Jackson GD ( 2008): Multi‐site voxel‐based morphometry: Methods and a feasibility demonstration with childhood absence epilepsy. NeuroImage 42 611–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavone P, Bianchini R, Trifiletti RR, Incorpora G, Pavone A, Parano E ( 2001): Neuropsychological assessment in children with absence epilepsy. Neurology 56: 1047–1051. [DOI] [PubMed] [Google Scholar]
- Polack PO, Mahon S, Chavez M, Charpier S ( 2009): Inactivation of the somatosensory cortex prevents paroxysmal oscillations in cortical and related thalamic neurons in a genetic model of absence epilepsy. Cereb Cortex (bhn237). [DOI] [PubMed] [Google Scholar]
- Rosso IM, Cintron CM, Steingard RJ, Renshaw PF, Young AD, Yurgelun‐Todd DA ( 2005): Amygdala and hippocampus volumes in pediatric major depression. Biol Psychiatry 57: 21–26. [DOI] [PubMed] [Google Scholar]
- Shattuck DW, Leahy RM ( 2002): BrainSuite: An automated cortical surface identification tool. Medical Image Analysis 6: 129–142. [DOI] [PubMed] [Google Scholar]
- Shaw P, Greenstein D, Lerch J, Clasen L, Lenroot R, Gogtay N, Evans A, Rapoport J, Giedd J ( 2006a) Intellectual ability and cortical development in children and adolescents. Nature 440: 676–679. [DOI] [PubMed] [Google Scholar]
- Shaw P, Lerch J, Greenstein D, Sharp W, Clasen L, Evans A, Giedd J, Castellanos FX, Rapoport J ( 2006b) Longitudinal mapping of cortical thickness and clinical outcome in children and adolescents with attention‐deficit/hyperactivity disorder. Arch Gen Psychiatry 63: 540–549. [DOI] [PubMed] [Google Scholar]
- Sled J, Pike G ( 1998): Standing‐wave and RF penetration artifacts caused by elliptic geometry: An electrodynamic analysis of MRI. IEEE Trans Med Imaging 17: 653–662. [DOI] [PubMed] [Google Scholar]
- Sowell E, Thompson PM, Welcome SE, Henkenius AL, Toga AW, Peterson BS ( 2003): Cortical abnormalities in children and adolescents with attention‐deficit hyperactivity disorder. Lancet 362: 1699–1707. [DOI] [PubMed] [Google Scholar]
- Szaflarski JP, Schmithorst VJ, Altaye M, Byars AW, Ret J, Plante E, Holland SK ( 2006): A longitudinal functional magnetic resonance imaging study of language development in children 5 to 11 years old. Ann Neurol 59: 796–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tosun D, Prince JL ( 2008): A geometry‐driven optical flow warping for spatial normalization of cortical surfaces. IEEE Trans Med Imaging [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tosun D, Rettmann ME, Resnick SM, Pham DL, Prince JL ( 2006): Cortical reconstruction using implicit surface evolution: Accuracy and precision analysis. NeuroImage 29: 838–852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tosun D, Duchesne S, Rolland Y, Toga AW, Verin M, Barillot C ( 2007): In Proceedings of MICCAI: Analysis of Cortical Morphometry in Differential Diagnosis of Parkinson's Plus Syndromes: Mapping Frontal Lobe Cortical Atrophy in Progressive Supranuclear Palsy Patients. Brisbane, Australia: pp 281–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trenerry MR, Jack CRJ, Ivnik RJ, Sharbrough FW, Cascino GD, Hirschorn KA, Marsh WR, Kelly PJ, Meyer FB ( 1993): MRI hippocampal volumes and memory function before and after temporal lobectomy. Neurology 43: 1800–1805. [DOI] [PubMed] [Google Scholar]
- Tucker D, Brown M, Luu P, Holmes GL ( 2007): Discharges in ventromedial frontal cortex during absence spells. Epilepsy Behav 11: 546–547. [DOI] [PubMed] [Google Scholar]
- Wechsler D ( 1991): Weschsler Intelligence Scale for Children, 3rd ed (WISC‐III). San Antonio: The Psychological Corporation. [Google Scholar]
- Williams J, Sharp G, Bates S, Griebel M, Lange B, Spence G, Thomas P ( 1996): Academic achievement and the behavioral ratings in children with absence and complex partial epilepsy. Educ Treat Children 19: 143–152. [Google Scholar]
- Wirrell EC, Camfield CS, Camfield PR, Dooley JM, Gordon KE, Smith B ( 1997): Long‐term psychosocial outcome in typical absence epilepsy. Sometimes a wolf in sheeps' clothing. Arch Pediatr Adolesc Med 151: 152–158. [DOI] [PubMed] [Google Scholar]
- Yezzi AJJ, Prince JL ( 2003): An Eulerian PDE approach for computing tissue thickness. IEEE Trans Med Imaging 22: 1332–1339. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional Supporting Information may be found in the online version of this article.
Supporting Figure 1
Supporting Information


