Summary
The syntheses of 2,6-bis[4-(4,5-dihydro-1H-imidazol-2-yl)phenyl]pyridine 7, 2-[4-(4,5-dihydro-1H-imidazol-2-yl)-phenyl]-6-[3-(4,5-dihydro-1H-imidazol-2-yl)phenyl]pyridine 8 and 2,6-bis[3-(4,5-dihydro-1H-imidazol-2-yl)phenyl]pyridine 9 in five steps from the appropriately substituted bromoacetophenone are described. 3,5-Bis[4-(4,5-dihydro-1H-imidazol-2-yl)phenyl]pyridine 13 is also reported, prepared in four steps from 4-bromophenylacetonitrile. The preparation of 2,5-bis[4-(4,5-dihydro-1H-imidazol-2-yl)-phenyl]pyridine 18 from 4-bromoacetophenone in six steps is presented. The dications bind to poly dA·dT in the order 7 > 13 > 18 > 8 > 9; the order of binding to poly A·U is 7 > 13 > 8 > 9; 18 essentially does not bind to the RNA model. Only 7 inhibits topoisomerase II at millimolar concentrations. The dicationic compounds that were tested against Pneumonocystis carinii in the immuno-suppressed rat model show only modest activity and are moderately toxic. Some of the compounds demonstrated modest anti-HIV-1 activity and selectivity in primary lymphocytes.
Keywords: pyridine, diaryldiamidine, DNA, RNA, topoisomerase II, HIV, PCP
Introduction
For a number of years, studies from our laboratories have examined the structural factors that influence the interaction of dicationic diaryldiamidines with nucleic acids [1-3] and the relationship between such interactions and the biological properties of diamidines [4]. The precise mode of binding of these types of dicationic molecules with DNA has been found to be extremely sensitive to structure and dramatic differences in binding mode and specificity have resulted from relatively minor changes in structure. The aromatic diamidines berenil and stilbamidine exhibit strong minor groove binding at sites which have three or more consecutive AT base pairs [5, 6]. However, the well-known DNA stain DAPI, 2-[4'-guanylphenyl]-6-guanylindole, which has long been known to bind in the minor groove of DNA at AT sites, has also been found to intercalate at GC sites [2]. In a related study, the nucleic-acid binding properties of a series of 2,5-diphenylfuran dications were found to vary in interaction with DNA from AT-selective minor groove binding to classical intercalation at GC sites and threading intercalation at GC sites, depending upon the intrinsic nature of the cationic groups [3]. A series of dications with six-membered ring nitrogen heterocycles as spacers between benzamidino groups has been found to bind to the minor groove of AT-rich regions of DNA and intercalate at GC sites [7]. Recently, the radius of curvature of groove-binding molecules has been demonstrated to be an important factor in determining the effectiveness of interaction of groove-binding dications with DNA [8]. In addition, certain diamidines, for example DAPI, several dicationic 2,5-diarylfurans and dicationic triazines, have been found to bind strongly to RNA [9].
We recently reported the synthesis and biological evaluation of a diaryl triazines which bind to nucleic acids [10]. In this article, we describe the synthesis of three sets of isomeric diarylpyridines, 2,6-diaryl-, 3,5-diaryl- and 2,5-diarylpyridines which have different intrinsic geometric relationships between the dicationic groups and therefore different radius of curvature values.
Chemistry
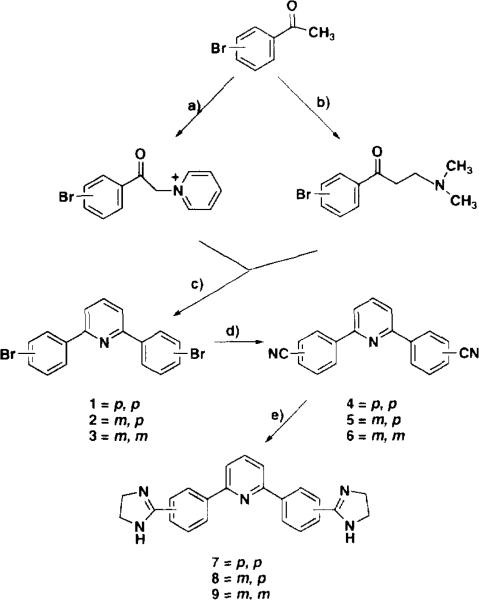
For the 2,6-diarylpyridine series, three different systems were selected for synthesis, which place the cationic centers of the molecules at different relative locations (see scheme 1). Each of these three 2,6-diarylpyridine systems were synthesized in a similar fashion starting from the appropriate bromo-substituted acetophenones. The approaches used, which are based on the Krohnke 2,6-diarylpyridine synthesis [11], are summarized in scheme 1 and lead to the three different 2,6-bis-[bromophenyl]pyridines (1-3). The various 2,6-bis-[bromophenyl]pyridines were allowed to react with copper(I) cyanide in refluxing dimethylformamide, which typically gave the corresponding bis-nitriles 4-6 in reasonable yields. As a result of the treatment of the bis-nitriles with ammonium hydroxide to remove copper ions during isolation, the product was often contaminated with low percentages of amide as indicated by infrared spectroscopy. Dehydration of the amide by treatment of the amide/nitrile mixtures with trifluoroacetic anhydride/pyridine [12] yielded amide-free bisnitriles. Efforts to prepare bis-amidines from the bisnitriles by the imidate ester route resulted, in our hands, in mixtures of amide and amidines which we were unable to separate. This problem was circumvented by preparation of cyclic amidines 7-9 by direct fusion of the bis-nitriles with the ethylenediamine hydrochloride [10, 13].
Scheme 1.
a) Br2/HOAc, pyridine; b) H2CO, HN(CH3)2, HCl, EtOH; c) NH4OAc, Ac2O; d) CuCN, DMF; e) NH2CH2CH2NH3+, 300°C.
Direct methods for synthesis of bis-3,5-diarylpyridines are limited. Routes involving palladium-catalyzed coupling [14] were considered but the method of Ohshiro [15] as outlined in scheme 2, worked satisfactorily. Reduction of 4-bromophenylacetonitrile with DIBAL yielded the corresponding 4-bromophenylacetaldehyde, which was not isolated but was directly converted into the enamine 10 by reaction with morpholine [16]. The remarkable reaction of the enamine 10 with 1,3,5-tri-t-butylhexahydra-1,3,5-triazine in p-xylene gave an approximately 60% yield of 3,5-bis[4'-bromophenyl]pyridine 11. Apparently, on refiuxing in p-xylene, 1,3,5-tri-t-butylhexahydra-1,3,5-triazine serves as an in situ source of N-t-butylmethanimine or the equivalent [15]. The bis-bromophenylpyridine 11 was converted into the bis-nitrile 12 and ultimately into the bisimidiazoline compound 13 by the approach previously described for the 2,6-diarylpyridine series.
