Abstract
Exposure to stress reliably activates the hypothalamo-pituitary-adrenocortical (HPA) axis response in rodents, which is significantly reduced (habituated) following repeated exposures. In the current study, it was first established that HPA axis response habituation to repeated loud noise lasted for at least four weeks in rats. In the next Experiment, a contextual extinction procedure following repeated loud noise exposures failed to restore the habituated HPA axis response. Although an additional study indicated some recovery of responses when the context was modified on a test day following habituation, this effect could be mostly attributed to the familiarity with the contextual cues. A final study confirmed that rats could distinguish between the contexts employed and further indicated that context pre-exposures reduce acute HPA axis responses to loud noise. These studies therefore provide no support for the hypothesis that contextual cues regulate HPA axis response habituation.
Keywords: CORT, ACTH, habituation, stress
Introduction
Repeated exposures to the same stimuli or situations routinely leads to decrements in several physiological and behavioral responses, a process generally termed habituation (Harris, 1943). As with habituation of behavioral responses (Christoffersen, 1997; Groves & Thompson, 1970; Horn & Hinde, 1970; Jordan, Strasser & McHale, 2000; Rankin et al., 2009), the neuroendocrine responses controlled by the hypothalamo-pituitary-adrenocortical (HPA) axis also undergo reliable habituation to moderate repeated stressful stimuli (homotypic) under many conditions (Grissom & Bhatnagar, 2009; Marti & Armario, 1998). One of the leading hypotheses in the habituation of behavioral responses to relatively innocuous stimuli involves the association of contextual cues with stimuli (unconditioned stimuli - US), and following repeated pairings or associations of the contextual cues with the US, the contextual cues automatically “prime” the retrieval of the US, which is proposed to inhibit the unconditioned responses normally triggered by the US (Wagner, 1979). Recently, the possibility that contextual cues are important components of the overall conditions that contribute to the regulation of habituated HPA axis responses has received some empirical support (Grissom, Iyer, Vining, & Bhatnagar, 2007). In this study, different groups of rats were exposed to repeated restraint stress (US) in rooms with distinct odors (contextual cues), but on the last day, one of those groups was restrained in a room with a novel odor. Under this contextual (odor) change, and in support of the associative theory of habituation, the habituated HPA axis response to restraint was restored to that displayed by animals stressed acutely, suggesting that contextual cues regulate the expression of HPA axis response habituation. However, one possibility that the above study did not test was the impact of the novel unfamiliar context on the habituated HPA axis response to the restraint. Several behavioral habituation studies have indeed described a reliable effect of contextual familiarity that can be confounded with contextual manipulations under some conditions (Evans and Hammon, 1983a, 1983b; Hall & Channel, 1985; Leaton & Jordon, 1978).
In this study, the possibility that contextual cues significantly regulate the expression of HPA axis response habituation to repeated stress was further explored, using several manipulations aimed at modifying the putative associations between contextual cues and the stress stimulus (US). Some of these studies required knowledge of the persistence or duration of HPA axis habituation after its establishment, so the initial study tested whether HPA axis habituation to repeated loud noise exposures could be expressed up to four weeks after the initial repeated exposure regimen. The next set of manipulations employed a classical contextual extinction procedure, by first establishing HPA axis habituation to repeated stress exposures in a specific context, repeatedly re-exposing the habituated rats to the context without the stressor, and then re-testing the habituated stressor (Bouton, 1993; Jordan, Strasser, & McHale, 2000; Marlin & Miller, 1981; Rankin, 2000; Tomsic, Pedreira, Romano, Hermitte & Maldonado, 1998). Additional studies involved changing several contextual cues on the test day following the establishment of HPA axis habituation to repeated stress exposures in a design similar to that described previously (Grissom et al., 2007), however, animals were tested in unfamiliar or familiar contexts in different studies. A final study assessed whether acute HPA axis responses would be modulated by contextual pre-exposures. Loud noise was employed as the stressor in these studies as it readily activates the HPA axis and reliably elevates both corticosterone (CORT) and adrenocorticotropic hormone (ACTH) release (Borrell, Torrellas, Guaza & Borrell, 1980; Campeau & Watson, 1997; Collu & Jequier, 1976; Henkin & Knigge, 1963; Irwin, Segal, Hauger & Smith, 1989; Segal, Kuszenski & Swick, 1989), and importantly, these responses habituate with repeated loud noise exposures (Armario, Castellanos & Balasch, 1984; Campeau, Dolan, Akil & Watson, 2002; Masini, Day & Campeau, 2008; Sasse, Greenwood, Masini, Nyhuis, Fleshner, Day & Campeau, 2008). The present studies indicate that the contexts are discernable, and that familiarity of the context in which the stressor is experienced appears to provide an important variable in determining overall HPA axis activity. Taken together, the results of these studies suggest that contextual cues are not necessary for the expression of HPA axis response habituation to repeated loud noise exposures.
Experiment 1
The duration of retention of HPA axis response habituation following repeated homotypic stress exposures has only been explored to a very limited extent. In one of the clearest tests of the duration of HPA axis habituation, repeatedly restrained rats displayed similar habituation one day and three weeks following the repeated restraint (Bhatnagar, Huber, Nowak, & Trotter, 2002), suggesting relatively long-term retention of habituated HPA axis responses. Experiment 1 examined whether the habituated CORT and ACTH release obtained after 7 repeated daily exposures to loud noise would be retained when animals were re-tested either 24 hrs, 1, 2, or 4 weeks after being habituated.
Materials and Method
Subjects
Thirty-two male Sprague-Dawley rats (Harlan, Indianapolis, IN) weighing 200–225 g and 2 months of age were used. Upon arrival, animals were grouped 4 to 5 in clear polycarbonate cages (48 × 27 × 20 cm) containing wood shavings and covered with wire lids providing food (rat chow [Harlan Teklad, Chow, 8640; Madison, WI]) and water ad libitum. They were kept on a controlled light-dark cycle (lights on at 7 a.m. and off at 7 p.m.), under constant humidity and temperature conditions. All experimental procedures were conducted between 8 a.m. and 12 p.m. to reduce variability due to circadian hormonal variations. All procedures were reviewed and approved by the Institutional Animal Care and Use Committee of the University of Colorado and conformed to the National Institutes of Health (1986) Guide for the Care and Use of Laboratory Animals.
Noise apparatus
Audiogenic stress was administered inside acoustic chambers, which consisted of ventilated double wooden (2.54 cm plywood board) chambers, with the outer chamber lined internally with 2.54 cm insulation (Celotex, Suffolk, England). The inner chamber was painted black, and its internal dimensions were 59.69 cm (w) × 38.10 cm (d) × 50.80 cm (h), which allowed placement of a polycarbonate rat home cage. Each chamber had a single 15.24 cm × 22.86 cm Optimus speaker (#12–1769 - 120 W RMS [Radio Shack, Fort Worth, TX]) in the middle of the ceiling. Lighting was provided by a fluorescent lamp (15 W) located in the upper left corner of the chamber. Noise was produced by a General Radio (#1381 [Concord, MA]) solid-state random-noise generator with the bandwidth set at 2 Hz-50 kHz. The output of the noise generator was amplified (Pyramid Studio Pro #PA-600X [Brooklyn, NY]) and fed to the speakers. Noise intensity was measured daily before and after noise presentations by placing a Radio Shack Realistic Sound Level Meter (A scale; #33–2050) in an empty home cage at several locations and taking an average of the different readings. The ambient/quiet noise level inside the chamber was approximately 60 decibels-A scale (dBA), and approximately 55 dBA in the rat colony.
Procedure
One week after arrival from the supplier, all animals were single housed in clear polycarbonate cages with wire lids (46 × 25 × 22 cm) and divided into four groups: 0 week (n = 8), 1 week (n = 8), 2 weeks (n = 8), and 4 weeks (n = 8). After at least 4 days acclimation to single housing conditions, rats were pre-exposed to the acoustic chambers for 30 min/day for 4 consecutive days prior to their initial noise exposure, to minimize transport and novel environment induced HPA axis activity. Animals in their individual home cages were carefully transported down a hallway on a cart, in groups of 8. In the experimental room, animals in their home cages were individually placed into the acoustic chambers without the presentation of noise for 30 min. After this 4-day acclimation period, the rats in their home cages were transported down the hallway and placed into the acoustic chambers and immediately exposed to 95 dBA white noise for 30 min, then removed from the chamber and taken back to the animal colony. This intensity of noise results in a submaximal HPA axis response (Burow, Day & Campeau, 2005), which readily habituates over repeated exposures (Campeau & Watson, 2002). Exposure to loud noise was repeated one each of 7 consecutive days. Animals in the 0 week group underwent the habituation procedure and received their 8th noise (test) exposure on the following day, immediately after habituation. Animals in the 1, 2 and 4 week groups underwent the habituation procedure and received their 8th noise (test) exposure 1, 2 or 4 weeks following the 7th noise exposure, respectively. All animals were killed via decapitation immediately following the 30-min noise exposure test. All experimental animals received their test noise exposure and were decapitated on the same day. It should be noted that animals in each group were habituated to the loud noise at slightly different ages in order to allow presentation of the noise exposure test on the same day and at the same age for all animals. This is most notable for animals in the 4 week group, who were habituated at 2 months of age and tested at 3 months of age.
Blood Collection
Blood samples were collected on noise exposure days 1, 3, and 7 by a small tail nick, and from trunk blood immediately following decapitation after the 8th noise exposure. Immediately after the 1st, 3rd, and 7th noise exposures, rats from each group were gently restrained using a clean towel. A small incision was then made in the lateral tail vein with the corner of a sterile razor blade. Approximately 400 μl of blood was collected from each animal, using heparinized hematocrit capillaries for CORT samples, and non-heparinized capillaries for ACTH samples. Blood was deposited into ice chilled 1.5 mL microcentrifuge tubes (for ACTH samples, microcentrifuge tubes contained 15 μl EDTA [20 mg/ml]). The whole procedure lasted less than 3 min from removal to return of the animal to its home cage. Blood collected via the lateral tail vein was centrifuged at 14,000 rpm for 2 min, and the plasma was then pipetted into 0.5 ml Ependorf microcentrifuge tubes and stored at −80°C until assayed. Immediately following the final 30 min noise test, all animals were killed by decapitation and trunk blood was collected into ice chilled BD-Vacutainers coated with EDTA and were centrifuged at 2000 rpm for 10 min. Plasma was then pipetted into ice chilled 0.5 ml Ependorf microcentrifuge tubes and stored at −80°C until assayed.
In order to assure that the different methods of blood collection employed in this study would not affect plasma ACTH and CORT measurements, plasma samples collected in Experiment 5 (described below) were taken via both methods. Importantly, when assayed side by side as described below, plasma samples from the same animal taken within 5 minutes via tail nick and then trunk (as described above) yielded similar values for ACTH and CORT (independent samples t-test assuming equal variances for ACTH, t(40) = 1.73, p > .05, and CORT, t(40) = 1.68; p > .05).
ACTH Radioimmunoassay (RIA)
The ACTH assay was performed according to the manufacturer’s protocol (kit # 27130-DiaSorin Inc., Stillwater, MN). The sensitivity of the assay ranged from 0–1500 pg/ml. All samples from this experiment were measured simultaneously to reduce interassay variability.
CORT Enzyme Linked ImmunoSorbent Assays (ELISA)
The CORT assay was performed according to the manufacturer’s protocol (kit #901–097 – AssayDesigns, Ann Arbor, MI). All samples from this experiment were measured simultaneously to reduce interassay variability. Optical density was then measured on a BioTek Elx808 microplate reader and CORT levels were calculated against a standard curve generated concurrently.
Data Analysis
ACTH and CORT values on exposure days 1, 3, and 7 were statistically analyzed using repeated measures analyses of variance (ANOVA), with the different retention interval periods (0, 1, 2 or 4 weeks) as a between-subjects variable and with days as the repeated measures variable. An additional repeated measures ANOVA on the 7th and test (8th) loud noise exposures with the different retention intervals as a between-subjects factor was performed to determine potential changes in CORT and ACTH release at the different retention intervals, as compared to the final habituation index (7th loud noise exposure).
Results
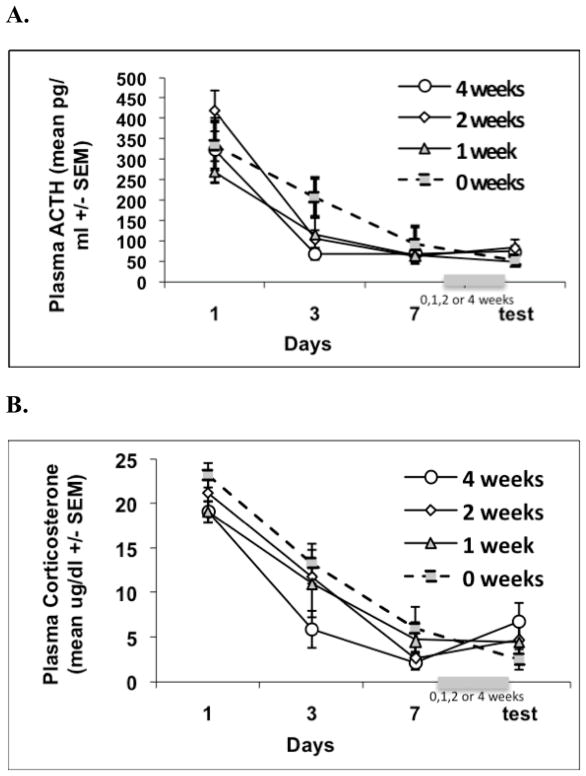
Repeated measures ANOVA on days 1, 3, and 7 revealed a significant days effect for both ACTH, F(2, 56) = 74.872, p < .0001, and CORT, F(2, 56) = 166.120, p < .0001, but no days × group effects, F(3, 28) = .357, p > .05 and F(3, 28) = 1.233, p > .05, respectively. There were no significant differences between groups for ACTH, F(3, 28) < 1, p = .471, or CORT, F(3, 28) < 1, p = .695, as shown in Figure 1. Repeated measures ANOVA between responses on the 7th and test (8th) loud noise exposures for ACTH revealed no difference between days, F(1, 29) < 1, p = .544, and no days × group interaction effects. A similar repeated measures ANOVA on CORT levels revealed no difference between days, F(1, 29) < 1, p = .365, and no significant days × group interaction effects (p > .05).
Figure 1.
Graphs show the mean plasma ACTH(A) and corticosterone (B) responses (±1 standard error of the mean) over the course of Experiment 1. Experiment 1 examined whether habituated CORT and ACTH release obtained after 7 repeated daily exposures to loud noise would be retained when animals were re-tested either 24 hrs, 1, 2, or 4 weeks after being habituated. Plasma endocrine values did not vary significantly between the groups on days 1, 3, 7 or test noise exposures.
Discussion
The results of Experiment 1 suggest that up to four weeks following 7 repeated exposures to 95 dBA loud noise, significant CORT and ACTH response habituation is retained. The results of this study are consistent with our prior findings with repeated loud noise, in which the robust CORT and ACTH responses induced by the initial noise exposure become increasingly smaller over repeated exposure days, to a point when, after 7 exposures, these responses were often indistinguishable from non-stressed control rats (Campeau et al., 2002; Day, Masini, & Campeau, 2009; Masini et al., 2008; Sasse et al., 2008). Furthermore, none of the groups displayed significant differences in CORT or ACTH levels between the seventh noise exposure and the test exposure, suggesting very limited, if any, spontaneous recovery of these responses up to four weeks after initial repeated noise exposures. These results are in general agreement with retention of habituation to other homotypic stressors, such as restraint (Bhatnagar et al., 2002), and strongly suggest that stress habituation is retained for periods of at least a month after the initial stress experience.
Experiment 2
As stated in the Introduction, recent evidence suggests that contextual cues may modulate the expression of response habituation to stress (Grissom et al., 2007), which fits with an associative view of habituation in general (Wagner, 1979). If associations between contextual cues and stress stimuli are necessary for the expression of habituated responses, including the neuroendocrine HPA axis responses, modulation of these associations should modify habituated responses. One procedure which could demonstrate such modification of associations would be to extinguish the contextual cues (Bouton & King, 1983; Bouton, 1993) following habituation to repeated loud noise exposures, such that the contextual cues would no longer prime retrieval of the loud noise stimulus memory (Wagner, 1979), thus some or all of the initial stressor-induced responses would be reinstated or restored. This procedure would be expected to reinstate higher HPA axis responses, compared to animals given no re-exposure to contextual cues in the absence of loud noise, as has been demonstrated for reinstatement of habituated behavioral responses in different organisms (Jordan et al., 2000; Rankin, 2000; Tomsic et al., 1998). Experiment 2 was therefore designed to examine whether, in habituated animals, re-exposure to the context in which loud noise exposures took place would reinstate larger noise-induced ACTH and CORT responses in habituated rats.