Scheme 2.
a) DIBAL, morpholine; b) t-Bu-N = CH2, see text; c) CuCN, DMF; d) , 300°C.
Synthetic methods for preparation of bis-2,5-diarylpyridines are also limited. In this case also, palladium-catalyzed coupling approaches were not chosen. The unusual method of Chalk [17] (scheme 3) was successfully employed. This approach requires a 1,4-diaryl-1,3-butadiyne, which was made starting with a conventional approach involving the reaction of phosphorus pentachloride with 4-bromoacetophenone to yield a mixture of chloro compounds, 1,1-dichloro-1-( 4'-bromophenyl)ethane and α-chloro-4'-bromostyrene. On dehydrohalogenation the mixture gave 4-bromophenylethyne 14 [18]. Oxidative coupling of the arylethyne yielded 1,4-bis[4'-bromophenyl]-1,3-butadiyne 15 [19]. The 1,3-butadiyne 15 undergoes reaction with phenylethylamine in a remarkable process [17], in which, in effect, phenylethylamine serves as a source for methanimine or the equivalent, to yield 2,5-[4'-bromophenyl]pyridine 16 in approximately 30% yield. 2,5-Bis[bromophenyl]-pyridine 16 was converted into the bis-nitrile derivative 17, which was transformed into the corresponding bis-imidazoline compound 18 following procedures developed for the synthesis of both series of diarylpyridines previously described.
Scheme 3.
a) PCl5; b) KOH, EtOH; c) Cu(OAc)2, MeOH, pyridine; d) 145°C, C6H5CH2CH2NH2; e) CuCN, DMF; f) , 300°C.
A monocationic 2,6-diarylpyridine was needed as a control compound for nucleic-acid binding studies. Consequently, 2-phenyl-6-[4-(4,5-dihydro-1H-2-ylimidazolyl)phenyl]pyridine 19 was prepared using the same approach employed for the synthesis of 7.
Biological results and discussion
The results from various biological evaluations of the diarylpyridines are presented in table I. The binding of the dicationic pyridines with DNA and RNA was evaluated by measuring the increase in thermal melting temperature (ΔTm) on complex formation with poly dA·dT and poly A·U, respectively [9]. Compounds 7 and 13 have the radius of curvature values that are the most favorable for complementing the minor groove and their ΔTm values on binding to poly dA·dT are the largest (24 and 22.8). The somewhat smaller ΔTm value for 13 is consistent with the fact that the 4-proton of 13 is likely to interfere with a tight fit in the minor groove on complex formation as well as produce a twist in the benzamidine ring pyridine ring bond. The compounds 8 and 9 have cationic centers located at the meta positions of the benzamidine rings consequently resulting in a more acute radius of curvature. A similar result was noted by Cory et al for some meta-substituted pentamidine analogs [8]. Interestingly, the more linear molecule 18, with a large radius of curvature, binds relatively strongly to DNA suggesting that the minor groove is more accommodating to approximately linear molecules than to acutely curved ones. Intercalation of 18 with DNA cannot be excluded on the basis of the current data. Since the intercalation sites on both DNA and RNA are quite similar [20] and since 18 essentially does not bind to RNA (vide infra), it seems unlikely that 18 intercalates with DNA.
Table I.
Biological evaluation of dicationic diarylpyridines.
 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Compound | ΔTm (DNA)a | ΔTm (RNA)b | IC50 (μM) 50% topoisomerase II inhibition (μM) |
EC50 (μM) antigiardial activitye | Anti-HIV activityf | IC50g 3H-dThd uptake | In vivo activity against Pneumocystis cariniih |
|||
| Dose (mg/kg per day) | Toxicity | Cyst/g lung (% of control) | ||||||||
| G lambliac | P cariniid | |||||||||
| 7 | > 24 | 12.9 | 12.5 | 0.5 | 0.9 | 0.23 | 8.3 | 5 | 1+ | 45.0 |
| 8 | 12.3 | 6.8 | 12.5 | 80 | 20.8 | 2.7 | 7.4 | – | – | – |
| 9 | 10.8 | 5.3 | 25 | 90 | 5.6 | 3.0 | 34.8 | – | – | – |
| 13 | 22.8 | 8.7 | 25–50 | 15 | 8.1 | 3.2 | 40.7 | 5 | 3+ | 26.4 |
| 18 | 20.0 | 0.7 | 25 | 15 | 2.7 | 1.8 | 34.8 | 5 | 0 | 61.3 |
| 19 | 0.2 | 0.6 | – | – | – | – | – | – | – | – |
| Pentamidine | 12.8 | 0 | > 500 | > 100 | – | 7.8 | 7.5 | 10 | 2+ | 5.0 |
| Saline | – | – | – | – | – | – | – | – | 0 | 100 |
Increase in thermal melting of poly dA·dT see ref [9].
Increase in thermal melting of poly A·U, see ref [9].
Inhibition of topoisomerase II isolated from G lamblia, see ref [25].
Inhibition of topoisomerase II isolated from P carinii, see ref [22].
Evaluation of dications against G lamblia see ref [26].
Evaluation of the dicationics against HIV-1 (strain LAV) in human PBM cells as described in ref [21]. EC50 is the median effective concentration (μM).
H-dThd uptake in PBM cells; IC50 is median inhibitory concentration [21].
Evaluation of iv dosage of the dications against P carinii in rats as described in ref [27].
The binding to RNA by diaryl dications related to those under investigation here is thought to be by intercalation [9]. The 2,6-diarylpyridine 7 exhibits the largest ΔTm value on binding to RNA of this series of dicationic pyridines. Molecular modeling suggests a good fit in the RNA intercalation cavity for molecules of this shape [20]. The 3,5-diaryl pyridine analog 13 is of similar shape to that of 7, but the van-der-Waals interactions between the 4-proton on the pyridine ring and the ortho-protons on the benzamidine rings cause a torsion angle twist which would reduce favorable stacking interactions and is presumably reflected in the lower ΔTm value. The two compounds with meta cationic centers 8 and 9 give only modest ΔTm values, which apparently reflects the overall poorer fit of these shaped molecules in the RNA intercalation site. The approximately linear molecule 18, again shows different properties, but it essentially does not bind to RNA. Presumably, the way this molecule interacts with RNA is in a bayonet insertion manner which would provide very poor stacking interactions and the cationic centers would be well away from the backbone phosphate groups.
In view of the moderately strong RNA interactions of some of the dicationic pyridines, the compounds were evaluated against HIV-1 in a previously described cell culture screen [21]. Significant anti-HIY activity was noted for 7, the compound with the highest RNA affinity. Cell toxicity was apparent for the dicationic pyridines tested, although some of the compounds had a therapeutic index of greater than 10, suggesting that these compounds are modest selective inhibitors of HIV.
Other diaryl diamidines have been found to inhibit microbial topoisomerases [22, 23] and consequently the pyridines were evaluated against topoisomerase II from Giardia lamblia and Pneumonocystis carinii (see table I). Moderate inhibition of these enzymes was noted, however, only 7 was active at micromolar concentrations against topoisomerase II from P carinii. Several of the dicationic pyridines were evaluated against P carinii pneumonia in the immunosuppressed rat model [24]. The pyridines tested were only modestly effective at 5 mg/kg dosage and most showed moderate toxicity.
Experimental protocols
2,6-Bis[4-bromophenyl]pyridine 1
α-[N-Pyridinium]-4'-bromoacetophenone bromide [28] (14.28 g, 0.04 mol), β-[[N,N-dimethylamino]-4'-bromopropiophenone hydrochloride [29] (11.7 g, 0.04 mol), ammonium acetate (30.8 g, 0.5 mol) in a mixture of 60 ml glacial acetic acid and 15 ml acetic anhydride were heated at reflux for 2 h. The reaction mixture was cooled and 200 ml cold water were added; the resulting brown precipitate was collected by filtration, washed with water and dried. The solid was washed with 50 ml ethanol/ether (1:1) to yield 8.7 g (56%) of an off-white solid, mp 180–181°C. IR (KBr) (cm-1) 1560, 1519, 1449, 780. 1H-NMR (DMSO-d6/90°C) (ppm, J values in Hz) 8.08 (d, 4H, J = 8.79), 7 94–7.82 (m, 3H), 7.65 (d, 4H, J = 8.79). 13C-NMR (DMSO-d6/90°C) (ppm) 154.3, 137.7, 137.39, 131.03, 128.04, 122.2, 118.42. MS m/e 389 (M+).
2-[4-Bromophenyl]-6-[3-bromophenyl]pyridine 2
α-[N-Pyridinium]-4-bromoacetophenone bromide [28] and β-[N,N-dimethylamino]-3-bromopropiophenone hydrochloride [30] were allowed to react as described above for 1 to give 9.3 g (60%) yield of 2, mp 68–69°C. IR (KBr) (cm–1) 1559, 1450, 769. 1H-NMR (DMSO-d6/35°C) (ppm, J values in Hz) 8.34 (dd, 1H, J = 1.46, J = 1.96), 8.16 (dd, 1H, J = 1.46, J = 1.96, J = 8.3), 8.12 (dd, 2H, J = 1.95, J = 8.79), 7.94–7.97 (m, 3H), 7.68 (dd, 2H, J = 1.95, J = 8.79), 7.62 (td, 1H, J = 1.46. J = 1.96, J = 8.3), 7.46 (dd, 1H, J = 7.8, J = 8.3). 13C (DMSO-d6/35°C) (ppm) 154.5, 154.0, 140.7, 138.5, 137.5, 131.8, 131.6, l30.8, 129.0, 128.5, 125.5, 122.8, 122.2, 119.4, 119.3, MS m/e 389 (M+).
2,6-Bis[3-hromophenyl]pyridine 3
α-[N-Pyridinium]-3'-bromoacetophenone bromide [31, 32] and β-[N,N-dimethylamino]-3'-bromopropiophenone hydrochloride [30] were allowed to react as described above for 1. The oily mass that separated was chromatographed over neutral Al2O3, using benzene/EtOAc (95:5) to give a yield of 7.9 g (50%) of 3, mp 36–37°C. IR (KBr) (cm–1) 1569, 1462, 1185, 1069, 797, 763. 1H-NMR (DMSO/45°C) (ppm, J values in Hz) 8.31 (s. 2H), 8.14 (d, 2H, J = 7.81), 8.02–7.84 (m, 3H), 7.62 (d, 2H, J = 7.81), 7.45 (t, 2H, J = 7.82). 13C-NMR (DMSO-d6/45°C) (ppm) 154.0, 140.7, 138.5, 131.8, 130.8, 129.0, 125.5, 122.2, 119.7. MS m/e 389 (M+).
3,5-Bis(4-bromophenyl)pyridine 11
To a mechanically stirred solution 24.8 g (0.13 mol) of 4-bromophenylacetonitrile in 100 ml dry toluene under nitrogen, a mixture of 162 ml 1 M DIBAL and 30 ml toluene was added dropwise, during which time the temperature was maintained below –5°C. After addition of DIBAL was complete (ca 1 h), the reaction mixture was stirred for an additional 1.5 h at –5°C. To the stirred solution, ethanol (50 ml) was added dropwise while maintaining the temperature below –5°C. While continuing to stir, 300 ml of 1 M H2SO4 was added slowly to the reaction mixture during which time the temperature was maintained between 0 and 5°C. The mixture was allowed to slowly warm to room temperature during which time the product appeared as a gummy solid which adhered to the flask. Toluene (100 ml) was added after stirring, the phases were separated and the organic layer was washed with a saturated sodium chloride solution (2 × 100 ml). The toluene solution (yellow) was added to 50 ml morpholine with stirring. After ca 10 min, the color changed to red and the reaction mixture was allowed to stand overnight. The solvent and excess morpholine were removed under reduced pressure and the residue was dissolved in cyclohexane. After concentration, 15 g (43%) of crude solid (mp 120–132°) was collected by filtration. Recrystallization from ethanol yielded 7.8 g (23%, mp 130–135°C) of 2-[N-morpholino]-4'-bromostyrene (10) [16].