Materials and Methods
Subjects
Twenty-four male Sprague-Dawley rats (Harlan, Indianapolis, IN) weighing 200–225 g and 2 months of age were used. Animals were housed in the same manner as rats in Experiment 1.
Noise apparatus
Audiogenic stress was administered in the same chambers and in the same manner as described in Experiment 1.
Procedure
One week after arrival from the supplier, all animals were single housed in clear polycarbonate cages with wire lids (46 × 25 × 22 cm) and divided into three groups: habituated (H; n = 8), habituated and re-exposed to the noise chamber (H-NC; n = 8), and habituated and re-exposed to the noise chamber room but not the noise chambers themselves (H-C; n = 8). After 4 days acclimation to single housing conditions, all groups were pre-exposed to the acoustic chambers for 30 min/day for 4 days prior to their initial loud noise exposure in the same manner described in Experiment 1. After this acclimation period, the rats in their home cages were transported down the hallway and placed into the acoustic chambers and immediately exposed to 95 dBA white noise for 30 min, then removed from the chamber and taken back to the animal colony (after tail nicks on days 1, 3, and 7). Loud noise sessions were repeated for 7 consecutive days. Beginning on day 8, rats were either wheeled down the corridor and placed in the noise chambers for 30 min without receiving noise (H-NC), wheeled down the corridor and placed on a counter in the room with the noise chambers for 30 min (H-C), or were left in the animal colony without further manipulation (H) for 13 consecutive days. On day 21 (14 days after the end of repeated loud noise exposures), all rats were again exposed to 95dBA noise for 30 min and immediately killed by decapitation. The results of Experiment 1 indicated that the simple passage of time (2 weeks) in the H group would not restore the habituated CORT and ACTH responses to loud noise.
Blood Collection
Blood samples were collected on noise exposure days 1, 3, and 7 by a small tail nick, and following the 8th (test) noise exposure via decapitation, using the same protocol described in Experiment 1.
ACTH Radioimmunoassay (RIA)
The ACTH assay was performed according to the manufacturer’s protocol (kit # 27130-DiaSorin Inc., Stillwater, MN), as described in Experiment 1.
CORT Enzyme Linked ImmunoSorbent Assays (ELISA)
The CORT assay was performed according to the manufacturer’s protocol (kit #901–097 –AssayDesigns, Ann Arbor, MI), as described in Experiment 1.
Data Analysis
Values for CORT and ACTH were statistically analyzed as described in Experiment 1, with the different extinction manipulations (H, H-C, and H-NC) as the between-subjects variables and with re-exposure days as the repeated measures variable.
Results
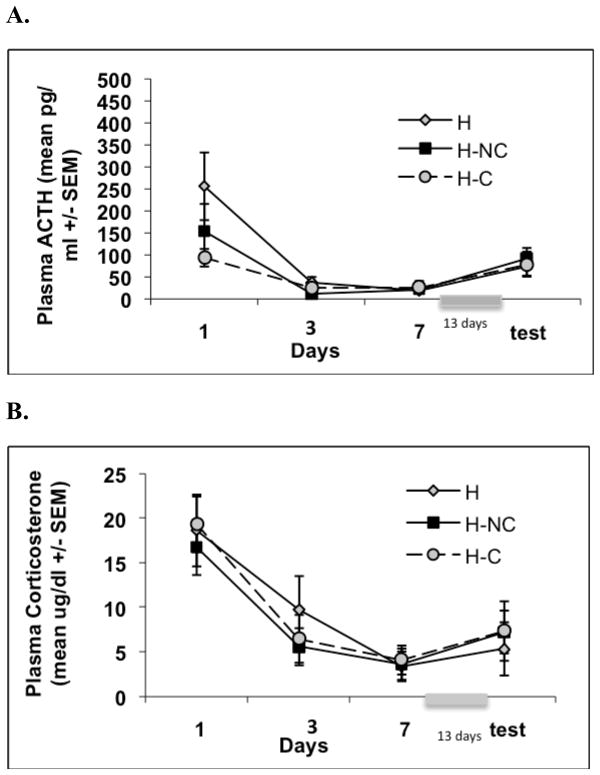
Repeated measures ANOVA on days 1, 3, and 7 revealed a significant days effect for both ACTH, F(2, 42) = 19.877, p < .001, and CORT, F(2, 42) = 51.742, p < .001, but no days X group interaction effects for either measure, F(2, 21) < 1, p > .05 and F(2, 21) < 1, p > .05, respectively. Repeated measures ANOVA on ACTH and CORT responses between noise exposure days 7 and test (8th noise) did not reveal significant differences between responses on these days, F(2, 21) < 1, p = .827 and F(2, 21) < 1, p = .756, respectively. There were no significant differences between groups on these days for ACTH, F(2, 21) < 1, p = .835, or CORT responses, F(2, 21) < 1, p = .943, as shown in Figure 2.
Figure 2.
Graphs show mean plasma ACTH(A) and corticosterone (B) responses (±1 standard error of the mean) over the course of Experiment 2. Experiment 2 examined whether, in habituated animals, re-exposure to the context in which loud noise exposures took place would reinstate larger noise-induced ACTH and CORT responses in habituated rats. Plasma endocrine values did not differ between H (rats habituated and kept in vivarium), H-NC (rats habituated and re-exposed to noise chambers without noise), and H-C (rats habituated and repeatedly placed on the counter in the noise chamber room) groups on days 1, 3, 7 or test.
Discussion
The results of Experiment 2 suggest that habituated ACTH and CORT responses were not reinstated by repeatedly re-exposing habituated animals to the associated context in the absence of loud noise. The different groups of rats again displayed significant habituation to the repeated noise exposures, and by the 7th loud noise session, no differences were observed in CORT or ACTH levels between any of the groups. Importantly, ACTH and CORT responses to the 8th (test) noise exposure were not significantly elevated compared to the 7th loud noise exposure, replicating the results of Experiment 1, and suggesting no effects of the extinction procedure on expression of habituated ACTH and CORT responses. Conceivably, the four pre-exposure sessions to the contextual cues prior to loud noise exposure might have produced latent inhibition effects and poorer retention of the habituated responses (Rankin, 2000; Tomsic et al., 1998), but the lack of differences between any of the groups argues against this possibility. Furthermore, unpublished data from a different study in our laboratory suggests that animals pre-exposed to a context for four days do not differ from non-pre-exposed animals in acute ACTH or CORT responsiveness to loud noise presentation (Nyhuis, Sasse, Masini, Day, & Campeau, unpublished data). Therefore, the results of the present study provide no support for the hypothesis that an association between contextual cues and stress stimuli are necessary for the expression of long-term habituated HPA axis responses.
Experiment 3
The results of Experiment 2 did not provide supportive evidence that contextual cues modulate the expression of habituated HPA axis responses. It is conceivable that contextual extinction is ineffective in modulating the association between contextual cues and stress stimuli. For instance, whereas contextual extinction procedures are often reported to modulate behavioral responding in many classes of paradigms (Bouton & King, 1983; Hall & Honey, 1990; Jordan et al., 2000; Rankin, 2000; Tomsic et al., 1998), there have been failures to demonstrate contextual extinction effects under some conditions (Baker & Mercier, 1982; Marlin & Miller, 1981). A manipulation which modifies contextual cues between repeated stimulus exposure sessions and the test session has been used to test the role of contextual cues in the expression of several habituated behavioral responses (Evans & Hammond, 1983a, 1983b; Hall & Channel, 1985; Hall & Honey, 1989; Honey, Pye, Lightbown, Rey & Hall, 1992; Jordan et al., 2000; Marlin & Miller, 1981; Rankin, 2000; Tomsic et al., 1998). This is also the procedure employed by Grissom et al. (2007) in their unique demonstration of the contextual specificity of HPA axis habituation to repeated restraint stress. In the present study, two distinct contextual environments were employed in order to test whether HPA axis habituation to repeated loud noise exposures in one context would transfer to a different context.
Materials and Methods
Subjects
Twenty-four male Sprague-Dawley rats (Harlan, Indianapolis, IN) weighing 200–225 g and 2 months of age were used. Animals were housed in the same manner as rats in Experiment 1.
Contexts and Noise Apparatus
Loud noise (105 dBA) was generated, amplified, and presented as described in Experiment 1, but inside one of two contexts differing on the basis of several static cues. This study was carried out several years prior to the other studies in this report, which explains the slightly different noise intensity employed (105 dBA) compared to the other studies, but HPA axis habituation at this intensity is also very reliable (Campeau et al., 2002), and should not detract from testing the effects of contextual shifts on habituated responses. Context A consisted of the same chambers as described in Experiment 1. Context B consisted of the same chambers as Context A, with the following differences: the inner chambers were lined with aluminum foil (different material), the 15-W incandescent lights were covered with red tissue paper, which provided lighting of different color and intensity, the floor angle was changed to 10° (different angle), and 1 ml of Pinesol™ was deposited on a small piece of paper towel and placed in a round plastic dish (10 cm diameter) in the right corner of the inner chamber (different odor). Context A and B were located in the same experimental room, but on opposite sides of the room. Noise intensity was measured daily before and after noise presentations by placing a Radio Shack Realistic Sound Level Meter (A scale; #33–2050) inside an empty polycarbonate cage and taking an average of the different readings.
Procedure
One week after arrival from the supplier, all animals were single housed in clear polycarbonate cages with wire lids (46 × 25 × 22 cm) and divided into four groups: Habituated in home cage in Context A and tested in Context A (A/A; n = 6), habituated in home cage in Context A and tested in Context B (A/B; n = 6), habituated in Context B and tested in Context B (B/B; n = 6), and habituated in Context B and tested in Context A (B/A; n = 6). After 4 days of acclimation to single housing conditions, all groups were pre-exposed only to the context in which they were to be exposed to loud noise for 30 min/day for 4 days prior to the beginning of loud noise exposures. Animals in their individual home cages were carefully transported down a hallway on a cart, in groups of 8. Animals were then individually placed into the appropriate context in their home cages, without the presentation of noise, for 30 min on each of four consecutive days. After this acclimation period, animals continued to be placed into the appropriate context for an additional 13 days, during which they received 105 dBA white noise for 30 min. On day 14, all rats were exposed to 105 dBA loud noise, but depending on experimental groups, they either received noise in the context in which they had previously experienced loud noise (groups A/A and B/B), or in the alternate context in which they were never exposed to or received noise (groups A/B and B/A). All animals were killed via decapitation immediately following the 30 min loud noise exposure on day 14.
Blood Collection
Blood samples were collected immediately following noise on days 1, 4, and 8 by a small tail nick, and following loud noise exposure on day 14 via decapitation (trunk blood), following the same protocol described in Experiment 1.
CORT Enzyme Linked ImmunoSorbent Assays (ELISA)
The CORT assay was performed according to the manufacturer’s protocol (kit #901–097 – AssayDesigns, Ann Arbor, MI), as described in Experiment 1. This study was performed before a reliable ACTH assay was available in the laboratory, so ACTH values are not available for Experiment 3.
Data Analysis
ACTH and CORT values on exposure days 1, 3, 7 and test were statistically analyzed using repeated measures analyses of variance (ANOVA), with the different contextual manipulations (training and testing contexts A and B) as the between-subjects factors (2 × 2 factorial design) and with days as the repeated measures variable. Additionally, one way factorial ANOVAs were conducted on days 1, 8 and 14 of loud noise exposures.
Results
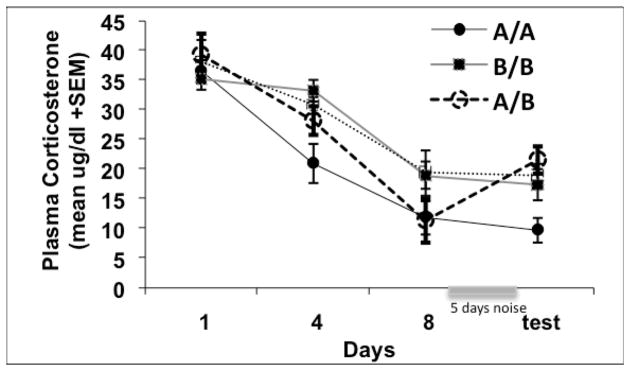
As shown in Figure 3, corticosterone habituation developed over the course of the repeated loud noise exposures in both Contexts A and B, as determined by a significant effect of days in a repeated measures ANOVA over exposure days 1, 4, 8 and 14, F(3, 57) = 60.46, p < .001. However, a days × training context interaction F(3,57) = 2.79, p = .049, and an overall training context effect, F(1, 19) = 7.79, p = .012, suggested that habituation in Context A was greater than in Context B. While a one way factorial ANOVA on day 1 indicated no significant effects of training context (p > .05), similar ANOVAs on days 8 and 14 revealed significant effects of training context on day 8, F(1,20) = 5.58, p = .028, a significant effect of testing context on day 14, F(1, 20) = 5.60, p = .028, and a significant training × testing context interaction, F(1, 20) = 9.45, p = .006 indicating that only the A to B switch group differed significantly during the test.
Figure 3.
Graphs show mean plasma corticosterone responses (±1 standard error of the mean) over the course of Experiment 3. Experiment 3 examined whether HPA axis habituation to repeated loud noise exposures in one context would transfer to a different context. Plasma CORT responses of animals in context A habituated to a greater extent than animals in context B (p < .05). Furthermore, animals switched from Context A to B following 13 consecutive noise exposures, but not Context B to A, showed increased CORT levels from the 8th to the 14th (test) noise exposure (p < .05). The y-axis range for this graph is larger because a greater intensity of noise was used in this particular study, thus the CORT responses were exaggerated.
Discussion
An important finding of Experiment 3 indicates that although the loud noise stimulus was physically and nominally identical in the two contexts, different overall habituation of CORT responses were obtained in the two contexts. It is conceivable that this difference was due to slight differences in standing waves generated in the differentially lined chambers (mat painted wood vs. wood covered aluminum foil). However, the initial corticosterone responses in the two contexts did not differ. It was the rate and final levels of CORT response habituation that were altered in the different contexts. Furthermore, whereas CORT levels were not measured on the last repeated noise exposure (day 13), observation of the two groups habituated and tested in the same contexts (A/A and B/B) showed respectively no differences in CORT levels between the 8th and the 14th loud noise exposure, indicating that the day 8 CORT measurements provided a good index of the final CORT habituation level.
Importantly, the switch of contextual cues during the test exposure to loud noise following habituation of the CORT response provided some mixed evidence for the hypothesis that contextual cues modulate habituated CORT responses. While the change of contextual cues from Context A to Context B during the test noise exposure produced a significant increase in CORT levels compared to the 8th noise exposure, the switch from Context B to Context A did not elevate CORT levels to the loud noise test above and beyond that observed to the 8th noise exposure. According to the contextual modulation hypothesis of habituation, both conditions should have elevated or restored CORT release to a loud noise exposure in environments with contextual cues never before associated with loud noise (Jordan et al., 2000; Wagner, 1979). The lack of contextual modulation of the habituated CORT response in group B/A provides a challenge for this hypothesis. And whereas the question of whether the contextual changes were prominent enough to be discriminated by the rats could be raised, it needs to be pointed out that if the contexts were different enough for group A/B to modulate the expression of habituated CORT release, the contextual discrimination should also have been sufficient to work in the B/A transition.
The pattern of results obtained in Experiment 3 might be explained by the observation obtained in behavioral habituation studies, in which the familiarity of contextual cues can significantly modify habituated responses (Evans & Hammond, 1983a, 1983b; Hall & Channel, 1985; Hall & Honey, 1989; Honey et al., 1992). According to this interpretation, Context B was most novel in aspects of olfactory (different smell than normal) and somatosensory (cage at a different angle) stimulation, which might explain the reduced CORT habituation observed in this context, as well as the apparent recovery of CORT response in the group switched to Context B following habituation in context A (A/B). The reason why the switch from Context B to Context A did not elevate or restore habituated CORT responses could have been due to the relative lack of novelty experienced in Context A given the similarity of odor and cage angle in this relatively standard environment, more similar to the vivarium environment. This possibility was assessed in Experiment 4.
Experiment 4
Experiment 3 provided only limited support for the hypothesis that contextual cues modulate the expression of HPA axis habituation in a context switch design. As discussed above, it could be argued that the most proximal contextual cues provided by the rats’ home cages in Experiment 3 were not manipulated, and therefore, simply changing the more distal cues such as chamber lining, lighting, angle, and odor provided only limited cue changes, which were most prominent in the Context A to Context B shift that did provide some restoration of habituated CORT release on the loud noise test. Alternatively, the major variable responsible for the finding of a significant context switch effect, but only from Context A to Context B, was the relative novelty of Context B on the final loud noise test. Under these conditions, novel contextual cues may provide the important variable determining the recovery of habituated HPA axis responses, just as exposure of rats to novel environments elevates HPA axis responses in many situations (Bassett, Cairncross & King, 1973; Hennessy, Levin & Levine, 1977; Pfister, 1979). This interpretation would also support a number of reports indicating that familiarity of the test context significantly modulates the expression of various habituated behavioral responses (Evans & Hammond, 1983a, 1983b; Hall & Channel, 1985; Hall & Honey, 1989; Honey et al., 1992). The present study was therefore designed to determine whether the manipulation of more proximal contextual cues compared to those in Experiment 3 would modulate the expression of HPA axis responses to a loud noise test following habituation, while simultaneously familiarizing rats to both experimental contexts equally in order to minimize the potential effects of novelty.