To a 37% formaldehyde solution (135 ml) was added drop-wise 96 ml of t-butylamine during which time the temperature was maintained near 5°C [33]. After the addition was complete, the mixture was allowed to slowly warm to room temperature and the mixture was stirred for 2 h. The organic layer was separated and allowed to stand over solid KOH for 3 h and decanted and was placed over fresh solid KOH. The process was repeated 5 times and ultimately 45 g (58%) of liquid hexahydra-1,3,5-tri-t-butyl-1,3.5-triazine was obtained, which was used without further purification in the next step.
A mixture of 10 (7.6 g, 0.028 mol) and 1,3,5-tri-t-butyl-hexahydra-1,3,5-triazine (1.18 g, 0.014 mol) in 100 ml p-xylene was allowed to reflux under nitrogen for 70 h (moni tored by TLC) [15]. The solvent was removed under reduced pressure and the residue was recrystallized from approximately boiling ethanol to yield 3.5 g (66%), mp 213–215°C, of 11. IR (KBr) (cm–1), 1468, 1381, 824. 1H-NMR (DMSO-d6) (ppm, J values in Hz) 8.88 (d, 2H, J = 1.95), 8.3 (dd, 1H, J = 2.44, J = 1.95), 7.8 (d, 4H, J = 8.79), 7.7 (d, 4H, J = 8.79). 13C-NMR (DMSO-d6) (ppm) 146.1, 135.6, 134.1, 131.3, 128.6, 121.3. MS m/e 389 (M+).
2,5-Bis(4-bromophenyl)pyridine 16
Phosphorus pentachloride (228.5 g, 1.1 mol) was added to well-stirred 4-bromoacetophenone (190 g, 0.95 mol). The mixture was slowly warmed and the reaction became vigorous and HCl evolved. The mixture was stirred at 70°C for 1.5 h and distilled under reduced pressure. The fraction that boiled between 123–127°C/18 mm Hg was collected (130 g). This fraction was a mixture of α-chloro-4'-bromostyrene and 1,1-dichloro-1-[4-bromophenyl]ethane and it was directly dehydrohalogenated [17].
To a well-stirred mixture of the above chloro compounds (105 g), 500 ml of 25% KOH in ethanol was slowly added; external cooling was employed to maintain the temperature under 40°C. After addition of the ethanolic potassium hydroxide was complete, the mixture was heated at reflux for 4 h. A large amount of salt separated and it was removed by hot filtration. The solid was washed with ether and the combined organic phases were evaporated under reduced pressure. The residue was dissolved in ether. The ether was washed with HCl solution to neutralize any remaining base. The ether layer was washed with brine, dried over anhydrous sodium sulfate and then removed in vacuo. The remaining oil was distilled 118–120°C/18 mm Hg and the distillate crystallized on cooling to give 14, mp 63–64°C (40 g, 40%); lit mp 64–65°C [18].
A solution of 4-bromophenylethyne (14) (31.9 g, 0.176 mol) in 50 ml ethanol was added to a stirred slurry of copper(II) acetate dihydrate (49.5 g, 0.232 mol) in 180 ml of pyridine/ methanol (1:1). Additional pyridine/methanol (150 ml:30 ml) was added to facilitate stirring and the mixture was allowed to reflux for 2 h. The reaction mixture was cooled and carefully added to 550 ml conc H2SO4 with external cooling and stirring. The resulting solid was collected by filtration. The solid was slurried in 75 ml of ethanol/water (50:50) and 40 g of silver nitrate in 75 ml of ethanol/water (50:50) was added. The mixture was stirred at room temperature for 0.5 h and filtered. The solid was washed with water, dried and placed in a Soxlet device and extracted with benzene (24 h). Evaporation of benzene gave 24.1 g (76%) of off-white solid 15 which melted at 260°C (lit value [33] 260°C).
A mixture of 14.4 g (0.04 mol) of 1,4-bis[4-bromophenyl]-1,3-butadiyne (15) and 35 ml phenylethylamine were heated at 145°C for 13 h (nitrogen atmosphere). After cooling, benzene (500 ml) was added. The benzene solution was washed with 10% acetic acid (3 × 200 ml), 5% sodium hydroxide (2 × 200 ml) and water (3 × 100 ml). The organic phase was dried (MgSO4) and the solvent removed under reduced pressure to yield a red oil which on trituration with acetone afforded 16 as pale-yellow crystals 4.5 g (28%), mp 245–247°C. IR (KBr) (cm–1), 1457, 1064, 814. 1H-NMR (DMSO-d6, 80°C) (ppm, J values in Hz) δ 8.96 1H, d, J = 2.4), 8.15 (1H, dd, J = 2.4, J = 8.3) 8.09 (2H, d, J = 8.8), 8.03 (1H, d, J = 8.3), 7.74 (2H, d, J = 8.8), 7.67 (2H, d, J = 8.8). 13C-NMR (DMSO-d6, 80°C) (ppm) 153.7, 146.8, 137.0, 135.5, 134.3, 132.8, 131.3, 131.0, 128.1, 127.9, 122.1, 121.1, 119.4. MS (m/e) 389.
2,6-Bis[4-cyanophenyl]pyridine 4
A mixture of 2,6-bis[4-bromophenyl]pyridine (1) (7.8 g, 0.02 mol) and copper(I) cyanide (7.2 g, 0.08 mol) in 75 ml dry DMF was heated at reflux with stirring for 10δ15 h (monitored by TLC). The hot reaction mixture was poured into a stirred slurry of ice in water. The resulting yellow-brown solid was filtered, suspended in 300 ml of 10% NH4OH, stirred for 0.5 h and filtered. The solid was washed repeatedly with water until the washings were no longer blue. The resultant cream-colored solid was dried in vacuo. Infrared spectroscopy indicated partial hydrolysis of nitrile to amide. The mixture of solids was suspended in 40 ml anhydrous THF and 4.5 ml pyridine was added. To this mixture, 5 ml trifluoroacetic acid anhydride was carefully added (exothermic). The mixture (under nitrogen) was stirred for 10 h and poured into 10% NaHCO3 slowly with stirring and cooling. The resultant solid was filtered, washed with water until neutral and dried in vacuo. The solid was placed in a sox let device and extracted with acetone for 24 h. The resulting solid obtained after removal of acetone was dissolved in dioxane and passed through a short (1 inch) column of neutral alumnia; the solid 4 obtained was 3.3 g (58%) mp 234δ235°C. IR (KBr) (cm–1) 2224, 1602, 1590, 1450, 796. 1H-NMR (DMSO-d6/30°C) (ppm, J values in Hz) 8.4 (d, 4H, J = 8.3), 8.14δ8.08 (m, 3H), 7.97 (d, 4H, J = 8.3). 13C-NMR (DMSO-d6/35°C) (ppm) 153.8, 142.2, 138.3, 132.1, 127.0,120.28, 118.04. 111.44. MS (m/e) 281 (M+).