Materials and Methods
Subjects
Thirty-two male Sprague-Dawley rats (Harlan, Indianapolis, IN) weighing 200–225 g and 2 months of age were used. Animals were housed in the same manner as rats in Experiment 1.
Noise apparatus
Loud noise stress was administered inside one of two contexts. The first context (Context A) consisted of the same chambers and in the same manner as described in Experiment 1, where the rats’ home cages were placed in the chambers for noise exposures. In the second context (Context C), loud noise was administered inside acoustic chambers which were identical to those used in context A, but with slightly different internal dimensions [59.69 cm (w) × 38.10 cm (d) × 38.10 cm (h)] and located in a different experimental room. The ambient/quiet noise level inside the chamber was approximately 60 decibels-A scale (dBA). In context C, animals were removed from their home cages and placed into Rubbermaid (#2963 [Fairlawn, OH]) blue round plastic buckets with the dimensions 30.5 (h) × 28.2 (w) × 26.7 (d) cm. The buckets were covered with wire mesh lids that did not contain food or water. 500 μl of Safeway brand pine scented all purpose cleaner (#S2696 [Pleasanton, CA]) soaked on a paper towel was placed inside the noise chamber in the back right corner in Context C. Noise intensity was measured daily before and after noise presentations by placing a Radio Shack Realistic Sound Level Meter (A scale; #33–2050) inside of the bucket at several locations and taking an average of the different readings.
Procedure
One week after arrival from the supplier, all animals were single housed in clear polycarbonate cages with wire lids (46 × 25 × 22 cm) and divided into four groups: Habituated and tested in Context A (A/A; n = 8), habituated in Context A and tested in Context C (bucket - A/C; n = 8), habituated and tested in Context C (C/C; n = 8), and habituated in Context C and tested in Context A (C/A; n = 8). In addition, on each day of habituation and acclimation all rats were exposed to the alternate context, without noise, for 30 min. After 4 days of acclimation to single housing conditions, all groups were pre-exposed to each of the two contexts for 30 min/day for 4 days prior to their initial noise exposure. Animals in their individual home cages were carefully transported down a hallway on large racks. All animals remained in the behavioral corridor in a room adjacent to the experimental rooms each day from 8 a.m. until 1 p.m. Animals were transported from this room to the noise chamber rooms on a cart in groups of 8. Animals were then individually placed into the acoustic chambers in their home cage in Context A or were removed from their home cages and placed into the buckets in Context C in an adjacent experimental room, without the presentation of noise for 30 min each. After this acclimation period, animals continued to be placed into each of the two contexts for 30 min every day. Depending on experimental group, animals either received 95 dBA white noise for 30 min in their home cage in Context A, or in the buckets of Context C. After animals were exposed to each of the two contexts, they were placed back in the holding room on a rack and remained there until all rats had been run through each context. This habituation procedure was repeated for 7 consecutive days. Animals were alternately placed into context A or B first each day, did not always immediately switch from one context to the next within the same day (i.e.- rather than being placed directly from context A into context B or vice versa every day, animals would be placed in context A or B for 30 min, placed back in the holding room for a minimum of 30 min., and then removed from the holding room and placed in the second context every other day), and each animal was placed in contexts during various times between 8 a.m. and 1 p.m. On day 8, depending on experimental groups, animals either received loud noise in the context in which they were habituated to loud noise (A/A and C/C), or in the context in which they had been familiarized but had never experienced loud noise (A/C and C/A). All animals were killed via decapitation immediately following the 8th (test) 30 min loud noise exposure.
Blood Collection
Blood samples were collected immediately following the 1st, 3rd, and 7th loud noise exposures by a small tail nick, and following the 8th noise exposure by collection of trunk blood following decapitation, as described in Experiment 1.
ACTH Radioimmunoassay (RIA)
The ACTH assay was performed according to the manufacturer’s protocol (kit # 27130-DiaSorin Inc., Stillwater, MN), as described in Experiment 1.
CORT Enzyme Linked ImmunoSorbent Assays (ELISA)
The CORT assay was performed according to the manufacturer’s protocol (kit #901–097 – AssayDesigns, Ann Arbor, MI), as described in Experiment 1.
Data Analysis
Values for CORT and ACTH were statistically analyzed as described in Experiment 3, with the different contextual manipulations (training and testing contexts A and C) as the between-subjects factors (2 × 2 factorial design) and with days as the repeated measures variable.
Results
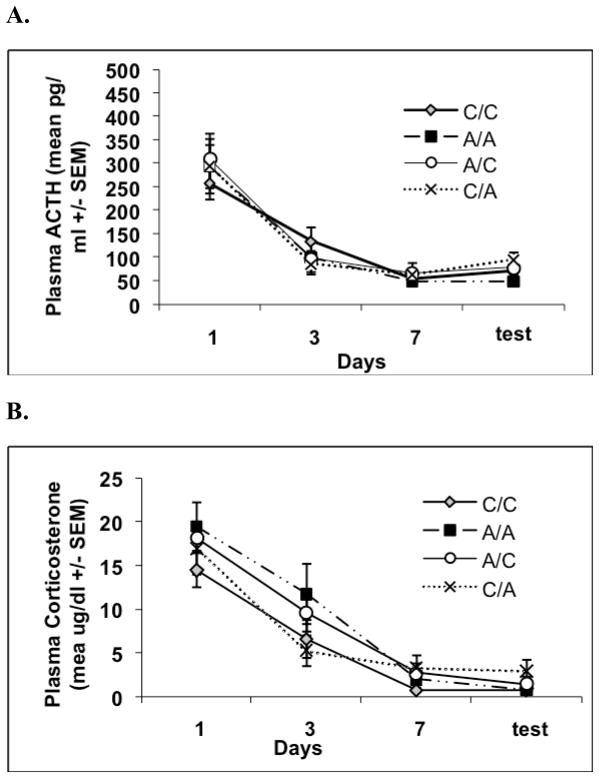
Two animals were excluded from all analyses because they had no endocrine response to the noise exposure throughout the course of the entire study. Repeated measures ANOVA on days 1, 3, 7 and 8 (test) indicated significant habituation as revealed by a significant days effect for both ACTH, F(3, 78) = 72.40, p < .0001, and CORT, F(3, 78) = 79.60, p < .0001, but no days X group interaction effects for either measure, as shown in Figure 4.
Figure 4.
Graphs show mean plasma ACTH(A) and corticosterone (B) responses (±1 standard error of the mean) over the course of Experiment 4. Experiment 4 examined whether manipulation of more proximal contextual cues compared to those in Experiment 3 would modulate the expression of HPA axis responses to a loud noise test following habituation, while simultaneously familiarizing rats to both experimental contexts equally in order to minimize the potential effects of novelty. Plasma ACTH and corticosterone values did not significantly differ between groups on days 1, 3, 7 or test noise exposures.
Discussion
The results of Experiment 4 suggest that habituated CORT and ACTH responses obtained in one context transferred completely when loud noise was presented in a different, but familiar context. All groups displayed significant and similar habituation of CORT and ACTH responses after 7 days of repeated loud noise exposures. Moreover, there was no recovery of CORT or ACTH responses between the 7th and test (8th) loud noise exposures in any of the groups, whether the test was performed in the habituated context or a distinct context never previously paired with loud noise. Given that the two contexts (A and C) were likely more distinct in this study as compared to those in Experiment 3, the results suggest that contextual cues do not readily modulate expression of habituated HPA axis responses to repeated loud noise stress. Given these negative results, it was deemed important to assess whether context A and C were different enough to be discriminated by rats. For this purpose, a contextual pre-exposure paradigm on acute HPA axis responses to loud noise was tested in the following study.