2[4-Cyanophenyl]-6-[3-cyanophenyl]pyridine 5
2[4-Bromophenyl]-6-13-bromophenyl]pyridine (2) was allowed to react with copper(I) cyanide as described above for 4 to give 3.7 g (65%) of 5, mp 202–203°C. IR (KBr) (cm–1), 2222, 1576, 1445, 800. 1H-NMR DMSO-d6/35°C) (ppm, J values in Hz) 8.61 (dd, 1H, J = 1.46, J = 1.96), 8.52 (td, 1H, J = 1.46, J = 1.96, J = 8.3), 8.4 (dd, 2H, J = 1.95, J = 8.79), 8.1 (m, 3H), 7.96 (dd, 2H, J = 1.95, J = 8.79), 7.92 (td, 1H, J = 1.46, J = 1.96, J = 8.3), 7.72 (t, 1H, J = 7.91). 13C-NMR (DMSO-d6) (ppm) 153.7, 153.6, 142.2, 139.2, 138.5, 132.5, 132.4, 131.1, 130.1, 129.8, 129.7, 127.3, 120.2, 120.1, 118.4. 111.9, 111.6. MS m/e 281 (M+).
2,6-Bis[3-cyanophenyl]pyridine 6
2,6-Bis[3-bromophenyl]pyridine (3) was allowed to react with copper(I) cyanide as described above for 4 to yield 2.9 g (52%) of 6, mp 176–177°C. IR (KBr) (cm–1) 2227, 1560, 1458, 783. 1H-NMR (DMSO/45°C) (ppm, J values in Hz) 8.63 (t, 2H, J = 1.47), 8.55 (td, 2H, J = 1.47, J = 7.82), 8.08 (m, 3H), 7.92 (td, 2H, J 1.47, J = 7.82), 7.73 (t. 2H, J = 7.82). 13C-NMR (DMSO-d6/45°C) (ppm) 153.7, 139.4, 138.8, 132.6, 131.2, 130.1, 129.9, 120.1, 118.6, 112.0. MS m/e 281 (M+).
3,5-Bis(4-cyanophenyl)pyridine 12
3,5-Bis(4-bromophenyl)pyridine (11) was allowed to react with copper(I) cyanide as described above for 4 to yield 3.1 g (55%) mp 294–296°C. IR (KBr) (cm–1) 2225, 1604, 1507, 855. 1H-NMR (DMSO-d6) (ppm, J values in Hz) 9.05 (d, 2H, J = 1.9), 8.48 (dd, 1H, J = 2.44, J = 1.9), 8.08 (d, 4H, J = 8.3), 7.98 (d, 4H, J = 8.79). 13C-NMR (DMSO-d6) (ppm), 147.8, 141.2, 134.0, 133.0, 132.9, 128.1, 118.6, 111.0. MS m/e 281 (M+).
2,5-Bis(4-cyanophenyl)pyridine 17
2.5-Bis(4-bromophenyl)pyridine (16) was allowed to react with copper(I) cyanide as described above for 4 to yield 3.0 g (53%) of 17, mp 264–266°C. IR (KBr) (cm–1) 2225, 1573, 1459, 1440, 702, 678. 1H-NMR (DMSO-d6/80°C) (ppm, J values in Hz) 9.09 (d, 1H, J = 1.95), 8.32 (d, 2H, J = 8.79), 8.3 (dd, 1H, J = 1.95, J = 8.32), 8.17 (d, 1H, J = 8.3), 8.02 (d, 2H, J = 8.79), 7.93 (d, 4H, J = 8.79). 13C-NMR (DMSO-d6/80°C) (ppm) 153.6, 147.5, 141.8, 140.6, 139.3, 135.1, 133.0, 132.3, 132.0, 127.2, 126.8, 120.5, 117.9, 111.4, 110.7. MS m/e 281 (M+).
2,6-Bis[4-(4,5-dihydro-1H-imidazol-2-yl)phenyl]pyridine 7
The bis-cyano compound 4 (0.56 g, 0.002 mol) was added to molten ethylenediamine hydrochloride (1.4 g, 0.01 mol) in a 25 ml round-bottomed flask. The mixture was stirred at 300–305°C for 10 min, during which time ammonia was evolved. The crude product was dissolved in boiling water (ca 25 ml) and filtered hot. The filtrate was concentrated with a stream of nitrogen to a volume of ca 5 ml and the solid which formed was collected by filtration. The yellow-brown solid was dissolved in 15 ml water, and the pH was adjusted to ca 10 by addition of 2 M NaOH with cooling and stirring. The resulting free base 7 was collected by filtration and recrystallization from hot ethanol yielded 0.33 g (45%) mp > 360°C. Anal calc for: C23H21N5: C, 75.17; H, 5.76; N, 19.06. Found: C, 74.96; H, 5.79; N, 18.97. IR (KBr) (cm–1) 3215, 2930, 2851, 1615, 1615, 1600. 1H-NMR (DMSO-d6, 100°C) (ppm, J values in Hz) 8.23 (d, 4H, J = 8.3), 8.0–7.91 (m, 7H), 3.66 (s, 8H). 13C-NMR (DMSO-d6, 100°C) (ppm), 162.9, 154.9, 139.8, 137.7, 130.9, 127.1, 125.9, 118.9, 49.2. MS m/e 367 (M+).
The free base 7 was suspended in 10–15 ml anhydrous ethanol saturated with HCl and warmed at gentle reflux for 4 h. The excess ethanol was then removed under reduced pressure and the resultant solid was triturated with anhydrous ether. The solid was collected by filtration, washed with ether and dried in vacuo at 80°C for 24 h to yield 0.3 g (36%) mp > 360°C. Anal calc for: C23H21N5·2HCl·H2O: C, 60.26; H, 5.50; N, 15.28. Found: C, 60.29; H, 5.51; N, 15.30. IR (KBr) (cm–1) 3412, 3128, 2969, 1618, 1452, 1366, 1280, 1032, 861, 811, 739, 666. 1H-NMR (DMSO-d6) (ppm, J values in Hz) 4.05 (s, 8H), 8.14 (t, 1H, J = 5.86), 8.21 (d, 2H, J = 8.30), 8.28 (d, 4H, J = 8.31), 8.50 (d, 4H, J = 8.31), 11.02 (brs, 4H). 13C-NMR (DMSO-d6) (ppm) 44.3, 121.0, 122.6, 127.2, 129.3, 143.6, 154.2, 164.4.