Experiment 5
Experiments 3 and 4 provided very little support for the hypothesis that contextual cues regulate the expression of HPA axis habituation in context switch designs. However, it could be argued that consistencies in the experimental procedure such as time of day when the rats were tested or daily transportation to the experimental rooms by the same experimenter may have provided all rats with important situational cues other than the manipulated static contextual cues to which rats were exposed. These additional cues may have masked or overridden the intended contextual manipulations of the different contexts employed in the experiments described above. It would therefore be important to establish whether animals could distinguish between the two contexts used in Experiment 4. Experiment 5 was designed to address these issues by evaluating whether context pre-exposures, with similar situational cues (i.e., time of day, transportation, and experimenter), could be shown to produce a reduction of the acute HPA axis responses to loud noise when experienced in a pre-exposed as compared to a novel context.
Materials and Methods
Subjects
Thirty-four male Sprague-Dawley rats (Harlan, Indianapolis, IN) weighing 200–225 g and 2 months of age were used. Animals were housed in the same manner as rats in Experiment 1.
Noise apparatus
Loud noise stress was administered in Context C, in the same manner described in experiment 4.
Procedure
Immediately upon arrival from the supplier, all animals were single housed in clear polycarbonate cages with wire lids (46 × 25 × 22 cm) and divided into five groups: the 12-Same group was pre-exposed for 11 days in Context C and exposed to noise on day 12 (test) in Context C (12-S; n = 6), the 12-Different group was pre-exposed for 11 days in Context A (as described previously) and exposed to noise on day 12 (test) in Context C (12-D; n = 6), The Control-12 group was pre-exposed for 12 days to Context C (C-12; n = 8), the Control-0 group was given no context pre-exposures and placed in Context C on test day (C-0; n = 8), and the Acute group was given no pre-exposures and exposed to noise in Context C on the test day (n = 6). After 4 days of acclimation to single housing conditions, all animals in their individual home cages were carefully transported down a hallway on large racks. All animals remained in the behavioral corridor in a room adjacent to the experimental rooms each day from 8 a.m. until 1 p.m. Animals in the 12-D, C-12, C-0, and acute groups were briefly handled each day to control for the additional handling of animals in the 12-S group when being placed into the buckets of context C. All animals in the 12-S group were pre-exposed to context C for 30 min/day for 11 days prior to their acute noise exposure in context C. All animals in the 12-D group were pre-exposed to context A for 30 min/day for 11 days prior to their acute noise exposure in context C. Animals were transported from this room to the noise chamber rooms on a cart in groups of 6 to 8. Depending on group, animals were then individually placed into the acoustic chambers in their home cage in context A or were removed from their home cages and placed into the buckets in context C in an adjacent experimental room, without the presentation of noise for 30 min each. On day 12, control animals (C-12 and C-0 groups) were placed into the buckets without noise exposure for 30 min, and all test animals (12-S, 12-D, and Acute) received 95 dBA white noise for 30 min in Context C. All animals were killed via decapitation immediately following the 30 min context or loud noise exposure on day 12.
Blood Collection
Blood samples were collected immediately following the acute noise exposure. In order to compare the two methods of blood collection employed in the previous experiments, samples were taken both by a small tail nick immediately followed by collection of trunk blood following decapitation for 21 animals in this study. The remaining 13 animals were rapidly decapitated and trunk blood collected on the test day. All ACTH and CORT comparisons discussed in this section were made using trunk blood.
ACTH Radioimmunoassay (RIA)
The ACTH assay was performed according to the manufacturer’s protocol (kit # 27130-DiaSorin Inc., Stillwater, MN), as described in Experiment 1.
CORT Enzyme Linked ImmunoSorbent Assays (ELISA)
The CORT assay was performed according to the manufacturer’s protocol (kit #901–097 – AssayDesigns, Ann Arbor, MI), as described in Experiment 1.
Data Analysis
ACTH and CORT values for no noise controls were statistically analyzed using an independent samples t-test with equal variances assumed, with significance set at p < .05. ACTH and CORT values following acute noise exposure were statistically analyzed using one-way analyses of variance (ANOVA), with the different group manipulations as a between-subjects variable. Group differences were evaluated using Tukey’s honestly significant difference (HSD) multiple means comparisons, with significance set at p < .05 for all tests.
Results
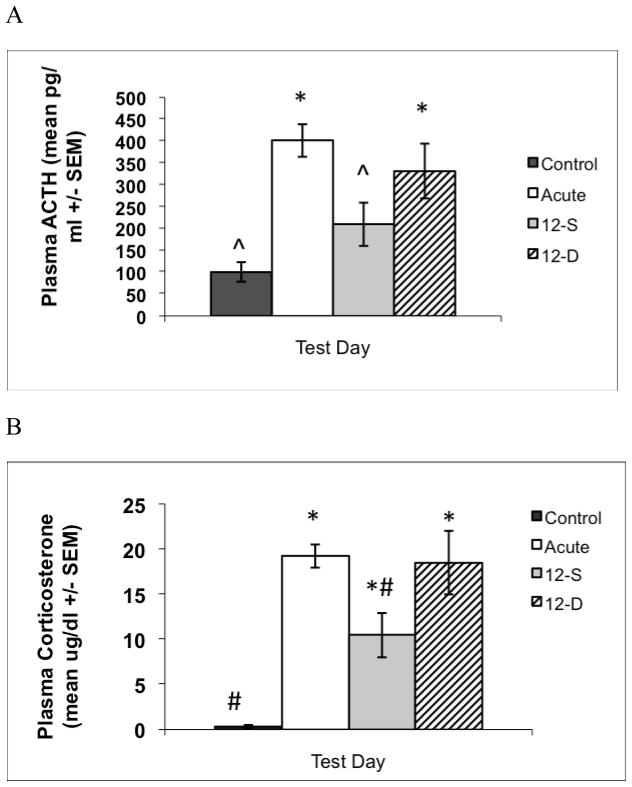
Independent samples t-test assuming equal variances on C-0 and C-12 samples revealed no significant differences between control groups for ACTH, t(14) < 1, p > .05, or CORT, t(14) = 1.49, p > .05. Therefore, these groups were combined into one group (control) for all further analyses. One-way ANOVA following the acute noise exposure revealed a group effect for ACTH, F(3, 33) = 14.13, p < .0001, as shown in Figure 5. Post hoc comparisons revealed that the acute group displayed significantly higher plasma ACTH response than the control group (Tukey’s HSD; p’s < .05). Although post hoc analysis revealed no difference between the 12-D and 12-S groups, there were differences between the 12-S group and the Acute group but not the control group, whereas the 12-D group differed from the Control but not the Acute group (Tukey’s HSD; p’s < .05). One-way ANOVA following the acute noise exposure revealed a group effect for CORT, F(3,33) = 39.13, p < .0001, as shown in Figure 5. Post hoc comparisons revealed that the Acute group displayed a significantly higher plasma CORT response than the Control group and the 12-S group, and that the 12-D group displayed higher plasma CORT than the 12-S and Control groups (Tukey’s HSD; p’s < .05). Furthermore, the Control group displayed significantly lower plasma CORT than the 12-S group (Tukey’s HSD; p’s < .05).
Figure 5.
Graphs show mean plasma ACTH (A) and corticosterone (B) responses (±1 standard error of the mean) on the test day of Experiment 5. This study examined whether animals could distinguish between contexts A and C despite other situational constancies in the experimental protocol for all groups. Rats in the 12-S group (which were pre-exposed to the noise context) but not the 12-D group (which were not pre-exposed to the noise context) displayed a lower plasma ACTH and CORT response to acute noise in context C as compared to animals in the Acute group. * indicates a significant difference from Control group, ^ indicates a significant difference from Acute group, and # indicates a significant difference from both the Acute and 12-D groups (Tukey’s HSD; p’s < .05).
Discussion
The results of Experiment 5 suggest that pre-exposure to the context in which a stressor is to be later experienced can reduce the acute HPA axis responses to this stressor, at least for audiogenic stress. This effect is furthermore specific to the pre-exposed context, as pre-exposure to a different context does not produce an attenuated HPA axis response. Because of this context specificity, the results of Experiment 5 further indicate that rats were able to distinguish between contexts A and C despite constancies in some other situational cues (relative time of day, transportation, and experimenter) for all groups. Animals in the 12-S group were familiarized to context C for eleven 30 min sessions prior to noise exposure, whereas animals in the 12-D group were familiarized to context A for eleven 30 min sessions prior to noise exposure in context C. Animals in the 12-S group, but not the 12-D group, displayed a lower plasma ACTH and CORT response to acute noise in context C as compared to animals in the Acute group. This effect is likely due a contextual pre-exposure effect, in which the rats’ previous innocuous experience in context C (the eleven pre-exposures) attenuated the acute ACTH and CORT response to loud noise in this context. However, animals in the 12-D group did not display a reduced plasma ACTH or CORT response as compared to controls, indicating that pre-exposure to context A, the experimenter, or other situational cues, were not sufficient to produce HPA axis attenuation in these animals. Importantly, these results indicate that rats were able to discriminate between context A and context C, regardless of the fact that aspects of the experimental protocol were similar for all animals.
General Discussion
The current series of experiments was designed to further characterize habituation of HPA axis responses to repeated stress, employing loud noise, which readily elevates CORT and ACTH plasma levels in rats (Borrell, et al., 1980; Campeau & Watson, 1997; Collu & Jequier, 1976; Henkin & Knigge, 1963; Irwin et al., 1989; Segal et al., 1989). The hypothesis that contextual cues modulate the expression of habituated HPA axis responses following stress habituation was tested, given a recent report that habituated corticosterone responses to repeated restraint were modulated by contextual cues (Grissom et al., 2007). It was first demonstrated in Experiment 1 that rats exhibit significantly reduced HPA axis responses to repeated loud noise exposures, with limited, if any, recovery after up to 4 weeks following the initial loud noise habituation. It is unlikely that this effect is based on auditory sensory deficits due to repeated exposures to loud noise because studies with a similar number of loud noise exposures of even louder intensities of white noise do not significantly modify sensitive indices of auditory functions, such as auditory-evoked brainstem potentials (Campeau et al., 2002), or the amplitude of the acoustic startle reflex and its prepulse facilitation or inhibition (Masini et al., 2008). This long-term retention of HPA axis response habituation to repeated loud noise exposures is consistent with the results of a prior study employing repeated restraint exposures (Bhatnagar et al., 2002).
The long-term retention of HPA axis response habituation with little or no recovery made it possible to assess the possibility that re-exposure of rats to the context in which they had initially experienced the loud noise would later re-establish HPA axis responsiveness to a subsequent loud noise exposure. This classic extinction procedure is effective in modulating other types of associative aversive and appetitive memories (Bouton & King, 1983; Bouton, 1993), and has been shown to be effective in modulating behavioral response habituation under some conditions (Jordan et al., 2000; Rankin et al., 2000; Tomsic et al., 1998), but not others (Marlin & Miller, 1981). The results of Experiment 2 indicated that habituated HPA axis responses are insensitive to extinction of contextual cues under the experimental parameters employed. This is one of the first attempts at modifying contextual cues with extinction procedures in the determination of HPA axis response modification following repeated stress exposures. The fact that rats were always treated in their home cages within the experimental chambers may have reduced the effectiveness of the contextual extinction procedures attempted, providing a limited test of putative contextual modulation of HPA axis response habituation. This possibility could eventually be tested in a design avoiding treatments of experimental animals in their home cages.
In order to test more directly the putative influence of contextual cues on the expression of HPA axis response habituation, Experiments 3 and 4 sought to modify contextual cues between the initial repeated loud noise exposures and the test exposure. These studies do not support the hypothesis that contextual cues modulate habituated HPA axis responses, as modifications of contextual cues failed to reliably and reproducibly restore HPA axis responses. The results of Experiment 5, however, established that context pre-exposure could inhibit acute loud noise induced HPA axis responses, and that this inhibition was context specific, demonstrating the discernability of the contexts employed in these studies. Together, the lack of effect of the extinction and discernable context switch procedures do not support the hypothesis that context-US associations (Wagner, 1979) are necessary for the expression of stress habituation. Furthermore, under this view, in the extreme case in which context would never change, long-term habituation should not develop; the context would provide no predictive information, as is the case for USs that are equally probable in the presence or absence of conditioned stimuli (Rescorla, 1968). In a recent study, however, long-term habituation to repeated loud noise was observed in rats housed in the same context for the entire duration of the study (8 days; Masini et al., 2008).
The lack of contextual modulation of habituated HPA axis responses is in keeping with several behavioral studies of habituation that have also reported a lack of modulation of habituated responses using contextual modification procedures (Evans & Hammond, 1983a, 1983b; Hall & Channel, 1985; Hall & Honey; 1989, Honey et al., 1992; Leaton, 1974; Leaton & Jordon, 1978; Marlin & Miller, 1981). A key factor revealed by some of these studies established the importance of controlling for familiarity of the contextual cues, in that the recovery of habituated responses in modified contexts disappears when contexts are made equally familiar (Hall & Channel, 1985; Hall & Honey, 1989; Honey et al., 1992). This factor could account for the results observed in Experiment 3, in which the most novel context (Context B) produced a modest level of habituated HPA axis response recovery, an effect that was not observed when the different contexts were made equally familiar in Experiment 4. This might also explain the pattern of results of Grissom et al. (2007), in which complete recovery of a habituated corticosterone (but not ACTH) response was observed in a room with a novel unfamiliar odor. However, a number of studies using behavioral indices of habituation have also demonstrated some recovery of habituated responses when controlling for context familiarity (Evans & Hammond, 1983a, 1983b; Jordan et al., 2000; Rankin, 2000). Therefore, the exact conditions under which habituated responses may come under the control of contextual cues remain to be defined, and this conclusion currently generalizes to repeated homotypic stress habituation, until additional studies clearly define the role of novelty, context, and perhaps stressor significance (Evans & Hammond, 1983a, 1983b) in this process. The present results do not rule out the possibility that under some conditions, associative factors control habituated stress responses. However, they reinforce the possibility that some forms of habituation are independent from associative contextual regulation (Jordan et al., 2000; Marlin & Miller, 1981), the importance of determining the contribution of associative and non-associative factors in experimental habituation models, and the likely possibility that these two forms of habituation are mediated by distinct underlying neural processes.
Acknowledgments
We would like to acknowledge the technical expertise of Jessica Babb and Ryan Newsom in the processing of blood samples in some of the studies. We also thank Melissa Noel for her help in carrying out Experiment 3. These studies were supported by grants from the National Institute of Mental Health, K02 MH068016 and R01 MH077152 (awarded to Serge Campeau).
Footnotes
Publisher's Disclaimer: The following manuscript is the final accepted manuscript. It has not been subjected to the final copyediting, fact-checking, and proofreading required for formal publication. It is not the definitive, publisher-authenticated version. The American Psychological Association and its Council of Editors disclaim any responsibility or liabilities for errors or omissions of this manuscript version, any version derived from this manuscript by NIH, or other third parties. The published version is available at www.apa.org/pubs/journals/bne
References
- Armario A, Castellanos JM, Balasch J. Adaptation of anterior pituitary hormones to chronic noise stress in male rats. Behavioral and Neural Biology. 1984;41:71–76. doi: 10.1016/s0163-1047(84)90745-3. [DOI] [PubMed] [Google Scholar]
- Bhatnagar S, Huber R, Nowak N, Trotter P. Lesions of the posterior paraventricular thalamus block habituation of hypothalamic-pituitary-adrenal responses to repeated restraint. Journal of Neuroscience. 2002;14:403–410. doi: 10.1046/j.0007-1331.2002.00792.x. [DOI] [PubMed] [Google Scholar]
- Baker AG, Mercier P. Extinction of the context and latent inhibition. Learning and Motivation. 1982;13:391–416. [Google Scholar]
- Bassett JR, Cairncross KD, King MG. Parameters of novelty, shock predictability and response contingency in corticosterone release in the rat. Physiology & Behavior. 1973;10:901–907. doi: 10.1016/0031-9384(73)90060-7. [DOI] [PubMed] [Google Scholar]
- Borrell J, Torrellas A, Guaza C, Borrell S. Sound stimulation and its effects on the pituitary-adrenocortical function and brain catecholamines in rats. Neuroendocrinology. 1980;31:53–59. doi: 10.1159/000123050. [DOI] [PubMed] [Google Scholar]