2-[4-(4,5-Dihydro-1H-imidazol-2-yl)phenyl]-6-[3-(4,5-dihydro-1H-imidazol-2-yl)phenyl]pyridine 8
The bis-cyano compound 5 was reacted with ethylenediamine hydrochloride as described above to yield 0.3 g (40%) of the free base 8, mp 254–255°C. Anal calc for: C23H21N5·0.25 H2O: C, 74.26; H, 5.69; N, 18.83. Found: C, 74.89; H, 6.01; N, 18.80. 1H-NMR (DMSO-d6, 90°C) (ppm, J values in Hz), 8.6 (dd, 1H, J = 1.5, J = 1.9), 8.3–8.24 (m, 3H), 8.04–7.9 (br, 2H), 3.66 (s, 4H), 3.64 (s, 4H). 13C-NMR (DMSO-d6, 90°C) (ppm), 163.3, 163.0, 155.3, 154.9, 138.5, 138.1, 131.2, 131.1, 128.4, 128.1, 127.6, 127.3, 126.1, 125.1, 119.1, 49.6, 49.5. MS m/e 367 (M+).
The salt (mp > 360°C) was prepared as described above. Anal calc for: C23H21N5·3HCl: C, 57.93; H, 5.07; N, 14.68. Found: C, 57.65; H, 5.38; N, 14.59. IR (KBr) (Cm–1), 3411, 3189, 3089, 2967, 1617, 1594, 1566, 1372, 1278, 1033, 805, 706. 1H-NMR (DMSO-d6, 90°C) (ppm, J values in Hz) 11.08 (br, 4H), 8.95 (br, 1H), 8.6 (d, 1H, J = 7.8), 8.49 (d, 2H, 7.8), 8.29–8.06 (m, 6H), 7.79 (dd, 1H, J = 7.8, J = 7.3), 4.06 (s, 4H), 4.04 (s, 4H). 13C-NMR (DMSO-d6/90°C) (ppm) 164.7, 164.3, 154.2, 153.8, 143.4, 139.3, 138.2, 131.9, 129.1, 128.8, 128.7, 126.8, 126.7, 122.5, 122.1, 120.2, 120.0, 44.0.
2,6-Bis[3-(-4,5-dihydro-1H-imidazol-2-yl)phenyl]pyridine 9
The bis-cyano compound 6 was reacted with ethylenediamine dihydrochloride as described above to yield 0.25 g (34%) mp 126–127°C. Anal calc for: C23H21N5.0.25 H1O: C, 74.26; H, 5.69; N, 18.83. Found: C, 74.01; H, 5.88; N, 18.62. IR (KBr) (cm–1) 3216, 2930, 2865, 1611, 1570, 1492. 1H-NMR (DMSO-d6, 100°C) (ppm. J values in Hz) 8.58 (t, 2H, J = 1.5), 8.29 (dd, 2H, J = 7.8, J = 1.0), 8.02 (m, 3H), 7.93 (dd, 2H, J = 6.35, J = 1.0), 7.58 (dd, 2H, J = 8.3, J = 7.81), 3.66 (s, 8H). 13C-NMR (DMSO-d6, 60°C) (ppm), 163.3, 155.3, 138.5, 138.1, 131.0, 128.2, 127.6, 125.1, 119.0, 49.5. MS m/e 367 (M+).
The salt was prepared as described above to yield 0.2 g (35%), mp 330°C (dec). Anal calc for: C23H21N5·3HCl: C, 57.93; H, 5.07; N, 14.68. Found: C, 57.65; H, 5.38; N, 14.59. IR (KBr) (cm–1) 3397, 3103, 3000, 1618, 1570, 1366, 1285, 1035, 796, 704. 1H-NMR (DMSO-d6/l00°C) (ppm, J values in Hz) 9.05 (s, 2H), 8.61 (d, 2H. J = 7.8), 8.18–1.10 (m, 5H), 7.79 (t. 2H, J = 7.8), 4.07 (s, 8H). 13C-NMR (DMSO-d6, 100°C) (ppm) 164.7, 154.1, 139.2, 132.1, 129.2, 128.6, 126.9, 122.5, 119.7, 44.0.
2,5-Bis-4-(4,5-dihydro-1H-imidazol-2-yl)phenyl)pyridine 13
The bis-cyano compound 12 was reacted with ethylenediamine hydrochloride as described above to yield 0.33 g (45%) of the free base 13, mp > 360°C. Anal calc for: C23H21N5: C, 75.17; H, 5.76; N, 19.06. Found: C. 75.09; H, 5.80; N, 18.99. IR (KBr) (cm–1) 3196, 2924, 1610, 1600, 1521, 1490. 1H-NMR (DMSO-d6, 110°C) (ppm, J values in Hz) 9.03 (d, 1H, J = 1.85), 8.20 (dd, 1H, J = 1.85, J = 8.49), 8.15 (d, 2H, J = 8.3). 8.05 (d, 1H, J = 8.49), 7.97 (d, 2H, J = 8.3), 7.94 (d, 2H, J = 8.3), 7.81 (d, 2H, J = 8.3), 3.67 (s, 8H). 13C-NMR (not obtained; not soluble). MS m/e 367 (M+).
The salt (mp > 360°C) was prepared as described above. Anal calc for C23H21N5: C, 75.17; H, 5.76; N, 19.06. Found: C, 75.09; H, 5.80; N, 18.99. IR (KBr) 3390, 3117, 2965, 1611, 1590, 1360, 1273, 1028, 831, 739. 1H-NMR (DMSO-d6/110°C) (ppm, J values in Hz) 9.03 (d, 1H, J = 1.9), 8.20 (dd, 1H, J = 1.9, J = 8.5), 8.15 (d, 2H, J = 8.3), 8.05 (d, 1H, J = 8.5),7.49 (d, 2H, J = 8.3), 7.94 (d, 2H, J = 8.3), 7.81 (d, 2H, J = 8.3), 3.67 (s, 8H). 13C-NMR (not obtained: not soluble).