- Bouton ME. Context, time, and memory retrieval in the interference paradigms of Pavlovian learning. Psychological Bulletin. 1993;114:80–99. doi: 10.1037/0033-2909.114.1.80. [DOI] [PubMed] [Google Scholar]
- Bouton ME, King DA. Contextual control of the extinction of conditioned fear: Tests for the associative value of the context. Journal of Experimental Psychology: Animal Behavior Processes. 1983;9:248–265. [PubMed] [Google Scholar]
- Burow A, Day EW, Campeau S. A detailed characterization of loud noise stress: Intensity analysis of hypothalamo–pituitary–adrenocortical axis and brain activation. Brain Research. 2005;1061:63–73. doi: 10.1016/j.brainres.2005.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campeau S, Dolan D, Akil H, Watson SJ., Jr c-fos mRNA induction in acute and chronic audiogenic stress: Possible role of the orbitofrontal cortex in habituation. Stress. 2002;5:121–130. doi: 10.1080/10253890290027895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campeau S, Watson SJ. Neuroendocrine and behavioral responses and brain pattern of c-fos induction associated with audiogenic stress. Journal of Neuroendocrinology. 1997;9:577–588. doi: 10.1046/j.1365-2826.1997.00593.x. [DOI] [PubMed] [Google Scholar]
- Christoffersen GRJ. Habituation: Events in the history of its characterization and linkage to synaptic depression. A new proposed kinetic criterion for its identification. Progress in Neurobiology. 1997;53:45–66. doi: 10.1016/s0301-0082(97)00031-2. [DOI] [PubMed] [Google Scholar]
- Collu R, Jequier JC. Pituitary response to auditory stress: Effects of treatment with a-methyl-p-tyrosine. Usefulness of a factorial mixed design for statistical analysis. Canadian Journal of Physiology and Pharmacology. 1976;54:596–602. doi: 10.1139/y76-082. [DOI] [PubMed] [Google Scholar]
- Day HE, Masini CV, Campeau S. Reversible inactivation of the auditory thalamus disrupts HPA axis habituation to repeated loud noise stress exposures. Brain Research. 2009;1276:123–30. doi: 10.1016/j.brainres.2009.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans JGM, Hammond GR. Habituation and recovery of orienting in rats as a function of stimulus significance. Animal Learning and Behavior. 1983a;11:424–430. [Google Scholar]
- Evans JGM, Hammond GR. Differential generalization of habituation across contexts as a function of stimulus significance. Animal Learning & Behavior. 1983b;11:431–434. [Google Scholar]
- Grissom N, Bhatnagar S. Habituation to repeated stress: get used to it. Neurobiology of Learning and Memory. 2009;92:215–224. doi: 10.1016/j.nlm.2008.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grissom N, Iyer V, Vining C, Bhatnagar S. The physical context of previous stress exposure modifies hypothalamic-pituitary-adrenal responses to a subsequent homotypic stress. Hormones and Behavior. 2007;51:95–103. doi: 10.1016/j.yhbeh.2006.08.011. [DOI] [PubMed] [Google Scholar]
- Groves PM, Thompson RF. Habituation: A dual-process theory. Psychological Review. 1970;77:419–450. doi: 10.1037/h0029810. [DOI] [PubMed] [Google Scholar]
- Hall G, Channell S. Differential effects of contextual change on latent inhibition and on the habituation of an orienting response. Journal of Experimental Psychology: Animal Behavior Processes. 1985;11:470–481. [Google Scholar]
- Hall G, Honey RC. Contextual effects in conditioning, latent inhibition, and habituation: Associative and retrieval functions of contextual cues. Journal of Experimental Psychology: Animal Behavior Processes. 1989;15:232–241. [Google Scholar]
- Harris JD. Habituatory response decrement in the intact organism. Psychological Bulletin. 1943;40:385–422. [Google Scholar]
- Henkin RI, Knigge KM. Effect of sound on the hypothalamic-pituitary-adrenal axis. American Journal of Physiology. 1963;204:910–914. doi: 10.1152/ajplegacy.1963.204.4.710. [DOI] [PubMed] [Google Scholar]
- Hennessy JW, Levin R, Levine S. Influence of experiential factors and gonadal hormones on pituitary-adrenal response of the mouse to novelty and electric shock. Journal of Comparative and Physiological Psychology. 1977;91:770–777. doi: 10.1037/h0077368. [DOI] [PubMed] [Google Scholar]
- Honey RC, Pye C, Lightbown Y, Rey V, Hall G. Contextual factors in neophobia and its habituation: The role of absolute and relative novelty. Quarterly Journal of Experimental Psychology B. 1992;45:327–347. [PubMed] [Google Scholar]
- Horn G, Hinde RA. Short-term changes in neural activity and behaviour: A conference sponsored by King’s College Research Centre, Cambridge. Cambridge University Press; Cambridge, UK: 1970. p. 628. [Google Scholar]
- Irwin MR, Segal DS, Hauger RL, Smith TL. Individual behavioral and neuroendocrine differences in responsiveness to audiogenic stress. Pharmacology, Biochemistry & Behavior. 1989;32:913–917. doi: 10.1016/0091-3057(89)90058-0. [DOI] [PubMed] [Google Scholar]
- Jokinen J, Nordstrom P. HPA axis hyperactivity as suicide predictor in elderly mood disorder inpatients. Psychoneuroendocrinology. 2008;33:1387–1393. doi: 10.1016/j.psyneuen.2008.07.012. [DOI] [PubMed] [Google Scholar]
- Jordan WP, Strasser HC, McHale L. Contextual control of long-term habituation in rats. Journal of Experimental Psychology: Animal Behavior Processes. 2000;26:323–339. doi: 10.1037//0097-7403.26.3.323. [DOI] [PubMed] [Google Scholar]
- Leaton RN. Long-term retention of the habituation of lick suppression in rats. Journal of Comparative and Physiological Psychology. 1974;87:1157–1164. doi: 10.1037/h0037596. [DOI] [PubMed] [Google Scholar]
- Leaton RN, Jordan WP. Habituation of the EEG arousal response in rats: Short- and long-term effects, frequency specificity, and wake-sleep transfer. Journal of Comparative and Physiological Psychology. 1978;92:803–814. doi: 10.1037/h0077538. [DOI] [PubMed] [Google Scholar]
- Lubow RE, Moore AU. Latent inhibition: Effect of non reinforced preexposure to the conditioned stimulus. Journal of Comparative Physiology & Psychology. 1959;52:415–419. doi: 10.1037/h0046700. [DOI] [PubMed] [Google Scholar]
- Marlin NA, Miller RR. Associations to contextual stimuli as a determinant of long-term habituation. Journal of Experimental Psychology: Animal Behavior Processes. 1981;7:313–333. [PubMed] [Google Scholar]
- Marti O, Armario A. Anterior pituitary response to stress: time-related changes and adaptation. International Journal of Developmental Neuroscience. 1998;16:241–260. doi: 10.1016/s0736-5748(98)00030-6. [DOI] [PubMed] [Google Scholar]
- Masini CV, Day HE, Campeau S. Long-term habituation to repeated loud noise is impaired by relatively short interstressor intervals in rats. Behavioral Neuroscience. 2008;122:210–223. doi: 10.1037/0735-7044.122.1.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfister HP. The glucocorticosterone response to novelty as a psychological stressor. Physiology & Behavior. 23:649–652. doi: 10.1016/0031-9384(79)90154-9. [DOI] [PubMed] [Google Scholar]
- Rankin CH. Context conditioning in habituation in the nematode Caenorhabditis elegans. Behavioral Neuroscience. 2000;114:496–505. [PubMed] [Google Scholar]
- Rankin CH, Abrams T, Barry RJ, Bhatnagar S, Clayton DF, Colombo J, Coppola G, Geyer MA, Glanzman DL, Marsland S, McSweeney FK, Wilson DA, Wu CF, Thompson RF. Habituation revisited: An updated and revised description of the behavioral characteristics of habituation. Neurobiology of Learning and Memory. 2009;92:135–138. doi: 10.1016/j.nlm.2008.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rescorla RA. Probability of shock in the presence and absence of CS in fear conditioning. Journal of Comparative and Physiological Psychology. 1968;66:1–5. doi: 10.1037/h0025984. [DOI] [PubMed] [Google Scholar]
- Sasse SK, Greenwood BN, Masini CV, Nyhuis TJ, Fleshner M, Day HEW, Campeau S. Chronic voluntary wheel running facilitates corticosterone response habituation to repeated audiogenic stress exposure in male rats. Stress. 2008;11:425–437. doi: 10.1080/10253890801887453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segal DS, Kuczenski R, Swick D. Audiogenic stress response: behavioral characteristics and underlying monoamine mechanisms. Journal of Neural Transmission. 1989;75:31–50. doi: 10.1007/BF01250642. [DOI] [PubMed] [Google Scholar]
- Tomsic D, Pedreira ME, Romano A, Hermitte G, Maldonado H. Context-US association as a determinant of long-term habituation in the crab Chasmagnathus. Animal Learning & Behavior. 1998;26:196–209. [Google Scholar]
- Wagner AR. Habituation and memory. In: Dickinson A, Boakes RA, editors. Mechanisms of Learning and Motivation. Erlbaum; Hillsdale, NJ: 1979. pp. 53–82. [Google Scholar]