3,5-Bis[4-(4,5-dihydro-1H-imidazol-2-yl)phenylpyridine 18
The bis-cyano compound 17 was reacted with ethylenediamine hydrochloride as described above to yield 0.35 g (47%) of the free base 18, mp 326–327°C. Anal calc for: C23H21N5: C, 75.17; H, 5.76: N, 19.06. Found: C, 75.08; H, 5.78; N, 19.03. IR (KBr) (cm–1) 3168, 2936, 2863, 1617, 1600, 1552. 1H-NMR (DMSO-d6, 95°C) (ppm, J values in Hz), 8.91 (d, 2H, J = 1.8). 8.35 (dd, 1H, J = 1.83, J = 2.4), 7.96 (d, 4H, J = 7.94), 7.88 (d. 4H, J = 8.55), 3.65 (s, 8H). 13C-NMR (DMSO-d6, 95°C) (ppm) 162.9, 146.4. 138.1, 134.7, 131.6, 130.2, 127.4, 126.4, 49.3. MS m/e 367 (M+).
The salt (mp > 360°C) was prepared as described above. Anal calc for C23H21N5: C, 75.17; H, 5.76; N, 19.06. Found: C, 75.08; H, 5.78; N, 19.03. IR (KBr) (cm–1) 3411, 3111, 2967, 1617, 1600, 1511, 1394, 1366, 1278, 1027, 839, 661. 1H-NMR (DMSO-d6/95°C) (ppm, J values in Hz) 8.91 (d, 2H. J = 1.83). 8.35 (t, 1H, J = 1.83,.1 = 2.44). 7.96 (d, 4H, J = 7.9), 7.88 (d, 4H, J = 8.5), 3.65 (s, 8H). 13C-NMR (DMSO-d6/95°C) (ppm) 162.9, 146.4, 138.1, 134.7, 131.6, 130.2, 127.4, 126.4, 49.3.
2-Phenyl-6-[4-bromophenyl]pyridine
A mixture of α-[N-pyridinium]acetophenone bromide (5.56 g, 0.02 mol), β-(N,N-dimethylamino)-4-bromopropiophenone hydrochloride (5.8 g, 0.02 mol). ammonium acetate (15.4 g, 0.25 mol) in 35 ml acetic acid and 10.0 ml acetic anhydride were heated at reflux for 2 h. On pouring into ice-water, a brown solid formed, which after recrystallization from ethanol/ether (1:3) yielded 3.2 g (53%) of white solid; mp 113–114°C. IR (KBr) 3064, 1598, 1573, 1446, 1170, 1070, 1007, 807, 696 cm–1. 1H-NMR (DMSO-d6) (ppm, J values in Hz) 8.19 (d, 1H, J = 1.5), 8.14 (d, 2H, J = 8.8), 7.93–7.89 (m, 3H). 7.69 (d, 2H, J = 8.3), 7.54–7.45 (m, 3H). 13C-NMR (DMSO-d6) (ppm) 155.6, 154.3, 138.4, 138.2, 137.7, 131.5, 129.0, 128.6, 128.4, 126.5, 122.6, 119.0, 118.6. MS m/e 310 (M+).
2-Phenyl-6-[4-cyanophenyl]pyridine
The monobromo compound (3.1 g, 0.01 mol) above and copper(I) cyanide (1.8 g, 0.02 mol) in 20 ml dry DMF was heated at reflux for 15 h. Work-up as described for 4 gave, after recrystallization from CHCl3/ether (1:3), 1.8 g (69%) of off-white solid; mp 108–109°C. IR (KBr) 3070, 223, 1605, 1585, 1563, 1446, 1270, 1017, 987, 812, 760 cm–1. 1H-NMR (DMSO-d6) (ppm, J values in Hz) 8.33 (d, 2H, J = 8.8), 8.16 (d, 2H, J = 8.3), 7.9–7.87 (m, 5H), 7.53–7.44 (m, 3H). 13C-NMR (DMSO-d6) (ppm) 155.8, 153.4, 142.6, 138.1, 138.0, 132.2, 128.8, 128.3, 127.0, 126.3, 119.5, 119.2, 118.3, 111.3. MS (m/e) 256 (M+).
2-Phenyl-6-[4-(4,5-dihydro-1H-imidazol-2-yl)phenyl]pyridine 19
The monocyano compound above (0.62 g, 0.002 mol) was added to molten ethylenediamine hydrochloride (0.7 g, 0.002 mol) and the temperature was maintained at ca 300°C for 10 min. The mixture was allowed to cool and the solid was dissolved in hot water, filtered, and the aqueous solution was concentrated under a stream of nitrogen. The solid thus obtained was filtered and redissolved in 10 ml water and made basic with 2 M NaOH, the resulting solid was washed with water, dried and recrystallized from ethanol to yield 0.37 g (S7%) of white solid, mp 198–199°C, IR (KBr) 3178, 3034, 2929, 2861, 1604, 1563, 1515, 1422, 1379, 1272, 980, 828, 740 cm–1. 1H-NMR (DMSO-d6) (ppm, J values in Hz) 8.96 (d, 1H, J = 4.89), 8.53 (br, 2H), 8.39 (d, 2H, J = 8.3), 8.04–8.01 (m, 3H), 7.56–7.55 (br, m, 4H), 3.67 (s, 4H). 13C-NMR (DMSO-d6) (ppm) 163.3, 163.1, 158.7, 139.8, 139.7, 132.6, 130.8, 128.6, 127.7, 126.9, 115.2, 49.4. Mass m/e 299 (M+).
The free base (0.28 g, 0.001 mol) dissolved in 10 ml absolute ethanol and treated with 10 ml ethanol saturated with HCl was heated at ca 60°C for 10 min. The solution was diluted with 50 ml dry ether and the resulting off-white solid was filtered, dried in vacuo at 75°C for 12 h, yield 0.3 g (84%), mp > 330°C. Anal calc for: C20H17N3·2HCl: C, 64.52; H, 5.14; N, 11.28. Found: C, 64.48; H, 5.30; N, 11.21. IR (KBr) 3065, 2991, 1611, 1567, 1469, 1384, 1280, 1029, 828, 739, 675 cm–1. 1H-NMR (DMSO-d6) (ppm, J values in Hz) 11.23 (s, 2H), 9.0 (d, 1H, J = 5.4), 8.56–8.49 (m, 4H), 8.36 (d, 2H, J = 8.8), 8.12 (d, 1H, 5.4). 7.56–7.53 (m, 4H), 6.2 (brs, 1H), 4.02 (s, 4H). 13C-NMR (DMSO-dh) (ppm) 163.9, 163.4, 161.1, 159.0, 141.5, 141.3, 136.9,130.9, 129.4, 128.6, 127.8, 127.5, 124.1, 115.7, 44.3.
Acknowledgments
This work was supported by NIH Grants NIAID AI-27196, AI-33363, and by the Georgia VA Research Center for AIDS and HIV infection. An award by the Chemical Instrumental Program of NSF (CHE 8409599) provided partial support for acquisition of the Varian VXR400 spectrometer. We appreciate the technical assistance of W Brake and B Bender with the animal model for P carinii.
References
- 1.Wilson WD, Tanious FA, Buczak H, et al. In: Structure and Function. Vol I, Nucleic Acids. Sarma RH, Sarma MH, editors. Adenine Press; New York. USA: 1992. pp. 83–105. [Google Scholar]
- 2.Wilson WD, Tanious FA, Barton HJ, Strekowski L, Boykin DW, Jones RL. J Am Chem Soc. 1989;111:5008–5010. [Google Scholar]
- 3.Wilson WD, Tanious FA, Buczak H, Venkatramanan MK, Das BP, Boykin DW. In: Jerusalem Symposia on Quantum Chemistry and Biochemistry. Pullman B, Jortner J, editors. Vol. 23. Kluwer Academic Publishers; The Netherlands: 1990. pp. 331–353. [Google Scholar]
- 4.Das BP, Boykin DW. J Med Chem. 1977;20:531–536. doi: 10.1021/jm00214a014. [DOI] [PubMed] [Google Scholar]
- 5.Zimmer C, Wahnert U. Prog Biophys Mol Biol. 1986;47:31–112. doi: 10.1016/0079-6107(86)90005-2. [DOI] [PubMed] [Google Scholar]
- 6.Neidle S, Pearl LH, Shelly JV. Biochem J. 1987;243:1–13. doi: 10.1042/bj2430001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tanious FA, Spychala J, Kumar A, Greene K, Boykin DW, Wilson WD. J Biomol Structure Dynamics. 1994;11:1063–1076. doi: 10.1080/07391102.1994.10508053. [DOI] [PubMed] [Google Scholar]
- 8.Cory M, Tidwell RR, Fairley T. J Med Chem. 1992;35:431–438. doi: 10.1021/jm00081a003. [DOI] [PubMed] [Google Scholar]
- 9.Wilson WD, Ratmeyer L, Zhao M, Strekowski L, Boykin DW. Biochemistry. 1993;32:4098–4104. doi: 10.1021/bi00066a035. [DOI] [PubMed] [Google Scholar]
- 10.Spychala J, Boykin DW, Wilson WD, et al. Eur J Med Chem. 1994;29:363–367. doi: 10.1016/0223-5234(94)90061-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Krohnke F, Zecher W. Angew Chem Int Ed Engl. 1962;1:626–632. [Google Scholar]
- 12.Campagne F, Carosti A, Casini G. Tetrahedron Lett 1813-1816. 1977 [Google Scholar]
- 13.Oxley P, Short WF. J Chem Soc 147-152. 1946 [Google Scholar]
- 14.Tilley JW, Zawoiski S. J Org Chem. 1988;53:386–390. [Google Scholar]
- 15.Komatsu M, Takamatsu S, Uesaka M, Yamamoto S, Ohshiro Y, Agawa T. J Org Chem. 1984;49:2691–2699. [Google Scholar]
- 16.Biere H, Schröder E, Ahrens H, Kapp JF, Böttcher I. Eur J Med Chem. 1982;17:27–34. [Google Scholar]
- 17.Chalk AJ. Tetrahedron. 1974;30:1387–1391. [Google Scholar]
- 18.Dufraisse C, Dequesnes A. Bull Soc Chim Fr. 1931;49:1880–1882. [Google Scholar]
- 19.Campbell ID, Eglinton G. Org Syn Coll. 1973;5:517–520. [Google Scholar]
- 20.Veal JM, Wilson WD. J Biomol Structure Dynamics. 1991;6:1119–1145. doi: 10.1080/07391102.1991.10507875. [DOI] [PubMed] [Google Scholar]
- 21.Dixon DW, Kim MS, Kumar V, Obara G, Marzilli W, Schinazi RF. Antiviral Chem Chemother. 1992;3:279–283. [Google Scholar]
- 22.Dykstra CC, Tidwell RR. J Protozool. 1991;38:78S–81S. [PubMed] [Google Scholar]
- 23.Dykstra CC, McClemon DR, Elwell LP, Tidwell RR. Antimicrob Agents Chemother. 1994;38:1890–1898. doi: 10.1128/aac.38.9.1890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tidwell RR, Jones SK, Naimen NA, et al. Antimicrob Agents Chemother. 1993;37:1713–1716. doi: 10.1128/aac.37.8.1713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bell CA, Dykstra CC, Naimen NA, Cory M, Fairley TA, Tidwell RR. Antimicrob Agents Chemother. 1993;37:2668–2673. doi: 10.1128/aac.37.12.2668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bell CA, Cory M, Fairley TA, Hall JE, Tidwell RR. Antimicrob Agents Chemother. 1991;35:1099–1107. doi: 10.1128/aac.35.6.1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tidwell RR, Jones SK, Naimen NA, et al. Antimicrob Agents Chemother. 1993;37:1713–1716. doi: 10.1128/aac.37.8.1713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Babcock SH, Nakamura FI, Fuson RC. J Am Chem Soc. 1932;54:4407–4409. [Google Scholar]
- 29.Knott EB. J Chem Soc. 1947:1190–1195. [Google Scholar]
- 30.Plastino E, Loprieno N, Bugian A, Tenerini J. 1962 Italian patent 637.371.; Chem Abst. 1963;60:P479c. [Google Scholar]
- 31.Lehn JM, Riddell FG, Price BJ, Sutherland IO. J Chem Soc (B) 1967:387–390. [Google Scholar]
- 32.Jones RAY, Katritzky AR, Snarey M. J Chem Soc (B) 1970:135–138. [Google Scholar]
- 33.Uchida A, Nakazawa T, Kondo I, Iwata N, Matsuda S. J Org Chem. 1972;37:3749–3750. [Google Scholar]